- 1Central Laboratory, Clinical Medical College and Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 2Department of Endocrinology, Clinical Medical College and Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 3Clinical Medical College of Chengdu University, Chengdu, Sichuan, China
Background: The relationships of the rs17782313 polymorphism near melanocortin 4 receptor gene (MC4R) and the rs8192678 polymorphism in peroxisome proliferator-activated receptor gamma coactivator 1 alpha gene (PGC1α) with metabolic abnormalities have been explored in many populations around the world, but the findings were not all consistent and sometimes even a bit contradictory.
Methods: Electronic databases including Medline, Scopus, Embase, Web of Science, CNKI and Google Scholar were checked for studies that met the inclusion criteria. Data were carefully extracted from eligible studies. Standardized mean differences (SMDs) were calculated by using a random-effects model to examine the differences in the indexes of obesity, glucometabolic disorder and dyslipidemia between the genotypes of the rs17782313 and rs8192678 polymorphisms. Cochran’s Q-statistic test and Begg’s test were employed to identify heterogeneity among studies and publication bias, respectively.
Results: Fifty studies (58,716 subjects) and 51 studies (18,660 subjects) were respectively included in the pooled meta-analyses for the rs17782313 and rs8192678 polymorphisms. The C-allele carriers of the rs17782313 polymorphism had a higher average level of body mass index (SMD = 0.21 kg/m2, 95% confidence interval [95% CI] = 0.12 to 0.29 kg/m2, p < 0.001), waist circumference (SMD = 0.14 cm, 95% CI = 0.06 to 0.23 cm, p < 0.001) and blood glucose (SMD = 0.09 mg/dL, 95% CI = 0.02 to 0.16 mg/dL, p = 0.01) than the TT homozygotes. Regarding the rs8192678 polymorphism, no significant associations with the indexes of obesity, glucometabolic disorder and dyslipidemia were detected. However, significant correlations between the rs8192678 polymorphism and multiple glucometabolic indexes were observed in subgroup analyses stratified by sex, age, ethnicity and health status.
Conclusion: The meta-analysis demonstrates that the C allele of the MC4R rs17782313 polymorphism confers a higher risk of obesity and hyperglycemia, and the PGC1α rs8192678 polymorphism is weakly correlated with glucometabolic disorder. These findings may partly explain the relationships between these variants and diabetes as well as cardiovascular disease.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022373543.
Introduction
Cardiovascular disease (CVD) remains the number one killer among the world’s population, and the mortality and severe disability rates are still on the increase in some countries such as China (1–3). Metabolic disorders have been recognized as the most important risk factors for CVD, accounting for approximately 50% of the population-attributable risk (4). Genetic variations play important roles in the occurrence and progression of both metabolic disorders and CVD (5–7). In recent years, more and more susceptibility genes as well as genetic polymorphisms to metabolic disorders and CVD have been explored and confirmed in vitro and in vivo studies. The genes and their polymorphisms from the melanocortin system and peroxisome proliferator activated receptor signaling pathway have been extensively investigated with regard to their relations with obesity, metabolic abnormalities and CVD (8–12).
Melanocortin 4 receptor (MC4R), a member of the G protein-coupled receptor family and with alpha-melanocyte-stimulating hormone (a-MSH) as its ligand, is mainly distributed in the ventromedial hypothalamus and is a crucial modulator of food intake and energy homeostasis (13). Human MC4R gene is situated at the 18q21 region of chromosome 18. The rs17782313 polymorphism, located approximately 190 kilobases downstream of the MC4R gene, is a single nucleotide variation changed from thymine (T) to cytosine (C). The C allele of the rs17782313 variant was widely reported to be linked to metabolic disorders such as obesity, hyperglycemia and dyslipidemia in children (14, 15) and adults (16–24). Peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) is a coactivator of peroxisome proliferator-activated receptor-γ (PPARγ) and possibly of several other transcriptional factors such as estrogen-related receptor, nuclear respiratory factor 1 and hypoxia inducible factor 1 alpha (25–28). PGC1α plays a crucial role in mitochondrial biogenesis, fatty acid oxidation and thermogenesis (29–31). A missense variant rs8192678 (also known as +1564G/A or p.Gly482Ser), located in exon 8 of PGC1α gene, is formed by a single-nucleotide variation from guanine (G) to adenine (A), leading to the amino acid substitution from glycine to serine. A series of studies have demonstrated that the A allele of the rs8192678 polymorphism is associated with an elevated risk of adiposity (32–34) and type 2 diabetes mellitus (T2DM) (35–37).
However, there are many inconsistencies and contradictions in the published data on these two polymorphic sites and metabolic abnormalities. Some researchers reported that C allele of the rs17782313 polymorphism is correlated with a higher level of body mass index (BMI) (38–43), waist circumference (WC) (40, 44), waist-to-hip ratio (WHR) (23), glucose (GLU) (16–18), insulin (INS) (14), triglyceride (TG) (19–21), total cholesterol (TC) (21) or low-density lipoprotein cholesterol (LDL-C) (21), and a lower level of high-density lipoprotein cholesterol (HDL-C) (45), while the experimental data obtained by other investigators could not support these conclusions (46–53). There were also lots of disagreements on the relations between the PGC1α rs8192678 variant and the indexes of obesity, glucometabolic disorder and dyslipidemia. Some researchers claimed that the individuals carrying the A allele of the rs8192678 variant had a higher level of BMI (54), WHR (33, 54), GLU (55–57), INS (58–61) and homeostasis model assessment of insulin resistance (HOMA-IR) (58–61), TG (62–66), TC (67) or LDL-C (66, 67), and a lower level of HDL-C (55, 65, 66) than the noncarriers, but other scientists failed to replicate these findings in their well-designed studies and thereby had to report no associations of the rs8192678 variant with the metabolic indexes (68–74).
The systematic review and meta-analysis was conducted to clarify the relationships between the rs17782313 and rs8192678 variants and the indexes of obesity, glucometabolic disorder and dyslipidemia. The obtained findings can help clarify the interrelationships among the rs17782313 and rs8192678 variants, metabolic abnormalities and CVD.
Methods
Literature search for eligible studies
This meta-analysis has been registered and supported by PROSPERO International Prospective Register of Systematic Reviews (identifier CRD42022373543), following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement. Electronic databases such as Medline, Scopus, Embase, Web of Science, CNKI and Google Scholar were thoroughly retrieved from 1980 to February 2023. The literature search keywords were as follows: (“melanocortin 4 receptor” or “MC4R”) and (“peroxisome proliferator-activated receptor gamma coactivator 1 alpha” or “PGC1α” or “PPARGC1A”) and (“rs17782313” or “rs8192678” or “variant” or “polymorphism” or “variance” or “mutation”) and (“body mass index” or “BMI” or “waist circumference” or “WC” or “waist-to-hip ratio” or “WHR”) and (“glucose” or “GLU” or “insulin” or “INS” or “homeostasis model assessment of insulin resistance” or “HOMA-IR”) and (“lipid” or “triglyceride” or “TG” or “total cholesterol” or “TC” or “low-density lipoprotein cholesterol” or “LDL-C” or “high-density lipoprotein cholesterol” or “HDL-C”). The parameters assessed in the meta-analysis include three obesity indexes (WHR, WC and BMI), three glucometabolic disorder indexes (HOMA-IR, INS and GLU), and four dyslipidemia indexes (HDL-C, LDL-C, TC and TG). Any studies that explored the relations of the rs17782313 and rs8192678 variants with the ten indexes were screened and reviewed.
Inclusion and exclusion criteria
Inclusion criteria: 1) The genotype distribution and sample size were presented clearly and accurately; 2) One or more of the ten variables (BMI, WC, WHR, GLU, INS, HOMA-IR, TG, TC, LDL-C, and HDL-C) were reported; 3) Data format of the variables were shown as mean/standard deviation or mean/standard error. Exclusion criteria: 1) Repeatedly published data of the variables; 2) Low quality data; 3) Case reports or conference abstracts.
Data extraction
Data were carefully checked and verified after extraction from the enrolled articles. The data extracted from each article were as follows: title, year of publication, author’s names, age, gender, ethnicity, health status, sample size, and means as well as standard deviations/standard errors by genotypes. Standard deviation was calculated if standard error was given. Units used for the variables included kg/m2 (BMI), cm (WC), μmol/μL (INS) and mg/dL (TG, TC, LDL-C, HDL-C and GLU), and units were converted if other units were used.
Meta-analysis
The STATA software package (College Station, TX, USA) was employed in this systematic review and meta-analysis. Most of the included studies presented their research results in a dominant way, therefore the present meta-analysis also adopted the dominant model (i.e., [CC + CT] vs. TT for the rs17782313 polymorphism and [AA + AG] vs. GG for the rs8192678 polymorphism). Subgroup analyses were conducted according to gender, age, ethnicity and health condition. Each subgroup was defined as a comparison in the meta-analysis, and subgroup analyses were performed with three or more comparisons to ensure statistical power. Standardized mean difference (SMD) was employed to express the differences in the indexes of obesity, glucometabolic disorder and dyslipidemia between the genotypes of the rs17782313 and rs8192678 polymorphisms. The random effects model usually provides a more reliable analysis result, so this study selected the random effects model for analysis. Cochran’s Q-statistic test was used to assess heterogeneity among the studies, and Galbraith plot was drawn to discover the potential sources of heterogeneity. Subgroup analyses were performed, and the subgroups were classified by gender (males and females), age (adults and children/adolescents), ethnicity (European Caucasians, South Americans, East Asians and West Asians), and health condition (overweight/obesity, T2DM, polycystic ovary syndrome, hypertension and general/control subjects). Begg’s test was conducted to evaluate publication bias, and the trim and fill method was used to assess the potential influence of the missing studies on the pooled result if there was a publication bias. All analyses were two-tailed, and p ≤ 0.05 was considered as statistically significant.
