- 1Department of Urology, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Background: The purpose of this study was to investigate the correlation between serum 25(OH)D concentrations and all-cause mortality in patients with kidney stone disease (KSD) as the effects of a deficiency in 25-hydroxyvitamin D on KSD patients are currently unclear.
Methods: For our prospective cohort study, we included 2,916 participants from the National Health and Nutrition Examination Survey (NHANES) 2007-2018. The National Death Index (NDI) was utilized to identify all causes of death and cause-specific mortality until December 31, 2018. We calculated hazard ratios (HR) and 95% confidence intervals (CIs) using multivariate Cox regression models.
Results: During the 18,859 person-years of follow-up, a total of 375 fatalities occurred, including 83 deaths from cardiovascular disease (CVD) and 79 deaths from cancer. At baseline, individuals with higher blood 25(OH)D concentrations had lower levels of glucose, glycohemoglobin, CRP, and insulin, as well as higher levels of HDL cholesterol (P < 0.01). In the fully adjusted model (Model 3), compared to the group with the lowest 25(OH)D concentrations, those with serum 25(OH)D concentrations ≥75 nmol/L had hazard ratios (HRs) and 95% confidence intervals (CIs) of 0.48 (0.26, 0.87) for all-cause mortality (P=0.02, P for trend = 0.02). The association between serum 25(OH)D concentrations and all-cause mortality in KSD patients was found to be significantly non-linear. A 7% decrease in the risk of death from all causes was observed for each unit-nmol/L increase in serum 25(OH)D concentrations when the concentrations were below 27.7 nmol/L (P < 0.05).
Conclusion: Based on the findings, KSD patients with insufficient serum 25(OH)D concentrations were at a higher risk of all-cause mortality. Therefore, it is crucial to maintain sufficient blood 25(OH)D concentrations and prevent 25(OH)D insufficiency in order to extend the lifespan of KSD patients.
Introduction
Kidney stone disease (KSD) is a highly prevalent and increasingly common disease worldwide, with an estimated prevalence of 1.7-14.8% (1). Recent NHANES studies have demonstrated a rise in the prevalence of KSD, particularly among women, with their prevalence increasing from 6.5% to 9.4% (2). Furthermore, KSD can affect individuals of various age groups (2, 3), and the presence of kidney stones can lead to various complications, such as renal colic, obstruction, renal function impairment, and, in severe cases, sepsis. As a result, KSD places a significant burden on the global public health system, with inpatient stays for KSD accounting for 0.5% of all hospital stays (4, 5). Currently, there are several effective treatment options available for kidney stone disease (KSD). However, one significant challenge in managing KSD is the high recurrence rate of stones after their removal (6). The cost associated with KSD exceeds $10 billion annually in the United States alone, and this value is expected to increase each year as the incidence of KSD rises (7).
In addition to the direct medical burden associated with KSD, patients with KSD are more likely to develop cardiovascular disease (CVD), chronic kidney disease (CKD), and renal insufficiency (8–10). Furthermore, patients with KSD also have an increased burden of comorbid conditions, including dyslipidemia, hypertension, diabetes, and metabolic syndrome (11–14). Both ailments are chronic disorders distinguished by their gradual progression over an extended duration. The production of reactive oxygen species (ROS) and the emergence of oxidative stress (OS) might signify a common route (15). Moreover, additional elements such as elevated sodium consumption, dietary intake of animal-derived proteins, and irregularities in purine metabolism (excessive dietary protein, overexpression of pivotal enzymes) could exert a significant impact (14). Although KSD is not typically considered a life-threatening disease, it can still lead to death, and the number of deaths is increasing with the rising incidence rate in England (5). Complications associated with stones, particularly urosepsis, are the primary factor contributing to mortality (5, 16). Given the association with CVD, the higher risk of death in KSD patients may be primarily due to CVD mortality (8, 16, 17). Therefore, in addition to adequate preoperative preparation for KSD to avoid death related to stone complications, it is crucial to identify controllable risk factors that contribute to or accelerate disease-related mortality.
The main active state of vitamin D in our body is 25-hydroxyvitamin D, which primarily regulates calcium and phosphorus metabolism (18). It is generally known that 25(OH)D facilitates calcium absorption and digestion, which, in turn, increases urinary calcium content and theoretically enables increased kidney stone formation. Despite previous research indicating that elevated concentrations of 25(OH)D and vitamin D supplementation did not increase the risk of KSD (19, 20), recent high-quality studies have demonstrated that both can actually increase the risk of developing KSD (21–23). On the other hand, a deficiency in serum 25(OH)D may increase the risk of developing various diseases, such as CVD, hypertension, obesity, diabetes, and chronic kidney disease (24–26). Higher concentrations of serum 25(OH)D have been proven to be associated with reduced all-cause and cardiovascular disease mortality in various populations, including those with diabetes, osteoporosis, the elderly, and cardiovascular disease (27–31). A review of relevant studies found that vitamin D supplementation only statistically decreased the risk of cancer-related deaths. However, it did not reduce overall mortality or improve cardiovascular disease outcomes (32).
Although serum 25(OH)D has shown potential benefits in reducing mortality risk, there is a lack of research on its effects in the KSD population. To address this gap, our study aimed to evaluate the impact of serum 25(OH)D concentration on mortality in a prospective cohort of KSD patients from the National Health and Nutrition Examination Survey (NHANES) database. Through this investigation, we aim to provide insight into the potential benefits of serum 25(OH)D in reducing mortality risk among KSD patients.
Methods
Study population
The NHANES project aims to assess the health and nutritional status of the American population through a combination of physical tests and interviews. In our study, we utilized data from this program to investigate the potential impact of serum 25(OH)D on all-cause mortality in adults with KSD.
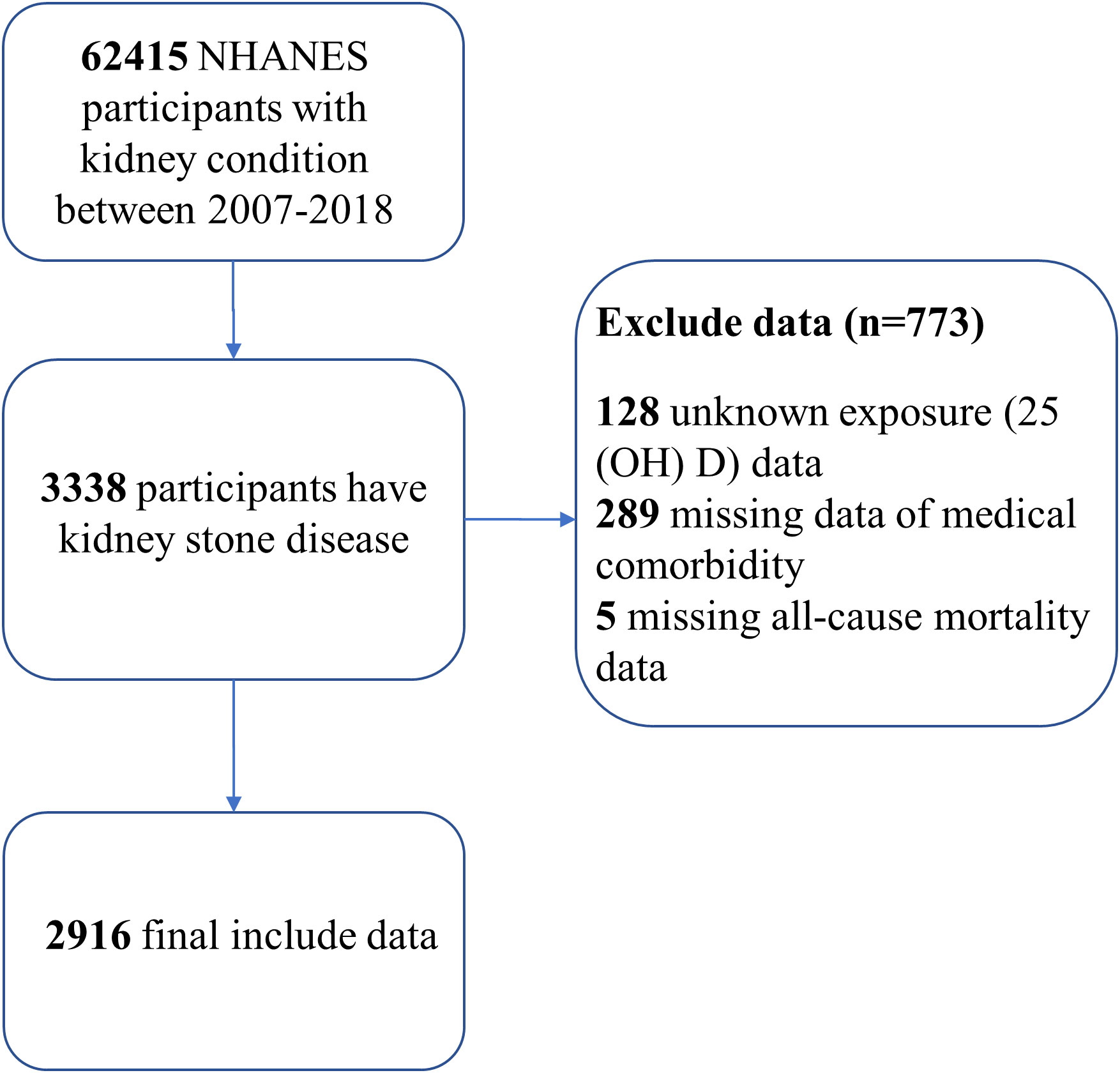
Our research utilized data from six NHANES cycles spanning from 2007 to 2018. We included individuals aged 20 years or older who had answered a questionnaire regarding their history of kidney stones (“Have you ever had kidney stones?”) (33). Using a standardized liquid chromatography–tandem mass spectrometry (LC-MS/MS) technique, we determined the concentrations of serum 25(OH)D. We excluded participants with unknown exposure to 25(OH)D data (n=128), missing medical comorbidity data (n=289), and those without death follow-up data (n=5). As a result, our analytical cohort consisted of 2916 participants with kidney stones (Figure 1).
