- 1Department of Stomatology, Shengli Oilfield Central Hospital, Dongying, China
- 2Department of Gynaecology, Shengli Oilfield Central Hospital, Dongying, China
- 3Department of Stomatology, The Affiliated Hospital of Qingdao University, Qingdao, China
Objective: Progesterone (PG) is an important sex steroid hormone commonly administered to protect the endometrium in perimenopausal women. The present study aimed to explore differential responses of periodontitis to PG in perimenopausal women who did or did not undergo scaling and root planing (SRP).
Methods: A total of 129 perimenopausal women with mild-to-moderate periodontitis were enrolled and underwent treatment as follows: SRP (n = 35); SRP + PG (n = 34); PG (n = 31); and no treatment (s) (n = 29). Pocket probing depth (PPD), clinical attachment level (CAL), sulcus bleeding index (SBI), and bleeding on probing (BOP) were measured using periodontal probes. Three inflammatory markers, including C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-α) in gingival crevicular fluid (GCF) were measured using ELISA techniques.
Results: PPD, CAL, SBI, BOP, and levels of inflammatory factors in GCF were all significantly decreased in perimenopausal women with periodontitis after SRP. In patients who did not undergo SRP, 6 months of PG treatment significantly elevated PPD, SBI, BOP, and GCF levels of CRP, IL-6, and TNF-α. In contrast, PG exhibited inhibitory effects on periodontal inflammation in patients who underwent SRP, evidenced by significantly decreased BOP and IL-6, and slightly decreased SBI, CRP, and TNF-α. PG-induced changes dissipated 6 months after withdrawal of PG (at 12 months).
Conclusions: Among perimenopausal women with periodontitis, PG enhanced periodontal inflammation in the absence of SRP but inhibited periodontal inflammation in those who underwent SRP.
Introductions
Periodontitis is a common inflammatory disease characterized by progressive destruction of the periodontal ligament and alveolar bone (1). Globally, the total prevalence of periodontitis has increased by 99.0% (2), and the age-standardized prevalence of severe periodontitis increased by 8.44% from 1990 to 2019 (3). The etiology of periodontitis is multifactorial and various risk factors have been identified, including old age, smoking, and diabetes. Mental disorders, drug abuse, poor dental care, and unhealthy dietary and lifestyle habits are also associated with periodontitis (4). Dynamic interactions between specific bacterial pathogens and the host inflammatory and immune responses are involved in the pathogenesis of periodontitis (5). Clinically, the principle of treating periodontitis is primarily to block the inflammatory process by controlling infection (6). Home care, and scaling and root planing (SRP) are the primary nonsurgical strategies, and regular long-term supportive therapy is crucial for good outcomes (7). If not treated appropriately and/or, in a timely manner, periodontitis may ultimately lead to tooth loss, thus greatly reducing quality of life (7, 8).
Because the periodontium is a target tissue for sex hormones, hormone-related events in females, such as pregnancy and menopause, are also important factors involved in the occurrence and progression of periodontitis (9–11). Perimenopause represents a transitional period of fluctuation(s) in sex hormone levels, indicating the initiation of reproductive senescence (12). Obvious physiological, emotional, and psychological changes occur in women during the perimenopausal period, along with a series of other problematic symptoms (13, 14). Notably, periodontal diseases, including periodontitis, tend to occur during the perimenopausal period because changes in sex hormone levels significantly influence the inflammatory response, bone resorption, and bone mineral density (15, 16). Gil-Montoya et al. reported that moderate or severe periodontitis presents in 52.6% of perimenopausal women, and that low bone mineral density in females > 58 years of age is positively associated with the onset of periodontitis (10). Chandra et al. reported that the perimenopausal-postmenopausal transition leads to an increasing oxidative stress in periodontal tissues and gingival crevicular fluid (GCF) (17). Manual intervention during perimenopause, therefore, is necessary to reduce the risk for periodontitis.
Estrogen and progesterone (PG) are the two most important sex hormones in females. During the normal menstrual cycle, PG physiologically antagonizes the effects of estrogen (18, 19). However, the production of PG is limited due to the absence of corpus luteum in the perimenopausal period (20). Without sufficient PG, estrogen may stimulate endometrial proliferation, leading to endometrial hyperplasia and carcinoma (21). Thus, PG is commonly administered to protect the endometrium in perimenopausal women. Interestingly, evidence has supported multiple roles for PG in the periodontal environment. PG can promote bone formation, exhibiting potential for the prevention and treatment of menopause-related osteoporosis (22). However, PG enhances chronic reactions by acting as an immunosuppressant in gingival tissues, resulting in exaggerated inflammation (23–25). Nevertheless, the specific role of PG in periodontitis among perimenopausal women has rarely been reported.
