- Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
Gestational diabetes mellitus is one of the most common endocrine diseases that occur during pregnancy. Disorders of blood glucose metabolism during pregnancy can increase the risk of adverse pregnancy outcomes, such as pregnancy-related hypertension, preeclampsia, eclampsia, miscarriage, macrosomia, and neonatal hypoglycemia. Continuous glucose monitoring (CGM) can safely and effectively monitor blood glucose changes in patients with gestational hyperglycemia, thereby reducing adverse pregnancy outcomes. Hence, this article aimed to provide a comprehensive review of the progress and indications for using CGM in pregnant patients with diabetes. CGM can reduce blood glucose fluctuations and the occurrence of serious hypoglycemia and hyperglycemia events and can provide time in range (TIR). TIR is an important indicator of blood glucose level. Patients with a higher TIR during pregnancy have better gestational outcomes.
1 Introduction
Diabetes is a common clinical complication of pregnancy, including gestational diabetes mellitus (GDM) and preexisting diabetes. Among these, GDM is the predominant type, accounting for 80–90% of pregnancies with hyperglycemia. According to the International Association of Diabetes and Pregnancy Study Groups (IADPSG), the global incidence of GDM is estimated to be 17.8% (1). Recent studies have shown that maternal pre-pregnancy body mass index is a potential modifiable risk factor for GDM. Moreover, this study showed that the incidence of GDM increased significantly with age. For women under 35 years of age, the prevalence of GDM is 16.4% in normal-weight, 23.0% in overweight, and 38.5% in obese women. For women over 35 years of age, the prevalence of GDM is 20.4%, 37.2%, and 51.4%, respectively (2).
With economic development and improvement in living standards, the prevalence of GDM has increased over the years (3), leading to increased adverse pregnancy outcomes in mothers and their offspring. For mothers, the incidences of dystocia, miscarriage, and eclampsia has increased (4). In the long term, the risk of type 2 diabetes mellitus (T2DM) in women with a history of GDM is nearly 10 times higher than that in women with normal blood glucose during pregnancy (5). The risks of macrosomia, neonatal hypoglycemia, hyperbilirubinemia, and neonatal respiratory distress syndrome are significantly increased in the offspring of women with GDM (4). A prospective study in 10–14-year-old children showed that the offspring of mothers with untreated GDM are at a high risk of impaired glucose tolerance (IGT). Among mothers with GDM, 10.6% of the children had IGT, whereas only 5.0% of the children of mothers without GDM had IGT. GDM is independently associated with children’s IGT (6). Therefore, monitoring and maintaining normal blood glucose levels during pregnancy is essential.
Currently, the commonly used clinical blood glucose monitoring methods include self-monitoring of blood glucose (SMBG), continuous glucose monitoring (CGM), hemoglobin A1c (HbA1c), and glycosylated albumin (GA). Many studies have recently shown that CGM is beneficial and widely used for the clinical treatment of patients with gestational diabetes. CGM can be real-time (rt-CGM) and intermittently scanned (is-CGM). It can continuously monitor glucose levels in subcutaneous tissue fluids and automatically record blood glucose levels at regular intervals to reflect blood glucose fluctuations accurately. CGM is employed for patients with diabetes during pregnancy, offering a more effective management approach in clinical settings. It enables clinicians to make better treatment selections and adjustments for patients, leading to optimal blood glucose control and improved pregnancy outcomes. This article reviews the use of CGM in pregnant women with diabetes.
2 Classification of pregnancy hyperglycemia
According to the American Diabetes Association (ADA) guidelines for 2023, pregnancy with hyperglycemia is categorized as GDM and preexisting diabetes (7).
GDM refers to a mild abnormality in glucose metabolism during pregnancy; however, the blood glucose level does not reach that of overt diabetes. During pregnancy, an increase in progesterone, cortisol, prolactin, and human placental hormone levels leads to the gradual aggravation of insulin resistance. Patients with GDM lack sufficient insulin production to combat the aggravation of insulin resistance, which leads to hyperglycemia. According to the diagnostic cut-off point established by IADPSG, GDM diagnostic criteria are: 75-g oral glucose tolerance test (OGTT) at any time during pregnancy, fasting blood glucose ≥ 5.1 mmol/L, 1-h OGTT blood glucose ≥ 10.0 mmol/L, and 2-h OGTT blood glucose ≥ 8.5 mmol/L. GDM can be diagnosed if one of the above mentioned blood glucose levels reaches the standard (8–10).
Pre-existing diabetes in pregnancy includes type 1 diabetes (T1DM), T2DM, or a special type of diabetes diagnosed before pregnancy, which is associated with the most severe hyperglycemia during pregnancy (8, 9). Pregnant women with T1DM have a higher risk of hypoglycemia and diabetic ketoacidosis than those with T2DM.The risk of hypertension and other comorbidities may be as high or higher in patients with T2DM than in those with T1DM (7).
3 Blood glucose monitoring of gestational diabetes
3.1 Hemoglobin A1c and glycosylated albumin
HbA1c reflects the average blood glucose level in the last 2–3 months (11). During pregnancy, red blood cell renewal is physiologically accelerated and the demand for iron increases exponentially (12), leading to a physiological decrease in HbA1c (13). In addition, increased vitamin C intake during pregnancy reduces HbA1c levels (14). Therefore, evaluating blood glucose control in patients with GDM using HbA1c levels is not accurate, as it can only serve as a supplementary reference for SMBG. Although several observational studies have shown that the level of HbA1c before pregnancy is associated with adverse pregnancy outcomes, such as fetal congenital malformation, premature delivery, preeclampsia, and perinatal death (15–17), the association between HbA1c level during the second trimester and adverse pregnancy outcomes has not been demonstrated (18, 19).
