- 1Department of Metabolic Medicine, Graduate School of Medicine, Osaka University, Suita, Japan
- 2Department of Lifestyle Medicine, Graduate School of Medicine, Osaka University, Suita, Japan
- 3Department of Diabetes Care Medicine, Graduate School of Medicine, Osaka University, Suita, Japan
Aims: The excess deposition of intra-pancreatic fat deposition (IPFD) has been reported to be associated with type 2 diabetes, chronic pancreatitis, and pancreatic ductal adenocarcinoma. In the current study, we aimed to identify a relationship between lifestyle factors and IPFD.
Materials and methods: 99 patients admitted to the Osaka University Hospital who had undergone abdominal computed tomography were selected. We evaluated the mean computed tomography values of the pancreas and spleen and then calculated IPFD score. Multiple regression analyses were used to assess the associations between IPFD score and lifestyle factors.
Results: Fast eating speed, late-night eating, and early morning awakening were significantly associated with a high IPFD score after adjusting for age, sex, diabetes status and Body Mass Index (p=0.04, 0.01, 0.01, respectively).
Conclusion: The current study has elucidated the significant associations of fast eating speed, late-night eating, and early morning awakening with IPFD.
1 Introduction
With the significant surge in the prevalence of obesity, organ dysfunction linked to ectopic fat accumulation is receiving a lot of attention in these days. Ectopic fat accumulation in the pancreas is termed “intra-pancreatic fat deposition” (IPFD) (1). A small amount of intra-pancreatic fat is a constituent of the normal human pancreas (1) and the amount of intra-pancreatic fat increases with age and more common in people of Asian descent (1). The excess of IPFD, which is termed “fatty pancreas disease (FPD)”, has been significantly associated with type 2 diabetes mellitus (2, 3), chronic pancreatitis (4), and pancreatic ductal adenocarcinoma (5).
Several previous studies have reported an association between lifestyle factors and IPFD (1). A randomized controlled trial showed that the use of the Mediterranean diet resulted in a significantly lower IPFD (6). In patients with post-pancreatitis, it was also reported that smoking and alcohol consumption may be associated with IPFD (7).
To elucidate whether IPFD is associated with specific dietary habits and other lifestyle factors, we previously reviewed medical records from the Osaka University Hospital, and reported that the consumption of two meals per day was associated with IPFD in patients with type 2 diabetes mellitus (8). In the current study, we conducted a prospective survey to assess more detailed lifestyle factors through the use of multiple questionnaires and examined the association between lifestyle factors and IPFD.
2 Methods
2.1 Study design and participants
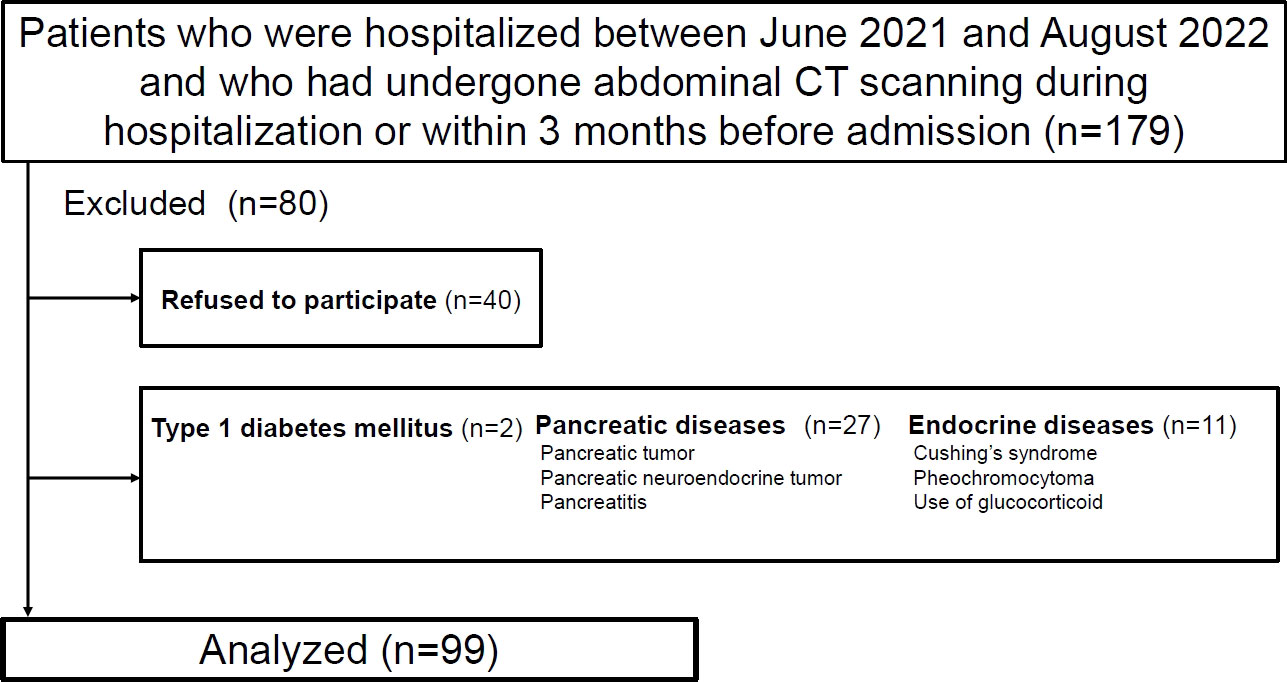
The participants were patients who were admitted in the Department of Metabolic Medicine, Osaka University Hospital, between June 2021 and August 2022, and had undergone abdominal computed tomography (CT) scans for underlying diseases during their hospital stay or within 3 months before admission. The sample size was set according to our previous research (8). We excluded patients with type 1 diabetes mellitus, pancreatic diseases (e.g., pancreatic tumors or pancreatitis), endocrine diseases (e.g., Cushing syndrome or pheochromocytoma), or taking oral corticosteroid (prednisolone 10 mg or more). Diagnosis of pancreatic cancer and pancreatitis was made by experienced radiologists. The study was approved by the institutional ethics review board of Osaka University Hospital (approval number: 21062). The participants were informed about the study and gave written consent to participate.
