
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 29 September 2023
Sec. Bone Research
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1212570
Aims: Evidence on the association between the risk of new-onset osteoporosis and oral anticoagulants remains controversial. We aimed to compare the risk of osteoporosis associated with the use of direct oral anticoagulants (DOACs) with that associated with warfarin use.
Methods: Studies published up to 15 March 2023 that investigated the association between the use of DOACs and warfarin and the incidence of osteoporosis were identified by online searches in PubMed, Embase, the Cochrane Library, and Web of Science conducted by two independent investigators. Random-effects or fixed-effect models were employed to synthesize hazard ratios (HRs)/relative ratios (RRs) with 95% confidence intervals (CIs) for estimating the risk of osteoporosis correlated with DOAC and warfarin prescriptions (PROSPERO No. CRD42023401199).
Results: Our meta-analysis ultimately included four studies involving 74,338 patients. The results suggested that DOAC use was associated with a significantly lower incidence of new-onset osteoporosis than warfarin use (pooled HR: 0.71, 95% CI: 0.57 to 0.88, p < 0.001, I2: 85.1%). Subanalyses revealed that rivaroxaban was associated with a lower risk of osteoporosis than both warfarin and dabigatran. In addition, DOACs were associated with a lower risk of developing osteoporosis than warfarin in both male and female patients, in patients with atrial fibrillation (AF), and in patients who underwent therapy for > 365 days.
Conclusion: DOAC users experienced a lower incidence of osteoporosis than warfarin users. This study may give us insight into safe anticoagulation strategies for patients who are at high risk of developing osteoporosis.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42023401199.
Warfarin, a vitamin K antagonist, is a traditional oral anticoagulant that has been used in many situations for the prevention or treatment of thromboembolic diseases or events. Warfarin can produce an anticoagulant effect by inhibiting the γ-carboxylation of coagulation factors II, VII, IX, and X (1). Studies have reported that there is a correlation between warfarin and hypocarboxylated osteocalcin, which is associated with low bone mineral density (BMD) (2, 3). Moreover, several studies indicate that long-term warfarin use is associated with reduced BMD and increased risk of developing osteoporosis and osteoporotic fractures (4–7). However, no significant association between warfarin use and osteoporosis has been identified in other studies (8, 9). Recently, direct oral anticoagulant (DOAC) therapy has been considerably expanded for clinical use, especially for the prevention of embolic phenomena in patients with atrial fibrillation (AF) (10, 11) and the treatment and prevention of venous thrombosis and pulmonary thromboembolism (12, 13). DOACs are non-vitamin K antagonists and include factor Xa inhibitors (apixaban and rivaroxaban) and thrombin inhibitors (dabigatran). The efficacy of DOACs has been described as equal or superior to that of warfarin, and patients receiving DOACs have required less anticoagulant monitoring (14–16). Moreover, many studies have demonstrated that DOAC users have a lower risk of developing osteoporosis than warfarin users (17–19). Nalevaiko et al. (20) recently indicated that patients using warfarin had a lower BMD and more degraded bone microarchitecture than patients using DOACs. In addition, it was reported that DOACs were associated with a significantly lower risk of osteoporotic fractures than warfarin use (17, 21). Our previous study suggested that the use of DOACs generated a lower risk of experiencing fractures than the use of warfarin in AF patients, particularly those with a history of osteoporosis (22). However, Lucenteforte et al. found no significant difference between the association of osteoporotic fractures with DOACs and their association with warfarin (23).
As oral anticoagulants are commonly prescribed for older patients who are vulnerable to thromboembolic events, the possible risks of osteoporosis constitute a vital clinical issue. Therefore, we conducted a meta-analysis to determine the correlation of DOACs vs. warfarin with the risk of osteoporosis by pooling the data from the included observational studies. These studies may indicate preferable anticoagulation strategies for patients at high risk of developing osteoporosis.
The meta-analysis and systematic review were carried out in accordance with the PRISMA guidelines (24) and the MOOSE statements (Supplementary Table 1) (25). The meta-analysis was registered at the PROSPERO website (https://www.crd.york.ac.uk/PROSPERO/; CRD42023401199).
