- 1Department of Nuclear Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, China
- 2Department of Ultrasound Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, China
Background: Sorafenib included in Chinese medical insurance is the earliest targeted drug for radioactive iodine refractory differentiated thyroid cancer (RR-DTC). This study is to further demonstrate the clinical efficacy and safety of sorafenib used in Zhujiang Hospital of Southern Medical University.
Methods: RR-DTC patients treated at our Department of Nuclear Medicine in Zhujiang Hospital of Southern Medical University (October 2017–May 2020) were retrospectively analyzed. Treatment effects, progression-free survival (PFS), and adverse effects (AEs) during medication were evaluated.
Results: Of the 31 patients included, 26 patients were evaluated for efficacy with a median follow-up time of 17.5 months (4.0–51.0 months). The disease control rate (DCR) was 57.7% (n = 15) and the objective response rate (ORR) was 26.9% (n = 7). Most patients with disease control had thyroglobulin decreases of more than 60% (p = 0.004), ORRs were favorable in patients with lung metastasis and lung-only metastasis (p = 0.010 and 0.001, respectively). The PFS of the 26 patients analyzed was 16.5 months (95%CI: 14.41 –23.90 months). In the subgroup analysis, female, patients with lung-only metastasis, hand-foot skin syndrome (HFS), and thyroglobulin response ≥ 60% observed longer PFS (p = 0.038, 0.045, 0.035, and 0.000, respectively), while patients with bone metastasis had lower PFS (p = 0.035). The most common toxicity profile was HFS (93.5%), followed by diarrhea (83.9%), alopecia (74.2%). All the side effects were mainly grade 1–2. Grade 3–4 adverse reactions were more common in diarrhea and HFS.
Conclusions: Sorafenib has promising efficacy in RR-DTC, especially in patients with lung metastasis and lung-only metastasis. The AEs of sorafenib were generally mild, and the main AE was HFS.
Introduction
Thyroid cancer is a common endocrine tumor, ranking seventh among all cancer types reported in China in 2022, with the fastest-growing incidence (1, 2). Differentiated thyroid cancer (DTC) is accounted for 95% of all histological types of thyroid cancer, including papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and Hürthle cell cancer (3). In general, thyroid cancer presents an excellent prognosis with standard management including surgery, radioactive iodine (RAI) treatment, and thyroid-stimulating hormone suppression therapy (4–6). However, about 20% and 10% of DTC patients are at risk of local recurrence and distant metastases respectively, and two-thirds of them lose the ability of RAI uptake initially or gradually, which was known as radioactive iodine refractory differentiated thyroid cancer (RR-DTC) (7). As limited treatment options result in poor prognosis, the 10-year survival rate is less than 10% in RR-DTC patients (8, 9).
Sorafenib, approved by the U.S. Food and Drug Administration and the State Food and Drug Administration of China for the treatment of RR-DTC, is an oral tyrosine kinase inhibitor (TKI) inhibiting vascular endothelial growth factor receptors (VEGFRs), RAF, RET, and platelet-derived growth factor receptor beta signaling (10–12). Compared with the placebo, sorafenib improved the progression-free survival (PFS) of RR-DTC patients showing efficacy and safety in DECISION trial (13). Real-world studies not only can reflect the effects of drugs and provide more important health condition information about patients in their daily life but also provide a more scientific basis guiding physicians’ clinical decision. Further demonstration of the clinical efficacy and safety of sorafenib used in different populations, centers, and age groups is essential.
Although many real-world studies on the treatment of RR-DTC with sorafenib have been reported (14–19), there are a few studies on the efficacy and adverse effects (AEs) of sorafenib at a standard dose of 400mg twice daily in Zhujiang Hospital of Southern Medical University. Our research not only analyzed the efficacy and AEs of sorafenib at a standard dose of 400mg twice daily for the treatment of RR-DTC patients at our Department of Nuclear Medicine from October 2017 to May 2020 but also summarized the real-world studies on sorafenib reported to date in the Discussion section.
Material and methods
Patients diagnosed with RR-DTC at our Department of Nuclear Medicine in Zhujiang Hospital of Southern Medical University from October 2017 to May 2020 were retrospectively analyzed. All patients were given sorafenib at the standard dose of 400mg, twice a day, and then the dose was adjusted according to the patient’s tolerance to side effects. Patients who received other treatments while taking sorafenib or were interrupted in follow-up were excluded. RR-DTC was defined as follows (if one of the followings is satisfied): 1) at least one target lesion without RAI uptake (never or ever); 2) progression of a target lesion despite significant RAI concentration; 3) cumulative RAI dose ≥ 22.3 GBq. Imaging examinations were performed every 3 to 6 months to assess efficacy, AEs were assessed monthly. The Response Evaluation Criteria in Solid Tumors version 1.1 was used to evaluate treatment efficacy (20): disease control rate (DCR) referred to complete response (CR) plus partial response (PR) plus stable disease (SD), objective response rate (ORR) was CR plus PR, and progression-free survival (PFS) was defined as the time from initiation of sorafenib to progression or death. Common Terminology Criteria for Adverse Events Version 5.0 was used to evaluate AEs and guide patients to adjust their medication in response to side effects: if patients had grade 1–2 AEs, the dose could be appropriately adjusted according to the patient’s ability to tolerate the AEs, if the patients had grade 3 or above AEs, the medication would be suspended until the AEs returned to grade 1–2. (Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 15 May 2020).
Baseline characteristics of patients were described: quantitative data were reported as mean or median, and categorical variables as numbers with percentages. Waterfall plots were used to present the best tumor response. The PFS was calculated using the Kaplan–Meier method, and a log-rank test was performed to compare the difference in the PFS of variables. Cox proportional hazard model was used to estimate prognostic factors associated with PFS. All data were analyzed using SPSS version 26.0, and a p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Table 1 summarizes the clinicopathological profiles of 31 retrospective patients with a median age of 58 years (18–79 years) when starting sorafenib, most of whom had PTC (61.3%). Twenty-four (77.4%) patients had distant metastases, half of whom had bone metastases and 16 had lung metastases. Of the 31 patients, five were excluded for the following reasons: three had only one radiological examination, one was absent of any measurable target lesion, and one underwent surgery during sorafenib therapy. The other 26 patients were eligible for radiological response assessment with a median follow-up of 17.5 months (4.0–51.0 months). AEs during sorafenib administration were recorded in 31 patients with a median follow-up of 18 months (2.0–51.0 months).