In the present study, perimenopausal women with periodontitis who were treated with SRP and/or PG (6 months) were enrolled. Differential responses of periodontitis to PG were evaluated in patients who did or did not undergone SRP. The study aimed to reveal the differential effects of routine PG intervention on the progression of periodontitis among perimenopausal women, in the presence or absence of SRP.
Methods
Subjects
This was a prospective cohort study. A total of 76 perimenopausal women (45–55 years of age) with mild-to-moderate chronic periodontitis and requiring PG were screened from the Department of Gynecology, Shengli Oilfield Central Hospital (Dongying, Shandong, China) between January 2021 and January 2022. Correspondingly, 79 perimenopausal women with mild-to-moderate chronic periodontitis who did not receive PG were recruited from the Physical Examination Center of the authors’ hospital in the same period. Periodontitis was diagnosed according to the Classification of Periodontal and Peri-Implant Diseases and Conditions described in 2017 (26). Inclusion criteria were as follows: fulfill the diagnostic criteria for mild-to-moderate chronic periodontitis; ≥ 20 teeth in the mouth; and 45–55 years of age at the perimenopausal period. Individuals with a history of periodontal therapy within 1 year before enrollment, medication history of antibiotics and/or immunosuppressants within 6 months, systemic and/or infectious diseases, and/or smoking history were excluded from this study. During the one-year study period, 5 patients were lost to follow-up. Ultimately, 129 patients were grouped as follows: SRP combined with 6 months of PG (SRP + PG [n = 34]); 6 months of PG (Control + PG [n = 31]); SRP (n = 35); and no treatment (Control [n = 29]). A flowchart illustrating the study design is presented in Figure 1. The present study was approved by the Ethics Committee of Shengli Oilfield Central Hospital in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients (Q/ZXYY-ZY-YWB-LL201819).
Treatments
For 2 weeks before the first periodontal examination and, until the end of this study, all participants were uniformly instructed to brush their teeth using a soft toothbrush twice per day in accordance with the BASS Technique. SRP was performed immediately after the periodontal examinations at baseline and at 6 and 12 months. As a personalized strategy based on clinical characteristics, risk factors, and status of oral plaque, supportive periodontal therapy was maintained for at least 1 year, aimed to prevent periodontal reinfection and progression of periodontitis, avoid tooth loss, and timely detection and treatment of other oral disorders. For perimenopausal patients who required PG supplementation, dihydroxyprogesterone tablet (10 mg) was taken orally after the first periodontal examination. The PG intervention began on the 15th day of menstruation every month and lasted for 6 months, twice per day, for 10 days per month.
Periodontal examination
Periodontal examinations were performed at baseline and at 6 and 12 months. Periodontal parameters, including pocket probing depth (PPD), clinical attachment level (CAL), sulcus bleeding index (SBI), and bleeding on probing (BOP), were measured using UNC 15 periodontal probes (Hu-Friedy, Chicago, IL, USA) in each tooth at six sites (mesio-, mid-, disto-buccal, and mesio-, mid-, disto-lingual points). PPD represents the distance from the gingival margin to the bottom of the gingival pocket, and CAL represents the distance from the bottom of the gingival pocket to the cementoenamel junction. SBI is scored according to six grades described by Muhlemann and Son in 1971 (27). BOP represents bleeding rate of each tooth after probing. Periodontal parameters were blindly examined by two senior stomatologists.
Measurement of inflammatory markers
Inflammatory markers were measured 1 week before the first periodontal examination and 1 week after the reexaminations (6 and 12 months). GCF samples were collected using paper strips from 6 target teeth (11, 16, 26, 31, 36, and 46) at the mesial, buccal, lingual, and distal sites. The levels of CRP, IL-6, and TNF-α in GCF were measured using commercially available ELISA kits (ThermoFisher Scientific, Waltham, MA, USA) in accordance with manufacturer’s instructions.
