
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 30 October 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1214651
 Yan Li1,2,3,4,5
Yan Li1,2,3,4,5 Xiaoqin Wu6
Xiaoqin Wu6 Yongfeng Song1,2,3,4,5,7
Yongfeng Song1,2,3,4,5,7 Peipei Wang7
Peipei Wang7 Bofei Zhang7
Bofei Zhang7 Bingzhou Guo8
Bingzhou Guo8 Ziwei Liu7
Ziwei Liu7 Yafei Wu1,2,3,4,5,7
Yafei Wu1,2,3,4,5,7 Shanshan Shao1,2,3,4,5,7
Shanshan Shao1,2,3,4,5,7 Yiping Cheng1,2,3,4,5,7*
Yiping Cheng1,2,3,4,5,7* Honglin Guo1,2,3,4,5,7*
Honglin Guo1,2,3,4,5,7* Xiude Fan1,2,3,4,5,7*
Xiude Fan1,2,3,4,5,7* Jiajun Zhao1,2,3,4,5,7*
Jiajun Zhao1,2,3,4,5,7*Purpose: Patients with digestive system cancers (DSCs) are at a high risk for hospitalizations; however, the risk factors for readmission remain unknown. Here, we established a retrospective cohort study to assess the association between metabolic obesity phenotypes and readmission risks of DSC.
Experimental design: A total of 142,753 and 74,566 patients at index hospitalization were ultimately selected from the Nationwide Readmissions Database (NRD) 2018 to establish the 30-day and 180-day readmission cohorts, respectively. The study population was classified into four groups: metabolically healthy non-obese (MHNO), metabolically healthy obese (MHO), metabolically unhealthy non-obese (MUNO), and metabolically unhealthy obese (MUO). Multivariate Cox regression analysis was used to estimate the effect of metabolic obesity phenotypes on DSC readmission.
Results: The MUNO phenotype had 1.147-fold (95% CI: 1.066, 1.235; p < 0.001) increased 180-day readmission risks in patients with neoplasm of the upper digestive tract. The MUNO phenotype had 1.073-fold (95% CI: 1.027, 1.121; p = 0.002) increased 30-day readmission risks and 1.067-fold (95% CI: 1.021, 1.115; p = 0.004) increased 180-day readmission risks in patients with neoplasm of the lower digestive tract. The MUNO and MUO phenotypes were independent risk factors of readmission in patients with liver or pancreatic neoplasm. Metabolic obesity status was independently associated with a high risk of severe and unplanned hospitalization within 30 days or 180 days.
Conclusion: Both obesity and metabolic abnormalities are associated with a high risk for the poor prognosis of DSC patients. The effect of metabolic categories on the short- or long-term readmission of liver or pancreas cancers may be stronger than that of obesity.
The fast-growing incidence of digestive system cancers (DSCs) worldwide is juxtaposed with a notable increase in mortality. A status report on the global burden of cancer worldwide using the Global Cancer Statistics 2018 (1) estimated that major DSCs (esophagus cancer, stomach cancer, colorectal cancer, liver cancer, and pancreas cancer) come in at 29.6% of newly diagnosed cancers and 39.0% of all cancer deaths. Therefore, DSCs have brought a substantial and heavy global health burden (2, 3). Of note, cancer patients are at a high risk of readmission due to the inherent complexity of their medical, comorbid, and psychosocial conditions (4). Furthermore, unplanned readmissions may cost the Centers for Medicare & Medicaid Services (CMS) an estimated >$17 billion annually (5, 6). While some inevitable readmissions occur as a result of the natural course of the disease, a proportion can be intervened in advance by identifying factors associated with hospital readmission for these high-risk patients early so that effective prevention and intervention strategies can be established to optimize short-term and long-term clinical outcomes while reducing costs (7, 8).
Obesity increases the hospital readmission risk of various diseases, such as acute myocardial infarction (9), end-stage renal disease (10), and colorectal cancer after surgery (11). However, recent studies now report that the obesity paradox exists and may even be extended to postoperative outcomes following robotic-assisted surgery for rectal cancer, that is, obesity did not impact the readmission of such patients (12). So, what accounts for this? Of note, not all obesity phenotypes are created equally, and obesity often naturally coexists with insulin resistance, dyslipidemia, and hypertension (13). However, it also cannot be ignored that obesity is not always associated with metabolic abnormalities. A metabolically healthy phenotype, known as metabolically healthy obesity (MHO), may occur in approximately 25%–30% of the obese population (14–17). Kabat GC et al. have yielded arrestive results that metabolically unhealthy normal weight (MUNW) phenotypes were associated with an increased risk of colorectal cancer, while other phenotypes showed no association (18). Therefore, the combination of obesity phenotype and different metabolic characteristics seems to be more accurate than obesity alone in predicting the readmission risk of diseases.
However, no study has yet systematically examined the association between metabolically defined obesity type and the short- or long-term readmission risk of DSC (neoplasm of the esophagus, stomach, duodenum, small intestine, large intestine, rectum, anal canal, anus, liver, and pancreas). The purpose of this study is to understand this important knowledge gap in two readmission cohorts by combining obesity and metabolic health status into a composite variable to assess the individual and joint effects of the two exposures and provide a reference for clinical prevention and readmission reduction programs.
We utilized the Nationwide Readmissions Database (NRD) 2018 to study 30-day readmission and 180-day readmission among patients with four major categories of DSCs, namely, neoplasm of the upper digestive tract, lower digestive tract, liver, and pancreas. The NRD, as a part of the Healthcare Cost and Utilization Project (HCUP), contains all-payer hospital inpatient stays data from 28 geographically dispersed states, representing 51% of the total U.S. population and 49% of all U.S. hospitalizations to generate national estimates of readmissions to hospital (19). Using a reliable and verified linkage number, patients are identified to track across the hospitals.
Using the corresponding International Classification of Diseases-10th Revision-Clinical Modification (ICD-10-CM) diagnostic and procedure codes, a total of 12,928,231 patients who were admitted to the hospital in 2018 were identified in the NRD database. There are no ethical aspects to this study.
For the 30-day readmission cohort, admissions in December (N = 923,585) were excluded, and for the estimation of the 180-day readmission, admission in the months of July, August, September, October, November, and December (N = 5,784,907) were excluded, as admission in the next year cannot be tracked in the NRD. We also excluded patients with 1) age less than 18 at the time of index hospitalization, 2) non-digestive system cancer, 3) transfer from another hospital, and 4) low body weight. In total, 142,753 and 74,566 participants with a primary or secondary discharge diagnosis of digestive system cancer at the time of index hospitalization were ultimately selected for inclusion in the short- or long-term readmission risk analysis, respectively. The details of the inclusion and exclusion criteria are described in Figure 1. Index hospitalization was defined as the whole hospital stay during which the index stay occurred. If a patient had two or more hospitalizations, the first hospitalization was considered as the index hospitalization.