Results
Characteristics of the included studies
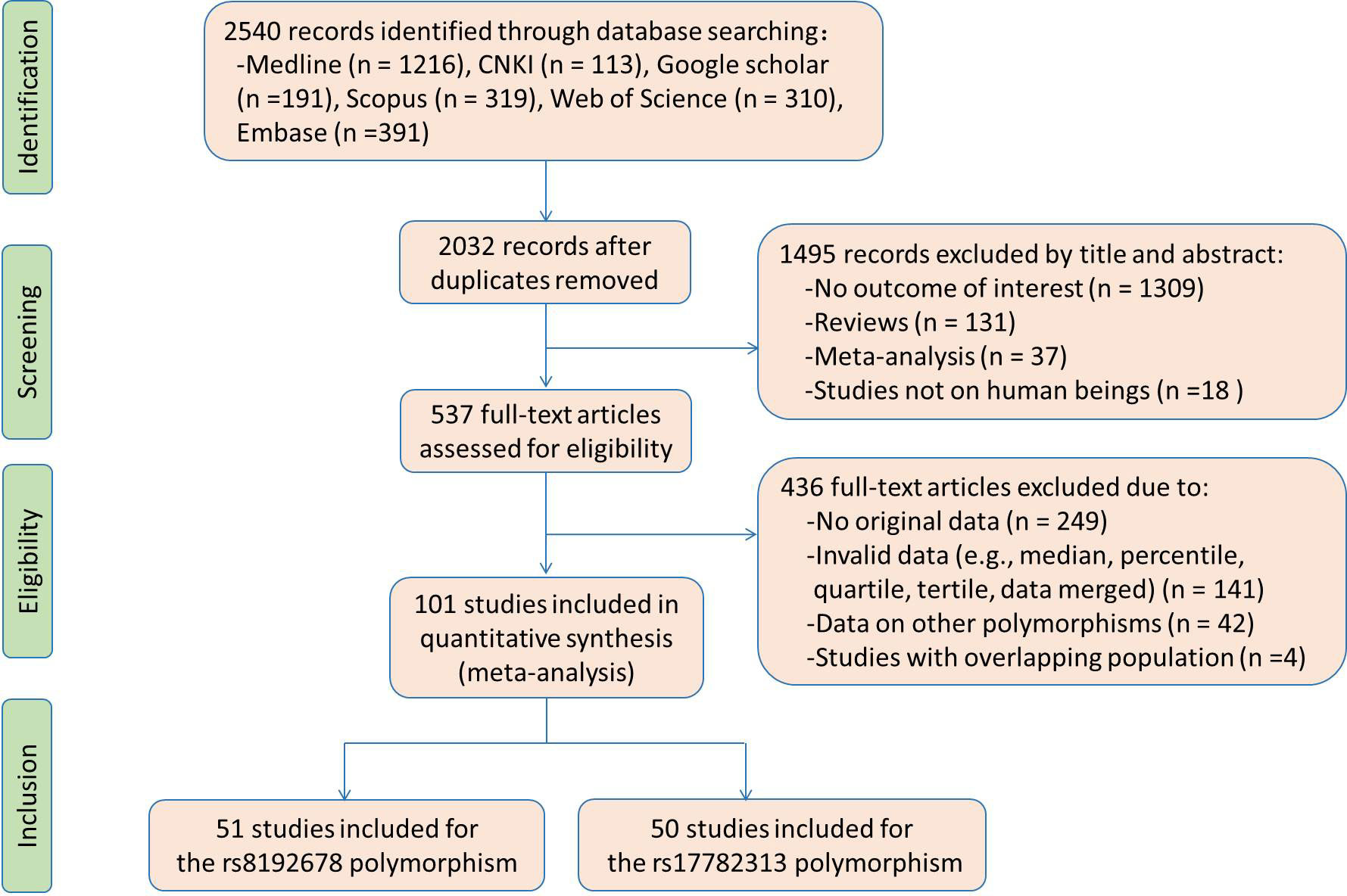
Flow diagram of the literature search is displayed in Figure 1. Fifty studies and 51 studies were respectively enrolled for the rs17782313 and rs8192678 polymorphisms, and the reference list of the included studies is presented in Table S1. Characteristics of the studies included for the rs17782313 variant are shown in Table S2. The included studies were published from 2009 to 2022, and written either in Chinese (2 articles) or English language (48 articles). Twenty-three studies, 12 studies, 7 studies and 2 studies involved European Caucasians, East Asians, West Asians and South Asians, respectively. Twenty-seven studies, 12 studies, 5 studies, and 3 studies involved overweight/obesity, T2DM, hypertension and hyperlipidemia, respectively. One study only involved males, 9 studies only involved females, and the rest studies involved both genders. The study populations in 14 studies were divided into subgroups based on gender and health condition, and each subgroup served as an independent comparison in the analysis. Original data of the indexes by genotypes of the rs17782313 polymorphism are shown in Tables S3-S5. Forty-five studies, 24 studies, 12 studies, 26 studies, 15 studies, 14 studies, 22 studies, 22 studies, 21 studies and 22 studies presented the data for BMI, WC, WHR, GLU, INS, HOMA-IR, TG, TC, LDL-C and HDL-C, respectively. A total of 65 comparisons were distinguished among the 50 studies included for the rs17782313 polymorphism, and 56 comparisons, 30 comparisons, 15 comparisons, 30 comparisons, 18 comparisons, 16 comparisons, 27 comparisons, 27 comparisons, 27 comparisons and 28 comparisons were included to analyze the differences in BMI, WC, WHR, GLU, INS, HOMA-IR, TG, TC, LDL-C and HDL-C, respectively. Fifty-eight thousand seven hundred and sixteen subjects were enrolled in the meta-analysis for the rs17782313 polymorphism. Fifty-nine percent of these subjects had the TT genotype, and the rest had the CT or CC genotype.
Characteristics of the studies included for the rs8192678 variant are shown in Table S6. The included studies were published from 2001 to 2022, and written either in Chinese (13 articles) or English language (38 articles). Thirty studies, 12 studies, 3 studies and 4 studies involved European Caucasians, East Asians, South Americans and West Asians, respectively. Eleven studies, 13 studies, 4 studies, 2 studies, 2 studies and 2 studies involved overweight/obesity, T2DM, polycystic ovary syndrome, nonalcoholic fatty liver disease, hypertension and metabolic syndrome, respectively. Four studies only involved males, 6 studies only involved females, and the other studies involved both genders. There were 17 studies in which the study populations were divided into subgroups based on gender and health condition, and each subgroup was served as an independent comparison in the analysis. Original data of the indexes by genotypes of the rs8192678 polymorphism are shown in Tables S7-S9. Forty-seven studies, 14 studies, 12 studies, 33 studies, 22 studies, 18 studies, 36 studies, 34 studies, 28 studies and 34 studies provided the data for BMI, WC, WHR, GLU, INS, HOMA-IR, TG, TC, LDL-C and HDL-C, respectively. Seventy-one comparisons were distinguished among the 51 studies included for the rs8192678 polymorphism, and 64 comparisons, 16 comparisons, 21 comparisons, 43 comparisons, 30 comparisons, 25 comparisons, 47 comparisons, 45 comparisons, 36 comparisons and 45 comparisons were respectively included to analyze the differences in BMI, WC, WHR, GLU, INS, HOMA-IR, TG, TC, LDL-C and HDL-C. Eighteen thousand six hundred and sixty subjects were enrolled in the meta-analysis for the rs8192678 variant. Thirty-nine percent of these subjects had the GG genotype, and the rest subjects had the AA or AG genotype.
Associations of the MC4R rs17782313 polymorphism with the indexes of obesity, glucometabolic disorder and dyslipidemia
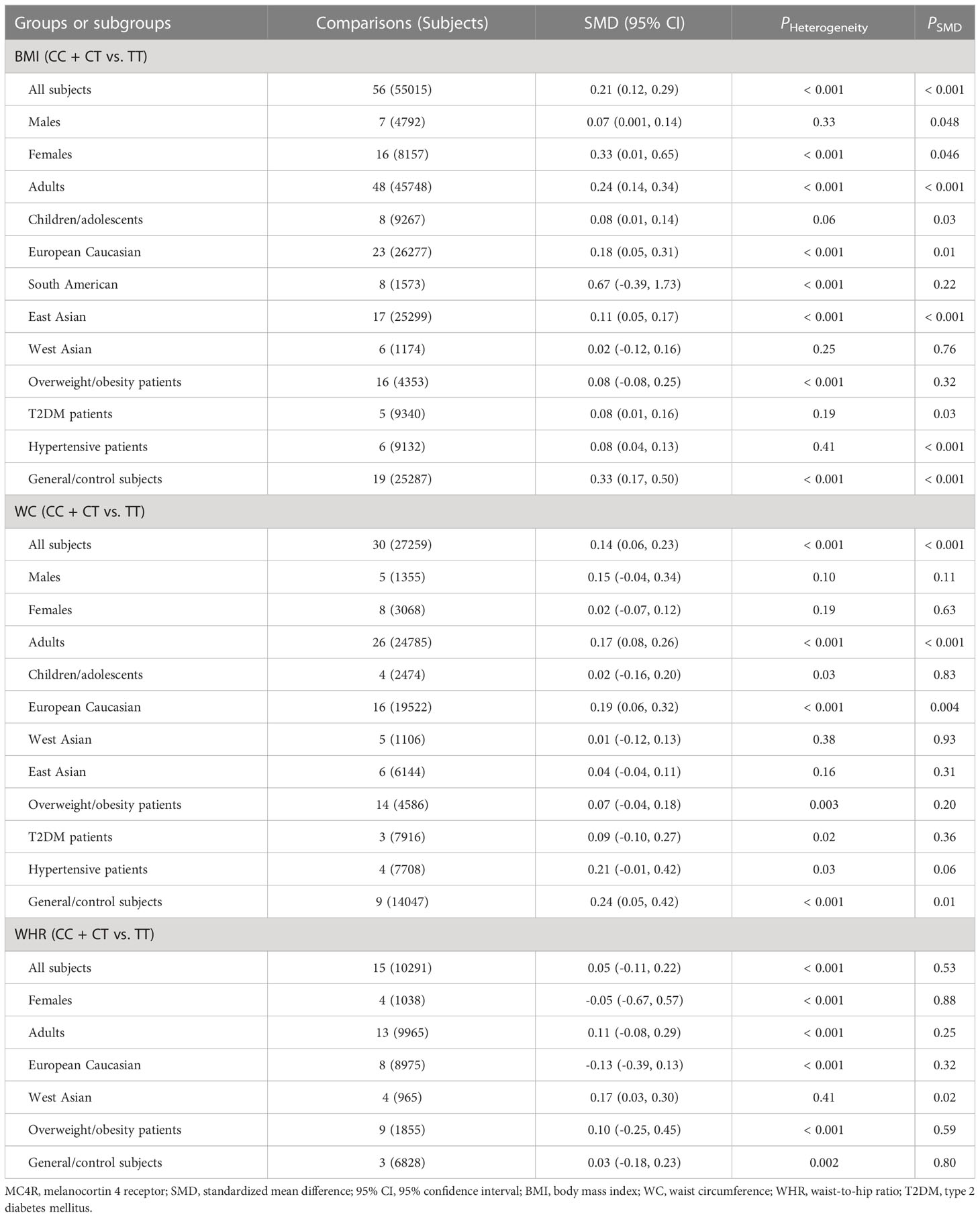
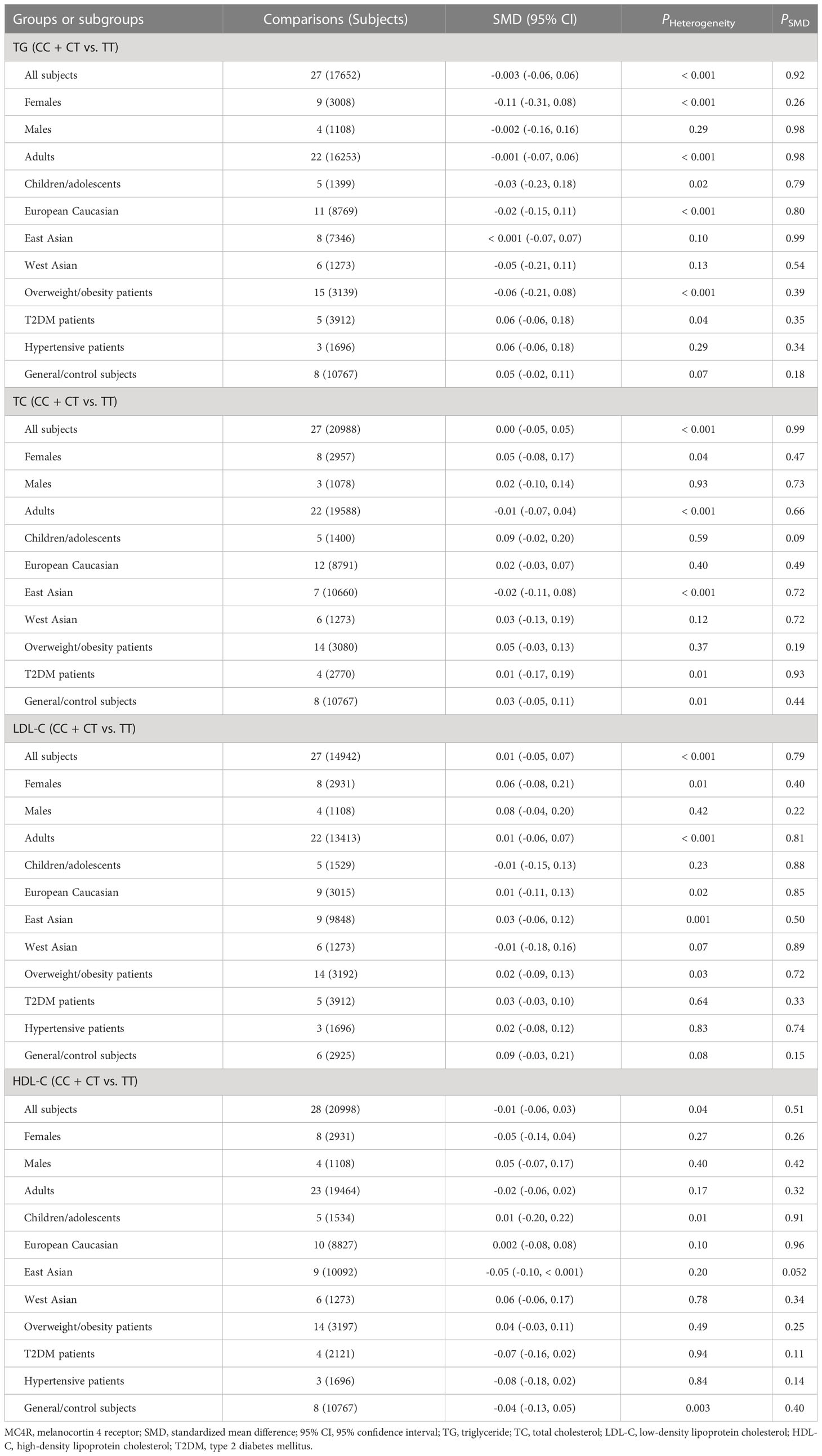
The associations of the rs17782313 polymorphism with the indexes of obesity, glucometabolic disorder and dyslipidemia are shown in Tables 1–3; Figures 2–4, S1–S7. The pooled meta-analyses displayed that individuals carrying the C allele of the rs17782313 variant had a higher level of BMI (SMD = 0.21 kg/m2, 95% confidence interval [95% CI] = 0.12 to 0.29 kg/m2, p < 0.001) (Table 1; Figure 2), WC (SMD = 0.14 cm, 95% CI = 0.06 to 0.23 cm, p < 0.001) (Table 1, Figure 3) or blood glucose (SMD = 0.09 mg/dL, 95% CI = 0.02 to 0.16 mg/dL, p = 0.01) (Table 2; Figure 4) than the TT homozygotes in the overall population. No significant associations of the rs17782313 variant with the levels of WHR, INS, HOMA-IR, TG, TC, LDL-C and HDL-C were detected in the overall population (Tables 1–3; Figures S1–S7).