Two investigators (YML and XPX) searched PubMed, Embase, Web of Science, and the Cochrane Library databases for eligible studies published before 15 March 2023. The search items included “osteoporosis”, “bone mineral density”, “direct oral anticoagulant”, “non-vitamin K antagonist”, “vitamin K antagonist”, and “warfarin”. The detailed search strategies are presented in Supplementary Table 2. The reference lists of all included studies and relevant reviews were also screened to identify additional studies. In addition, unpublished articles were identified from the ClinicalTrials.gov website, gray literature, and through consultation with experts in the field.
Studies were included if they fulfilled the following inclusion criteria: (1) patients had been prescribed a DOAC or warfarin for the first time; (2) the study aimed to evaluate the association between the risk of developing osteoporosis and DOAC use compared with warfarin use; (3) the study provided sufficient data to pool the results, such as hazard ratios (HRs) or relative risks (RRs) with 95% confidence intervals (CIs); and (4) the study design was an observational study. Patients who initiated osteoporosis medication were also considered to have osteoporosis. The exclusion criteria were as follows: (1) duplicate reports; (2) case reports, editorials, reviews, and meta-analyses; and (3) animal or molecular biology studies.
Two investigators (YML and XPX) independently screened the articles by the titles and abstracts. Subsequently, the full texts were obtained to identify eligible studies. Disagreements were resolved by extensive discussion or consultation when necessary.
The data were extracted using a standardized table. The collected data included the first author, year of publication, region, number of patients, study period, study participants, and study outcomes. For outcome data, the HRs or RRs with 95% CIs were extracted to synthesize the results. Two investigators (YML and XPX) independently conducted the extraction process. Discrepancies were resolved by discussion. Consultation with a third reviewer (TCY) was sought when necessary. Furthermore, we evaluated the quality of the included observational studies using the Newcastle–Ottawa Scale (NOS) (26). Studies with a score of eight or more were regarded as being of high quality (27).
For the primary analysis, the risk of developing osteoporosis in DOAC users was compared with that in warfarin users. HRs or RRs with corresponding 95% CIs were collected to calculate pooled outcomes. Pooled HRs/RRs were presented for estimation in this meta-analysis. Heterogeneity was assessed by using the Q statistic and the I2 statistic. Values of p < 0.05 and I2 > 50% indicated significant heterogeneity across the included studies. Subsequently, random-effects models were employed to pool the results of HRs or RRs and corresponding 95% CIs for osteoporosis when heterogeneity was statistically significant. Fixed-effect models were used when heterogeneity was not statistically significant. Furthermore, we conducted subgroup meta-analyses based on individual DOACs, comparisons among each DOAC, gender, duration of therapy, and patients with AF or an unspecified condition. In addition, sensitivity analyses were performed to test whether or not the included studies had a high risk of bias and to examine the robustness of the results by removing individual studies. Publication bias was assessed using a funnel plot, Begg’s rank correlation, and Egger’s weighted regression methods. A two-tailed p < 0.05 was considered statistically significant. All analyses were conducted using STATA software, version 12.0 (StataCorp, TX, USA).
The database search identified 1,046 potentially relevant articles (Supplementary Table 2). A total of 98 duplicate articles were removed and 924 articles were excluded after their titles and abstracts were scanned. Subsequently, we selected the remaining potentially eligible 24 articles for full-text review, and eventually determined that four studies fulfilled the inclusion criteria; these were included in this meta-analysis (17–19, 28). The article search and selection process is summarized in the literature flow diagram (Figure 1).
There were four studies included in our meta-analysis (17–19, 28). Detailed study characteristics are provided in Table 1. These four studies were retrospective observational studies and the study duration ranged from 4 to 8 years. This meta-analysis involved 74,338 patients who had been prescribed DOACs or warfarin for the first time. Among these participants, 3,017 developed osteoporosis, of whom 1,741 were DOAC users (3.7%) and 1,276 were warfarin users (4.9%) (Supplementary Table 3). The baseline characteristics of the participants in each study are summarized in Table 2. The average age of these patients was over 70 years. According to the NOS guidelines, these four studies had NOS scores between eight and nine, indicating high quality (Supplementary Table 4).
There were four studies involving a total of 74,338 patients included in the data analyses. The pooled results indicated that DOAC use was associated with a significantly lower risk of new-onset osteoporosis than warfarin use (pooled HR: 0.71, 95% CI: 0.57 to 0.88, p < 0.001, I2: 85.1%; Figure 2). This result was based on a random effects model because the heterogeneity between studies was significant.