Ascertainment of mortality
To determine the mortality status of our participants, we utilized specific study identifiers (SEQN) and probability matching to the National Death Index (NDI) as of December 31, 2018. The National Center for Health Statistics provided further details regarding the matching procedure.
In addition to determining mortality status, we also utilized the 10th revision to the international statistical classification of diseases (ICD-10) to identify disease-specific deaths. Our study’s primary findings focused on mortality from all causes and from specific diseases.
Assessment of covariates
Data were collected in cycles from 2007 to 2018, including information on sociodemographic traits, physical characteristics, health issues, lifestyle traits, and lab results. Sociodemographic traits included age (less than 60 years and 60 years or older), gender, race (non-Hispanic white, non-Hispanic black, Hispanic, and other), marital status (married or with partner, single), educational attainment (less than high school, high school, and greater than high school), and family poverty ratio (less than 1.3, 1.3 to less than 3.5, and 3.5 or greater). Leisure-time physical activity, defined as engaging in moderate-to-strenuous recreational physical activity during a typical week, was a key lifestyle attribute (2). BMI was selected as the body measurement parameter and was categorized according to clinical guidelines for overweight and obesity: less than 25.0 kg/m2, 25.0-29.9 kg/m2, and 30 kg/m2 or greater. Smoking status was classified as current smoker or non-smoker. Drinking status was determined by whether the individual consumed 12 or more alcoholic beverages per year. A self-reported medical history of cancer, diabetes, hypertension, cardiovascular disease, and stroke was also collected.
The 2007-2018 NHANES also included laboratory blood tests, which were conducted using strict procedures for blood collection and analysis. This study focused on several cardiometabolic markers, including plasma glucose, triglycerides (TG), uric acid, cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), C-reactive protein (CRP), glycohemoglobin (HbA1c), and insulin, which were measured at baseline.
Statistical analysis
To ensure nationally representative estimates, we followed the NHANES analytical reporting criteria and utilized survey analysis methodologies (34). Referring to the Endocrine Society Clinical Practice guidelines, the concentration of serum 25(OH)D was categorized into four groups (<25.0 nmol/L, 25.0–49.9 nmol/L, 50.0–74.9 nmol/L, and ≥75.0 nmol/L) (35). All statistical analyses were performed using STATA version 16.0 and Empower Stats software. To address missing data, we employed the chained equation technique and repeated imputation based on five replicates. Categorical and dichotomous data were presented as percentages, while continuous variables were reported as mean and standard deviation (SD). In order to assess the disparities in clinical characteristics across various groups, we employed chi-square tests for categorical variables and utilized a weighted linear regression model for continuous variables. Person-years were calculated from baseline until the date of death, loss to follow-up, or December 31, 2018, whichever occurred first. We used multivariate Cox regression models to calculate hazard ratios (HR) and 95% confidence intervals (CIs). We constructed three models: (1) non-adjusted; (2) adjusted for age, gender, race, BMI, educational attainment, leisure-time physical activity, marital, family poverty ratio, smoking, and alcohol; and (3) adjusted for age, gender, race, BMI, educational attainment, leisure-time physical activity, marital, family poverty ratio, smoking, alcohol, diabetes, hypertension, stroke, and cardiovascular disease. We conducted additional subgroup analyses to investigate the association between serum 25(OH)D and all-cause mortality and tested for P-values for interactions within each subgroup. Weighted generalized additive model regression and smoothed curve fitting were used to explore the relationship between serum 25(OH)D and all-cause mortality in greater detail. If a non-linear correlation was identified, a two-piecewise linear regression would be used to determine the effect threshold.
Results
Baseline characteristics of participants
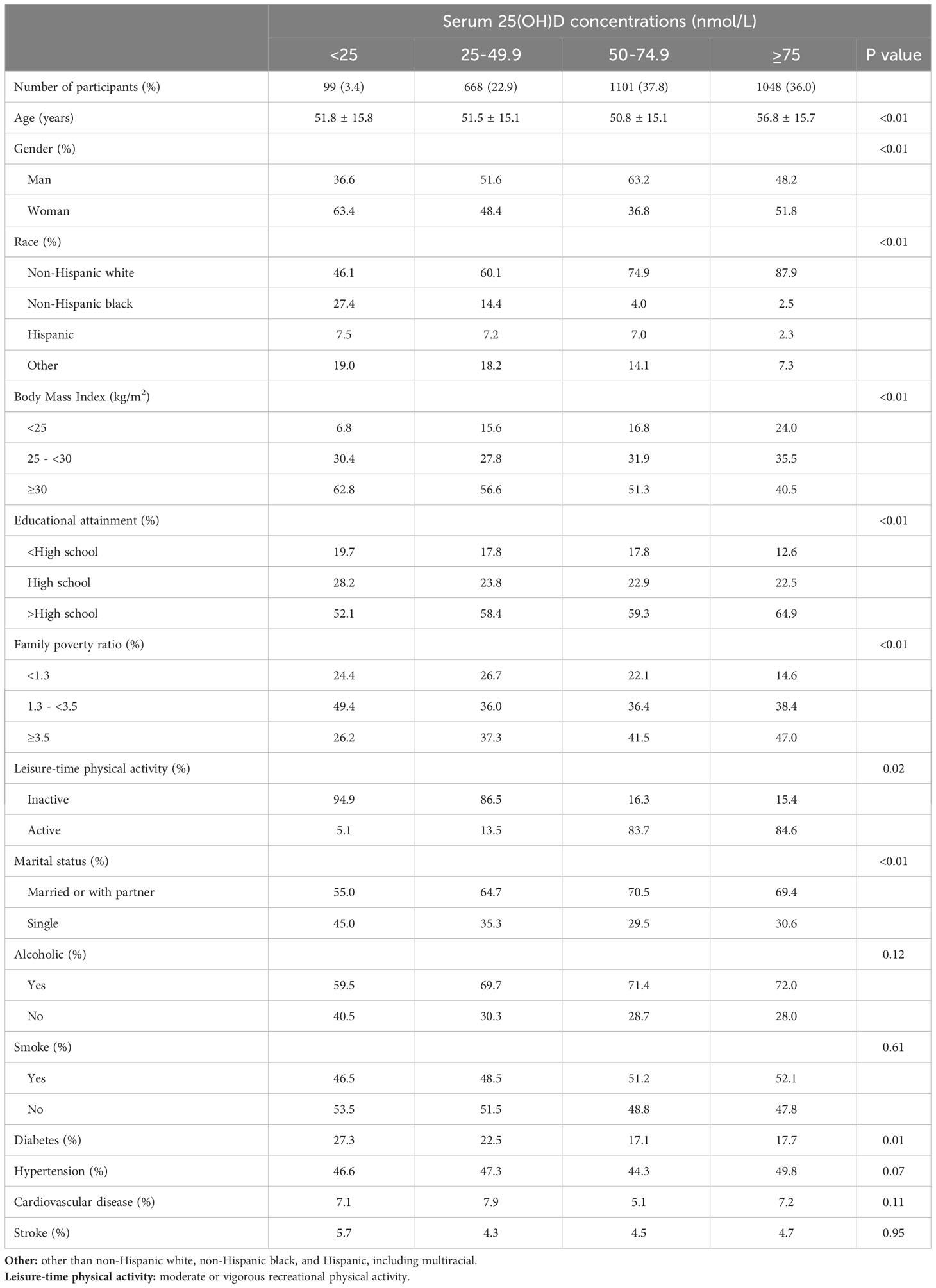
Among the 2916 adults with KSD, the median age was 58.0 years (interquartile range 26.0 years), and 44.4% were women. Among this group, 26.3% had a deficiency of 25(OH)D (<50 nmol/L), and 64.1% had a deficiency of 25(OH)D (<75 nmol/L). Table 1 displays the weighted sociodemographic characteristics and some disease conditions of the four groups. Participants with better serum 25(OH)D status had an older age, a higher percentage of non-Hispanic whites, higher diploma and household earnings, and were less likely to have diabetes or be overweight (P<0.01). When comparing cardiometabolic biomarkers, participants with better serum 25(OH)D status were likely to have worse glucose, glycohemoglobin, C-reactive protein (CRP), and insulin and higher HDL-cholesterol (P < 0.01, Table 2).
