
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 20 December 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1216160
 Tanawan Kongmalai1,2,3
Tanawan Kongmalai1,2,3 Phorntida Hadnorntun2
Phorntida Hadnorntun2 Pattara Leelahavarong2
Pattara Leelahavarong2 Pinkawas Kongmalai4
Pinkawas Kongmalai4 Varalak Srinonprasert1,2,5
Varalak Srinonprasert1,2,5 Srisakul Chirakarnjanakorn6
Srisakul Chirakarnjanakorn6 Usa Chaikledkaew1,7
Usa Chaikledkaew1,7 Gareth McKay8
Gareth McKay8 John Attia9
John Attia9 Ammarin Thakkinstian1,10*
Ammarin Thakkinstian1,10*Background: In patients with type 2 diabetes (T2D) and a history of heart failure (HF), sodium–glucose cotransporter-2 inhibitors (SGLT2is) have demonstrated cardiovascular (CV) benefits. However, the comparative efficacy of individual SGLT2is remains uncertain. This network meta-analysis (NMA) compared the efficacy and safety of five SGLT2is (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and sotagliflozin) on CV outcomes in these patients.
Materials and methods: PubMed, Embase, and the Cochrane Central Register of Controlled Trials were searched up to September 23, 2022, to identify all randomized controlled trials (RCTs) comparing SGLT2is to placebo in T2D patients with HF. The main outcomes included composite CV death/heart failure hospitalization (HFH), HFH, CV death, all-cause mortality, and adverse events. Pairwise and NMA approaches were applied.
Results: Our analysis included 11 RCTs with a total of 20,438 patients with T2D and HF. All SGLT2is significantly reduced HFH compared to standard of care (SoC) alone. “Add-on” SGLT2is, except ertugliflozin, significantly reduced composite CV death/HFH relative to SoC alone. Moreover, canagliflozin had lower composite CV death/HFH compared to dapagliflozin. Based on the surface under the cumulative ranking curve (SUCRA), the top-ranked SGLT2is for reducing HFH were canagliflozin (95.5%), sotagliflozin (66.0%), and empagliflozin (57.2%). Head-to-head comparisons found no significant differences between individual SGLT2is in reducing CV death. “Add-on” SGLT2is reduced all-cause mortality compared with SoC alone, although only dapagliflozin was statistically significant. No SGLT2is were significantly associated with serious adverse events. A sensitivity analysis focusing on HF-specific trials found that dapagliflozin, empagliflozin, and sotagliflozin significantly reduced composite CV death/HFH, consistent with the main analysis. However, no significant differences were identified from their head-to-head comparisons in the NMA. The SUCRA indicated that sotagliflozin had the highest probability of reducing composite CV death/HFH (97.6%), followed by empagliflozin (58.4%) and dapagliflozin (44.0%).
Conclusion: SGLT2is significantly reduce the composite CV death/HFH outcome. Among them, canagliflozin may be considered the preferred treatment for patients with diabetes and a history of heart failure, but it may also be associated with an increased risk of any adverse events compared to other SGLT2is. However, a sensitivity analysis focusing on HF-specific trials identified sotagliflozin as the most likely agent to reduce CV death/HFH, followed by empagliflozin and dapagliflozin.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022353754.
Heart failure (HF) is a prevalent and debilitating complication of type 2 diabetes (T2D), contributing to increase morbidity and mortality in affected individuals. Worldwide, more than 26 million people are affected by this condition (1, 2), and T2D is a well-established risk factor with approximately 10%–30% of T2D patients aged over 70 years reported to have had HF (3).
Comorbid T2D with cardiovascular disease (CVD) is associated with higher mortality (4), highlighting the importance of reducing the risk of CVD in T2D management. A previous systematic review and meta-analysis (SRMA) indicated that intensive glucose lowering was not significantly associated with CVD risk reduction but conversely increased HF by 47% (5). Novel strategies are therefore necessary to improve prognosis and lower mortality in patients with T2D.
Sodium–glucose cotransporter-2 inhibitors (SGLT2is) are relatively recent oral anti-diabetic drugs (OADs) that decrease renal tubular glucose reabsorption (6). Although they provide modest glycemic control, cardiometabolic and hemodynamic improvements are evident with a low risk of hypoglycemia (7, 8). As a result, SGLT2is are strongly recommended in clinical practice guidelines for HF (9, 10). Although individual SGLT2is have similar mechanistic effects, pharmacological variations have resulted in variable efficacy and safety in cardiovascular outcome trials (CVOTs) (11, 12), making the most appropriate choice of SGLT2i challenging. For instance, sotagliflozin has the lowest SGLT2/SGLT1 selectivity, canagliflozin has the lowest oral bioavailability, empagliflozin has the highest SGLT2 protein selectivity, and ertugliflozin has the highest oral bioavailability (13). Although several studies have shown the benefits of SGLT2is in reducing heart failure hospitalization (HFH) in T2D (14–19), the effects reported for each SGLT2i vary, and the effects on cardiovascular (CV) and all-cause mortality were inconsistent (14–21).
The associated costs of SGLT2is also limit their accessibility, especially in limited-resource settings. The cost for SGLT2i therapy in the United States ranged from $405.98 to $426.27/person/month, with an out-of-pocket cost of $36.76 to $56.64/person/month (22). However, given the variable individual medication pricing, an improved understanding of individual SGLT2i efficacy and safety will inform treatment decisions. Direct head-to-head comparisons of all SGLT2is are unlikely; a network meta-analysis (NMA) may provide indirect comparisons and a ranking of the efficacy and safety of individual SGLT2is.
Although several trials were conducted in patients with T2D, only ~10% had previously reported HF (19, 23, 24), in contrast to the ~50% of HF patients who had previously reported T2D (14, 15, 17, 20, 25). None of these studies were sufficiently powered to evaluate SGLT2i efficacy in HF-T2D patients. Previous SRMAs have evaluated the efficacy of SGLT2is in various patient groups (26–29), although none have specifically targeted HF-T2D or used an NMA approach. Therefore, this NMA was conducted to compare the CV benefits and adverse events (AEs) associated with individual SGLT2is in HF-T2D patients. Specifically, we aimed to determine which SGLT2i provides the greatest efficacy in reducing cardiovascular events in this patient population.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (30) and was registered in PROSPERO (CRD42022353754).
Three electronic databases, PubMed, Embase, and the Cochrane Central Register of Controlled Trials, were searched from inception to August 15, 2022, with an update on September 23, 2022, without language restrictions. The search terms and strategies are provided in Supplementary Table S1. The titles and abstracts were reviewed by two independent reviewers (TK and PH), and disagreements were resolved with a third reviewer (PL). The inclusion criteria included i) randomized controlled trials (RCTs) or their subgroup or post-hoc analyses of SGLT2is in HF-T2D, ii) compared SGLT2is with standard of care (SoC), and iii) included any outcome of interest (i.e., composite CV death/HFH, HFH, CV death, and all-cause mortality).
Two reviewers (TK and PH) independently extracted the data; disagreements were adjudicated by PL. The data extractions included i) study characteristics (i.e., study participants and number, study design, follow-up period, age, sex, baseline ejection fraction (EF), HF type (preserved/reduced EF), functional class (New York Heart Association (NYHA) Functional Classification), and other concomitant medications); (ii) interventions (SGLT2i type, dose, and duration); and (iii) outcomes (i.e., composite CV death/HFH, HFH, CV death, all-cause mortality, and AEs).