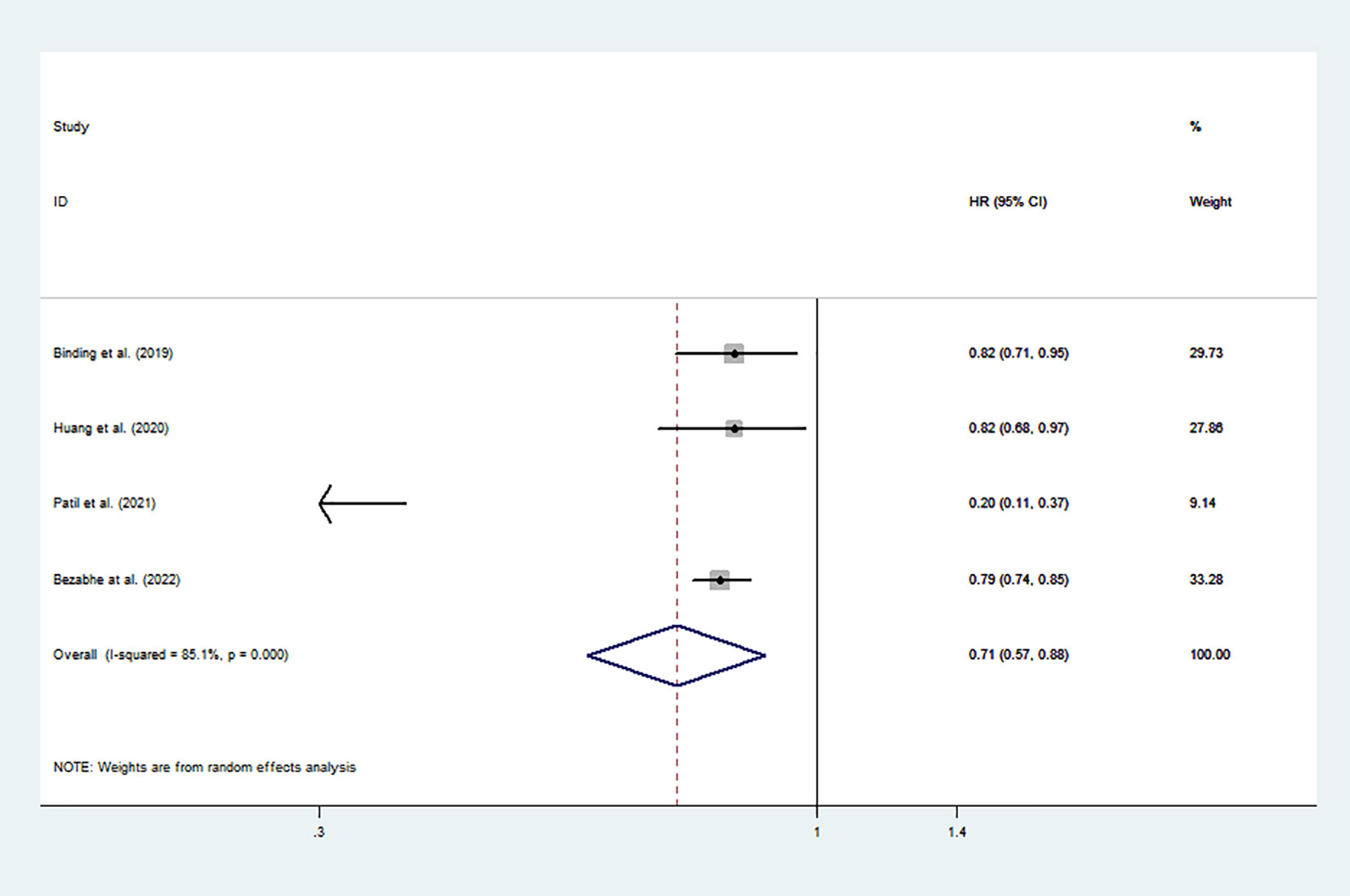
Figure 2 Risk of osteoporosis associated with all DOACs vs. warfarin. HR, hazard ratio; CI, confidence interval; DOACs, direct oral anticoagulants.