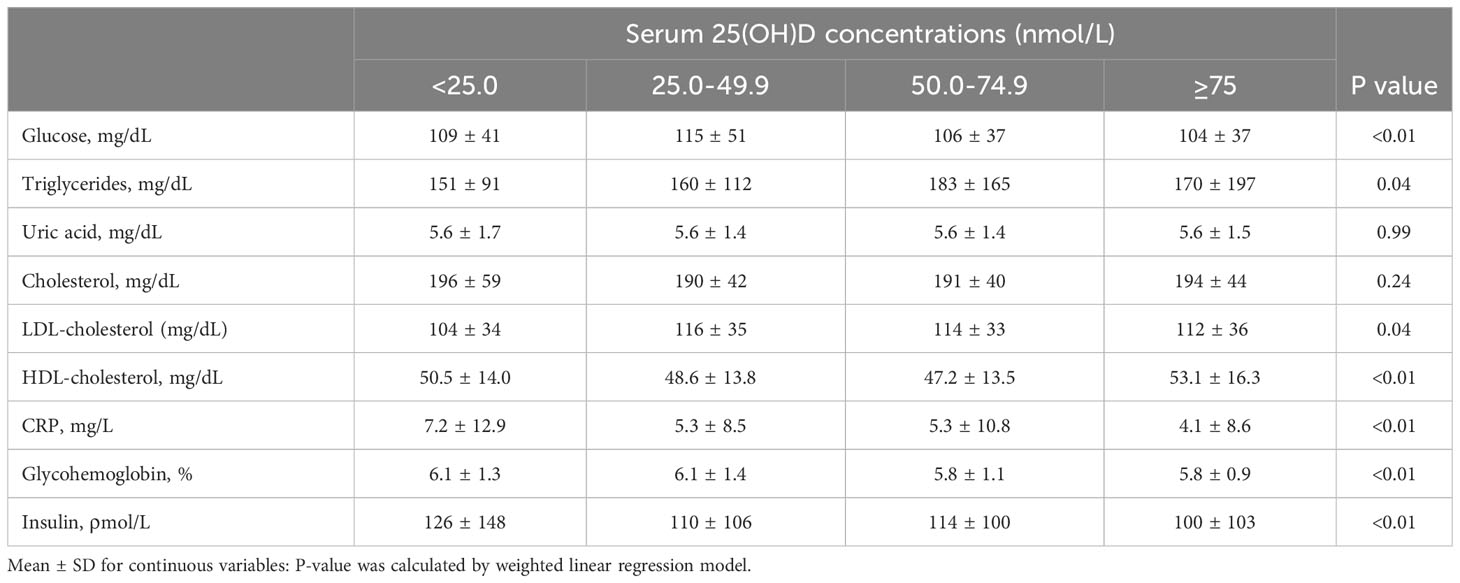
Table 2 Baseline levels of cardiometabolic markers according to serum 25(OH)D concentrations among participants with kidney stones.
Relationships of 25(OH)D concentration with mortality
During the average follow-up of 6.4 years (range, 0.17-13.2 years), encompassing a total of 18,859 person-years, there were 375 all-cause fatalities. We conducted three Cox regression models, as presented in Table 3, to investigate the independent impact of serum 25(OH)D status on mortality. No relationship between serum vitamin D concentration and mortality was observed before adjustment. In adjusted model, we discovered a negative association between serum 25(OH)D concentrations and all-cause mortality. The hazard ratios (HRs) and 95% confidence intervals (CIs) of Model 3 for serum 25(OH)D severe deficiency to sufficient (25.00, 25.00-49.99, 50.00-74.99, and 75.00 nmol/L) were, respectively, 1.00 (reference), 0.62 (0.34, 1.13), 0.63 (0.35, 1.13), and 0.48 (0.26,0.87) and P for trend was 0.02. For CVD or cancer mortality (P trend = 0.68), the HR and 95% CI were not statistically significant, and P for trend was 0.68 and 0.08 respectively. Adjustments for LDL, HDL, TG, and CRP did not significantly alter the results (Table 4).
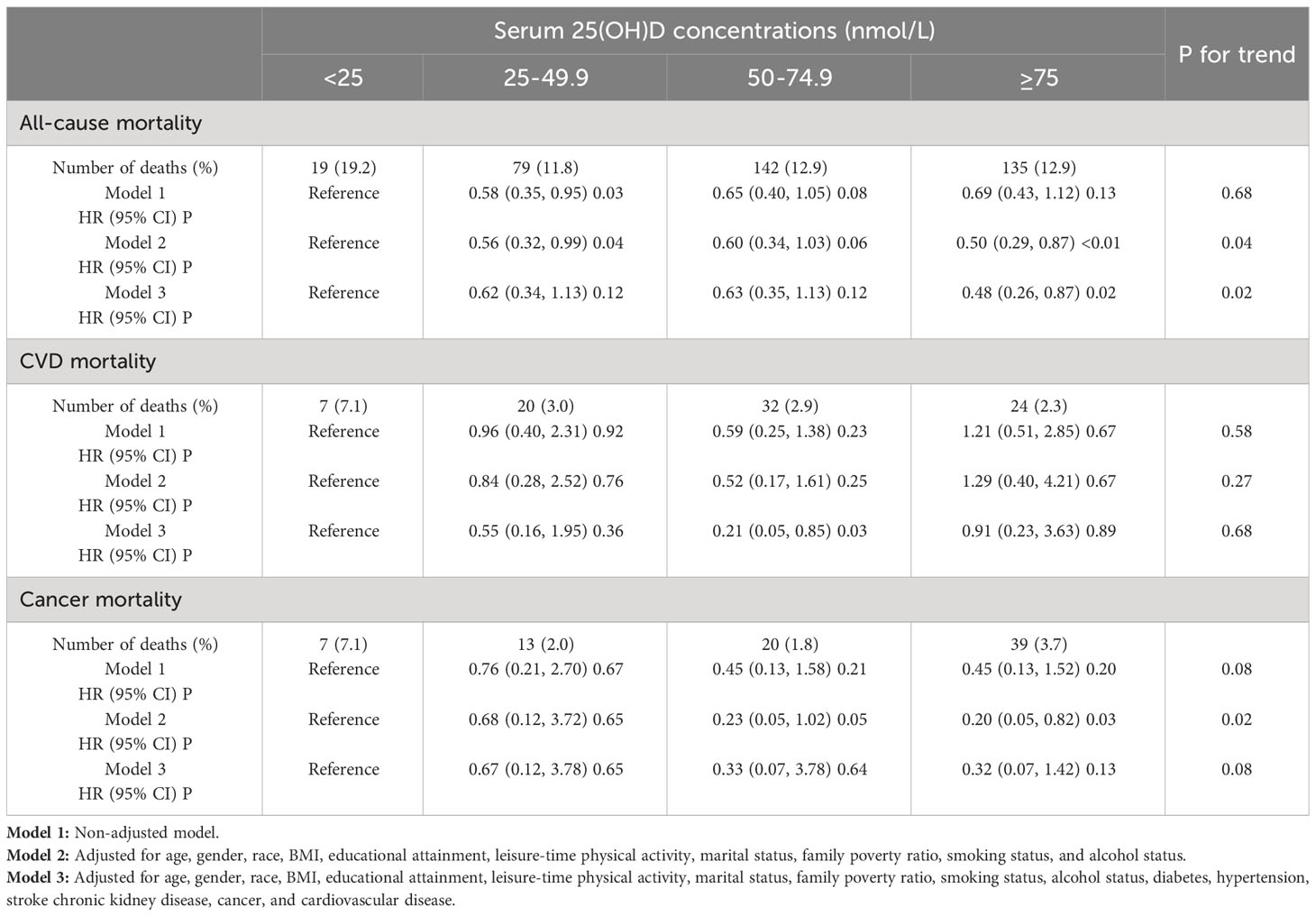
Table 3 HR (95% CIs) for all-cause and cause-specific mortality according to serum 25(OH)D concentrations among participants with kidney stones.