2.2 Assessment of lifestyle factors
We collected information on lifestyle factors through self-administered questionnaires distributed to the patients during the hospitalization period. For dietary habits, a validated food frequency questionnaire developed by the Japan Public Health Center (JPHC) (9, 10), including 128 food items and 13 beverage items, was used. For exercise habits, the International Physical Activity Questionnaire short form (11) was used to yield a score in MET-hours/week. The participants were also asked about the number of meals consumed per day, snacking habits, timing of each meal, and eating speed. Information about eating speed and sleeping habits (sleep duration, frequency of difficulty falling asleep, mid-awakening, early morning awakening and deep sleep disorder) were collected using a questionnaire based on the JPHC-NEXT interview form (12). The questionnaire on these lifestyle factors is shown in Supporting Information Table S1. Smoking were assessed by the Brinkman index (number of cigarettes consumed per day multiplied by years of smoking) (13). Late-night eating was defined as eating after 9:00 p.m., and fast eating was defined as those who responded that their eating speed was fast/very fast according to previous study (14). Short sleep duration was defined as sleeping 5 hours or less per day (15).
2.3 Measurement of ectopic fat accumulation in the pancreas and liver
In order to measure the extent of IPFD and intra-hepatic lipid accumulation (IHLA), unenhanced CT values were utilized, which have been demonstrated to exhibit strong correlation with histologically determined organ fat content (16). As previously reported (8, 17), we determined the CT values of the pancreas (P) by calculating the mean value of three 1cm2 regions of pancreas (head, body, and tail). The measurements were carefully conducted by excluding the pancreatic duct and margins from the selected areas. Likewise, the CT values of the liver (L) was determined by calculating the mean CT value of three 1cm2 regions of liver (anterior, posterior, and lateral). The CT values of the spleen (S) was determined by calculating the mean CT value of three 1cm2 regions of spleen (upper, middle, and lower). Subsequently, by following previous studies, we quantified IPFD by calculating the difference between the mean pancreatic values and the mean splenic values (P-S) (8, 17, 18). Similarly, we quantified IHLA by calculating the difference between the mean liver values and the mean splenic values (L-S) (17, 19). Lower CT values indicate higher levels of fat accumulation. Measurements were performed by an experienced physician blinded to each patient’s information. To determine inter-observer variability in IPFD and IHLA measurements, another physician randomly selected 27 patients and independently measured their IPFD and IHLA. The intraclass correlation coefficients (ICC) and Bland-Altman plot between these two physicians were evaluated for these measurements. All unenhanced CT scanning was performed with a slice thickness of 5 mm. The images were analyzed using Aquarius Net Viewer Version 4.4 (TeraRecon, Inc., Tokyo, Japan).
2.4 Covariate assessment
The following data was obtained from the medical records at the time of participants’ admission: age; sex; height; body weight; Hemoglobin A1c (HbA1c); fasting plasma glucose; triglycerides (TG); high-density lipoprotein-cholesterol (HDL-C); and low-density lipoprotein-cholesterol (LDL-C) concentrations. Blood samples were obtained in the morning, prior to breakfast, on the day following admission. Diabetes mellitus were diagnosed according to Japanese diagnostic criteria (20).