Obesity was defined as body mass index (BMI) ≥25 kg/m (2). Metabolically unhealthy status was defined as having at least one of the following three components of metabolic syndrome (20) except for waist circumference collinear with BMI: 1) hyperglycemia; 2) hyperlipidemia: high serum triglyceride (TG) levels or high high-density lipoprotein (HDL)-cholesterol levels, etc.; and 3) hypertension. Metabolic health status was defined as having none of the above three components of metabolic syndrome. These diagnoses identified by the ICD-10-CM codes are listed in Supplementary Table 1. Furthermore, we used a combination of obesity and metabolic status to classify individuals into metabolic obesity phenotypes as follows: 1) MHNO: metabolically healthy non-obese, metabolic health status with BMI <25 kg/m (2); 2) MHO: metabolically healthy obese, metabolic health status with BMI ≥25 kg/m (2); 3) MUNO: metabolically unhealthy non-obese, metabolically unhealthy status with BMI <25 kg/m (2); and 4) MUO: metabolically unhealthy obese, metabolically unhealthy status with BMI ≥25 kg/m (2).
DSC in our study refers to all types of neoplasm combined including neoplasm of the esophagus, stomach, duodenum, small intestine, large intestine, rectum, anal canal, anus, liver, and pancreas. Neoplasm of the upper digestive tract was defined as neoplasm combined including neoplasm of the esophagus, stomach, and duodenum. Neoplasm of the lower digestive tract included the small intestine, large intestine, rectum, anal canal, and anus. Furthermore, patient demographics, including age, sex, income quartile based on household income of the patient’s zip code, patient location, length of stay (LOS), primary expected payment source (Medicare/Medicaid, private insurance, self-pay, and other insurance types), and common comorbidities to calculate Charlson’s comorbidity, were also obtained from the NRD database according to the corresponding code.
Our primary outcomes of interest were short- (30-day) or long-term (180-day) readmission risk of DSC after discharge from index hospitalization. Secondary outcomes of interest included the following: 1) severe DSC-related readmission (LOS >7 days) and 2) unplanned hospitalization.
In addition, we counted the top 4 main reasons for readmission of patients with different metabolic obesity phenotypes of DSC based on primary diagnosis codes.
To better elucidate the effect of obesity, hyperglycemia, hyperlipidemia, and hypertension on the short- or long-term readmission risk of DSC, the study population was divided into 10 groups and 8 groups twice for comparisons. The first grouping included those with 1) non-obese and no metabolic abnormality, 2) non-obese and simple hyperglycemia, 3) non-obese and simple hypertension, 4) non-obese and simple hyperlipidemia, 5) non-obese and multiple metabolic abnormalities, 6) obese and no metabolic abnormality, 7) obese and simple hyperglycemia, 8) obese and simple hypertension, 9) obese and simple hyperlipidemia, and 10) obese and multiple metabolic abnormalities. The secondary grouping included 1) non-obese and no metabolic abnormality, 2) non-obese and one metabolic syndrome component, 3) non-obese and two metabolic syndrome components, 4) non-obese and three metabolic syndrome components, 5) obese and no metabolic abnormality, 6) obese and one metabolic syndrome component, 7) obese and two metabolic syndrome components, and 8) obese and three metabolic syndrome components.
We used descriptive statistics to compare patient demographic characteristics and the prevalence of DSC across four groups of metabolic obesity phenotypes. Continuous variables were expressed as median ± standard deviation and categorical variables as frequency counts and percentages. The chi-square test was used to compare categorical variables, while variance testing was used to compare continuous variables. The multivariable Cox regression model was performed to identify higher risk subgroups of the 30-day or 180-day readmission of DSC and secondary outcomes of interest, using MHNO, a low-risk group, as the reference. To report adjusted hazard ratios (HRs) for DSC, age, sex, primary expected payment source, patient location, Deyo–Charlson comorbidity index, LOS at index hospitalization, and severe hospitalization at index hospitalization were selected as covariates. Kaplan–Meier curves for the probability of outcomes were obtained for the four phenotypes of different DSCs. R Studio (2022.12.0 + 353), R (4.1.1), and SPSS 25.0 software (SPSS Inc., Chicago, IL, USA) were used to conduct all statistical analyses in our study. A two-sided p-value <0.05 was considered significant.
In Table 1, we described the sociodemographic and common comorbidity characteristics and prevalence of DSC in patients with different metabolic obesity phenotypes. Patients in the MHO categories had the lowest age level and male proportions in the 30-day and 180-day readmission cohorts. Compared with patients with MHNO or MHO in the two cohorts, those in the MUNO or MUO groups were older, which indicates that older patients with digestive cancers may have poorer metabolic status. Medicare/Medicaid and private insurance were the main way of hospitalization payment for the majority of DSC patients. The distribution of median household income and residence of patients with different phenotypes showed no difference. As expected, participants with MUNO or MUO tended to have more common comorbidities, such as myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, hemiplegia, moderate/severe renal disease, leukemia, lymphoma, and acquired immune deficiency syndrome (AIDS). Individuals with the MHNO phenotypes had significantly shorter LOS and fewer proportion of severe hospitalizations at index hospitalization compared with those with the MHO, MUNO, and MUO phenotypes (all p < 0.05). Compared with the MHO or MUO phenotypes, the MHNO and MUNO phenotypes accounted for a higher proportion of individuals with neoplasm of the upper digestive tract. Furthermore, the proportion of non-obese subjects in patients with neoplasm of the upper digestive tract was higher than that of obese subjects, regardless of metabolic status. Participants in the MUNO category had the highest proportion of patients with neoplasm of the liver and pancreas and the lowest proportion of patients with neoplasm of the lower digestive tract than those in the other three categories (Table 1).