Interventions included individual SGLT2is, i.e., dapagliflozin (5 and 10 mg), canagliflozin (100 and 300 mg), empagliflozin (10 and 25 mg), sotagliflozin (200 and 400 mg), and ertugliflozin (5 and 15 mg).
Comparators included placebo or SoC for HF. HF treatment included device therapies, such as implantable cardioverter defibrillators and cardiac resynchronization therapy if indicated, in addition to medications, including diuretics, beta-blockers, mineralocorticoid receptor antagonists (MRAs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and sacubitril–valsartan.
The primary outcome included composite CV death/HFH originally defined by individual RCTs. Secondary outcomes included HFH, CV death, all-cause mortality, and any AEs (i.e., volume depletion, acute kidney injury (AKI), urinary tract infection, genital tract infection, diabetic ketoacidosis (DKA), and bone fracture) in addition to serious AEs (SAEs). SAEs were defined as i) death or immediate life-threatening event, ii) persistent or clinically significant disability or incapacity, iii) events requiring hospitalization, iv) events related to a congenital anomaly or birth defect, or v) deemed serious for any other reason (14, 31, 32).
Two authors (TK and PK) independently assessed the risk of bias (RoB) using the Cochrane Risk of Bias tool version 2 (RoB2) based on five domains: randomization process, deviations from the intended protocol, missing outcome data, measurement of the outcomes, and selection of the reported result. Disagreements were adjudicated by PL. The overall quality was graded as high, with some concern and a low risk of bias (33).
Effect sizes (i.e., unstandardized mean difference (USMD) and risk ratio (RR)) along with 95% confidence intervals (CIs) were estimated for continuous data and dichotomous outcomes. Heterogeneity was assessed using the Q-test and I2 statistics. If heterogeneity was present (Q test <0.1 or I2 > 50% (34)), a meta-analysis (MA) random-effects model was used; otherwise, a fixed-effects model was considered. A meta-regression investigated the heterogeneity source by fitting each co-variable in the model including age, sex, baseline EF, HF type (reduced or preserved EF), functional class (NYHA), SoC with any HF treatment (i.e., MRA, renin–angiotensin system inhibitor [RASi], and angiotensin receptor/neprilysin inhibitor [ARNI]), treatment duration, and acute/chronic HF.
A two-stage NMA was applied as follows. First, a relative treatment effect (i.e., lnRR and USMD) was estimated with common variance–covariance. Second, treatment effects were pooled across studies using a multivariate MA with a consistency model. Transitivity was evaluated by exploring patient characteristics between comparisons or intervention arms, where appropriate. The inconsistency assumption was assessed using a global design-by-treatment interaction model, if applicable. Relative treatment effects were ranked using a rankogram and surface under the cumulative ranking curve (SUCRA). Publication bias was assessed using Egger’s test and adjusted comparison funnel plots, which, if asymmetrical, were evaluated further using a contour-enhanced funnel plot.
Subgroup/sensitivity analyses were pre-planned by HF type (preserved and reduced EF) and concomitant use of ARNI, MRA, and RAS blockade as SoC, if data were available. Furthermore, clustered-ranking plots were used to evaluate and rank risks and benefits associated with individual SGLT2is.
STATA version 17 (StataCorp, College Station, TX, USA) was used for all analyses. A significance threshold of p < 0.05 was considered, except for the heterogeneity and Egger’s tests, where a p-value <0.10 was used.
A total of 7,952 articles were identified, but only 12 met the eligibility criteria. An updated search conducted on September 23, 2022, revealed one additional study (25). Among the 13 publications, two (14, 35) were from SOLOIST-WHF, and two were from EMPA-REG (31, 36), each reporting different outcomes of interest. This resulted in 13 articles drawn from 11 RCTs for inclusion within this NMA (see Figure 1). Among these, four RCTs assessed SGLT2is in patients with HF, with or without T2D (DAPA-HF (32), EMPEROR-reduced (37), EMPEROR-preserved (20), and DELIVER (25)), and two RCTs originally recruited patients with HF and T2D at baseline (CANONICAL (38) and SOLOIST-WHF (14, 35), hereafter referred to as “HF-specific” trials), while five RCTs (EMPA-REG outcome (31, 36), CANVAS (24), DECLARE-TIMI 58 (39), VERTIS-CV (40), and SCORED (41)) reported HF outcomes in subgroup or post-hoc analyses in T2D patients, hereafter called “T2D-specific” trials.
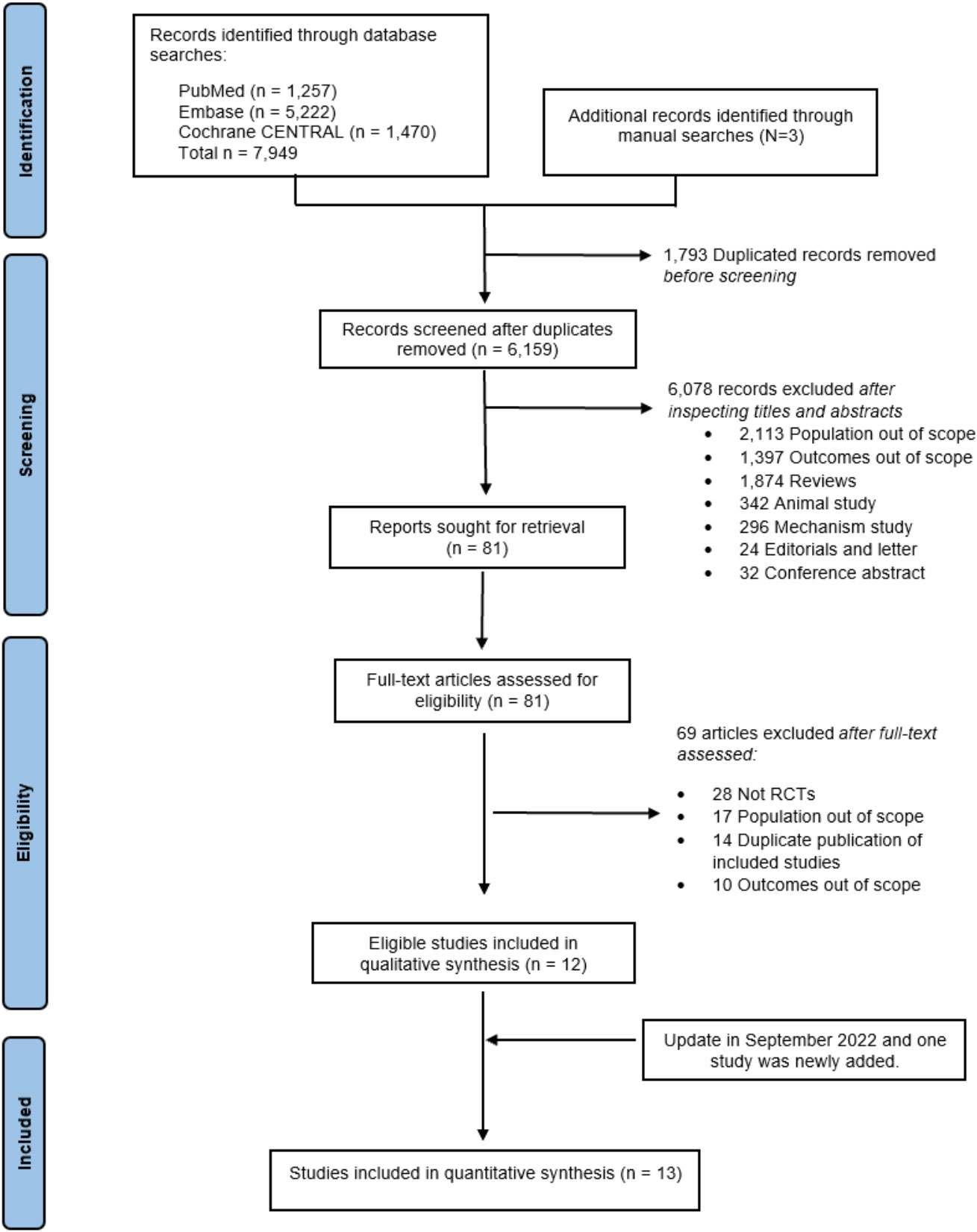
Figure 1 PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study characteristics are summarized in Table 1; the median age was 67.3 years, and the percentage of female was 2.3 to 43.9, with a median follow-up time of 0.5 to 3.6 years (mean 1.7 years). There was evidence of elevated N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) in all HF-specific trials, but no reports in T2D-specific trials. NYHA was mainly class II. The HF medications at baseline are shown in Supplementary TableS2.