In the subanalyses comparing each DOAC with warfarin, the results showed that rivaroxaban users tended to have a lower risk of new-onset osteoporosis than warfarin users (pooled HR: 0.74, 95% CI: 0.48 to 0.80, p < 0.001, I2: 0.0%). However, there was no significant difference in the risk of osteoporosis associated with apixaban and dabigatran compared with that associated with warfarin (Table 3).
Two studies (18, 28) reported the risk of osteoporosis associated with each individual DOAC, and the combined results showed that in AF patients, there was a lower risk of new-onset osteoporosis associated with rivaroxaban than with dabigatran (pooled HR: 0.74, 95% CI: 0.56 to 0.96, p = 0.03, I2: 74.5%). In AF patients, neither dabigatran nor rivaroxaban use was significantly associated with the risk of developing osteoporosis compared with apixaban (Table 3).
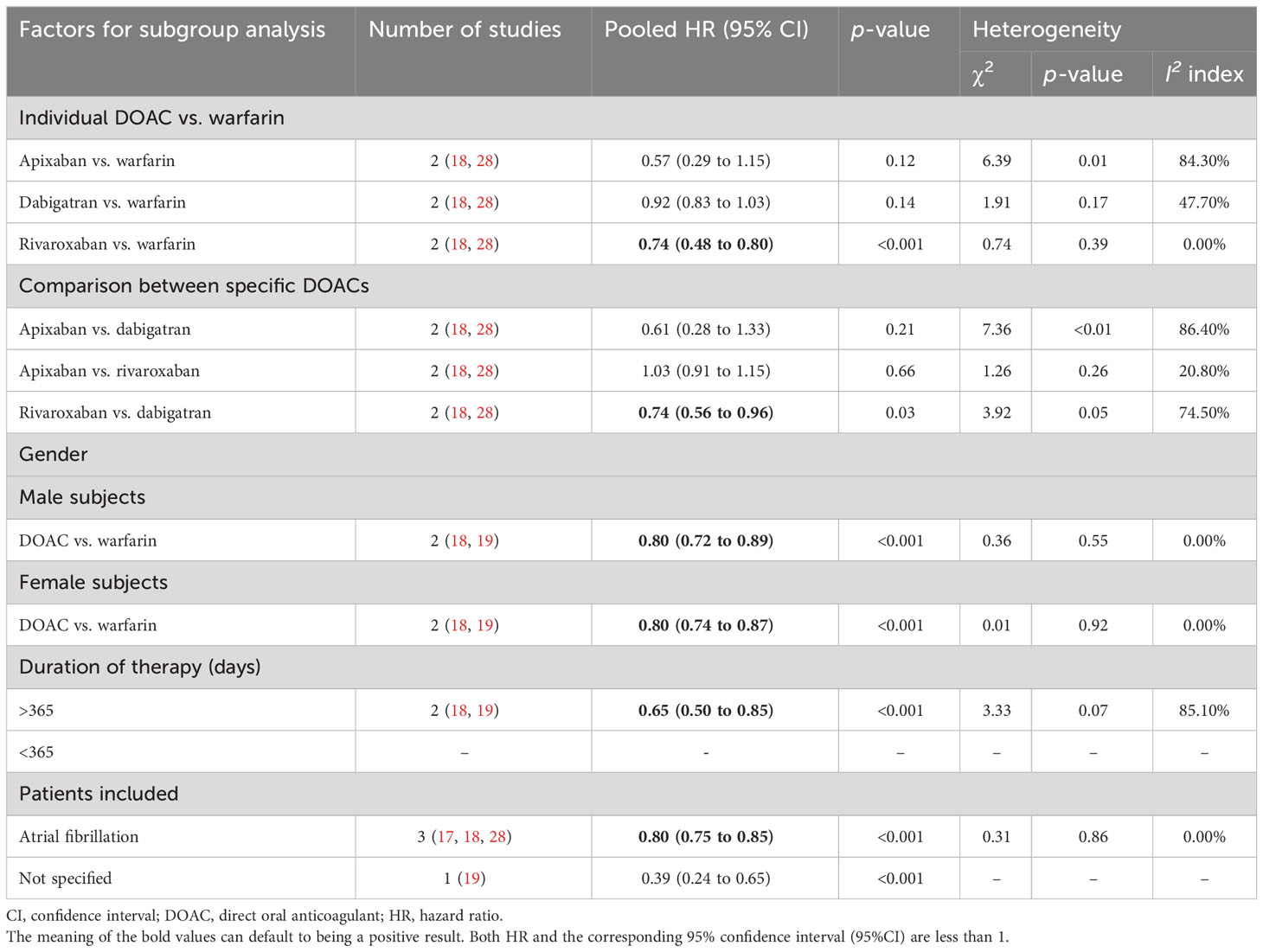
Table 3 Subgroup meta-analyses for the risk of osteoporosis in patients treated with different oral anticoagulants.