Efficacy of sorafenib
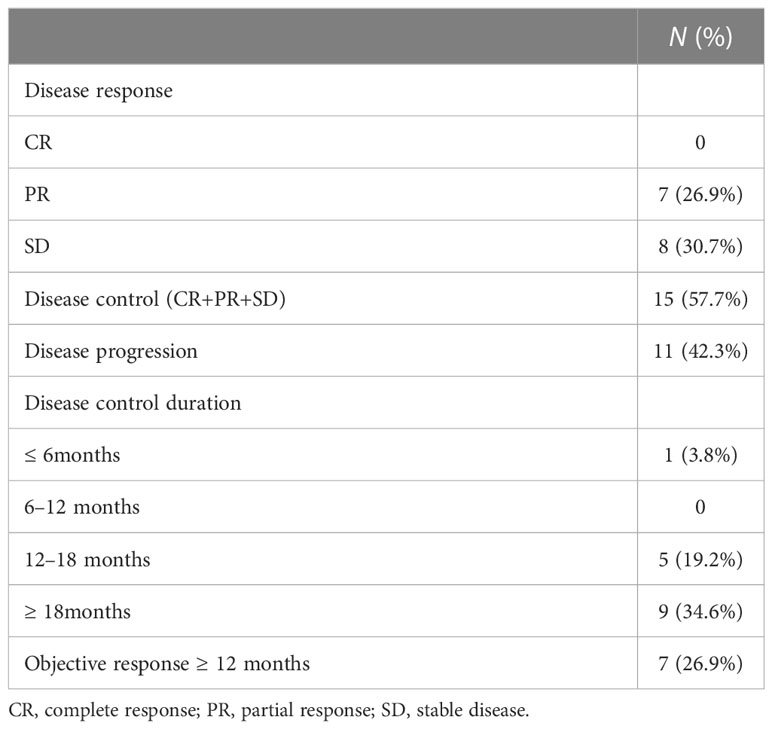
As presented in Table 2, no one achieved CR, 26.9% (n = 7) of patients achieved PR and all exceeded 12 months, and 30.7% (n = 8) had SD. The DCR was 57.7% (n = 15), with 14 cases controlled for ≥ 12 months, nine cases for ≥ 18 months, and three cases for ≥ 24 months. Eleven (42.3%) had documented disease progression at the end of follow-up, three of whom recorded new metastases rather than enlargement of target lesions. Figure 1 demonstrates the best changes in target lesions.
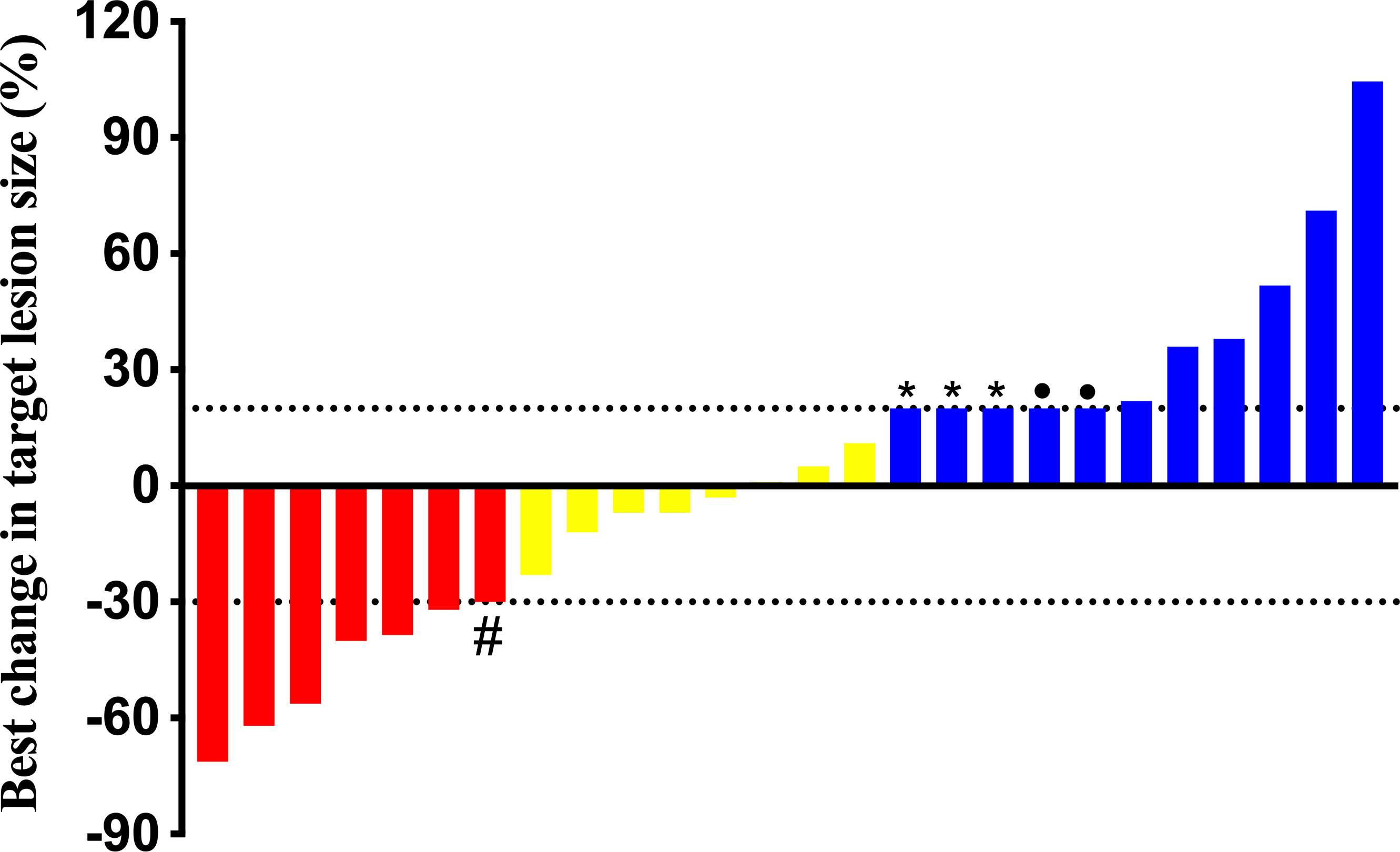
Figure 1 Best change in target lesions of 26 patients. #: This patient’s chest computed tomography scan showed micro-nodules scattered throughout the lungs which couldn’t be determined by the Response Evaluation Criteria in Solid Tumors version 1.1, but we found a significant reduction in his lung micro-nodules and a 91.4% reduction in thyroglobulin after follow-up, thus we considered a partial response. *: All three patients were found with new lesions. •:The two patients were died to pulmonary infection and bone related events, respectively.