Statistical analysis
PPD was used as the primary variable to calculate the sample size (24). To achieve 90% power with a 95% confidence interval (α = 0.05), the minimum sample size was 11. Statistical analyses were performed using SPSS version 21.0 (IBM Corporation, Armonk, NY, USA). Continuous data with normal distribution are expressed as mean ± standard deviation. Differences among multiple groups were analyzed using two-way analysis of variance and Tukey’s test for pairwise comparisons. Differences with P < 0.05 were considered to be statistically significant.
Results
Effects of PG on periodontal destruction in women with periodontitis who did or did not undergo SRP
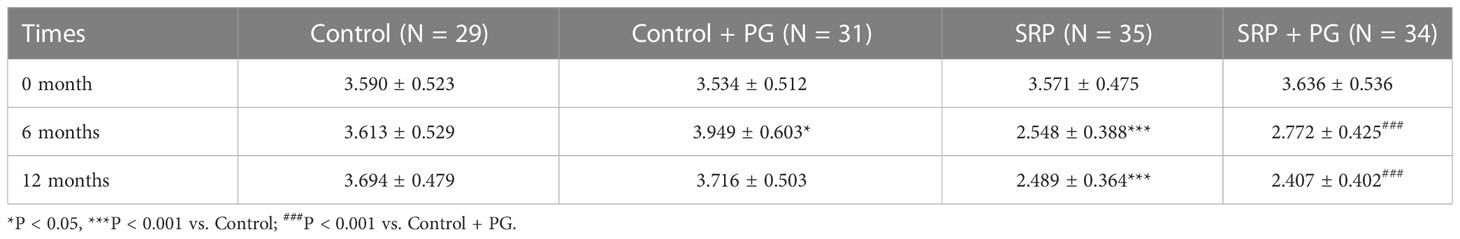
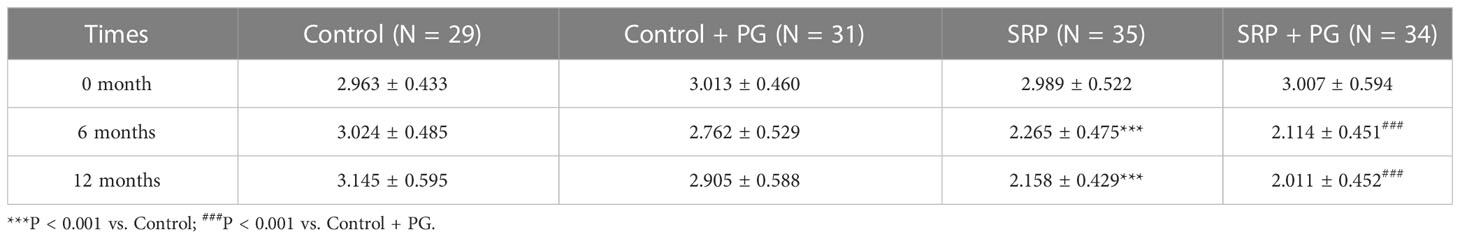
Two key periodontal parameters—PPD and CAL—were used to evaluate periodontal destruction among different groups of women with periodontitis. As shown in Table 1, PPD was significantly lower in the SRP group than in the control group, and was also significantly lower in the SRP + PG group than in the control + PG group at 6 and 12 months (P < 0.001). In control patients who did not undergo SRP, PPD was significantly increased after a 6-month intervention with PG (P < 0.05). However, PPD partially decreased 6 months after withdrawal of PG and was not significantly different between the control and control + PG groups at 12 months. In patients who underwent SRP, PPD was slightly increased after 6 months of PG, although the difference was not statistically significant. At 12 months, PPD in the SRP + PG group decreased to a level similar to that of the SRP group. Consistent with PPD, CAL was significantly decreased by SRP with or without PG (P < 0.001). Additionally, intervention with PG slightly decreased CAL among patients, regardless of whether they underwent SRP, at 6 and 12 months; however, the differences were not statistically significant (Table 2).