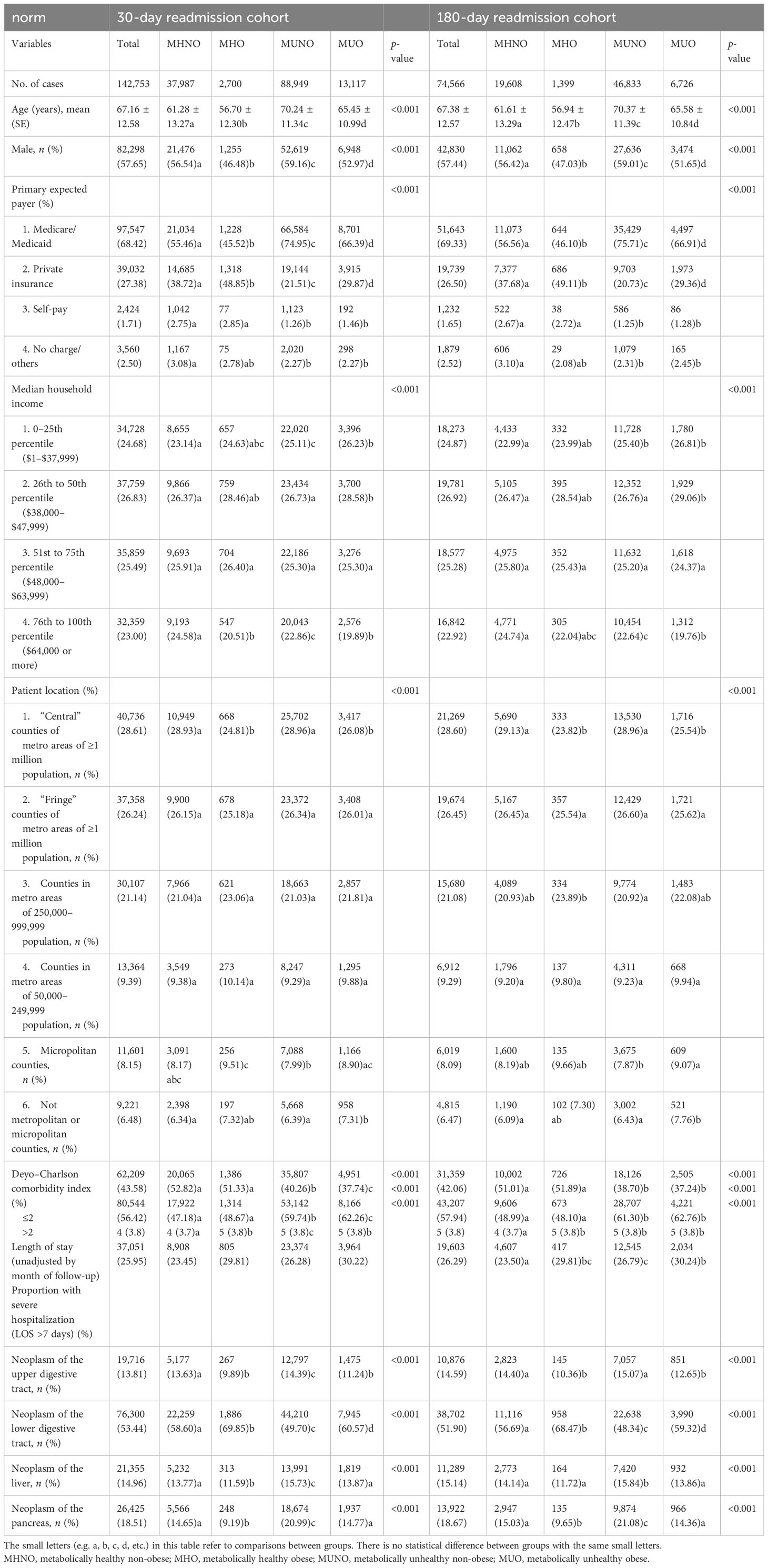
Table 1 Baseline characteristics of the study population of digestive system cancer patients from the NRD, classified by different metabolic obesity phenotypes.
Patients were followed over 26.63 ± 7.85 days after index hospitalization in the 30-day readmission cohort. For neoplasm of the upper digestive tract, the MHO phenotype had a significantly higher rate of readmission within 30 days (p < 0.01), as compared with the other three phenotypes (Supplementary Table 2, Figure 2). Compared with the MHNO phenotype, higher 30-day readmission rates were observed in the MUNO and MUO phenotypes in patients with neoplasm of the lower digestive tract. For neoplasm of the liver, there was no significant difference in readmission rates among the four phenotypes. The MUO phenotype in patients with neoplasm of the pancreas had a higher rate of 30-day readmission than the MHNO and MUNO phenotypes.
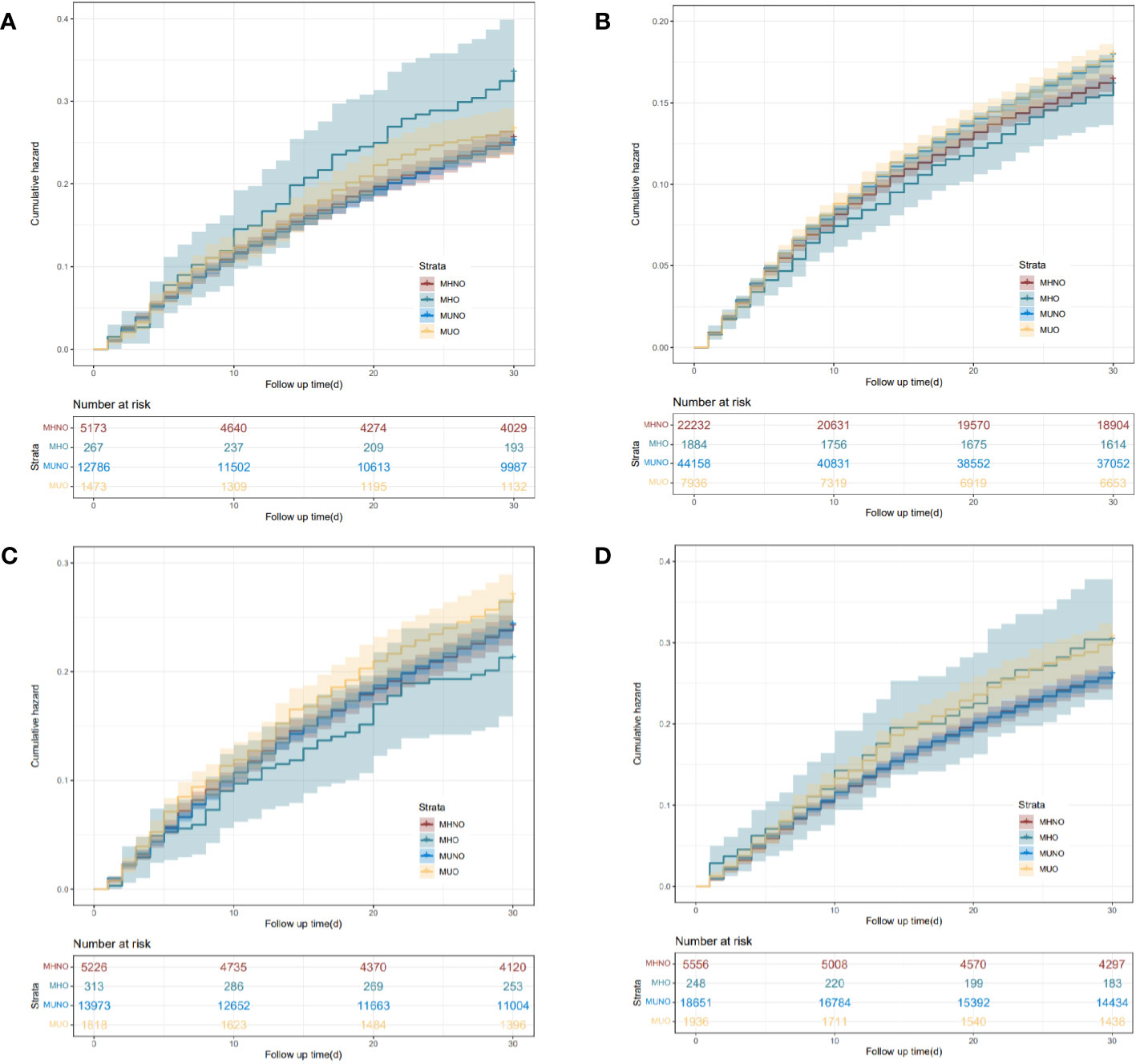
Figure 2 Kaplan–Meier curves of the 30-day readmission for the four phenotypes of different digestive system cancers. (A) Neoplasm of the upper digestive tract. (B) Neoplasm of the lower digestive tract. (C) Neoplasm of the liver. (D) Neoplasm of the pancreas. MHNO, metabolically healthy non-obese; MHO, metabolically healthy obese; MUNO, metabolically unhealthy non-obese; MUO, metabolically unhealthy obese.
