- 1Department of Nutrition and Food Hygiene, School of Public Health, Tianjin Medical University, Tianjin, China
- 2Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, China
- 3Qingdao Municipal Center for Disease Control and Prevention, Qingdao, China
- 4Qingdao Institute of Preventive Medicine, Qingdao, China
- 5Tianjin Key Laboratory of Environment, Nutrition and Public Health, Tianjin, China
- 6Tianjin Key Laboratory of Hormones and Development (Ministry of Health), Tianjin, China
Background: The relationship between normal thyroid-stimulating hormone (TSH) levels and thyroid disease in adults remains controversial. This study aimed to investigate the correlation between serum TSH levels, particularly those falling within the normal range, and thyroid diseases in Chinese adults, including thyroid nodules (TN), goiter (GR), and thyroid antibody positivity.
Materials and methods: This research was a cross-sectional study conducted in an adult population in Tianjin, China. Thyroid volume (Tvol) and TN were assessed using thyroid ultrasonography. Fasting venous blood and spot urine samples were collected to evaluate thyroid function and iodine status.
Results: A total of 2460 subjects participated in the survey. The prevalence of thyroid dysfunction was 9.76%, and abnormal TSH levels were found to potentially increase the risk of GR and thyroid antibody positivity in adults. A total of 2220 subjects with TSH within the normal reference range were included in the further study. In these patients, Tvol decreased as TSH levels increased, in both men and women (P < 0.0001). Low TSH levels (0.27–1.41 IU/mL) were identified as a risk factor for TN (odds ratio [OR], 1.46; 95% CI: 1.14-1.87) and GR (OR 5.90, 95% CI 2.27-15.3). Upon stratification by sex and age, the risk of TN was found to be higher in women and elderly individuals (≥60 years old), while the risk of GR was found to be higher in men and younger individuals (<60 years old). High TSH levels (2.55–4.2 IU/mL) were identified as a risk factor for thyroid antibody positivity (OR, 1.53; 95% CI: 1.11-2.10). Men and younger individuals with high TSH levels exhibited a higher risk of thyroid antibody positivity.
Conclusion: In adults with normal TSH levels, low TSH levels were associated with an increased risk of TN and GR, whereas high TSH levels were associated with thyroid antibody positivity. The research also suggests that adults whose TSH levels at upper or lower limits of the normal range should be reviewed regularly.
1 Introduction
The incidence of thyroid diseases has been increasing in recent years. Thyroid-stimulating hormone (TSH), the most sensitive indicator reflecting thyroid function, is produced by the adenohypophysis and regulated by the hypothalamic-adenohypophysis-thyroid axis. As a stimulator of thyroid growth, TSH is closely associated with the occurrence of various thyroid disorders.
Thyroid nodules (TN) are common thyroid conditions, with a reported prevalence of 4%–7% through palpation, and a prevalence of 13%–67% by high-resolution ultrasonography (1). The American Association of Clinical Endocrinologists recommends that TSH should be reevaluated within 12–24 months based on clinical characteristics and changes in nodules following the initial TSH assessment (2). A study involving 1990 adults in Korea found that TSH level was negatively associated with TN, with patients with TN having significantly higher TSH levels than those without TN (3). In addition, high TSH levels within the normal range may be an early indicator of hypothyroidism and are linked to an increased risk of autoimmune thyroid disease (4). Previous studies have found that thyroid peroxidase antibody (TPOAb) and/or thyroglobulin autoantibody (TgAb) levels significantly increase with increasing TSH levels (5, 6). In China, a study aimed at establishing new reference values for thyroid volume (Tvol) showed that Tvol was negatively correlated with TSH levels. Some studies have also reported that serum TSH levels in adults with goiter (GR) were significantly lower than those in individuals without GR (7). These findings highlight the impact of TSH level fluctuations on the occurrence of thyroid diseases.
TSH levels play a significant role in the development of thyroid diseases through various mechanisms. Clarifying the relationship between TSH levels and thyroid diseases is helpful to the diagnosis of thyroid diseases in clinic. In addition, whether the TSH levels in the normal range can predict the future risk of thyroid diseases is also a topic worthy of attention. This study aims to investigate whether serum TSH levels, especially within the normal range, are associated with common thyroid disorders, including TN, GR, and thyroid antibody positivity.
2 Materials and methods
2.1 Subjects
A cross-sectional study was conducted from March to October 2015 in Tianjin, China. Participants were chosen through random cluster sampling. To fulfill the research requirements, the subject selection criteria for this study were as follows (1): age > 18 years (2), Han nationality, and (3) residency in Tianjin for >5 years. Subjects who received iodine-containing contrast media or iodine-containing medications within the previous 3 months, or subjects who were undergoing treatment with thyroid hormone or anti-thyroidal drugs for thyroid-related issues were excluded. Pregnant and lactating women were also excluded. The purpose and content of the study were explained in detail to the participating adults. All subjects signed an informed consent. This study was approved by the Ethics Committee of the China Medical University (serial number: IRB [2013]115).
2.2 Anthropometric measurements
Each subject was instructed to accurately measure their height and weight. The researchers calculated the body mass index (BMI) for each participant by squaring their weight and dividing it by their height (kg/m2). Furthermore, each subject was required to provide a 5-mL fasting venous blood sample to assess thyroid function, including TSH, free thyroxine (FT4), and serum free triiodothyronine (FT3), as well as TgAb and TPOAb. TSH, FT4, and FT3 levels were determined using the ADVIA Centaur automatic chemiluminescence immunoassay (Siemens Healthcare Diagnostics, Gwynedd, United Kingdom). In cases where TSH levels fell within the normal range, FT3 and FT4 were not measured additionally, whereas if TSH levels were abnormal, FT3 and FT4 were measured. TgAb and TPOAb levels were determined through a chemiluminescence reaction using the IMMULITE 2000 system (Siemens Healthcare Diagnostics Inc, Gwynedd, United Kingdom).
2.3 Urinary iodine
Subjects were instructed to collect 5 mL of mid-course spot urine using a plastic centrifuge tube. Urinary iodine was quantified as the urinary iodine concentration (UIC), which was determined through catalytic spectrophotometry with arsenic and cerium. Four levels of freeze-dried human urine reference material (GBW09108l, GBW09110n, GBW09111a, and GBW09112a; National Reference Laboratory for Iodine Deficiency Disorders, Beijing) were run together with each batch of samples, and the average standard iodine concentrations were 68 (range: 59–77) μg/L, 195 (range: 185–205) μg/L, and 558 (range: 541–575) μg/L. The coefficient of variation for UIC ranged from 0.1% to 4.7%.