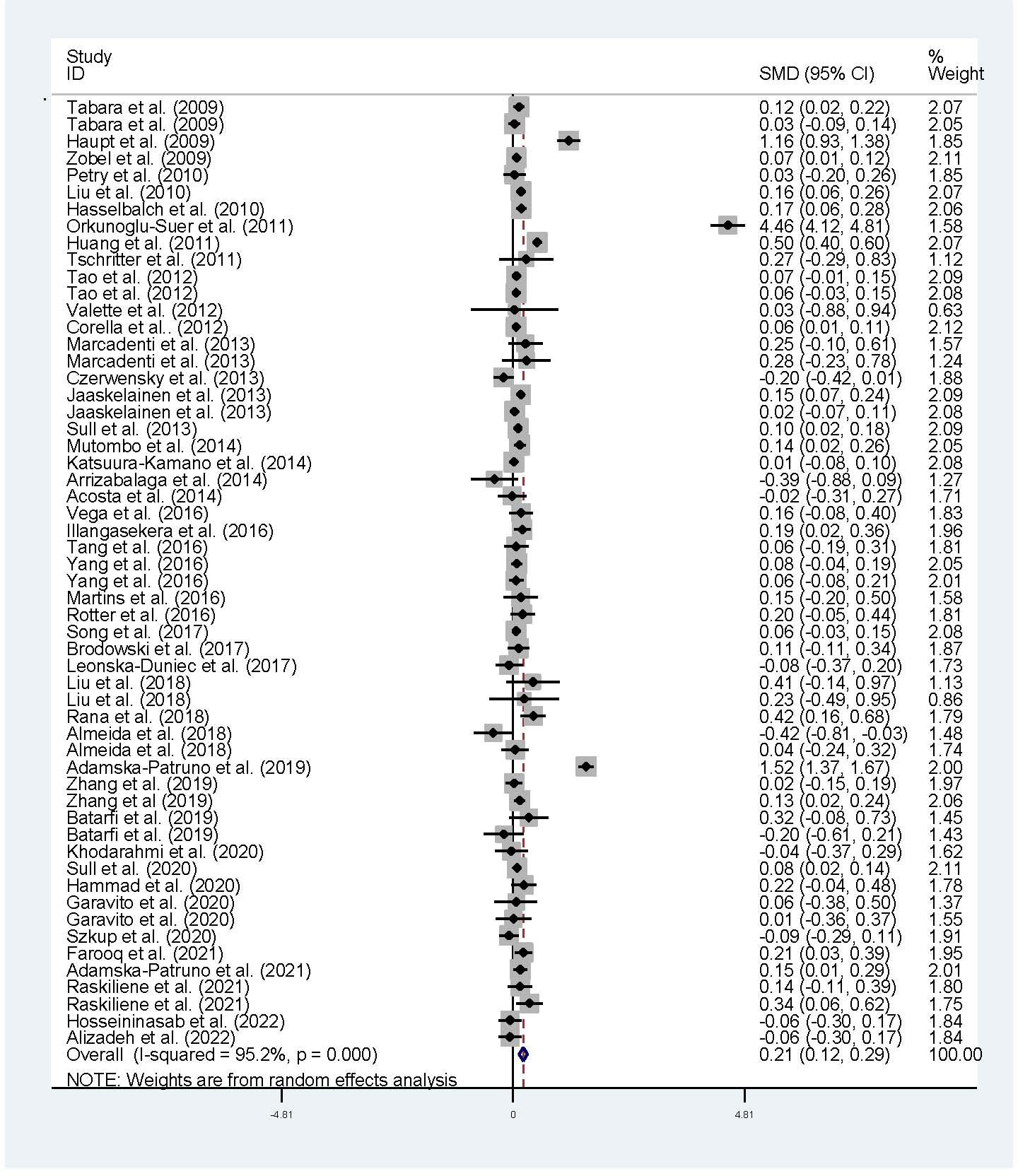
Figure 2 Forest plot of the association analysis between the MC4R rs17782313 polymorphism and BMI. MC4R, melanocortin 4 receptor, SMD, standardized mean difference: 95% CI, 95% confidence interval; BMI, body mass index.
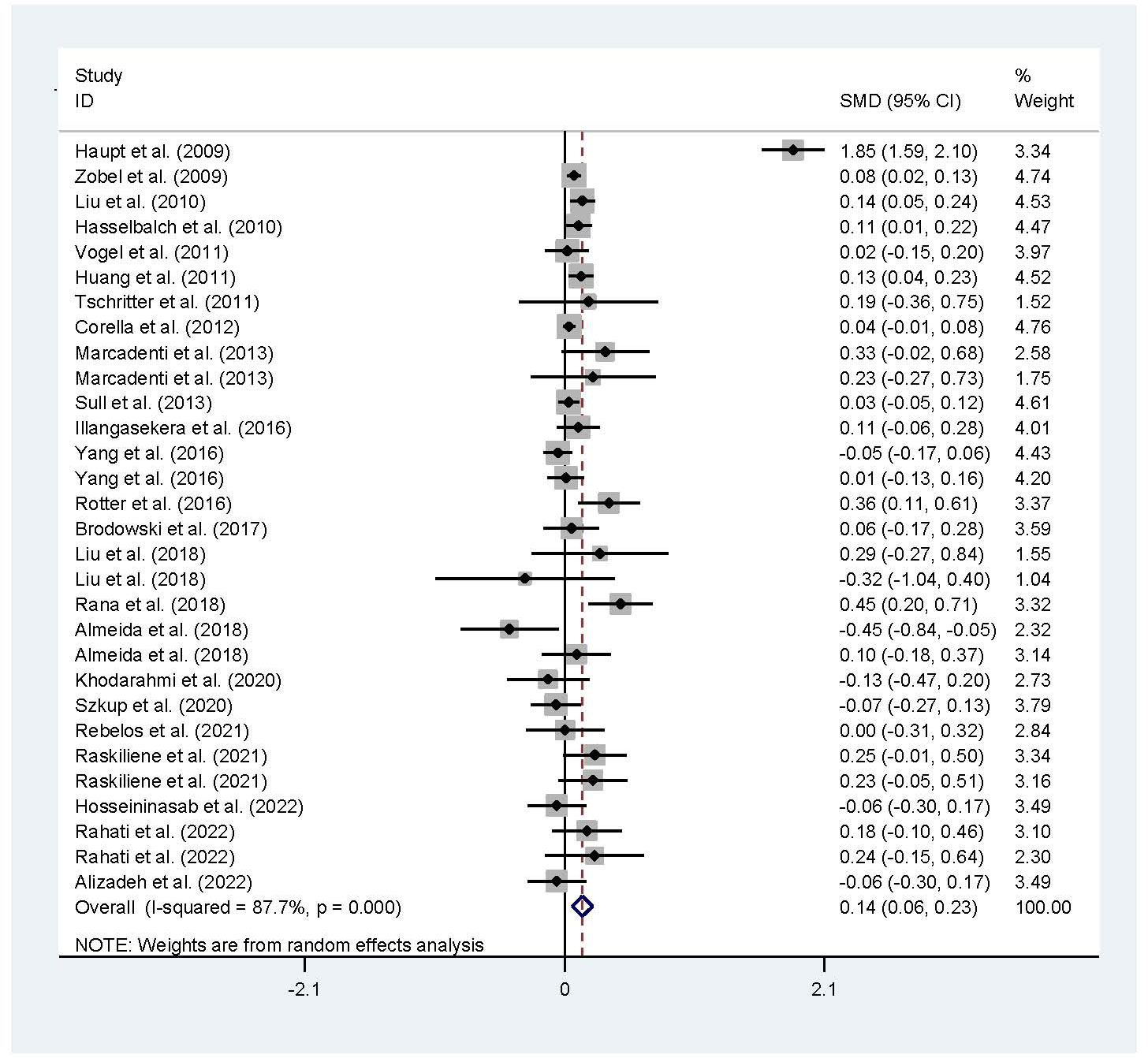
Figure 3 Forest plot of the association analysis between the rs177782313 polymorphism and WC. MC4R, melanocortin 4 receptor; SMD, standardized mean difference: 95%, CI, 95% confidence interval; WC, waist circumtances.