Effects of PG on periodontal inflammation among women with periodontitis who did or did not undergo SRP
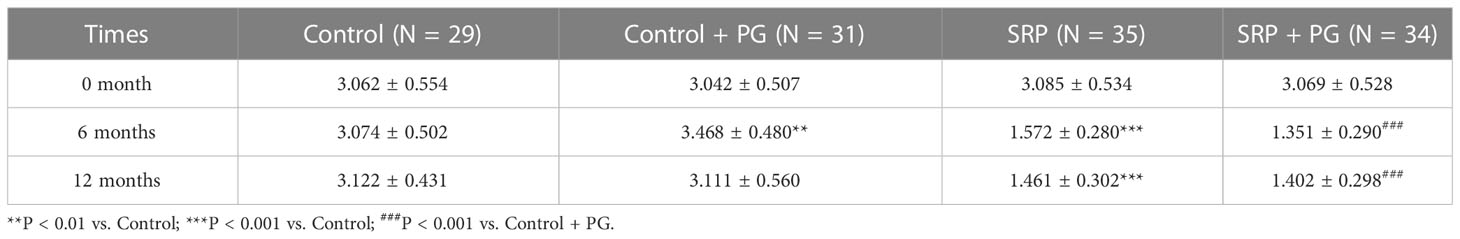
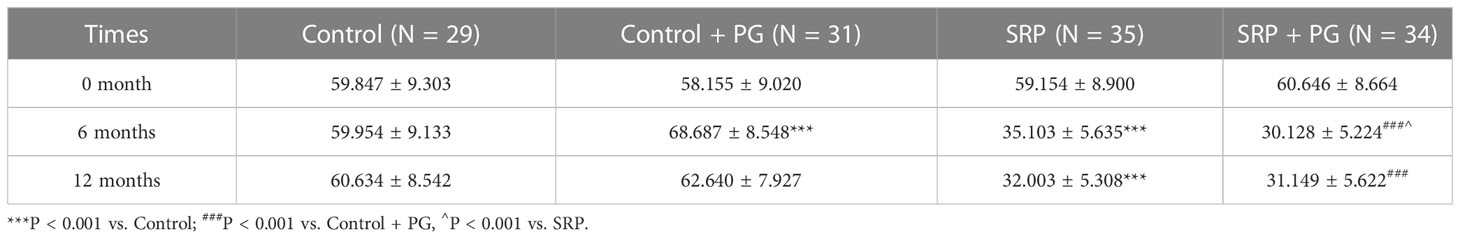
SBI and BOP, two important indicators of periodontal inflammation, were evaluated. In the presence or absence of PG, both SBI and BOP were significantly reduced by SRP in women with periodontitis at 6 and 12 months (P < 0.001). Intervention with PG for 6 months significantly increased SBI and BOP in control patients who did not undergo SRP (P < 0.01). Six months after withdrawal of PG (at 12 months), SBI and BOP in the control + PG group decreased to levels similar to those of the control group. In contrast, PG treatment for 6 months significantly decreased BOP (P < 0.05) and slightly decreased SBI in patients who underwent SRP. No significant differences were observed in BOP and SBI between the SRP and SRP + PG groups at 12 months (Tables 3, 4).
Effects of PG on inflammatory indictors in women with periodontitis who did or did not undergo SRP
To further identify the differential effects of PG on periodontal inflammation in the presence or absence of SRP, the levels of three inflammatory indicators in GCF, including CRP, IL-6, and TNF-α, were measured. As shown in Figures 2A–C, GCF levels of CRP, IL-6, and TNF-α were significantly decreased by SRP with or without PG (P < 0.001). In control patients who did not undergo SRP, the levels of CRP, IL-6, and TNF-α were significantly increased after 6 months of PG treatment (P < 0.05). However, these indicators decreased 6 months after withdrawal of PG (at 12 months) and were not significantly different between the SRP and SRP + PG groups. In contrast with control patients, intervention with PG for 6 months significantly decreased IL-6 level (P < 0.05), and slightly decreased CRP and TNF-α levels in patients who underwent SRP. Six months after withdrawal of PG, none of these indicators were significantly different between the SRP and SRP + PG groups.

Figure 2 GCF levels of CRP (A), IL-6 (B), and TNF-α (C) in women with periodontitis undergoing SRP and/or PG (6 months). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Control; ###P < 0.001 vs. Control + PG; ^P < 0.05 vs. SRP.