2.5 Statistical analysis
The P-S and L-S values were square root transformed to normalize their distributions and then multiplicated to the range of 0–100 (with 0 representing lowest fat deposition and 100 as highest fat deposition). The numbers were defined as “IPFD score” and “IHLA score”, respectively. Multiple regression analyses were conducted to assess the associations between each lifestyle factor and the scores of IPFD and IHLA, adjusting for covariates including age (continuous), sex (male or female), diabetes status (presence of diabetes mellitus), and body mass index (BMI, continuous). Least squares geometric mean concentrations were calculated for lifestyle factors. The results were showed as Model 1, not adjusted; Model 2, adjusted for age and sex; Model 3, adjusted for age, sex, and diabetes status; and Model 4, adjusted for age, sex, diabetes status, and body mass index. In addition, to evaluate generalizability, sensitivity analysis was performed by stratifying by the presence or absence of diabetes. Each macro-nutrient and micro-nutrient intake provided by FFQ is adjusted for total energy intake by residual method. Statistical analyses were performed using R version 4.2.2. (https://www.r-project.org/) and two-sided p-value < 0.05 was considered to represent statistical significance. Any missing data were excluded from the statistical analyses.
3 Results
3.1 Participant characteristics
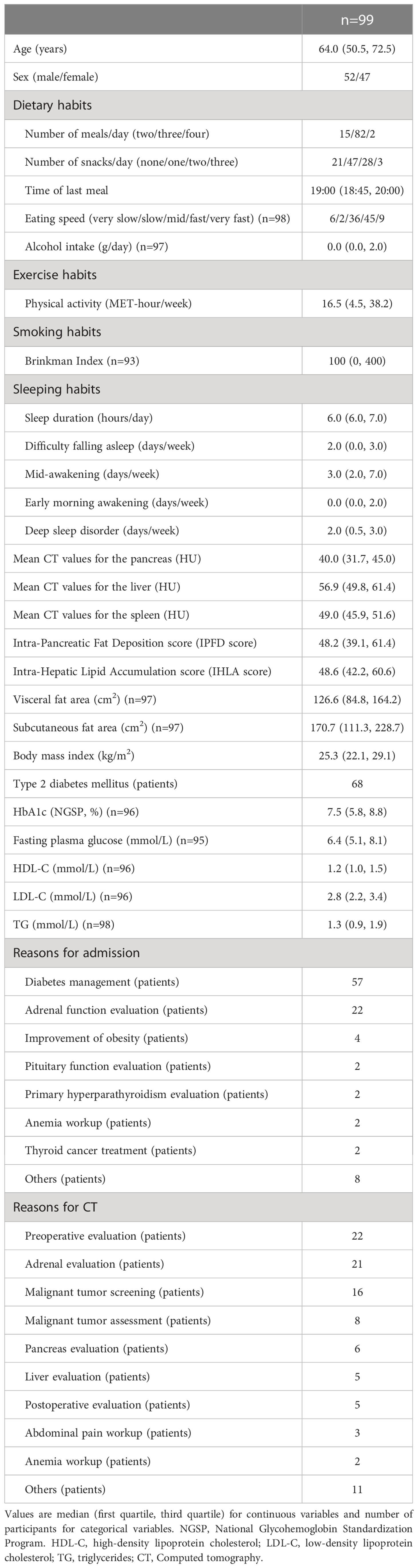
Ultimately, 99 patients were enrolled in the study (Figure 1). Table 1 presents the clinical characteristics of the participants upon hospitalization. The median age was 64 years and median BMI was 25.3 kg/m2. The median CT values for the pancreas, liver and spleen were 40.0 HU, 56.9 HU and 49.0 HU, respectively. ICC (2,1) for IPFD (P-S) was 0.86 (95%CI: 0.71-0.93) and ICC (2,1) for IHLA (L-S) was 0.98 (95%CI: 0.96-0.99). Bland-Altman plots for IPFD and IHLA were shown in Supporting Information Figures S1, 2. For both measurements, more than 95% values were included within the limits of agreement. No significant fixed errors (p = 0.08 and 0.41, respectively) and proportional errors (p = 0.59 and 0.39, respectively) were observed. IPFD score 18.0 and IHLA score 18.7 correspond to 1SD.
3.2 Relationship between lifestyle factors and IPFD
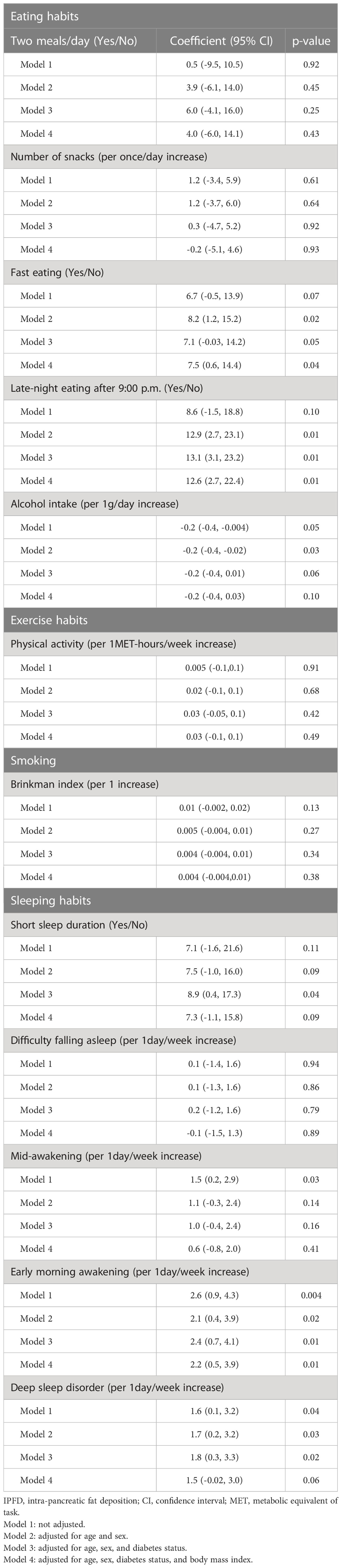
We examined the associations of IPFD score with lifestyle factors (Table 2) and nutrient intakes (Supporting Information Table S2). Fast eating speed, late-night eating, low alcohol intake, early morning awakening and deep sleep disorder were significantly associated with high IPFD score after adjusting for age and sex (p=0.02, 0.01, 0.03, 0.02 and 0.03, respectively). After further adjustment for diabetes status and BMI, fast eating speed, late-night eating, and early morning awakening were significantly associated with high IPFD score (p=0.04, 0.01 and 0.01, respectively). For nutrient intakes, vitamin B12 intake tended to be negatively associated with IPFD in the age, sex, diabetes, and BMI adjusted model (p=0.05).