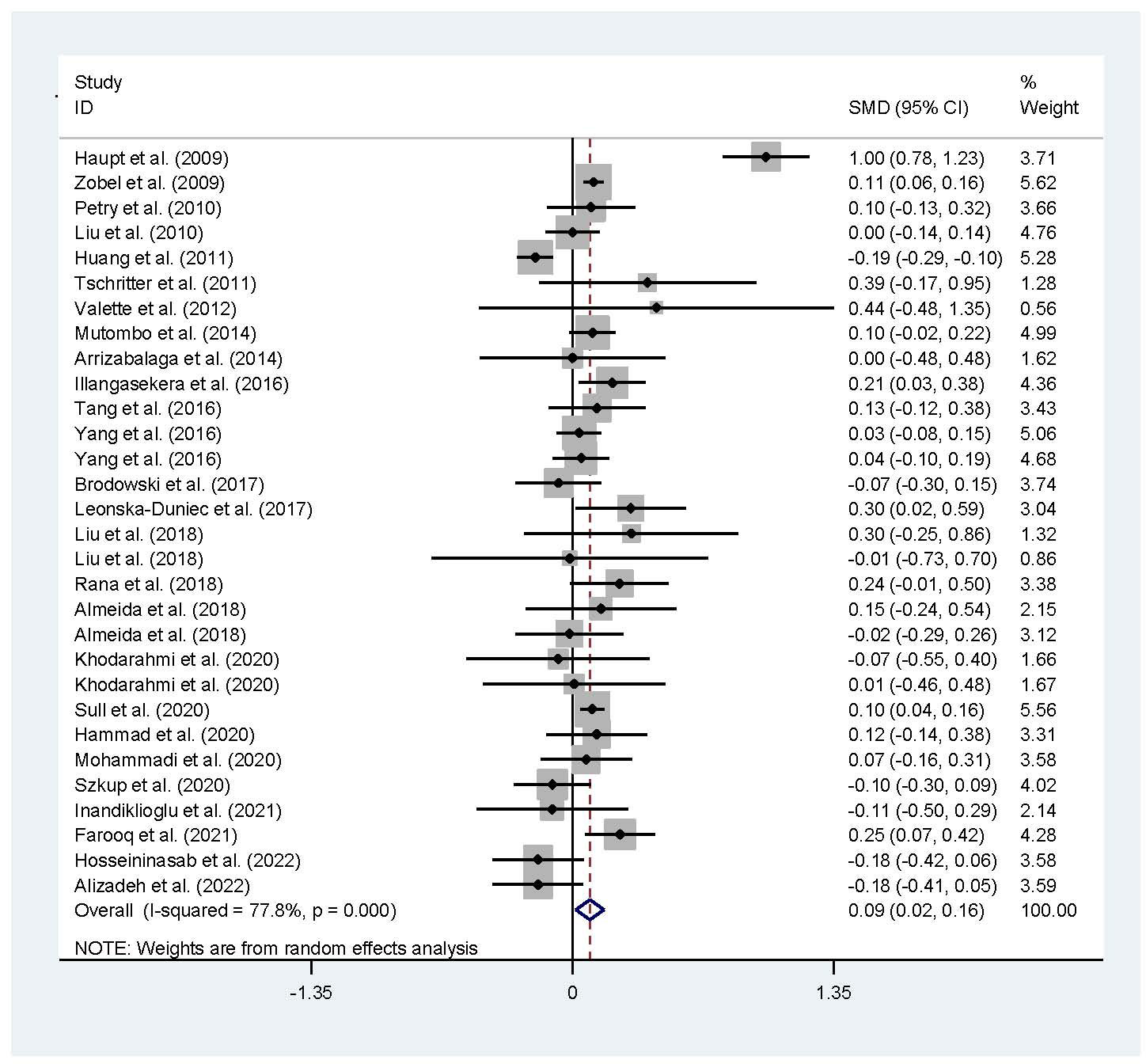
Figure 4 Forest plot of the association analysis between the MC4R rs17782313 polymorphism and blood glucose. MC4R, melanocortin 4 receptor; SMD, standardized mean difference; 95% CI, 95% confidence interval.
The interactions of the rs17782313 polymorphism with age, ethnicity and health condition on the indexes of obesity and glucometabolic disorder were observed (Tables 1, 2). The C allele of the rs17782313 variant is correlated with a higher level of WC (SMD = 0.17 cm, 95% CI = 0.08 to 0.26 cm, p < 0.001) or GLU (SMD = 0.10 mg/dL, 95% CI = 0.02 to 0.19 mg/dL, p = 0.02) in adults, but not in children/adolescents. The C allele of the rs17782313 polymorphism is associated with a higher level of BMI in European Caucasians (SMD = 0.18 kg/m2, 95% CI = 0.05 to 0.31 kg/m2, p = 0.01) and East Asians (SMD = 0.11 kg/m2, 95% CI = 0.05 to 0.17 kg/m2, p < 0.001), but not in South Americans and West Asians. Similarly, the C allele of the rs17782313 polymorphism is associated with higher levels of WC (SMD = 0.19 cm, 95% CI = 0.06 to 0.32 cm, p = 0.004) and GLU (SMD = 0.15 mg/dL, 95% CI = 0.01 to 0.29 mg/dL, p = 0.03) in European Caucasians, but not in any other ethnicities. The C allele of the rs17782313 polymorphism is associated with a higher level of WHR (SMD = 0.17, 95% CI = 0.03 to 0.30, p = 0.02) in West Asians, but not in the whole population or any other ethnicities. The C allele of the rs17782313 polymorphism is associated with a higher level of BMI in T2DM patients (SMD = 0.08 kg/m2, 95% CI = 0.01 to 0.16 kg/m2, p = 0.03), hypertensive patients (SMD = 0.08 kg/m2, 95% CI = 0.04 to 0.13 kg/m2, p < 0.001) and general/control subjects (SMD = 0.33 kg/m2, 95% CI = 0.17 to 0.50 kg/m2, p < 0.001), but not in overweight/obesity patients. Notably, the C allele of the rs17782313 variant is correlated with a higher level of WC (SMD = 0.24 cm, 95% CI = 0.05 to 0.42 cm, p = 0.01) or GLU (SMD = 0.15 mg/dL, 95% CI = 0.03 to 0.27 mg/dL, p = 0.01) in general/control subjects, but not in any of the patient populations.
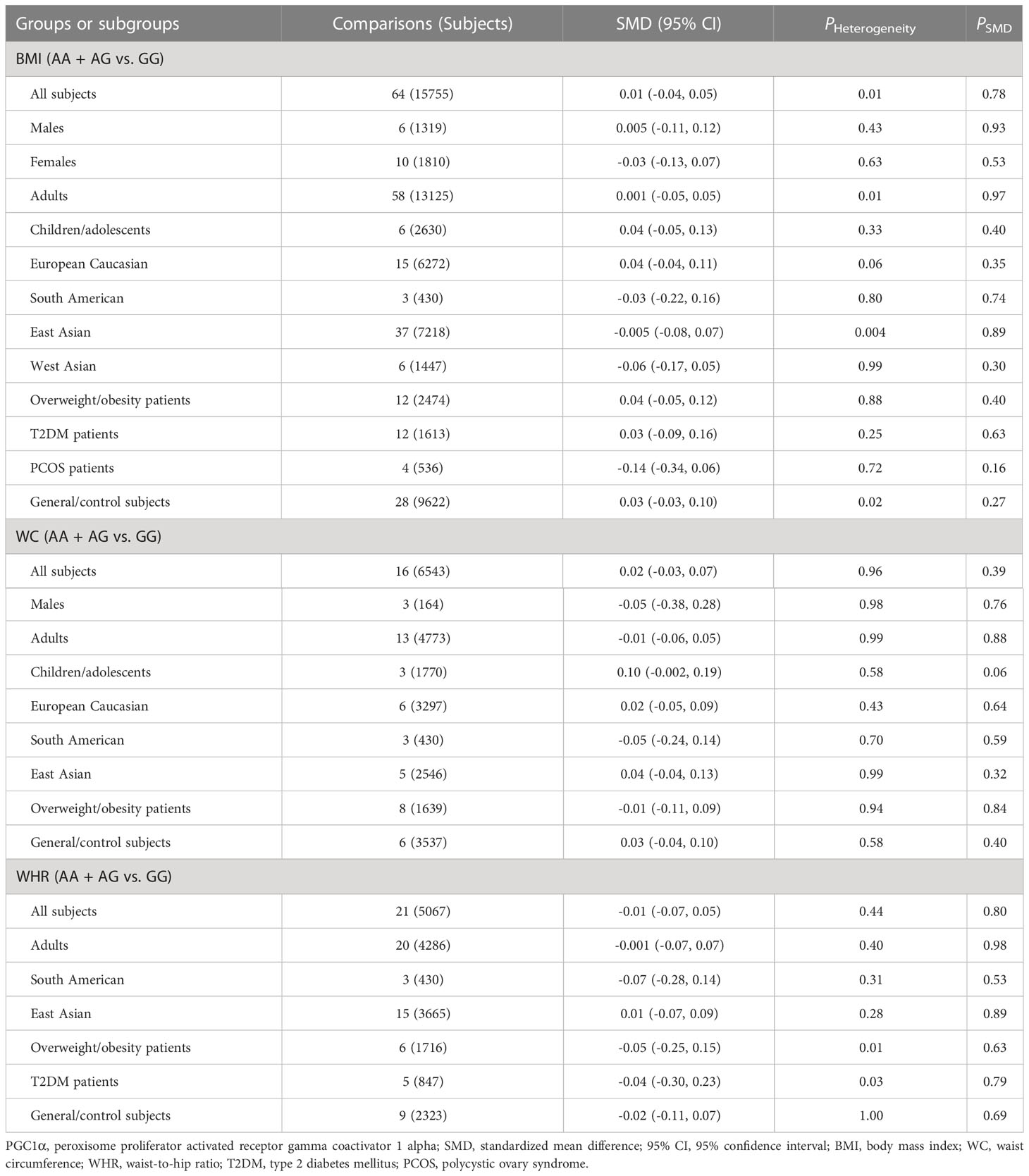
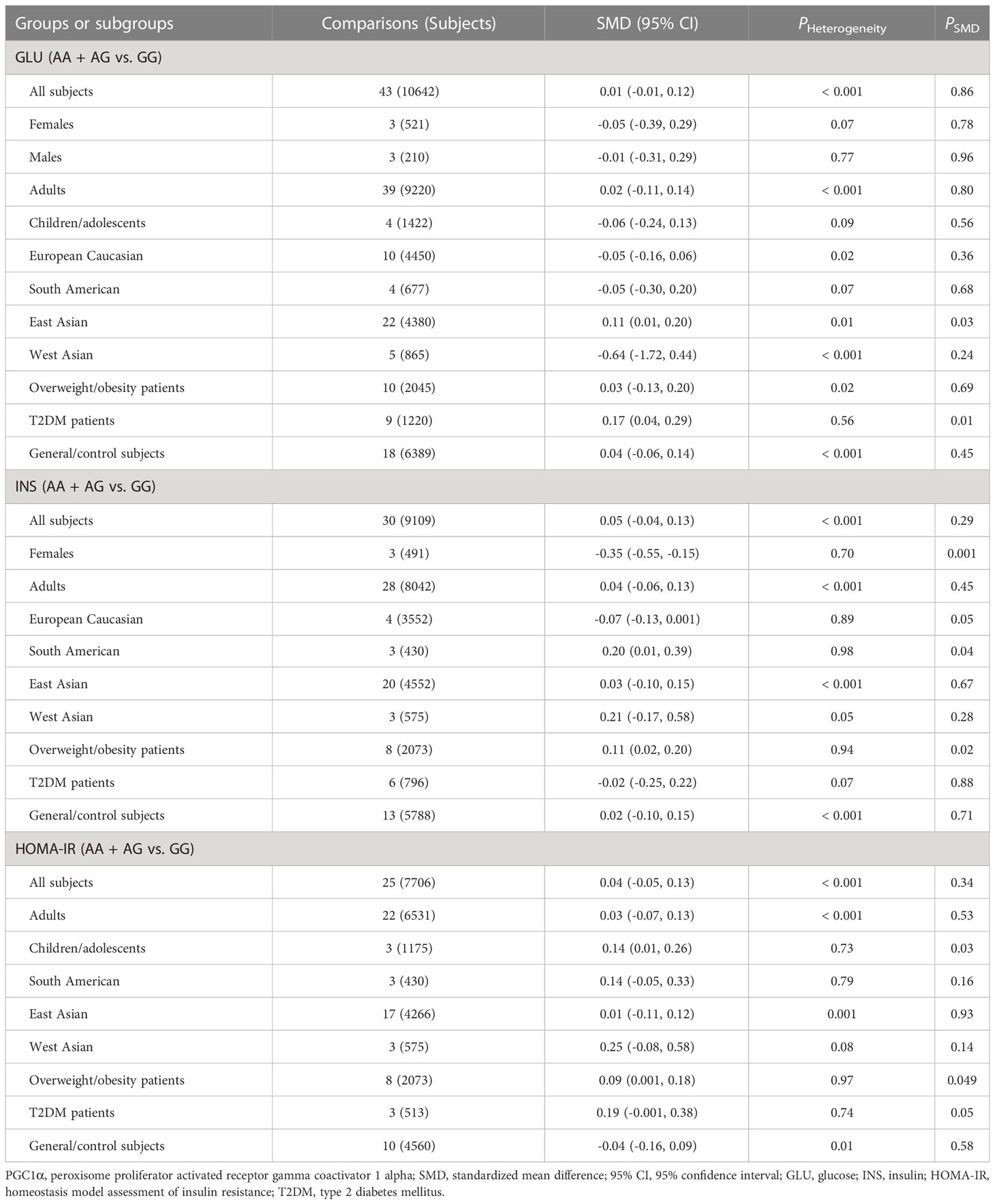
Associations of the PGC1α rs8192678 polymorphism with the indexes of obesity, glucometabolic disorder and dyslipidemia
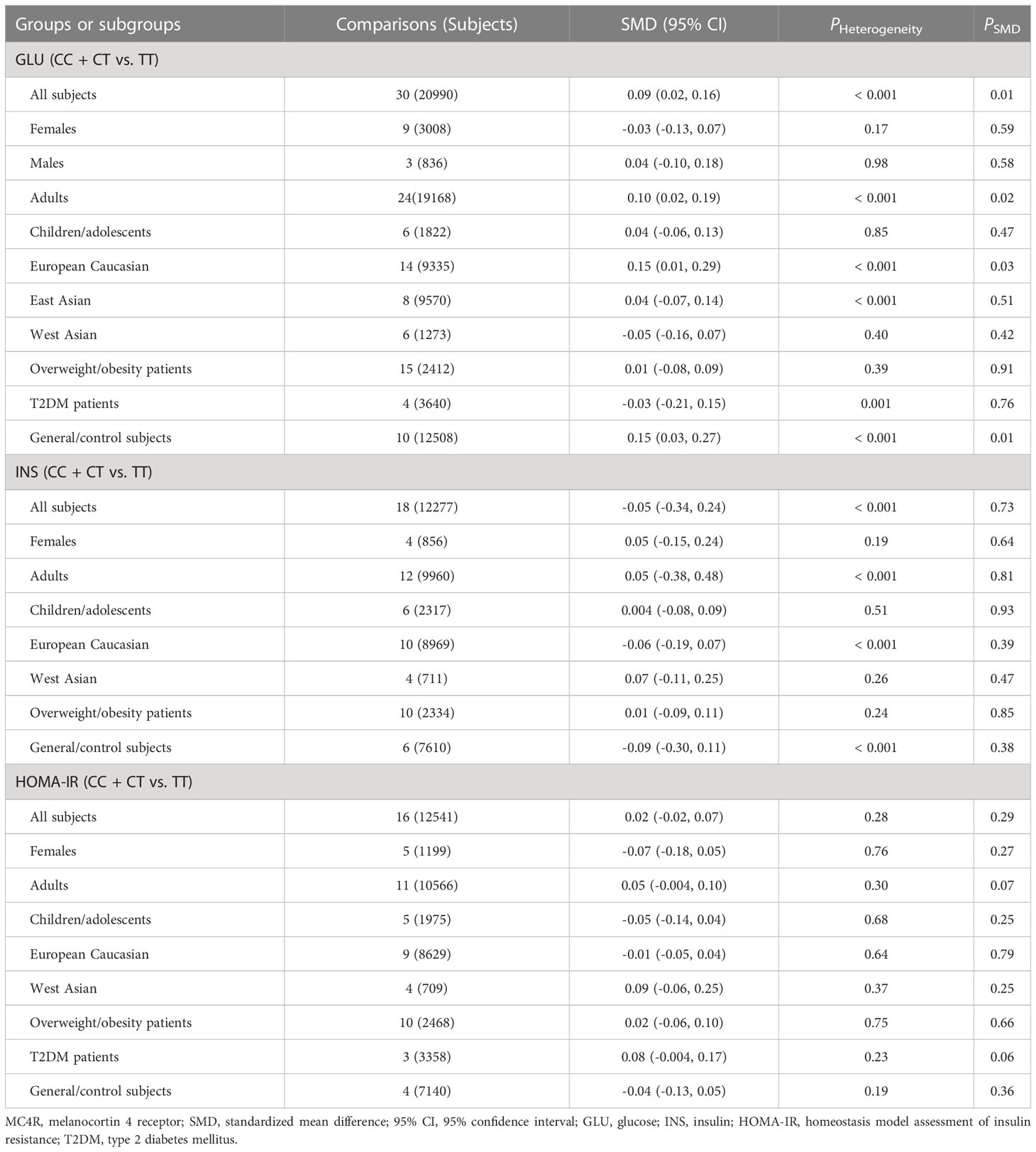
As indicated in Tables 4–6 and Figures S8-S17, there are no significant correlations being detected between the rs8192678 polymorphism and any of the ten variables of obesity, glucometabolic disorder and dyslipidemia in the pooled meta-analyses in the overall population. However, significant associations between the rs8192678 polymorphism and glucometabolic disorder indexes as well as LDL-C were found in the subgroup analyses (Tables 5, 6). The A-allele carriers of the rs8192678 polymorphism had a higher level of GLU in East Asians (SMD = 0.11 mg/dL, 95% CI = 0.01 to 0.20 mg/dL, p = 0.03) and T2DM patients (SMD = 0.17 mg/dL, 95% CI = 0.04 to 0.29 mg/dL, p = 0.01), a higher level of INS in South Americans (SMD = 0.20 μU/mL, 95% CI = 0.01 to 0.39 μU/mL, p = 0.04) and overweight/obesity patients (SMD = 0.11 μU/mL, 95% CI = 0.02 to 0.20 μU/mL, p = 0.02), and a higher level of HOMA-IR in children/adolescents (SMD = 0.14, 95% CI = 0.01 to 0.26, p = 0.03), overweight/obesity patients (SMD = 0.09, 95% CI = 0.001 to 0.18, p = 0.049) as well as T2DM patents (SMD = 0.19, 95% CI = -0.001 to 0.38, p = 0.05) than the GG homozygotes. Somehow, the A-allele carriers of the rs8192678 polymorphism had a lower level of INS than the GG homozygotes in females (SMD = -0.35 μU/mL, 95% CI = -0.55 to -0.15 μU/mL, p = 0.001) and European Caucasians (SMD = -0.07 μU/mL, 95% CI = -0.13 to 0.001 μU/mL, p = 0.05). Notably, the A-allele carriers of the rs8192678 polymorphism were found to have a higher level of LDL-C (SMD = 0.12 mg/dL, 95% CI = 0.04 to 0.20 mg/dL, p < 0.01) than the GG homozygotes in East Asians.
Heterogeneity analysis
Significant heterogeneity was found in the pooled meta-analyses for the rs17782313 and rs8192678 polymorphisms in the overall population (Tables 1-6). Sources of heterogeneity were successfully identified by using Galbraith plots. The heterogeneity was effectively removed or decreased after excluding the outlier studies, while the results of the pooled meta-analyses were not altered significantly except the association of the rs17782313 polymorphism with WHR, which became significant when the outlier studies were excluded (SMD = 0.15, 95% CI = 0.09 to 0.22, PHeterogeneity = 0.24, PSMD < 0.001).