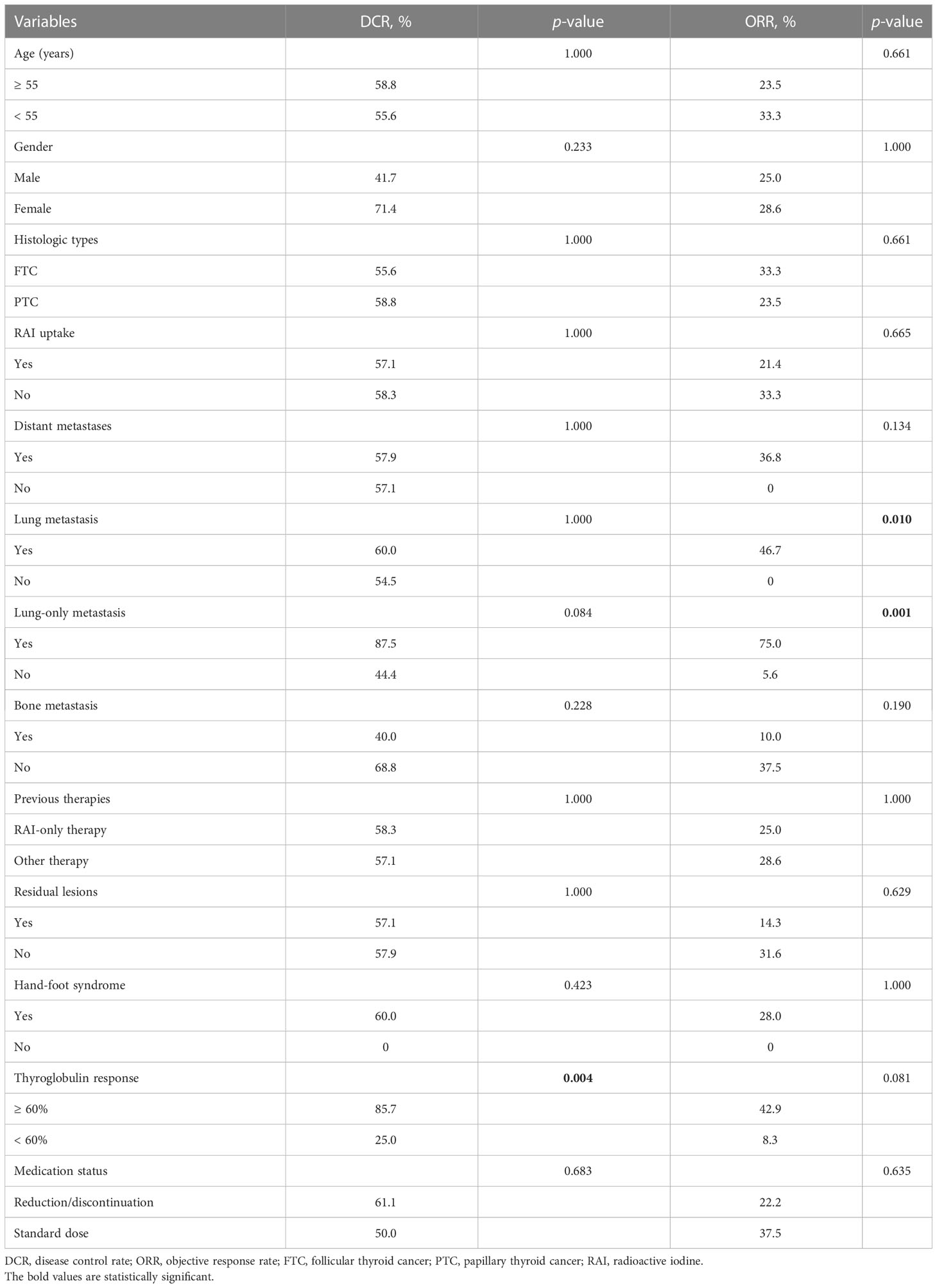
As illustrated in Table 3, there were no significant differences in DCRs and ORRs according to age, gender, histologic types, RAI uptake, distant metastases, bone metastasis, previous treatment modality, residual lesions, and hand-foot skin syndrome (HFS). Notably, DCRs were superior in patients with a reduction of ≥ 60% in thyroglobulin (Tg) (p = 0.004). ORRs were favorable in patients with lung metastasis and lung-only metastasis (p = 0.010 and 0.001, respectively).
The median PFS of all 26 evaluable patients was 16.5 months (95%CI: 14.41–23.90 months) (Figure 2). There were no significant differences in PFS concerning age, histologic type, RAI uptake, distant metastases, lung metastasis, previous treatment modality, and residual lesions as indicated in Table 4. PFS was better in female and patients with lung-only metastasis, HFS, and Tg response ≥ 60% (p = 0.038, 0.045, 0.007, and 0.000, respectively); however, it was worse in patients with bone metastasis (p = 0.035). Nineteen patients with distant metastasis were divided into groups with and without bone metastasis, and survival analysis showed a worse PFS with the former (p = 0.062) (Table 4; Figure 3).
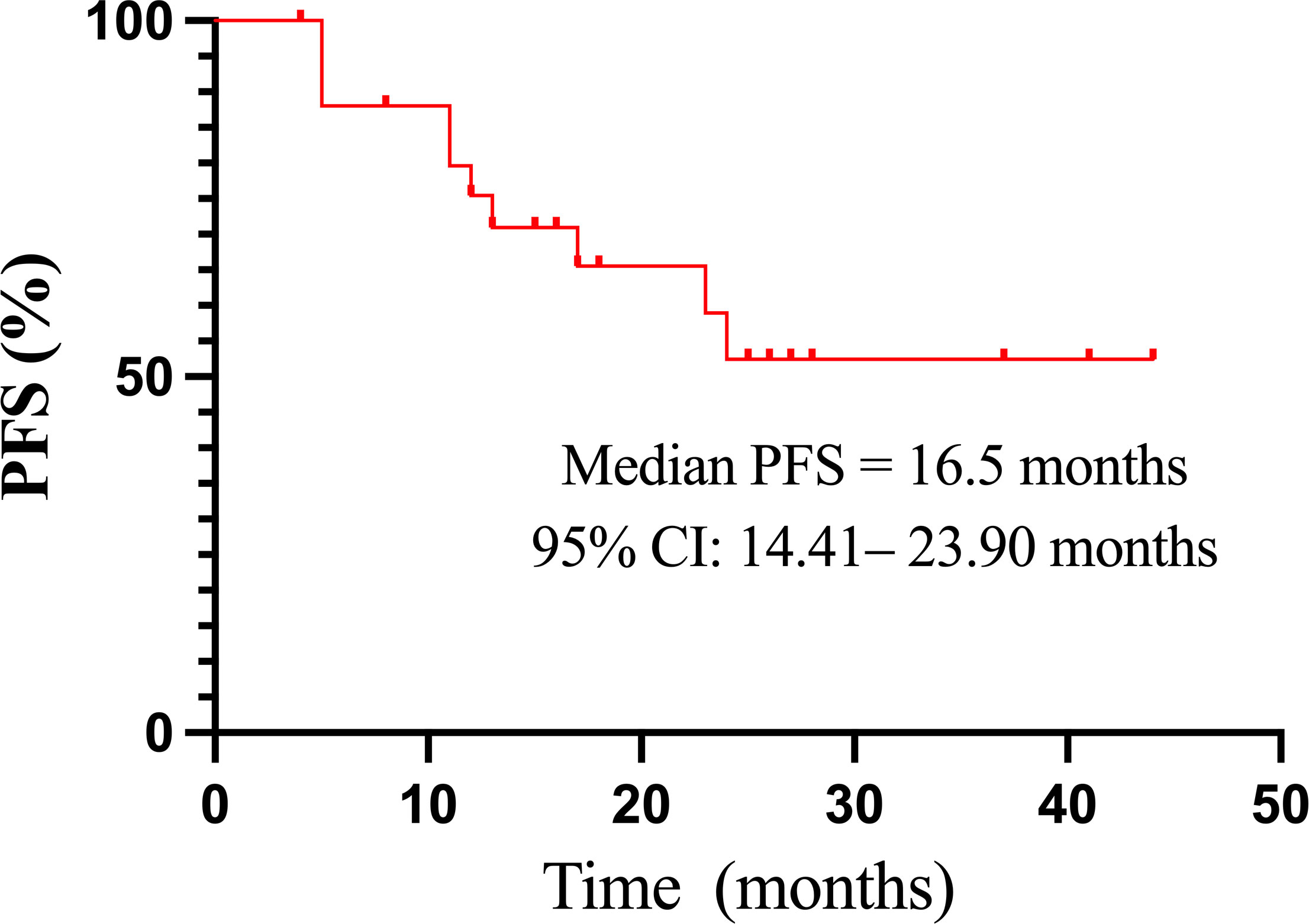
Figure 2 The Kaplan–Meier curve of PFS in 26 patients treated with sorafenib. CI, confidence interval; PFS, progression-free survival.
