- 1Department of Endocrinology, Xiyuan Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 2Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Shandong First Medical University, Jinan, China
Aim: This study aims to assess the association between sodium–glucose cotransporter type-2 inhibitor (SGLT-2i) treatment and muscle atrophy in patients with type 2 diabetes mellitus (T2DM).
Methods: We searched six databases from 1 January 2012 to 1 May 2023, without language restrictions. The primary outcome was muscle. Secondary outcomes were weight loss, weakness, malaise, or fatigue. Subgroup analyses were performed according to different definitions of muscle, treatment duration, and measurement methods. The quality of the studies was assessed using the Cochrane tool. The quality of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tool.
Results: Nineteen randomized controlled trials (RCTs) involving 1,482 participants were included. Compared with the control group, a meta-analysis showed that T2DM participants in the group treated with SGLT-2i demonstrated statistically significant reductions in lean body mass of 0.66 (95% confidence interval (CI), −1.05 to −0.27; p = 0.0009) and skeletal muscle mass of 0.35 (95% CI, −0.66 to −0.04; p = 0.03). No deaths or serious adverse events were reported. The quality of evidence in the included trials was low.
Conclusions: SGLT-2i may lead to a reduction in muscle strength in the treatment of T2DM compared to the control group. However, there is still a lack of high-quality evidence to evaluate muscle atrophy caused by SGLT-2i.
Systematic review registration: https://inplasy.com/inplasy-2022-12-0061/, identifier 2022120061.
Introduction
The global incidence of type 2 diabetes mellitus (T2DM) is very high, and the International Diabetes Federation (IDF) estimates that approximately 463 million people suffered from T2DM in 2019. This number will reach 700 million by the year 2045 (1). T2DM and its complications will contribute substantially to the global rates of death and disability. Commonly used treatments include lifestyle interventions, pharmacological interventions, and bariatric surgery (2).
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) represent a new type of oral hypoglycemic agent (3). The world’s first SGLT-2i, dapagliflozin, was approved in 2012 for treating T2DM in Europe, with nearly 10 types of SGLT-2i in clinical use up to the present (4). The incidence and mortality from T2DM are considered to be mainly related to chronic cardiovascular complications. SGLT-2i lowers blood glucose mainly by promoting urinary glucose excretion and can assist in reducing weight, blood pressure, and cardiovascular and renal risks (5–9). The pathophysiology of the cardioprotective outcome produced by the application of SGLT-2i is not known. Possible mechanisms of action include its role in anti-inflammatory and oxidative stress pathways (10). A recent review summarized animal studies and clinical trials of SGLT-2i analogs, which showed that they have pharmacological effects in the treatment of diabetes by attenuating oxidative stress associated with T2DM, which may explain their cardiovascular benefits and have promising applications in the treatment of T2DM (11). A previous large cardiovascular outcomes trial investigated the efficacy of empagliflozin in 7,020 patients with T2DM and established ASCVD and reported a significant 38% reduction in cardiovascular mortality and a 32% reduction in heart failure. This review not only mentioned empagliflozin but also mentioned the pharmacological effects of other SGLT-2i on diabetes (11). Their pharmacological effects are similar. Standards of Medical Care in Diabetes-2021 stated that among patients with type 2 diabetes who have established atherosclerotic cardiovascular disease (ASCVD) or indicators of high risk, established kidney disease, or heart failure, a SGLT-2i is recommended as part of a glucose-lowering regimen independent of A1C and in consideration of patient-specific factors (12). Additionally, the latest guidelines of the American Diabetes Association (ADA) recommend that SGLT-2i be actively recommended to all patients with T2DM who also have comorbid cardiovascular disease (13). As can be seen from the above, SGLT-2i has gained great importance in the treatment of type 2 diabetes patients with cardiovascular and other diseases.
Symptoms of muscle atrophy and weakness were observed during T2DM treatments with SGLT-2i. Some patients experience improvement or complete regression of these symptoms after discontinuing SGLT-2i (14–17). Additional case reports suggest a potential relationship between SGLT-2i use and myopathy episodes, both of which raise concerns about SGLT-2i-induced muscle atrophy. However, current clinical trials involving the effects of SGLT-2i on muscles have shown inconsistent results. Several clinical trials have shown beneficial or insubstantial effects of SGLT-2i application on muscle (18, 19), whereas others have reached the opposite conclusion (20). Overall, the actual effect of SGLT-2i on muscles is not known.
The aim of this systematic review and meta-analysis was to evaluate the association between SGLT-2i and muscle atrophy when treating patients with T2DM, with the aim of providing a basis for clinical practice and new directions for research in this field.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines (21, 22). The protocol was registered in INPLASY (2022120061). The DOI number is 10.37766/inplasy2022.12.0061.
Search strategy
We searched six databases, including PubMed, Web of Science, Embase, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), and Wan Fang Database, for randomized controlled trials of SGLT-2i for T2DM from 1 January 2012 to 1 May 2023. A detailed search strategy is presented in Supplementary Material 1.