Discussion
PG is a physiological partner of estrogen and is widely administered for a series of indications in females, such as assisted reproductive technology, anovulatory menstrual cycle, contraception during lactation, and postmenopausal endometrial hyperplasia (28). PG is commonly used to antagonize the adverse effects of estrogen (18, 19, 21). In this study, responses of the periodontal environment to PG were evaluated in perimenopausal women with periodontitis. Results demonstrated that a 6-month intervention with PG significantly increased PPD, SBI, and BOP. These results indicate an adverse role of PG in the periodontal environment, which is similar to previous findings in pregnant women. For example, Taani et al. found higher gingival index and PPD in pregnant women with high PG level than in non-pregnant women (29). Raga et al. revealed that decreasing PG after delivery is associated with a reduction in BOP, PPD, and CAL in pregnant women without periodontal treatment (30). Gil et al. reported decreased PG, BOP, and PPD levels in pregnant women after childbirth (31). Potential mechanisms underlying the adverse effects of PG on the periodontium are twofold. First, PG induces the hyperproliferation of periodontal ligament cells, contributing to gingival hyperplasia (32, 33). Second, PG exacerbates inflammation initiated by dental plaque (23). In addition, we found that CAL tended to decrease with PG by the intervention, although the difference was not significant. This phenomenon may be explained by the fact that PG inhibits alveolar bone loss in patients with periodontitis. PG is a “link” between the ovaries and bone in women. More specifically, PG level is positively associated with bone formation and negatively associated with bone resorption in perimenopausal women (34). Therefore, the protective effects of PG on the alveolar bone may offset its adverse effects on the periodontium. In addition, results of this study revealed that the effects of PG on the above periodontal parameters disappeared 6 months after withdrawal of PG. This phenomenon indicates the effects of PG on periodontitis are instantaneous.
SRP is a common nonsurgical strategy to clean subgingival biofilms and is indisputably effective for the treatment of periodontitis (35, 36). In this study, SRP significantly decreased PPD, CAL, SBI, and BOP, demonstrating its efficacy in improving periodontitis in perimenopausal women. Interestingly, we found that CAL and SBI were slightly decreased, and BOP was significantly decreased after 6 months of PG treatment in patients who underwent SRP. These results indicate that PG plays an anti-inflammatory role in the periodontal environment after SRP. These findings are consistent with those of previous studies investigating many other disorders. For example, PG inhibits inflammation, neovascularization, and neurogenesis during endometriosis (37). PG inhibits airway inflammation by synergistically interacting with glucocorticoids (38). PG suppresses inflammation and apoptosis to protect the brain from hypoxic-ischemic damage (39). Notably, the anti-inflammatory effect of PG in the presence of SRP was contrary to its pro-inflammatory effect in the absence of SRP. This difference may be attributed to the status of dental plaque. Dental plaque is a diverse microbial community present on the tooth surface, and its accumulation is a leading risk factor for the onset of periodontitis (40). Wu et al. reported that PG exacerbates periodontal inflammation initiated by dental plaque (23). Therefore, when dental plaque is cleared by SRP, PG may inhibit inflammation. In addition, after SRP treatment, we found a slight elevation in PPD in patients treated with PG, although the difference was not significant. This result is similar to findings in patients who did not undergo SRP and indicates that the promoting effect of PG on gingival hyperplasia persists after SRP.
In the mouth, various immune regulators are involved in the defense response against pathogenic bacteria, including pro-inflammatory cytokines, matrix metalloproteinases, and reactive oxygen species, all of which contribute to periodontal damage (41). IL-6 and TNF-α are common inflammatory cytokines that are elevated in patients with periodontitis (42). Our previous study found that 3 months of PG treatment elevates GCF levels of IL-6 and TNF-α in women with periodontitis (43). Accordingly, this study also revealed that IL-6 and TNF-α levels were increased by 6 months of treatment with PG in patients who did not undergo SRP. As the mainstay of periodontal therapy, SRP is associated with reduced GCF levels of inflammatory cytokines (44). In this study, SRP significantly decreased the GCF levels of IL-6 and TNF-α, which supports the anti-inflammatory effect of SRP in periodontitis. Of note, a significant decrease in IL-6 level and a slight decrease in TNF-α level were induced by PG in patients who underwent SRP. These results indicate that PG exerts an anti-inflammatory effect in the presence of SRP, which is different from its pro-inflammatory effect in the absence of SRP. These findings also support changes in SBI and BOP in patients with periodontitis, demonstrating that PG plays a “double-edged” role in periodontal inflammation by regulating inflammatory cytokines. In addition, CRP is an acute-phase protein produced in response to infection or damage and an inflammatory marker (45). Elevated CRP level is closely associated with the onset of periodontitis (46). Evidence has demonstrated that SRP is effective in reducing CRP level (47), which is consistent with results obtained in this study. In addition, Gil et al. reported that CRP level increases during pregnancy with high PG level, which is positively correlated with BOP (31). Consistent with BOP, CRP level also significantly increased 6 months after PG in patients who did not undergo SRP. In contrast, CRP was slightly decreased by PG in patients who underwent SRP. Therefore, CRP also exhibits different responses to PG in perimenopausal women with periodontitis in the presence or absence of SRP.