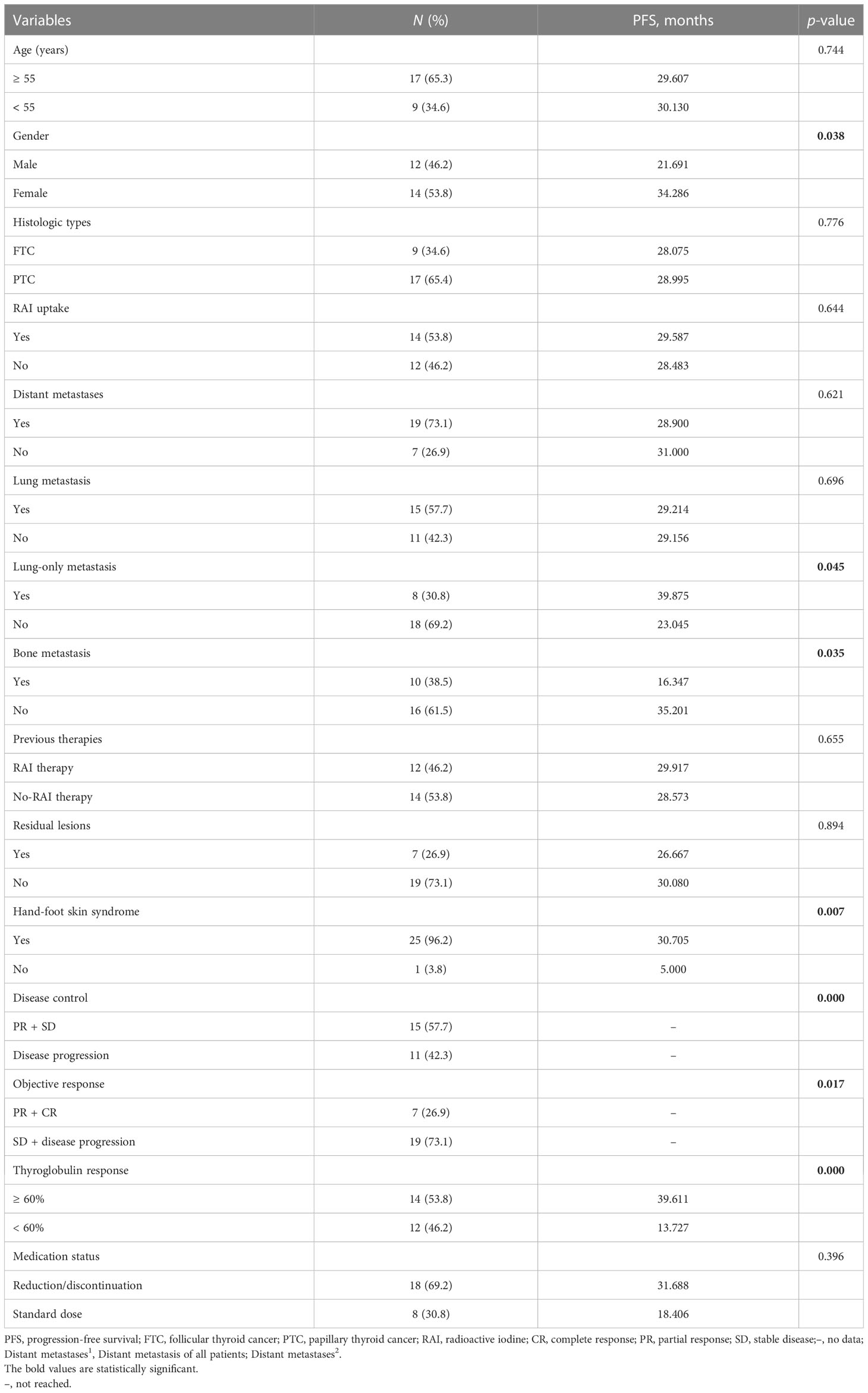
Table 4 Univariate analyses of potential prognostic factors associated with the PFS of patients treated with sorafenib.
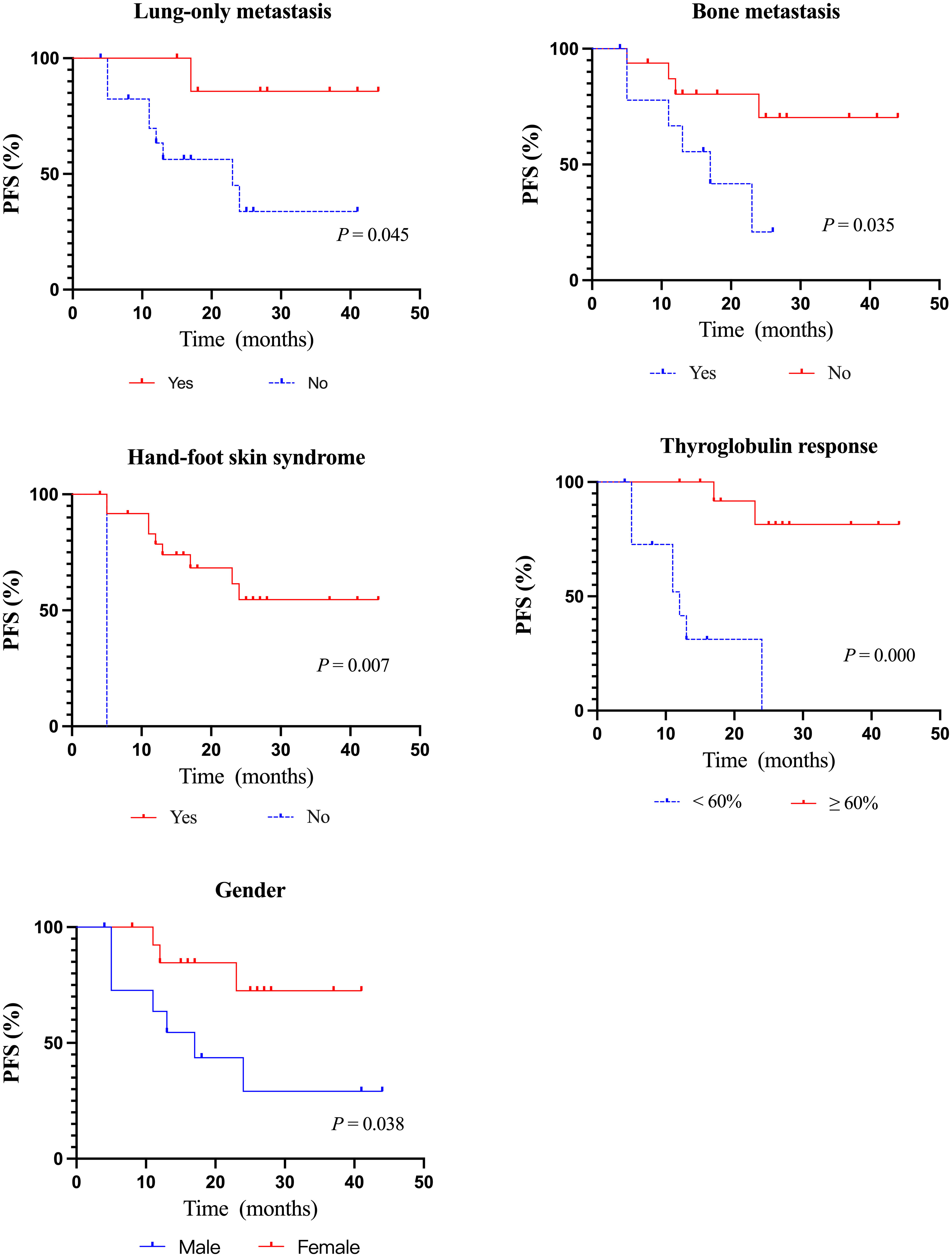
Figure 3 Kaplan–Meier curves of PFS according to clinicopathological features. PFS, progression-free survival.