Two studies (18, 19) compared the risk of osteoporosis between DOAC and warfarin in both male and female users. For the overall comparison between DOACs and warfarin, the combined results indicated that the risk of developing osteoporosis decreased in patients prescribed DOACs compared with those prescribed warfarin, regardless of gender (pooled HR: 0.80, 95% CI: 0.72 to 0.89, p < 0.001, I2: 0.00% for male patients, and pooled HR: 0.80, 95% CI: 0.74 to 0.87, p < 0.001, I2: 0.0% for female patients; Table 3). In addition, in the analyses stratified by treatment duration, DOACs were associated with a lower risk of osteoporosis than warfarin in patients who had used the medication for more than 1 year (duration of therapy > 365 days).
Only patients with non-valvular AF participated in three studies (17, 18, 28), and patients’ disease statuses were not clearly stated in one study (19). The results indicated that DOACs were associated with a lower risk of new-onset osteoporosis than warfarin in patients with non-valvular AF (HR: 0.80, 95% CI: 0.75 to 0.85, p < 0.001, I2: 0.0%; Table 3).
After removing one study at a time in the sensitivity analyses, the risk of new-onset osteoporosis for DOAC users vs. warfarin users appeared to be stable and robust (Supplementary Table 5; Supplementary Figure 1). In this meta-analysis, there was no obvious publication bias based on the funnel plots (Supplementary Figure 2), Begg’s test (p = 0.50), or Egger’s test (p = 0.53).
This meta-analysis based on four observational studies indicated that patients prescribed DOACs were at a significantly lower risk of experiencing new-onset osteoporosis than those prescribed warfarin. This meta-analysis was based on significant heterogeneity (I2: 85.1%). In addition, the subanalyses for each DOAC vs. warfarin revealed that rivaroxaban use was associated with a significantly lower risk of new-onset osteoporosis than warfarin and dabigatran use. In addition, DOACs tended to be associated with a lower risk of new-onset osteoporosis than warfarin in both male and female patients and in patients with non-valvular AF. Among patients treated for more than 365 days, the association between DOAC use and a lower incidence of osteoporosis appeared to be stronger than that between warfarin use and a lower incidence of osteoporosis.
Several reasons may explain why the risk of developing osteoporosis is lower in patients prescribed DOACs than in those prescribed warfarin. Three typical vitamin K-dependent proteins, namely osteocalcin, matrix Gla protein, and growth arrest-specific protein, are closely related to the maintenance of bone strength (29). Considering that vitamin K is essential for the γ-carboxylation of these proteins, warfarin could control the functions of these proteins and subsequently lead to low BMD and osteoporosis (30). Moreover, long-term warfarin exposure at clinically relevant doses was found to increase osteoclast numbers and decrease the numbers and activity levels of osteoblasts (31). In contrast, given that DOACs are oral anticoagulants based on vitamin K-independent processes, they theoretically have a lesser effect on bone health. An in vivo study demonstrated that patients in the dabigatran-treated group had an increased bone volume and decreased trabecular separation compared to those in the warfarin-treated group (32). In addition, an experimental study indicated that rivaroxaban treatment did not impair femur fracture healing in a rat model (33), and Gigi et al. (34) revealed that rivaroxaban inhibited osteoblast metabolism. These studies revealed that DOACs may have a protective effect on bone health. Accordingly, the risk of developing osteoporosis may decrease in DOAC users compared with warfarin users.