Following up 129.18 ± 72.55 days, no difference was found in the long-term readmission rate among the four metabolic health statuses in patients with neoplasm of the upper digestive tract and lower digestive tract. However, unlike the short-term readmission rate, the long-term readmission rate of liver cancer patients with the MUO phenotype was higher than that of patients with the MHNO phenotype. For neoplasm of the pancreas, differences in long-term readmission rates among the four phenotypic patients were the same as the differences in short-term readmission rates (Supplementary Table 2, Figure 3).
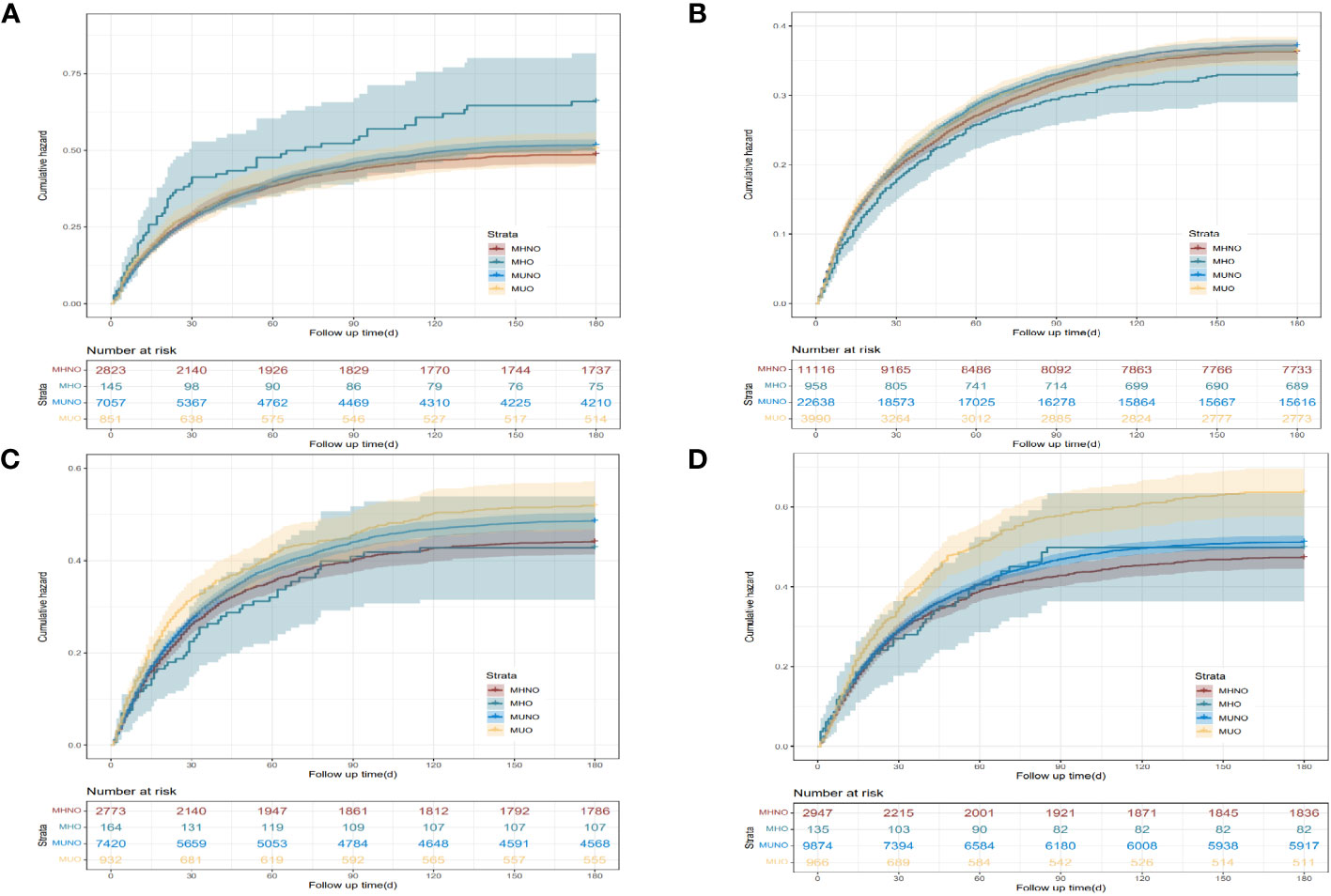
Figure 3 Kaplan–Meier curves of the 180-day readmission for the four phenotypes of different digestive system cancer. (A) Neoplasm of the upper digestive tract. (B) Neoplasm of the lower digestive tract. (C) Neoplasm of the liver. (D) Neoplasm of the pancreas. MHNO, metabolically healthy non-obese; MHO, metabolically healthy obese; MUNO, metabolically unhealthy non-obese; MUO, metabolically unhealthy obese.