Table 4 Hazard ratios (95% CIs) of all-cause mortality according to serum 25(OH)D concentrations among KSD with further adjustment of blood lipids and CRP.
The detection of non-linear relationships
The adjusted smooth curve model (Model 3) revealed a non-linear association (Figure 2). To further investigate the nonlinear relationship between serum 25(OH)D concentrations and all-cause and CVD mortality in patients with KSD, the threshold effect analysis revealed the inflection points for all-cause mortality were 27.7 nmol/L. For serum 25(OH)D concentrations below 27.70 nmol/L, each unit increase was associated with a 7% reduction in the risk of all-cause mortality (HR 0.93; 95% CI: 0.88, 0.99) (Table 5). When serum 25(OH)D concentrations exceeded 27.70 nmol/L, there was no association with all-cause mortality (HR 0.99; 95% CI: 0.99, 1.00).
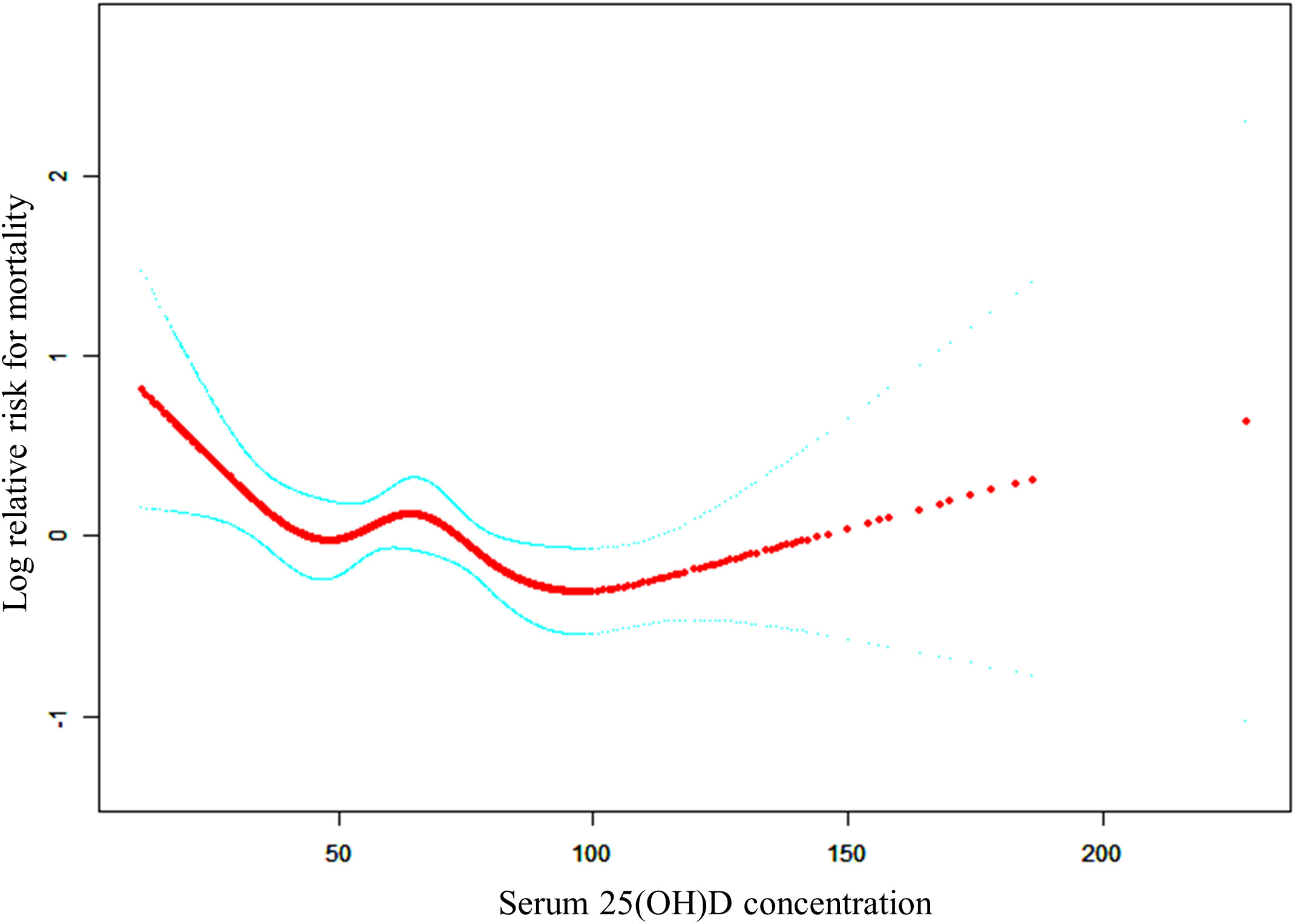
Figure 2 The non-linear relationship between serum 25(OH)D concentrations and the risk of all-cause mortality.
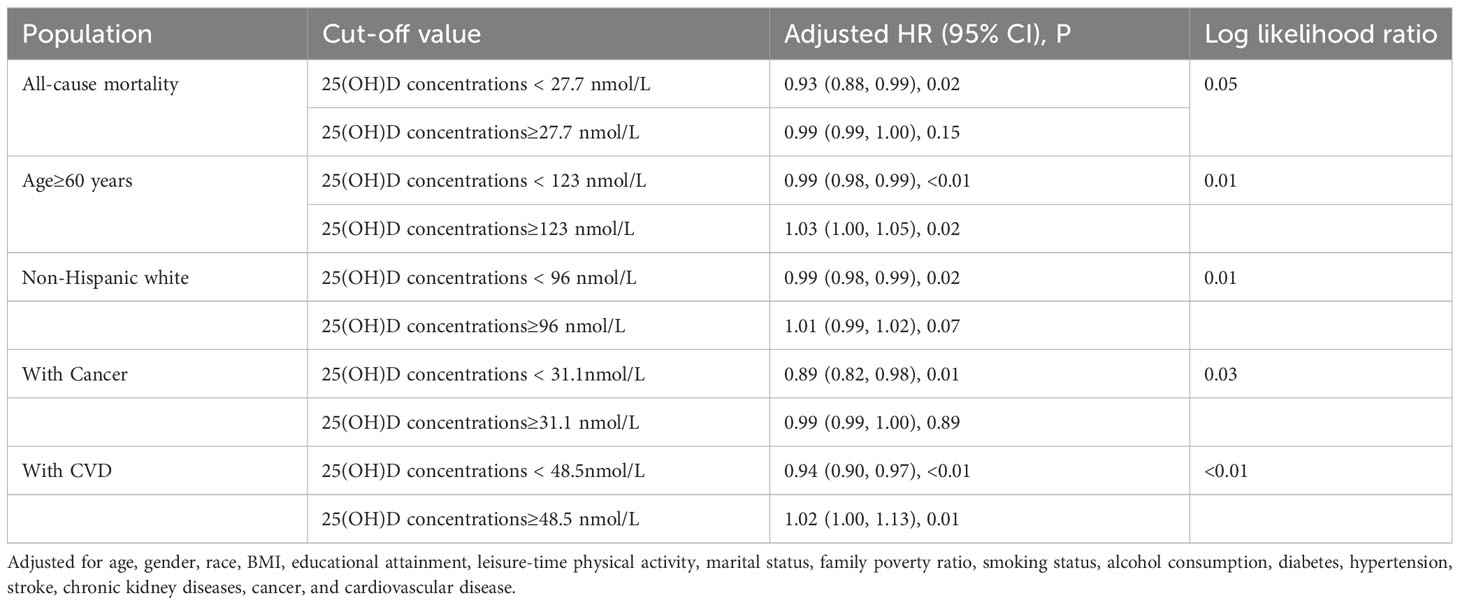
Table 5 Threshold effect analysis of serum 25(OH)D concentrations on all-cause and cancer mortality in kidney stone patients.