Recently, a cross-sectional study showed that lower BMDs and trabecular bone scores (TBSs) were seen in patients on anticoagulants than in those not on anticoagulants, which was more pronounced with warfarin use than DOACs. This conclusion was inconsistent with the findings of a study of two retrospective cohorts conducted by Bezabhe et al. (35). In addition, a study using multi-methodological data mining demonstrated an association between warfarin use (but not DOACs) and osteoporosis, regardless of gender differences (36). As we mentioned previously, long-term warfarin use may have a negative effect on BMD (4–7). Our pooled results revealed that, in patients who had received anticoagulants for more than 365 days, there was a lower incidence of osteoporosis in those who used DOACs than in those who used warfarin. Despite the patients in their study having a therapy duration of over 548 days, the conclusions of Bezabhe et al. (28) were consistent with ours. There are also many studies showing a significant association between DOAC use and a lower risk of developing fractures in AF patients than between fractures in AF patients and warfarin use. The primary outcomes included all clinical and hospitalized fractures. For instance, Lau et al. concluded that the use of dabigatran compared with warfarin was associated with a lower risk of osteoporotic fractures in patients with AF (36). Recently, a meta-analysis conducted by Huang et al. indicated that DOAC users tended to have a lower incidence of hip fractures than warfarin users (37). Our previous meta-analysis also suggested that, among AF patients, DOAC users experienced a lower risk of fracture than warfarin users, especially among those patients with a history of osteoporosis (22). We included a recent study and conducted a meta-analysis to compare the risk of new-onset osteoporosis between DOACs and warfarin for the first time. Although our results showed that the incidence of osteoporosis in DOAC users was lower than that in warfarin users, the administration of DOACs or warfarin in elderly patients should generally be based on the risk of ischemic stroke, bleeding status, and affordability rather than the risk of osteoporosis. Therefore, patients taking oral anticoagulants need physical exercise, vitamin D supplements, and calcium supplements to protect against osteoporosis and osteoporotic fractures (38).
This meta-analysis has several strengths. First, we included observational studies that involved a large number of participants and compared the risk of developing osteoporosis in patients receiving DOACs with that in those receiving warfarin based on pooled data. In addition, we analyzed the risk of osteoporosis associated with each DOAC compared with that associated with warfarin. Second, the studies included in this meta-analysis had a relatively long study duration for the emergence of osteoporosis.
This meta-analysis has several limitations. First, due to unmeasured confounders, observational studies are prone to bias. Although sophisticated analysis methods, such as propensity score matching, were employed in the included studies, unmeasured confounders remained. Second, the included observational studies were based on routinely collected electronic health records that were not designed to study osteoporosis, which may have resulted in misclassification bias in the outcomes. For example, BMD data were not available for baseline characteristics in all studies. Finally, a high degree of heterogeneity exists in this meta-analysis, which may limit the findings. Our subanalyses indicated that an individual DOAC could potentially contribute to heterogeneity. Therefore, our results cannot completely determine causality in the association between DOACs or warfarin and the risk of osteoporosis, and this conclusion should be interpreted with caution.
This meta-analysis demonstrated that new DOAC users experienced a lower incidence of osteoporosis than warfarin users. Among all the oral anticoagulants, rivaroxaban was associated with a lower risk of osteoporosis than both warfarin and dabigatran. In addition, DOAC use was significantly associated with a lower risk of osteoporosis than warfarin use in both male and female patients, and in patients with non-valvular AF. Further randomized controlled trials are needed to determine the comparative risk of osteoporosis for different DOACs and warfarin to provide safe anticoagulation options for patients with risk factors for osteoporosis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YL designed the study, conducted statistical analysis of the data, and drafted the manuscript. XX, SB, QZ, QS, and YS contributed to the writing and discussed the manuscript. TY provided critical suggestions and contributed significantly to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant number 31970090).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1212570/full#supplementary-material