2.4 Thyroid ultrasonography
Tvol was measured by professionally trained operators using a 7.5 MHz transducer (Madison Portable B-SA-600). During the procedure, subjects remained seated with their upper bodies straight and fully exposing their necks. The operator systematically scanned the thyroid gland in an up-and-down, left-to-right fashion, recording the maximum width of the thyroid gland. Subsequently, longitudinal scanning was conducted first on the left side and then on the right side, recording both the maximum depth and maximum length of the thyroid lateral lobe. The unilateral volume was calculated using the formula: Tvol (mL) = 0.479 × width × depth × length (mm)/1000, and the total Tvol was determined as the sum of the left and right thyroid volumes.
2.5 Definition and diagnostic
According to the Diagnostic Criteria for Endemic Goiter (WS-2007), Tvol exceeding 25.0 mL for men and 18.0 mL for women was considered indicative of GR. TN have been described as additional abnormalities in ultrasonic structures. The reference range for TSH provided by the Clinical Laboratory of Tianjin Medical University General Hospital was 0.27–4.2 IU/mL. In this study, when serum TSH levels fell within this normal range, the thyroid function of the subjects was deemed normal. In addition, thyroid antibody positivity was defined as having TPOAb > 34 IU/mL and/or TgAb > 115 IU/mL.
2.6 Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics (version 26). The Kolmogorov-Smirnov test was used to test the normality of continuous variables. Non-normally distributed data were presented as median (interquartile range, IQR). Categorical variables were described as ratios and percentages. The Mann–Whitney U test and the Kruskal–Wallis test were used to compare non-normally distributed data. The chi-square test was used to compare the prevalence of TN, GR, and thyroid antibody positivity. Trend tests were performed to evaluate changes in UIC, Tvol, TN, and GR and the antibody positivity rate across the TSH quartiles. Univariate logistic analysis was used to assess whether TSH levels falling below the 25th percentile or above the 75th percentile were associated with thyroid diseases. An interaction test was employed to analyze the interaction between sex, age, and TSH levels on the prevalence of thyroid diseases. P < 0.05 was considered to be statistically significant.
3 Results
3.1 General population characteristics
A total of 2460 subjects were enrolled. The prevalence of thyroid dysfunction was 9.76%, including 16 patients with hyperthyroidism and subclinical hyperthyroidism and 224 patients with hypothyroidism and subclinical hypothyroidism. After excluding 240 subjects with abnormal TSH levels, a total of 2220 adults with normal thyroid function were included for further analysis. The baseline data for the subjects are presented in Table 1. There were no significant differences in age distribution between men and women (P = 0.48). Men had a higher BMI than women (P < 0.0001). The median (IQR) UIC was 135 (81.8-208) μg/L, which indicated adequate iodine nutrition in the population. The median (IQR) Tvol was 9.07 (7.17-11.6) mL. The medians (IQR) of TSH in men and women were 1.81 (1.32–2.39) IU/mL and 2.05 (1.53–2.75) IU/mL, respectively. Women exhibited a higher prevalence of TN and thyroid antibody positivity than men.
3.2 Prevalence rate of thyroid diseases in quartiles of different TSH
The percentages of thyroid diseases across different TSH quartiles are depicted in Figure 1. Tvol in men from TSH-Q1 to TSH-Q4 was 11.2 mL, 10.4 mL,9.86 mL, and 8.99 mL (P < 0.0001). Similarly, in women, Tvol was 8.26 mL, 7.91 mL, 7.58 mL, and 7.45 mL (P < 0.0001; Table 2). With the increase of TSH levels in men and women, the prevalence of GR had a downward trend. In women, the proportion of TN in the TSH-Q1 group was higher than that in the TSH-Q2 group and TSH-Q3 group. In addition, the prevalence rate of thyroid antibody positivity in TSH-Q4 group was significantly higher than that in TSH-Q2 group in men. With the increase of TSH levels in women, the prevalence of thyroid antibody positivity had an increasing trend. We analyzed the continuous characteristics of the different TSH level groups and presented the corresponding P values for trends in Table 2.
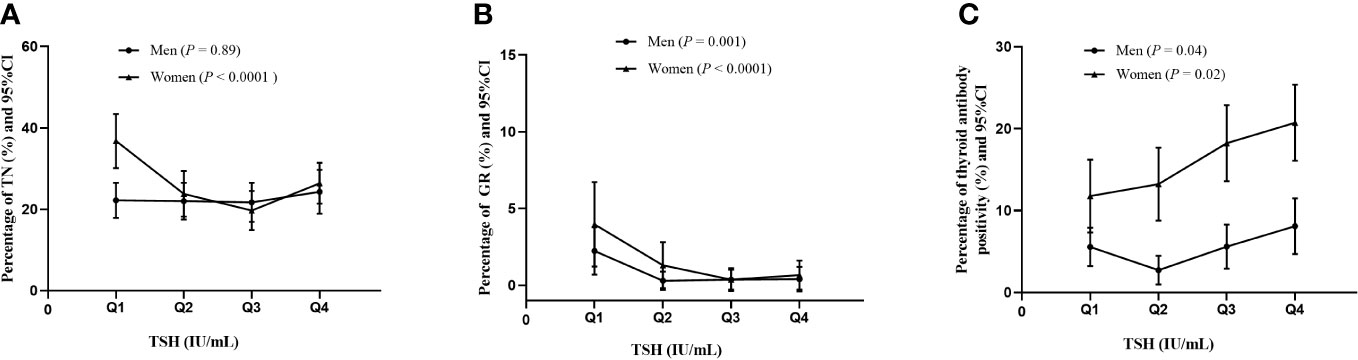
Figure 1 Percentage of thyroid diseases in men and women according to TSH quartiles. TN (A), GR (B), thyroid antibody positivity (C).