GA represents the blood glucose level within 2–3 weeks (20). An increase in GA levels can be observed in GDM (21), and GA can be used as a supplementary test for GDM diagnosis and blood glucose control monitoring (22). However, with increasing gestational age, GA continues to decrease, and the detection of GA has limited value in diagnosing gestational diabetes and predicting adverse pregnancy outcomes (23).
3.2 Self blood glucose monitoring
SMBG includes daily self-monitoring of fasting and postprandial blood glucose levels. The target values recommended by the ADA are as follows: fasting blood glucose < 5.3 mmol/L, 1-h postprandial blood glucose < 7.8 mmol/L, or 2-h postprandial blood glucose < 6.7 mmol/L (7). However, owing to multiple measurements of SMBG during pregnancy, long-term compliance is poor (24); hence, the fluctuation of blood glucose levels and the time spent within the target range cannot be readily displayed or interpreted. Errors often occur during clinical treatment processes, and new indicators are urgently needed.
3.3 Continuous glucose monitoring
CGM is an effective means of evaluating the fluctuation range of daytime and nighttime blood glucose levels in patients with diabetes. In the past decade, CGM has been proven to exhibit similar accuracy to that of SMBG (25) and can provide better treatment optimization, as well as indicate the trend of blood glucose, owing to its high test frequency (26). CGM can comprehensively analyze the patients’ blood glucose changes and provide information to patients and clinicians more intuitively by presenting an ambulatory glucose profile (AGP) and trend arrows. More importantly, CGM can also provide an alarm to help avoid serious hypoglycemic and hyperglycemic crises. CGM can improve the mental health and quality of life of patients by reducing the pain associated with fingertip blood sampling, thus improving compliance (27, 28). With its wide adoption in clinical practice, CGM can improve HbA1c and reduce glucose variability in patients with T1DM (29) and is more suitable for treatment monitoring than the use of SMBG in patients with T2DM (30). CGM is also widely used in patients with preexisting T1DM and T2DM during pregnancy and can improve gestational outcomes (31). Among women with GDM, CGM can provide a more comprehensive assessment of nocturnal hyperglycemia and improve the targeting of GDM interventions (32). CGM is also better than SMBG in detecting hypoglycemic episodes, which may improve maternal and fetal outcomes (26). Moreover, patient compliance is higher in CGM than in SMBG. In a prospective study, patient compliance in the CGM group was as high as 90%, which was significantly higher than that in the SMBG group (14). Therefore, CGM is recommended for patients with preexisting diabetes in pregnancy (especially T1DM complicated with pregnancy), GDM requiring insulin treatment, large blood glucose fluctuation, and potential nighttime hypoglycemia (33, 34).
In addition, a recent prospective cohort study of 73 women showed that CGM was well accepted among patients, could better demonstrate the blood glucose control of patients with GDM, and revealed the potential misdiagnosis of OGTT in GDM (35).Another pilot study conducted by the same team, involving 107 women, further validated the potential of CGM in detecting OGTT misdiagnosis. Additionally, CGM was more acceptable than OGTT to the participants (36).
4 Classification of CGM
4.1 Real-time continuous glucose monitoring
The rt-CGM system can provide a comprehensive glucose status for 3–14 days based on different needs. The device comprises a glucose-sensing device based on tiny glucose oxidase-filled electrodes and a glucose monitor connected by a cable. The system measures glucose concentration in the interstitial fluid every 5 min, continuously monitors glucose level for 24 h, and then forms an AGP. Rt-CGM has been extensively studied in patients with diabetes, and its clinical practicality has been demonstrated. It can detect postprandial hyperglycemia, nocturnal hyperglycemia, and hypoglycemia, which have not been previously reported. Rt-CGM displays not only glucose data in real time but also uses “arrows” to indicate the direction and rate of glucose changes, providing high and low blood glucose alarms and warnings. It can also provide data synchronization to enable timely intervention by the doctors and patients, thereby reducing the occurrence of serious hypoglycemia and hyperglycemia events (37–39). Moreover, CGM can improve the accuracy and effectiveness of clinical decision-making in patients with preexisting diabetes during pregnancy (40); however, the current rt-CGM system partially relies on SMBG for calibration.
4.2 Intermittently scanned continuous glucose monitoring
The current is-CGM system, also known as the instant glucose monitoring system, tracks glucose concentration in the human interstitial fluid approximately once every minute and requires scanning near the sensor placed on the skin to retrieve the data. Flash glucose monitoring is a typical example of is-CGM, which was identified by ADA in 2019 as a method that can replace SMBG for blood glucose monitoring (4). When the user scans the sensor, the current blood glucose value is recorded and retrospective reports for blood glucose data and related parameters, such as time in range (TIR), are generated (41). The is-CGM can be used for up to 14 days and does not need calibration with SMBG; however, it cannot deliver alerts (42).
Some studies have compared the two types of CGM and found that both is-CGM and rt-CGM can improve TIR, while rt-CGM has a greater percentage of TIR and can significantly reduce the incidence of hypoglycemia (43). When switching from is-CGM (FreeStyle Libre version 1) to rt-CGM (Dexcom G4) in 18 adult patients with T1DM, without changing insulin therapy management, there was an increase in TIR, a decrease in time below average (TBR), and no change in time above average (TAR) (44). Another study showed that in pregnant women with T1DM, no differences in TIR and TAR were observed, but women monitored by rt-CGM had a lower TBR compared to those monitored by is-CGM (45). Therefore, rt-CGM is more suitable for reducing the occurrence of hypoglycemia.