Publication bias
Publication bias was observed in the association analysis between the rs17782313 variant and BMI, as well as between the rs8192678 polymorphism and GLU or TG. However, no significant changes were found for all after adjustment by the trim-and-fill method.
Discussion
MC4R and PGC1α play critical roles in energy homeostasis, and are implicated in the pathogenesis of metabolic diseases including obesity, diabetes, hepatic steatosis and CVD (73–80). A number of scientific reports have suggested that the MC4R rs17782313 and PGC1α rs8192678 polymorphisms are associated with the onset of T2DM and CVD (81–86), which prompted us to conduct this meta-analysis to clarify the relationships of the two polymorphic variants with the indexes of obesity, glucometabolic disorder and dyslipidemia since all of these indexes are closely related to T2DM and CVD. The present meta-analysis demonstrates that the C allele of the rs17782313 polymorphism near MC4R is associated with a higher level of BMI, WC or GLU, but not with blood lipids, which is in agreement with the previous findings that the C allele of the rs17782313 variant is strongly correlated with an elevated risk of obesity and T2DM (87–89), but weakly with CVD (90). In addition, we found that the PGC1α rs8192678 polymorphism is associated with glucometabolic disorder indexes in some specific populations such as East and West Asians, Europeans, and overweight/obesity and T2DM patients although this variant is not correlated with the indexes of obesity, dyslipidemia and glucometabolic disorder in the pooled meta-analyses in the overall population.
Although the rs17782313 polymorphism is just localized near, but not within the MC4R gene, its impact on obesity risk seems to be greater than those polymorphic loci located in the MC4R gene. There were many more studies in the literature reporting the effect of the rs17782313 polymorphism on obesity risk compared to several MC4R exonic polymorphisms such as V103I, I251L, S127L and K73R. Nearly 90% of the studies reported that the rs17782313 polymorphism has a significant impact on obesity risk, while only 50% of the studies on average reported that MC4R exonic polymorphisms are significantly related to obesity risk (data not shown). Interestingly, another polymorphic site near the MC4R gene, rs12970134, was also found to be strongly associated with obesity risk, with 24 studies out of 25 studies reporting a significant impact of the rs12970134 polymorphism on obesity risk (data not shown).
The mechanisms underlying the correlations of the rs17782313 polymorphism with obesity as well as hyperglycemia may be that this variant leads to abnormal expression of MC4R as it is located downstream of the MC4R gene without any amino acid alteration in MC4R receptor. Indeed, Tang and colleagues (91) conducted MeQTL and eQTL analyses to explore the effects of the rs17782313 polymorphism on DNA methylation and MC4R expression, and found that the C allele of the rs17782313 polymorphism is associated with a decreased methylation state in the promoter region of MC4R (p = 1.7 × 10-4) and an increased mRNA expression level of MC4R (p = 1.9 × 10-3). We used GTEx Analysis Release V8 (dbGaP Accession phs000424.v8.p2) to assess the impact of the rs17782313 polymorphism on the mRNA expression pattern of MC4R, and the results displayed that the MC4R mRNA level increases orderly with the TT, TC and CC genotypes in brain nucleus accumbens and putamen, but decreases orderly in cerebellum (data not shown, p < 0.05 for all). However, Lauria et al. (92) did not detect a significant link between the rs17782313 polymorphism and the MC4R mRNA expression level in peripheral blood cells. The relation between the rs17782313 polymorphism and MC4R expression pattern remains to be examined. Several studies have shown that MC4R can promote the expression of brain-derived neurotrophic factor (BDNF), and activate the AMPK-SIRT1-PGC-1α pathway (93–95). Therefore, BDNF and sirtuin 1 may mediate the association between the MC4R rs17782313 polymorphism and obesity as well as hyperglycemia, which requires further investigation to confirm.