Eligibility criteria
Studies were included in the systematic screening if they met the following criteria: (1) the study was conducted in adults with T2DM; (2) the use of SGLT-2i, including canagliflozin, dapagliflozin, empagliflozin, tofogliflozin, ipragliflozin, luseogliflozin, and so on, had been prescribed to patients with T2DM; (3) single or add-on therapy with SGLT-2i as an intervention, with no restrictions on dosage or frequency of use; (4) data on at least one of the outcome indicators of muscle mass, skeletal muscle mass, fat-free mass, or lean body mass was provided, with information on the mean and standard deviation of the change in the above outcome indicators; and (5) the design was a randomized controlled trial. Studies were excluded if they included (1) pregnant women, (2) adults without diabetes, (3) SGLT-2i treatment prior to the intervention, (4) combination formulations of SGLT-2i in fixed-dosage combinations with other commonly used drugs, (5) an intervention period of less than 4 weeks, (6) a lack of required outcome data, (7) nonrandomized controlled trials, and (8) duplicate reports.
Study selection
The retrieved studies were imported into NoteExpress, and duplicate records were removed. The title, abstract, and full-text screening were independently evaluated by three reviewers (Chengdong Xia, Yufeng Han, and Chunhui Yin). Any disagreements between the three reviewers were resolved through discussion with a fourth reviewer or a senior author.
Data extraction
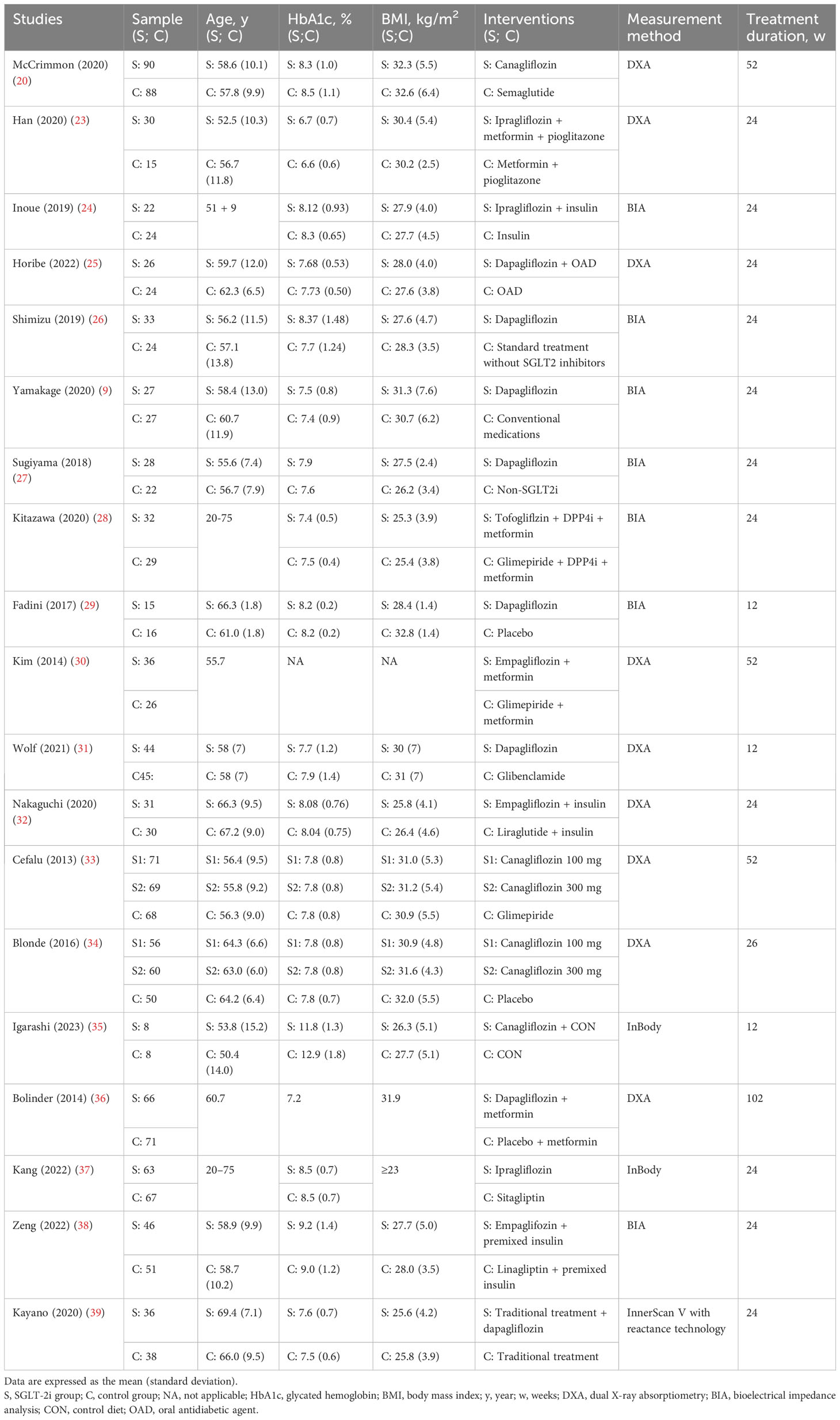
Table 1 lists the trial-level characteristics of the 19 studies, including the first author, year of publication, sample size, baseline characteristics of the participants (age, HbA1c, and body mass index), interventions and controls, treatment duration, and measurement method. The risk factors evaluated in this meta-analysis were the mean and standard deviation of the measures of change before and after the intervention. The definitions were largely similar (40). The data were extracted independently by three authors (Chengdong Xia, Yufeng Han, and Chunhui Yin). Disagreements were resolved through discussion with a fourth reviewer or senior author.