On multivariable analysis, adjusting for age, sex, primary expected payer, patient location, Deyo–Charlson comorbidity index, LOS at index hospitalization, and severe hospitalization at index hospitalization, the MHO, MUNO, and MUO phenotypes were short- and long-term readmission risk factors of DSC compared with the MHNO phenotype (Supplementary Table 3).
Specifically, the MUNO phenotype had 1.147-fold (95% CI: 1.066, 1.235; p < 0.001) increased risks of the 180-day readmission in patients with neoplasm of the upper digestive tract (Supplementary Table 3). The MUNO phenotype had 1.073-fold (95% CI: 1.027, 1.121; p = 0.002) increased risks of the 30-day readmission (Supplementary Table 3) and 1.067-fold (p = 0.004) increased risks of the 180-day readmission (Supplementary Table 3) in patients with neoplasm of the lower digestive tract, whereas the MHO phenotype was a protective factor for the 180-day readmission in these patients with cancer [aHR, 0.873 (95% CI, 0.771, 0.989), p = 0.033]. The MUNO and MUO phenotypes were independent risk factors of short- and long-term readmission in patients with liver or pancreatic neoplasm (p < 0.05; Supplementary Table 3).
To explore the effect of obesity, hyperlipidemia, hyperglycemia, and hypertension on the short- or long-term readmission risk of DSC, we divided the study population into 10 groups for comparison. Consistently, no group was a risk factor for the 30-day readmission in patients with neoplasm of the upper digestive tract (Supplementary Table 4, Supplementary Figure 1A). Non-obese and simple hypertension abnormalities as well as non-obese and multiple metabolic abnormalities were risk factors for the 180-day readmission in these patients (Supplementary Table 4, Supplementary Figure 2A). For patients with neoplasm of the lower digestive tract, non-obese and simple hypertension abnormalities as well as non-obese and multiple metabolic abnormalities were risk factors for the 30-day readmission. Non-obese and simple hypertension abnormalities as well as non-obese and multiple metabolic abnormalities were risk factors for the 180-day readmission, while non-obese and simple hyperlipidemia abnormalities as well as obese and no metabolic abnormality were protective factors for the 180-day readmission (Supplementary Table 4, Supplementary Figures 1B, 2B). Non-obese and simple hyperglycemia, non-obese and simple hyperlipidemia, non-obese and multiple metabolic abnormalities, and obese and multiple metabolic abnormalities were independent risk factors of short- and long-term readmission in patients with neoplasm of the liver (Supplementary Table 4, Supplementary Figures 1C, 2C). Non-obese and multiple metabolic abnormalities as well as obese and multiple metabolic abnormalities were risk factors for the 30-day and 180-day readmission in patients with neoplasm of the pancreas (Supplementary Table 4, Supplementary Figures 1D, 2D).
Furthermore, after grouping the patients according to the number of metabolic abnormalities, this independent impact of non-obese and two to three types of metabolic abnormality phenotypes on the risk of the 30-day and 180-day readmission was observed in patients with neoplasm of the lower digestive tract, liver, and pancreas. Also, being obese and the three types of metabolic abnormality phenotypes were risk factors for the 30-day and 180-day readmission in patients with neoplasm of the liver and pancreas (Supplementary Table 5, Supplementary Figures 3, 4).
For neoplasm of the upper digestive tract, the MUNO phenotype was not a risk factor for the 180-day severe hospitalization within 180 days but a risk factor for unplanned hospitalization within 180 days (Supplementary Tables 6, 7).
Compared with the MHNO phenotype, higher 30-day readmission rates were shown in the MUNO and MUO phenotypes in patients with neoplasm of the lower digestive tract. Higher 30-day severe readmission rates were shown in the MUO phenotype in patients with neoplasm of the lower digestive tract compared with the MHO phenotype (Supplementary Table 6), while the 30-day unplanned hospitalization rate of the MUNO and MUO phenotypes was higher than that of the MHNO and MHO phenotypes in patients with neoplasm of the lower digestive tract (Supplementary Table 2).
For neoplasm of the liver, the MUNO and MUO phenotypes were risk factors for severe hospitalizations (Supplementary Table 6) and unplanned hospitalization within 30 days and 180 days compared with the MHNO phenotype (Supplementary Table 7).
For neoplasm of the pancreas, the MUNO and MUO phenotypes were risk factors for the 180-day severe hospitalizations (Supplementary Table 6) and the 30-day or 180-day unplanned hospitalization (Supplementary Table 7), compared with the MHNO phenotype.
In the top 4 diagnosis positions, the most frequent ICD-10 diagnoses for the 30-day readmissions are displayed in Figure 4. Concerning diagnoses not referencing DSC or metabolic obesity status, the top 4 most common diagnoses associated with the 30-day readmissions in the MHNO, MHO, and MUNO groups were sepsis, anemia, acute kidney failure, and gastroesophageal reflux disease. In contrast, in the MUO group, these diagnoses were acute kidney failure, sepsis, anemia, and gastroesophageal reflux disease (Figure 4A).
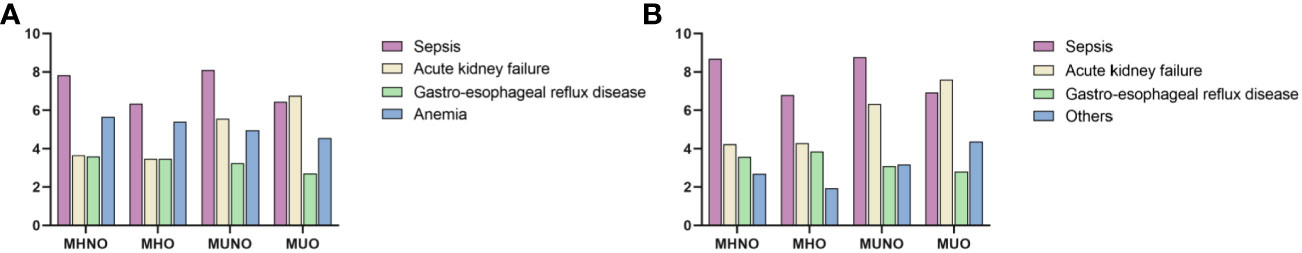
Figure 4 Reasons for hospitalization by metabolic obesity status. (A) The four most common diagnoses associated with the 30-day readmissions. (B) The four most common diagnoses associated with the 180-day readmissions. MHNO, metabolically healthy non-obese; MHO, metabolically healthy obese; MUNO, metabolically unhealthy non-obese; MUO, metabolically unhealthy obese.