3.3 Relationship between lifestyle factors and IHLA
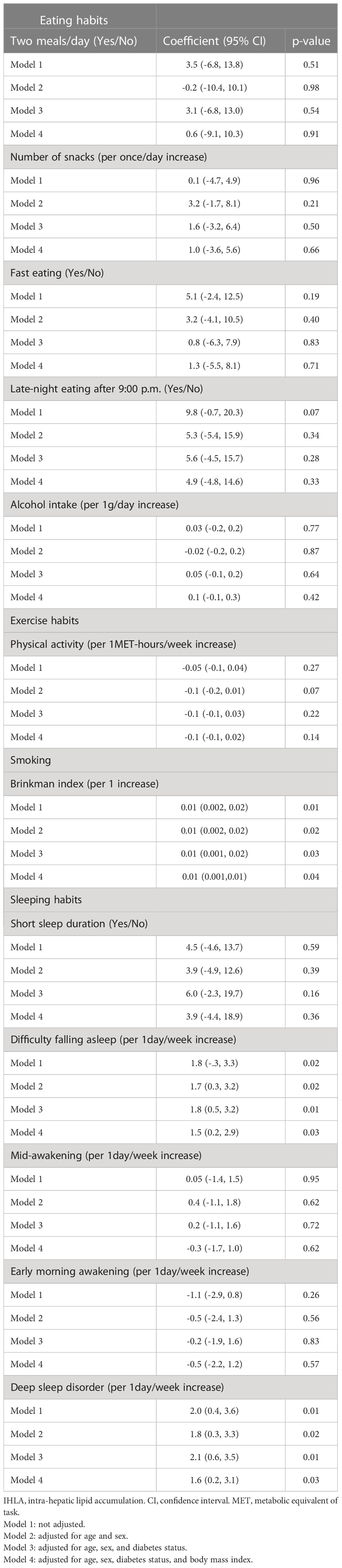
In addition to IPFD, we examined the associations of IHLA score with lifestyle factors (Table 3) and nutrient intakes (Supporting Information Table S3). After adjusting for age, sex, diabetes status, and BMI, Brinkman index, difficulty falling asleep, deep sleep disorder were significantly associated with high IHLA score (p=0.04, 0.03, 0.03, respectively). Fast eating speed, late-night eating, and early morning awakening, which were significantly associated with IPFD score, were not associated with IHLA score. For nutrient intakes, while negative associations were observed between intakes of potassium, β-carotene, vitamin B6, vitamin C, and folate and IHLA in the univariate model (p=0.02, 0.03, 0.01, 0.04, 0.02, respectively), all these associations were attenuated after adjusted for age and sex.