Stratified analyses
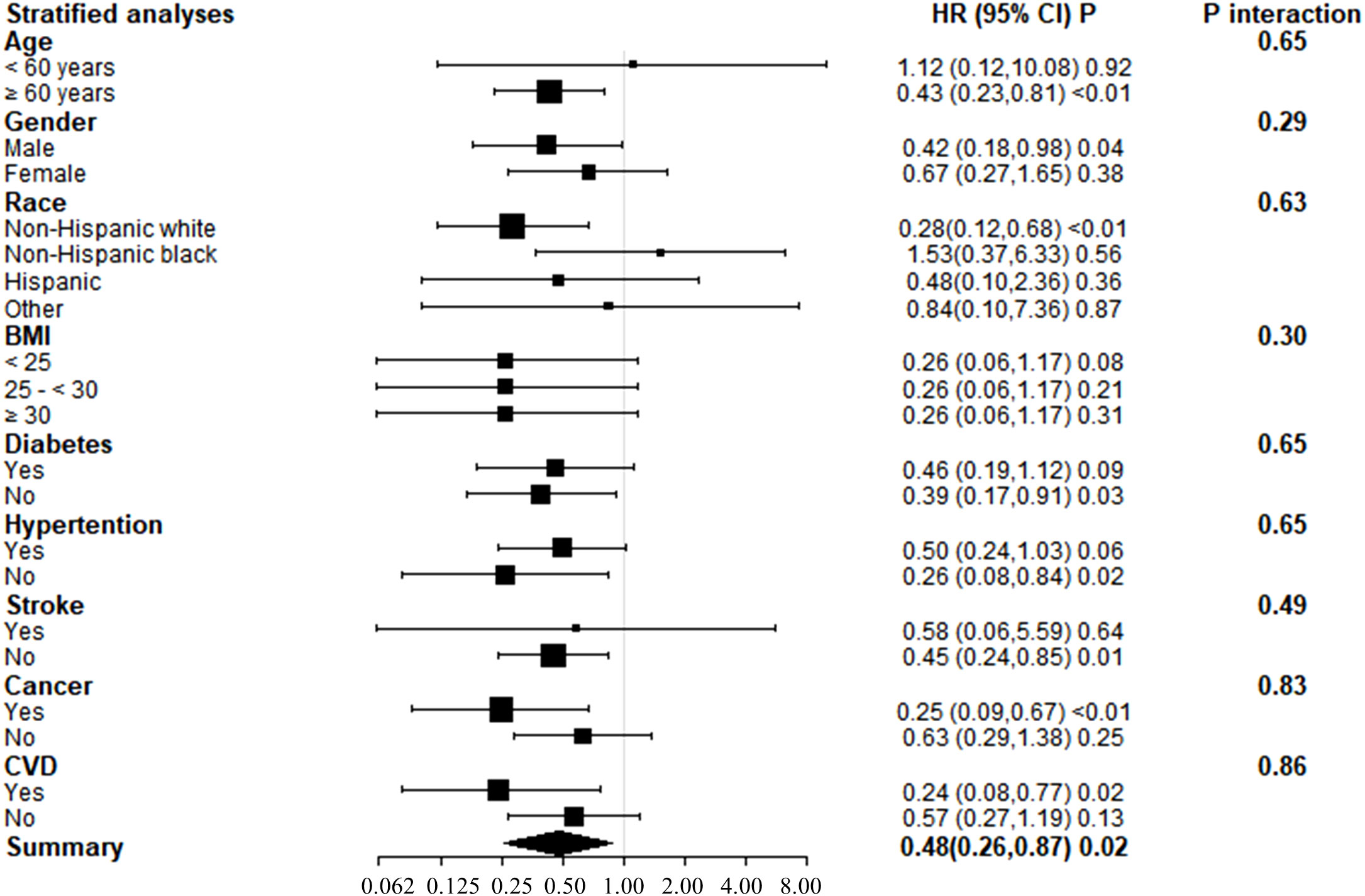
Subgroup analyses of adjusted model 3 were conducted to determine the benefit of increased serum 25(OH)D concentrations on KSD patient survival, stratified by age, gender, race, BMI, diabetes, hypertension, stroke, cancer, and CVD (Figure 3). Serum 25(OH)D concentrations and stratification factors did not show significant interaction. Although the interaction was not clear, our findings revealed stronger inverse associations between 25(OH)D concentrations and all-cause mortality in older adults (>60 years), non-Hispanic whites, and patients with cancer or cardiovascular disease. The threshold effect analysis found the inflection points were 123 nmol/L, 31.0nmol/L, 31.1nmol/L, and 48.5nmol/L in participants ≥ 60 years old, who were Non-Hispanic white, with cancer, and with CVD, respectively (Table 5). For individuals over the age of 60 and Non-Hispanic white individuals, a decrease of 1 unit in serum 25(OH)D concentrations, when below 123.0 nmol/L or 96.0 nmol/L, respectively, was associated with a 1% decrease in the risk of all-cause mortality. The hazard ratios were 0.98 (95% CI 0.98, 0.99) and 0.99 (95% CI 0.98, 0.99) for the two groups, respectively.
Sensitivity analysis
The results remained robust in sensitivity analyses that excluded cases with less than 1 or 2 years of follow-up (Supplementary Table S1). The fully adjusted hazard ratio (HR) of all-cause mortality rate for serum 25(OH)D concentrations ≥75 nmol/L relative to <25nmol/L, excluding deaths at 1 year, was 0.56 (95% CI: 0.33, 0.97), and excluding deaths at 2 years, it was 0.52 (95% CI: 0.30, 0.92), compared to 0.48 (95% CI: 0.26, 0.87) for all deaths. In a calculation that took into account the number of deaths at > 3 y, the estimated HR for the all-cause mortality rate was 0.57 (95% CI: 0.31, 1.04, P=0.07).
Discussion
In this study, we were able to determine the impact of 25(OH)D concentrations on all-cause mortality in the KSD population using follow-up data from the NHANES death cohort. We found that higher concentrations of serum 25(OH)D were strongly associated with lower all-cause mortality within a certain range, independent of traditional risk factors such as lifestyle, BMI, cardiometabolic indicators, diabetes, hypertension, stroke, CVD, CKD, and cancer. This conclusion was further supported by other stratified analyses. In addition, KSD patients with higher 25(OH)D concentrations had lower levels of inflammatory biomarkers and better lipid profiles. Even after accounting for these factors, 25(OH)D still had an impact on all-cause mortality. Our findings suggest potential therapeutic interventions and may serve as dietary guidelines and clinical intervention references for reducing all-cause mortality in KSD patients.
Low serum 25(OH)D concentrations, especially 25(OH)D deficiency, have been associated with numerous diseases and all-cause mortality (18, 24–28, 36). Recently, a large study provided real-world evidence to support the effectiveness of vitamin D supplementation in reducing both overall mortality and mortality related to specific causes (37). As a result, there has been a significant increase in the prescription of vitamin D, even for healthy individuals. However, a recent meta-analysis of randomized clinical trials and a Cochrane review have shown that vitamin D and calcium supplementation did not have the benefit of reducing all-cause mortality but reduced the risk of cancer death (32, 38, 39). Moreover, the RCT found a significant reduction in cancer mortality after omission of the first one and two years of data (40). It indicated that the long-term effects of vitamin D supplementation play a more significant role in reducing the risk of death from cancer. Additionally, epidemiological studies have found that taking vitamin D supplements increases the risk of KSD in predisposed individuals (22, 23, 38). Our study indicated that raising vitamin D concentrations alone may not be sufficient to prevent CVD or cancer death in KSD patients, although it was associated with all-cause mortality. Other factors may play a more significant role in the development of CVD or cancer death in this population. The conflicting results may be due to differences in study populations, interventions, outcome definitions, and analytical methods. Therefore, it is crucial to consider these differences when drawing conclusions about the appropriate concentrations of vitamin D for overall health and longevity to avoid inappropriate comparisons. Our study suggests that there is an optimal range of vitamin D concentrations for health, and both low and high concentrations may increase the risk of mortality. Once the serum vitamin D concentration reaches a specific threshold (27.7nmol/L in our study) through supplements, the mortality rate will not be further decreased. Previous studies have been conducted on individuals who are either at high risk for cardiovascular disease or the general population, but there have been no investigations on the role of vitamin D in mortality among individuals with KSD who are more prone to developing chronic kidney disease (25, 41).