Notably, sepsis was the most common of the 180-day admission diagnoses not referencing DSC or metabolic obesity status in those with MHNO, MHO, and MUNO phenotypes. Acute kidney failure was the most common of these diagnoses in those with MUO. In addition, obstructive sleep apnea was one of the four main causes of the 180-day readmission in patients with MHO. Atherosclerotic heart disease was also the main cause of the 180-day readmissions for patients with the MUNO phenotype (Figure 4B).
Based on this nationally representative longitudinal study using NRD, we made several crucial observations regarding the prognostic impact of metabolic obesity phenotypes in hospitalized individuals with four major categories of DSC. First, the MHO, MUNO, and MUO phenotypes were risk factors for the short- or long-term readmission of DSC compared with the MHNO phenotype, after adjustment for age, sex, and other confounding factors. More precisely, the effect of metabolism on the short- or long-term readmission of patients with liver or pancreas cancers may be stronger than that of obesity, highlighting the importance of abnormal metabolic status modification regardless of obesity status. Second, we observed that metabolic obesity status was independently associated with a significantly high risk of severe hospitalizations and unplanned hospitalization within 30 days or 180 days. Third, the most common non-tumor-related reason for readmission in DSC patients with the MHNO, MHO, and MUNO phenotypes was sepsis, whereas acute renal failure was the leading cause of the short- or long-term readmission for MUO phenotypes. Overall, this is the first large-scale study to systematically assess the impact of metabolic obesity status on short- and long-term clinical outcomes in patients with DSC, which might provide a guideline for the calibration of readmission risk stratification in these individuals.
Epidemiological data show the links between obesity and esophageal or gastric cancer (21). Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort with a mean follow-up of 14 years demonstrated that abdominal obesity was positively associated with upper gastrointestinal cancers (22). Although the high association between the two has been well studied, little is known regarding readmission rates in patients with metabolically defined obesity type. Our study fills the gap in knowledge of the effect of both obesity and metabolic abnormalities on readmission rates in patients with DSC. The 1.362-fold increased short-term readmission risk of the MHO phenotype among patients with neoplasm of the upper digestive tract in our multivariable models was both statistically and clinically significant, while other phenotypes had no significant effect on 30-day readmission in these patients. Moreover, the MUNO phenotype had 1.147-fold increased risks of long-term readmission in patients with neoplasm of the upper digestive tract, compared with the MHO, MHNO, and MUO phenotypes. These data suggest that obesity may have an important effect on short-term readmission in patients with neoplasm of the upper digestive tract, yet metabolic factors have a greater impact on long-term readmission in these patients. Our findings illustrate the need to improve the management and transitional care in patients with different metabolic obesity statuses.
Despite the association between obesity or BMI and patient outcomes after laparoscopic colorectal resection has been studied (23), whether metabolically defined obesity types affect the risk of rehospitalization of lower digestive tract tumors has not been systematically assessed. This undoubtedly brings excessive burden to clinical practice. Under this urgent situation, our retrospective cohort study revealed that metabolically unhealthy non-obese status was a significant risk factor for readmission in patients with lower digestive tract tumors, including the small intestine, large intestine, rectum, anal canal, and anus. One study previously demonstrated the detrimental effect of metabolic abnormalities on the prognosis of colorectal cancer, with significantly worse survival, recurrence, and liver metastases, suggesting that metabolic abnormalities are important prognostic factors for colorectal cancer (24). In addition, hypertension and high triglyceride levels were also identified as important risk factors for postoperative complications of colorectal cancer after surgery (25). These associations may be due to a multifactorial mechanism involving the action of inflammatory cytokines and adipokines, such as adiponectin, leptin, IGF-1, and others (26). Surprisingly, while obesity has been identified as a risk factor for multiple cancers (27), metabolically healthy obese status could reduce long-term readmission rates of patients with lower digestive tract tumors in our cohort. This phenomenon has been well described as the “obesity paradox” (28). We assume that progression of the cancer, such as cachexia and poor appetite, results in the inability to maintain body weight so that individuals with low BMI have higher rates of readmission, prolonged lengths of stay, and other worse prognosis.
We identified that liver or pancreas cancer patients with abnormal metabolic status were more likely to be readmitted to the hospital, independently of obesity status, highlighting that the effect of metabolic status on the development of liver or pancreas cancers may be stronger than that of obesity. Previous studies reported that all BMI categories in metabolically unhealthy individuals were associated with a high risk of liver cancer (17); the risk of liver cancer increased with the number of metabolic syndrome components in subjects not chronically infected with hepatitis viruses (29). Studies have found that lipids, which are metabolized primarily in the liver, play a crucial role in liver physiology and the pathological progression of diseases such as hepatocellular carcinoma. The progression of hepatocellular carcinoma is associated with inflammation and complex metabolic reprogramming. Drugs targeting lipid metabolism can interfere with lipid metabolism in the tumor and tumor microenvironment and become a new approach to the treatment of liver cancer. Liver cancer patients with hypertension had a high mortality (30). Structural activation of tyrosine kinases, cell overproliferation, and changes in growth factor receptors have been suggested as potential mechanisms by which hypertension may increase occurrence risk and poor prognosis of liver cancers (29, 31). Both prediabetes (32) and diabetes mellitus (33) are also found to be associated with high risks of liver cancers, which is consistent with our subgroup analysis of readmission risk. On the other hand, Chung HS et al. demonstrated that the metabolically unhealthy phenotypes (MUNO and MUO) significantly increased the risk of pancreatic cancer, whereas obese individuals with the metabolically healthy phenotypes did not (34). Chronic inflammation with changes in the concentration of inflammatory cytokines and the infiltration of pancreatic immunosuppressive cells can explain the association between metabolic abnormalities and the incidence and prognosis of pancreatic cancer (35), but more research is urgently needed to elucidate the underlying pathophysiological mechanisms. In combination with the metabolic obesity phenotype, clinical markers of liver or pancreatic cancer may effectively help screen patients at a high risk of readmission.