Conclusion
SRP relieved periodontitis in perimenopausal women. Intervention with PG instantaneously enhanced periodontal inflammation in the absence of SRP but inhibited it in the presence of SRP. Active SRP is recommended for perimenopausal women with periodontitis, and PG can further enhance the inhibitory effect of SRP on inflammation. However, this study is limited by an insufficient sample size. As such, the mechanisms of action of PG in periodontitis related to dental plaque need to be further explored.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shengli Oilfield Central Hospital (Q/ZXYY-ZY-YWBLL-201819). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design: SY, YM, FN, and JQ; Data collection: SY, YM, LC, FN, and JQ; Data analysis and interpretation: SY, YM, and JL; Drafting the manuscript: SY and YM; Revising the manuscript critically for important intellectual content: FN and JQ; Funding acquisition: YM; All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Shandong Medical and Health Science and Technology Development Plan Project (202208010497).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hirtz C, O'Flynn R, Voisin PM, Deville de Periere D, Lehmann S, Guedes S, et al. The potential impact of salivary peptides in periodontitis. Crit Rev Clin Lab Sci (2021) 58(7):479–92. doi: 10.1080/10408363.2021.1907298
2. Wu L, Zhang SQ, Zhao L, Ren ZH, Hu CY. Global, regional, and national burden of periodontitis from 1990 to 2019: Results from the Global Burden of Disease study 2019. J Periodontol (2022) 93(10):1445–54. doi: 10.1002/JPER.21-0469
3. Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990-2019: An analysis of the Global Burden of Disease Study 2019. J Clin Periodontol (2021) 48(9):1165–88. doi: 10.1111/jcpe.13506
4. Darby I. Risk factors for periodontitis & peri-implantitis. Periodontol 2000. (2022) 90(1):9–12. doi: 10.1111/prd.12447
5. Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. (2017) 75(1):7–23. doi: 10.1111/prd.12221
6. Nunez J, Vignoletti F, Caffesse RG, Sanz M. Cellular therapy in periodontal regeneration. Periodontol 2000. (2019) 79(1):107–16. doi: 10.1111/prd.12250
7. Kwon T, Lamster IB, Levin L. Current Concepts in the Management of Periodontitis. Int Dent J (2021) 71(6):462–76. doi: 10.1111/idj.12630
8. Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet (2019) 394(10194):249–60. doi: 10.1016/S0140-6736(19)31146-8
9. Sharma N, Sharma RK, Tewari S, Chauhan M, Bhatia A. Association of periodontal inflammation, systemic inflammation, and duration of menopausal years in postmenopausal women. Quintessence Int (2018) 49(2):123–31. doi: 10.3290/j.qi.a39512
10. Gil-Montoya JA, Garrido-Martinez M, Barrios-Rodriguez R, Ramos-Garcia P, Lenouvel D, Montes-Castillo C, et al. Association between low bone mineral density and periodontitis in generally healthy perimenopausal women. J Periodontol (2021) 92(1):95–103. doi: 10.1002/JPER.20-0029
11. Romandini M, Shin HS, Romandini P, Lafori A, Cordaro M. Hormone-related events and periodontitis in women. J Clin Periodontol (2020) 47(4):429–41. doi: 10.1111/jcpe.13248
12. Bacon ER, Mishra A, Wang Y, Desai MK, Yin F, Brinton RD. Neuroendocrine aging precedes perimenopause and is regulated by DNA methylation. Neurobiol Aging. (2019) 74:213–24. doi: 10.1016/j.neurobiolaging.2018.09.029
13. Troia L, Martone S, Morgante G, Luisi S. Management of perimenopause disorders: hormonal treatment. Gynecol Endocrinol (2021) 37(3):195–200. doi: 10.1080/09513590.2020.1852544
14. Johnson A, Roberts L, Elkins G. Complementary and Alternative Medicine for Menopause. J Evid Based Integr Med (2019) 24:2515690X19829380. doi: 10.1177/2515690X19829380