5 CGM indicators
In clinical practice, patients are recommended to wear CGM for 14 days. For patients with T1DM, 12–15 days of monitoring every 3 months can more accurately assess the level of blood glucose control (46, 47).
The CGM measurement value includes three key indicators: TIR (the proportion of time when the blood glucose is 3.9–10.0 mmol/L), TBR (proportion of time when blood glucose is <3.9 mmol/L), and TAR (proportion of time when blood glucose is>10.0 mmol/L). The main objective of effective and safe glucose control is to increase the TIR while reducing the TAR and TBR (48). Beck et al. found that in patients with diabetes mellitus, the probability of developing diabetic retinopathy and microalbuminuria increased by 64% and 40%, respectively, for every 10% reduction in TIR (49, 50). A study conducted among 141 pregnant women showed that among those with T2DM or GDM who utilized CGM, approximately 40% had TIR ≤ 70% and a higher likelihood of adverse neonatal and maternal outcomes compared to those with TIR > 70% (51). Murphy et al. pointed out that every 5% reduction in TIR and 5% increase in TAR in the second and third trimesters will increase the risk of being older than the gestational age, neonatal hypoglycemia, and admission to the neonatal intensive care unit (52). Therefore, it is necessary to improve the TIR levels in patients. In 2019, the TIR International Consensus recommended a TIR control target of >70% in pregnant women with T1DM. However, TIR control targets should be personalized. Patients with GDM and pregnant women with T2DM require more stringent targets and greater attention to overnight glucose (53).
In addition, common indicators of CGM include glucose management indicators, also called estimating A1C (54), blood glucose change rate [CV, target ≤ 36% (55)], and glycemic variability (GV). Patients with GDM risk factors have higher CV, and the corresponding incidence of adverse pregnancy outcomes is higher (56). GV in early pregnancy can be used as a potential predictor of subsequent GDM diagnosis. The mean amplitude of glycemic excursion, which is derived from GV, was significantly higher in patients with GDM (57).
6 CGM can better control blood glucose and improve pregnancy outcomes
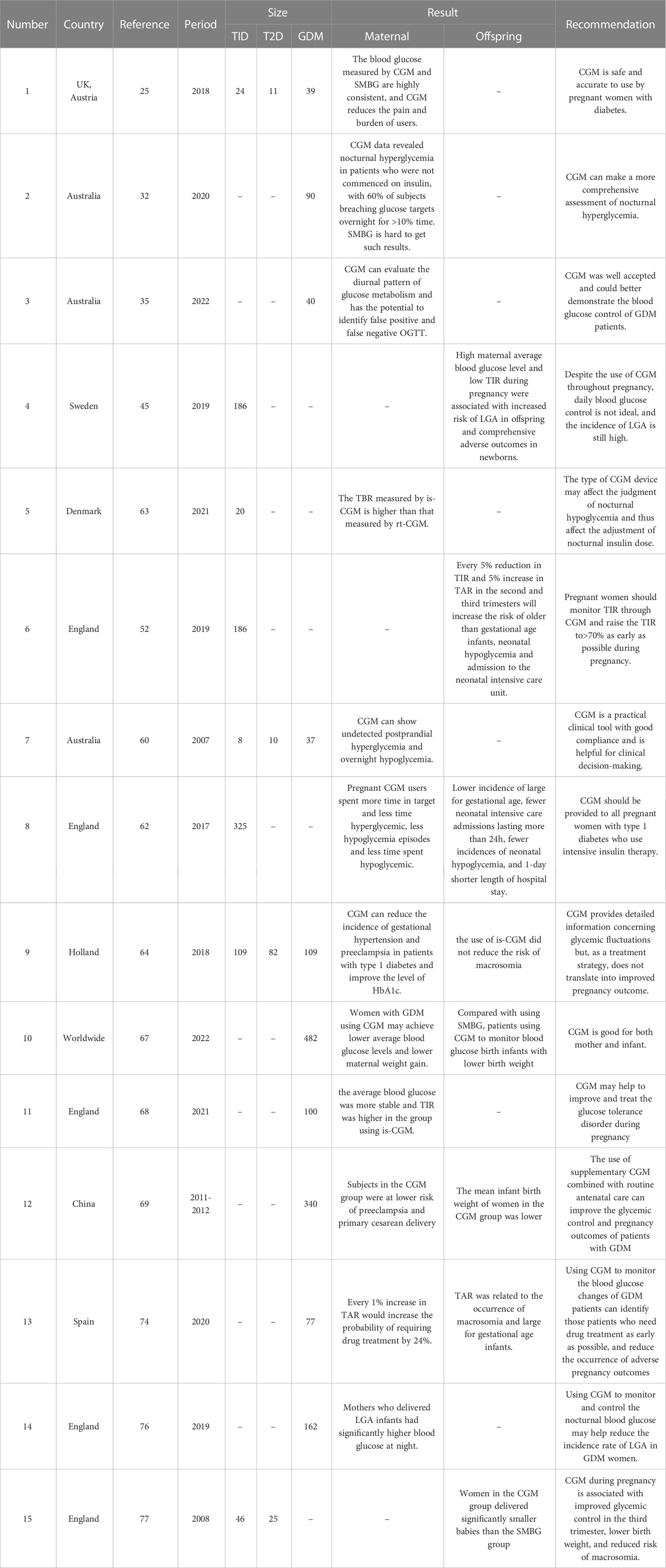
Gestational diabetes increases the risk of pregnancy-related complications, such as hypertension, preeclampsia, eclampsia, premature rupture of membranes, cesarean section, postpartum hemorrhage, and intrauterine infection (58). Therefore, the management of blood glucose levels during pregnancy is very important for reducing adverse pregnancy outcomes. As shown in Table 1, many studies have reported that CGM can reduce adverse pregnancy outcomes. CGM provides patients with intuitive information on changes in blood glucose levels, enabling them to change their lifestyle and participate in treatment (59). Currently, CGM is being increasingly used in patients with gestational diabetes.