Ten of the 11 studies (n = 20,191) reported a composite CV death/HFH outcome. A direct MA approach was applied to pool the treatment effects for dapagliflozin (25, 32, 39) (n = 6,932), empagliflozin (20, 31, 37) (n = 5,500), and sotagliflozin (14, 41) (n = 4,505) relative to SoC; canagliflozin (24) (n = 1,461) and ertugliflozin (40) (n = 1,958) were not pooled, as these were single studies. Dapagliflozin, empagliflozin, and sotagliflozin were associated with significant reductions in composite CV death/HFH with pooled RRs (95% CI;I2) of 0.80 (0.72–0.87; I2 = 8.48%), 0.79 (0.71–0.88; I2 = 0.00%), and 0.74 (0.68–0.81; I2 = 29.38%), respectively (see Figure 2). Overall pooled SGLT2i effects were also significantly associated with reduced composite CV death/HFH with an RR (95% CI) of 0.77 (0.73–0.81; I2 = 12.81%).
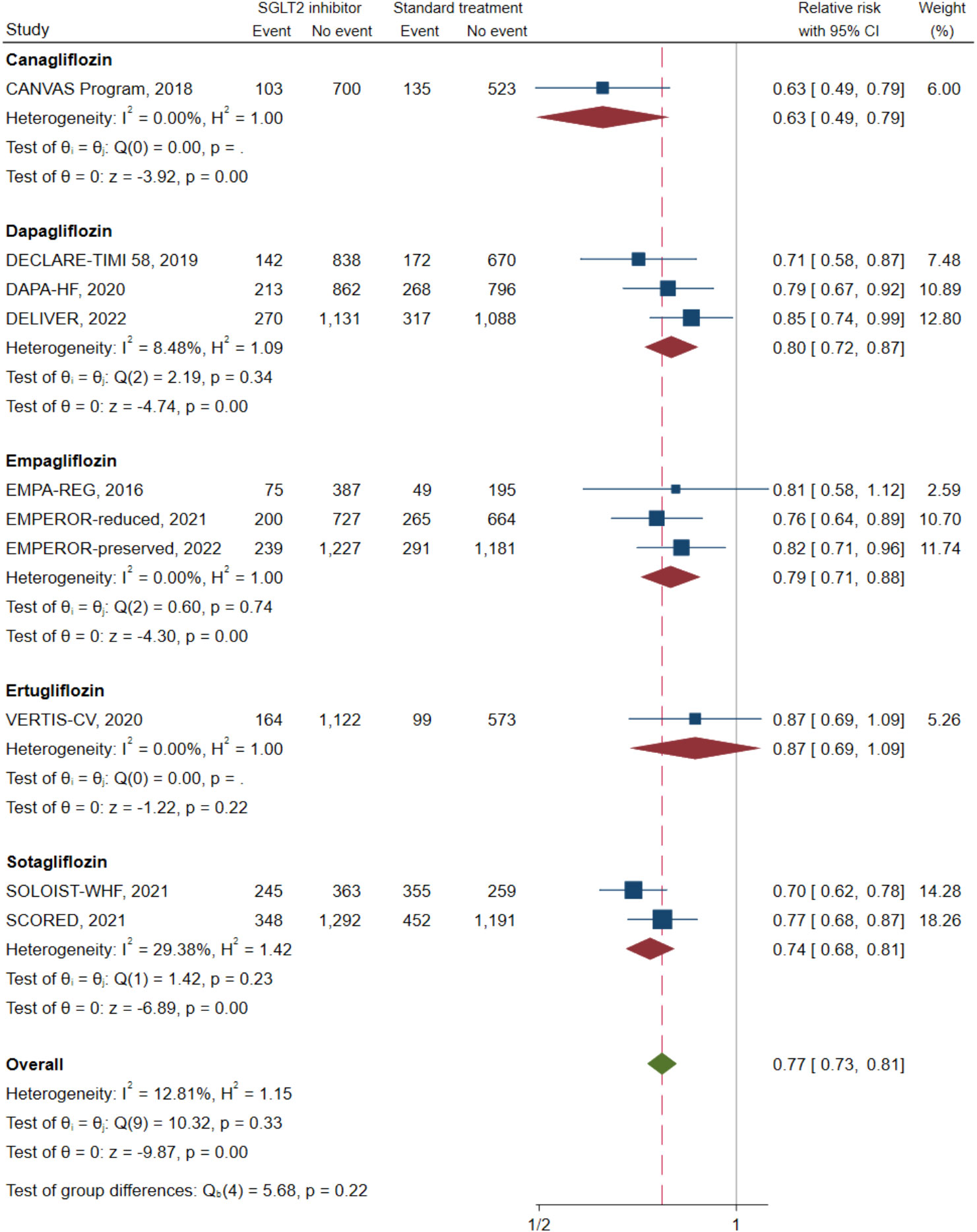
Figure 2 Composite cardiovascular death or heart failure hospitalization in type 2 diabetes with heart failure patients receiving SGLT2 inhibitors versus standard of care. SGLT2, sodium–glucose cotransporter-2.
The NMA (see Figure 3A) indicated that with the exception of ertugliflozin, these “add-on” medications were associated with a significantly lowered RR of a composite CV death/HFH outcome between 13% and 37% relative to SoC (see Table 2). Of the SGLT2is, both canagliflozin and sotagliflozin had significantly lower composite CV death/HFH compared to dapagliflozin with pooled RRs (95% CI) of 0.75 (0.59, 0.97) and 0.88 (0.78, 1.00), respectively (see Table 2). The SUCRA ranking identified “add-on” canagliflozin to SoC as the best for reducing composite CV death/HFH (SUCRA 95.9%), followed by sotagliflozin (SUCRA 77.4%) and empagliflozin (SUCRA 53.2%) (see Supplementary Table S3, Supplementary Figure S1A).

