- 1Neural Signaling and Circuit Plasticity Group, Department of Biology, KU Leuven, Leuven, Belgium
- 2Functional Genomics and Proteomics Group, Department of Biology, KU Leuven, Leuven, Belgium
In vertebrates, thyrostimulin is a highly conserved glycoprotein hormone that, besides thyroid stimulating hormone (TSH), is a potent ligand of the TSH receptor. Thyrostimulin is considered the most ancestral glycoprotein hormone and orthologs of its subunits, GPA2 and GPB5, are widely conserved across vertebrate and invertebrate animals. Unlike TSH, however, the functions of the thyrostimulin neuroendocrine system remain largely unexplored. Here, we identify a functional thyrostimulin-like signaling system in Caenorhabditis elegans. We show that orthologs of GPA2 and GPB5, together with thyrotropin-releasing hormone (TRH) related neuropeptides, constitute a neuroendocrine pathway that promotes growth in C. elegans. GPA2/GPB5 signaling is required for normal body size and acts through activation of the glycoprotein hormone receptor ortholog FSHR-1. C. elegans GPA2 and GPB5 increase cAMP signaling by FSHR-1 in vitro. Both subunits are expressed in enteric neurons and promote growth by signaling to their receptor in glial cells and the intestine. Impaired GPA2/GPB5 signaling causes bloating of the intestinal lumen. In addition, mutants lacking thyrostimulin-like signaling show an increased defecation cycle period. Our study suggests that the thyrostimulin GPA2/GPB5 pathway is an ancient enteric neuroendocrine system that regulates intestinal function in ecdysozoans, and may ancestrally have been involved in the control of organismal growth.
Introduction
Glycoprotein hormones are key neuroendocrine factors that control diverse physiological processes, such as development, reproduction, energy homeostasis and growth (1, 2). In vertebrates, five glycoprotein hormones have been described that are all formed by the heterodimerization of two cysteine-knot containing polypeptides, an alpha (GPA) and beta (GPB) subunit, which are non-covalently associated (1, 3–5). Four of the glycoprotein hormones, including thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), luteinizing hormone (LH) and chorionic gonadotropin (CG), share a common alpha subunit, GPA1, that associates with hormone-specific beta subunits (TSHβ, FSHβ, LHβ and CGβ) (3, 6, 7). A fifth glycoprotein hormone, called thyrostimulin, was discovered more recently and is comprised of a unique glycoprotein alpha 2 (GPA2) and glycoprotein beta 5 (GPB5) subunit (8, 9). Thyrostimulin GPA2 and GPB5 subunits are widely conserved across bilaterian animals, whereas the conservation of GPA1 and other GPB polypeptides is restricted to vertebrates (1, 8, 10). Orthologs of GPA2 and GPB5 have been identified both in vertebrates and invertebrates, including arthropods (11–14), nematodes (9, 15), annelids (16, 17), mollusks (18–20), urochordates (21, 22), and cephalochordates (21, 23, 24). Thyrostimulin is therefore considered the most ancestral glycoprotein hormone, from which other vertebrate glycoprotein hormone systems most likely evolved through gene duplication (3, 7, 8).
In contrast to the well-studied TSH and gonadotropin endocrine systems, the physiological role of thyrostimulin remains largely unexplored. In 2002, a receptor for human thyrostimulin was identified (10). The heterodimeric GPA2/GPB5 glycoprotein has been shown to activate the human TSH receptor (TSHR), but not FSH or LH/CG receptors, and is capable of inducing receptor-mediated cyclic adenosine monophosphate (cAMP) production more potently than human TSH itself (10, 25). Like TSH, GPA2/GPB5 increases thyroid hormone secretion in rats and, based on its TSHR- and thyroid-stimulating activity, was named thyrostimulin (10). Later studies in basal vertebrates, urochordates, cephalochordates, and insects revealed that the interaction of thyrostimulin with TSHR orthologs is widely conserved (14, 23, 26–29). Glycoprotein hormone receptors belong to a subfamily of leucine-rich repeat containing G protein-coupled receptors (LGRs) that are conserved across divergent animal phyla (7, 8, 30, 31). Thyrostimulin orthologs were found to activate LGRs related to TSHRs in lampreys, tunicates, amphioxus, the fruit fly Drosophila melanogaster and the mosquito Aedes aegypti (14, 23, 26–29), indicating that the evolutionary origin of this glycoprotein hormone-receptor system dates back to a common ancestor of bilaterian animals.
To date, few studies have investigated the physiological role of thyrostimulin in vertebrates. These suggest that thyrostimulin has pleiotropic functions, as it is involved in reproduction (32), skeletal development (33, 34), immune responses (35, 36) and thyroxine production (10, 37). In invertebrates, the functions of thyrostimulin-like signaling have so far been studied only in arthropods. GPA2/GPB5 or its receptor have been implicated in the control of reproduction, development, feeding, diuresis and osmoregulation in insects (12, 38–40). In the prawn Macrobrachium rosenberghii, knockdown of GPA2/GPB5 or its predicted receptor affects female reproduction (41). The mechanisms by which GPA2/GPB5 regulates these biological processes as well as its suggested hormonal functions in other invertebrates, however, are still undetermined.
Due to its well-defined nervous system and extensive genetic toolbox, the nematode Caenorhabditis elegans is an excellent model to study neuroendocrine systems. Several vertebrate endocrine factors, like gonadotropin-releasing hormone (GnRH) and thyrotropin-releasing hormone (TRH), have been identified in C. elegans and were found to have conserved physiological roles, such as in the control of reproduction and growth (42, 43). Phylogenetic analyses also revealed orthologs of the thyrostimulin GPA2 and GPB5 subunits in the C. elegans genome (9, 15, 21, 40), which carry a cysteine knot motif of six cysteine residues that is typical of glycoprotein hormone subunits (44, 45). Recent analysis of the crystal structure of C. elegans GPA2/GPB5 validates its structural similarity to vertebrate glycoprotein hormones that was previously found by comparative genomic analyses (3, 8, 46). In addition, C. elegans has only one receptor ortholog of the vertebrate glycoprotein hormone receptors. This C. elegans receptor, designated as FSHR-1, belongs to the same LGR subfamily as the vertebrate thyrostimulin receptors (7, 8), but is still orphan. Here, we identify C. elegans GPA2 and GPB5 as ligands of FSHR-1. Using CRISPR/Cas9 reverse genetics, we functionally characterize the thyrostimulin-related system in C. elegans and demonstrate that GPA2/GPB5 signaling, together with TRH-related neuropeptides, regulates growth and intestinal function.
Results
C. elegans thyrostimulin-like GPA2/GPB5 orthologs regulate growth
Phylogenetic analysis revealed two orthologs of thyrostimulin GPA2 and GPB5 subunits in the C. elegans genome (8, 9). These are encoded by the genes flr-2 and T23B12.8 (15, 21), which we called gpla-1 and gplb-1 (glycoprotein hormone-like alpha and beta), respectively. GPLA-1 and GPLB-1 resemble vertebrate GPA2 and GPB5 and are widely conserved in nematodes (Figures S1, S2). In C. elegans, mutations in gpla-1 have been reported to suppress slow growing of several growth-defective mutants (15, 47). We therefore hypothesized that thyrostimulin-like signaling may influence C. elegans body size. To test this, we generated complete gene knockouts of gpla-1 and gplb-1 using CRISPR/Cas9 gene editing (Figure 1A) and investigated if these null mutants show body size defects at day 1 of adulthood (65 hours post L1 arrest). The generated gpla-1 (ibt1) and gplb-1 (ibt4) knockout mutants display a significantly shorter body length compared to wild-type animals (Figures 1B, C). This size defect persists for at least 5 days into adulthood, suggesting that it results from defective growth rather than a delay in the timing of development (Figure S3A). Additionally, worms lacking gpla-1 and gplb-1 have a reduced body width compared to wild type (Figure S3B). We also measured body sizes of two independently generated gpla-1 mutants, carrying the ut5 and ok2127 alleles (Figure 1A). The gpla-1 (ut5) mutant carries a substitution of the glycine residue between the second and third cysteine (G63E), which is highly conserved and important for proper cysteine knot formation (45). The gpla-1 (ok2127) mutant contains a large deletion, removing the start codon and the complete first exon of the gene (Figure 1A). We found that these mutants also display a decreased body length compared to wild-type animals. Mutants carrying the ut5 allele show a reduction in body width as well (Figures S3C, D). The defect in length, however, is most pronounced in all gpla-1 and gplb-1 mutants. Therefore, we quantified body length in further experiments. Expressing wild-type gpla-1 and gplb-1 under control of their endogenous promoter in their respective mutant background fully restored body length (Figures 1B, C), indicating that both GPA2 and GPB5 orthologs are required for normal body size.