There is disagreement regarding the role of 25(OH)D in KSD. Previous studies from the Third NHANES have shown that high concentrations of 25(OH)D were not associated with KSD, and kidney stone prevalence did not increase the risk of all-cause and CVD death (19, 42). However, a meta-analysis reported that there was a significant increase in circulating 25(OH)D concentrations only in hypercalciuria stone formers (65.7 nmol/L vs 54.6 nmol/L for hypercalciuria stone formers and controls, respectively), while there was no significant elevation in 25(OH)D concentrations of total stone formers (72.8 nmol/L vs 79.3 nmol/L for total stone formers and controls, respectively) (23). The evidence supporting the role of serum 25(OH)D and vitamin D supplementation in promoting kidney stone formation primarily comes from a Mendelian study, which has the specific ability to establish causal relationships. This study indicates that the impact of vitamin D supplements was limited to the duration of the experimental period, while higher concentrations of 25(OH)D circulating throughout one’s lifetime have a more significant influence on the occurrence of kidney stones (21). Long-term supplementation with vitamin D alone may increase the risk of KSD by increasing 25(OH)D concentrations. The conflicting results among studies regarding the relationship between vitamin D and kidney stone formation may be attributed to the interference of major risk factors associated with kidney stone formation. For example, other nutritional factors, including dietary protein, carbohydrates, oxalates, calcium, and sodium chloride, can also regulate the risk of urinary conditions and increase the risk of kidney stone formation (43). Although there is controversy regarding the conclusion that supplementing vitamin D alone increases the risk of hypercalciuria and kidney stones (44, 45), it is clear that there is an increased risk of kidney stone formation when vitamin D is supplemented along with calcium (22, 46). Additionally, strong evidence has shown that low urine output and dehydration are the common risks of all stone types (6, 47). This is because adequate fluid intake helps to dilute the abnormal urine components that contribute to stone formation, such as hypercalciuria, hyperoxaluria, and other factors. It is our belief that individuals with a genetic susceptibility to high serum vitamin D concentrations are more prone to developing hypercalciuria, thereby increasing the risk of kidney stones. A recent meta-analysis has also found that supplemental vitamin D doses of 3200-4000 IU/d may lead to an increased risk of hypercalcemia and other adverse events, but only in a small subset of individuals (48). Similarly, the higher risk of dying from any cause, including CVD, in KSD patients may be related to unique population characteristics and related comorbidities such as diabetes and CVD (42). To better understand how vitamin D affects all-cause and specific mortality in the KSD population, we conducted this study and found that 25(OH)D insufficiency (<27.7nmol/L) was associated with an increased risk of all-cause mortality in the KSD population after adjusting for unique demographics and associated comorbidities. However, this association was not present for CVD and cancer-specific causes of death.
There are various factors that could account for the decrease in all-cause mortality risk, particularly cardiovascular disease mortality, associated with a reduction in 25(OH)D concentrations. As previously mentioned, low serum concentrations of 25(OH)D have been linked to numerous diseases that increase the risk of premature death, including hypertension, dyslipidemia, diabetes, chronic kidney disease, and, notably, cardiovascular disease (18, 25, 26, 49–51). Hence, the findings from the two additional studies conducted on individuals with diabetes or osteoarthritis indicate that a deficiency in 25(OH)D primarily elevates the risk of mortality from cardiovascular disease (27, 28). On other hand, meat is an important source of vitamin D. Vegetarians and vegans have been shown to have lower plasma concentrations of 25(OH)D compared to individuals who consume meat and fish (52). Additionally, red meat and processed meat increase the risk of chronic diseases such as diabetes, chronic kidney disease, and coronary heart disease (53–55). Hence, we should also take into consideration whether the population that shows an increase in serum vitamin D concentrations due to meat consumption may obscure the health effects of vitamin D. The mechanism by which 25(OH)D reduces the risk of mortality from cardiovascular disease may involve the inhibition of renin synthesis, reduction of angiotensin II production, improvement of pancreatic beta-cell function, endothelial protective effects, and immunomodulatory activities, as suggested by previous research (18). Furthermore, 25(OH)D may improve adverse cholesterol metabolism, reduce inflammatory parameters, and enhance myocardial function and glucose metabolism (56, 57).
Interestingly, it was observed that in the KSD population with 25(OH)D insufficiency, there was no statistically significant association between 25(OH)D and mortality from cardiovascular disease. Our study revealed that individuals with KSD who had lower concentrations of 25(OH)D also had higher levels of CRP, indicating that the impact of 25(OH)D on mortality may be partially mediated through inflammatory pathways. Moreover, there is mounting evidence that 25(OH)D deficiency may heighten the risk of fatal events resulting from infections (58–61). A recent systematic review on mortality in KSD patients revealed that 21% of the reported mortality cases out of 2550 were related to surgical procedures, with sepsis being one of the primary causes of mortality (16). In fact, more than half of the deaths in patients who underwent intervention for KSD were attributed to sepsis (62). It is worth noting that other causes of death, such as cardiac-related, respiratory-related, and multiorgan failure, could also be attributed to sepsis. Based on this finding, it is possible that the higher mortality rate in KSD patients may be due to the fact that infections resulting from stone obstruction or intervention are more likely to progress to sepsis. Vitamin D, which can have activating or inhibiting effects on immune responses in the body, has been linked to susceptibility to infections. Most studies have demonstrated a strong association between sepsis and vitamin D deficiency, with sepsis patients who have low concentrations of vitamin D being more prone to developing secondary infections (63–66). However, rather than being a direct cause of mortality, vitamin D deficiency may be an indicator of other unfavorable health outcomes that could lead to premature death. Vitamin D also exerts an influence on other diseases related to the urinary system. Numerous studies have demonstrated the positive impact of vitamin D on reducing prostate volume and PSA levels and improving symptoms associated with benign prostatic hyperplasia (BPH) (67–69). In addition, vitamin D deficiency increases the risk of overactive bladder and urinary incontinence, and vitamin D supplementation reduces the risk of urinary incontinence (70). This can also explain why studies have observed lower concentrations of vitamin D in men with lower urinary tract symptoms (LUTS) (71). The impact of vitamin D is well described in certain tumors; however, the findings regarding the role of vitamin D in prostate cancer and serum PSA levels are contradictory and lack consistency (72, 73). Moreover, there has a possible relationship between vitamin D and urinary tract infection (UTI), erectile dysfunction (ED), and the male reproductive system (74–78). Therefore, further research is needed to elucidate how 25(OH)D impacts mortality in KSD patients.
Our study is the first to investigate the correlation between serum 25(OH)D concentrations and all-cause mortality in a large, nationally representative sample of KSD patients, and we were able to control for a range of potential confounding variables using comprehensive data from the NHANES survey. However, there are several limitations to our study. Firstly, we relied on self-reported and recalled disease data, which may have introduced memory bias. Secondly, as NHANES is a cross-sectional survey, we were unable to track changes in confounding variables over time, especially the serum 25(OH)D concentration. Supplementary Table 1 also confirms that the strength of our study results weakens or even loses statistical significance with increasing follow-up time. Finally, we were unable to determine the impact of kidney stone location, size, and composition on all-cause mortality. Further research that includes repeated follow-up and additional variables is needed to better understand the relationship between variations in 25(OH)D concentrations and mortality risk in KSD patients.
Conclusion
Our study found that after adjusting for multiple variables, serum 25(OH)D insufficiency (less than 27.7nmol/L) was associated with an increased risk of all-cause mortality in KSD patients, but this association was not observed for specific causes of death such as CVD and cancer. This effect may be due in part to the inflammatory pathways of vitamin D. Our results suggest that monitoring and preventing serum 25(OH)D deficiency may have potential benefits in reducing premature death in KSD patients.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
Approval of the study from the National Center of Health and Statistics Research ethics review board was waived because the research relied on publicly used, de-identified secondary data.
Author contributions
HC and ZZ made contributions to the conception and design of the work; MG analyzed and interpreted the data and drafted the work; ML and JC substantively revised the work. All authors have approved the submitted version and agreed both to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
This work was supported by the National Natural Science Foundation of China (81770705 to HC) and the Central South University Independent Exploration and Innovation Project for Graduate Students (2021zzts0348 to ZZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1207943/full#supplementary-material
Abbreviations
KSD, Kidney stone disease; CVD, Cardiovascular disease; 25(OH)D, 25-Hydroxyvitamin D; 95% CI, 95% confidence interval; HR, Hazard ratio; NHANES, National Health and Nutrition Examination Survey; HDL, High-density lipoprotein cholesterol; TG, Triglycerides; LDL, Low-density lipoprotein cholesterol; OA, Osteoarthritis; CKD, chronic kidney disease.