Statistical analysis
We assessed the risk of bias in each included study based on the Cochrane risk-of-bias tool, which consists of the following aspects: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. For each of these aspects, the assessment tool has three options: “low risk of bias,” “unclear risk of bias,” and “high risk of bias.” (41) In addition, we used the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) system to assess the quality of evidence for primary outcomes (42).
Data synthesis
We used Review Manager 5.4 to synthesize the extracted data. Muscle-related outcome indicators were considered continuous variables and analyzed using standardized mean differences (SMD) and 95% confidence intervals (95% CI).
Subgroup analysis and investigation of heterogeneity
Heterogeneity between the studies was assessed using the I2 statistic. When heterogeneity was not significant (I2 < 50%), a fixed-effects model was used to synthesize the data. When heterogeneity was significant (I2 < 50%), a random-effects model was used.
Subgroup analyses were performed according to different definitions of muscle, the SGLT-2i measurement method, and treatment duration. In addition, sensitivity analyses were performed to assess the robustness of the meta-analysis by excluding trials with poor methodological quality (those with insufficient randomization methods and trials with selective reporting bias).
One of the subgroup analyses was performed based on different definitions of muscle: lean body mass, skeletal muscle mass, fat-free mass, and muscle mass (Supplementary Figures 1, 4 in Supplementary Material 2). Lean body mass was defined as body weight without fat minus total bone mass; skeletal muscle mass was defined as lean body mass minus connective tissue, skin, and other organ mass; and fat-free mass was defined as total body weight minus total fat mass (as defined in each original study) (40).
Results
A total of 16,727 studies were identified through our search, and 19 were included (Figure 1).
Study characteristics
In this systematic review, 19 RCTs involving 1,482 participants were included. Three studies (23, 26, 37) were conducted in adults with T2DM combined with nonalcoholic fatty liver disease, and the remaining 16 studies (9, 20, 24, 25, 27–36, 38, 39) were conducted in adults with T2DM. Fourteen studies (9, 23–29, 31, 32, 35, 37–39) had a treatment duration of 24 weeks, whereas the remaining five studies (20, 30, 33, 34, 36) had treatment durations beyond 24 weeks. Eight studies (20, 23, 30–34, 36) used dual X-ray absorptiometry (DXA) to measure lean body mass, six studies (9, 26, 27, 28, 29, 38) used bioelectrical impedance analysis (BIA) to measure skeletal muscle mass, one study (24) used DXA to measure lean body mass and used BIA to measure muscle mass, one study (25) used DXA to measure fat-free mass and used BIA to measure muscle mass, one study (35) used InBody to measure muscle mass, one study (37) used InBody to measure lean body mass, and one study (39) used InnerScan V with reactance technology to measure lean body mass. The sample sizes ranged from 15 to 90 patients in each study.
Risk of bias assessment
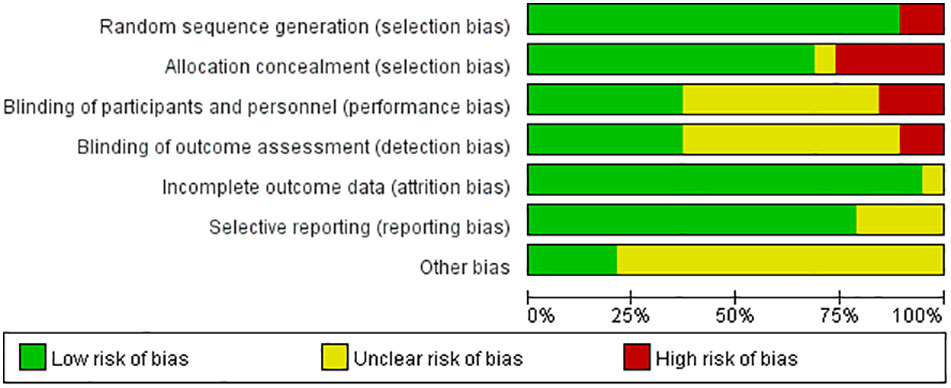
Most studies demonstrated a low or unclear risk of bias, mainly owing to a lack of blinding of the study personnel and participants (8/19, 42.1%), no blinding of the outcome assessment (10/19, 52.6%), and other unclear biases (14/19, 73.7%) (Figure 2).