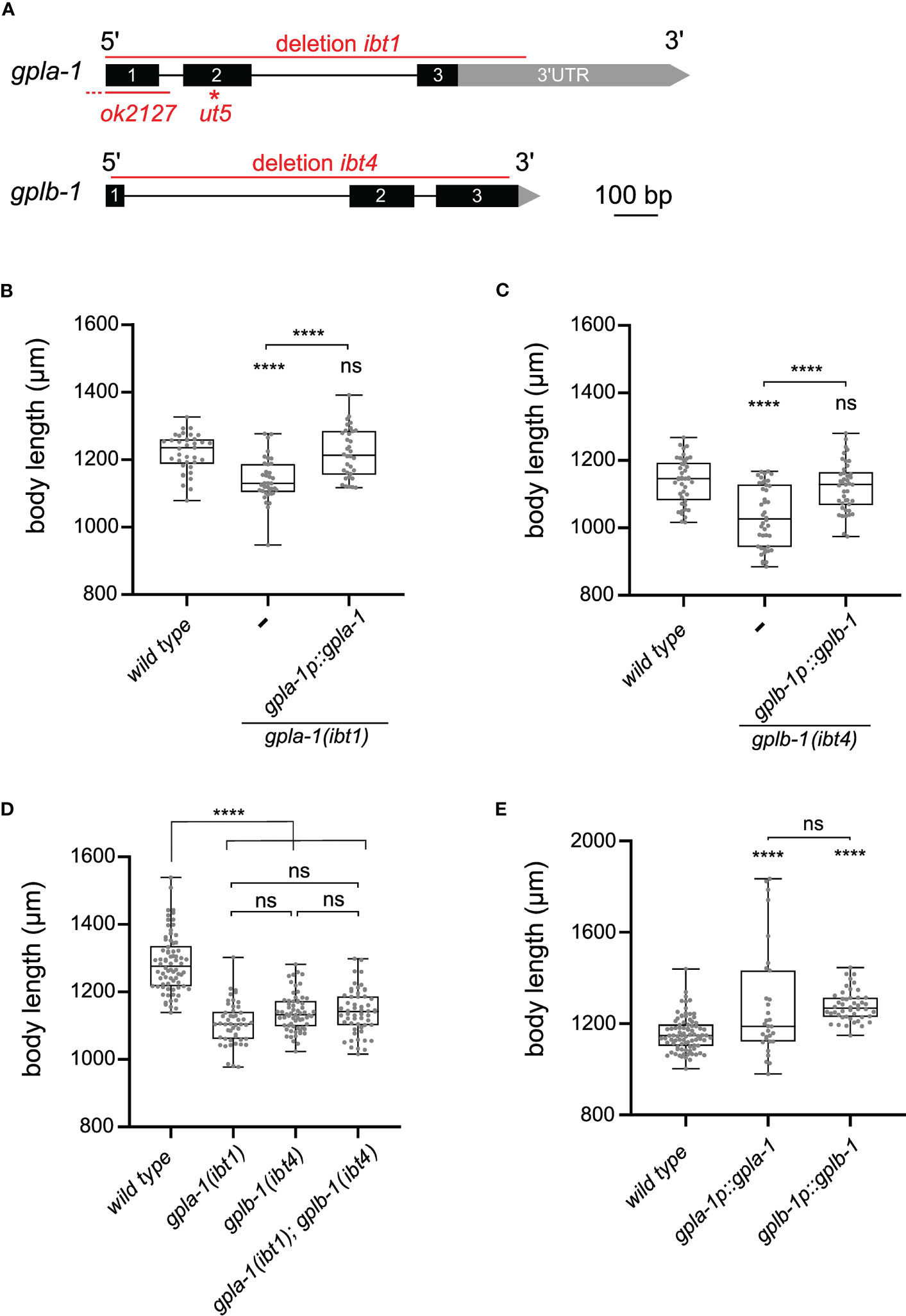
Figure 1 Thyrostimulin-like signaling promotes C. elegans growth. (A) Structure of gpla-1 and gplb-1 transcripts. Black boxes represent exons; black lines indicate intronic regions; and grey boxes and arrows represent 3’UTR sequences. Red lines mark the positions of the available ut5 and ok2127 mutant alleles, and the ibt1 and ibt4 deletions created by CRISPR/Cas9 gene editing. (B, C) Null mutants of gpla-1 and gplb-1 have a significantly shorter body length than wild-type animals at day 1 of adulthood. Restoring wild-type gpla-1 (B) and gplb-1 (C) expression under the control of their respective endogenous promoter rescues the body size defect. (D) Double mutants of gpla-1 and gplb-1 do not significantly differ in body length from the single mutants. (E) Overexpressing wild-type gpla-1 and gplb-1 significantly increases body length compared to wild type. Boxplots indicate 25th (lower boundary), 50th (central line), and 75th (upper boundary) percentiles. Whiskers show minimum and maximum values. For (B–E), each genotype was tested in at least 2 assays with 10–30 animals per trial. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001; ns, not significant.
To determine whether GPLA-1 and GPLB-1 act in the same growth-regulating pathway, we quantified the body length of double mutants. Mutants lacking both genes did not significantly differ in length from the single mutants (Figure 1D). This suggests that gpla-1 and gplb-1 indeed act in the same genetic pathway to control body size. Overexpression of each individual gene increased the length of wild-type worms (Figure 1E), suggesting that GPLA-1 and GPLB-1 are sufficient to induce growth.
GPLA-1/GPA2 and GPLB-1/GPB5 control body size through the glycoprotein hormone receptor ortholog FSHR-1
To unravel how thyrostimulin-like signaling regulates C. elegans body size, we aimed to identify its target receptor. Since glycoprotein hormones typically bind to LGRs (8), we hypothesized that GPLA-1 and GPLB-1 may signal through FSHR-1, which is the sole glycoprotein hormone receptor ortholog in C. elegans (48). To determine whether C. elegans thyrostimulin can activate FSHR-1, we used a cAMP-based receptor activation assay in human embryonic kidney (HEK) cells. We co-expressed FSHR-1 with a cAMP response element (CRE)-luciferase reporter in HEK cells and quantified its activation by assessing bioluminescence levels in the presence of luciferin substrate (Figure 2A). Consistent with previous reports (48), we observed basal activity of FSHR-1, as bioluminescent cAMP signals were higher in receptor-expressing cells than in cells transfected with an empty control vector (Figure S4A). However, challenging cells with recombinant GPLA-1 and GPLB-1 proteins significantly increased cAMP signaling by FSHR-1 (Figures 2B–D). To generate recombinant GPLA-1/GPLB-1, HEK293 cells were first transfected with expression vectors encoding His-tagged GPLA-1 and non-tagged GPLB-1. Tagging of GPLA-1 did not affect protein function as transgenic C. elegans expressing endogenous His-GPLA-1 showed normal body size (Figure S4B). Recombinant proteins purified from cells expressing both subunits significantly increased cAMP signaling by FSHR-1, suggesting that GPLA-1/GPLB-1 is a ligand of FSHR-1 (Figure 2B). We also tested whether GPLA-1 and GPLB-1 proteins could activate FSHR-1 individually. For this, recombinant proteins were purified from HEK293 cells transfected with an expression vector encoding only one of the two subunits. We found that GPLA-1 as well as GPLB-1 increased cAMP signaling by FSHR-1 (Figures 2C, D), which indicates that each subunit alone is capable of activating the receptor. None of the purified recombinant proteins elicited a significant cAMP response in cells transfected with an empty control vector instead of the FSHR-1 expression plasmid (Figure S4C). Taken together, these results suggest that GPLA-1 and GPLB-1 are cognate ligands of FSHR-1.
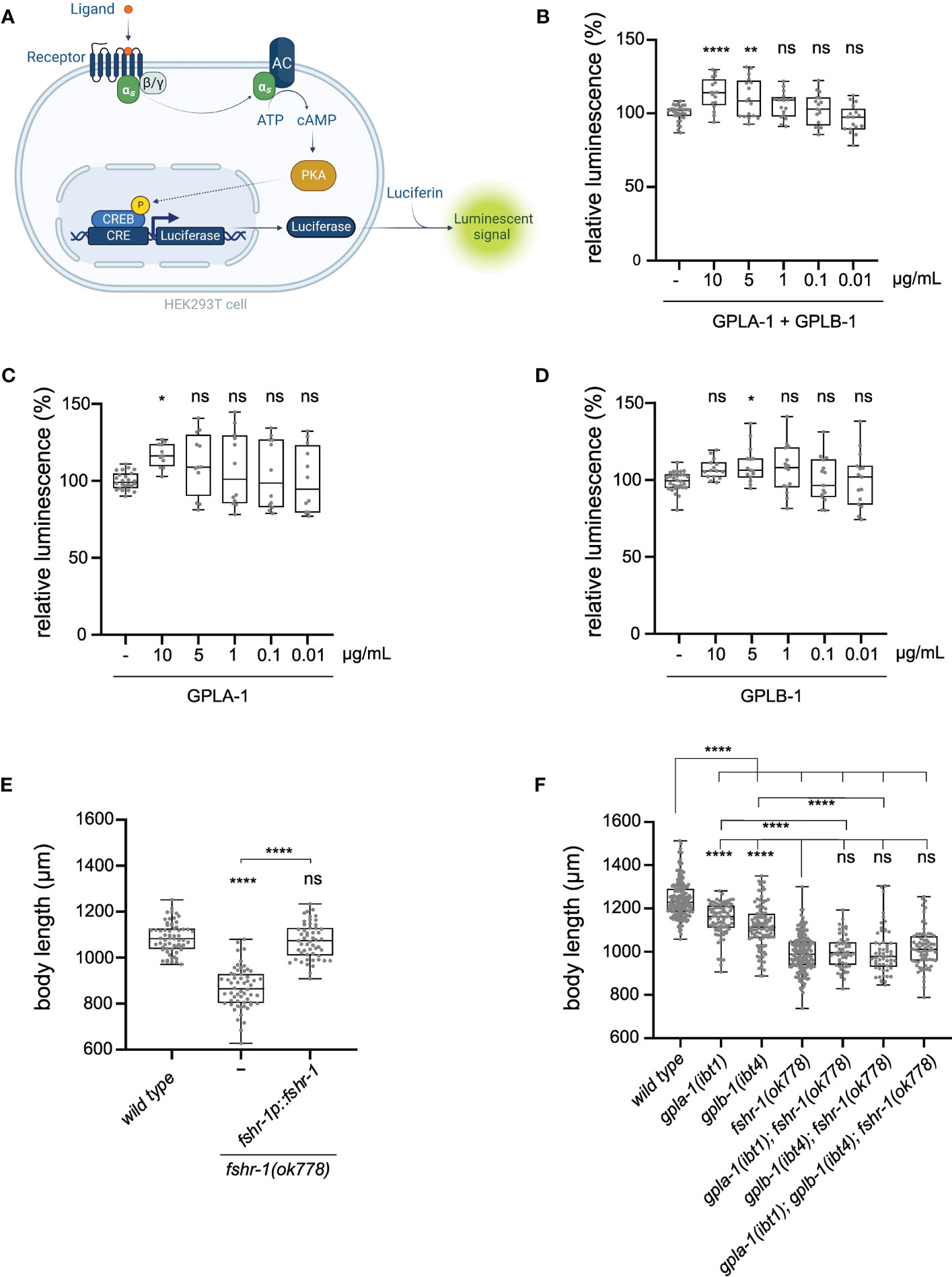
Figure 2 C. elegans GPLA-1 and GPLB-1 regulate growth through the glycoprotein hormone receptor ortholog FSHR-1. (A) Luminescence-based cAMP assay for measuring GPCR activation. FSHR-1 is co-expressed with the CRE(6x)-luciferase reporter in HEK cells. Receptor activation initiates the production of cAMP by adenylyl cyclase (AC), which is monitored by the cAMP-sensitive biosensor luciferase in the presence of luciferin substrate. Created with BioRender.com. (B–D) Recombinant GPLA-1 and GPLB-1 proteins activate FSHR-1 in a cellular assay described in panel (A). Data are shown as relative luminescence after normalization to the ligand-free control. One-way ANOVA with Dunnett’s multiple comparisons test. n ≥ 4 assays. (E) fshr-1 mutants are significantly shorter than wild-type animals, which is rescued by expressing wild-type fshr-1 under its endogenous promoter. (F) Double and triple mutants of gpla-1, gplb-1 and fshr-1 do not significantly differ in body length from fshr-1 single mutants. Body size data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. Boxplots indicate 25th (lower boundary), 50th (central line), and 75th (upper boundary) percentiles. Whiskers show minimum and maximum values. For (E, F), each genotype was tested in at least 3 assays with 20-30 animals per trial. *P < 0.05; **P < 0.01; ****P < 0.0001; ns, not significant.