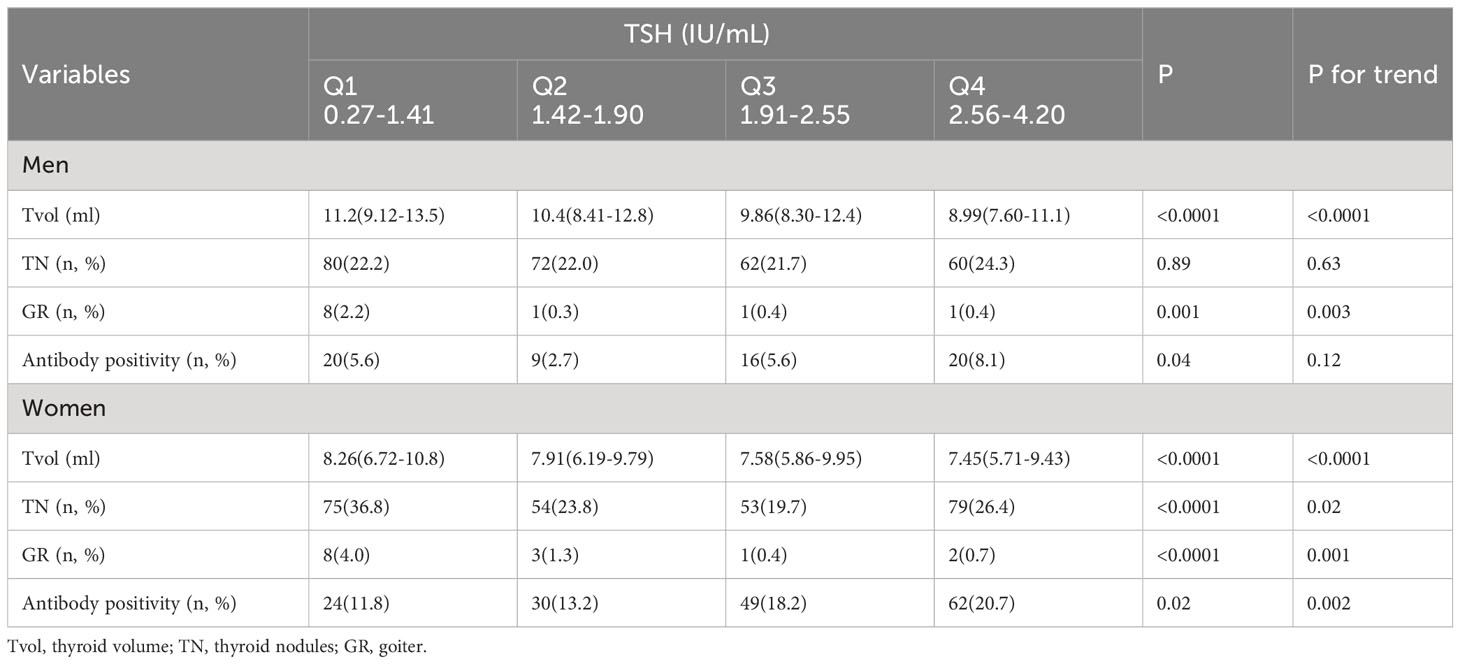
Table 2 Characteristics of men and women subjects between TSH level which were divided into four groups.
3.3 Relationship between abnormal TSH and thyroid diseases
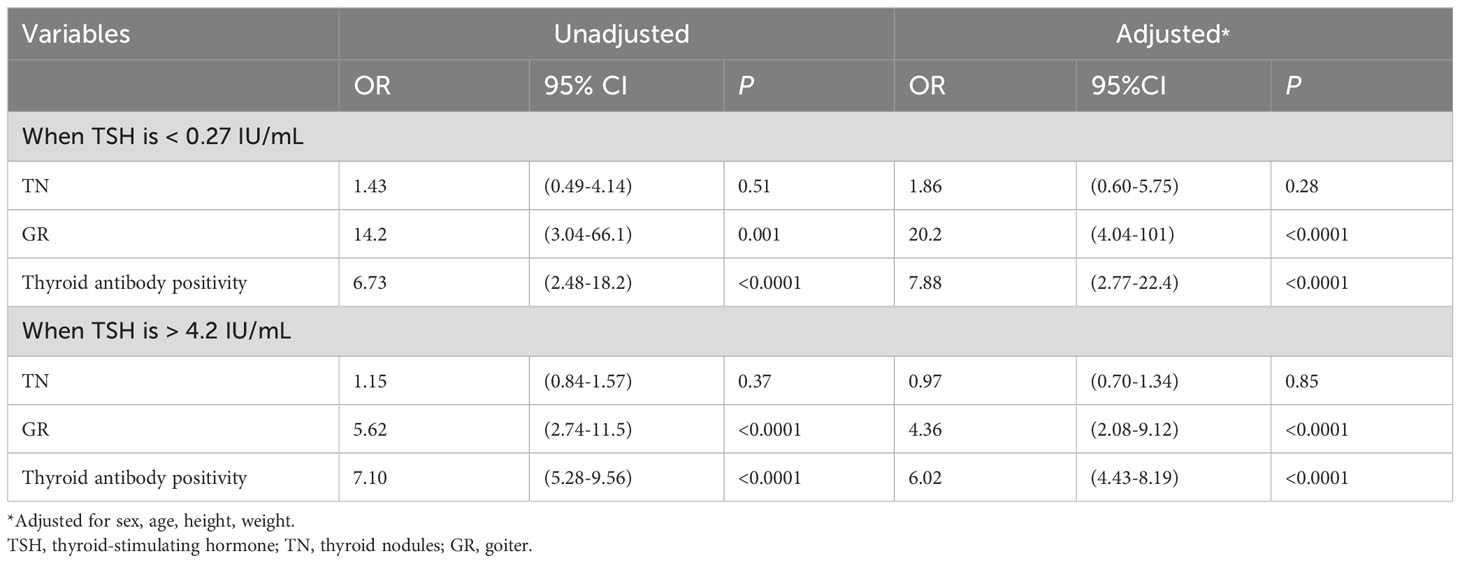
The relationship between abnormal TSH levels and TN, GR, and thyroid antibody positivity was investigated (Table 3). We defined the normal TSH range (0.27–4.2 IU/mL) as the reference point. Logistic regression analysis revealed that after adjusting for sex, age, height and weight, TSH < 0.27 IU/mL or TSH > 4.2 IU/mL may elevate the risk of GR and thyroid antibody positivity. Specifically, the risk of GR in adults with TSH < 0.27 IU/mL and TSH > 4.2 IU/mL was 20.2 and 4.36 times higher, respectively, than in individuals with normal TSH levels. Similarly, the risk of thyroid antibody positivity in adults with TSH < 0.27 IU/mL and TSH > 4.2 IU/mL was 7.88 and 6.02 times higher, respectively, than in those with normal TSH levels.