In a prospective study in Australia, 68 consecutive blood glucose monitoring examinations were conducted in 55 pregnant women. Sixty-two percent of the results provided important information for altering clinical management decisions, including postprandial and nocturnal hypoglycemia, and 77% of the participants acknowledged that CGM provided more benefits than inconvenience (60). CGM is a practical clinical tool with good compliance and is helpful in clinical decision-making.
The use of CGM is more suitable for the control of blood glucose levels, reduction of blood glucose fluctuations, and improvement of TIR in mothers with preexisting diabetes during pregnancy. Patients with T1DM have a high risk of developing severe hypoglycemia, which can have serious adverse effects on both the mother and fetus during pregnancy. Using CGM allows detection of glycemia fluctuations that might have gone unnoticed with intermittent blood glucose monitoring (61). An international study titled the CONCEPTT divided 325 women with T1DM into two groups. Only capillary blood glucose levels were monitored in one group, and CGM-assisted capillary blood glucose levels were monitored for the other group. Pregnant women who underwent CGM had a higher TIR and lower TAR and TBR. This report suggests that CGM should be administered to all pregnant women with T1DM receiving intensive insulin therapy (62). Viralnshah et al. conducted a prospective study and collected CGM data from 27 women with T1DM during pregnancy and found that TIR was significantly negatively correlated with HbA1c. For every 10% increase in TIR, HbA1c decreased by 0.3%, and the correlation between TIR and HbA1c in the second and third trimesters was stronger than that in the first trimester (r = -0.4) (63). Therefore, we assumed that CGM is suitable for pregnant women with T1DM, as it can help control blood glucose better.
A prospective study including 300 patients with gestational hyperglycemia found that CGM could reduce the incidence of gestational hypertension and preeclampsia in patients with T1DM and improve the level of HbA1c (64). However, although CGM can reduce the incidence of hypertensive disorders that complicate pregnancy in patients with diabetes, it does not significantly reduce the incidence of preeclampsia; the impact of CGM on preeclampsia remains to be discussed (65). Therefore, more robust evidence is required to confirm the effectiveness of CGM in improving pregnancy outcomes.
Although the blood glucose level in patients with GDM is much lower than that in patients with preexisting diabetes during pregnancy, its adverse effects on the future of the mother and fetus should not be underestimated. A follow-up study in Asia showed that women with a history of GDM had a high risk of developing T2DM in the future, and this risk increased with age (66).
García-Moreno et al. searched and screened a large number of studies and conducted a meta-analysis of 482 patients. Compared to women using traditional blood glucose monitoring methods, women with GDM using CGM may have lower average blood glucose levels, lower maternal weight gain, and lower birth weight of infants (67).
Majewska et al. recruited 100 women diagnosed with GDM and randomly assigned them to is-CGM and SMBG groups. The average blood glucose and total insulin resistance levels were determined. The average blood glucose was more stable and total insulin resistance was higher in the group using CGM, which may help to improve and treat glucose tolerance disorder during pregnancy (68).
One study found that the application of the CGM system can reduce the daily blood glucose fluctuation of patients with GDM by more than 25%, and the valley value of hyperglycemia can be significantly reduced (69, 70). This shows that CGM can better control blood glucose fluctuations and avoid excessive increases in blood glucose levels in patients with GDM. Compared to SMBG, CGM can reduce the average blood glucose level, increase the amplitude of maternal and infant birth weights, and improve pregnancy outcomes (68).
A randomized crossover study aimed to determine how the distribution of dietary carbohydrates affects blood glucose levels in women with GDM. CGM was used to monitor the blood glucose levels of 12 women with GDM undergoing diet treatment. The study concluded that “50% carbohydrate distribution in the morning is beneficial for reducing blood glucose and improving insulin sensitivity of women with GDM; however, it resulted in higher blood glucose variability.” Thus, women with GDM should reasonably manage their diet (71).
7 CGM improves perinatal outcomes
In patients with gestational diabetes, blood glucose level increases, leading to excessive glucose passing through the placenta and stimulating the pancreatic islets. This stimulation causes the fetus to produce excess insulin, resulting in increased synthesis of protein and fat in the fetus, consequently resulting in the development of a large baby (72). In addition, owing to excessive insulin production, hypoglycemia can occur easily when the fetus separates from the mother during childbirth. If glucose is not supplemented in time, the incidence of hypoglycemia increases. Both hyperglycemia and hyperinsulinemia can reduce the surface-active substance of fetal lung type II cells, hindering the growth of the fetal lung and affecting its normal development. This condition can lead to neonatal respiratory distress syndrome (73). Poor blood glucose control during pregnancy can result in adverse perinatal outcomes. As shown in Table 1, several studies have reported that CGM reduces adverse perinatal outcomes.
In a prospective study, CGM was used to monitor blood glucose changes in 77 patients with GDM at 26–32 weeks of gestation for 6days. The pattern of hyperglycemia before, after, and at night and its correlation with maternal and fetal complications and drug treatment were analyzed. TAR was related to the occurrence of macrosomia and large-for-gestational-age (LGA) infants. Every 1% increase in TAR increased the probability of requiring drug treatment by 24%. Using CGM to monitor blood glucose changes in patients with GDM enables identification of patients who require drug treatment at an early stage. This proactive approach can help reduce the incidence of adverse pregnancy outcomes, such as macrosomia (74).