1. Tew BY, Hong TB, Otto-Duessel M, Elix C, Castro E, He M, et al. Vitamin K epoxide reductase regulation of androgen receptor activity. Oncotarget (2017) 8(8):13818–31. doi: 10.18632/oncotarget.14639
2. Sugiyama T, Kugimiya F, Kono S, Kim YT, Oda H. Warfarin use and fracture risk: an evidence-based mechanistic insight. Osteoporos Int (2015) 26(3):1231–2. doi: 10.1007/s00198-014-2912-1
3. Rubinacci A. Expanding the functional spectrum of vitamin K in bone. Focus on: "Vitamin K promotes mineralization, osteoblast to osteocyte transition, and an anti-catabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms". Am J Physiol Cell Physiol (2009) 297(6):C1336–8. doi: 10.1152/ajpcell.00452.2009
4. Marques JVO, Nalevaiko JZ, Oliveira MF, Raetsch AWP, Marques GL, Petterle RR, et al. Trabecular bone score (TBS) and bone mineral density in patients with long-term therapy with warfarin. Arch Osteoporos (2020) 15(1):102. doi: 10.1007/s11657-020-00770-z
5. Rezaieyazdi Z, Falsoleiman H, Khajehdaluee M, Saghafi M, Mokhtari-Amirmajdi E. Reduced bone density in patients on long-term warfarin. Int J Rheum Dis (2009) 12(2):130–5. doi: 10.1111/j.1756-185X.2009.01395.x
6. Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF. Risk of osteoporotic fracture in elderly patients taking warfarin: Results from the National Registry of Atrial Fibrillation 2. Arch Intern Med (2006) 166(2):241–6. doi: 10.1001/archinte.166.2.241
7. Barnes C, Newall F, Ignjatovic V, Wong P, Cameron F, Jones G, et al. Reduced bone density in children on long-term warfarin. Pediatr Res (2005) 57(4):578–81. doi: 10.1203/01.PDR.0000155943.07244.04
8. Woo C, Chang LL, Ewing SK, Bauer DC, , Osteoporotic Fractures in Men Study G. Single-point assessment of warfarin use and risk of osteoporosis in elderly men. J Am Geriatr Soc (2008) 56(7):1171–6. doi: 10.1111/j.1532-5415.2008.01786.x
9. Jamal SA, Browner WS, Bauer DC, Cummings SR. Warfarin use and risk for osteoporosis in elderly women. Study of osteoporotic fractures research group. Ann Intern Med (1998) 128(10):829–32. doi: 10.7326/0003-4819-128-10-199805150-00006
10. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al. The changing landscape for stroke prevention in AF: Findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol (2017) 69(7):777–85. doi: 10.1016/j.jacc.2016.11.061
11. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation (2019) 140(2):e125–51. doi: 10.1161/CIR.0000000000000665
12. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: A major contributor to global disease burden. Arterioscler Thromb Vasc Biol (2014) 34(11):2363–71. doi: 10.1161/ATVBAHA.114.304488
13. Middeldorp S, Prins MH, Hutten BA. Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism. Cochrane Database Syst Rev (2014) 2014(8):CD001367. doi: 10.1002/14651858.CD001367.pub3
14. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med (2009) 361(12):1139–51. doi: 10.1056/NEJMoa0905561
15. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med (2011) 365(11):981–92. doi: 10.1056/NEJMoa1107039
16. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med (2011) 364(9):806–17. doi: 10.1056/NEJMoa1007432
17. Binding C, Bjerring Olesen J, Abrahamsen B, Staerk L, Gislason G, Nissen Bonde A. Osteoporotic fractures in patients with atrial fibrillation treated with conventional versus direct anticoagulants. J Am Coll Cardiol (2019) 74(17):2150–8. doi: 10.1016/j.jacc.2019.08.1025
18. Huang HK, Liu PP, Hsu JY, Lin SM, Peng CC, Wang JH, et al. Risk of osteoporosis in patients with atrial fibrillation using non-vitamin K antagonist oral anticoagulants or warfarin. J Am Heart Assoc (2020) 9(2):e013845. doi: 10.1161/JAHA.119.013845
19. Patil T, Hobson J. Risk of new-onset osteoporosis in single-center veteran population receiving direct oral anticoagulants versus warfarin. Thromb Res (2021) 200:56–63. doi: 10.1016/j.thromres.2021.01.019