Effect of SGLT-2i on lean body mass
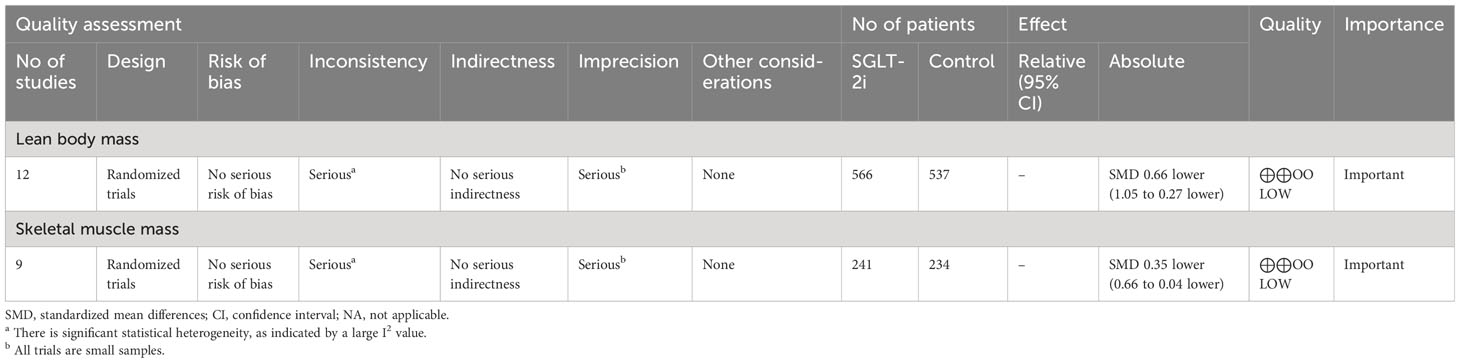
Twelve studies (20, 23–25, 28, 30–34, 36, 37) with 1,103 participants were included to compare the differences in lean body mass between the SGLT-2i and control groups. The meta-analysis results of the random-effects model showed that lean body mass was reduced by 0.66 (95% CI, −1.05, −0.27; p = 0.0009) in the SGLT-2i group compared to the control group (Figure 3A), indicating that SGLT-2i significantly reduced lean body mass in patients with T2DM. The certainty of evidence was low (Table 2). Because the heterogeneity was significant (I2 = 89%), we performed three subgroup analyses based on muscle definition, treatment duration, and measurement method (Supplementary Figures 1, 2 and 3 in Supplementary Material 2). The results showed that lean body mass (SMD = −0.41; 95% CI, −0.77 to −0.04; p = 0.03) or fat-free mass (SMD = −0.70; 95% CI, −1.54 to 0.15; p = 0.11) or muscle mass (SMD = −3.27; 95% CI, −4.22 to −2.32; p < 0.00001) decreased, which indicated that lean body mass and muscle mass showed statistically significant reductions. We also performed subgroup analyses based on the treatment duration. The results showed that treatment duration within 24 weeks (SMD = −0.93; 95% CI, −1.60 to −0.26; p = 0.007) or beyond 24 weeks (SMD = −0.36; 95% CI, −0.80 to 0.07; p = 0.10) reduced lean body mass, and treatment duration within 24 weeks caused a statistically significant reduction in lean body mass. Our subgroup analysis also showed that compared with the control group, the lean body mass in DXA (SMD = −0.67; 95% CI, −1.12 to −0.23; p = 0.003) or BIA (SMD = −1.30; 95% CI, −1.86 to −0.74; p < 0.00001) or InBody (SMD = −0.03; 95% CI, −0.37 to 0.31; p = 0.86) decreased, which indicated that DXA and BIA both showed statistically significant reductions in lean body mass. Removing each study individually failed to alter the results of the meta-analysis.

Figure 3 Meta-analysis of changes in lean body mass (A) and skeletal muscle mass (B) between the SGLT-2i group and the control group.
Effect of SGLT-2i on skeletal muscle mass
Nine studies (9, 24–27, 29, 35, 38, 39) with 475 participants were included to compare differences in skeletal muscle mass between the SGLT-2i and control groups. The meta-analysis results of the random-effects model showed that skeletal muscle mass was reduced by 0.35 (95% CI, −0.66, −0.04; p = 0.03) in the group treated with SGLT-2i for T2DM compared with the control group (Figure 3B). The certainty of evidence was low (Table 2). Because the heterogeneity was significant (I2 = 63%) and all studies including skeletal muscle mass as an outcome metric had a treatment duration of 24 weeks, we performed two subgroup analyses based on different definitions of muscle and measurement methods (Supplementary Figures 4, 5 in Supplementary Material 2). The results of subgroup analyses based on different definitions of muscle showed that skeletal muscle mass (SMD = −0.06; 95% CI, −0.31 to 0.20; p = 0.67) or fat-free mass (SMD = −0.57; 95% CI, −1.29 to 0.15; p = 0.12) or muscle mass (SMD = −0.66; 95% CI, −1.30 to −0.01; p = 0.05) decreased, which indicated the muscle mass showed a statistically significant reduction. Subgroup analyses were performed using this measurement method. The results showed that compared with the control group, the skeletal muscle mass in BIA (SMD = −0.40; 95% CI, −0.79 to −0.01; p = 0.04) or InBody (SMD = −0.15; 95% CI, −0.57 to 0.26; p = 0.46) decreased, which indicated that BIA showed a statistically significant reduction in skeletal muscle mass. Removing each study individually failed to alter the results of the meta-analysis.
Adverse events
The most commonly reported adverse effects in the 19 studies were urinary tract and genital infections; however, these were not serious. No deaths were reported in any of the included studies.
Discussion
Summary of results
We conducted an extensive literature search and identified 19 studies (1,482 participants). Treatment with SGLT-2i in patients with T2DM resulted in statistically significant reductions in lean body mass and skeletal muscle mass compared to the controls. In a more detailed subgroup analysis of different definitions of muscle, meaningful muscle loss occurred only in lean body and muscle mass. In the subgroup analysis, lean body mass showed statistically significant reductions based on DXA and BIA measurements; skeletal muscle mass showed a statistically significant decrease in BIA measurements; and both showed a meaningless decrease in InBody measurements. In the subgroup analysis based on treatment duration, lean body mass significantly decreased at treatment duration within 24 weeks. No sources of heterogeneity were found. There was no evidence that SGLT-2i intervention led to death or serious side effects. However, the quality of evidence in the included trials was moderate to low.