The PGC1α rs8192678 polymorphism has also been shown to affect the expression and function of PGC-1α. Taghvaei et al. (96) demonstrated that the substitution of glycine with serine at the rs8192678 polymorphism has a destabilizing effect on PGC-1α by computational analysis. The influences of multiple genetic and environmental factors on PGC-1α expression in skeletal muscles were investigated in Swedish twins, and the researchers observed that PGC-1α gene expression decreased with age and was modulated by the rs8192678 polymorphism (97). Chen et al. (98) explored the direct role of the rs8192678 polymorphism in altering the actions of PGC-1α on hepatocyte fat deposition and found that the hepatocytes with the Ser/Ser genotype exhibited a significant elevation in palmitate-induced fat deposition compared to the hepatocytes with the Gly/Gly genotype. In addition, the Ser/Ser genotype of the rs8192678 polymorphism caused a significant decrease in the expression of carnitine palmitoyl transferase 1a, a rate-limiting enzyme in fatty acid oxidation that transfers long-chain fatty acids into mitochondria for oxidation (98).
Significant heterogeneity was existed in the pooled meta-analyses of the rs17782313 polymorphism with BMI, WC and GLU. The outlier studies were figured out by observing Galbraith plots. No significant alterations in SMDs as well as 95% CIs were observed after removing the outlier studies, which indicates that the associations between the rs17782313 variant and these indexes are very robust. WHR, an indicator of abdominal obesity, was shown to be significantly associated with the rs17782313 polymorphism after exclusion of the outlier studies. It suggests that the significant correlation between the rs17782313 polymorphism and WHR has been masked by the extensive heterogeneity among studies. Significant heterogeneity was also detected in the association analyses between the rs8192678 polymorphism and the indexes of obesity, glucometabolic disorder and dyslipidemia. The outlier studies were identified and excluded, but there were still no significant correlations between the rs8192678 polymorphism and all of these indexes in the whole population, which indicates that the associations of the rs8192678 polymorphism with obesity, diabetes and dyslipidemia are weak, although PGC1α works as an activator of PPARγ, an important modulator of glucose and lipid metabolism (7).
There are several limitations to current study. Firstly, this meta-analysis only included the studies that published in Chinese or English as it is difficult to obtain the full articles written in various languages. Secondly, the interactions of the rs17782313 and rs8192678 polymorphisms with other genetic variants or non-genetic factors other than age, gender, ethnicity and health condition on metabolic abnormalities were not investigated in this meta-analysis due to lack of original data from the enrolled studies. A large number of genes, diets, physical activities and environmental factors are implicated in the pathogenesis of metabolic disorders such as dyslipidemia, diabetes and obesity. More precise results could be obtained if gene-gene interactions were explored, or stratification analyses according to diets, physical activities and environmental factors were conducted.
In conclusion, the C allele of the rs17782313 polymorphism near MC4R confers a higher risk of obesity and hyperglycemia, and the A allele of the rs8192678 polymorphism is associated with glucometabolic disorder in some specific populations. These findings can provide a direct link between the rs17782313 and rs8192678 polymorphisms and T2DM as well as CVD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YS, YZ and SL conceived of the systematic review and meta-analysis, participated in the design, analyzed the data, and drafted the manuscript. HN, XW, XL, JW and ML carried out the literature search, collected the data, and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The project was supported by the Natural Science Foundation of Sichuan, China [2022NSFSC0740], and the Research Project of Clinical Medical College and Affiliated Hospital of Chengdu University [YYB2017002].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1210455/full#supplementary-material
References
1. Mehta LS, Velarde GP, Lewey J, Sharma G, Bond RM, Navas-Acien A, et al. Cardiovascular disease risk factors in women: the impact of race and ethnicity: A scientific statement from the american heart association. Circulation (2023) 147(19):1471–87. doi: 10.1161/CIR.0000000000001139
2. DOmanski MJ, Wu CO, Tian X, Hasan AA, Ma X, Huang Y, et al. Association of incident cardiovascular disease with time course and cumulative exposure to multiple risk factors. J Am Coll Cardiol (2023) 81(12):1151–61. doi: 10.1016/j.jacc.2023.01.024
3. Ma H, Wang X, Xue Q, Li X, Liang Z, Heianza Y, et al. Cardiovascular health and life expectancy among adults in the United States. Circulation (2023) 147(15):1137–46. doi: 10.1161/CIRCULATIONAHA.122.062457
4. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet (2004) 364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9
5. Li S, Zhang Y, Xu W, Lv Z, Xu L, Zhao Z, et al. C Allele of the PPARδ+294T>C polymorphism confers a higher risk of hypercholesterolemia, but not obesity and insulin resistance: A systematic review and meta-analysis. Horm Metab Res (2023) 55(5):355–66. doi: 10.1055/a-2043-7707
6. Li S, He C, Nie H, Pang Q, Wang R, Zeng Z, et al. G Allele of the rs1801282 Polymorphism in PPARγ Gene Confers an Increased Risk of Obesity and Hypercholesterolemia, While T Allele of the rs3856806 Polymorphism Displays a Protective Role Against Dyslipidemia: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) (2022) 13:919087. doi: 10.3389/fendo.2022.919087
7. Song Y, Li S, He C. PPARγ Gene polymorphisms, metabolic disorders, and coronary artery disease. Front Cardiovasc Med (2022) 9:808929. doi: 10.3389/fcvm.2022.808929
8. Castro GV, Latorre AFS, Korndorfer FP, de Carlos Back LK, Lofgren SE. The impact of variants in four genes: MC4R, FTO, PPARG and PPARGC1A in overweight and obesity in a large sample of the Brazilian population. Biochem Genet (2021) 59(6):1666–79. doi: 10.1007/s10528-021-10079-2
9. Bakhashab S, Filimban N, Altall RM, Nassir R, Qusti SY, Alqahtani MH, et al. The Effect Sizes of PPARγ rs1801282, FTO rs9939609, and MC4R rs2229616 Variants on Type 2 Diabetes Mellitus Risk among the Western Saudi Population: A Cross-Sectional Prospective Study. Genes (Basel) (2020) 11(1):98. doi: 10.3390/genes11010098
10. Szkup M, Owczarek AJ, Schneider-Matyka D, Brodowski J, Łój B, Grochans E. Associations between the components of metabolic syndrome and the polymorphisms in the peroxisome proliferator-activated receptor gamma (PPAR-γ), the fat mass and obesity-associated (FTO), and the melanocortin-4 receptor (MC4R) genes. Aging (Albany NY) (2018) 10(1):72–82. doi: 10.18632/aging.101360
11. Rotter I, Skonieczna-Żydecka K, Kosik-Bogacka D, Adler G, Rył A, Laszczyńska M. Relationships between FTO rs9939609, MC4R rs17782313, and PPARγ rs1801282 polymorphisms and the occurrence of selected metabolic and hormonal disorders in middle-aged and elderly men - a preliminary study. Clin Interv Aging (2016) 11:1723–32. doi: 10.2147/CIA.S120253
12. Song Y, Raheel TM, Jia A, Dai G, Liu L, Long X, et al. rs10865710 polymorphism in PPARG promoter is associated with the severity of type 2 diabetes mellitus and coronary artery disease in a Chinese population. Postgrad Med J (1164) 2022:98. doi: 10.1136/postgradmedj-2021-140354
13. Singh U, Saito K, Khan MZ, Jiang J, Toth BA, Rodeghiero SR, et al. Collateralizing ventral subiculum melanocortin 4 receptor circuits regulate energy balance and food motivation. Physiol Behav (2023) 262:114105. doi: 10.1016/j.physbeh.2023.114105
14. Vogel CI, Boes T, Reinehr T, Roth CL, Scherag S, Scherag A, et al. Common variants near MC4R: exploring gender effects in overweight and obese children and adolescents participating in a lifestyle intervention. Obes Facts (2011) 4(1):67–75. doi: 10.1159/000324557
15. Liu G, Zhu H, Lagou V, Gutin B, Barbeau P, Treiber FA, et al. Common variants near melanocortin 4 receptor are associated with general and visceral adiposity in European- and African-American youth. J Pediatr (2010) 156(4):598–605. doi: 10.1016/j.jpeds.2009.10.037
16. Sull JW, Kim G, Jee SH. Association of MC4R (rs17782313) with diabetes and cardiovascular disease in Korean men and women. BMC Med Genet (2020) 21(1):160. doi: 10.1186/s12881-020-01100-3
17. Leońska-Duniec A, Jastrzębski Z, Zarębska A, Smółka W, Cięszczyk P. Impact of the polymorphism near MC4R (rs17782313) on obesity- and metabolic-related traits in women participating in an aerobic training program. J Hum Kinet (2017) 58:111–9. doi: 10.1515/hukin-2017-0073
18. Farooq S, Rana S, Siddiqui AJ, Iqbal A, Musharraf SG. Association of metabolites with obesity based on two gene variants, MC4R rs17782313 and BDNF rs6265. Biochim Biophys Acta Mol Basis Dis (2021) 1867(7):166144. doi: 10.1016/j.bbadis.2021.166144
19. Katsuura-Kamano S, Uemura H, Arisawa K, Yamaguchi M, Hamajima N, Wakai K, et al. A polymorphism near MC4R gene (rs17782313) is associated with serum triglyceride levels in the general Japanese population: the J-MICC Study. Endocrine (2014) 47(1):81–9. doi: 10.1007/s12020-014-0306-y
20. Yang J, Gao Q, Gao X, Tao X, Cai H, Fan Y, et al. Melanocortin-4 receptor rs17782313 polymorphisms are associated with serum triglycerides in older Chinese women. Asia Pac J Clin Nutr (2016) 25(1):213–9. doi: 10.6133/apjcn.2016.25.1.18
21. Brodowski J, Szkup M, Jurczak A, Wieder-Huszla S, Brodowska A, Laszczyńska M, et al. Searching for the relationship between the parameters of metabolic syndrome and the rs17782313 (T>C) polymorphism of the MC4R gene in postmenopausal women. Clin Interv Aging (2017) 12:549–55. doi: 10.2147/CIA.S129874
22. Huang W, Sun Y, Sun J. Combined effects of FTO rs9939609 and MC4R rs17782313 on obesity and BMI in Chinese Han populations. Endocrine (2011) 39(1):69–74. doi: 10.1007/s12020-010-9413-6
23. Rana S, Rahmani S, Mirza S. MC4R variant rs17782313 and manifestation of obese phenotype in Pakistani females. RSC Adv (2018) 8(30):16957–72. doi: 10.1039/c8ra00695d