20. Nalevaiko JZ, Marques JVO, Oliveira MF, Raetsch AWP, Marques GL, Petterle RR, et al. Bone density and quality in patients treated with direct-acting oral anticoagulants versus warfarin. Bone (2021) 150:116000. doi: 10.1016/j.bone.2021.116000
21. Lau WCY, Cheung CL, Man KKC, Chan EW, Sing CW, Lip GYH, et al. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: A population-based cohort study. Ann Intern Med (2020) 173(1):1–9. doi: 10.7326/M19-3671
22. Xie X, Liu Y, Li J, Gu F, Zhang K, Sui Z, et al. Fracture risks in patients with atrial fibrillation treated with different oral anticoagulants: A meta-analysis and systematic review. Age Ageing (2022) 51(1):1–10. doi: 10.1093/ageing/afab264
23. Lucenteforte E, Bettiol A, Lombardi N, Mugelli A, Vannacci A. Risk of bone fractures among users of oral anticoagulants: An administrative database cohort study. Eur J Intern Med (2017) 44:e30–1. doi: 10.1016/j.ejim.2017.07.022
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
25. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. meta-analysis of observational studies in Epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
27. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: Systematic review of observational studies and meta-analysis. BMJ (2012) 344:e536. doi: 10.1136/bmj.e536
28. Bezabhe WM, Radford J, Wimmer BC, Salahudeen MS, Bindoff I, Peterson GM. Incidence of osteoporosis in primary care patients with atrial fibrillation receiving different oral anticoagulants. J Clin Med (2022) 11(21):6348. doi: 10.3390/jcm11216438
29. Wen L, Chen J, Duan L, Li S. Vitamin Kdependent proteins involved in bone and cardiovascular health (Review). Mol Med Rep (2018) 18(1):3–15. doi: 10.3892/mmr.2018.8940
30. Price PA, Williamson MK. Effects of warfarin on bone. Studies on the vitamin K-dependent protein of rat bone. J Biol Chem (1981) 256(24):12754–9. doi: 10.1016/S0021-9258(18)42959-6
31. Simon RR, Beaudin SM, Johnston M, Walton KJ, Shaughnessy SG. Long-term treatment with sodium warfarin results in decreased femoral bone strength and cancellous bone volume in rats. Thromb Res (2002) 105(4):353–8. doi: 10.1016/s0049-3848(02)00035-x
32. Fusaro M, Dalle Carbonare L, Dusso A, Arcidiacono MV, Valenti MT, Aghi A, et al. Differential effects of dabigatran and warfarin on bone volume and structure in rats with normal renal function. PLoS One (2015) 10(8):e0133847. doi: 10.1371/journal.pone.0133847
33. Kluter T, Weuster M, Bruggemann S, Menzdorf L, Fitschen-Oestern S, Steubesand N, et al. Rivaroxaban does not impair fracture healing in a rat femur fracture model: An experimental study. BMC Musculoskelet Disord (2015) 16:79. doi: 10.1186/s12891-015-0502-9
34. Gigi R, Salai M, Dolkart O, Chechik O, Katzburg S, Stern N, et al. The effects of direct factor Xa inhibitor (Rivaroxaban) on the human osteoblastic cell line SaOS2. Connect Tissue Res (2012) 53(6):446–50. doi: 10.3109/03008207.2012.711867
35. Bezabhe WM, Radford J, Wimmer BC, Salahudeen MS, Bindoff I, Ling T, et al. Risk of osteoporosis in patients with atrial fibrillation with and without oral anticoagulant therapy. Expert Rev Clin Pharmacol (2022) 15(8):1003–10. doi: 10.1080/17512433.2022.2103540
36. Yokoyama S, Ieda S, Nagano M, Nakagawa C, Iwase M, Hosomi K, et al. Association between oral anticoagulants and osteoporosis: Real-world data mining using a multi-methodological approach. Int J Med Sci (2020) 17(4):471–9. doi: 10.7150/ijms.39523
37. Huang HK, Yeh JI, Loh CH. Hip fracture risk in patients with atrial fibrillation receiving oral anticoagulants: A meta-analysis based on current observational studies. Eur Heart J (2020) 41(30):2919–20. doi: 10.1093/eurheartj/ehaa361
Keywords: direct oral anticoagulant, warfarin, osteoporosis, atrial fibrillation, meta-analysis
Citation: Liu Y, Xie X, Bi S, Zhang Q, Song Q, Sun Y and Yu T (2023) Risk of osteoporosis in patients treated with direct oral anticoagulants vs. warfarin: an analysis of observational studies. Front. Endocrinol. 14:1212570. doi: 10.3389/fendo.2023.1212570
Received: 26 April 2023; Accepted: 07 August 2023;
Published: 29 September 2023.
Edited by:
Roberta Okamoto, São Paulo State University, BrazilReviewed by:
Omer Iqbal, Loyola University Chicago, United StatesCopyright © 2023 Liu, Xie, Bi, Zhang, Song, Sun and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiecheng Yu, eXV0Y0BqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.