As FSHR-1 is activated by GPLA-1 and GPLB-1 in vitro, we asked if this receptor is involved in growth regulation. We observed that a loss-of-function mutant of fshr-1 suffers from severe growth defects that persist throughout adulthood (Figures 2E, S3A). Expressing wild-type fshr-1 under the control of its endogenous promoter fully restores this growth defect, indicating that this receptor, like its activating ligands GPLA-1 and GPLB-1, is required for growth (Figure 2E). Although body length was more strongly reduced in fshr-1 animals than in mutants lacking the thyrostimulin subunits, double and triple mutants of gpla-1, gplb-1 and their receptor fshr-1 were not shorter than the fshr-1 single mutant (Figure 2F). This suggests they all act in the same growth-regulating pathway in vivo, consistent with our finding that FSHR-1 is a receptor for GPLA-1 and GPLB-1 in vitro.
C. elegans GPA2/GPB5 and FSHR-1 are expressed in enteric neurons and the intestine
To gain further insight into the role of thyrostimulin-like signaling in growth regulation, we investigated the expression patterns of gpla-1, gplb-1 and fshr-1. Fluorescent reporter transgenes, which rescue body length defects (Figures 1B, C, 2E), showed expression in multiple pharyngeal neurons and tissues associated with feeding and digestive processes. Consistent with single-cell RNA sequencing data and previous reporter analyses (15, 49, 50), we found that gpla-1 is predominantly expressed in neurons associated with the gastrointestinal tract (Figures 3A, B), which are part of the enteric nervous system in C. elegans (50–52). These include the pharyngeal neurons M1, M5, I5 and NSM, as well as the excitatory motor neurons AVL and DVB that control enteric muscle contractions in the hindgut during defecation (53–55). We also observed expression of gplb-1 in the enteric DVB neuron (Figure 3D). In addition, both gpla-1 and gplb-1 reporter transgenes show fluorescence in the RME motor neurons that innervate the head muscles (Figures 3A, C). Using a reporter transgene for GABAergic neurons, we confirmed that gpla-1 and gplb-1 are both expressed in the GABAergic DVB and RME neurons (Figure S5). In addition, the gplb-1 reporter transgene shows expression in non-neuronal tissues, including the head mesodermal cell (hmc) and the enteric muscles of the hindgut, which are involved in defecation (53–57). Taken together, these results indicate that expression of gpla-1 and gplb-1 co-localizes in the RME neurons in the head and the enteric neuron DVB in the tail, whereas no overlapping expression is seen in the pharyngeal neurons (gpla-1) or hmc and the enteric muscles (gplb-1).
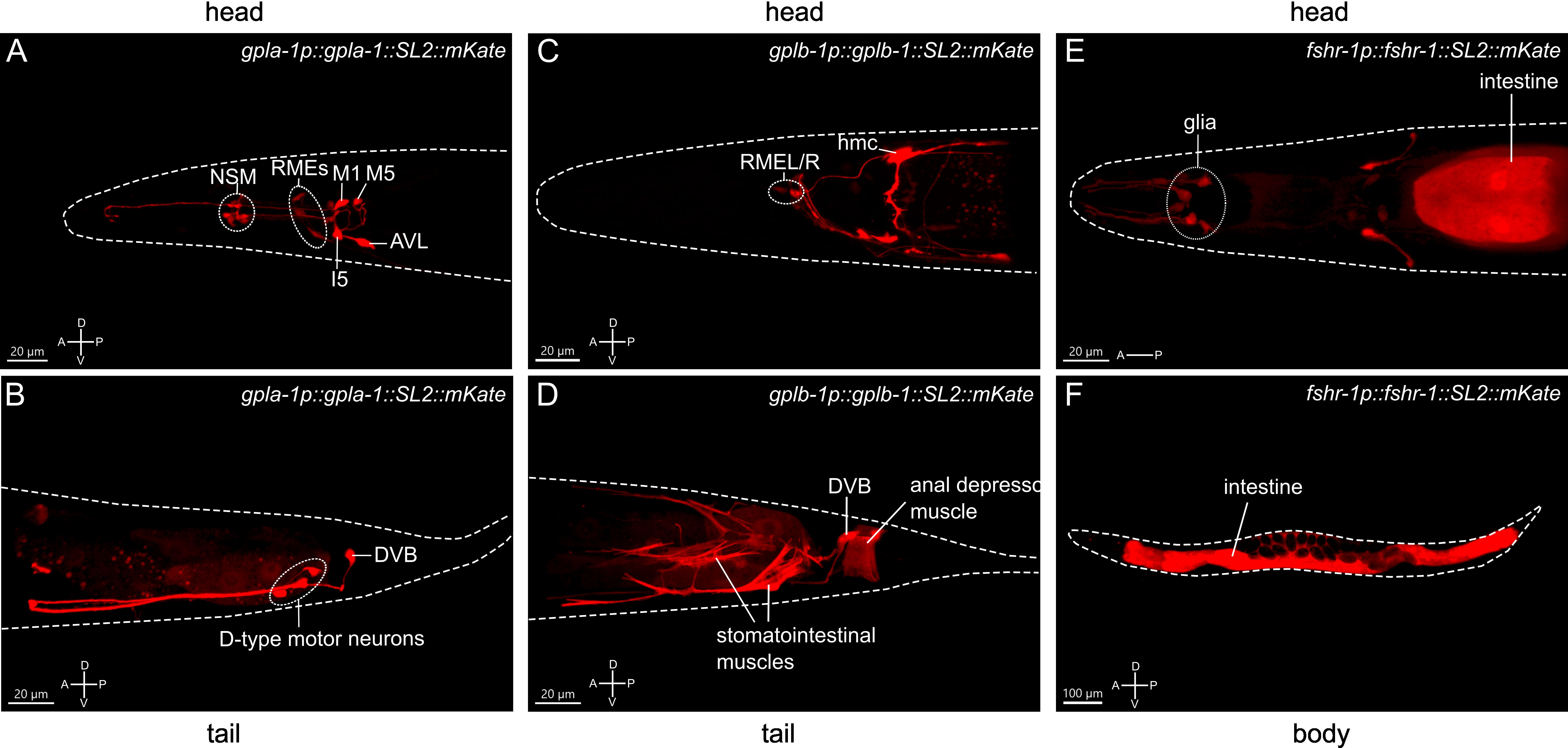
Figure 3 Expression patterns of the C. elegans thyrostimulin-like subunits GPLA-1/GPA2 and GPLB-1/GPB5 and their receptor FSHR-1. Representative confocal Z-stack projections of the regions showing expression of an mKate reporter transgene for gpla-1 (A, B), gplb-1 (C, D) and fshr-1 (E, F). A, anterior; P, posterior; D, dorsal; V, ventral orientation.
A reporter construct expressing fshr-1 under its endogenous promoter recapitulated the reported expression of the receptor in the intestine and in multiple neurons in the head (49, 58–60). In addition, we observed expression of fshr-1 in several cells close to the anterior pharyngeal bulbus that, based on position and morphology of their projections, are most likely glial cells (Figures 3E, F). Together, these expression patterns suggest that thyrostimulin-like signaling may influence body size by controlling intestinal or neural function.
Intestinal and glial glycoprotein hormone receptor signaling controls body size
Next, we asked in which tissues fshr-1 is required for normal body size. Expressing wild-type fshr-1 under control of the intestine-specific ges-1 (61–63) or the pan-glial mir-228 (64) promoters fully rescued the body size defect of fshr-1 mutants (Figure 4A). By contrast, pan-neuronal expression using the rab-3 (65, 66) promoter partially restored body size (Figure 4A). Since additional expression of this promoter has been reported in the intestine (67, 68), we generated a second transgene for pan-neuronal expression of wild-type fshr-1 using the rgef-1 promoter (69). This transgene did not restore the body size defect of fshr-1 mutants (Figure 4A); however, it remains possible that fshr-1 expression levels under control of the rgef-1 promoter were not sufficient to rescue body size. Taken together, our results show that FSHR-1 regulates growth in the intestine and glial cells, although we cannot exclude that FSHR-1 signaling in neurons may influence body size as well.