The dose was adjusted according to the patient’s tolerance to side effects. Five patients discontinued the drug for AEs and resumed the drug after the AEs were alleviated, with the longest interval of 6 months. The others’ minimum maintenance dose was 200mg daily. There were no significant differences in DCR, ORR, and PFS between patients with dose reduction or withdrawal of sorafenib and patients with continuous treatment (Tables 3, 4).
Safety and AEs
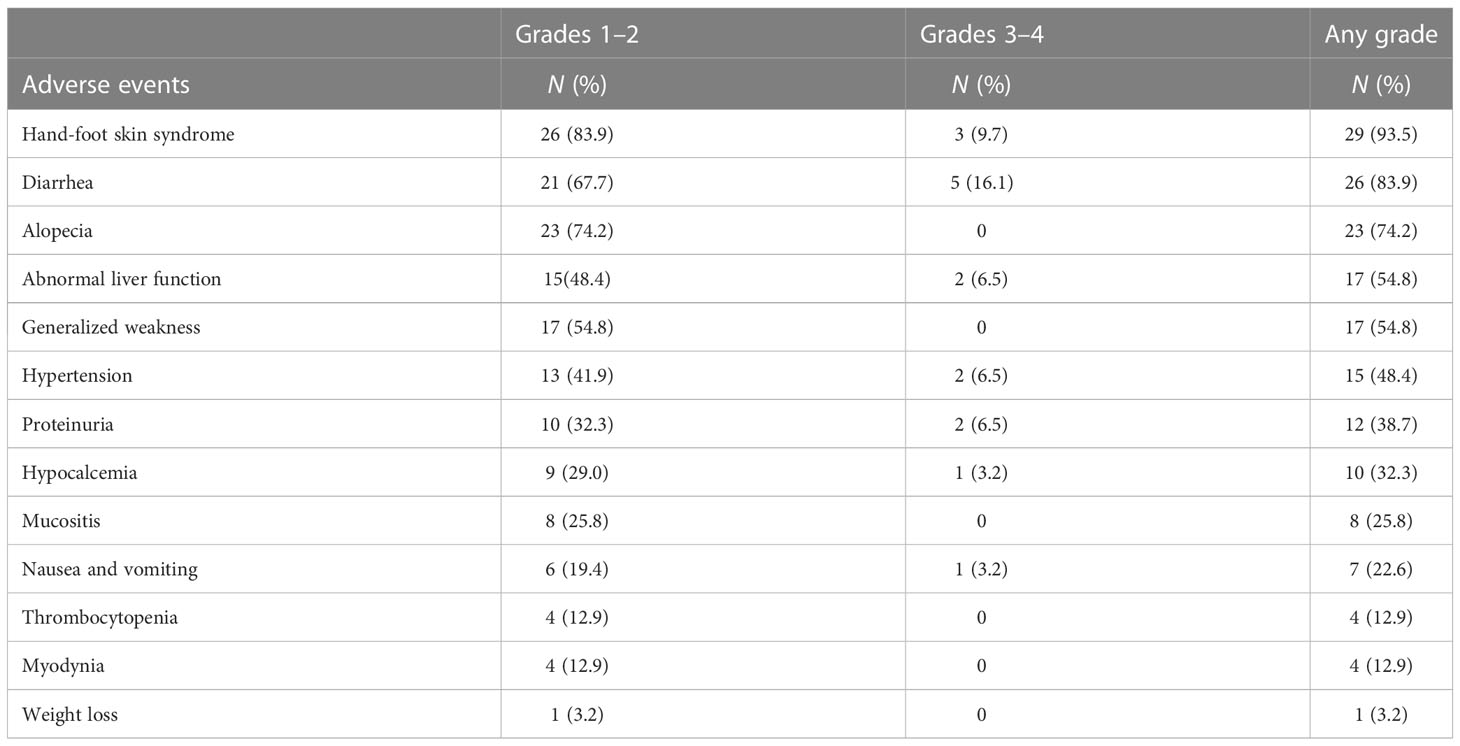
AEs were documented in all patients as revealed in Table 5. The most frequent AE of any grade was HFS (93.5%), followed by diarrhea (83.9%), alopecia (74.2%), and so on. These AEs were mainly grades 1–2. Similarly, diarrhea (16.1%) and HFS (9.7%) were the most prevalent among grades 3–4. Moreover, a few uncommon AEs such as weight loss (n = 1), myodynia (n = 4), and thrombocytopenia (n = 4) were reported. Most side effects were persistent, especially HFS, diarrhea, and alopecia, with the longest lasting 44 months. The median duration of HFS was 18 months (range: 0.5–44 months), the median duration of diarrhea was 16.5 months (range: 1–44 months), and the median duration of alopecia was 12.0 months (range: 2–44 months). Grade 3-4 AEs were common with HFS, diarrhea and abnormal liver function for a maximum duration of 38 months. Nineteen (61.3%) patients had drug reductions due to AEs (some patients had more than one AEs: HFS, n = 19; diarrhea, n = 5; abnormal liver function, n = 1; proteinuria, n = 1).