3.4 Sensitivity analysis
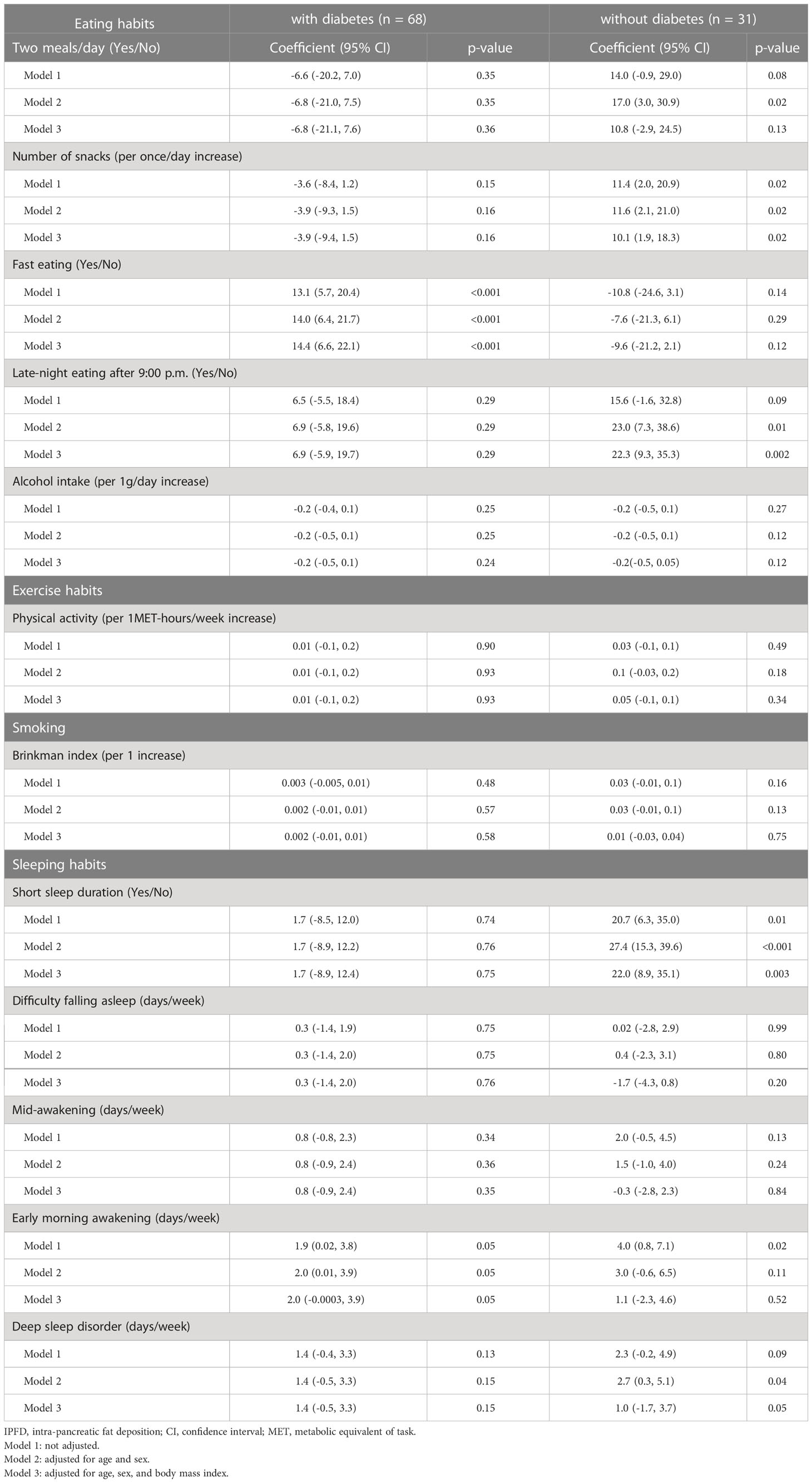
Considering the possibility that IPFD changes depending on the presence or absence of diabetes, analysis was performed separately in the patients with and without diabetes (Table 4). In the patients with diabetes, fast eating speed were significantly associated with IPFD score (p<0.001), after adjusting for age, sex, and BMI. In the patients without diabetes, number of snacks, late-night eating and short sleep duration were significantly associated with IPFD score (p=0.02, 0.002 and 0.003, respectively), after adjusting for age, sex, and BMI.
4 Discussion
In the current study, we elucidated that fast eating speed, late-night eating and early morning awakening were significantly associated only with IPFD, not with IHLA, independently of age, sex, diabetes status, and BMI. To our knowledge, this is the first study to report the associations of dietary and sleeping habits with IPFD.
Several mechanisms may account for the association between fast eating and IPFD. Eating speed has been associated with obesity (21), metabolic syndrome (22), and the risk of type 2 diabetes mellitus (14). Fast eating causes a smaller postprandial decrease in ghrelin levels and a smaller increase in glucagon-like peptide-1 levels than does slow eating (23, 24), which result in postprandial hyperglycemia (25). As IPFD may accumulate because of a paracrine action of pancreatic local insulin secretion (17, 26), postprandial hyperglycemia and local hyperinsulinemia may explain the association between fast eating and IPFD, independent of the effects of obesity and IHLA. In fact, when we examined the association separately in the patients with and without diabetes, fast eating was significantly associated with IPFD only in patients with diabetes, who were prone to postprandial hyperglycemia.
The habit of late-night eating was also significantly associated with high IPFD score. Late-night eating leads to an elevation in hunger upon waking and a reduction in serum leptin levels (27). It also diminishes energy expenditure and core body temperature during wakefulness (27). In addition, it modifies adipose tissue gene expression favoring increased lipid storage (27) and decreases the thermic effect of food (28). Late-night eating has been reported to increase postprandial blood glucose level to the evening meal and the subsequent breakfast (29), which may lead to increased IPFD.