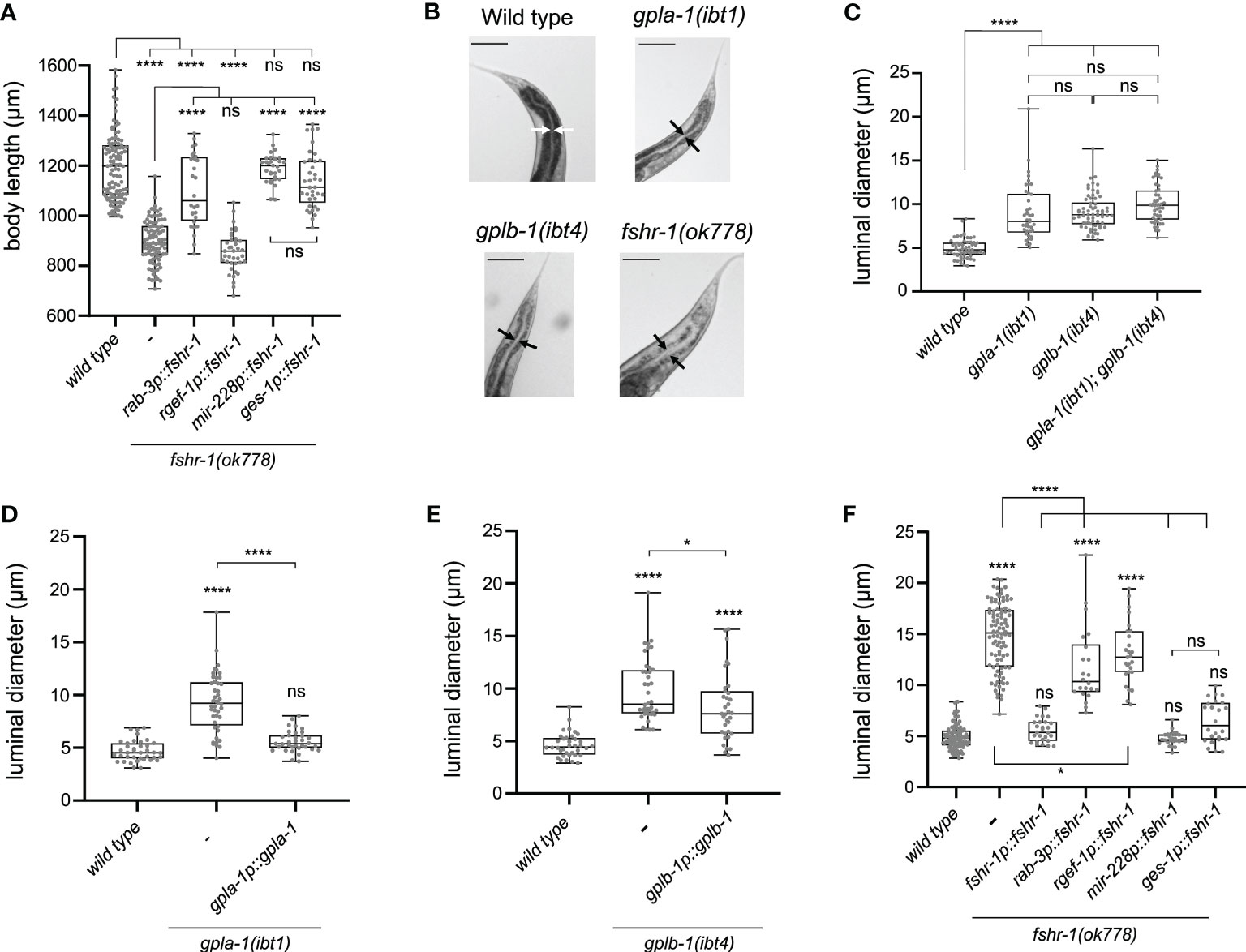
Figure 4 Thyrostimulin-like signaling promotes growth by regulating intestinal function. (A) Expression of wild-type fshr-1 under control of the pan-glial promoter mir-228p or the intestinal promoter ges-1p fully restores the body size defect of fshr-1 mutants. Expression of wild-type fshr-1 under the pan-neuronal rab-3 promoter partially rescues body size, whereas expression from the pan-neuronal rgef-1 promoter does not. (B) Mutants deficient in thyrostimulin signaling show intestinal bloating. Scale bars represent 100 μm. (C) The intestinal lumen of gpla-1 and gplb-1 single and double mutants is significantly wider than that of wild-type animals. (D, E) Restoring wild-type gpla-1 (D) and gplb-1 (E) expression rescues the intestinal bloating phenotype. (F) Luminal size of the intestine is significantly increased in fshr-1 mutants, and expressing wild-type copies of fshr-1 under control of an endogenous promoter (fshr-1p), pan-glial promoter (mir-228p), or intestinal promoter (ges-1p) fully rescues intestinal bloating. Pan-neuronal fshr-1 expression controlled by rab-3p or rgef-1p partially restores luminal size of the intestine. Boxplots indicate 25th (lower boundary), 50th (central line), and 75th (upper boundary) percentiles. Whiskers show minimum and maximum values. For (A) and (C–F), each genotype was tested in at least 2 assays with 20-30 animals per trial. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. *P < 0.05; ****P < 0.0001; ns, not significant.
Mutants deficient in thyrostimulin-like signaling show defecation defects and intestinal bloating
Our expression analysis and rescue experiments suggest that thyrostimulin-like signaling regulates intestinal function. To test this hypothesis, we investigated the anatomy of the intestine and measured the size of the intestinal lumen. The lumen of the intestine was bloated to a similar extent in single and double mutants of gpla-1 and gplb-1 (Figures 4B, C). The bloating phenotype was fully rescued by expressing wild-type copies of gpla-1 and partially restored by expressing wild-type gplb-1 (Figures 4D, E). Mutants lacking fshr-1 also displayed a severely bloated intestinal lumen, which was rescued by restoring wild-type fshr-1 expression under its endogenous promoter (Figures 4B, F). The lumen of double and triple mutants of gpla-1, gplb-1 and their receptor fshr-1 was not wider than that of the fshr-1 single mutant (Figures S6D–F), suggesting that they act in the same pathway controlling distention of the intestinal lumen. Tissue-specific expression of wild-type fshr-1 in either the intestine or glial cells fully rescued luminal bloating, whereas pan-neuronal expression under control of the rab-3 or rgef-1 promoter partially restored the luminal size (Figure 4F). Distention of the intestinal lumen is thus regulated by fshr-1 in the intestinal and glial cells, and in part by FSHR-1 signaling in neurons.
Since gpla-1 and gplb-1 are predominantly expressed in enteric neurons and tissues controlling the defecation motor program, we hypothesized that thyrostimulin signaling may influence growth by regulating defecation behavior. To test this, we first examined whether mutants defective in the defecation motor program also show growth defects. In C. elegans, the defecation motor program is a rhythmic behavior with a period of about 50 seconds, which ultimately results in contraction of the enteric muscles in the hindgut and the subsequent expulsion of digested food from the intestine (53–55). Mutants for unc-25 and exp-1 are deficient in synaptic GABAergic transmission, which drives enteric muscle contraction, and show severe defecation defects (70–72). We found that unc-25(e156) and exp-1(ox276) mutants are also significantly smaller than wild-type animals (Figure S6A), suggesting that defecation defects correlate with impaired growth in these mutants. Next, we asked whether mutants defective in thyrostimulin-like signaling show defects in defecation behavior. We found that the contraction frequency of the anterior body wall muscles (aBoc) did not differ from wild-type aBoc frequency (Figure S6B). However, the average length of the defecation cycle between consecutive contractions of the posterior body wall muscles (pBoc) was significantly longer in mutants for gpla-1, gplb-1, and fshr-1 compared to the cycle length of wild-type animals (Figure S6C). Taken together, these findings suggest that thyrostimulin-like neuroendocrine signaling regulates growth by controlling intestinal function.
GPA2/GPB5 and TRH-like neuropeptide signaling act in the same pathway regulating growth
In vertebrates, glycoprotein hormone signaling is regulated by hypothalamic factors that control the release of hormones from the pituitary (2, 73). TRH is a highly conserved releasing factor that regulates growth and metabolism across bilaterian animals (2, 43, 73–75). In C. elegans TRH-related neuropeptides promote growth and mutants lacking the TRH-like precursor gene trh-1 have a smaller body size than wild-type animals (43). We therefore asked whether TRH- and thyrostimulin-like signaling systems may interact to control C. elegans growth.
To test this hypothesis, we compared the body size of single and double mutants lacking trh-1 and the thyrostimulin subunit orthologs gpla-1 and gplb-1. Double mutants defective in both signaling systems did not significantly differ in length compared to the single mutants (Figure 5A). Similar to thyrostimulin mutants, worms lacking TRH-1 also displayed intestinal bloating (Figures 5B, C). Although the effect is more severe in mutants lacking thyrostimulin-like signaling, the extent of luminal bloating in double mutants of gpla-1, gplb-1 and trh-1 did not significantly differ from single gpla-1 or gplb-1 mutants (Figures 5D, E). These results indicate that trh-1 and gpla-1/gplb-1 act in the same growth-stimulating pathway in vivo.
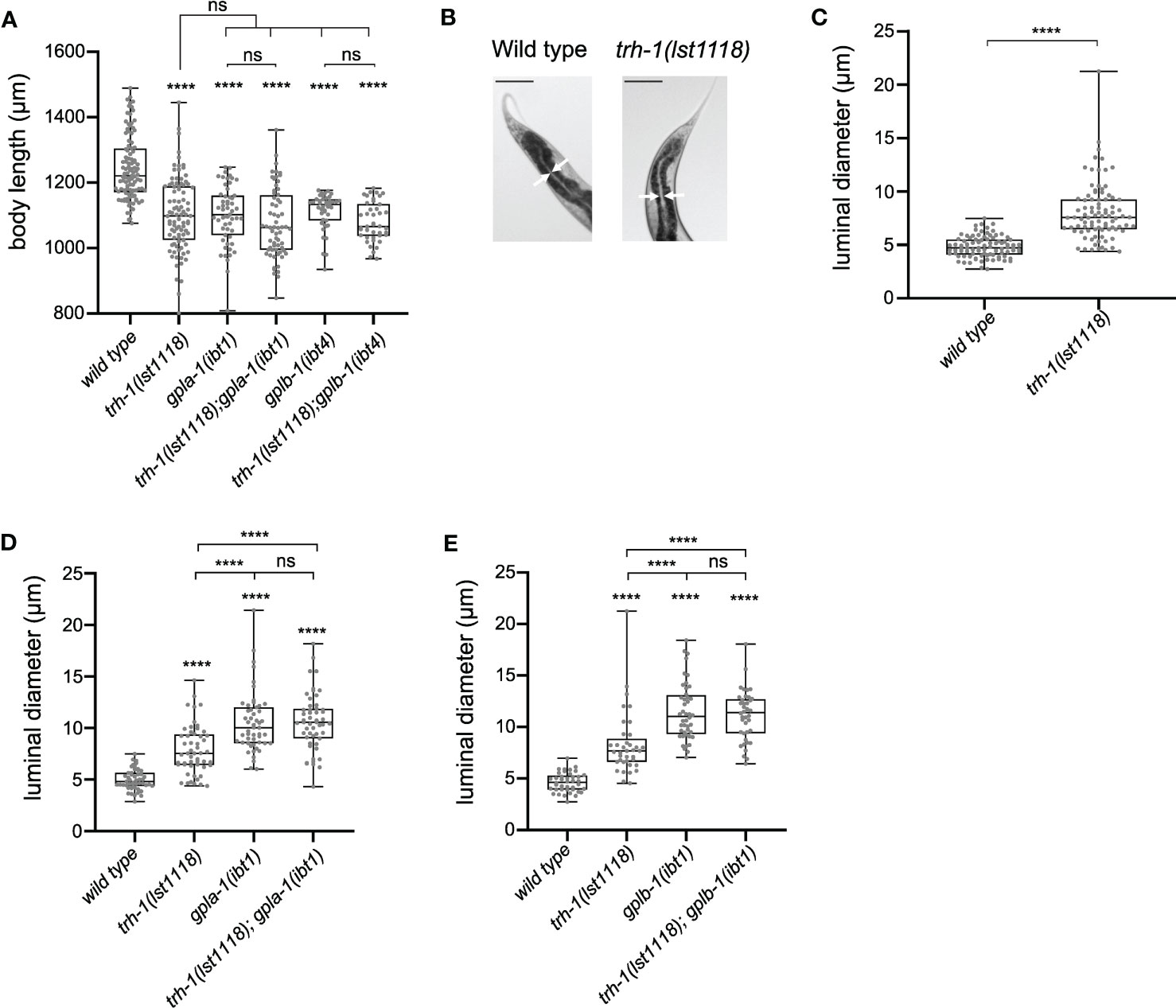
Figure 5 TRH-like neuropeptides and GPA2/GPB5 signaling act in the same growth-regulating pathway. (A) Double mutants defective in both the TRH-like neuropeptide precursor trh-1 and thyrostimulin gpla-1 or gplb-1 do not differ in body length from the single mutants. (B, C) Mutants lacking trh-1 show an increased luminal size of the intestine. Scale bars represent 100 μm. (D, E) Luminal width of double mutants lacking trh-1 and gpla-1 (D) or gplb-1 (E) does not significantly differ from the width of single gpla-1 or gplb-1 mutants. Boxplots indicate 25th (lower boundary), 50th (central line), and 75th (upper boundary) percentiles. Whiskers show minimum and maximum values. For (A) and (C-E), each genotype was tested in at least 2 assays with 20-30 animals per trial. Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test. ****P < 0.0001; ns, not significant.