Typical cases
Patients 1, 2, and 3 were diagnosed with RR-DTC on 08/09/2018, 06/20/2018, and 08/30/2019, respectively, and started taking sorafenib using 400 mg twice daily, followed by regular follow-up. Only lung metastases were found in all of them, and all target lesions were lung metastases. As depicted in Figure 4, at the end of the follow-up, their chest computed tomography scans (12/21/2021, 03/01/2022, and 12/18/2019, respectively) showed that their target lesions significantly reduced by ≥ 30% or even disappeared compared with the baseline data, hence they were evaluated as PR.
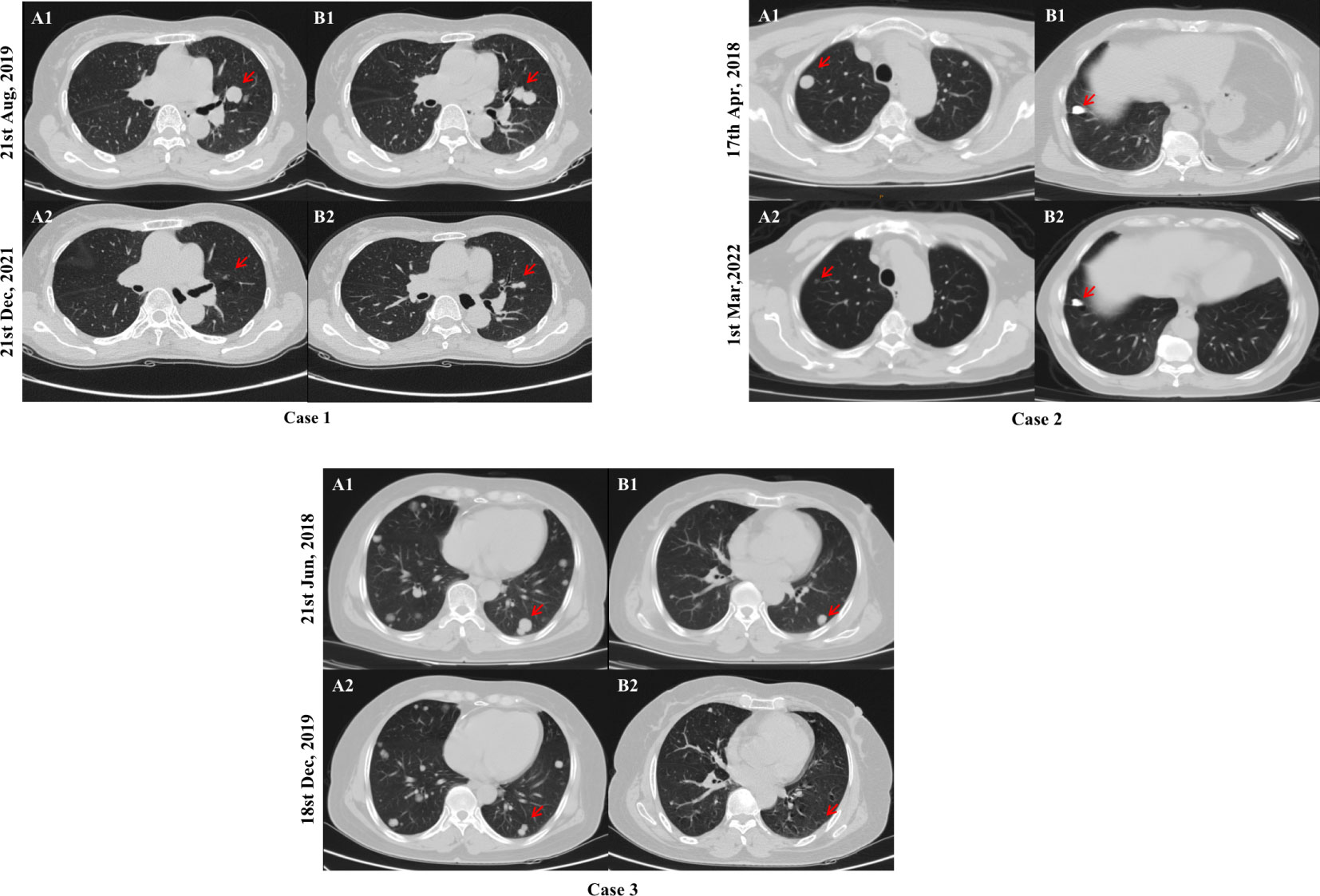
Figure 4 Comparison of chest computed tomography scans of typical cases. After 28, 46, and 17 months of sorafenib treatment, a significant reduction in the size of target lesions was observed in cases 1, 2, and 3, respectively. Chest computed tomography scans at baseline (A1 and B1) and the end of follow-up (A2 and B2) are shown above. The red arrows indicates the location of the target lesions at baseline and the last follow-up.
Discussion
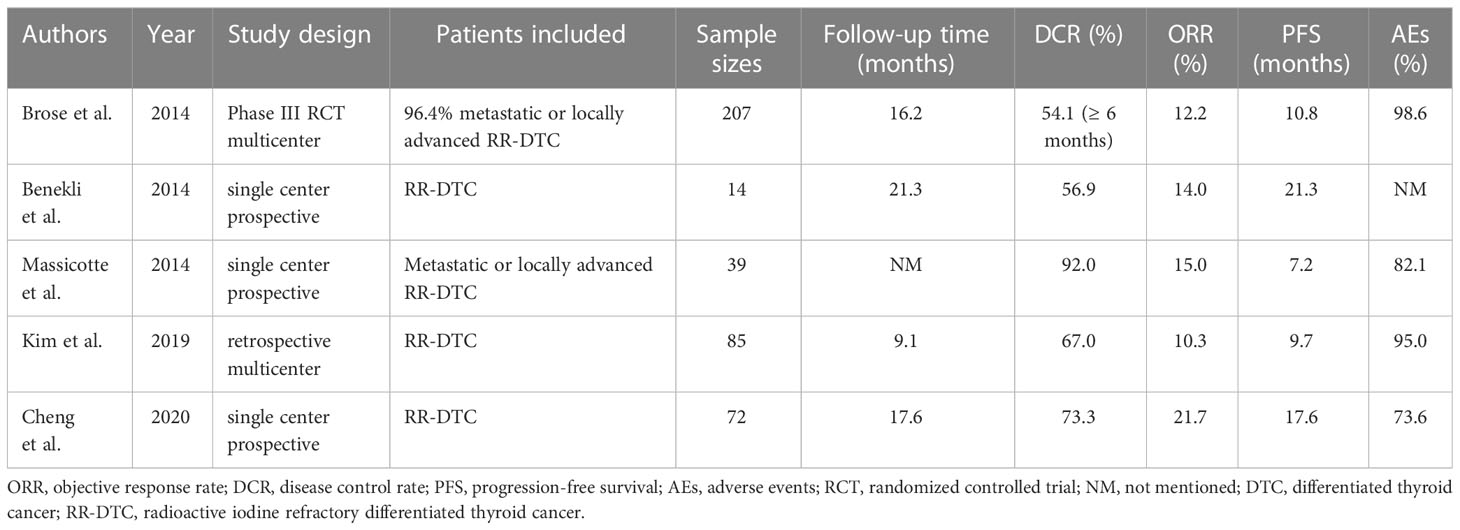
As shown in Table 6, we summarize the real-world studies reported so far and the DECISION trial (13, 14, 16, 18, 19). The DCR of our study was 57.7%, similar to most of the above studies. However, the ORR in our study was slightly improved to 26.9% compared with those reported by Benekli et al., Massicotte et al., Kim et al., and Cheng et al. (14.0%,15.0%, 10.3%, and 21.7%, respectively). Furthermore, the median PFS in our study was 16.5 months, longer than those of DECISION, Kim et al., and Massicotte et al. (10.8, 9.7, and 7.2 months, respectively) (13, 14, 18), which may be due to the different tumor burdens of patients included such as those with advanced disease or distant metastases (14, 16, 18). However, the median PFS of the studies by Cheng et al. and Benekli et al. (17.6 and 21.3 months, respectively) was longer than ours, and a longer follow-up was found in the study of Cheng et al. than ours (median follow-up: 25.1 months versus 17.5 months) possibly resulting in longer PFS. Overall, both the clinical trial and real-world studies have demonstrated considerable efficacy in treating RR-DTC with sorafenib.