15. Shu L, Guan SM, Fu SM, Guo T, Cao M, Ding Y. Estrogen modulates cytokine expression in human periodontal ligament cells. J Dent Res (2008) 87(2):142–7. doi: 10.1177/154405910808700214
16. Li L, Wang Z. Ovarian Aging and Osteoporosis. Adv Exp Med Biol (2018) 1086:199–215. doi: 10.1007/978-981-13-1117-8_13
17. Chandra RV, Sailaja S, Reddy AA. Estimation of tissue and crevicular fluid oxidative stress marker in premenopausal, perimenopausal and postmenopausal women with chronic periodontitis. Gerodontology (2017) 34(3):382–9. doi: 10.1111/ger.12279
18. Gompel A. Progesterone, progestins and the endometrium in perimenopause and in menopausal hormone therapy. Climacteric (2018) 21(4):321–5. doi: 10.1080/13697137.2018.1446932
19. Parry BL. Optimal management of perimenopausal depression. Int J Womens Health (2010) 2:143–51. doi: 10.2147/IJWH.S7155
20. Nicula R, Costin N. Management of endometrial modifications in perimenopausal women. Clujul Med (2015) 88(2):101–10. doi: 10.15386/cjmed-421
21. Vaidya S, Lakhey M, Sharma PK, Hirachand S, Lama S, Kc S. Histopathological pattern of abnormal uterine bleeding in endometrial biopsies. Nepal Med Coll J (2013) 15(1):74–7.
22. Prior JC. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric (2018) 21(4):366–74. doi: 10.1080/13697137.2018.1467400
23. Wu M, Chen SW, Jiang SY. Relationship between gingival inflammation and pregnancy. Mediators Inflamm (2015) 2015:623427. doi: 10.1155/2015/623427
24. Man Y, Sun L, Qin J, Zhang X, Yan S, Niu F. Exogenous progesterone short-termly affects the periodontal environment in perimenopausal women. Oral Dis (2022) 29(4):1795–801. doi: 10.1111/odi.14133
25. Ojanotko-Harri AO, Harri MP, Hurttia HM, Sewon LA. Altered tissue metabolism of progesterone in pregnancy gingivitis and granuloma. J Clin Periodontol (1991) 18(4):262–6. doi: 10.1111/j.1600-051X.1991.tb00425.x
26. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol (2018) 89(Suppl 1):S159–S72. doi: 10.1002/JPER.18-0006
27. Muhlemann HR, Son S. Gingival sulcus bleeding–a leading symptom in initial gingivitis. Helv Odontol Acta (1971) 15(2):107–13.
28. Sitruk-Ware R. Non-clinical studies of progesterone. Climacteric (2018) 21(4):315–20. doi: 10.1080/13697137.2018.1463982
29. Taani DQ, Habashneh R, Hammad MM, Batieha A. The periodontal status of pregnant women and its relationship with socio-demographic and clinical variables. J Oral Rehabil. (2003) 30(4):440–5. doi: 10.1046/j.1365-2842.2003.01058.x
30. Raga LG, Minguez I, Caffesse R, Llambes F. Changes in Periodontal Parameters and C-Reactive Protein After Pregnancy. J Periodontol (2016) 87(12):1388–95. doi: 10.1902/jop.2016.160093
31. Gil L, Minguez I, Caffesse R, Llambes F. Periodontal Disease in Pregnancy: The Influence of General Factors and Inflammatory Mediators. Oral Health Prev Dent. (2019) 17(1):69–73. doi: 10.3290/j.ohpd.a41981
32. Gungormus M, Akgul HM, Yilmaz AB, Dagistanli S, Erciyas K. Generalized gingival hyperplasia occurring during pregnancy. J Int Med Res (2002) 30(3):353–5. doi: 10.1177/147323000203000320
33. Yuan G, Cai C, Dai J, Liu Y, Zhang R, Dai Y, et al. Progesterone modulates the proliferation and differentiation of human periodontal ligament cells. Calcif Tissue Int (2010) 87(2):158–67. doi: 10.1007/s00223-010-9377-9
34. Seifert-Klauss V, Schmidmayr M, Hobmaier E, Wimmer T. Progesterone and bone: a closer link than previously realized. Climacteric (2012) 15 Suppl 1:26–31. doi: 10.3109/13697137.2012.669530
35. Herrera D. Scaling and Root Planning is Recommended in the Nonsurgical Treatment of Chronic Periodontitis. J Evid Based Dent Pract (2016) 16(1):56–8. doi: 10.1016/j.jebdp.2016.01.005