Implications for clinical research
Some studies have shown that SGLT-2i may induce or worsen muscle atrophy in patients with T2DM (14, 43–45). SGLT-2i promotes glucose excretion through urine primarily by inhibiting the reabsorption of glucose from the proximal tubules, thereby lowering blood glucose levels. However, SGLT-2i may increase energy expenditure and hypoxia in the kidney medulla. Hypoxia and a low-glucose environment can stimulate gluconeogenesis in the liver, leading to lipolysis, which reduces body fat mass (8, 46). The underlying mechanism of muscle atrophy in T2DM patients caused by SGLT-2i may be related to the fact that SGLT-2i activates gluconeogenesis and promotes lipolysis, as well as facilitates the breakdown of skeletal muscle proteins into amino acids that are supplied to the liver as substrates. However, the exact mechanism remains debatable. As the balance between vitamin D (Vit-D) and parathyroid hormone (PTH) is considered a key regulator of muscle strength, it is unclear whether SGLT-2i can disrupt this balance and cause muscle atrophy (47). Therefore, it is necessary to further explore its specific mechanisms in clinical studies.
The findings of this meta-analysis indicated that SGLT-2i resulted in statistically significant reductions in lean body mass and skeletal muscle mass. This is nearly identical to the results of a previously published study (48). That study showed meaningful and significant changes in skeletal muscle mass after SGLT-2i treatment, with a meaningless reduction in lean body mass. The latter was confirmed through our analysis. Our analysis demonstrated that following treatment with SGLT-2i, lean body mass was significantly reduced. Compared to a previous study (48), we performed more in-depth subgroup analyses based on muscle definition, measurement methods, and treatment durations. Based on the subgroup analysis, we believe that there is a clinical need to standardize the metrics that reflect muscle gain or loss so that data can be extracted and analyzed more accurately to guide clinical practice. Our data suggest that using DXA or BIA to measure lean body mass or skeletal muscle mass is more accurate than using InBody measurements. It should be noted that there are very few studies using InBody as a measurement method. Muscle loss in patients treated with SGLT-2i within 24 weeks should be closely evaluated. There is significant heterogeneity between the results of different clinical studies. Whether this heterogeneity is related to individual drug characteristics, measurement methods, or treatment cycles remains unknown and deserves further study.
Muscles and bones are closely integrated. SGLT-2i may cause muscle atrophy, which appears to be a potential factor for increased fracture risk (49–51). Researchers have suggested that the fracture risk observed in elderly patients treated with SGLT-2i does not appear to be directly related to its effect on the bone and that the effect of SGLT-2i on bone metabolism and bone turnover may be indirect (52). SGLT-2i-induced hypovolemia and hyponatremia may induce fatigue, weakness, or other psychosomatic and neurological symptoms. Muscle atrophy is a major feature of frailty (45), which can further contribute to debilitation and seriously jeopardize the health and function of the elderly. Such outcomes can lead to increased clinical adverse events such as falls, fractures, and incapacitation, severely affecting their quality of life and increasing the risk of death. Several speculations point to the possibility that fractures related to falls are secondary to factors such as weakness, upright hypotension, or postural dizziness (53, 54). Further studies are necessary to determine whether SGLT-2i-induced muscle atrophy is associated with an increased risk of fracture.
In addition, studies have shown that muscle function can be balanced and enhanced through physical activity and exercise, including resistance training, aerobic training, and whole-body vibrational therapy (55). We suggest that future clinical trials on the relationship between SGLT-2i and muscle function should consider physical exercise and exercise intensity as possible influencing factors.
Implications for clinical practice
SGLT-2i may cause muscle atrophy, and sarcopenia is a serious consequence of muscle atrophy. Therefore, we recommend that clinicians conduct a thorough assessment of patients before drug administration, including physical indicators such as age, weight, body mass index, muscle mass, muscle strength, and muscle fat infiltration (56,) and use SGLT-2i with caution, especially in certain high-risk groups of patients with T2DM and sarcopenia (57).
The European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AMGS) both propose muscle mass, muscle strength, and physical function as diagnostic criteria for sarcopenia (58, 59). Declines in muscle strength and body functions are a result of the loss of muscle mass and have an adverse effect on prognoses. T2DM is one of the risk factors for sarcopenia. SGLT-2i should be used in T2DM patients with attention to the risk of sarcopenia and can be used to evaluate indicators of muscle mass, muscle strength, and somatic function and thereby more comprehensively detail the possible effects of SGLT-2i treatment on muscles. In addition to DXA or BIA, diagnostic B-mode ultrasonography (60), MRI, and CT can be considered.
Several investigators have focused on the correlation between SGLT-2i and muscle atrophy. However, comprehensive and systematic research supported by sufficient data from clinical trials is still lacking. We call on researchers to focus on this issue and provide more high-quality evidence to guide future clinical practice.
Strengths and limitations
This systematic review of the association between SGLT-2i and muscle atrophy in the treatment of T2DM provides data to answer the current question of whether SGLT-2i has harmful, insubstantial, or beneficial effects on muscles while reducing fat mass, lowering body weight, and altering the body composition of patients. We also evaluated the robustness of the meta-analysis using sensitivity analysis. Compared with a previous study (48), we went a step further and performed a subgroup analysis based on muscle definition, measurement methods, and treatment duration. We searched two Chinese databases to increase the breadth of this data.