Figure 3 The network plot of the included studies. (A) Composite cardiovascular (CV) death or heart failure hospitalization (HFH). (B) Heart failure hospitalization (HFH). (C) Cardiovascular death (CV death). (D) All-cause mortality. The size of the nodes indicates the total sample size of the associated intervention (blue circles). The thickness of each line represents a direct comparison between two therapies and corresponds to the number of trials that examined each comparison.
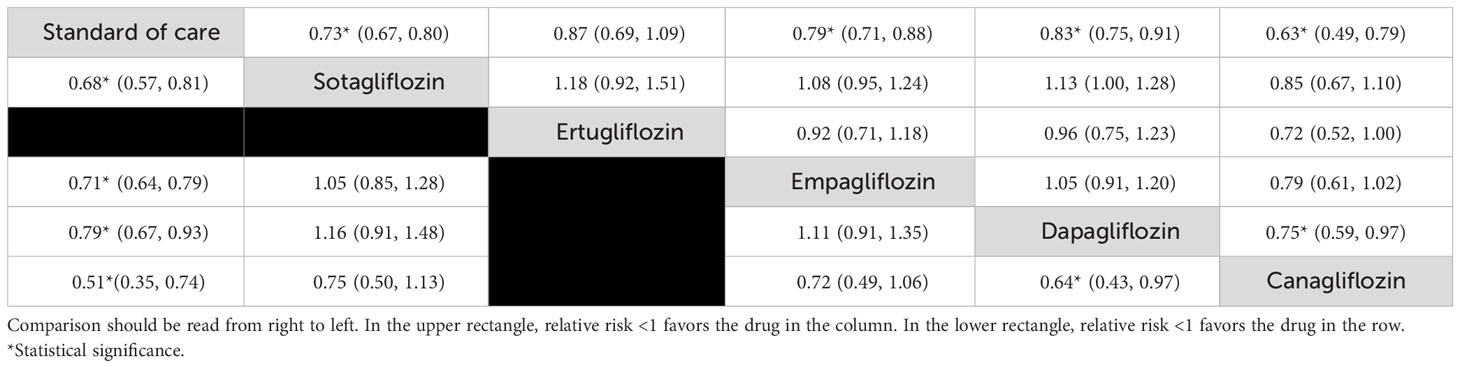
Table 2 Relative treatment effect comparison (95% CI) for composite cardiovascular death or heart failure hospitalization (HFH) (upper triangle) and HFH (lower triangle).
Of the eight studies (n = 12,391) that reported HFH outcomes, two (32, 39) (n = 4,126), one (14) (n = 1,222), two (24, 38) (n = 1,543), and three (20, 31, 37) (n = 5,500) studies investigated dapagliflozin, sotagliflozin, canagliflozin, and empagliflozin, respectively (see Supplementary Figure S2A). Canagliflozin, dapagliflozin, and empagliflozin were associated with a significant reduction in HFH relative to SoC with pooled RRs (95% CI; I2) of 0.51 (0.35–0.74; I2 = 0.00%), 0.79 (0.67–0.93; I2 = 0.00%), and 0.71 (0.64–0.79; I2 = 11.98%), respectively. Pooling of all SGLT2is provided an RR (95% CI;I2) of 0.71 (0.71(0.66-0.76) I2 = 6.64%).
The NMA (see Figure 3B) indicated that “add-on” canagliflozin, sotagliflozin, empagliflozin, and dapagliflozin treatment led to significant reductions in HFH with RRs (95% CI) of 0.51 (0.35, 0.74), 0.68 (0.57, 0.81), 0.71 (0.64, 0.79), and 0.79 (0.67, 0.93), respectively (Table 2). Furthermore, canagliflozin was associated with significant reductions in HFH compared to dapagliflozin [RR (95% CI) 0.64 (0.43, 0.97)]. The SUCRA indicated that the top three ranked SGLT2is were canagliflozin (95.5%), sotagliflozin (66.0%), and empagliflozin (57.2%) (see Supplementary Table S3, Supplementary Figure S2B).
Of the nine studies (n = 14,349) that reported CV death as an outcome, an MA approach was applied across two (32, 39) (n = 4,126), two (24, 38) (n = 1,543), and three (20, 31, 37) (n = 5,500) studies investigating dapagliflozin, canagliflozin, and empagliflozin relative to SoC, respectively (see Supplementary Figure S3A). Add-on canagliflozin, dapagliflozin, and empagliflozin were not associated with significantly reduced CV death, with pooled RRs (95% CI; I2) of 0.78 (0.57–1.06; I2 = 0.00%), 0.89 (0.74–1.06; I2 = 40.37%), and 0.92 (0.79–1.09; I2 = 0.00%), respectively. However, overall pooling for all SGLT2is was associated with a significant reduction in CV death with an RR (95% CI) of 0.90 (0.81–0.99) without evidence of heterogeneity (I2 = 0.00%).
The NMA showed that “add-on” SGLT2is tended to reduce CV death compared to SoC alone, although individually none were significant (see Table 3, Figure 3C). The top three ranked interventions were canagliflozin (81.5%), dapagliflozin (60.0%), and sotagliflozin (52.9%) (see Supplementary Table S3, Supplementary Figure S3B).
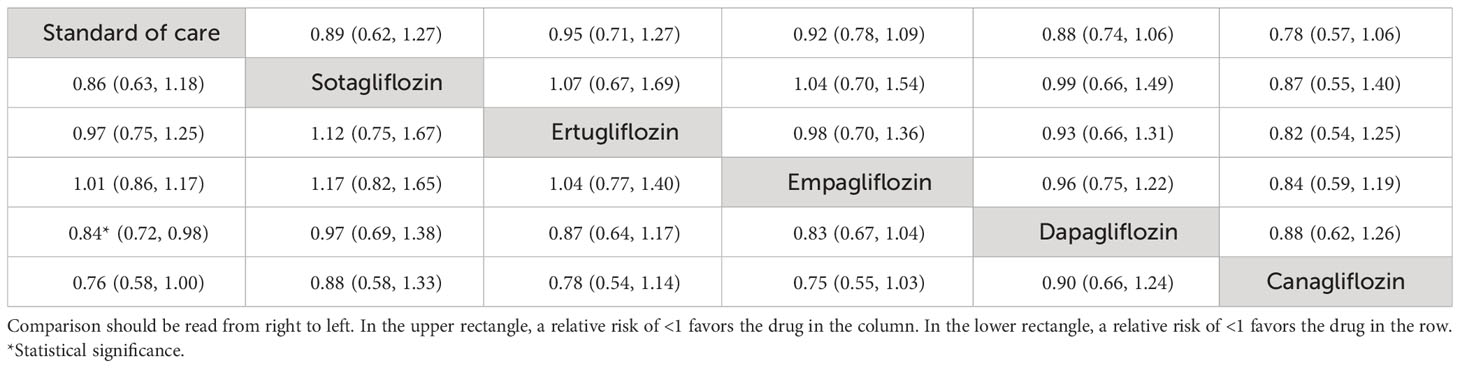
Table 3 Relative treatment effect comparisons (95% CI) for cardiovascular death (upper triangle) and all-cause mortality (lower triangle).