24. Raskiliene A, SMalinskiene A, Kriaucioniene V, Lesauskaite V, Petkeviciene J. Associations of MC4R, LEP, and LEPR polymorphisms with obesity-related parameters in childhood and adulthood. Genes (Basel) (2021) 12(6):949. doi: 10.3390/genes12060949
25. Niu N, Li H, Du X, Wang C, Li J, Yang J, et al. Effects of NRF-1 and PGC-1α cooperation on HIF-1α and rat cardiomyocyte apoptosis under hypoxia. Gene (2022) 834:146565. doi: 10.1016/j.gene.2022.146565
26. Papatheodorou I, Makrecka-Kuka M, Kuka J, Liepinsh E, Dambrova M, Lazou A. Pharmacological activation of PPARβ/δ preserves mitochondrial respiratory function in ischemia/reperfusion via stimulation of fatty acid oxidation-linked respiration and PGC-1α/NRF-1 signaling. Front Endocrinol (Lausanne) (2022) 13:941822. doi: 10.3389/fendo.2022.941822
27. Sannigrahi MK, Rajagopalan P, Lai L, Liu X, Sahu V, Nakagawa H, et al. HPV E6 regulates therapy responses in oropharyngeal cancer by repressing the PGC-1α/ERRα axis. JCI Insight (2022) 7(18):e159600. doi: 10.1172/jci.insight.159600
28. Brown EL, Foletta VC, Wright CR, Sepulveda PV, Konstantopoulos N, Sanigorski A, et al. PGC-1α and PGC-1β Increase protein synthesis via ERRα in C2C12 myotubes. Front Physiol (2018) 9:1336. doi: 10.3389/fphys.2018.01336
29. Sun X, Ping Y, Li X, Mao Y, Chen Y, Shi L, et al. Activation of PGC-1α-dependent mitochondrial biogenesis supports therapeutic effects of silibinin against type I diabetic periodontitis. J Clin Periodontol (2023) 50(7):964–79. doi: 10.1111/jcpe.13811
30. Liu L, Ning X, Wei L, Zhou Y, Zhao L, Ma F, et al. Twist1 downregulation of PGC-1α decreases fatty acid oxidation in tubular epithelial cells, leading to kidney fibrosis. Theranostics (2022) 12(8):3758–75. doi: 10.7150/thno.71722
31. Chen D, Duan Y, Yu S, Zhang X, Li N, Li J. Rutaecarpine Promotes Adipose Thermogenesis and Protects against HFD-Induced Obesity via AMPK/PGC-1α Pathway. Pharm (Basel) (2022) 15(4):469. doi: 10.3390/ph15040469
32. Ridderstråle M, Johansson LE, Rastam L, Lindblad U. Increased risk of obesity associated with the variant allele of the PPARGC1A Gly482Ser polymorphism in physically inactive elderly men. Diabetologia (2006) 49(3):496–500. doi: 10.1007/s00125-005-0129-8
33. Weng SW, Lin TK, Wang PW, Chen IY, Lee HC, Chen SD, et al. Gly482Ser polymorphism in the peroxisome proliferator-activated receptor gamma coactivator-1alpha gene is associated with oxidative stress and abdominal obesity. Metabolism (2010) 59(4):581–6. doi: 10.1016/j.metabol
34. Myles S, Lea RA, Ohashi J, Chambers GK, Weiss JG, Hardouin E, et al. Testing the thrifty gene hypothesis: the Gly482Ser variant in PPARGC1A is associated with BMI in Tongans. BMC Med Genet (2011) 12:10. doi: 10.1186/1471-2350-12-10
35. Sun L, Yang Z, Jin F, Zhu XQ, Qu YC, Shi XH, et al. The Gly482Ser variant of the PPARGC1 gene is associated with Type 2 diabetes mellitus in northern Chinese, especially men. Diabetes Med (2006) 23(10):1085–92. doi: 10.1111/j.1464-5491.2006.01949.x
36. Du F, Yang KJ, Piao LS. Correlation between PPARGC1A gene rs8192678 G>A polymorphism and susceptibility to type-2 diabetes. Open Life Sci (2019) 14:43–52. doi: 10.1515/biol-2019-0006
37. Xia W, Chen N, Peng W, Jia X, Yu Y, Wu X, et al. Systematic meta-analysis revealed an association of PGC-1α rs8192678 polymorphism in type 2 diabetes mellitus. Dis Markers. (2019) 2019:2970401. doi: 10.1155/2019/2970401
38. Batarfi AA, Filimban N, Bajouh OS, Dallol A, Chaudhary AG, Bakhashab S. MC4R variants rs12970134 and rs17782313 are associated with obese polycystic ovary syndrome patients in the Western region of Saudi Arabia. BMC Med Genet (2019) 20(1):144. doi: 10.1186/s12881-019-0876-x
39. Adamska-Patruno E, Bauer W, Bielska D, Fiedorczuk J, Moroz M, Krasowska U, et al. An association between diet and MC4R genetic polymorphism, in relation to obesity and metabolic parameters-A cross sectional population-based study. Int J Mol Sci (2021) 22(21):12044. doi: 10.3390/ijms222112044
40. Zobel DP, Andreasen CH, Grarup N, Eiberg H, Sørensen TI, Sandbaek A, et al. Variants near MC4R are associated with obesity and influence obesity-related quantitative traits in a population of middle-aged people: studies of 14,940 Danes. Diabetes (2009) 58(3):757–64. doi: 10.2337/db08-0620
41. Orkunoglu-Suer FE, Harmon BT, Gordish-Dressman H, Clarkson PM, Thompson PD, Angelopoulos TJ, et al. MC4R variant is associated with BMI but not response to resistance training in young females. Obes (Silver Spring) (2011) 19(3):662–6. doi: 10.1038/oby.2010.180
42. Illangasekera YA, Kumarasiri RP, Fernando DJ, Dalton CF. Association of FTO and near MC4R variants with obesity measures in urban and rural dwelling Sri Lankans. Obes Res Clin Pract (2016) 10 Suppl 1:S117–24. doi: 10.1016/j.orcp.2016.02.003
43. Zhang Y, Ren H, Wang Q, Deng W, Yue W, Yan H, et al. Chinese Antipsychotics Pharmacogenomics Consortium. Testing the role of genetic variation of the MC4R gene in Chinese population in antipsychotic-induced metabolic disturbance. Sci China Life Sci (2019) 62(4):535–43. doi: 10.1007/s11427-018-9489-x
44. Rahati S, Qorbani M, Naghavi A, Pishva H. Association and interaction of the MC4R rs17782313 polymorphism with plasma ghrelin, GLP-1, cortisol, food intake and eating behaviors in overweight/obese Iranian adults. BMC Endocr Disord (2022) 22(1):234. doi: 10.1186/s12902-022-01129-w
45. Garavito P, Mosquera-Heredia MI, Fang L, Payares F, Ruiz M, Arias I, et al. Polymorphisms of leptin-melanocortin system genes associated with obesity in an adult population from Barranquilla. Biomedica (2020) 40(2):257–69. doi: 10.7705/biomedica.4827
46. Rebelos E, Honka MJ, Ekblad L, Bucci M, Hannukainen JC, Fernandes Silva L, et al. The Obesity Risk SNP (rs17782313) near the MC4R Gene Is Not Associated with Brain Glucose Uptake during Insulin Clamp-A Study in Finns. J Clin Med (2021) 10(6):1312. doi: 10.3390/jcm10061312
47. Alizadeh S, Pooyan S, Mirzababaei A, Arghavani H, Hasani H, Mirzaei K. Interaction of MC4R rs17782313 variants and dietary carbohydrate quantity and quality on basal metabolic rate and general and central obesity in overweight/obese women: a cross-sectional study. BMC Endocr Disord (2022) 22(1):121. doi: 10.1186/s12902-022-01023-5
48. Hammad MM, Abu-Farha M, Hebbar P, Cherian P, Al Khairi I, Melhem M, et al. MC4R variant rs17782313 associates with increased levels of DNAJC27, ghrelin, and visfatin and correlates with obesity and hypertension in a Kuwaiti cohort. Front Endocrinol (Lausanne) (2020) 11:437. doi: 10.3389/fendo.2020.00437
49. Mohammadi M, Khodarahmi M, Kahroba H, Farhangi MA. Dietary patterns interact with the variations of 18q21. 23 rs17782313 locus on regulation of hypothalamic-pituitary axis hormones and cardio-metabolic risk factors in obesity. Eat Weight Disord (2020) 25(5):1447–59. doi: 10.1007/s40519-020-00855-1
50. Khodarahmi M, Kahroba H, Jafarabadi MA, Mesgari-Abbasi M, Farhangi MA. Dietary quality indices modifies the effects of melanocortin-4 receptor (MC4R) rs17782313 polymorphism on cardio-metabolic risk factors and hypothalamic hormones in obese adults. BMC Cardiovasc Disord (2020) 20(1):57. doi: 10.1186/s12872-020-01366-8
51. Hosseininasab D, Mirzababaei A, Abaj F, Firoozi R, Clark CCT, Mirzaei K. Are there any interactions between modified Nordic-style diet score and MC4R polymorphism on cardiovascular risk factors among overweight and obese women? A cross-sectional study. BMC Endocr Disord (2022) 22(1):221. doi: 10.1186/s12902-022-01132-1
52. Inandiklioğlu N, Yaşar A. Association between rs1421085 and rs9939609 Polymorphisms of Fat Mass and Obesity-Associated Gene with High-Density Lipoprotein Cholesterol and Triglyceride in Obese Turkish Children and Adolescents. J Pediatr Genet (2021) 10(1):9–15. doi: 10.1055/s-0040-1713154
53. Szkup M, Brodowski J, Jurczak A, Stanisławska M, Grochans E. Seeking genetic determinants of selected metabolic disorders in women aged 45-60. Ann Agric Environ Med (2020) 27(3):407–12. doi: 10.26444/aaem/112579
54. Shan L, Wang C, Ji LN, Yang Z, Zhu Q. Association of PGC-1α gene Gly482Ser polymorphism with type 2 diabetes mellitus. Chin J Diabetes. (2006) 14(2):103–4. doi: 10.3321/j.issn:1006-6187.2006.02.009
55. Sun L, Zheng CG, Lv ZP, Hu CY, Huang ZZ, Liang QH, et al. Association of peroxisome proliferator-activated receptor gamma coactivator-1 Gly482Serwithapolipoprotein and the longevityand metabolic traits of Hans in Guangxi Yongfu. Chin J Geriatrics. (2013) 32(3):300–4. doi: 10.3760/cma.j.issn
56. Geloneze SR, Geloneze B, Morari J, Matos-Souza JR, Lima MM, Chaim EA, et al. PGC-1α gene Gly482Ser polymorphism predicts improved metabolic, inflammatory and vascular outcomes following bariatric surgery. Int J Obes (Lond) (2012) 36(3):363–8. doi: 10.1038/ijo.2011.176
57. Okauchi Y, Iwahashi H, Okita K, Yuan M, Matsuda M, Tanaka T, et al. PGC-1alpha Gly482Ser polymorphism is associated with the plasma adiponectin level in type 2 diabetic men. Endocr J (2008) 55(6):991–7. doi: 10.1507/endocrj.k08e-070
58. Hara K, Tobe K, Okada T, Kadowaki H, Akanuma Y, Ito C, et al. A genetic variation in the PGC-1 gene could confer insulin resistance and susceptibility to Type II diabetes. Diabetologia (2002) 45(5):740–3. doi: 10.1007/s00125-002-0803-z
59. Goyenechea E, Crujeiras AB, Abete I, Parra D, Martínez JA. Enhanced short-term improvement of insulin response to a low-caloric diet in obese carriers the Gly482Ser variant of the PGC-1alpha gene. Diabetes Res Clin Pract (2008) 82(2):190–6. doi: 10.1016/j.diabres.2008.08.011
60. Lin YC, Chang PF, Chang MH, Ni YH. A common variant in the peroxisome proliferator-activated receptor-γ coactivator-1α gene is associated with nonalcoholic fatty liver disease in obese children. Am J Clin Nutr (2013) 97(2):326–31. doi: 10.3945/ajcn.112.046417
61. Ha CD, Cho JK, Han T, Lee SH, Kang HS. Relationship of PGC-1α gene polymorphism with insulin resistance syndrome in Korean children. Asia Pac J Public Health (2015) 27(2):NP544–51. doi: 10.1177/1010539513477685
62. Song J, Jia WP, Fang QC, Zhang R, Hu C, Lu JX, et al. Relationg between peroxisome proliferator-activated receptor-γ coactivator-1α Gly482Ser variant and glucose and lipid metabolism in Chinese subjects and patients. Shangha iMed J (2007) 30(7):485–9. doi: CNKI:SUN:SHYX.0.2007-07-007