Our review has some limitations. The results showed excessive heterogeneity, suggesting significant variability among the samples, which may have impacted the overall estimates. Subgroup analyses were performed based on different definitions of muscle, measurement methods, and treatment duration for each outcome indicator. However, subgroup analyses based on treatment duration were missing for the skeletal muscle quality group because the treatment duration of all studies using skeletal muscle quality as an outcome index was within 24 weeks. Furthermore, negligible muscle atrophy may have contributed to statistical bias. Owing to the large number of studies screened, it was uncertain whether all studies that met the criteria were included in the meta-analysis. However, we believe that these methodological limitations do not affect the overall conclusions of this meta-analysis. In addition, the studies included in this meta-analysis did not consider muscle-related indicators as primary outcomes.
Conclusions
Our systematic review and meta-analysis suggest that treatment with SGLT-2i in patients with T2DM may lead to muscle loss. As mentioned above, this study had some limitations. To fully evaluate the possible effects of SGLT-2i treatment on the muscles of patients with T2DM, a large-sample, multicenter, and well-designed randomized controlled trial involving measures of muscle mass, muscle strength, and physical function is required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CX, YH, and MY conceived and designed the study. CX and YH drafted the manuscript. CX, YH, and CY were responsible for searching and selecting studies. CX, YH, and CY participated in data extraction and assessed the study quality. RG, ZL, and YD prepared forms and pictures and performed the statistical analyses. CX and YH wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to Dr. Yu for his contribution to the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1220516/full#supplementary-material
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina (Kaunas). (2019) 55(9):546. doi: 10.3390/medicina55090546
3. Li CX, Liang S, Gao L, Liu H. Cardiovascular outcomes associated with SGLT-2 inhibitors versus other glucoselowering drugs in patients with type 2 diabetes: A real-world systematic review and meta-analysis. PloS One (2021) 16(2):e0244689. doi: 10.1371/journal.pone.0244689
4. Vivian EM, PharmD MS, BC-ADM CDE. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context. (2014) 3:212264. doi: 10.7573/dic.212264
5. American Diabetes Association Professional Practice Committee. Addendum. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care (2022) 45(9):2182–4. doi: 10.2337/dc22-ad08a
6. Cao H, Liu T, Wang L, Ji Q. Comparative efficacy of novel antidiabetic drugs on cardiovascular and renal outcomes in patients with diabetic kidney disease: A systematic review and network meta-analysis. Diabetes Obes Metab (2022) 24(8):1448–57. doi: 10.1111/dom.14702
7. Sasaki T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig (2019) 10(2):193–5. doi: 10.1111/jdi.12966
8. Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab (2019) 21(5):1237–50. doi: 10.1111/dom.13648
9. Yamakage H, Tanaka M, Inoue T, Odori S, Kusakabe T, Satoh-Asahara N. Effects of dapagliflozin on the serum levels of fibroblast growth factor 21 and myokines and muscle mass in Japanese patients with type 2 diabetes: A randomized, controlled trial. J Diabetes Investig (2020) 11(3):653–61. doi: 10.1111/jdi.13179
10. Giri Ravindran S, Kakarla M, Ausaja Gambo M, Yousri Salama M, Haidar Ismail N, Tavalla P, et al. The effects of sodium-glucose cotransporter-2 inhibitors (SLGT-2i) on cardiovascular and renal outcomes in non-diabetic patients: A systematic review. Cureus. (2022) 14(5):e25476. doi: 10.7759/cureus.25476
11. Andreadi A, Muscoli S, Tajmir R, Meloni M, Muscoli C, Ilari S, et al. Recent pharmacological options in type 2 diabetes and synergic mechanism in cardiovascular disease. Int J Mol Sci (2023) 24(2):1646. doi: 10.3390/ijms24021646
12. American Diabetes Association. Standards of medical care in diabetes-2021 abridged for primary care providers. Clin Diabetes (2021) 39(1):14–43. doi: 10.2337/cd21-as01
13. American Diabetes Association. Introduction. Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S1–2. doi: 10.2337/dc22-Sint
14. Yasuda M, Iizuka K, Kato T, Liu Y, Takao K, Nonomura K, et al. Sodium-glucose cotransporter 2 inhibitor and sarcopenia in a lean elderly adult with type 2 diabetes: A case report. J Diabetes Investig (2020) 11(3):745–7. doi: 10.1111/jdi.13137
15. Stella M, Biassoni E, Fiorillo C, Grandis M, Mattioli F, Del Sette M. A case of anti-HMGCR myopathy triggered by sodium/glucose co-transporter 2 (SGLT2) inhibitors. Neurol Sci (2022) 43(7):4567–70. doi: 10.1007/s10072-022-06046-3