Discussion
Phylogenetic analyses show a clear conservation of thyrostimulin GPA2 and GPB5 polypeptides across Protostomia and Deuterostomia, but their physiological roles remain elusive (7–9, 14, 26, 29, 46). In invertebrates, GPA2/GPB5 orthologs have only been functionally characterized in arthropods (12, 38–40). Here, we identify a thyrostimulin-like neuroendocrine system in the nematode C. elegans and uncover a conserved role of this signaling pathway in the control of growth. Our findings expand our understanding of the evolutionary history and physiological actions of GPA2/GPB5 glycoprotein hormone signaling, for which we delineate a potentially ancestral role in regulating intestinal function.
The orphan receptor FSHR-1 is the sole glycoprotein hormone receptor ortholog identified in C. elegans, which belongs to the type A LGR family (7, 8, 48). We show that C. elegans GPLA-1/GPA2 and GPLB-1/GPB5 are cognate ligands of FSHR-1 in vivo and are able to functionally activate this receptor in vitro. Our results indicate that FSHR-1 has basal receptor activity, like other thyrostimulin receptors (28, 29, 76–78). Previous work identified a sequence motif in the third intracellular loop of FSHR-1 that is likely responsible for ligand-independent signaling (48). In addition, we show that GPLA-1 and GPLB-1 activate FSHR-1, as receptor-mediated cAMP signaling increases when both subunits are applied together or alone. These findings support the evolutionary conservation of a functional thyrostimulin-like neuroendocrine system from nematodes to humans.
In vertebrates, the beta subunits of classical glycoprotein hormones (GPB1 – 4) have a carboxytail extension, referred to as the “seatbelt”, that wraps around the alpha subunit to stabilize heterodimer configuration (3, 79, 80). However, thyrostimulin GPB5 polypeptides lack the seatbelt structure (3, 9, 81), and whether heterodimerization is required for GPA2/GPB5 signaling is debated. In vitro studies of thyrostimulin receptors in humans, insects and Amphioxus found potent receptor activation only with heterodimeric or tethered GPA2/GPB5 proteins, although human GPB5 also activates TSHRs at 100-fold higher concentrations than the heterodimer (14, 23, 25, 28, 82). In addition, differences in the expression patterns of GPA2 and GPB5, both in vertebrates and invertebrates, suggest that the two subunits may have monomeric or homodimeric functions (21, 25, 27, 29, 38, 82). These findings have raised the question as to what the ancestral configuration of GPA2 and GPB5 hormones might have been and whether each subunit may also signal independently. Recent analysis of the crystal structure of C. elegans GPLA-1/GPLB-1 revealed that the two subunits indeed heterodimerize in solution (46). However, GPLA-1 and GPLB-1 were also found to be more similar in structure as compared to the alpha and beta subunits of human CG and FSH (46). Based on this similarity, Gong and colleagues hypothesized that the heterodimer may not be the only functional form and that thyrostimulin-like subunits may have evolved from a monomeric or homodimeric antecedent (46). Several of our results support this hypothesis: First, we show that cAMP signaling by FSHR-1 increases when GPLA-1 and GPLB-1 are applied together or separately. Although the structure of these recombinant proteins remains to be determined, our results suggest that both GPLA-1/GPLB-1 and the individual subunits are capable of activating FSHR-1. The interaction of FSHR-1 with GPLA-1 is further corroborated by a recent study from Wang and colleagues (83), in which GPLA-1 was co-immunoprecipitated with the extracellular domain of FSHR-1 from transgenic C. elegans, suggesting that GPLA-1 alone can also bind FSHR-1 in vivo. Second, we find that the expression patterns of gpla-1 and gplb-1 partially overlap. This suggests that GPLA-1 and GPLB-1 can be co-released from some cells, such as the enteric DVB neuron and the RME motor neurons, but may also signal independently from cells expressing only one of the two subunits. Whether GPLA-1 and GPLB-1 signal as monomers, homodimers or heterodimers in vivo remains to be understood. Our genetic analysis of the thyrostimulin-like system shows that both GPLA-1 and GPLB-1 are required for normal body size and act together, possibly as heterodimers, in the same growth-regulating pathway in vivo. Based on our receptor activation and expression analysis, we speculate that GPLA-1 and GPLB-1 may also exert independent functions. While expression of both subunits co-localizes in multiple cells, gpla-1 is additionally expressed in pharyngeal neurons and may have separate roles in the regulation of feeding behavior. GPLB-1 may independently act as a neuroendocrine signal from non-neuronal tissues, such as the enteric muscles and the epithelial-like hmc cell.
The role of thyrostimulin GPA2/GPB5 orthologs in protostomes is largely unknown. A physiological role in growth and development, diuresis, and reproduction has previously been described in arthropods (11–13, 38, 39). In insects, the thyrostimulin receptor ortholog LGR1, like C. elegans FSHR-1, is abundantly expressed in the alimentary canal (13, 38). In the adult mosquito, GPA2/GBP5 influences ion transport across the hindgut by promoting potassium secretion and inhibiting natriuresis (13). Thus, GPA2/GBP5 signaling may be ancestrally involved in regulating intestinal function, at least in ecdysozoans. In addition, knockdown of GPA2/GPB5 or their receptor has been shown to affect vitellogenesis in female prawns and leads to reduced reproductive success in male mosquitos (39, 41). Thyrostimulin-like signaling may also have a role in regulating hermaphrodite reproduction in C. elegans, as previous research revealed that FSHR-1 is involved in germline development and fertility (84). In vertebrates, GPA2 and GPB5 are found in reproductive tissues as well (10, 25, 82), and a thyrostimulin-TSHR paracrine signaling system has been identified in the mammalian ovary (32). Besides a reproductive function, knockout of GPB5 in mice increases bone mineralization and volume, suggesting that thyrostimulin signaling is also involved in regulating mineral balance in vertebrates (33).
In C. elegans, we found that thyrostimulin-like signaling promotes growth via activation of FSHR-1 in the intestine and glial cells. Restoring expression of fshr-1 in either cell type fully rescues the body size defect of fshr-1 mutants. Because extrachromosomal multi-copy transgenes may result in tissue-specific overexpression of fshr-1, it remains elusive whether FSHR-1 signaling in the glial or intestinal cells alone is sufficient to restore body size. Nevertheless, our results indicate that both glial and intestinal FSHR-1 signaling regulate growth as well as luminal width of the intestine.
In vertebrates, the thyrostimulin receptor TSHR is found to be expressed in glia (85, 86). In C. elegans, body size is also regulated by sensory amphid neurons in the head that are closely associated with glial cells (87). Based on our reporter analysis, indicating that GPLA-1 and GPLB-1 are expressed in several enteric neurons, we further investigated the effects of thyrostimulin-like signaling on intestinal function. Our results show that GPLA-1/GPLB-1 and their receptor regulate the cycle period of defecation as well as distention of the intestinal lumen. Loss of GPLA-1/GPLB-1 or FSHR-1 results in intestinal bloating, which may be caused by different factors. For example, widening of the intestinal lumen has been observed in C. elegans upon pathogenic infection and in mutants defective in the defecation motor program, due to the accumulation of intestinal content or bacterial colonization of the gut (62, 88, 89). In some cases, undigested microbial cells can reach the intestine, accumulate in the lumen, and undergo cell divisions that result in a swollen lumen (90). Moreover, intermediate filaments (IFs), localized to the apical side of intestinal cells, provide structural support to the intestinal lumen and regulate the luminal distortions that accompany food intake and movement along the digestive tract. Absence of these IFs alters the morphology of the epithelial cells, resulting in luminal bloating (90, 91). We speculate that the intestinal bloating of thyrostimulin-defective mutants results from the buildup of intestinal content, as we find that the defecation cycle period is significantly longer in gpla-1, gplb-1 and fshr-1 mutants than in wild type animals. The defecation motor program has important functions in the uptake of dietary nutrients. For example, defecation defects have been linked with aberrant fat metabolism and a reduced uptake of fatty acids from the intestinal lumen (92). Such nutritional deficits may impair growth. Indeed, we find that other mutants defective in the defecation motor program, like thyrostimulin mutants, have a shorter body length.
How thyrostimulin-like signaling might regulate the periodicity of the defecation cycle remains to be determined. The defecation behavior is controlled by motor actions, including aBoc, and by a cycle period timer. Altering either the motor program or the period timer does not necessarily affect the other, since they are functionally distinct controllers of defecation (93). Previous research revealed that the period is set by spontaneous calcium oscillations in the intestinal cells. These trigger pH oscillations and neuropeptide release in the gut, which mainly control the execution of the defecation motor program (62, 94, 95). Mutations that disrupt intestinal calcium or pH oscillations cause defects in period length and variability (55, 94, 96). In addition, cycle length is influenced by various environmental and internal factors, such as temperature (97), food (53), reactive oxygen species (56), and infection (98). We speculate that GPLA-1/GPLB-1 signaling, through activation of FSHR-1, might regulate rhythmic calcium or pH signaling in intestinal cells. This is supported by a previous study in which loss of GPLA-1 and the putative lipid-binding protein GHI-1 was shown to partially suppress defecation defects in mutants lacking flr-1, an acid-sensing member of the Degenerin/Epithelial Sodium Channel (DEG/ENaC) superfamily that is required for intestinal calcium and pH oscillations (15, 99). Interestingly, changes in intestinal pH also affect pathogen susceptibility, and mutants of both fshr-1 and gpla-1 were previously found to be more susceptible to infection (15, 60, 100). FSHR-1 has been shown to protect C. elegans from pathogenic bacteria by inducing pathogen avoidance, upregulating stress response genes, and delaying intestinal accumulation of ingested pathogens (59, 60, 88, 101), although it remains unknown whether these functions are mediated by GPLA-1/GPLB-1 signaling. In addition, FSHR-1 has been implicated in host responses to oxidative and freezing-thawing stress (60, 83). Whether FSHR-1 regulates the defecation motor program upon infection or oxidative stress has not yet been determined, but it is conceivable that thyrostimulin-like signaling may also regulate protective immune and stress responses in C. elegans.