Of the eight studies (n = 12,493) that reported all-cause mortality outcomes, two (32, 39) (n = 4,126), two (24, 38) (n = 1,543), and two (20, 31) (n = 3,644) studies investigated dapagliflozin, canagliflozin, and empagliflozin relative to SoC, respectively. Only “add-on” dapagliflozin was associated with significant reductions in all-cause mortality with an RR of 0.84 (0.72, 0.98; I2 = 0.00), while the remaining SGLT2is were not significant. Collectively, the overall pooled effect of SGLT2is was associated with significantly reduced all-cause mortality with an RR (95% CI) of 0.90 (0.82, 0.99) with no evidence of heterogeneity (I2 = 0.00%) (Supplementary Figure S4A).
The NMA (Figure 3D) showed that “add-on” dapagliflozin and canagliflozin significantly reduced all-cause mortality compared to SoC alone with RRs (95% CI) of 0.84 (0.72, 0.98) and 0.76 (0.58, 1.00) (Table 3). The top-ranked interventions by SUCRA were canagliflozin (86.1%), dapagliflozin (72.6%), and sotagliflozin (59.4%) (see Supplementary Table S3, Supplementary Figure S4B).
Seven studies (n = 12,303) reported SAEs associated with dapagliflozin (32, 39) (n = 4,123), sotagliflozin (14) (n = 1,216), canagliflozin (24) (n = 1,461), and empagliflozin (20, 31, 37) (n = 5,503). Dapagliflozin and empagliflozin were associated with a lower risk of SAEs than SoC with pooled RRs (95% CI;I2) of 0.87 (0.80–0.95; I2 = 0.00%) and 0.89 (0.85–0.94; I2 = 0.00%), respectively. The overall pooled SGLT2i RR associated with SAEs compared with SoC was 0.87 (95% CI: 0.83, 0.91), I2 = 22.31% (see Supplementary Figure S5A). The NMA (Supplementary Figure S5B) identified canagliflozin, dapagliflozin, and empagliflozin were associated with a significantly reduced risk of SAEs compared to SoC with RRs (95% CI) of 0.81 (0.75–0.87), 0.87 (0.80–0.95), and 0.89 (0.85–0.94), respectively. Canagliflozin was also borderline significant when compared with empagliflozin [RR 0.90 (0.82–0.99)] and sotagliflozin [RR 0.85 (0.73–1.00)] (see Supplementary Table S4) Supplementary Figure S5C and Supplementary Table S3 show the probability of serious AEs of each SGLT2i.
Eight studies (n = 12,377) reported outcomes related to any AEs. Of these, a direct MA approach was applied for canagliflozin (24, 38) (n = 1543), dapagliflozin (32, 39) (n = 4,123), and empagliflozin (20, 31, 37) (n = 5,495). None of the three SGLT2is were significantly associated with any AE outcomes with pooled RRs (95% CI; I2) of 1.36 (0.93–1.98; I2 = 69.22%), 1.05 (0.96–1.15; I2 = 0.00%), and 1.04 (0.91–1.19; I2 = 27.08%). The overall pooled RRs across all SGLT2is was 1.07 (95% CI: (0.99-1.15)), I2 = 24.98% (see Supplementary Figure S5E). The NMA (Supplementary Figure S5F) indicated that canagliflozin was associated with a significantly increased risk of any adverse event with RR of (95% CI) of 1.50 (1.01–2.23), 1.45 (0.96–2.2), and 1.42 (0.95–2.13) compared with SoC, empagliflozin, and dapagliflozin, respectively (see Supplementary Table S5). Canagliflozin was also associated with the highest probability of any AEs (89.5%), followed by sotagliflozin (71.0%) and dapagliflozin (43.4%) (see Supplementary Table S3, Supplementary Figure S5G).
Clustered ranking suggested that canagliflozin and sotagliflozin offered the best efficacy in reducing HFH and composite CV death/HFH, although both had higher risks associated with any AE. Empagliflozin was associated with a high probability of reducing HFH and the lowest probability of any AEs (Figure 4).
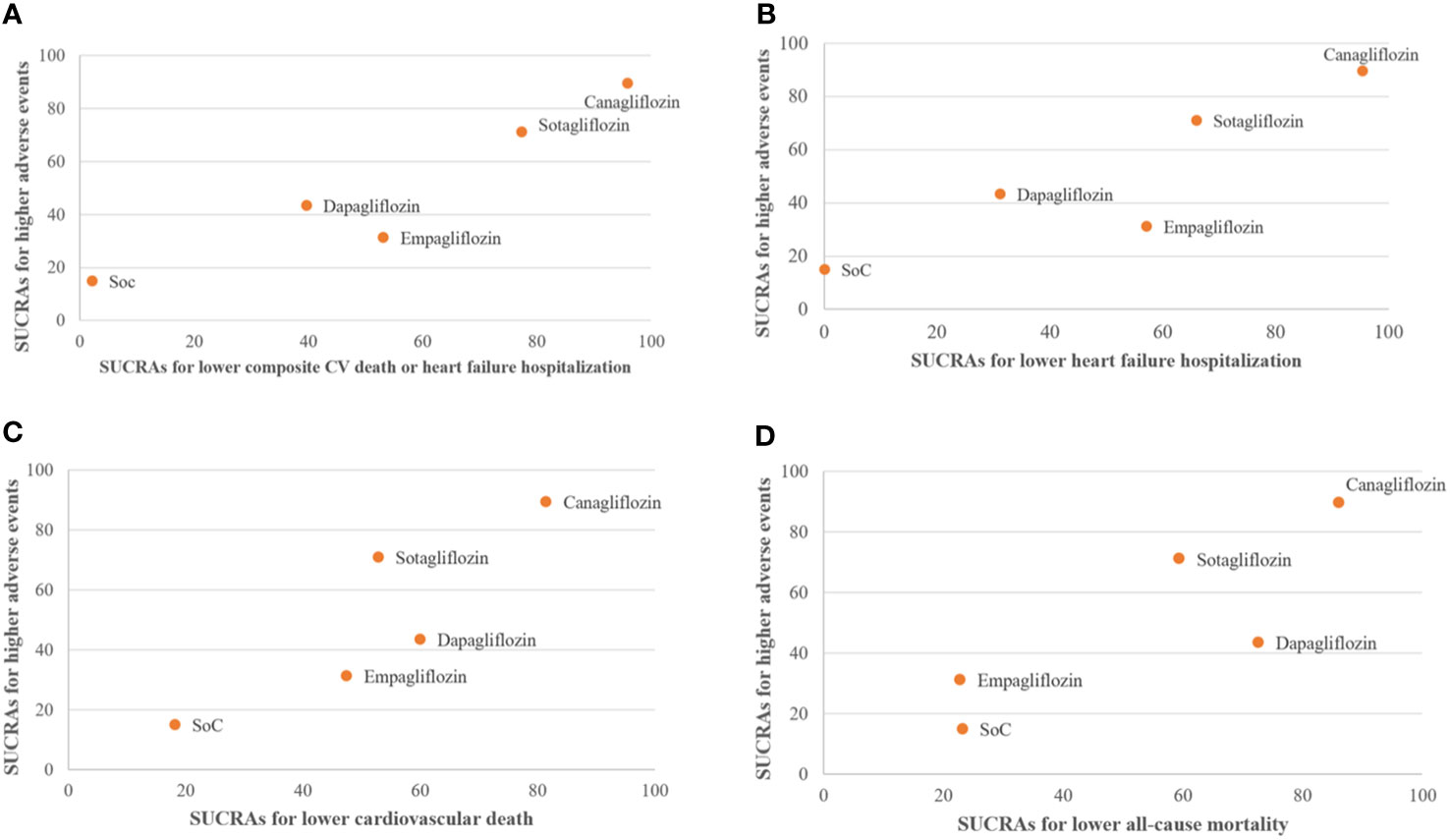
Figure 4 Clustered ranking plot of standard of care (SoC), canagliflozin, dapagliflozin, empagliflozin, and sotagliflozin showing the surface under the cumulative ranking curves (SUCRAs) for the highest probability of any adverse events (AEs) versus the SUCRAs for the highest probability of improving cardiovascular outcomes. (A) Composite cardiovascular (CV) death or heart failure hospitalization. (B) Heart failure hospitalization. (C) CV death. (D) All-cause mortality. Intervention lying in the upper right corners are associated with the higher- of probability of treatment efficacy and "higher" probability of AEs.