We have previously reported that skipping meals, which causes postprandial hyperglycemia following refeeding (30), was associated with IPFD in the patients with type 2 diabetes mellitus (8). Although in this study we did not observe an association between meal skipping and diabetes status possibly because of the small case number (only six patients with type 2 diabetes mellitus consumed two meals), consistent with other studies, our results suggest that avoiding eating patterns that cause postprandial hyperglycemia and hyperinsulinemia is important in terms of lowering the risk of IPFD.
In this study, early morning awakening, deep sleep disorder, and short sleep duration were associated with high IPFD score after adjusting for age, sex, and diabetes status. With further adjustment for BMI, the association between early morning awakening and high IPFD score remained significant. Early morning awakening, deep sleep disorder, and short sleep duration are known to be a risk factor for obesity (31, 32) and type 2 diabetes mellitus (33). They are linked to elevated levels of growth hormone and ghrelin, as well as reduced levels of leptin (34). Elevated growth hormone levels have been observed to hinder insulin receptors, causing insulin resistance (35). On the other hand, low levels of leptin and high levels of ghrelin are associated with an increased risk of obesity, either by reducing feelings of fullness or by stimulating appetite (36). Therefore, it is speculated that the association between IPFD and early morning awakening, deep sleep disorder, and short sleep duration may in part be explained by obesity. However, in the current study, the association between early morning awakening and IPFD remained significant after adjusting for BMI. A possible reason for this is that early morning awakening is a symptom of circadian rhythm sleep-wake disorders (37). The circadian rhythm is influenced by light exposure and diet, and it plays a crucial role in regulating metabolism (38). Circadian rhythm disturbances has been reported to be associated with elevated levels of both blood glucose and insulin (39), and this mechanism may explain the association between IPFD and circadian rhythm sleep-wake disorders.
For nutrient intakes, vitamin B6, folate, potassium, β-carotene, and vitamin C were negatively associated with IHLA in the univariate model, and vitamin B12 tended to be negatively associated with IPFD in the multivariate adjusted model. Vitamin B6, vitamin B12, and folate are involved in methionine metabolism, and their deficiency causes a decrease in phosphatidylcholine synthesis, which leads to accumulate ectopic fat (40). In addition, it has been reported that vitamin C and carotenoids may reduce fatty liver due to their antioxidant and anti-inflammatory effects (41). These mechanisms may explain the associations of nutrient intakes with IHLA and IPFD elucidated in this study.
There are several limitations of the present study that should be noted. First, this study used a cross-sectional design. The results can only show association and not causation. To demonstrate the causal relationship between lifestyle habits and IPFD, further longitudinal studies and intervention studies are needed. Also, residual confounding due to unmeasured variables or imprecise measurement of confounding should be considered. Second, the assessment of lifestyle is based on self-reported questionnaires. The results may not accurately reflect the patient’s actual lifestyle. Third, there was no histological confirmation of IPFD. However, the assessment method employed in this study has been validated in several prior studies, which demonstrated significant correlations between CT values and histologically determined fat quantities (16). Fourth, as the individuals included in this study were patients who were admitted to the hospital and received CT scans, generalizability may be limited. Fifth, the CT machines used in this study were not identical and scans obtained from imaging conditions with different tube voltages were included. However, this effect is thought to be minor because the CT values were adjusted by subtracting the spleen values (as a reference) for the assessment of ectopic fat.
In conclusion, the current study has elucidated the significant associations of fast eating speed, late-night eating, and early morning awakening with IPFD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Osaka University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KM and MB conceived and designed the study and performed statistical analysis. KM, MB, SK, AN, HO, SM, CI, YH, YF, AT, TN, and JK interpreted the data. KM and MB drafted the manuscript. KM and MB are the guarantors of this work and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. IS and JK critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Grants-in-Aid for Scientific Research [grant number 21K17660, 21K08529].