It has been estimated that patients with cancer have a 10-fold higher risk of sepsis than those without cancer (36), and cancer-related sepsis is associated with significantly increased mortality at all ages (37). We found that sepsis was the most common 180-day admission diagnosis that did not involve digestive cancer or metabolic obesity status among patients with MHNO, MHO, and MUNO phenotypes. Therefore, early recognition and improved management of sepsis may be beneficial to decrease the adverse outcomes, such as readmission and death in patients with digestive tract tumors. In addition, acute kidney failure was the most common of the readmission diagnoses in MUO individuals who participated in our studies. The interplay between cancer and acute kidney injury is intricate (38), and it should be remembered that acute kidney injury in a cancer setting is a serious complication of the disease course and clinicians should pay attention to the prevention and monitoring.
Several limitations should be noted in our study. First, the identification of digestive tract tumors and metabolic obesity status using ICD-10-CM diagnosis codes may be subject to potential misclassification. Second, this study is a retrospective cohort study design. Although we adjusted for potential confounders, the risk of unmeasured or unconsidered confounders that could affect the results cannot be excluded.
In summary, we observed that the MHO, MUNO, and MUO phenotypes were higher risk factors for poor prognosis, such as short- or long-term readmission, severe hospitalization, and unplanned hospitalization of patients with DSC, compared with those with the MHNO phenotype. The effect of metabolic status on the short- or long-term readmission of liver or pancreas cancer may be stronger than that of obesity, highlighting that clinical intervention should focus on not only obesity but also metabolic abnormalities. Sepsis and acute kidney injury were the leading causes of admission diagnosis in patients with DSC, independent of digestive cancer or metabolic obesity status. Future studies should focus on the identification of novel screening guidelines for the readmission risk of patients with DSC according to various metabolic obesity phenotypes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
JZ created the study protocol. XF contributed to the analysis plan. YC and YL wrote the first draft of the manuscript. XW, SS, YS, and HG revised the manuscript to create the final version. HG contributed to the design of the study protocol and reviewed the manuscript. XF contributed to the data analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China (2017YFC1309800), the National Natural Science Foundation of China (Nos. 82130025 and 91957209), the China Postdoctoral Science Foundation (2023TQ0202), and the Shandong Postdoctoral Science Foundation (SDBX2023035).
We kindly acknowledge the participants and researchers who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1214651/full#supplementary-material
Supplementary Figure 1 | Kaplan–Meier curves of the relationships between obesity, hyperglycemia, hyperlipidemia, and hypertension and the 30-day READMISSION of digestive system cancer in study population. (A) Neoplasm of upper digestive tract. (B) Neoplasm of lower digestive tract. (C) Neoplasm of liver. (D) Neoplasm of pancreas.
Supplementary Figure 2 | Kaplan–Meier curves of the relationships between obesity, hyperglycemia, hyperlipidemia, and hypertension and the 180-day READMISSION of digestive system cancer in study population. (A) Neoplasm of upper digestive tract. (B) Neoplasm of lower digestive tract. (C) Neoplasm of liver. (D) Neoplasm of pancreas.
Supplementary Figure 3 | Kaplan–Meier curves of the relationships between obesity with the number of metabolic abnormalities and the 30-day READMISSION of digestive system cancer in study population. (A) Neoplasm of upper digestive tract. (B) Neoplasm of lower digestive tract. (C) Neoplasm of liver. (D) Neoplasm of pancreas.
Supplementary Figure 4 | Kaplan–Meier curves of the relationships between obesity with the number of metabolic abnormalities and the 180-day READMISSION of digestive system cancer in study population. (A) Neoplasm of upper digestive tract. (B) Neoplasm of lower digestive tract. (C) Neoplasm of liver. (D) Neoplasm of pancreas. MHNO, metabolically healthy nonobese; MHO, metabolically healthy obese; MUNO, metabolically unhealthy nonobese; MUO, metabolically unhealthy obese.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Zhang Y, Song M, Chan AT, Eva S, Brian M, Meir J, et al. Unrestrained eating behavior and risk of digestive system cancers: a prospective cohort study. Am J Clin Nutr (2021) 114(5):1612–24. doi: 10.1093/ajcn/nqab235
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
4. Granda-Cameron C, Behta M, Hovinga M, Rundio A, Mintzer D. Risk factors associated with unplanned hospital readmissions in adults with cancer. Oncol Nurs forum. (2015) 42(3):E257–268. doi: 10.1188/15.ONF.E257-E268
5. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. New Engl J Med (2009) 360(14):1418–28. doi: 10.1056/NEJMsa0803563
6. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. New Engl J Med (2016) 374(16):1543–51. doi: 10.1056/NEJMsa1513024
7. Halfon P, Eggli Y, Prêtre-Rohrbach I, Meylan D, Marazzi A, Burnand B. Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care (2006) 44(11):972–81. doi: 10.1097/01.mlr.0000228002.43688.c2
8. Kim JW, Mannalithara A, Sehgal M, Mithal A, Singh G, Ladabaum U. A nationwide analysis of readmission rates after colorectal cancer surgery in the US in the Era of the Affordable Care Act. Am J Surg (2020) 220(4):1015–22. doi: 10.1016/j.amjsurg.2020.04.013
9. Schuller KA. Is obesity a risk factor for readmission after acute myocardial infarction? J healthcare Qual Res (2020) 35(1):4–11. doi: 10.1016/j.jhqr.2019.09.002
10. Siracuse JJ, Shah NK, Peacock MR, Tahhan G, Kalish JA, Rybin D, et al. Thirty-day and 90-day hospital readmission after outpatient upper extremity hemodialysis access creation. J Vasc Surg (2017) 65(5):1376–82. doi: 10.1016/j.jvs.2016.11.023