In mammals, the hypothalamic neuropeptide TRH is a releasing factor for growth-promoting hormones, like GH and TSH from the pituitary gland (74). Vertebrate TSH and thyrostimulin both induce the release of growth-stimulating thyroid hormones (2, 10, 25). Evidence for the existence of these hormones in nematodes is lacking (102). However, we find that GPLA-1/GPLB-1 and TRH-related neuropeptides, encoded by the trh-1 gene, act in the same genetic pathway to regulate body size in C. elegans. Mutations in trh-1 also cause bloating of the intestine, although to a lesser extent than mutants defective in GPLA-1/GPLB-1 signaling, suggesting that other factors interact with thyrostimulin-like signaling as well. TRH-like neuropeptides and their receptors are widely conserved across protostomian and deuterostomian animals (43, 103–105). It will be interesting to see whether the TRH system influences the release of invertebrate glycoprotein hormones, similar to its function in vertebrates, and to further unravel the growth pathways that interact with GPA2/GPB5 signaling in the control of body size. Candidate pathways include transforming growth factor β (TGF-β) signaling, mediated by the TGF-β ligand DBL-1 and the Sma/Mab pathway, which are important regulators of body size in C. elegans (106). Additionally, the insulin/insulin-like growth factor 1 (IGF-1) pathway promotes cell growth in C. elegans, and its receptor DAF-2 controls body size in a food-dependent manner (107).
Taken together, our findings lend further support to the ancient origin of the thyrostimulin GPA2/GPB5 glycoprotein hormone system that may have evolved from mono- or homodimeric antecedents predating the split of the vertebrate lineage. Our genetic analysis of the GPA2/GPB5 system in C. elegans suggests that this neuroendocrine axis together with TRH-like neuropeptides constitute a conserved pathway that regulates growth. Our results also provide a molecular basis for further investigations into a potentially ancestral role of GPA2/GPB5 signaling in regulating intestinal function.
Materials and methods
C. elegans strains and maintenance
All C. elegans strains were maintained at 20°C on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 bacteria. All experiments were performed using 1-day adult hermaphrodites, unless mentioned otherwise. Wild-type (N2-Bristol), IBE7 flr-2 (ut5), IBE24 flr-2 (ok2127), IBE1 fshr-1 (ok778) and EG1285 lin-15B&lin-15A (n765) oxIs12 [unc-47p::GFP + lin-15(+)] strains were obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota). CB156 unc-25 (e156) and EG276 exp-1 (ox276) strains were a kind gift from the lab of W.R. Schafer (Laboratory of Molecular Biology, Cambridge, UK). Deletion alleles for gpla-1 (ibt1) and gplb-1 (ibt4) were obtained by CRISPR/Cas9 genome editing, as described below. A full list of strains used in this study can be found in Table S1.
Molecular biology
All fluorescent reporter and rescue constructs were made using the MultiSite Gateway Three-Fragment cloning system (Invitrogen). Genomic DNA or cDNA sequences of fshr-1 (a isoform), gpla-1, and gplb-1 (a isoform) were cloned in front of a SL2 trans-splicing site and fluorescent reporter gene.
Putative promoter sequences for fshr-1 (3110 bp), gpla-1 (4058 bp), and gplb-1 (3589 bp) were cloned from genomic DNA of wild-type C. elegans. Tissue-specific rescue transgenes were generated using promoter regions of rab-3 (1208 bp) (65, 66), rgef-1 (3660 bp) (69), ges-1 (3313 bp, kind gift from the lab of W.R. Schafer, Cambridge, UK) (61–63), and mir-228 (2217 bp) (64). All constructs have a let-858 3’UTR except the rgef-1 rescue construct, which was made using a tbb-2 3’UTR.
The plasmid for heterologous expression of fshr-1 in HEK cells was obtained by directionally cloning the fshr-1 cDNA into the pcDNA3.1/V5-His-TOPO vector (Invitrogen). The cDNA sequence of the fshr-1a gene isoform was amplified by PCR using cDNA from mix-staged wild-type C. elegans as template. The forward primer included a ‘CACC’ sequence at the 5’ end that introduced a partial Kozak sequence for increased translation efficiency in mammalian cells.
CRISPR/Cas9 gene editing
The gpla-1(ibt1) and gplb-1(ibt4) knockout alleles were generated by CRISPR/Cas9-mediated deletion of the gpla-1 or gplb-1 open reading frames. For each gene, two crRNA sequences were designed by looking for PAM sites (NGG) that were in the vicinity of the double stranded break site. After analysis of putative off-target sites (http://crispor.tefor.net/), we selected the highest scoring sequences based on their predicted on-target activities. Repair templates were designed to be in-frame and were codon-optimized for C. elegans (https://www.genscript.com/tools/codon-frequency-table). To simplify selection of the successfully edited worms, we used a Co-CRISPR technique in which the dpy-10 gene was also knocked out by CRISPR editing (108). We used the CRISPR/Cas9 protocol as described by Mello et al. (108) for the preparation of injection mixes containing all required components (Table S2). After injection of young adult hermaphrodites, F1 progeny that showed the rolling and/or dumpy phenotype were transferred to individual NGM plates and checked for gene deletion by PCR and subsequent gel-electrophoresis. Successful deletion of the genes was confirmed by sequencing.
The gpla-1 (ibt3 [His::GPLA-1]) knockin allele was made by CRISPR/Cas9 mediated insertion of a His-tag at the N-terminus of the gpla-1 open reading frame, using a similar strategy as described above and the crRNA and repair template listed in Table S2.
Below, the sequences of the gene knockouts and knockins are shown with flanks on the (-) strand. Exons are in grey, with start and stop codons in red, the remaining gene sequences after CRISPR gene editing are underlined, and with the inserted gene sequence in blue.
gpla-1(ibt1) deletion:
ttctttaaattctatttttaacttttcagcctataccttctcaaatgATGGGCTCCAAAGCACGAGCACGACGACGTTTAAGTTGTTTTTTAAGCGTTTTTGTTGTGACATGCTTATTACAGTACTGCACAGCAGGTGTTACTAAGAATAATAGTTGCAAAAAAGTTGgtacgtcacgaaatctactaaacttcgatcagtgtcctttaaatttttttttcagGAGTGGAGGAACTTATAGATGAAGAAGGCTGTGATTTGATGATAATTCGAATCAATCGATGCAGTGGGCATTGCTTCTCATTTACATTTCCTAATCCCTTAACGAAAAAATATTCAGTGCATGCGAAGTGCTGCCGGATGGTTGAATGGGAAATGgttagtattttttaactacagaaatgacttctgaaaatttataacaaagttattacggaagccaaaattctgggaatgtgttttgcgcaacatgtaaaaaaaatctcgtagcgaaagctacagtaattctctaaataactactgtagtgcttgcgtcgaattacggacttgatttgcgatatatccttcgtttctctgtattactttctcatttttgttttttttttaaacattctatcgaaaaattgatgattaattcatttcgaaagccgagcccgtaaatcgtcacaagcgctacagtagtcatgtaaagaattactgtatcgctacgagatgttttaatattgttttccaagaatcaatttttatttttcagCTTGAAACAGAATTAAAATGTTCCAAAGGAAACCGAAATCTTCGAATACCATCTGCAACACAATGTGAATGTTTTGATTGTCTTGTTCGATAGttgcagtttccatctctttcatttctcattcttgaatctcttgagttttacctgcataatagatttaatttattttctctcctccagtgccacattcatccacatttccataaaatctcgttcccttaattatttctataataatctcgttgatacgggcatctaaaaatgcaaaaaatcaaagcgaaagaaggatgactgacataaattgtctcaa
gplb-1(ibt4) deletion:
tgttgacgttaagctcttttgaaaaaaATGCTTATATTTCCCGTGATCACTATACTTCATATATTTTTGgtaactttttgaaatatcttctaaaatgaatttaatatgtaacaatattctaacaaaatttttaatattgatcagatagtttttccaaatttaagtattaaccaggcgcgaaattttccgaattttaggccaaaaatacggtgcccggtctcgacacgaatttttttattaggtaaaaatgggtgtgtgcctttaaagagtactgtaactttaaactttcgttgctgcggaacttttgtcgacttttcatagctattagataaaaataaaaaaatattcaatattttcaacaaatctttagaaaaactatgtaaaatcgataaaaattctgcaacaaaaatttgaagttacagtactctttaaaggcacacactcgattgtatttaacaaaaaaagtcgtgtcgagaccggttaccgtaatttttgcgcaaatcggaataatttcgcgcttgggtaataagcatcacatctccaactaatttaaaagcaaaagtgtgatttttaaattcagATTTCGGTTGAATCTGGAAAAGAATGCGAGTTTGCAATGCGATTGGTCCCAGGATTCAATCCACTTCGTCAAGTTGATGCAAATGGAAAAGAATGCCGAGGAAACGTGGAATTGCCATTTTGCAAGGGTTACTGTAAGACTAGCGAGgtgaatttccttttttttccgattcaaaaataatccaattaaatttcagAGTGGCACCCATGGCTTTCCACCACGAGTTCAAAATAGTAAAGTGTGCACATTGGTCACCACTTCAACTCGAAAAGTAGTTCTTGATGATTGTGATGATGGAGCCGATGAGAGTGTCAAGTTTGTAATGGTTCCACATGGAACTGATTGTGAATGTTCTGCAGTTCCACTTGAACAACATCATTCATAAattatcatacattcattcaaaaattcatcgaataaataaaagttttgtg
gpla-1(ibt3 [His::GPLA-1]) insertion:
ttctttaaattctatttttaacttttcagcctataccttctcaaatgATGCATCACCATCACCATCACGGCTCCAAAGCACGAGCACGACGACGTTTAAGTTGTTTTTTAAGCGTTTTTGTTGTGACATGCTTATTACAGTACTGCACAGCAGGTGTTACTAAGAATAATAGTTGCAAAAAAGTTGgtacgtcacgaaatctactaaacttcgatcagtgektcctttaaatttttttttcagGAGTGGAGGAACTTATAGATGAAGAAGGCTGTGATTTGATGATAATTCGAATCAATCGATGCAGTGGGCATTGCTTCTCATTTACATTTCCTAATCCCTTAACGAAAAAATATTCAGTGCATGCGAAGTGCTGCCGGATGGTTGAATGGGAAATGgttagtattttttaactacagaaatgacttctgaaaatttataacaaagttattacggaagccaaaattctgggaatgtgttttgcgcaacatgtaaaaaaaatctcgtagcgaaagctacagtaattctctaaataactactgtagtgcttgcgtcgaattacggacttgatttgcgatatatccttcgtttctctgtattactttctcatttttgttttttttttaaacattctatcgaaaaattgatgattaattcatttcgaagccgagcccgtaaatcgtcacaagcgctacagtagtcatgtaaagaattactgtatcgctacgagatgttttaatattgttttccaagaatcaatttttatttttcagCTTGAAACAGAATTAAAATGTTCCAAAGGAAACCGAAATCTTCGAATACCATCTGCAACACAATGTGAATGTTTTGATTGTCTTGTTCGATAGttgcagtttccatctctttcatttctcattcttgaatctcttgagttttacctgcataatagatttaatttattttctctcctccagtgccacattcatccacatttccataaaatctcgttcccttaattatttctataataatctcgttgatacgggcatctaaaaatgcaaaaaatcaaagcgaaagaaggatgactgacataaattgtctcaa
Transgenesis and expression pattern analysis
Germline transformations were carried out by injecting constructs into the syncytial gonad of young adult worms together with a co-injection marker (myo-2p::mCherry, unc-122p::dsRed, or unc-122p::GFP) and 1-kb DNA ladder (Thermo Scientific) as carrier DNA.