63. Jin J, Ding G, Bao H, Chen Y, Han Y, Zhao F, et al. Correlation between PPAR gene polymorphisms and primary nephrotic syndrome in children. PPAR Res (2013) 2013:927915. doi: 10.1155/2013/927915
64. Queiroz EM, Cândido AP, Castro IM, Bastos AQ, MaChado-Coelho GL, Freitas RN. IGF2, LEPR, POMC, PPARG, and PPARGC1 gene variants are associated with obesity-related risk phenotypes in Brazilian children and adolescents. Braz J Med Biol Res (2015) 48(7):595–602. doi: 10.1590/1414-431X20154155
65. Sun Y, Liu HJ, Cai Q, Xu XR. Correlation between Gly482Ser polymorphism of PGC-1α gene and metabolic syndrome in pilots. Acad J Chin PLA Med Sch (2013) 34(9):913–8. doi: 10.3969/j.issn.2095-5227.2013.09.004
66. Wang YB, Yu YC, Li Z, Wang C, Wang JY, Wu GT. Study on the relationship between polymorphisms of peroxisome proliferators-activated receptor-gamma coactivator-1alpha gene and type 2 diabetes in Shanghai Hans in China. Chin J Med Genet (2005) 22(4):453–6. doi: 10.3760/j.issn:1003-9406.2005.04.025
67. Zhang SL, Lu WS, Yan L, Wu MC, Xu MT, Chen LH, et al. Association between peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene polymorphisms and type 2 diabetes in southern Chinese population: role of altered interaction with myocyte enhancer factor 2C. Chin Med J (Engl) (2007) 120(21):1878–85. doi: 10.1097/00029330-200711010-00005
68. Tobina T, Mori Y, Doi Y, Nakayama F, Kiyonaga A, Tanaka H. Peroxisome proliferator-activated receptor gamma co-activator 1 gene Gly482Ser polymorphism is associated with the response of low-density lipoprotein cholesterol concentrations to exercise training in elderly Japanese. J Physiol Sci (2017) 67(5):595–602. doi: 10.1007/s12576-016-0491-y
69. Tai CM, Huang CK, Tu HP, Hwang JC, Yeh ML, Huang CF, et al. Interactions of a PPARGC1α Variant and a PNPLA3 variant affect nonalcoholic steatohepatitis in severely obese Taiwanese patients. Med (Baltimore) (2016) 95(12):e3120. doi: 10.1097/MD.0000000000003120
70. Reddy TV, Govatati S, Deenadayal M, Shivaji S, Bhanoori M. Polymorphisms in the TFAM and PGC1-α genes and their association with polycystic ovary syndrome among South Indian women. Gene (2018) 641:129–36. doi: 10.1016/j.gene.2017.10.010
71. Csép K, Szigeti E, Vitai M, Korányi L. The PPARGC1α - Gly482Ser polymorphism (rs8192678) and the metabolic syndrome in a central rOmanian population. Acta Endocrinol (Buchar) (2017) 13(2):161–7. doi: 10.4183/aeb.2017.161
72. Ramos-Lopez O, Riezu-Boj JI, Milagro FI, Goni L, Cuervo M, Martinez JA. Association of the Gly482Ser PPARGC1A gene variant with different cholesterol outcomes in response to two energy-restricted diets in subjects with excessive weight. Nutrition (2018) 47:83–9. doi: 10.1016/j.nut.2017.10.008
73. Zehsaz F, Abbasi Soltani H, Hazrati R, Farhangi N, Monfaredan A, Ghahramani M. Association between the PPARa and PPARGCA gene variations and physical performance in non-trained male adolescents. Mol Biol Rep (2018) 45(6):2545–53. doi: 10.1007/s11033-018-4422-2
74. Zhang Q, Liu SS, Sun BB, Zhang M, Xin YN. Association of peroxisome proliferator-activated receptor-γ coactivator-1alpha rs8192678 single nucleotide polymorphisms with the risk of nonalcoholic fatty liver disease. J Clin Hepatol (2020) 36(9):2035–9. doi: 10.3969/j.issn.1001-5256.2020.09.025
75. Lotta LA, Mokrosiński J, Mendes de Oliveira E, Li C, Sharp SJ, Luan J, et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell (2019) 177(3):597–607.e9. doi: 10.1016/j.cell.2019.03.044
76. Wang N, Tong R, Xu J, Tian Y, Pan J, Cui J, et al. PDX1 and MC4R genetic polymorphisms are associated with type 2 diabetes mellitus risk in the Chinese Han population. BMC Med Genomics (2021) 14(1):249. doi: 10.1186/s12920-021-01037-3
77. Hao H, Lin R, Li Z, Shi W, Huang T, Niu J, et al. MC4R deficiency in pigs results in hyperphagia and ultimately hepatic steatosis without high-fat diet. Biochem Biophys Res Commun (2019) 520(3):651–6. doi: 10.1016/j.bbrc.2019.08.016
78. Xu L, Huang Z, Lo TH, Lee JTH, Yang R, Yan X, et al. Hepatic PRMT1 ameliorates diet-induced hepatic steatosis via induction of PGC-1α. Theranostics (2022) 12(6):2502–18. doi: 10.7150/thno.63824
79. Besseiche A, Riveline JP, Gautier JF, Bréant B, Blondeau B. Metabolic roles of PGC-1α and its implications for type 2 diabetes. Diabetes Metab (2015) 41(5):347–57. doi: 10.1016/j.diabet.2015.02.002
80. Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, et al. Microglial PGC-1α protects against ischemic brain injury by suppressing neuroinflammation. Genome Med (2021) 13(1):47. doi: 10.1186/s13073-021-00863-5
81. Ortega-Azorín C, Sorlí JV, Asensio EM, Coltell O, Martínez-González MÁ, Salas-Salvadó J, et al. Associations of the FTO rs9939609 and the MC4R rs17782313 polymorphisms with type 2 diabetes are modulated by diet, being higher when adherence to the Mediterranean diet pattern is low. Cardiovasc Diabetol (2012) 11:137. doi: 10.1186/1475-2840-11-137
82. Song Z, Qiu L, Hu Z, Liu J, Liu D, Hou D. Evaluation of the obesity genes FTO and MC4R for contribution to the risk of large artery atherosclerotic stroke in a chinese population. Obes Facts. (2016) 9(5):353–62. doi: 10.1159/000448588
83. Zhang Y, Xu W, Li X, Tang Y, Xie P, Ji Y, et al. Association between PPARGC1A gene polymorphisms and coronary artery disease in a Chinese population. Clin Exp Pharmacol Physiol (2008) 35(10):1172–7. doi: 10.1111/j.1440-1681.2008.04988.x
84. Yang Y, Mo X, Chen S, Lu X, Gu D. Association of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PPARGC1A) gene polymorphisms and type 2 diabetes mellitus: a meta-analysis. Diabetes Metab Res Rev (2011) 27(2):177–84. doi: 10.1002/dmrr.1158
85. Sharma R, Matharoo K, Kapoor R, Bhanwer AJS. Association of PGC-1α gene with type 2 diabetes in three unrelated endogamous groups of North-West India (Punjab): a case-control and meta-analysis study. Mol Genet Genomics (2018) 293(2):317–29. doi: 10.1007/s00438-017-1385-2
86. Yu K, Li L, Zhang L, Guo L, Wang C. Association between MC4R rs17782313 genotype and obesity: A meta-analysis. Gene (2020) 733:144372. doi: 10.1016/j.gene.2020.144372
87. Resende CMM, Silva HAMD, Campello CP, Ferraz LAA, de Lima ELS, Beserra MA, et al. Polymorphisms on rs9939609 FTO and rs17782313 MC4R genes in children and adolescent obesity: A systematic review. Nutrition (2021) 111474:91–2. doi: 10.1016/j.nut.2021.111474
88. Xi B, Chandak GR, Shen Y, Wang Q, Zhou D. Association between common polymorphism near the MC4R gene and obesity risk: a systematic review and meta-analysis. PloS One (2012) 7(9):e45731. doi: 10.1371/journal.pone.0045731
89. Xi B, Takeuchi F, Chandak GR, Kato N, Pan HW, AGEN-T2D Consortium;, et al. Common polymorphism near the MC4R gene is associated with type 2 diabetes: data from a meta-analysis of 123,373 individuals. Diabetologia (2012) 55(10):2660–6. doi: 10.1007/s00125-012-2655-5
90. Blauw LL, Noordam R, van der Laan SW, Trompet S, Kooijman S, van Heemst D, et al. Common genetic variation in MC4R does not affect atherosclerotic plaque phenotypes and cardiovascular disease outcomes. J Clin Med (2021) 10(5):932. doi: 10.3390/jcm10050932
91. Tang Y, Jin B, Zhou L, Lu W. MeQTL analysis of childhood obesity links epigenetics with a risk SNP rs17782313 near MC4R from meta-analysis. Oncotarget (2017) 8(2):2800–6. doi: 10.18632/oncotarget.13742
92. Lauria F, Siani A, Picó C, Ahrens W, Bammann K, De Henauw S, et al. A common variant and the transcript levels of MC4R gene are associated with adiposity in children: the IDEFICS study. J Clin Endocrinol Metab (2016) 101(11):4229–36. doi: 10.1210/jc.2016-1992
93. Caruso C, Carniglia L, Durand D, Gonzalez PV, Scimonelli TN, Lasaga M. Melanocortin 4 receptor activation induces brain-derived neurotrophic factor expression in rat astrocytes through cyclic AMP-protein kinase A pathway. Mol Cell Endocrinol (2012) 348(1):47–54. doi: 10.1016/j.mce.2011.07.036
94. Ramirez D, Saba J, Carniglia L, Durand D, Lasaga M, Caruso C. Melanocortin 4 receptor activates ERK-cFos pathway to increase brain-derived neurotrophic factor expression in rat astrocytes and hypothalamus. Mol Cell Endocrinol (2015) 411:28–37. doi: 10.1016/j.mce.2015.04.008
95. Zhang HH, Liu J, Qin GJ, Li XL, Du PJ, Hao X, et al. Melanocortin 4 receptor activation attenuates mitochondrial dysfunction in skeletal muscle of diabetic rats. J Cell Biochem (2017) 118(11):4072–9. doi: 10.1002/jcb.26062
96. Taghvaei S, Saremi L, Babaniamansour S. Computational analysis of gly482Ser single-nucleotide polymorphism in PPARGC1A gene associated with CAD, NAFLD, T2DM, obesity, hypertension, and metabolic diseases. PPAR Res (2021) 2021:5544233. doi: 10.1155/2021/5544233
97. Ling C, Poulsen P, Carlsson E, Ridderstråle M, Almgren P, Wojtaszewski J, et al. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest (2004) 114(10):1518–26. doi: 10.1172/JCI21889
98. Chen Y, Mu P, He S, Tang X, Guo X, Li H, et al. Gly482Ser mutation impairs the effects of peroxisome proliferator-activated receptor γ coactivator-1α on decreasing fat deposition and stimulating phosphoenolpyruvate carboxykinase expression in hepatocytes. Nutr Res (2013) 33(4):332–9. doi: 10.1016/j.nutres.2013.02.003
Keywords: MC4R, PGC1α, rs17782313, rs8192678, obesity, hyperglycemia
Citation: Zhang Y, Li S, Nie H, Wang X, Li X, Wen J, Li M and Song Y (2023) The rs17782313 polymorphism near MC4R gene confers a high risk of obesity and hyperglycemia, while PGC1α rs8192678 polymorphism is weakly correlated with glucometabolic disorder: a systematic review and meta-analysis. Front. Endocrinol. 14:1210455. doi: 10.3389/fendo.2023.1210455
Received: 22 April 2023; Accepted: 25 July 2023;
Published: 09 August 2023.
Edited by:
Patrick Osei-Owusu, Case Western Reserve University, United StatesReviewed by:
Ian James Martins, University of Western Australia, AustraliaRosaura Leis, University of Santiago de Compostela, Spain
Copyright © 2023 Zhang, Li, Nie, Wang, Li, Wen, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongyan Song, c29uZ3lvbmd5YW5AY2R1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Youjin Zhang1†
Youjin Zhang1† Yongyan Song
Yongyan Song