3.4 Relationship between normal TSH and thyroid diseases
The middle TSH group (with TSH levels ranging from 1.41 to 2.55 IU/mL) was regarded as the reference group. TSH levels below the 25th percentile were categorized as low TSH (TSH < 1.41 IU/mL), whereas levels above the 75th percentile were categorized as high TSH (TSH > 2.55 IU/mL). Regression analysis revealed that both low and high TSH levels can be risk factors for thyroid diseases (Table 4). The odds ratio (OR) for TN in adults with lower TSH levels was 1.46 compared to those with higher TSH level (1.41–2.55 IU/mL). Adults with low TSH levels were associated with an increased risk of GR (OR, 5.90; 95% CI, 2.27-15.3). Similarly, the risk of thyroid disease was significantly elevated in individuals with TSH > 2.55 IU/mL, with a significantly increased risk of antibody positivity (OR, 1.53; 95% CI, 1.11-2.10).
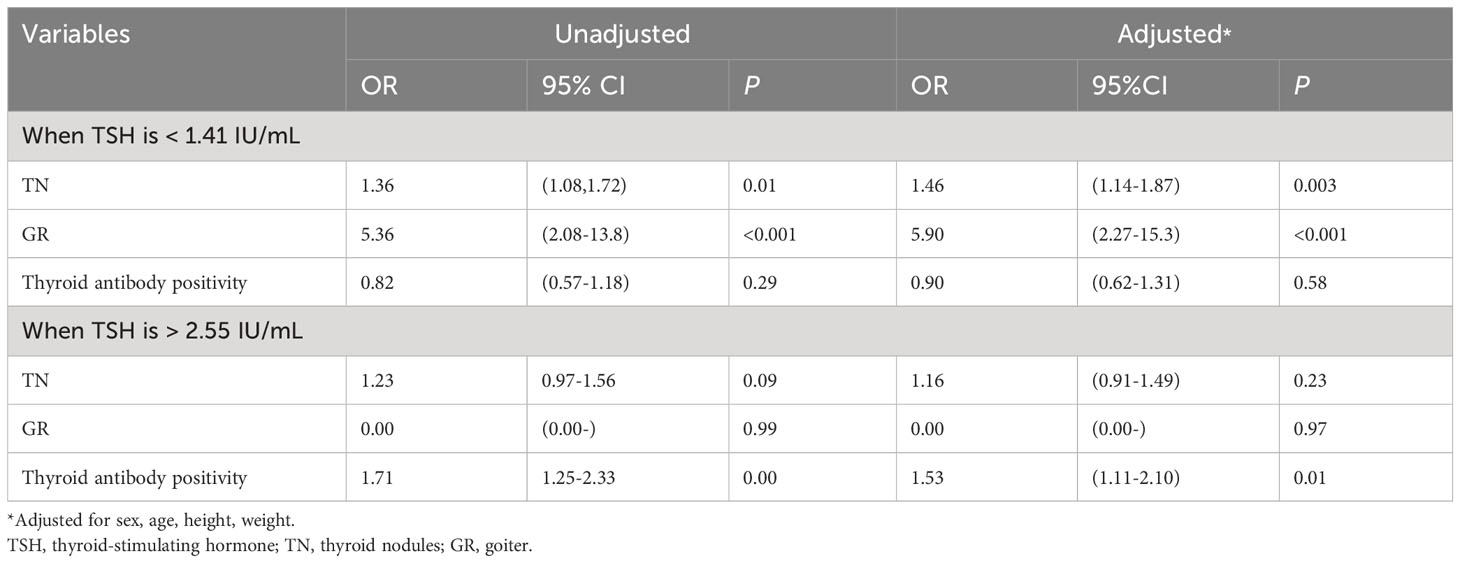
Table 4 Logistic analysis of TSH < 25 percentile or TSH > 75 percentile as a risk factor for thyroid disease.
Figure 2 and Table 5 displays the risk of thyroid diseases with low and high TSH levels stratified by sex and age (<60 years and ≥60 years). On the one hand, TN was significantly associated with low TSH levels in women (OR: 2.30, 95% CI, 1.57-3.36) but not in men. Furthermore, the risk was significantly higher in the ≥60-year-old group than in the <60-year-old group (OR: 1.75, 95% CI: 1.03-2.96 vs. OR: 1.34, 95% CI: 1.02-1.76). However, the interaction between age and low TSH levels was not statistically significant for the risk of TN (P for interaction = 0.98). Men or young people (<60 years old) with low TSH levels had a higher risk for GR than women or elderly people (≥60 years old). Considering the interaction of sex or age with low TSH levels, low TSH had no significant effect on GR (P for interaction = 0.66, P for interaction = 0.57, respectively). On the other hand, in young adults (<60-year-old group), thyroid antibody positivity was significantly correlated with high TSH levels (OR, 1.54; 95% CI, 1.08–2.20), but this association was not observed in older adults (≥60-year-old group). Furthermore, the risk of antibody positivity was notably higher in the men group (OR: 1.92, 95% CI: 1.04–3.54) than in the women group (OR: 1.39, 95% CI: 0.96-2.01), although the interaction between sex and high TSH was not statistically significant (P for interaction = 0.32).
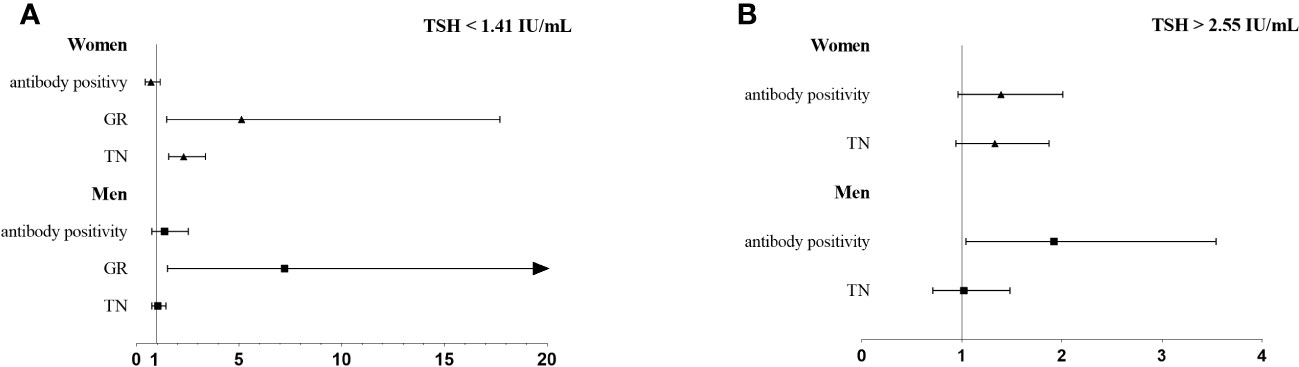
Figure 2 The association of thyroid diseases with TSH < 25 percentile (A) or TSH > 75 percentile (B) stratified by sex.