Expression patterns of fluorescent reporter transgenes were visualized by an Olympus confocal microscope (FluoView FV1000, IX81) and resulting Z-stack projections were created and analyzed with ImarisViewer (v9.7.2) software. Adult worms were immobilized by mounting in 50 mM Sodium Azide solution in M9 buffer on a 2% agarose pad and covered with a glass cover slip. Expression patterns were confirmed in at least two independent transgenic strains. DVB and RME expression was confirmed by crossing with marker strain EG1285, which marks GABAergic neurons (109). Other cells expressing gpla-1, gplb-1, or fshr-1 were identified based on their position and morphology.
Body size and luminal width quantification
Body size parameters (length, width, volume) of day one adults were measured using the custom WormSizer MATLAB script (https://github.com/jwatteyne/WormSizer). Synchronized L1 larvae were placed onto freshly seeded NGM plates one day post-synchronization and kept at 20°C until assaying. After 65 hours on food, 20 to 30 well-fed day one adults were transferred from the plates and anesthetized in 20 µL of 10 mM tetramisole hydrochloride solution (Sigma-Aldrich) in Milli-Q water on a 2% agarose pad. Images were captured with a ZEISS Axio Observer.Z1 at 5x magnification. Body size was measured from images of anesthetized day 1 adult worms on at least two independent days. After skeletonization, delineating the worm’s outline and midline, the worm’s total length and middle width were calculated using the calibration factor (pixels/µm) of the pictures. For the volume estimation, the midline of the worm was separated in 30 segments of the same size. The volume for each segment was determined by using the formula for the frustum of a cone, taking the natural shape of a worm into consideration. The summation of all these volumes was described as the worm’s total volume. To assess growth throughout adulthood, body size measurements were executed for 5 subsequent days (day 1 to day 5 adulthood) for at least two independent time periods.
Intestinal lumen diameter was quantified from images taken for body size measurements of day 1 adult worms. Using the ImageJ software (version 1.53s), luminal width was determined by measuring the average width (in µm) of the intestine at two different points in the posterior lumen. Body size parameters and luminal widths were plotted, and significance levels were calculated with GraphPad Prism 8 software.
Recombinant protein synthesis
Recombinant proteins were synthesized and purified by OriGene™ Technologies (Rockville, USA). To generate recombinant GPLA-1/GPLB-1, HEK293 cells were transfected with expression vectors encoding C. elegans N-terminal 6xHis-tagged GPLA-1 and non-tagged GPLB-1. Recombinant proteins were purified from cell lysate by affinity chromatography in PBS buffer (PBS and 10% glycerol, pH 7.3) and analyzed by Tricine-SDS-PAGE on a 8 – 20% gel in Tris-Glycine running buffer and by subsequent Western blot using anti-His monoclonal antibodies. A similar approach was used to synthesize individual GPLA-1 and GPLB-1 proteins. N-terminal 6xHis-tagged GPLA-1 was expressed in HEK293 cells and purified by affinity chromatography. Non-tagged GPLB-1 was expressed in HEK cells and purified using ion exchange chromatography. Both recombinant GPLA-1 and GPLB-1 protein samples were analyzed by Tricine-SDS-PAGE.
cAMP bioluminescence assay
The cAMP bioluminescence assay quantifies ligand-induced bioluminescent responses by measuring changes in intracellular cAMP levels after receptor activation. HEK293T cells were cultured in monolayer at 37°C in a humidified atmosphere with 5% CO2, in Dulbecco’s Modified Eagle’s Medium (DMEM)/Nutrient F-12 Ham (Gibco) supplemented with 10% heat inactivated Fetal Bovine Serum (FBS, Sigma-Aldrich) and 1% Penicillin/Streptomycin (Gibco). Co-transfection with the cAMP indicator CRE(6x)-luciferase and pcDNA3.1/fshr-1a was done in a 1:1 ratio when cells reached 60-70% confluency. Transfection medium contained Opti-MEM (Gibco, Life Technologies), CRE(6x)-luciferase and receptor plasmid DNA, Plus Reagent (Invitrogen) and Lipofectamine LTX (Invitrogen). One day post-transfection, fresh culture medium was added to the transfected cells. Two days post transfection, each well of a Bio-One CELLSTAR™ 96-well, Flat Bottom Microplate (Greiner) was loaded with a dilution series of recombinant protein compounds in 200 µM 3-isobutyl-1-methylxanthine (IBMX), which inhibits cAMP hydrolysis. Compound buffer (PBS with 10% glycerol) without hormone was added to the IBMX medium as a ligand-free negative control. Cells were detached, counted, pelleted and resuspended to a concentration of 10E-06 cells/mL in IBMX medium. Each well of the compound plate was supplemented with 50 µL of the cell suspension (50 000 cells/well) and the plate was incubated at 37°C for 3.5 h. Cells were then loaded with 100 µL SteadyLitePlus substrate (PerkinElmer) and incubated on a shaking plate for 15 minutes under dark conditions at RT. Finally, luminescence was measured twice for 5s (at 0s and 5s) per well at 469 nm on a Mithras LB 940 luminometer (Berthold Technologies). Measurements were performed in triplicate in at least 4 independent experiments. Luminescence values were plotted and significance levels were calculated with GraphPad Prism 8 software.
Defecation motor program assay
The defecation motor program (DMP) was analyzed as previously described (93). Animals were synchronized by letting day one adults lay eggs for 2-3 hours, before removing them from the NGM plates, and allowing the eggs to develop into day one adults. One day prior to the DMP assay, L4 animals were picked onto a freshly seeded NGM plate and all strains were blinded. After 15-17 hours, worms were acclimated on the microscope stage for ~10 minutes. At least 10 consecutive defecation cycles were observed from each animal on a Nikon stereomicroscope. Cycle length, aBoc and expulsion events were scored, and defecation behavior was logged using BORIS (Behavioral Observation Research Interactive Software). Animals were assayed alongside wild type and at least one defecation-defective control. The custom DefecationAnalysis R script was applied to measure the defecation parameters of day one adults (https://github.com/NathanDeFruyt/DefecationAnalysisBeetsLab). The length of one DMP cycle was defined by the time elapsed between two subsequent contractions of the posterior body wall muscles (pBocs). The average cycle length was calculated over 10 defecation cycles for each worm. aBoc frequency was defined as the ratio of aBoc over pBoc. Defecation parameters were plotted and significance levels were calculated with GraphPad Prism 8 software.
Sequence alignment of GPA2 and GPB5 orthologs
The H. Sapiens GPA2 and GPB5 and C. elegans GPLA-1 and GPLB-1 sequences were used as queries to identify sequences with highest similarity in selected model systems representative for their phylum by the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI) database. The multiple sequence alignment program Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) was used to align sequences, and conserved residues were identified using BoxShade (https://embnet.vital-it.ch/software/BOX_form.html).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
SK, MI, JW, LS and IB designed the research. SK, MI, SD and EV performed the experiments. SK, MI and IB analyzed data. IB and LS supervised the project and were responsible for funding acquisition. SK and IB wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Research Foundation Flanders (FWO) grant G0C0618N and KU Leuven Research Council grant C16/19/003 (to IB and LS). SD is a fellow of the Research Foundation Flanders (FWO) and JW is supported by a postdoctoral fellowship of the KU Leuven Research Council.