Studies have shown that soft tissue metastases such as lymph nodes, pleura, and pulmonary lesions presented better outcomes (16, 18, 21). Similarly, our study discovered that Sorafenib is more effective in lung metastases, all patients with PR were lung metastasis and patients with lung-only metastasis generally had a better prognosis such as typical cases shown in Figure 4, while those with bone metastasis presented a worse prognosis (Table 4). Besides, in the subgroup analysis of the relationship between PFS and clinicopathological features, as shown in Table 4, PFS was significantly better in patients with lung-only metastases and significantly worse in patients with bone metastases, in the subgroup analysis of PFS between the presence and absence of bone metastasis groups, the bone metastasis group tended to have worse PFS. We believe that the reasons are as follows: VEGF stimulates endothelial cell proliferation and tumor angiogenesis, playing an important role in the development and progression of thyroid cancer, and its expression level correlates with advanced disease (22, 23). Sorafenib is a multitargeted TKI whose core mechanism is to block the activity of VEGFR-2 and VEGFR-3 to inhibit tumor angiogenesis and cell proliferation. VEGFR is mainly expressed in soft tissues such as heart, lung, kidney, and skeletal muscle (24). Therefore, we estimate that lower VEGFR signal transduction in bone may be responsible for the poor efficacy of bone metastases in line with the study of Cheng et al. (16). Beyond that, pathophysiological processes in the bone microenvironment promote the rapid progression of bone lesions, and complex bone composition and structure make it difficult to be targeted, which leads to the rapid development of bone metastases (25–27). Therefore, patients with lung metastases respond relatively well to sorafenib treatment. For bone metastasis, anti-angiogenic therapy alone may be less effective, and a multimodal approach including local and systemic therapies should be considered. However, in this cohort, patients with bone metastasis just tended to have worse PFS, and the p value was still greater than 0.05, which still needs more data to further confirm.
In our cohort, male had worse PFS than female (Table 4; Figure 3). There are also various opinions on the relationship between gender and thyroid cancer prognosis. Some studies have shown that the prognosis of thyroid cancer in male patients is worse than that in female patients (28, 29), it may be related to the higher risk of radiation exposure and aggressive characteristics of male (28). However, there is no relevant study on the relationship between the efficacy of targeted drugs in RR-DTC treatment and gender, and we will further explore in our follow-up study.
Serum Tg is a well-recognized prognostic indicator for the presence of metastases or occult lesions after total thyroidectomy (6, 30, 31). However, RR-DTC lesions have various degrees of dedifferentiation, resulting in the loss of the ability to produce Tg; thus, it is controversial to use Tg to evaluate curative effects (32, 33). Our subgroup analysis revealed that patients with ≥ 60% decrease in Tg had longer PFS and better DCRs. Besides, patients in PR had a significant diminishment of the best change in Tg (more than a 90% decrease in six cases and a 58.2% decrease in one case). Kim et al. also found that a ≥ 60% decrease in Tg was associated with longer PFS and DCRs (14), and a similar conclusion was expounded by Cheng et al. (16). In contrast, Benekli et al. emphasized that Tg levels had no significance in prognosis or predicting response (19), and decreased or no change in Tg was observed in patients with advanced thyroid cancer treated with other TKIs (21, 34, 35). Whether the measurement of Tg can monitor therapeutic effects remains to be determined, but dynamic monitoring of changes in Tg such as the degree of Tg diminishment and Tg-doubling time can evaluate the efficacy of postoperative patients with thyroid cancer to a certain extent (31).
In our study, dose reduction and drug withdrawal occurred in 42.3% (n = 18) of patients due to AEs, mainly because of HFS, and a lower rate of AEs was found compared with the DECISION trial (93.1% versus 98.6%) (13), but a lower rate of AEs was found in the study of Cheng et al. (73.6%) than ours; therefore, a higher dose may result in a higher rate of AEs (16). Since common AEs were HFS, diarrhea, alopecia, abnormal liver function, generalized weakness, and hypertension, clinicians should closely monitor patients’ skin condition, blood pressure, and general situation when using sorafenib to avoid dose reduction or discontinuation.
It has been confirmed that patients treated with TKIs are at high risk of HFS (36). Virtually all patients had HFS (93.5%), most of them were grades 1–2, and only three were grades 3 and above. Moreover, the occurrence of HFS was correlated with better PFS in our analysis. Many studies have confirmed that HFS is associated with a higher cumulative sorafenib dose and with anti-VEGF and anti-VEGFR activities (17, 37). Since anti-angiogenesis is one of the main mechanisms of sorafenib in treating RR-DTC, HFS could be a significant therapeutic index during the administration of TKIs (38, 39). Thus, clinicians could estimate whether TKIs are starting to work based on the appearance of HFS.