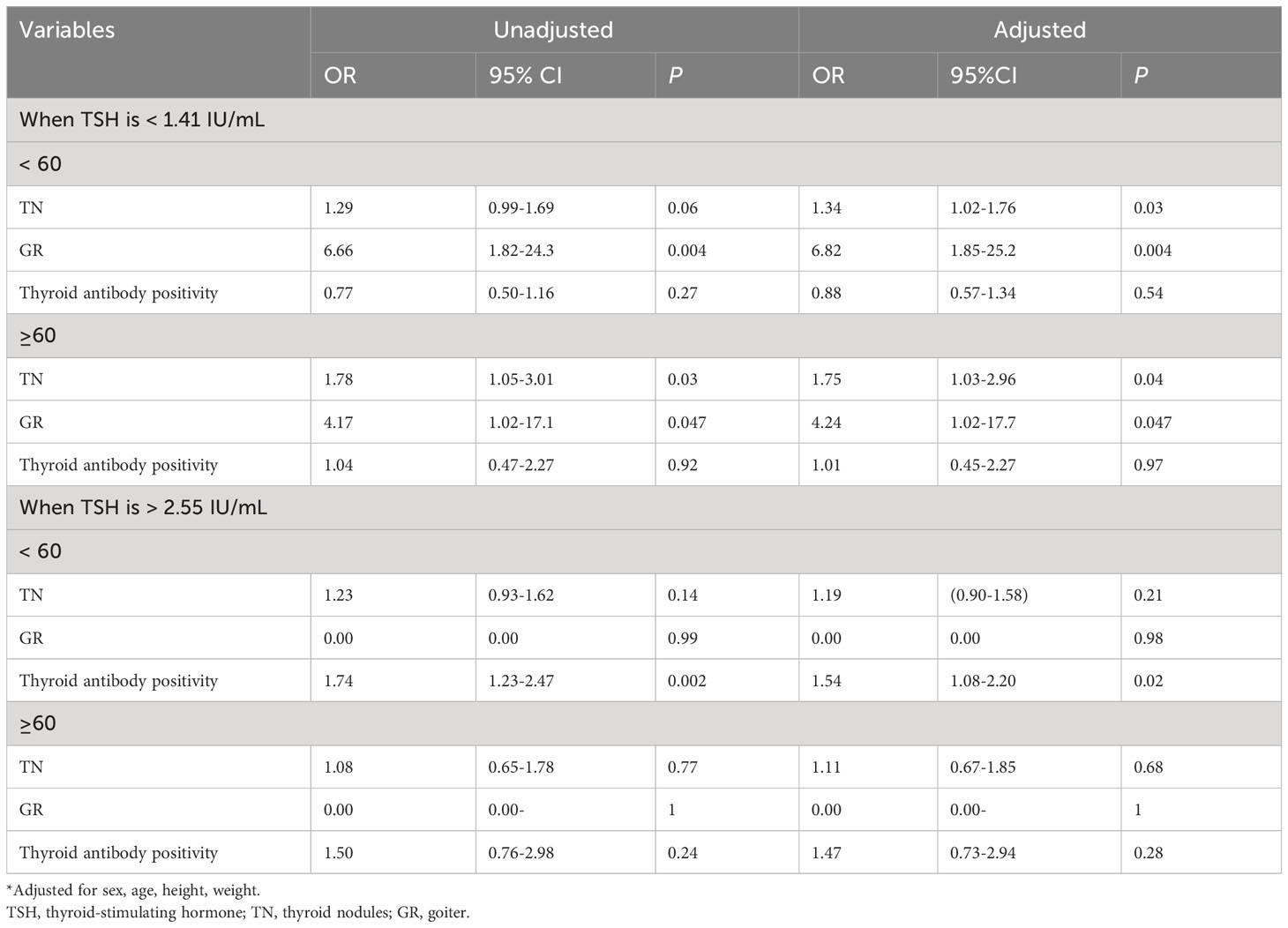
Table 5 The association of thyroid diseases with TSH < 25 percentile or TSH > 75 percentile stratified by age.
4 Discussion
Thyroid diseases represent the most prevalent group of disorders within the endocrine system. TSH levels play a significant role in the development of thyroid diseases through various mechanisms. The main cause of GR remains iodine deficiency (8). When dietary iodine intake is insufficient to synthesize thyroid hormones, serum thyroxine reduces, leading to increased TSH release by the pituitary gland. Elevated TSH stimulates the growth and metabolism of thyroid follicular cells, potentially causing GR. In addition, TSH binds to the TSH receptor on thyroid follicular epithelial cells, activates G proteins, and stimulates thyroid hyperplasia through the GS-AC-cAMP signaling system; the development of TN may increase as TSH levels increase. Additional studies have reported higher rates of TPOAb alone, TgAb alone, or at least one antibody positivity in both the low TSH and high TSH values groups than in euthyroid individuals (9). As demonstrated in our study, abnormal TSH levels were associated with an increased risk of GR and antibody positivity in adults. As the primary hormone regulating thyroid function, TSH is the most important indicator for the laboratory diagnosis of thyroid diseases. Therefore, it would also be particularly interesting to explore the future risk of thyroid disease in subjects with currently normal TSH levels. This study focused on the relationship between normal TSH levels and common thyroid diseases.