LGA infants are referred as newborns whose birth weight is above the 90th percentile of the average weight of infants at the same gestational age, which is closely related to the increase in maternal blood glucose. Long-term glucose metabolic dysfunction may increase the risk of macrosomia (75). A prospective observational study was conducted using CGM in 162 pregnant women with GDM for 7 days at 30–32 weeks of gestation. Using the blood glucose index and blood glucose variability measurements provided by CGM, functional data analysis showed that mothers who delivered LGA infants had significantly higher blood glucose levels at night. Monitoring and controlling nocturnal blood glucose levels may help further reduce the incidence rate of LGA infants in women with GDM (76).
The CONCEPTT study pointed out that compared with SMBG, patients who underwent CGM had significantly improved newborn health outcomes, including a reduced incidence of LGA infants, fewer neonatal intensive care inpatients lasting more than 24 h, a decreased occurrence of neonatal hypoglycemia, and a shortened hospitalization period by one day (62). The use of CGM during pregnancy in patients with T1DM is related to an improvement in neonatal outcomes, which may be attributed to a reduction in maternal hyperglycemia exposure.
Murphy et al. studied the effects of CGM on the offspring of pregnant women with T1DM (46 women) or T2DM (25 women). These women were randomly assigned to the CGM and standard prenatal treatment group (CGM+SMBG, 38 women) or the standard prenatal treatment group (SMBG, 33 women). Women in the CGM group, as measured by the median percentile of birth weight, eventually delivered significantly smaller babies than those in the SMBG group. However, no significant difference was observed between the two groups in terms of LGA infants, cesarean section, preeclampsia, or other indicators used to measure the incidence rate of newborns (77).
Similarly, Kristensen et al. conducted a prospective study of 186 pregnant women with T1DM in Sweden, 92 of whom underwent rt-CGM and 94 underwent is-CGM. The number of LGA infants was similar in rt-CGM and is-CGM users, and high maternal average blood glucose levels and low TIR during pregnancy were associated with an increased risk of LGA and comprehensive adverse outcomes in newborns. However, the rt-CGM group exhibited a lower TBR than the is-CGM group. Therefore, although the impact of rt-CGM on perinatal outcomes was not significantly different from that of is-CGM, rt-CGM was still more suitable for reducing the occurrence of hypoglycemia (45). However, another study showed that intermittent rt-CGM use during pregnancy did not improve blood glucose control or pregnancy outcomes in women with GDM (76).
In summary, there are still few controversial findings regarding CGM improving perinatal outcomes in patients with gestational diabetes. Therefore, a large number of prospective studies are needed to explore the effectiveness of CGM in improving perinatal outcomes in patients with gestational diabetes.
8 Summary
The prevalence of gestational diabetes is increasing with improvements in living standards. Blood glucose monitoring is the basis for GDM management. The goal of GDM treatment is to minimize maternal and fetal adverse events related to hyperglycemia or severe hypoglycemia. Several clinical studies have demonstrated that satisfactory glucose control during pregnancy effectively reduces maternal and infant complications. CGM can effectively monitor blood glucose changes in patients with diabetes during pregnancy, thereby providing better guidance for clinical treatment. Therefore, CGM is recommended for patients with preexisting diabetes in pregnancy (especially T1DM complicated with pregnancy), GDM requiring insulin treatment, large blood glucose fluctuations, and possible nighttime hypoglycemia. This article reviews the use of CGM in patients with diabetes during pregnancy, and many studies have confirmed that CGM can improve pregnancy outcomes. However, there is still some controversy about the impact of CGM on maternal and infant health, which necessitates further discussion and clarification using big data and large samples.
Author contributions
YS wrote the first draft of the manuscript and edited it. XZ summarized the manuscript and drew the Table 1. YB and CL reviewed literature and organized them. LZ performed critical revision of the literature and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This article was supported by “345 talent project plan” of Shengjing Hospital of China Medical University.
Acknowledgments
Thanks, Shengjing Hospital of China Medical University for giving financial support for this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADA, American Diabetes Association; AGP, ambulatory glucose profile; CGM, continuous glucose monitoring; GA, glycosylated albumin; GDM, gestational diabetes mellitus; GV, glycemic variability; HbA1C, hemoglobin A1c; IADPSG, International Association of Diabetes and Pregnancy Study Groups; IGT, impaired glucose tolerance; is-CGM, intermittently scanned continuous glucose monitoring; LGA, large-for-gestational-age; OGTT, oral glucose tolerance test; rt-CGM, real time continuous glucose monitoring; SMBG, self-monitoring of blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TAR, time above average; TBR, time below average; TIR, time in range.