As the sample size included in our study was not large and the median follow-up was only 17.5 months, no factors affecting PFS were identified in multivariate analysis. We will continue to follow up with patients for PFS and include more patients to analyze the factors influencing PFS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Zhujiang Hospital of Southern Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YL, WO and HF designed the study. YL and XX collected and analyzed the data and wrote the manuscript. YL, XX, and JL checked and corrected the data and manuscript. QZ, PC, LP, and ML participated in measuring and collecting the data. WO and HF reviewed and edited the manuscript and approved the final draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Bureau of Science and Technology of Guangzhou Municipality [grant numbers 2022 0101 1072].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) (2021) 134(7):783–91. doi: 10.1097/CM9.0000000000001474
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388(10061):2783–95. doi: 10.1016/S0140-6736(16)30172-6
4. Jayarangaiah A, Sidhu G, Brown J, Barrett-Campbell O, Bahtiyar G, Youssef I, et al. Therapeutic options for advanced thyroid cancer. Int J Clin Endocrinol Metab (2019) 5(1):26–34. doi: 10.17352/ijcem.000040
5. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30(12):1856–83. doi: 10.1093/annonc/mdz400
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
7. Liu J, Liu Y, Lin Y, Liang J. Radioactive iodine-refractory differentiated thyroid cancer and redifferentiation therapy. Endocrinol Metab (Seoul) (2019) 34(3):215–25. doi: 10.3803/EnM.2019.34.3.215
8. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocr Metab (2006) 91(8):2892–9. doi: 10.1210/jc.2005-2838
9. Cho SW, Choi HS, Yeom GJ, Lim JA, Moon JH, Park DJ, et al. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid (2014) 24(2):277–86. doi: 10.1089/thy.2012.0654
10. Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemoth Pharm (2007) 59(5):561–74. doi: 10.1007/s00280-006-0393-4
11. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther (2008) 7(10):3129–40. doi: 10.1158/1535-7163.MCT-08-0013
12. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res (2004) 64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443
13. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet (2014) 384(9940):319–28. doi: 10.1016/S0140-6736(14)60421-9
14. Kim M, Kim TH, Shin DY, Lim DJ, Kim EY, Kim WB, et al. Tertiary care experience of sorafenib in the treatment of progressive radioiodine-refractory differentiated thyroid carcinoma: a Korean multicenter study. Thyroid (2018) 28(3):340–8. doi: 10.1089/thy.2017.0356
15. Kim SY, Kim SM, Chang H, Kim BW, Lee YS, Chang HS, et al. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: real-world use of lenvatinib and sorafenib in Korea. Front Endocrinol (Lausanne) (2019) 10:384. doi: 10.3389/fendo.2019.00384
16. Cheng L, Fu H, Jin Y, Sa R, Chen L. Clinicopathological features predict outcomes in patients with radioiodine-refractory differentiated thyroid cancer treated with sorafenib: a real-world study. Oncologist (2020) 25(4):e668–e78. doi: 10.1634/theoncologist.2019-0633
17. Lin CY, Chang JS, Huang SM, Hung CJ, Hung CL, Chang CT, et al. Experience of sorafenib treatment in differentiated thyroid cancer from Taiwan. J Formos Med Assoc (2021) 120(1):189–95. doi: 10.1016/j.jfma.2020.04.021
18. Massicotte MH, Brassard M, Claude-Desroches M, Borget I, Bonichon F, Giraudet AL, et al. Tyrosine kinase inhibitor treatments in patients with metastatic thyroid carcinomas: a retrospective study of the TUTHYREF network. Eur J Endocrinol (2014) 170(4):575–82. doi: 10.1530/EJE-13-0825
19. Benekli M, Yalcin S, Ozkan M, Elkiran ET, Sevinc A, Cabuk D, et al. Efficacy of sorafenib in advanced differentiated and medullary thyroid cancer: experience in a Turkish population. Onco Targets Ther (2015) 8:1–5. doi: 10.2147/OTT.S70670
20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
21. Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol (2009) 161(6):923–31. doi: 10.1530/EJE-09-0702
22. Bunone G, Vigneri P, Mariani L, Butó S, Collini P, Pilotti S, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol (1999) 155(6):1967–76. doi: 10.1016/S0002-9440(10)65515-0
23. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab (2001) 86(2):656–8. doi: 10.1210/jcem.86.2.7226
24. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med (2003) 9(6):669–76. doi: 10.1038/nm0603-669
25. Ooi LL, Zheng Y, Stalgis-Bilinski K, Dunstan CR. The bone remodeling environment is a factor in breast cancer bone metastasis. Bone (2011) 48(1):66–70. doi: 10.1016/j.bone.2010.05.007
26. Quinn JM, Matsumura Y, Tarin D, McGee JO, Athanasou NA. Cellular and hormonal mechanisms associated with malignant bone resorption. Lab Invest (1994) 71(4):465–71.
27. Stapleton M, Sawamoto K, Alméciga-Díaz CJ, Mackenzie WG, Mason RW, Orii T, et al. Development of bone targeting drugs. Int J Mol Sci (2017) 18(7):1345. doi: 10.3390/ijms18071345
28. Remer LF, Lee CI, Picado O, Lew JI. Sex differences in papillary thyroid cancer. J Surg Res (2022) 271:163–70. doi: 10.1016/j.jss.2021.11.004
29. Akslen LA, Myking AO, Salvesen H, Varhaug JE. Prognostic importance of various clinicopathological features in papillary thyroid carcinoma. Eur J Cancer (1992) 29a(1):44–51. doi: 10.1016/0959-8049(93)90574-y
30. Kloos RT, Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab (2005) 90(9):5047–57. doi: 10.1210/jc.2005-0492
31. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid (2011) 21(7):707–16. doi: 10.1089/thy.2010.0355
32. Lamartina L, Anizan N, Dupuy C, Leboulleux S, Schlumberger M. Redifferentiation-facilitated radioiodine therapy in thyroid cancer. Endocr-Relat Cancer (2021) 28(10):T179–T91. doi: 10.1530/ERC-21-0024
33. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol (2014) 2(5):356–8. doi: 10.1016/S2213-8587(13)70215-8
34. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol (2010) 11(10):962–72. doi: 10.1016/S1470-2045(10)70203-5
35. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol (2008) 26(29):4708–13. doi: 10.1200/JCO.2007.15.9566
36. Ding F, Liu B, Wang Y. Risk of hand-foot skin reaction associated with vascular endothelial growth factor-tyrosine kinase inhibitors: a meta-analysis of 57 randomized controlled trials involving 24,956 patients. J Am Acad Dermatol (2020) 83(3):788–96. doi: 10.1016/j.jaad.2019.04.021
37. Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res (2009) 15(4):1411–6. doi: 10.1158/1078-0432.CCR-08-1141
38. Ogawa C, Morita M, Omura A, Noda T, Kubo A, Matsunaka T, et al. Hand-foot syndrome and post-progression treatment are the good predictors of better survival in advanced hepatocellular carcinoma treated with sorafenib: a multicenter study. Oncology (2017) 93:113–9. doi: 10.1159/000481241
Keywords: differentiated thyroid cancer, sorafenib, radioactive iodine refractory, real-world clinical study, efficacy and safety
Citation: Ling Y, Xiong X, Luo J, Zou Q, Chen P, Pan L, Long M, Feng H and Ouyang W (2023) The efficacy and safety in radioactive iodine refractory thyroid cancer patients treated with sorafenib. Front. Endocrinol. 14:1200932. doi: 10.3389/fendo.2023.1200932
Received: 05 April 2023; Accepted: 28 June 2023;
Published: 18 July 2023.
Edited by:
Jadwiga Maria Furmaniak, Independent researcher, Cardiff, United KingdomReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandLongfei Deng, Southwest University, China
Copyright © 2023 Ling, Xiong, Luo, Zou, Chen, Pan, Long, Feng and Ouyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Ouyang, b3l3MTk2M0BzaW5hLmNvbQ==; Huijuan Feng, ZmhqMDQwM0AxMjYuY29t
†These authors share first authorship
 Yuanna Ling1†
Yuanna Ling1† Xiaoli Xiong
Xiaoli Xiong Wei Ouyang
Wei Ouyang