In this study, the prevalence of TN was 26.1% in women and 22.4% in men, consistent with a report by Chen et al. (10), in which the prevalence rate of TN in Hangzhou in 2013 was 24.1% for men and 34.7% for women (11, 12). In addition, it is well known that women are more likely than men to develop TN, which is consistent with our findings. TSH significantly regulates the growth and differentiation of thyroid cells and may play a direct role in nodule formation (13). Currently, numerous studies have examined the relationship between thyroid function and TN, yet a definitive conclusion remains elusive. For instance, one study on the elderly concluded that TN in this age group had no correlation with laboratory thyroid function indicators (14). Polyps et al. pointed out that within the normal range, an increase in TSH levels is associated with an elevated risk of malignant tumors in TN (15). Wu et al. reported the same conclusion (16). In contrast, some studies have reported that TSH levels were negatively correlated with TN (3, 17). In this study, TSH levels and the prevalence of TN were analyzed in individuals with normal thyroid function, which revealed that TN prevalence was significantly higher in the TSH-Q1 group (0.27–1.41 IU/mL) than in other groups. This observation was consistent with the findings of Wu et al. (18), which found a correlation between the prevalence of TN and declining TSH levels, even after accounting for factors such as age, sex, smoking, alcohol consumption, and medication use. Moreover, we discovered that a TSH level of 0.27–1.41 IU/mL (low TSH level) was a risk factor for TN. A study on TN in Cyprus women showed that the median serum TSH level was lower in women with nodules than in those without nodules (1.4 mIU/L and 1.7 mIU/L, respectively), and the median serum TSH remained within the normal range (17). In addition, our study indicated that the prevalence of TN increased significantly only in the women’s group among those with low TSH levels, which is consistent with the findings of a Beijing adult study, indicating that women are independent risk factors for TN (19). Estrogen may play a role in the formation of TN, as it can influence thyroid tissue and stimulate the production of TSH. Estrogen receptors are expressed in both normal and tumor thyroid tissue, suggesting that estrogen may contribute to thyroid cell growth and nodule formation (19). We made a stratified analysis of men and women, with age of 50 as the cutoff point for stratification. Similar to the above results, the risk of TN only increased in women (Supplementary Tables 1, 2). In addition, we found that in populations with low TSH levels, the risk of TN was notably higher in adults aged >60 years than in those aged <60 years. However, regarding the prevalence of TN, there was no significant interaction between age and low TSH levels. This indicates that TSH is valuable in predicting the nature of TN, but further investigation is needed. Furthermore, we found that Tvol decreased with increasing TSH levels in both men and women, even within the normal TSH range. While some studies have demonstrated a clear correlation between TSH and Tvol (20, 21), others have not observed this correlation (22). In a cross-sectional and longitudinal analysis of obese children, GR was not associated with thyroid function parameters (23). In contrast, our study revealed that in addition to the negative correlation between TSH and Tvol, the risk of GR increased when TSH levels within the normal range were <1.41 IU/mL, with a higher risk in men than in women and in the young than in the elderly. A Lithuanian study reported a negative association between Tvol and TSH, regardless of the presence of TN, with patients with GR having lower TSH levels than those without GR (24). Other studies have also found a negative association between TSH levels and GR, with a reduced risk of GR at higher TSH levels (25, 26). These findings are consistent with the findings of our study. Available data suggest that women are more prone to developing GR than men, especially in iodine-deficient areas (27, 28). This difference may be attributed to the hormonal status of women, wherein estrogen downregulates the sodium/iodine symporter (29). Some authors have reported that sex-related differences become apparent after puberty, which indicated that sex hormones may play a role in Tvol (30). After stratified analysis of men and women with age of 50 as the cutoff point for stratification, it was found that the risk of GR was obviously increased when TSH was at a low level (0.27-1.41 IU/mL) for women over 50 years old (Supplementary Tables 1, 2). Advanced age has been identified as an independent risk factor for GR (31), with one study on factors related to the prevalence of GR in Turkish populations revealing that the prevalence of GR increased with age and reached its peak (38.4%) in those aged >70 years (25). A Danish study showed that the prevalence of GR increased with age but plateaued at 40–45 years old (29).
The presence of thyroid antibodies may serve as an early indicator of thyroid disease, particularly autoimmune thyroid disease (4). TPOAb and/or TgAb have been shown to significantly increase with increasing TSH levels and have been associated with an elevated risk of hypothyroidism (5, 6). Another study found that there was a positive correlation between TPOAb levels and TSH, suggesting that TPOAb and TSH levels may predict hypothyroidism risk, even when TSH levels fall within the normal laboratory reference range (32). In this study, TSH > 2.55 IU/mL within the normal range were associated with an increased risk of antibody positivity. Earlier studies by Jensen et al. (33) and Hollowell et al. (34) linked the presence of TPOAbs not only to a higher frequency of elevated TSH levels outside the normal range but also to a trend toward higher TSH levels within the normal range. This indicates that the presence of TPOAbs necessitate a compensatory increase in TSH levels to maintain normal thyroid function (32). Our study further revealed that while the incidence of thyroid antibody positivity was higher in women than in men, logistic regression analysis showed that men with high TSH levels were at a greater risk of antibody positivity than women. Furthermore, our results demonstrated that high TSH levels in young adults increased the risk of antibody positivity. A Dutch study reported that the risk of positive TPOAb levels decreased with increasing age (35). Several studies have reported that various subsets of T and B lymphocytes decrease with age (36–39), which may lead to a decrease in thyroid lymphocyte infiltration, subsequently lowering the production of TPOAb in older individuals.
We conducted an analysis based on a large-scale epidemiological survey to analyze the association of abnormal and normal TSH levels with common thyroid diseases. This study shows that abnormal TSH levels may increase the risk of GR and thyroid antibody positivity. In addition, our research results show that the normal TSH levels can also predict the future risk of thyroid diseases. This also suggests that adults with TSH at higher or lower levels within the normal range, especially those at upper or lower limits of the normal range, should be reviewed regularly. Nevertheless, several limitations need to be acknowledged. First, not all subjects underwent laboratory testing for FT4 and FT3; only adults with abnormal TSH levels were tested. Second, comorbidities and concomitant medications are common among the elderly. Certain drugs, such as estrogen, aspirin, non-steroidal anti-inflammatory drugs, and glucocorticoids, can impact thyroid hormones. Finally, this study was cross-sectional and did not account for time. Therefore, we cannot infer a causal relationship between TSH and TN, GR, and antibody positivity in the euthyroid population. More longitudinal cohort studies are needed to further investigate these associations.