36. Elashiry M, Morandini AC, Cornelius Timothius CJ, Ghaly M, Cutler CW. Selective Antimicrobial Therapies for Periodontitis: Win the "Battle and the War". Int J Mol Sci (2021) 22(12):6459. doi: 10.3390/ijms22126459
37. Reis FM, Coutinho LM, Vannuccini S, Batteux F, Chapron C, Petraglia F. Progesterone receptor ligands for the treatment of endometriosis: the mechanisms behind therapeutic success and failure. Hum Reprod Update. (2020) 26(4):565–85. doi: 10.1093/humupd/dmaa009
38. Fei X, Bao W, Zhang P, Zhang X, Zhang G, Zhang Y, et al. Inhalation of progesterone inhibits chronic airway inflammation of mice exposed to ozone. Mol Immunol (2017) 85:174–84. doi: 10.1016/j.molimm.2017.02.006
39. Li X, Zhang J, Zhu X, Wang P, Wang X, Li D. Progesterone reduces inflammation and apoptosis in neonatal rats with hypoxic ischemic brain damage through the PI3K/Akt pathway. Int J Clin Exp Med (2015) 8(5):8197–203.
40. Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque-induced gingival conditions. J Periodontol (2018) 89 Suppl 1:S17–27. doi: 10.1002/JPER.17-0095
41. Sczepanik FSC, Grossi ML, Casati M, Goldberg M, Glogauer M, Fine N, et al. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontol 2000. (2020) 84(1):45–68. doi: 10.1111/prd.12342
42. Cardoso EM, Reis C, Manzanares-Cespedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med (2018) 130(1):98–104. doi: 10.1080/00325481.2018.1396876
43. Man Y, Sun L, Qin J, Zhang X, Yan S, Niu F. Exogenous progesterone short-termly affects the periodontal environment in perimenopausal women. Oral Dis (2023) 29(4):1795–801. doi: 10.1111/odi.14133
44. Komatsu S, Oshikiri S, Nagano T, Yashima A, Matsushima Y, Shirakawa S, et al. Effects of One-Stage Full-Mouth Scaling and Root Planing with Azithromycin on Diabetes and Periodontal Disease: A Randomized Controlled Trial. Antibiotics (Basel) (2022) 11(9):1266. doi: 10.3390/antibiotics11091266
45. Machado V, Botelho J, Escalda C, Hussain SB, Luthra S, Mascarenhas P, et al. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front Immunol (2021) 12:706432. doi: 10.3389/fimmu.2021.706432
46. Bansal T, Pandey A DD, Asthana AK. C-Reactive Protein (CRP) and its Association with Periodontal Disease: A Brief Review. J Clin Diagn Res (2014) 8(7):ZE21–4. doi: 10.7860/JCDR/2014/8355.4646
47. Mohan M, Jhingran R, Bains VK, Gupta V, Madan R, Rizvi I, et al. Impact of scaling and root planing on C-reactive protein levels in gingival crevicular fluid and serum in chronic periodontitis patients with or without diabetes mellitus. J Periodontal Implant Sci (2014) 44(4):158–68. doi: 10.5051/jpis.2014.44.4.158
Keywords: progesterone, perimenopause, periodontitis, inflammation, scaling and root planning
Citation: Yan S, Man Y, Lu J, Cui L, Niu F and Qin J (2023) The “double-edged” role of progesterone in periodontitis among perimenopausal women undergoing or not undergoing scaling and root planing. Front. Endocrinol. 14:1224763. doi: 10.3389/fendo.2023.1224763
Received: 18 May 2023; Accepted: 26 July 2023;
Published: 14 August 2023.
Edited by:
Yunzhi Lin, First Affiliated Hospital of Fujian Medical University, ChinaReviewed by:
Sumanth Gunupati, Narayana Dental College and Hospital, IndiaGirish Suragimath, Krishna Institute of Medical Sciences Deemed University, India
Copyright © 2023 Yan, Man, Lu, Cui, Niu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyong Qin, cWluankxMDIzQDE2My5jb20=; Feifei Niu, c2x5dG5mZkAxMjYuY29t
†These authors have contributed equally to this work
 Shengjie Yan1†
Shengjie Yan1† Feifei Niu
Feifei Niu