Characteristics for each comparison, including age, sex, the strength of the medication, baseline EF, functional class, concurrent medications, and treatment duration, were explored across comparisons (see Supplementary Table S6). Age, percentage of females, and EF varied between comparisons. In a network of the composite CV death/HFH outcome, patients in the canagliflozin–SoC comparison were more likely to be younger and female compared to patients in other comparisons. In HFH, CV death, and all-cause mortality networks, patients in the canagliflozin–SoC comparison were more likely to be female and have a higher EF compared to other comparisons. Patients in the ertugliflozin–SoC comparison were more likely to be younger, while those in the sotagliflozin–SoC comparison were more likely to have had a shorter follow-up period for CV death and all-cause mortality networks.
The RoB was assessed by both reviewers (TK, PK) with 72.73% agreement (kappa 0.48, p = 0.03). Of the 11 RCTs, five were considered low risk, and six had some concerns; the major domain driving this tended to be the randomization process. Given that NMA focused on HF and T2D, studies that did not stratify analyses based on diabetes or HF were considered to have some concerns regarding randomization (Supplementary Figure S6).
Comparison-adjusted funnel plots indicated evidence of asymmetry associated with HFH, CV death, and all-cause mortality networks due to small-study effects from a single study (see Supplementary Figures S1B, S2C, S3C, S4C, S5D, S5H). However, this was due to a very small effect size.
Since the HF diagnostic criteria varied between HF-specific RCTs and post-hoc analyses of CV death/HFH death outcomes, we performed a sensitivity analysis that included only HF-specific trials at baseline. Consequently, only three SGLT2is, i.e., empagliflozin, dapagliflozin, and sotagliflozin, were retained within the analysis. The results showed that dapagliflozin, empagliflozin, and sotagliflozin significantly reduced composite CV death/HFH with corresponding RRs (95% CI) of 0.82 (0.74–0.92), 0.79 (0.71–0.89), and 0.70 (0.62–0.78); these RRs were comparable with the original analysis that included all HF-specific and T2D-specific trials with corresponding RRs of 0.80 (0.72–0.87), 0.79 (0.71–0.88), and 0.74 (0.68–0.81) (see Supplementary Figure S7). A NMA also identified add-on therapies in combination with these three SGLT2is to be significantly associated with reduced risk of composite CV death/HFH compared to SoC. However, no significant differences in head-to-head comparisons were identified (see Supplementary Table S7). Similar to our main analysis, SUCRA identified sotagliflozin with the highest probability of reducing composite CV death/HFH (97.6%), followed by empagliflozin (58.4%) and dapagliflozin (44.0%).
In addition, we performed a sensitivity analysis that excluded a single study with a small-study effect (sample size <100) and a treatment duration of less than 6 months (38) from the main analysis. However, our findings remained unchanged (see Supplementary Figure S8).
The Confidence in Network Meta-Analysis (CiNeMA) for each outcome is shown in Supplementary Table S8. The minimal clinically important differences for each outcome were set according to the Dutch guidelines committee T2D in primary care (42). CiNeMA indicated canagliflozin, sotagliflozin, and empagliflozin had very low confidence ratings for composite CV death/HFH. Within-study bias, reporting bias, and incoherence were the reasons for these downgrades. There was significant concern with incoherence given the lack of a closed loop within the network framework.
A NMA was conducted and revealed that when added to SoC, SGLT2is significantly reduce the composite outcomes of CV death/HFH. Notably, canagliflozin was the most effective, followed by sotagliflozin, while dapagliflozin and empagliflozin exhibited comparable efficacy. The addition of SGLT2is beyond SoC reduced CV death by between 8% and 22%. Only dapagliflozin and canagliflozin were associated with lower all-cause mortality compared to SoC. Importantly, we did not find any statistically significant associations between SGLT2is and adverse side effects or SAEs.
Our findings indicate that SGLT2is reduce composite CV death/HFH outcomes in patients with T2D and previously documented HF by approximately 20%. Although our study encompasses participants from both HF-specific trials and post-hoc analyses, our main findings and sensitivity analyses align with those previously reported in an SRMA that focused exclusively on HF-specific trials (43). Notably, the composite outcome of CV death/HFH was primarily influenced by HFH. In our analysis, canagliflozin and sotagliflozin ranked first and second, respectively, in reducing HFH, while they ranked first and third, respectively, in reducing CV death. This ranking is consistent with previous NMA findings (44), which support the notion that non-selective SGLT2is may offer greater advantages in treating HF compared to selective SGLT2is for reducing HFH (44). It is hypothesized that SGLT1 plays a pivotal role in glucose absorption in the intestines, and concurrent inhibition of SGLT1 and SGLT2 may further enhance renal sodium and glucose reabsorption. Furthermore, SGLT1 receptors are expressed in the human myocardium, and their upregulation has been observed in HF patients (45). However, the understanding of the role of SGLT1 cardiac expression and its interactions with SGLT2 in HF patients remains limited.
This study reveals that despite differences in chemical structure, pharmacokinetic and pharmacodynamic properties, as well as variations in SGLT1/SGLT2 receptor selectivity, all SGLT2is investigated in this study generally reduce the risk of HFH, consistent with previous SRMA results (8). We also observed little disparity in the efficacy of individual SGLT2is, with the exception of dapagliflozin, which exhibited a 36% higher rate of HFH compared to canagliflozin. As such, our findings support the beneficial effects of SGLT2is in reducing HFH as a class effect. Notably, the natriuretic and diuretic effects that lead to increased renal glucose excretion may have beneficial implications for endothelial progenitor cells, weight loss, improved myocardial energetics, adaptive cellular reprogramming, and reductions in both blood pressure and left ventricular hypertrophy (46–48).
Previous SRMAs have consistently reported a significant reduction of CV death in patients with T2D who were prescribed SGLT2is (HR 0.85, 95% CI 0.78–0.93, I2 = 64.5%, p = 0.02) (8) as well as in patients with HF with or without T2D (HR 0.87, 95% CI 0.79–0.95, p = 0.94) (43). Our study specifically focused on patients with comorbid HF and T2D, and our findings align with the previously reported evidence. Furthermore, our NMA highlights that canagliflozin and dapagliflozin provide the greatest reduction in the risk of CV death, corroborating earlier research (44). Interestingly, we did not observe any significant differences in the ability to reduce CV death between selective and non-selective SGLT2is.