11. Poelemeijer YQM, Lijftogt N, Detering R, Fiocco M, Tollenaar R, Wouters M. Obesity as a determinant of perioperative and postoperative outcome in patients following colorectal cancer surgery: A population-based study (2009-2016). Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol (2018) 44(12):1849–57. doi: 10.1016/j.ejso.2018.05.027
12. Duchalais E, Machairas N, Kelley SR, Landmann RG, Merchea A, Colibaseanu DT, et al. Does obesity impact postoperative outcomes following robotic-assisted surgery for rectal cancer? Surg endoscopy. (2018) 32(12):4886–92. doi: 10.1007/s00464-018-6247-4
13. Cheng Y, Han J, Li Q, Shi Y, Zhong F, Wu YW, et al. Metabolic obesity phenotypes: a friend or foe of digestive polyps?-An observational study based on National Inpatient Database. Metabolism: Clin Exp (2022) 132:155201. doi: 10.1016/j.metabol.2022.155201
14. Jung CH, Lee WJ, Song KH. Metabolically healthy obesity: a friend or foe? Korean J Internal Med (2017) 32(4):611–21. doi: 10.3904/kjim.2016.259
15. Stefan N, Kantartzis K, Machann J, Schicket F, Thame C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Internal Med (2008) 168(15):1609–16. doi: 10.1001/archinte.168.15.1609
16. Stefan N, Kantartzis K, Machann J, Schick F, Häring HU. Global trends in body-mass index. Lancet (London England) (2011) 377(9781):1917. doi: 10.1016/S0140-6736(11)60805-2
17. Nasereldin DS, White LJ, Hodge DO, Roberts LR, Patel T, Antwi SO. Association of metabolic health phenotypes, obesity, and hepatocellular carcinoma risk. Digestive liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver (2021) 54(7):964–72. doi: 10.1016/j.dld.2021.12.002
18. Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol (2017) 2(2):181–7. doi: 10.1001/jamacardio.2016.4792
19. Mitchell MA, Dhaliwal I, Mulpuru S, Amjadi K, Chee A. Early readmission to hospital in patients with cancer with Malignant pleural effusions: analysis of the nationwide readmissions database. Chest (2020) 157(2):435–45. doi: 10.1016/j.chest.2019.09.007
20. Shaharyar S, Roberson LL, Jamal O, Younus A, Blahaet MJ, Ali SS, et al. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy Brazilian population. J Obes (2015) 2015:178526. doi: 10.1155/2015/178526
21. Tay SW, Li JW, Fock KM. Diet and cancer of the esophagus and stomach. Curr Opin Gastroenterol (2021) 37(2):158–63. doi: 10.1097/MOG.0000000000000700
22. Sanikini H, Muller DC, Sophiea M, Rinaldi S, Agudo A, Duell EJ, et al. Anthropometric and reproductive factors and risk of esophageal and gastric cancer by subtype and subsite: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer (2020) 146(4):929–42. doi: 10.1002/ijc.32386
23. Hotouras A, Ribas Y, Zakeri SA, Nunes QM, Murphy J, Bhan C, et al. The influence of obesity and body mass index on the outcome of laparoscopic colorectal surgery: a systematic literature review. Colorectal Dis Off J Assoc Coloproctology Great Britain Ireland (2016) 18(10):O337–o366. doi: 10.1111/codi.13406
24. Shen Z, Ye Y, Bin L, Yin Y, Yang XD, Jiang KW, et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. Am J Surg (2010) 200(1):59–63. doi: 10.1016/j.amjsurg.2009.05.005
25. Lohsiriwat V, Pongsanguansuk W, Lertakyamanee N, Lohsiriwat D. Impact of metabolic syndrome on the short-term outcomes of colorectal cancer surgery. Dis colon Rectum (2010) 53(2):186–91. doi: 10.1007/DCR.0b013e3181bdbc32
26. Pais R, Silaghi H, Silaghi AC, Rusu ML, Dumitrascu DL. Metabolic syndrome and risk of subsequent colorectal cancer. World J gastroenterology. (2009) 15(41):5141–8. doi: 10.3748/wjg.15.5141
27. Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep (2019) 8(3):175–81. doi: 10.1007/s13668-019-00280-6
28. Lainscak M, von Haehling S, Doehner W, Anker SD. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle (2012) 3(1):1–4. doi: 10.1007/s13539-012-0059-5
29. Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, et al. Metabolic syndrome and hepatocellular carcinoma risk. Br J cancer. (2013) 108(1):222–8. doi: 10.1038/bjc.2012.492
30. Chiang CH, Lee LT, Hung SH, Lin WY, Hung HF, Yang WS, et al. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatol (Baltimore Md) (2014) 59(6):2207–15. doi: 10.1002/hep.27014
31. Hamet P. Cancer and hypertension: a potential for crosstalk? J hypertension. (1997) 15(12 Pt 2):1573–7. doi: 10.1097/00004872-199715120-00058
32. Huang Y, Cai X, Qiu M, Chen P, Tang HF, Hu YZ, et al. Prediabetes and the risk of cancer: a meta-analysis. Diabetologia (2014) 57(11):2261–9. doi: 10.1007/s00125-014-3361-2
33. El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterological Assoc (2006) 4(3):369–80. doi: 10.1016/j.cgh.2005.12.007
34. Chung HS, Lee JS, Song E, Kim JA, Roh E, Yu JH, et al. Effect of metabolic health and obesity phenotype on the risk of pancreatic cancer: A nationwide population-based cohort study. Cancer epidemiology Biomarkers Prev Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol (2021) 30(3):521–8. doi: 10.1158/1055-9965.EPI-20-1262
35. Stolzenberg-Solomon RZ, Graubard BI, Chari S, Paul L, Philip RT, Jarmo V, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. Jama (2005) 294(22):2872–8. doi: 10.1001/jama.294.22.2872
36. Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with Malignancy. Chest. (2006) 129(6):1432–40. doi: 10.1378/chest.129.6.1432
37. Mirouse A, Vigneron C, Llitjos JF, Chicheet JD, Mira JR, Mokart D, et al. Sepsis and cancer: an interplay of friends and foes. Am J Respir Crit Care Med (2020) 202(12):1625–35. doi: 10.1164/rccm.202004-1116TR
Keywords: obesity, metabolic, phenotype, digestive system, neoplasm
Citation: Li Y, Wu X, Song Y, Wang P, Zhang B, Guo B, Liu Z, Wu Y, Shao S, Cheng Y, Guo H, Fan X and Zhao J (2023) The short- and long-term readmission of four major categories of digestive system cancers: does obesity or metabolic disorder matter? Front. Endocrinol. 14:1214651. doi: 10.3389/fendo.2023.1214651
Received: 30 April 2023; Accepted: 28 September 2023;
Published: 30 October 2023.
Edited by:
Marco Falasca, Curtin University, AustraliaReviewed by:
Priyadarshani Dharia, Moderna Inc, United StatesCopyright © 2023 Li, Wu, Song, Wang, Zhang, Guo, Liu, Wu, Shao, Cheng, Guo, Fan and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiajun Zhao, amp6aGFvQHNkdS5lZHUuY24=; Xiude Fan, ZmFueGl1ZGV4anR1QDE2My5jb20=; Honglin Guo, Z3VvaG9uZ2xpbjUxMkAxNjMuY29t; Yiping Cheng, Y2hlbmd5aXBpbmc5M0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.