References
1. Mariotti C, Giovannini A, Reibaldi M, Nicolai M, Saitta A. 25-gauge vitrectomy combined with half-fluence photodynamic therapy for the treatment of juxtapapillary retinal capillary hemangioma: a case report. Case Rep Ophthalmol (2014) 5(2):162–7. doi: 10.1159/000363564
2. Mirabelli M, Tocci V, Donnici A, Giuliano S, Sarnelli P, Salatino A, et al. Maternal preconception body mass index overtakes age as a risk factor for gestational diabetes mellitus. J Clin Med (2023) 12(8). doi: 10.3390/jcm12082830
3. Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society, Geriatric Professional Committee of Beijing Medical Award Foundation, National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). [Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition)]. Zhonghua Nei Ke Za Zhi (2022) 61(1):12–50. doi: 10.3760/cma.j.cn112138-20211027-00751
4. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care (2019) 43(Supplement_1):S14–31. doi: 10.2337/dc20-S002
5. Juan J, Yang H-X, Su R-N, Kapur A. Diagnosis of gestational diabetes mellitus in China: perspective, progress and prospects. Maternal-Fetal Med (2019) 1(1):31–7. doi: 10.1097/FM9.0000000000000008
6. Lowe WL Jr., Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care (2019) 42(3):372–80. doi: 10.2337/dc18-1646
7. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 15. Management of diabetes in pregnancy: standards of care in diabetes—2023. Diabetes Care (2022) 46(Supplement_1):S254–66. doi: 10.2337/dc23-S015
8. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care (2010) 33(3):676–82. doi: 10.2337/dc10-0719
9. Gupta Y, Kalra B, Baruah MP, Singla R, Kalra S. Updated guidelines on screening for gestational diabetes. Int J Womens Health (2015) 7:539–50. doi: 10.2147/IJWH.S82046
10. Committee, A.D.A.P.P. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care (2021) 45(Supplement_1):S17–38. doi: 10.2337/dc22-S002
11. Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia (2007) 50(11):2239–44. doi: 10.1007/s00125-007-0803-0
12. Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care (2009) 33(3):509–11. doi: 10.2337/dc09-1954
13. Mosca A, Paleari R, Dalfrà MG, Di Cianni G, Cuccuru I, Pellegrini G, et al. Reference intervals for hemoglobin A1c in pregnant women: data from an Italian multicenter study. Clin Chem (2006) 52(6):1138–43. doi: 10.1373/clinchem.2005.064899
14. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet (2016) 388(10057):2254–63. doi: 10.1016/S0140-6736(16)31535-5
15. Guerin A, Nisenbaum R, Ray JG. Use of maternal GHb concentration to estimate the risk of congenital anoMalies in the offspring of women with prepregnancy diabetes. Diabetes Care (2007) 30(7):1920–5. doi: 10.2337/dc07-0278
16. Jensen DM, Korsholm L, Ovesen P, Beck-Nielsen H, Moelsted-Pedersen L, Westergaard JG, et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care (2009) 32(6):1046–8. doi: 10.2337/dc08-2061
17. Nielsen GL, Møller M, Sørensen HT. HbA1c in early diabetic pregnancy and pregnancy outcomes: a Danish population-based cohort study of 573 pregnancies in women with type 1 diabetes. Diabetes Care (2006) 29(12):2612–6. doi: 10.2337/dc06-0914
18. Hong JGS, Fadzleeyanna MYN, Omar SZ, Tan PC. HbA1c at term delivery and adverse pregnancy outcome. BMC Pregnancy Childbirth (2022) 22(1):679. doi: 10.1186/s12884-022-05000-7
19. Yamamoto JM, Kallas-Koeman MM, Butalia S, Lodha AK, Donovan LE. Large-for-gestational-age (LGA) neonate predicts a 2.5-fold increased odds of neonatal hypoglycaemia in women with type 1 diabetes. Diabetes Metab Res Rev (2017) 33(1). doi: 10.1002/dmrr.2824
20. Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta (2014) 433:96–104. doi: 10.1016/j.cca.2014.03.001
21. Mendes N, Alves M, Andrade R, Ribeiro RT, Papoila AL, Serrano F. Association between glycated albumin, fructosamine, and HbA1c with neonatal outcomes in a prospective cohort of women with gestational diabetes mellitus. Int J Gynaecol Obstet (2019) 146(3):326–32. doi: 10.1002/ijgo.12897
22. Toft JH, Bleskestad IH, Skadberg Ø, Gøransson LG, Økland I. Glycated albumin in pregnancy: LC-MS/MS-based reference interval in healthy, nulliparous Scandinavian women and its diagnostic accuracy in gestational diabetes mellitus. Scand J Clin Lab Invest (2022) 82(2):123–31. doi: 10.1080/00365513.2022.2033827
23. Dong Y, Zhai Y, Wang J, Chen Y, Xie X, Zhang C, et al. Glycated albumin in pregnancy: reference intervals establishment and its predictive value in adverse pregnancy outcomes. BMC Pregnancy Childbirth (2020) 20(1):12. doi: 10.1186/s12884-019-2704-x
24. Hsu CR, Chen YT, Sheu WH. Glycemic variability and diabetes retinopathy: a missing link. J Diabetes Complications (2015) 29(2):302–6. doi: 10.1016/j.jdiacomp.2014.11.013
25. Scott EM, Bilous RW, Kautzky-Willer A. Accuracy, user acceptability, and safety evaluation for the freeStyle libre flash glucose monitoring system when used by pregnant women with diabetes. Diabetes Technol Ther (2018) 20(3):180–8. doi: 10.1089/dia.2017.0386
26. Yu Q, Aris IM, Tan KH, Li LJ. Application and utility of continuous glucose monitoring in pregnancy: A systematic review. Front Endocrinol (2019) 10. doi: 10.3389/fendo.2019.00697
27. Runge AS, Kennedy L, Brown AS, Dove AE, Levine BJ, Koontz SP, et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin Diabetes (2018) 36(2):112–9. doi: 10.2337/cd17-0094
28. Gavin JR, Bailey CJ. Real-world studies support use of continuous glucose monitoring in type 1 and type 2 diabetes independently of treatment regimen. Diabetes Technol Ther (2021) 23(S3):S19–s27. doi: 10.1089/dia.2021.0211
29. Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther (2017) 19(S2):S55–s61. doi: 10.1089/dia.2017.0051
30. Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther (2017) 19(S2):S4–s11. doi: 10.1089/dia.2017.0024
31. Carreiro MP, Nogueira AI, Ribeiro-Oliveira A. Controversies and advances in gestational diabetes-an update in the era of continuous glucose monitoring. J Clin Med (2018) 7(2). doi: 10.3390/jcm7020011
32. Zaharieva DP, Teng JH, Ong ML, Lee MH, Paldus B, Jackson L, et al. Continuous glucose monitoring versus self-monitoring of blood glucose to assess glycemia in gestational diabetes. Diabetes Technol Ther (2020) 22(11):822–7. doi: 10.1089/dia.2020.0073
33. Hawkins JS. Glucose monitoring during pregnancy. Curr Diabetes Rep (2010) 10(3):229–34. doi: 10.1007/s11892-010-0111-9
34. Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J Diabetes (2021) 12(12):2036–49. doi: 10.4239/wjd.v12.i12.2036
35. Di Filippo D, Ahmadzai M, Chang MHY, Horgan K, Ong RM, Darling J, et al. Continuous glucose monitoring for the diagnosis of gestational diabetes mellitus: A pilot study. J Diabetes Res 2022 (2022) p:5142918. doi: 10.1155/2022/5142918
36. Di Filippo D, Henry A, Bell C, Haynes S, Chang MHY, Darling J, et al. A new continuous glucose monitor for the diagnosis of gestational diabetes mellitus: a pilot study. BMC Pregnancy Childbirth (2023) 23(1):186. doi: 10.1186/s12884-023-05496-7
37. Mian Z, Hermayer KL, Jenkins A. Continuous glucose monitoring: review of an innovation in diabetes management. Am J Med Sci (2019) 358(5):332–9. doi: 10.1016/j.amjms.2019.07.003
38. Kudva YC, Ahmann AJ, Bergenstal RM, Gavin JR 3rd, Kruger DF, Midyett LK, et al. Approach to using trend arrows in the freeStyle libre flash glucose monitoring systems in adults. J Endocr Soc (2018) 2(12):1320–37. doi: 10.1210/js.2018-00294
39. Tanenberg R, Bode B, Lane W, Levetan C, Mestman J, Harmel AP, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc (2004) 79(12):1521–6. doi: 10.4065/79.12.1521
40. Chen R, Yogev Y, Ben-Haroush A, Jovanovic L, Hod M, Phillip M. Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J Matern Fetal Neonatal Med (2003) 14(4):256–60. doi: 10.1080/jmf.14.4.256.260
41. Bruttomesso D, Laviola L, Avogaro A, Bonora E, Del Prato S, Frontoni S, et al. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: A consensus view of Italian diabetes experts using the Delphi method. Nutr Metab Cardiovasc Dis (2019) 29(5):421–31. doi: 10.1016/j.numecd.2019.01.018
42. Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care (2018) 41(11):2265–74. doi: 10.2337/dc18-1150
43. Hásková A, Radovnická L, Petruželková L, Parkin CG, Grunberger G, Horová E, et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care (2020) 43(11):2744–50. doi: 10.2337/dc20-0112
44. Préau Y, Galie S, Schaepelynck P, Armand M, Raccah D. Benefits of a switch from intermittently scanned continuous glucose monitoring (isCGM) to real-time (rt) CGM in diabetes type 1 suboptimal controlled patients in real-life: A one-year prospective study (§). Sensors (Basel) (2021) 21(18). doi: 10.3390/s21186131
45. Kristensen K, Ögge LE, Sengpiel V, Kjölhede K, Dotevall A, Elfvin A, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia (2019) 62(7):1143–53. doi: 10.1007/s00125-019-4850-0
46. Xing D, Kollman C, Beck RW, Tamborlane WV, Laffel L, Buckingham BA, et al. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther (2011) 13(3):351–8. doi: 10.1089/dia.2010.0156
47. Riddlesworth TD, Beck RW, Gal RL, Connor CG, Bergenstal RM, Lee S, et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther (2018) 20(4):314–6. doi: 10.1089/dia.2017.0455
48. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care (2019) 42(8):1593–603.
49. Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, et al. The relationships between time in range, hyperglycemia metrics, and hbA1c. J Diabetes Sci Technol (2019) 13(4):614–26. doi: 10.1177/1932296818822496
50. Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care (2019) 42(3):400–5. doi: 10.2337/dc18-1444
51. Bitar G, Cornthwaite JA, Sadek S, Ghorayeb T, Daye N, Nazeer S, et al. Continuous glucose monitoring and time in range: association with adverse outcomes among people with type 2 or gestational diabetes mellitus. Am J Perinatol (2023). doi: 10.1055/s-0043-1764208
52. Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia (2019) 62(7):1123–8. doi: 10.1007/s00125-019-4904-3
53. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care (2019) 42(8):1593–603. doi: 10.2337/dci19-0028
54. Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose management indicator (GMI): A new term for estimating A1C from continuous glucose monitoring. Diabetes Care (2018) 41(11):2275–80. doi: 10.2337/dc18-1581
55. Monnier L, Colette C, Wojtusciszyn A, Dejager S, Renard E, Molinari N, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care (2017) 40(7):832–8. doi: 10.2337/dc16-1769
56. Gáborová M, Doničová V, Bačová I, Pallayová M, Bona M, Peregrim I, et al. Glycaemic variability and risk factors of pregnant women with and without gestational diabetes mellitus measured by continuous glucose monitoring. Int J Environ Res Public Health (2021) 18(7). doi: 10.3390/ijerph18073402
57. Quah PL, Tan LK, Lek N, Thain S, Tan KH. Glycemic variability in early pregnancy may predict a subsequent diagnosis of gestational diabetes. Diabetes Metab Syndr Obes (2022) 15:4065–74. doi: 10.2147/DMSO.S379616
58. Yang H. Current situation and countermeasures of diagnosis and treatment of gestational diabetes in China Chinese Journal of Practical Gynecology and Obstetrics. (2013).