5 Conclusion
In summary, our study suggests that lower TSH levels (0.27-1.41 IU/mL) were associated with an increased risk of TN and GR, while higher TSH levels (2.55-4.20 IU/mL) were associated with thyroid antibody positivity. A larger sample size cohort study is needed to investigate the relationship between normal TSH levels and thyroid diseases. In addition, the research also suggests that adults whose TSH levels at upper or lower limits of the normal range should be reviewed regularly.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the China Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FJ, LT and WZ designed the research. SL, WG and QM conducted the research and collected the data. MZ and HW performed the laboratory analysis. SL and WG performed the statistical analysis. SL and WG wrote the paper. All authors have read and approved the final manuscript.
Funding
This study was supported by the Tianjin Education Committee Social Science Major Project (Grant no. 2017JWZD35), Tianjin Natural Science Foundation (Grant no. 20JCZDJC00080) and Danone Nutrition Research and Education Fund (Grant no. DIC2020-05).
Acknowledgments
We thank all the subjects and the researchers for their cooperation and participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1204552/full#supplementary-material
Abbreviations
BMI, body mass index; CV, coefficient of variation; FT4, free thyroxine; FT3, serum free triiodothyronine; GR, goiter; IQR, interquartile range; OR, odds ratio; TgAb, thyroglobulin autoantibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid-stimulating hormone; TN, thyroid nodules; Tvol, thyroid volume; UIC, urinary iodine concentration.
References
1. Sohn SY, Kim HJ, Jang HW, Kim SW, Chung JH. Lack of association between high serum thyroid-stimulating hormone level and risk of papillary thyroid microcarcinomas. Head Neck (2014) 36(1):43–6. doi: 10.1002/hed.23252
2. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedüs L, et al. American association of clinical endocrinologists, american college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocrine practice: Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists (2016) 22(5):622–39. doi: 10.4158/ep161208.GL
3. Shin J, Kim MH, Yoon KH, Kang MI, Cha BY, Lim DJ. Relationship between metabolic syndrome and thyroid nodules in healthy koreans. Korean J Internal Med (2016) 31(1):98–105. doi: 10.3904/kjim.2016.31.1.98
4. Ren R, Ma Y, Deng F, Li T, Wang H, Wei J, et al. Association between serum tsh levels and metabolic components in euthyroid subjects: A nationwide population-based study. Diabetes Metab syndrome obesity: Targets Ther (2019) 12:1563–9. doi: 10.2147/DMSO.S202769
5. Yan YR, Liu Y, Huang H, Lv QG, Gao XL, Jiang J, et al. Iodine nutrition and thyroid diseases in chengdu, China: an epidemiological study. QJM: monthly J Assoc Physicians (2015) 108(5):379–85. doi: 10.1093/qjmed/hcu216
6. Zhai X, Zhang L, Chen L, Lian X, Liu C, Shi B, et al. An age-specific serum thyrotropin reference range for the diagnosis of thyroid diseases in older adults: A cross-sectional survey in China. Thyroid: Off J Am Thyroid Assoc (2018) 28(12):1571–9. doi: 10.1089/thy.2017.0715
7. Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F, et al. The spectrum of thyroid disorders in an iodine-deficient community: the pescopagano survey. J Clin Endocrinol Metab (1999) 84(2):561–6. doi: 10.1210/jcem.84.2.5508
8. Santos JAR, Christoforou A, Trieu K, McKenzie BL, Downs S, Billot L, et al. Iodine fortification of foods and condiments, other than salt, for preventing iodine deficiency disorders. Cochrane Database systematic Rev (2019) 2(2):CD010734. doi: 10.1002/14651858.CD010734.pub2
9. O'Leary PC, Feddema PH, Michelangeli VP, Leedman PJ, Chew GT, Knuiman M, et al. Investigations of thyroid hormones and antibodies based on a community health survey: the busselton thyroid study. Clin Endocrinol (2006) 64(1):97–104. doi: 10.1111/j.1365-2265.2005.02424.x
10. Chen Z, Xu W, Huang Y, Jin X, Deng J, Zhu S, et al. Associations of noniodized salt and thyroid nodule among the chinese population: A large cross-sectional study. Am J Clin Nutr (2013) 98(3):684–92. doi: 10.3945/ajcn.112.054353
11. Blanc E, Ponce C, Brodschi D, Nepote A, Barreto A, Schnitman M, et al. Association between worse metabolic control and increased thyroid volume and nodular disease in elderly adults with metabolic syndrome. Metab syndrome related Disord (2015) 13(5):221–6. doi: 10.1089/met.2014.0158
12. Knudsen N, Laurberg P, Perrild H, Bülow I, Ovesen L, Jørgensen T. Risk factors for goiter and thyroid nodules. Thyroid: Off J Am Thyroid Assoc (2002) 12(10):879–88. doi: 10.1089/105072502761016502
13. Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (Tsh) receptor: interaction with tsh and autoantibodies. Endocrine Rev (1998) 19(6):673–716. doi: 10.1210/edrv.19.6.0352
14. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clinics North America (2007) 36(3):707–35. doi: 10.1016/j.ecl.2007.04.009
15. Polyzos SA, Kita M, Efstathiadou Z, Poulakos P, Slavakis A, Sofianou D, et al. Serum thyrotropin concentration as a biochemical predictor of thyroid Malignancy in patients presenting with thyroid nodules. J Cancer Res Clin Oncol (2008) 134(9):953–60. doi: 10.1007/s00432-008-0373-7
16. Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z, et al. Coexistence of thyroglobulin antibodies and thyroid peroxidase antibodies correlates with elevated thyroid-stimulating hormone level and advanced tumor stage of papillary thyroid cancer. Endocrine (2014) 46(3):554–60. doi: 10.1007/s12020-013-0121-x
17. Gaengler S, Andrianou XD, Piciu A, Charisiadis P, Zira C, Aristidou K, et al. Iodine status and thyroid nodules in females: A comparison of Cyprus and Romania. Public Health (2017) 143:37–43. doi: 10.1016/j.puhe.2016.10.027