Our findings demonstrate that SGLT2is can reduce all-cause mortality in patients with HF-T2D by approximately 10%. However, only dapagliflozin reached statistical significance, possibly due to the inclusion of two large-scale placebo-controlled RCTs (DECLARE-TIMI and DAPA-HF). The robust reduction in all-cause mortality observed in our study was predominantly driven by the DAPA-HF trial, which revealed a remarkable 17% reduction in all-cause mortality in patients with HF-prescribed dapagliflozin, with or without T2D. In contrast, while the EMPA-REG RCT demonstrated a significant reduction in all-cause mortality, only 9.9% of the patients had a history of cardiac failure at baseline. Moreover, empagliflozin exhibited no survival benefits in the EMPEROR-reduced and EMPEROR-preserved RCTs. Similarly, the impact of canagliflozin, ertugliflozin, and sotagliflozin on mortality outcomes in patients with T2D and HF at baseline was found to be minimal in the CANVAS, VERTIS-CV, and SOLOIST-WHF RCTs, respectively.
The safety profile of SGLT2is is firmly established, encompassing known risks such as mycotic genital infections, urinary tract infections, diabetic ketoacidosis, volume depletion, kidney impairment (16, 19, 23), and the risk of amputation (21). Our findings, as corroborated by our NMA, confirm that SAEs were notably absent across all individual SGLT2is analyzed. However, our analysis did reveal an increased risk of any adverse event associated with canagliflozin.
Although the benefit of SGLT2is in reducing HFH appears to be a class effect, our findings highlight variations among individual SGLT2is in reducing CV and all-cause death, and safety profiles, which may be attributed to several factors. First, each SGLT2i exhibits distinct properties including their selectivity for SGLT1/SGLT2 inhibition, particularly within cardiomyocytes, which could influence CV and renal effects. Second, the differences in the characteristics of the study populations, concomitant medications, the duration of treatments, and follow-up time may introduce elements of heterogeneity, potentially confounding the observed outcomes.
Our NMA has several strengths: first, this is the first NMA to address uncertainties regarding the ranking of CV benefits provided by individual SGLT2is for HF-T2D patients. Second, our NMA includes a broader evidence base, incorporating more RCTs and a larger cohort of HF-T2D patients in comparison to the most recent SRMA (43) (20,438 vs. 9,739). Third, we have considered all available SGLT2is (5 SGLT2is vs. 3 SGLT2is) and have included additional CV outcome measures, including HFH, CV death, and all-cause mortality in HF-T2D patients. These efforts enable us to comprehensively rank the clinical efficacy and safety profile of individual SGLT2is across all of the CV outcomes of interest.
We also recognize several limitations in our study. First, we employed aggregated study-level data rather than individual patient data, which limited our ability to explore additional baseline factors that might potentially confound outcomes, including concomitant drug used, EF, and the etiology of HF (ischemic or non-ischemic heart disease). Second, our study outcomes may have been influenced by differences in patient populations, study designs, and trial durations. For instance, the SOLOIST-WHF trial focused on T2D patients with more severe HF, enrolling participants either before or within 3 days of HFH, whereas other studies included T2D patients with chronic HF. Variances in the duration of participant follow-up were also observed with CANONICAL and SOLOIST-WHF, which monitored participants for less than 1 year, while other RCTs had longer follow-up periods. Third, our study encompassed both HF-specific and post-hoc analysis of CVOTs. We observed disparities in the diagnostic criteria for HF between HF-specific RCTs and post-hoc CVOTs. Specifically, all participants enrolled in HF-specific trials exhibited elevated brain natriuretic peptide or NT-proBNP levels, which are established HF diagnostic biomarkers, while diagnostic criteria in CVOTs were less strictly defined. Nevertheless, it is noteworthy that despite these potential confounding factors, the observed heterogeneity in our NMA remained low, and the results from a sensitivity analysis that focused solely on HF-specific trials were consistent with the findings of the overall analysis. Fourth, the efficacy of canagliflozin is primarily derived from the post-hoc analysis of CANVAS studies, which did not specifically focus on heart failure at baseline. These results should be interpreted with caution. Fifth, many of the treatment comparisons in our NMA exhibited low confidence levels, as assessed using the six-domain CINeMA tool. These findings underscore the significance of taking into account the uncertainty associated with these comparisons when drawing conclusions from our study.
SGLT2is significantly reduce the composite CV death/HFH outcome. Among them, canagliflozin may be considered the preferred treatment for patients with diabetes and a history of heart failure, but it may also be associated with an increased risk of any adverse events compared to other SGLT2is. However, a sensitivity analysis focusing on HF-specific trials identified sotagliflozin as the most likely agent to reduce CV death/HFH, followed by empagliflozin and dapagliflozin.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
TK contributed to research topic initiation, study design, data collection, data analysis, data interpretation, risk of bias assessment and writing of the manuscript. PK contributed to the risk of bias assessment. PH contributed to the data collection. PL supervised the data collection and risk of bias assessment. VS, SC, UC, GM, and JA provided critical feedback in the writing of the manuscript. AT contributed to research methods, data analysis, data interpretation and provided critical feedback in writing of the manuscript. All authors contributed to the article and approved the submitted version.
This work is part of the training towards a PhD degree in Health Technology Assessment (HTA), for which a scholarship was provided by Mahidol University and the International Decision Support Initiative (iDSI) through a grant awarded to UC (OPP1087363). This study was conducted as part of the International Decision Support Initiative (www.idsihealth.org), which supports countries in obtaining the best value of money from health spending. The iDSI receives funding support from the Bill & Melinda Gates Foundation, the UK Department for International Development, and the Rockefeller Foundation. In addition, this work was also supported by the Medicine Regulations Division, Food and Drug Administration, Ministry of Public Health, Thailand. This study was conducted at the request of the National List of Essential Medicine (NLEM). This manuscript is a part of the project “Economic evaluation of add-on SGLT2 inhibitor to standard treatment in type 2 diabetes with congestive heart failure in Thailand” which was used to support the policy-making process under the Subcommittee for the Development of the NLEM in Thailand through the Health Economic Working Group (HEWG) but the HEWG is not responsible for the study findings or their dissemination. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This paper represents the views of the authors.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1216160/full#supplementary-material
1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev (2017) 3(1):7–11. doi: 10.15420/cfr.2016:25:2
2. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail (2013) 6(3):606–19. doi: 10.1161/HHF.0b013e318291329a
3. Ceriello A, Catrinoiu D, Chandramouli C, Cosentino F, Dombrowsky AC, Itzhak B, et al. Heart failure in type 2 diabetes: current perspectives on screening, diagnosis and management. Cardiovasc Diabetol (2021) 20(1):218. doi: 10.1186/s12933-021-01408-1
4. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol (2018) 17(1):83. doi: 10.1186/s12933-018-0728-6
5. Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ (2011) 343:d4169. doi: 10.1136/bmj.d4169
6. Saisho Y. SGLT2 inhibitors: the star in the treatment of type 2 diabetes? Diseases (2020) 8(2):1–12. doi: 10.3390/diseases8020014
7. Giugliano D, Longo M, Scappaticcio L, Bellastella G, Maiorino MI, Esposito K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs. Cardiovasc Diabetol (2021) 20(1):236. doi: 10.1186/s12933-021-01430-3
8. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol (2021) 6(2):148–58. doi: 10.1001/jamacardio.2020.4511
9. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care (2023) 46(Supplement_1):S140–S57. doi: 10.2337/dc23-S009
10. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J (2021) 42(48):4901. doi: 10.1093/eurheartj/ehab670
11. Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol (2022) 21(1):83. doi: 10.1186/s12933-022-01512-w
12. Papakitsou I, Vougiouklakis G, Elisaf MS, Filippatos TD. Differential pharmacology and clinical utility of dapagliflozin in type 2 diabetes. Clin Pharmacol (2019) 11:133–43. doi: 10.2147/CPAA.S172353
13. Williams DM, Nawaz A, Evans M. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: are they all the same? A narrative review of cardiovascular outcome trials. Diabetes Ther (2021) 12(1):55–70. doi: 10.1007/s13300-020-00951-6
14. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med (2021) 384(2):117–28. doi: 10.1056/NEJMoa2030183
15. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
16. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med (2020) 383(15):1425–35. doi: 10.1056/NEJMoa2004967
17. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
18. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
19. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
20. Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, Ferreira JP, et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation (2022) 146(9):676–86. doi: 10.1161/CIRCULATIONAHA.122.059785
21. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med (2017) 377(7):644–57. doi: 10.1056/NEJMoa1611925
22. Aggarwal R, Vaduganathan M, Chiu N, Bhatt DL. Out-of-pocket costs for SGLT-2 (Sodium-glucose transport protein-2) inhibitors in the United States. Circ Heart Fail (2022) 15(3):e009099. doi: 10.1161/CIRCHEARTFAILURE.121.009099
23. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
24. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation (2018) 138(5):458–68. doi: 10.1161/CIRCULATIONAHA.118.034222
25. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med (2022) 387(12):1089–98. doi: 10.1056/NEJMoa2206286
26. Tsampasian V, Elghazaly H, Chattopadhyay R, Ali O, Corballis N, Chousou PA, et al. Sodium glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Prev Cardiol (2022) 29(6):e227–e9. doi: 10.1093/eurjpc/zwab189
27. Zhang A, Luo X, Meng H, Kang J, Qin G, Chen Y, et al. Sodium glucose cotransporter 2 inhibitors reduce the risk of heart failure hospitalization in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) (2020) 11:604250. doi: 10.3389/fendo.2020.604250
28. Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta-analysis. ESC Heart Fail (2022) 9(2):942–6. doi: 10.1002/ehf2.13805
29. Shrestha DB, Budhathoki P, Sedhai YR, Karki P, Gurung S, Raut S, et al. Sodium-glucose cotransporter-2 inhibitors in heart failure: an updated systematic review and meta-analysis of 13 randomized clinical trials including 14,618 patients with heart failure. J Cardiovasc Pharmacol (2021) 78(4):501–14. doi: 10.1097/FJC.0000000000001099
30. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
31. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J (2016) 37(19):1526–34. doi: 10.1093/eurheartj/ehv728
32. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA (2020) 323(14):1353–68. doi: 10.1001/jama.2020.1906
33. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
34. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
35. Szarek M, Bhatt DL, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Effect of sotagliflozin on total hospitalizations in patients with type 2 diabetes and worsening heart failure: A randomized trial. Ann Intern Med (2021) 174(8):1065–72. doi: 10.7326/M21-0651
36. Pellicori P, Ofstad AP, Fitchett D, Zeller C, Wanner C, George J, et al. Early benefits of empagliflozin in patients with or without heart failure: findings from EMPA-REG OUTCOME. ESC Heart Fail (2020) 7(6):3401–07. doi: 10.1002/ehf2.12891
37. Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-reduced trial. Circulation (2021) 143(4):337–49. doi: 10.1161/CIRCULATIONAHA.120.051824
38. Ueda T, Kasama S, Yamamoto M, Nakano T, Ueshima K, Morikawa Y, et al. Effect of the sodium-glucose cotransporter 2 inhibitor canagliflozin for heart failure with preserved ejection fraction in patients with type 2 diabetes. Circ Rep (2021) 3(8):440–8. doi: 10.1253/circrep.CR-21-0030
39. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation (2019) 139(22):2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130
40. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation (2020) 142(23):2205–15. doi: 10.1161/CIRCULATIONAHA.120.050255
41. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med (2021) 384(2):129–39. doi: 10.1056/NEJMoa2030186
42. Dankers M, Nelissen-Vrancken MHJMG, Hart BH, Lambooij AC, van Dijk L, Mantel-Teeuwisse AK. Alignment between outcomes and minimal clinically important differences in the Dutch type 2 diabetes mellitus guideline and healthcare professionals’ preferences. Pharmacol Res Perspective (2021) 9:1–12. doi: 10.1002/prp2.750
43. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet (2022) 400(10354):757–67. doi: 10.1016/S0140-6736(22)01429-5
44. Tager T, Frankenstein L, Atar D, Agewall S, Frey N, Grundtvig M, et al. Influence of receptor selectivity on benefits from SGLT2 inhibitors in patients with heart failure: a systematic review and head-to-head comparative efficacy network meta-analysis. Clin Res Cardiol (2022) 111(4):428–39. doi: 10.1007/s00392-021-01913-z
45. Sayour AA, Olah A, Ruppert M, Barta BA, Horvath EM, Benke K, et al. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc Diabetol (2020) 19(1):159. doi: 10.1186/s12933-020-01141-1
46. Verma S, Mazer CD, Bhatt DL, Raj SR, Yan AT, Verma A, et al. Empagliflozin and cardiovascular outcomes in patients with type 2 diabetes and left ventricular hypertrophy: A subanalysis of the EMPA-REG OUTCOME trial. Diabetes Care (2019) 42(3):e42–e4. doi: 10.2337/dc18-1959
47. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART cardioLink-6 randomized clinical trial. Circulation (2019) 140(21):1693–702. doi: 10.1161/CIRCULATIONAHA.119.042375
48. Opingari E, Verma S, Connelly KA, Mazer CD, Teoh H, Quan A, et al. The impact of empagliflozin on kidney injury molecule-1: a subanalysis of the Effects of Empagliflozin on Cardiac Structure, Function, and Circulating Biomarkers in Patients with Type 2 Diabetes CardioLink-6 trial. Nephrol Dial Transplant (2020) 35(5):895–7. doi: 10.1093/ndt/gfz294
Keywords: sodium-glucose cotransporter 2 inhibitor (SGLT2 inhibitor), congestive heart failure, cardiovascular disease, diabetes mellitus, systematic review, network meta-analysis
Citation: Kongmalai T, Hadnorntun P, Leelahavarong P, Kongmalai P, Srinonprasert V, Chirakarnjanakorn S, Chaikledkaew U, McKay G, Attia J and Thakkinstian A (2023) Comparative cardiovascular benefits of individual SGLT2 inhibitors in type 2 diabetes and heart failure: a systematic review and network meta-analysis of randomized controlled trials. Front. Endocrinol. 14:1216160. doi: 10.3389/fendo.2023.1216160
Received: 03 May 2023; Accepted: 17 November 2023;
Published: 20 December 2023.
Edited by:
Jean Paul Deslypere, Aesculape CRO, BelgiumReviewed by:
Emanuele Gallinoro, OLV Aalst, BelgiumCopyright © 2023 Kongmalai, Hadnorntun, Leelahavarong, Kongmalai, Srinonprasert, Chirakarnjanakorn, Chaikledkaew, McKay, Attia and Thakkinstian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ammarin Thakkinstian, QW1tYXJpbi50aGFAbWFoaWRvbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.