References
1. Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol (2017) 35:1301–20. doi: 10.1007/s00345-017-2008-6
2. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D'Andrea D, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007-2018 data. Eur Urol Focus (2021) 7:1468–75. doi: 10.1016/j.euf.2020.08.011
3. Chewcharat A, Curhan G. Trends in the prevalence of kidney stones in the United States from 2007 to 2016. Urolithiasis (2021) 49:27–39. doi: 10.1007/s00240-020-01210-w
4. Thongprayoon C, Krambeck AE, Rule AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol (2020) 16:736–46. doi: 10.1038/s41581-020-0320-7
5. Kum F, Mahmalji W, Hale J, Thomas K, Bultitude M, Glass J. Do stones still kill? An analysis of death from stone disease 1999-2013 in England and Wales. BJU Int (2016) 118:140–4. doi: 10.1111/bju.13409
6. Peerapen P, Thongboonkerd V. Kidney stone prevention. Adv Nutr (2023) 14:555–69. doi: 10.1016/j.advnut.2023.03.002
7. Kirkali Z, Rasooly R, Star RA, Rodgers GP. Urinary stone disease: progress, status, and needs. Urology (2015) 86:651–3. doi: 10.1016/j.urology.2015.07.006
8. Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, et al. History of kidney stones and the risk of coronary heart disease. JAMA (2013) 310:408–15. doi: 10.1001/jama.2013.8780
9. Zhe M, Hang Z. Nephrolithiasis as a risk factor of chronic kidney disease: a meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis (2017) 45:441–8. doi: 10.1007/s00240-016-0938-x
10. Shoag J, Halpern J, Goldfarb DS, Eisner BH. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol (2014) 192:1440–5. doi: 10.1016/j.juro.2014.05.117
11. Weinberg AE, Patel CJ, Chertow GM, Leppert JT. Diabetic severity and risk of kidney stone disease. Eur Urol (2014) 65:242–7. doi: 10.1016/j.eururo.2013.03.026
12. Hung JA, Li CH, Geng JH, Wu DW, Chen SC. Dyslipidemia increases the risk of incident kidney stone disease in a large Taiwanese population follow-up study. Nutrients (2022) 14:1339. doi: 10.3390/nu14071339
13. Kittanamongkolchai W, Mara KC, Mehta RA, Vaughan LE, Denic A, Knoedler JJ, et al. Risk of hypertension among first-time symptomatic kidney stone formers. Clin J Am Soc Nephrol (2017) 12:476–82. doi: 10.2215/CJN.06600616
14. Soligo M, Morlacco A, Zattoni F, Valotto C, De Giorgo G, Beltrami P. Metabolic syndrome and stone disease. Panminerva Med (2022) 64:344–58. doi: 10.23736/S0031-0808.21.04517-1
15. Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res (2012) 40:95–112. doi: 10.1007/s00240-011-0448-9
16. Whitehurst L, Jones P, Somani BK. Mortality from kidney stone disease (KSD) as reported in the literature over the last two decades: a systematic review. World J Urol (2019) 37:759–76. doi: 10.1007/s00345-018-2424-2
17. Rule AD, Roger VL, Melton 3, Bergstralh EJ, Li X, Peyser PA, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol (2010) 21:1641–4. doi: 10.1681/ASN.2010030253
19. Tang J, McFann KK, Chonchol MB. Association between serum 25-hydroxyvitamin D and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988-94. Nephrol Dial Transplant (2012) 27:4385–9. doi: 10.1093/ndt/gfs297
20. Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr (2016) 104:1039–51. doi: 10.3945/ajcn.116.134981
21. Jian Z, Huang Y, He Y, Jin X, Li H, Li S, et al. Genetically predicted lifelong circulating 25(OH)D levels are associated with serum calcium levels and kidney stone risk. J Clin Endocrinol Metab (2022) 107:e1159–66. doi: 10.1210/clinem/dgab758
22. Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, et al. Calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US preventive services task force. JAMA (2018) 319:1600–12. doi: 10.1001/jama.2017.21640
23. Letavernier E, Daudon M, Vitamin D. Hypercalciuria and kidney stones. Nutrients (2018) 10:366. doi: 10.3390/nu10030366
24. Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol (2013) 9:337–47. doi: 10.1038/nrneph.2013.74
25. Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis (2011) 58:374–82. doi: 10.1053/j.ajkd.2011.03.020
26. de Borst MH, de Boer RA, Stolk RP, Slaets JP, Wolffenbuttel BH, Navis G. Vitamin D deficiency: universal risk factor for multifactorial diseases? Curr Drug Targets (2011) 12:97–106. doi: 10.2174/138945011793591590
27. Xiao Q, Cai B, Yin A, Huo H, Lan K, Zhou G, et al. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the NHANES database prospective cohort study. BMC Med (2022) 20:308. doi: 10.1186/s12916-022-02510-1
28. Wan Z, Guo J, Pan A, Chen C, Liu L, Liu G. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care (2021) 44:350–7. doi: 10.2337/dc20-1485
29. Hu B, Chen J, Shi Y, Hou L. Association between serum 25(OH)D and risk of all-cause mortality in adults with prior cardiovascular disease: a cohort study from NHANES 2007-2018. BMC Cardiovasc Disord (2023) 23:240. doi: 10.1186/s12872-023-03257-0
30. Wang B, Cheng X, Fu S, Sun D, Zhang W, Liu W, et al. Associations of serum 25(OH)D, PTH, and beta-CTX levels with all-cause mortality in chinese community-dwelling centenarians. Nutrients (2022) 15:94. doi: 10.3390/nu15010094
31. Liu Y, Yang D, Shi F, Wang F, Liu X, Wen H, et al. Association of serum 25(OH)D, cadmium, CRP with all-cause, cause-specific mortality: A prospective cohort study. Front Nutr (2022) 9:803985. doi: 10.3389/fnut.2022.803985
32. Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ (2019) 366:l4673. doi: 10.1136/bmj.l4673
33. Shoag J, Eisner BH. Relationship between C-reactive protein and kidney stone prevalence. J Urol (2014) 191:372–5. doi: 10.1016/j.juro.2013.09.033
34. C.f.D.C.a. Prevention. About the national health and nutrition examination survey. United States: Centers for Disease Control and Prevention (2022).
35. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
36. van den Berg KS, Marijnissen RM, Brink RHSvd, Voshaar RCO, Hegeman JM. Adverse health outcomes in vitamin D supplementation trials for depression: A systematic review. Ageing Res Rev (2021) 71:101442. doi: 10.1016/j.arr.2021.101442
37. Sha S, Nguyen TMN, Kuznia S, Niedermaier T, Zhu A, Brenner H, et al. Real-world evidence for the effectiveness of vitamin D supplementation in reduction of total and cause-specific mortality. J Intern Med (2023) 293:384–97. doi: 10.1111/joim.13578
38. Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev (2014) 2014:CD000227. doi: 10.1002/14651858.CD000227.pub4
39. Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol (2019) 30:733–43. doi: 10.1093/annonc/mdz059
40. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med (2019) 380:33–44. doi: 10.1056/NEJMoa1809944
41. Rule AD, Bergstralh EJ, Melton 3LJ, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol (2009) 4:804–11. doi: 10.2215/CJN.05811108
42. Tang J, Mettler P, McFann K, Chonchol M. The association of prevalent kidney stone disease with mortality in US adults: the National Health and Nutrition Examination Survey III, 1988-1994. Am J Nephrol (2013) 37:501–6. doi: 10.1159/000350691
44. Malihi Z, Lawes CMM, Wu Z, Huang Y, Waayer D, Toop L, et al. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: results from a randomized controlled trial. Am J Clin Nutr (2019) 109:1578–87. doi: 10.1093/ajcn/nqy378
45. Billington EO, Burt LA, Rose MS, Davison EM, Gaudet S, Kan M, et al. Safety of high-dose vitamin D supplementation: secondary analysis of a randomized controlled trial. J Clin Endocrinol Metab (2020) 105:dgz212. doi: 10.1210/clinem/dgz212
46. Wallace RB, Wactawski-Wende J, O'Sullivan MJ, Larson JC, Cochrane B, Gass M, et al. Urinary tract stone occurrence in the Women's Health Initiative (WHI) randomized clinical trial of calcium and vitamin D supplements. Am J Clin Nutr (2011) 94:270–7. doi: 10.3945/ajcn.110.003350
47. Littlejohns TJ, Neal NL, Bradbury KE, Heers H, Allen NE, Turney BW. Fluid intake and dietary factors and the risk of incident kidney stones in UK biobank: A population-based prospective cohort study. Eur Urol Focus (2020) 6:752–61. doi: 10.1016/j.euf.2019.05.002
48. Zittermann A, Trummer C, Theiler-Schwetz V, Pilz S. Long-term supplementation with 3200 to 4000 IU of vitamin D daily and adverse events: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr (2023) 62:1833–44. doi: 10.1007/s00394-023-03124-w
49. Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care (2005) 28:1228–30. doi: 10.2337/diacare.28.5.1228
50. Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G. Vitamin D deficiency and risk of cardiovascular diseases: a narrative review. Clin Hypertens (2018) 24:9. doi: 10.1186/s40885-018-0094-4
51. Jung CH, Kim KJ, Kim BY, Kim CH, Kang SK, Mok JO. Relationship between vitamin D status and vascular complications in patients with type 2 diabetes mellitus. Nutr Res (2016) 36:117–24. doi: 10.1016/j.nutres.2015.11.008
52. Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr (2011) 14:340–6. doi: 10.1017/S1368980010002454
53. Al-Shaar L, Satija A, Wang DD, Rimm EB, Smith-Warner SA, Stampfer MJ, et al. Red meat intake and risk of coronary heart disease among US men: prospective cohort study. BMJ (2020) 371:m4141. doi: 10.1136/bmj.m4141
54. Yang X, Li Y, Wang C, Mao Z, Zhou W, Zhang L, et al. Meat and fish intake and type 2 diabetes: Dose-response meta-analysis of prospective cohort studies. Diabetes Metab (2020) 46:345–52. doi: 10.1016/j.diabet.2020.03.004
55. Mirmiran P, Yuzbashian E, Aghayan M, Mahdavi M, Asghari G, Azizi F. A prospective study of dietary meat intake and risk of incident chronic kidney disease. J Ren Nutr (2020) 30:111–8. doi: 10.1053/j.jrn.2019.06.008
56. Riek AE, Oh J, Bernal-Mizrachi C. 1,25(OH)2 vitamin D suppresses macrophage migration and reverses atherogenic cholesterol metabolism in type 2 diabetic patients. J Steroid Biochem Mol Biol (2013) 136:309–12. doi: 10.1016/j.jsbmb.2012.12.019
57. Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, et al. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol (2010) 5:905–11. doi: 10.2215/CJN.06510909
58. Mohan M, Cherian JJ, Sharma A. Exploring links between vitamin D deficiency and COVID-19. PloS Pathog (2020) 16:e1008874. doi: 10.1371/journal.ppat.1008874
59. Fernandez-Ruiz M, Corbella L, Morales-Cartagena A, Gonzalez E, Polanco N, Ruiz-Merlo T, et al. Vitamin D deficiency and infection risk in kidney transplant recipients: A single-center cohort study. Transpl Infect Dis (2018) 20:e12988. doi: 10.1111/tid.12988
60. Lang PO, Samaras N, Samaras D, Aspinall R. How important is vitamin D in preventing infections? Osteoporos Int (2013) 24:1537–53. doi: 10.1007/s00198-012-2204-6
61. White JH. Vitamin D metabolism and signaling in the immune system. Rev Endocr Metab Disord (2012) 13:21–9. doi: 10.1007/s11154-011-9195-z
62. Bhanot R, Pietropaolo A, Tokas T, Kallidonis P, Skolarikos A, Keller EX, et al. Predictors and strategies to avoid mortality following ureteroscopy for stone disease: A systematic review from european association of urologists sections of urolithiasis (EULIS) and uro-technology (ESUT). Eur Urol Focus (2022) 8:598–607. doi: 10.1016/j.euf.2021.02.014
63. Delrue C, Speeckaert R, Delanghe JR, Speeckaert MM. Vitamin D deficiency: an underestimated factor in sepsis? Int J Mol Sci (2023) 24:2924. doi: 10.3390/ijms24032924
64. Xiao K, Zhang DC, Hu Y, Song LC, Xu JQ, He WX, et al. Potential roles of vitamin D binding protein in attenuating liver injury in sepsis. Mil Med Res (2022) 9:4. doi: 10.1186/s40779-022-00365-4
65. Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med (2014) 42:97–107. doi: 10.1097/CCM.0b013e31829eb7af
66. Flynn L, Zimmerman LH, McNorton K, Dolman M, Tyburski J, Baylor A, et al. Effects of vitamin D deficiency in critically ill surgical patients. Am J Surg (2012) 203:379–82; discussion 382. doi: 10.1016/j.amjsurg.2011.09.012
67. Zendehdel A, Ansari M, Khatami F, Mansoursamaei S, Dialameh H. The effect of vitamin D supplementation on the progression of benign prostatic hyperplasia: A randomized controlled trial. Clin Nutr (2021) 40:3325–31. doi: 10.1016/j.clnu.2020.11.005
68. Chen Y, Xu H, Liu C, Gu M, Chen Q, Zhan M, et al. Therapeutic effects of 25-hydroxyvitamin D on the pathological process of benign prostatic hyperplasia: an in vitro evidence. Dis Markers (2021) 2021:4029470. doi: 10.1155/2021/4029470
69. Zhang W, Zheng X, Wang Y, Xiao H. Vitamin D deficiency as a potential marker of benign prostatic hyperplasia. Urology (2016) 97:212–8. doi: 10.1016/j.urology.2016.03.070
70. Zhang Q, Zhang Z, He X, Liu Z, Shen L, Long C, et al. Vitamin D levels and the risk of overactive bladder: a systematic review and meta-analysis. Nutr Rev (2023). doi: 10.1093/nutrit/nuad049
71. Elshazly MA, Sultan MF, Aboutaleb HA, Salem SM, Aziz MS, Abd Elbaky TM, et al. Vitamin D deficiency and lower urinary tract symptoms in males above 50 years of age. Urol Ann (2017) 9:170–3. doi: 10.4103/0974-7796.204192
72. Crocetto F, Barone B, D'Aguanno G, Falcone A, de Vivo R, Rienzo M, et al. a regulator of androgen levels, is not correlated to PSA serum levels in a cohort of the middle Italy region participating to a prostate cancer screening campaign. J Clin Med (2023) 12:1831. doi: 10.3390/jcm12051831
73. Krajewski W, Dziegala M, Kolodziej A, Dembowski J, Zdrojowy R, Vitamin D. and urological cancers. Cent Eur J Urol (2016) 69:139–47. doi: 10.5173/ceju.2016.784
74. Crafa A, Cannarella R, Barbagallo F, Leanza C, Palazzolo R, Flores HA, et al. Mechanisms suggesting a relationship between vitamin D and erectile dysfunction: an overview. Biomolecules (2023) 13:930. doi: 10.3390/biom13060930
75. Yuan C, Xiang L, Jian Z, Liao B. Vitamin D levels and risk of male factor infertility: A mendelian randomization study. World J Mens Health (2023) 41:640–8. doi: 10.5534/wjmh.220109
76. Li X, Yu Q, Qin F, Zhang B, Lu Y. Serum vitamin D level and the risk of urinary tract infection in children: A systematic review and meta-analysis. Front Public Health (2021) 9:637529. doi: 10.3389/fpubh.2021.637529
77. Cito G, Cocci A, Micelli E, Gabutti A, Russo GI, Coccia ME, et al. and male fertility: an updated review. World J Mens Health (2020) 38:164–77. doi: 10.5534/wjmh.190057
Keywords: kidney stones, 25-hydroxyvitamin D, National Health and Nutrition Examination Survey, all-cause mortality, inflammation
Citation: Gao M, Liu M, Chen J, Zhu Z and Chen H (2023) Association of serum 25-hydroxyvitamin D concentrations with all-cause mortality among individuals with kidney stone disease: the NHANES database prospective cohort study. Front. Endocrinol. 14:1207943. doi: 10.3389/fendo.2023.1207943
Received: 18 April 2023; Accepted: 08 September 2023;
Published: 03 October 2023.
Edited by:
Kyong Park, Yeungnam University, Republic of KoreaReviewed by:
Biagio Barone, Azienda Ospedaliera di Caserta, ItalyWilliam B. Grant, Sunlight Nutrition and Health Research Center, United States
Copyright © 2023 Gao, Liu, Chen, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zewu Zhu, emh1emV2QDE2My5jb20=; Hequn Chen, Y2hlbmhlcXVueHlAMTI2LmNvbQ==
 Meng Gao1,2
Meng Gao1,2 Minghui Liu
Minghui Liu Jinbo Chen
Jinbo Chen Zewu Zhu
Zewu Zhu Hequn Chen
Hequn Chen