18. Wu Z, Shen M. Advances in diagnosis and treatment for nodular goiter. Chin J Bases Clinics Gen Surg (2004) 11(06):483–5. doi: 10.3969/j.issn.1007-9424.2004.06.005
19. Jiang H, Tian Y, Yan W, Kong Y, Wang H, Wang A, et al. The prevalence of thyroid nodules and an analysis of related lifestyle factors in beijing communities. Int J Environ Res Public Health (2016) 13(4):442. doi: 10.3390/ijerph13040442
20. Duran AO, Anil C, Gursoy A, Nar A, Inanc M, Bozkurt O, et al. Thyroid volume in patients with glucose metabolism disorders. Arquivos brasileiros endocrinologia e metabologia (2014) 58(8):824–7. doi: 10.1590/0004-2730000003418
21. Ertek S, Cicero AF, Caglar O, Erdogan G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones (Athens Greece) (2010) 9(3):263–8. doi: 10.14310/horm.2002.1276
22. Sousa PA, Vaisman M, Carneiro JR, Guimarães L, Freitas H, Pinheiro MF, et al. Prevalence of goiter and thyroid nodular disease in patients with class iii obesity. Arquivos brasileiros endocrinologia e metabologia (2013) 57(2):120–5. doi: 10.1590/s0004-27302013000200004
23. Lass N, Barth A, Reinehr T. Thyroid volume and thyroid function parameters are independently associated with weight status in overweight children. Hormone Res paediatrics (2020) 93(5):279–86. doi: 10.1159/000509786
24. Dauksiene D, Petkeviciene J, Klumbiene J, Verkauskiene R, Vainikonyte-Kristapone J, Seibokaite A, et al. Factors associated with the prevalence of thyroid nodules and goiter in middle-aged euthyroid subjects. Int J Endocrinol (2017) 2017:8401518. doi: 10.1155/2017/8401518
25. Kocak M, Erem C, Deger O, Topbas M, Ersoz HO, Can E. Current prevalence of goiter determined by ultrasonography and associated risk factors in a formerly iodine-deficient area of Turkey. Endocrine (2014) 47(1):290–8. doi: 10.1007/s12020-013-0153-2
26. Derumeaux H, Valeix P, Castetbon K, Bensimon M, Boutron-Ruault MC, Arnaud J, et al. Association of selenium with thyroid volume and echostructure in 35- to 60-year-old french adults. Eur J Endocrinol (2003) 148(3):309–15. doi: 10.1530/eje.0.1480309
27. Roti E, Gardini E, D'Amato L, Salvi M, Robuschi G, Manfredi A, et al. Goiter size and thyroid function in an endemic goiter area in northern Italy. J Clin Endocrinol Metab (1986) 63(3):558–63. doi: 10.1210/jcem-63-3-558
28. Kimiagar M, Azizi F, Navai L, Yassai M, Nafarabadi T. Survey of iodine deficiency in a rural area near tehran: association of food intake and endemic goitre. Eur J Clin Nutr (1990) 44(1):17–22.
29. Knudsen N, Bülow I, Jorgensen T, Laurberg P, Ovesen L, Perrild H. Goitre prevalence and thyroid abnormalities at ultrasonography: A comparative epidemiological study in two regions with slightly different iodine status. Clin Endocrinol (2000) 53(4):479–85. doi: 10.1046/j.1365-2265.2000.01121.x
30. Fleury Y, Van Melle G, Woringer V, Gaillard RC, Portmann L. Sex-dependent variations and timing of thyroid growth during puberty. J Clin Endocrinol Metab (2001) 86(2):750–4. doi: 10.1210/jcem.86.2.7209
31. Völzke H, Schwahn C, Kohlmann T, Kramer A, Robinson DM, John U, et al. Risk factors for goiter in a previously iodine-deficient region. Exp Clin Endocrinol diabetes: Off journal German Soc Endocrinol [and] German Diabetes Assoc (2005) 113(9):507–15. doi: 10.1055/s-2005-865741
32. Roos A, Links TP, de Jong-van den Berg LT, Gans RO, Wolffenbuttel BH, Bakker SJ. Thyroid peroxidase antibodies, levels of thyroid stimulating hormone and development of hypothyroidism in euthyroid subjects. Eur J Internal Med (2010) 21(6):555–9. doi: 10.1016/j.ejim.2010.09.001
33. Jensen E, Hyltoft Petersen P, Blaabjerg O, Hansen PS, Brix TH, Kyvik KO, et al. Establishment of a serum thyroid stimulating hormone (Tsh) reference interval in healthy adults. Importance Environ Factors Including Thyroid Antibodies. Clin Chem Lab Med (2004) 42(7):824–32. doi: 10.1515/cclm.2004.136
34. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum tsh, T4, and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (Nhanes iii). J Clin Endocrinol Metab (2002) 87(2):489–99. doi: 10.1210/jcem.87.2.8182
35. Khan SR, Peeters RP, van Hagen PM, Dalm V, Chaker L. Determinants and clinical implications of thyroid peroxidase antibodies in middle-aged and elderly individuals: the rotterdam study. Thyroid: Off J Am Thyroid Assoc (2022) 32(1):78–89. doi: 10.1089/thy.2021.0403
36. Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients (2018) 10(10):1531. doi: 10.3390/nu10101531
37. Bulati M, Caruso C, Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by "Inflamm-ageing". Ageing Res Rev (2017) 36:125–36. doi: 10.1016/j.arr.2017.04.001
38. Apoil PA, Puissant-Lubrano B, Congy-Jolivet N, Peres M, Tkaczuk J, Roubinet F, et al. Influence of age, sex and hcmv-serostatus on blood lymphocyte subpopulations in healthy adults. Cell Immunol (2017) 314:42–53. doi: 10.1016/j.cellimm.2017.02.001
Keywords: thyroid-stimulating hormone, thyroid dysfunction, thyroid nodules, goiter, thyroid volume, thyroid antibody positivity
Citation: Li S, Guo W, Meng Q, Zhu M, Wei H, Ji F, Tan L and Zhang W (2023) The association between thyroid-stimulating hormone and thyroid nodules, goiter and thyroid antibody positivity. Front. Endocrinol. 14:1204552. doi: 10.3389/fendo.2023.1204552
Received: 19 June 2023; Accepted: 14 September 2023;
Published: 02 October 2023.
Edited by:
Noriyuki Koibuchi, Gunma University, JapanReviewed by:
Ana Valea, Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca, RomaniaHoonsung Choi, Chung-Ang University, Republic of Korea
Copyright © 2023 Li, Guo, Meng, Zhu, Wei, Ji, Tan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengying Ji, amZ5LTVAMTI2LmNvbQ==; Long Tan, dGFubG9uZ0B0bXUuZWR1LmNu; Wanqi Zhang, d3F6aGFuZ0B0bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Shaohan Li1†
Shaohan Li1† Wenxing Guo
Wenxing Guo Qi Meng
Qi Meng Wanqi Zhang
Wanqi Zhang