59. Alfadhli E, Osman E, Basri T. Use of a real time continuous glucose monitoring system as an educational tool for patients with gestational diabetes. Diabetol Metab Syndr (2016) 8:48. doi: 10.1186/s13098-016-0161-5
60. McLachlan K, Jenkins A, O'Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol (2007) 47(3):186–90. doi: 10.1111/j.1479-828X.2007.00716.x
61. Lenhard MJ, Kinsley BT. Insulin therapy for the treatment of type 1 diabetes during pregnancy. J Matern Fetal Neonatal Med (2014) 27(12):1270–5. doi: 10.3109/14767058.2013.864631
62. Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet (2017) 390(10110):2347–59. doi: 10.1016/S0140-6736(17)32400-5
63. Nørgaard SK, Mathiesen ER, Nørgaard K, Ringholm L. Comparison of glycemic metrics measured simultaneously by intermittently scanned continuous glucose monitoring and real-time continuous glucose monitoring in pregnant women with type 1 diabetes. Diabetes Technol Ther (2021) 23(10):665–72. doi: 10.1089/dia.2021.0109
64. Voormolen DN, DeVries JH, Sanson RME, Heringa MP, de Valk HW, Kok M, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): A multicentre randomized controlled trial. Diabetes Obes Metab (2018) 20(8):1894–902. doi: 10.1111/dom.13310
65. Jones LV, Ray A, Buckley BS, West HM. Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes. Cochrane Database Syst Rev (2019) 5(5):Cd009613. doi: 10.1002/14651858.CD009613.pub4
66. Li Z, Cheng Y, Wang D, Chen H, Chen H, Ming WK, et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review and meta-analysis of 170,139 women. J Diabetes Res 2020 (2020), 3076463. doi: 10.1155/2020/3076463
67. García-Moreno RM, Benítez-Valderrama P, Barquiel B, González Pérez-de-Villar N, Hillman N, Lora Pablos D, et al. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabetes Med (2022) 39(1):e14703. doi: 10.1111/dme.14703
68. Majewska A, Stanirowski P, Wielgoś M, Bomba-Opoń D. Flash glucose monitoring in gestational diabetes mellitus: study protocol for a randomised controlled trial. BMJ Open (2021) 11(3):e041486. doi: 10.1136/bmjopen-2020-041486
69. Yu F, Lv L, Liang Z, Wang Y, Wen J, Lin X, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab (2014) 99(12):4674–82. doi: 10.1210/jc.2013-4332
70. Sugiyama T, Nagao K, Metoki H, Nishigori H, Saito M, Tokunaga H, et al. Pregnancy outcomes of gestational diabetes mellitus according to pre-gestational BMI in a retrospective multi-institutional study in Japan. Endocr J (2014) 61(4):373–80. doi: 10.1507/endocrj.EJ13-0541
71. Rasmussen L, Christensen ML, Poulsen CW, Rud C, Christensen AS, Andersen JR, et al. Effect of high versus low carbohydrate intake in the morning on glycemic variability and glycemic control measured by continuous blood glucose monitoring in women with gestational diabetes mellitus-A randomized crossover study. Nutrients (2020) 12(2). doi: 10.3390/nu12020475
72. Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during pregnancy: A maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int J Mol Sci (2021) 22(6):2965. doi: 10.3390/ijms22062965
73. McGillick EV, Morrison JL, McMillen IC, Orgeig S. Intrafetal glucose infusion alters glucocorticoid signaling and reduces surfactant protein mRNA expression in the lung of the late-gestation sheep fetus. Am J Physiol Regul Integr Comp Physiol (2014) 307(5):R538–45. doi: 10.1152/ajpregu.00053.2014
74. Márquez-Pardo R, Torres-Barea I, Córdoba-Doña JA, Cruzado-Begines C, García-García-Doncel L, Aguilar-Diosdado M, et al. Continuous glucose monitoring and glycemic patterns in pregnant women with gestational diabetes mellitus. Diabetes Technol Ther (2020) 22(4):271–7. doi: 10.1089/dia.2019.0319
75. Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care (2013) 36(7):1877–83. doi: 10.2337/dc12-2360
76. Law GR, Alnaji A, Alrefaii L, Endersby D, Cartland SJ, Gilbey SG, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care (2019) 42(5):810–5. doi: 10.2337/dc18-2212
Keywords: gestational diabetes, continuous glucose monitoring, CGM, pregnancy outcome, perinatal outcome
Citation: Song Y, Zhai X, Bai Y, Liu C and Zhang L (2023) Progress and indication for use of continuous glucose monitoring in patients with diabetes in pregnancy: a review. Front. Endocrinol. 14:1218602. doi: 10.3389/fendo.2023.1218602
Received: 07 May 2023; Accepted: 07 August 2023;
Published: 23 August 2023.
Edited by:
Guoju Li, Qingdao Women and Children’s Hospital, ChinaReviewed by:
David Hill, Lawson Health Research Institute, CanadaMaria Masulli, Federico II University Hospital, Italy
Copyright © 2023 Song, Zhai, Bai, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Zhang, emhhbmdsZTE5ODYyNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yu Song
Yu Song Xiaodan Zhai
Xiaodan Zhai Yu Bai
Yu Bai