Acknowledgments
We express our gratitude to Mr. Hiroyuki Kurakami and the team at the Data Coordinating Center, Department of Medical Innovation, Osaka University, for their valuable input and specialized knowledge in assisting our research, and Edanz (http://jp.edanz.com/ac) for providing editing assistance with the draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1219579/full#supplementary-material
References
1. Petrov MS, Taylor R. Intra-pancreatic fat deposition: bringing hidden fat to the fore. Nat Rev Gastroenterol Hepatol (2022) 19(3):153–68. doi: 10.1038/s41575-021-00551-0
2. Singh RG, Yoon HD, Wu LM, Lu J, Plank LD, Petrov MS. Ectopic fat accumulation in the pancreas and its clinical relevance: A systematic review, meta-analysis, and meta-regression. Metabolism (2017) 69:1–13. doi: 10.1016/j.metabol.2016.12.012
3. Yamazaki H, Tauchi S, Wang J, Dohke M, Hanawa N, Kodama Y, et al. Longitudinal association of fatty pancreas with the incidence of type-2 diabetes in lean individuals: A 6-year computed tomography-based cohort study. J Gastroenterol (2020) 55(7):712–21. doi: 10.1007/s00535-020-01683-x
4. Tirkes T, Jeon CY, Li L, Joon AY, Seltman TA, Sankar M, et al. Association of pancreatic steatosis with chronic pancreatitis, obesity, and type 2 diabetes mellitus. Pancreas (2019) 48(3):420–6. doi: 10.1097/mpa.0000000000001252
5. Sreedhar UL, DeSouza SV, Park B, Petrov MS. A systematic review of intra-pancreatic fat deposition and pancreatic carcinogenesis. J Gastrointest Surg (2020) 24(11):2560–9. doi: 10.1007/s11605-019-04417-4
6. Gepner Y, Shelef I, Schwarzfuchs D, Zelicha H, Tene L, Yaskolka Meir A, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: central magnetic resonance imaging randomized controlled trial. Circulation (2018) 137(11):1143–57. doi: 10.1161/circulationaha.117.030501
7. Stuart CE, Ko J, Modesto AE, Alarcon Ramos GC, Bharmal SH, Cho J, et al. Implications of tobacco smoking and alcohol consumption on ectopic fat deposition in individuals after pancreatitis. Pancreas (2020) 49(7):924–34. doi: 10.1097/mpa.0000000000001600
8. Niki A, Baden MY, Kato S, Mitsushio K, Horii T, Ozawa H, et al. Consumption of two meals per day is associated with increased intrapancreatic fat deposition in patients with type 2 diabetes: A retrospective study. BMJ Open Diabetes Res Care (2022) 10(5). doi: 10.1136/bmjdrc-2022-002926
9. Ishihara J, Sobue T, Yamamoto S, Yoshimi I, Sasaki S, Kobayashi M, et al. Validity and reproducibility of a self-administered food frequency questionnaire in the Jphc study cohort ii: study design, participant profile and results in comparison with cohort I. J Epidemiol (2003) 13(1 Suppl):S134–47. doi: 10.2188/jea.13.1sup_134
10. Sasaki S, Kobayashi M, Tsugane S. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the Jphc study cohort I: comparison with dietary records for food groups. J Epidemiol (2003) 13(1 Suppl):S57–63. doi: 10.2188/jea.13.1sup_57
11. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc (2003) 35(8):1381–95. doi: 10.1249/01.Mss.0000078924.61453.Fb
12. Hanyuda A, Sawada N, Uchino M, Kawashima M, Yuki K, Tsubota K, et al. Relationship between unhealthy sleep status and dry eye symptoms in a japanese population: the Jphc-next study. Ocul Surf (2021) 21:306–12. doi: 10.1016/j.jtos.2021.04.001
13. Brinkman GL, Coates EO Jr. The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis (1963) 87:684–93. doi: 10.1164/arrd.1963.87.5.684
14. Kudo A, Asahi K, Satoh H, Iseki K, Moriyama T, Yamagata K, et al. Fast eating is a strong risk factor for new-onset diabetes among the Japanese general population. Sci Rep (2019) 9(1):8210. doi: 10.1038/s41598-019-44477-9
15. Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep Med Rev (2010) 14(4):239–47. doi: 10.1016/j.smrv.2009.08.001
16. Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, et al. Quantitative assessment of pancreatic fat by using unenhanced ct: pathologic correlation and clinical implications. Radiology (2014) 271(1):104–12. doi: 10.1148/radiol.13122883
17. Ishibashi C, Kozawa J, Hosakawa Y, Yoneda S, Kimura T, Fujita Y, et al. Pancreatic fat is related to the longitudinal decrease in the increment of C-peptide in glucagon stimulation test in type 2 diabetes patients. J Diabetes Investig (2020) 11(1):80–7. doi: 10.1111/jdi.13108
18. Yamazaki H, Tsuboya T, Katanuma A, Kodama Y, Tauchi S, Dohke M, et al. Lack of independent association between fatty pancreas and incidence of type 2 diabetes: 5-year Japanese cohort study. Diabetes Care (2016) 39(10):1677–83. doi: 10.2337/dc16-0074
19. Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced ct for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology (2007) 244(2):479–85. doi: 10.1148/radiol.2442061177
20. Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int (2020) 11(3):165–223. doi: 10.1007/s13340-020-00439-5