16. Gao F, Hall S, Bach LA. Myopathy secondary to empagliflozin therapy in type 2 diabetes. Endocrinol Diabetes Metab Case Rep (2020) 2020:20–0017. doi: 10.1530/EDM-20-0017
17. Kabadi UM. Marked weight loss, muscle wasting and fatigue on administration of empagliflozin in a subject with type 2 diabetes. Br J Med Med Res (2017) 21(5):1–7. doi: 10.9734/BJMMR/2017/33253
18. Sakamoto M, Goto Y, Nagayama A, Yano M, Sato S, Tajiri Y, et al. Two-year administration of sodium-glucose co-transporter 2 inhibitor brought about marked reduction of body fat independent of skeletal muscle amount or glycemic improvement in Japanese patients with type 2 diabetes. Diabetol Int (2021) 13(1):117–23. doi: 10.1007/s13340-021-00512-7
19. Koshizaka M, Ishikawa K, Ishibashi R, Maezawa Y, Sakamoto K, Uchida D, et al. Effects of ipragliflozin versus metformin in combination with sitagliptin on bone and muscle in Japanese patients with type 2 diabetes mellitus: Subanalysis of a prospective, randomized, controlled study (PRIME-V study). J Diabetes Investig (2021) 12(2):200–6. doi: 10.1111/jdi.13340
20. McCrimmon RJ, Catarig AM, Frias JP, Lausvig NL, le Roux CW, Thielke D, et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. (2020) 63(3):473–85. doi: 10.1007/s00125-019-05065-8
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). (2021) 74(9):790–9. doi: 10.1016/j.rec.2021.07.010
22. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
23. Han E, Lee YH, Lee BW, Kang ES, Cha BS. Ipragliflozin additively ameliorates non-alcoholic fatty liver disease in patients with type 2 diabetes controlled with metformin and pioglitazone: A 24-week randomized controlled trial. J Clin Med (2020) 9(1):259. doi: 10.3390/jcm9010259
24. Inoue H, Morino K, Ugi S, Tanaka-Mizuno S, Fuse K, Miyazawa I, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: A randomized clinical trial. J Diabetes Investig (2019) 10(4):1012–21. doi: 10.1111/jdi.12985
25. Horibe K, Morino K, Miyazawa I, Tanaka-Mizuno S, Kondo K, Sato D, et al. Metabolic changes induced by dapagliflozin, an SGLT2 inhibitor, in Japanese patients with type 2 diabetes treated by oral anti-diabetic agents: A randomized, clinical trial. Diabetes Res Clin Pract (2022) 186:109781. doi: 10.1016/j.diabres.2022.109781
26. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab (2019) 21(2):285–92. doi: 10.1111/dom.13520
27. Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, et al. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb (2018) 25(6):467–76. doi: 10.5551/jat.40873
28. Kitazawa T, Seino H, Ohashi H, Inazawa T, Inoue M, Ai M, et al. Comparison of tofogliflozin versus glimepiride as the third oral agent added to metformin plus a dipeptidyl peptidase-4 inhibitor in Japanese patients with type 2 diabetes: A randomized, 24-week, open-label, controlled trial (STOP-OB). Diabetes Obes Metab (2020) 22(9):1659–63. doi: 10.1111/dom.14059
29. Fadini GP, Bonora BM, Zatti G, Vitturi N, Iori E, Marescotti MC, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo-controlled trial. Cardiovasc Diabetol (2017) 16(1):42. doi: 10.1186/s12933-017-0529-3
30. Kim G, Ridderstra°le M, Andersen KR, Zeller C, Woerle HJ, Broedl UC. Effect of empagliflozin compared with glimepiride as add-on to metformin for 2 years on the amount and distribution of body fat in patients with type 2 diabetes. Diabetologia (2014) 57:330–1. doi: 10.1007/s00125-014-3355-0
31. Wolf VLW, Breder I, de Carvalho LSF, Soares AAS, Cintra RM, Barreto J, et al. Dapagliflozin increases the lean-to total mass ratio in type 2 diabetes mellitus. Nutr Diabetes. (2021) 11(1):17. doi: 10.1038/s41387-021-00160-5
32. Nakaguchi H, Kondo Y, Kyohara M, Konishi H, Oiwa K, Terauchi Y. Effects of liraglutide and empagliflozin added to insulin therapy in patients with type 2 diabetes: A randomized controlled study. J Diabetes Investig (2020) 11(6):1542–50. doi: 10.1111/jdi.13270
33. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. (2013) 382(9896):941–50. doi: 10.1016/S0140-6736(13)60683-2
34. Blonde L, Stenlöf K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med (2016) 128(4):371–80. doi: 10.1080/00325481.2016.1169894
35. Igarashi H, Uchino H, Kanaguchi M, Hisanaga K, Sato G, Yoshikawa F, et al. SGLT2 inhibitor versus carbohydrate-restricted isocaloric diet: reprogramming substrate oxidation in type 2 diabetes. Diabetol Metab Syndr (2023) 15(1):25. doi: 10.1186/s13098-023-00990-6
36. Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab (2014) 16(2):159–69. doi: 10.1111/dom.12189
37. Kang SM, Yun HM, Sohn M, Lim S. Vascular and metabolic effects of ipragliflozin versus sitagliptin (IVS) in type 2 diabetes treated with sulphonylurea and metformin: IVS study. Diabetes Obes Metab (2023) 25(7):1922–31. doi: 10.1111/dom.15056