21. Garcidueñas-Fimbres TE, Paz-Graniel I, Nishi SK, Salas-Salvadó J, Babio N. Eating speed, eating frequency, and their relationships with diet quality, adiposity, and metabolic syndrome, or its components. Nutrients (2021) 13(5). doi: 10.3390/nu13051687
22. Yuan SQ, Liu YM, Liang W, Li FF, Zeng Y, Liu YY, et al. Association between eating speed and metabolic syndrome: A systematic review and meta-analysis. Front Nutr (2021) 8:700936. doi: 10.3389/fnut.2021.700936
23. Reddy NL, Peng C, Carreira MC, Halder L, Hattersley J, Piya MK, et al. Enhanced thermic effect of food, postprandial nefa suppression and raised adiponectin in obese women who eat slowly. Clin Endocrinol (Oxf) (2015) 82(6):831–7. doi: 10.1111/cen.12652
24. Kokkinos A, le Roux CW, Alexiadou K, Tentolouris N, Vincent RP, Kyriaki D, et al. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide Yy and glucagon-like peptide-1. J Clin Endocrinol Metab (2010) 95(1):333–7. doi: 10.1210/jc.2009-1018
25. Saito Y, Kajiyama S, Nitta A, Miyawaki T, Matsumoto S, Ozasa N, et al. Eating fast has a significant impact on glycemic excursion in healthy women: randomized controlled cross-over trial. Nutrients (2020) 12(9). doi: 10.3390/nu12092767
26. Bhargava R, Senior PA, Ackerman TE, Ryan EA, Paty BW, Lakey JR, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes (2004) 53(5):1311–7. doi: 10.2337/diabetes.53.5.1311
27. Vujović N, Piron MJ, Qian J, Chellappa SL, Nedeltcheva A, Barr D, et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab (2022) 34(10):1486–98.e7. doi: 10.1016/j.cmet.2022.09.007
28. McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U.S.A. (2014) 111(48):17302–7. doi: 10.1073/pnas.1412021111
29. Sato M, Nakamura K, Ogata H, Miyashita A, Nagasaka S, Omi N, et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes Res Clin Pract (2011) 5(3):e169–266. doi: 10.1016/j.orcp.2011.02.001
30. Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until Noon Triggers Increased Postprandial Hyperglycemia and Impaired Insulin Response after Lunch and Dinner in Individuals with Type 2 Diabetes: A Randomized Clinical Trial. Diabetes Care (2015) 38(10):1820–6. doi: 10.2337/dc15-0761
31. Covassin N, Singh P, McCrady-Spitzer SK, St Louis EK, Calvin AD, Levine JA, et al. Effects of Experimental Sleep Restriction on Energy Intake, Energy Expenditure, And visceral obesity. J Am Coll Cardiol (2022) 79(13):1254–65. doi: 10.1016/j.jacc.2022.01.038
32. Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep (2008) 31(5):619–26. doi: 10.1093/sleep/31.5.619
33. Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev (2016) 30:11–24. doi: 10.1016/j.smrv.2015.10.002
34. Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) (2005) 99(5):2008–19. doi: 10.1152/japplphysiol.00660.2005
35. Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol Metab Clin North Am (2014) 43(1):75–102. doi: 10.1016/j.ecl.2013.10.005
36. Yeung AY, Tadi P. Physiology, Obesity Neurohormonal Appetite and Satiety Control. Statpearls. Treasure Island (FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
37. Sun SY, Chen GH. Treatment of Circadian rhythm sleep-wake disorders. Curr Neuropharmacol (2022) 20(6):1022–34. doi: 10.2174/1570159x19666210907122933
38. Jakubowicz D, Wainstein J, Tsameret S, Landau Z. Role of high energy breakfast "Big breakfast diet" in clock gene regulation of postprandial hyperglycemia and weight loss in type 2 diabetes. Nutrients (2021) 13(5). doi: 10.3390/nu13051558
39. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U.S.A. (2009) 106(11):4453–8. doi: 10.1073/pnas.0808180106
40. Kitagawa E, Ota Y, Hasegawa M, Nakagawa T, Hayakawa T. Accumulation of liver lipids induced by vitamin B(6) deficiency was effectively ameliorated by choline and, to a lesser extent, betaine. J Nutr Sci Vitaminol (Tokyo) (2019) 65(1):94–101. doi: 10.3177/jnsv.65.94
Keywords: lifestyle factors, ectopic fat, fast eating, intra-pancreatic fat deposition, late-night eating
Citation: Mitsushio K, Baden MY, Kato S, Niki A, Ozawa H, Motoda S, Ishibashi C, Hosokawa Y, Fujita Y, Tokunaga A, Nammo T, Kozawa J and Shimomura I (2023) Relationships between intra-pancreatic fat deposition and lifestyle factors: a cross-sectional study. Front. Endocrinol. 14:1219579. doi: 10.3389/fendo.2023.1219579
Received: 09 May 2023; Accepted: 11 July 2023;
Published: 27 July 2023.
Edited by:
Alexandre Gabarra Oliveira, São Paulo State University, BrazilReviewed by:
Chinmay Marathe, University of Adelaide, AustraliaStefania Camastra, University of Pisa, Italy
Copyright © 2023 Mitsushio, Baden, Kato, Niki, Ozawa, Motoda, Ishibashi, Hosokawa, Fujita, Tokunaga, Nammo, Kozawa and Shimomura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megu Y. Baden, bWJhZGVuQGVuZG1ldC5tZWQub3Nha2EtdS5hYy5qcA==
 Kento Mitsushio
Kento Mitsushio Megu Y. Baden
Megu Y. Baden Sarasa Kato1
Sarasa Kato1 Harutoshi Ozawa
Harutoshi Ozawa Chisaki Ishibashi
Chisaki Ishibashi Yukari Fujita
Yukari Fujita Takao Nammo
Takao Nammo Junji Kozawa
Junji Kozawa