38. Zeng YH, Liu SC, Lee CC, Sun FJ, Liu JJ. Effect of empagliflozin versus linagliptin on body composition in Asian patients with type 2 diabetes treated with premixed insulin. Sci Rep (2022) 12(1):17065. doi: 10.1038/s41598-022-21486-9
39. Kayano H, Koba S, HIrano T, Matsui T, Fukuoka H, Tsuijita H, et al. Dapagliflozin influences ventricular hemodynamics and exercise-induced pulmonary hypertension in type 2 diabetes patients- A randomized controlled trial. Circ J (2020) 84(10):1807–17. doi: 10.1253/circj.CJ-20-0341
40. Sargeant JA, Henson J, King JA, Yates T, Khunti K, Davies MJ. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab (Seoul). (2019) 34(3):247–62. doi: 10.3803/EnM.2019.34.3.247
41. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
42. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
43. Watanabe Y, Suzuki D, Kuribayashi N, Uchida D, Kato M, Ohashi H, et al. A randomized controlled trial of two diets enriched with protein or fat in patients with type 2 diabetes treated with dapagliflozin. Sci Rep (2021) 11(1):11350. doi: 10.1038/s41598-021-90879-z
44. Gupta R, Alcantara R, Popli T, Mahajan S, Tariq U, Dusaj RS, et al. Myopathy associated with statins and SGLT2 - A review of literature. Curr Probl Cardiol (2021) 46(4):100765. doi: 10.1016/j.cpcardiol.2020.100765
45. Post A, Groothof D, Eisenga MF, Bakker SJL. Sodium-glucose cotransporter 2 inhibitors and kidney outcomes: true renoprotection, loss of muscle mass or both? J Clin Med (2020) 9(5):1603. doi: 10.3390/jcm9051603
46. Watanabe Y, Kuribayashi N, Uchida D, Suzuki D, Kato M, Nagayama D, et al. Study Protocol for the Effects of Formula Diet with Dapagliflozin on Metabolic Improvement and Body Composition in Type 2 Diabetes Mellitus [published correction appears in Diabetes Ther. Diabetes Ther (2019) 10(1):311–21. doi: 10.1007/s13300-018-0555-5
47. Chang WT, Wu CH, Hsu LW, Chen PW, Yu JR, Chang CS, et al. Serum vitamin D, intact parathyroid hormone, and Fetuin A concentrations were associated with geriatric sarcopenia and cardiac hypertrophy. Sci Rep (2017) 7:40996. doi: 10.1038/srep40996
48. Pan R, Zhang Y, Wang R, Xu Y, Ji H, Zhao Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PloS One (2022) 17(12):e0279889. doi: 10.1371/journal.pone.0279889
49. Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab (2016) 101(1):44–51. doi: 10.1210/jc.2015-1860
50. Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int (2014) 85(4):962–71. doi: 10.1038/ki.2013.356
51. Lv XH, Cong XX, Nan JL, Lu XM, Zhu QL, Shen J, et al. Anti-diabetic drug canagliflozin hinders skeletal muscle regeneration in mice. Acta Pharmacol Sin (2022) 43(10):2651–65. doi: 10.1038/s41401-022-00878-7
52. Egger A, Kraenzlin ME, Meier C. Effects of incretin-based therapies and SGLT2 inhibitors on skeletal health. Curr Osteoporos Rep (2016) 14(6):345–50. doi: 10.1007/s11914-016-0337-9
53. Tembo MC, Mohebbi M, Holloway-Kew KL, Gaston J, Sui SX, Brennan-Olsen SL, et al. The contribution of musculoskeletal factors to physical frailty: a cross-sectional study. BMC Musculoskelet Disord (2021) 22(1):921. doi: 10.1186/s12891-021-04795-4
54. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol (2021) 17(9):534–48. doi: 10.1038/s41574-021-00512-2
55. Papadopoulou SK. Sarcopenia: A contemporary health problem among older adult populations. Nutrients. (2020) 12(5):1293. doi: 10.3390/nu12051293
56. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev (2013) 35:51–65. doi: 10.1093/epirev/mxs006
57. Sanz-Cánovas J, López-Sampalo A, Cobos-Palacios L, Ricci M, Hernández-Negrín H, Mancebo-Sevilla JJ, et al. Management of type 2 diabetes mellitus in elderly patients with frailty and/or sarcopenia. Int J Environ Res Public Health (2022) 19(14):8677. doi: 10.3390/ijerph19148677
58. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc (2020) 21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012
59. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48(4):601. doi: 10.1093/ageing/afz046
Keywords: SGLT-2i, muscle atrophy, type 2 diabetes mellitus, systematic review, meta-analysis
Citation: Xia C, Han Y, Yin C, Geng R, Liu Z, Du Y and Yu M (2023) Relationship between sodium–glucose cotransporter-2 inhibitors and muscle atrophy in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 14:1220516. doi: 10.3389/fendo.2023.1220516
Received: 10 May 2023; Accepted: 22 August 2023;
Published: 15 September 2023.
Edited by:
Qi Pan, Peking University, ChinaReviewed by:
Aikaterini Andreadi, University of Rome Tor Vergata, ItalyKomuraiah Myakala, Georgetown University Medical Center, United States
Copyright © 2023 Xia, Han, Yin, Geng, Liu, Du and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingkun Yu, SDEzNTYxNTEwMzE2QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Chengdong Xia
Chengdong Xia Yufeng Han
Yufeng Han Chunhui Yin
Chunhui Yin Ruyue Geng
Ruyue Geng Zhenfei Liu
Zhenfei Liu Yongle Du
Yongle Du Mingkun Yu
Mingkun Yu