Acknowledgments
We thank the Caenorhabditis Genetics Center (CGC, University of Minnesota), funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), for providing strains; all members of the Beets lab for experimental advice; N. De Fruyt for help on statistics; M. Christiaens and A. Kieswetter for technical assistance; and J. Kowalski, W.R Schafer, E. Kaulich and D. Walker for advice and reagents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1200407/full#supplementary-material
References
1. Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem (1981) 50(1):465–95. doi: 10.1146/annurev.bi.50.070181.002341
2. Norris DO, Carr JA. “Organization of the mammalian hypothalamus – pituitary axes” inVertebrate endocrinology. 5th ed. Elsevier (2013) 93–150. doi: 10.1016/B978-0-12-394815-1.00004-5
3. Cahoreau C, Klett D, Combarnous Y. Structure-function relationships of glycoprotein hormones and their subunits’ ancestors. Front Endocrinol (Lausanne) (2015) 6:26. doi: 10.3389/fendo.2015.00026
4. Ryan RJ, Charlesworth MC, McCormick DJ, Milius RP, Keutmann HT. The glycoprotein hormones: recent studies of structure-function relationships. FASEB J (1988) 2(11):2661–9. doi: 10.1096/fasebj.2.11.2456242
5. Pierce JG. Eli Lilly lecture: the subunits of pituitary thyrotropin–their relationship to other glycoprotein hormones. Endocrinology (1971) 89(6):1331–44. doi: 10.1210/endo-89-6-1331
6. Querat B. Unconventional actions of glycoprotein hormone subunits: a comprehensive review. Front Endocrinol (Lausanne) (2021) 12:731966. doi: 10.3389/fendo.2021.731966
7. Roch GJ, Sherwood NM. Glycoprotein hormones and their receptors emerged at the origin of metazoans. Genome Biol Evol (2014) 6(6):1466–79. doi: 10.1093/gbe/evu118
8. Park JI, Semyonov J, Chang CL, Hsu SYT. Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine (2005) 26(3):267–76. doi: 10.1385/endo:26:3:267
9. Hsu SY, Nakabayashi K, Bhalla A. Evolution of glycoprotein hormone subunit genes in bilateral metazoa: identification of two novel human glycoprotein hormone subunit family genes, GPA2 and GPB5. Mol Endocrinol (2002) 16(7):1538–51. doi: 10.1210/mend.16.7.0871
10. Nakabayashi K, Matsumi H, Bhalla A, Bae J, Mosselman S, Hsu S, et al. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J Clin Invest (2002) 109(11):1445–52. doi: 10.1172/JCI14340
11. Sellami A, Agricola HJ, Veenstra JA. Neuroendocrine cells in Drosophila melanogaster producing GPA2/GPB5, a hormone with homology to LH, FSH and TSH. Gen Comp Endocrinol (2011) 170(3):582–8. doi: 10.1016/j.ygcen.2010.11.015
12. Al-Dailami AN, Leyria J, Orchard I, Lange AB. Exploring the role of glycoprotein hormone GPA2/GPB5 in the medically important insect Rhodnius prolixus. Peptides (2022) 149:170710. doi: 10.1016/j.peptides.2021.170710
13. Paluzzi JP, Vanderveken M, O’Donnell MJ. The heterodimeric glycoprotein hormone, GPA2/GPB5, regulates ion transport across the hindgut of the adult mosquito, Aedes aegypti. PloS One (2014) 9(1):e86386. doi: 10.1371/journal.pone.0086386
14. Sudo S, Kuwabara Y, Park JI, Hsu SY, Hsueh AJW. Heterodimeric fly glycoprotein hormone-α2 (GPA2) and glycoprotein hormone-β5 (GPB5) activate fly leucine-rich repeat-containing G protein-coupled receptor-1 (DLGR1) and stimulation of human thyrotropin receptors by chimeric fly GPA2 and human GPB5. Endocrinology (2005) 146(8):3596–604. doi: 10.1210/en.2005-0317
15. Oishi A, Gengyo-Ando K, Mitani S, Mohri-Shiomi A, Kimura K, Ishihara T, et al. FLR-2, the glycoprotein hormone alpha subunit, is involved in the neural control of intestinal functions in Caenorhabditis elegans. Genes to Cells (2009) 14(10):1141–54. doi: 10.1111/j.1365-2443.2009.01341.x
16. Williams EA, Verasztó C, Jasek S, Conzelmann M, Shahidi R, Bauknecht P, et al. Synaptic and peptidergic connectome of a neurosecretory center in the annelid brain. Elife (2017) 6:e26349. doi: 10.7554/elife.26349
17. Andreatta G, Broyart C, Borghgraef C, Vadiwala K, Kozin V, Polo A, et al. Corazonin signaling integrates energy homeostasis and lunar phase to regulate aspects of growth and sexual maturation in Platynereis. Proc Natl Acad Sci USA (2020) 117(2):1097–106. doi: 10.1073/pnas.1910262116
18. Heyland A, Plachetzki D, Donelly E, Gunaratne D, Bobkova Y, Jacobson J, et al. Distinct expression patterns of glycoprotein hormone subunits in the lophotrochozoan Aplysia: implications for the evolution of neuroendocrine systems in animals. Endocrinology (2012) 153(11):5440–51. doi: 10.1210/en.2012-1677
19. Robinson SD, Li Q, Bandyopadhyay PK, Gajewiak J, Yandell M, Papenfuss AT, et al. Hormone-like peptides in the venoms of marine cone snails. Gen Comp Endocrinol (2017) 244:11–8. doi: 10.1016/j.ygcen.2015.07.012
20. Veenstra JA. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. Gen Comp Endocrinol (2010) 167(1):86–103. doi: 10.1016/j.ygcen.2010.02.010
21. dos Santos S, Bardet C, Bertrand S, Escriva H, Habert D, Querat B. Distinct expression patterns of glycoprotein hormone-α2 and -β5 in a basal chordate suggest independent developmental functions. Endocrinology (2009) 150(8):3815–22. doi: 10.1210/en.2008-1743
22. Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, et al. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Sci (1979) (2002) 298(5601):2157–67. doi: 10.1126/science.1080049
23. Wang P, Liu S, Yang Q, Liu Z, Zhang S. Functional characterization of thyrostimulin in amphioxus suggests an ancestral origin of the TH signaling pathway. Endocrinology (2018) 159(10):3536–48. doi: 10.1210/en.2018-00550
24. Tando Y, Kubokawa K. A homolog of the vertebrate thyrostimulin glycoprotein hormone α subunit (GPA2) is expressed in amphioxus neurons. Zool Sci (2009) 26(6):409–14. doi: 10.2108/zsj.26.409
25. Okada SL, Ellsworth JL, Durnam DM, Haugen HS, Holloway JL, Kelley ML, et al. A glycoprotein hormone expressed in corticotrophs exhibits unique binding properties on thyroid-stimulating hormone receptor. Mol Endocrinol (2006) 20(2):414–25. doi: 10.1210/me.2005-0270
26. Sower SA, Decatur WA, Hausken KN, Marquis TJ, Barton SL, Gargan J, et al. Emergence of an ancestral glycoprotein hormone in the pituitary of the sea lamprey, a basal vertebrate. Endocrinology (2015) 156(8):3026–37. doi: 10.1210/en.2014-1797
27. Hausken KN, Tizon B, Shpilman M, Barton S, Decatur W, Plachetzki D, et al. Cloning and characterization of a second lamprey pituitary glycoprotein hormone, thyrostimulin (GpA2/GpB5). Gen Comp Endocrinol (2018) 264:16–27. doi: 10.1016/j.ygcen.2018.04.010
28. Rocco DA, Paluzzi JP. Expression profiling, downstream signaling, and inter-subunit interactions of GPA2/GPB5 in the adult mosquito aedes aegypti. Front Endocrinol (Lausanne) (2020) 11:158. doi: 10.3389/fendo.2020.00158
29. Yang LK, Zhang J, Liu D, Han TY, Qin QS, Wang AQ, et al. Ancestral glycoprotein hormone and its cognate receptor present in primitive chordate ascidian: molecular identification and functional characterization. Int J Biol Macromol (2023) 229:401–12. doi: 10.1016/j.ijbiomac.2022.12.297
30. Nothacker HP, Grimmelikhuijzen CJP. Molecular cloning of a novel, putative G protein-coupled receptor from sea anemones structurally related to members of the FSH, TSH, LH/CG receptor family from mammals. Biochem Biophys Res Commun (1993) 197(3):1062–9. doi: 10.1006/bbrc.1993.2586
31. Tensen CP, van Kesteren ER, Planta RJ, Cox KJA, Burke JF, Van Heerikhuizen H, et al. A G protein-coupled receptor with low density lipoprotein-binding motifs suggests a role for lipoproteins in G-linked signal transduction. Proc Natl Acad Sci USA (1994) 91(11):4816–20. doi: 10.1073/pnas.91.11.4816
32. Sun SC, Hsu PJ, Wu FJ, Li SH, Lu CH, Luo CW. Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J Biol Chem (2010) 285(6):3758–65. doi: 10.1074/jbc.M109.066266
33. Bassett JHD, van der Spek A, Logan JG, Gogakos A, Bagchi-Chakraborty J, Murphy E, et al. Thyrostimulin regulates osteoblastic bone formation during early skeletal development. Endocrinology (2015) 156(9):3098–113. doi: 10.1210/en.2014-1943
34. Macdonald LE, Wortley KE, Gowen LC, Anderson KD, Murray JD, Poueymirou WT, et al. Resistance to diet-induced obesity in mice globally overexpressing OGH/GPB5. Proc Natl Acad Sci USA (2005) 102(7):2496–501. doi: 10.1073/pnas.0409849102
35. Suzuki C, Nagasaki H, Okajima Y, Suga H, Ozaki N, Arima H, et al. Inflammatory cytokines regulate glycoprotein subunit β5 of thyrostimulin through nuclear factor-κB. Endocrinology (2009) 150(5):2237–43. doi: 10.1210/en.2008-0823
36. van Zeijl CJJ, Surovtseva Ov, Wiersinga WM, Fliers E, Boelen A. Acute inflammation increases pituitary and hypothalamic glycoprotein hormone subunit B5 mRNA expression in association with decreased thyrotrophin receptor mRNA expression in mice. J Neuroendocrinol (2011) 23(4):310–9. doi: 10.1111/J.1365-2826.2011.02116.X
37. van Zeijl CJJ, Surovtseva Ov, Wiersinga WM, Boelen A, Fliers E. Transient hypothyroxinemia in juvenile glycoprotein hormone subunit B5 knock-out mice. Mol Cell Endocrinol (2010) 321(2):231–8. doi: 10.1016/j.mce.2010.03.002
38. Vandersmissen HP, van Hiel MB, van Loy T, Vleugels R, vanden Broeck J, Silencing D. Melanogaster lgr1 impairs transition from larval to pupal stage. Gen Comp Endocrinol (2014) 209:135–47. doi: 10.1016/j.ygcen.2014.08.006
39. Rocco DA, Garcia ASG, Scudeler EL, dos Santos DC, Nóbrega RH, Paluzzi JP. Glycoprotein hormone receptor knockdown leads to reduced reproductive success in male Aedes aegypti. Front Physiol (2019) 10:266(MAR). doi: 10.3389/fphys.2019.00266
40. Rocco DA, Paluzzi JP. Functional role of the heterodimeric glycoprotein hormone, GPA2/GPB5, and its receptor, LGR1: an invertebrate perspective. Gen Comp Endocrinol (2016) 234:20–7. doi: 10.1016/j.ygcen.2015.12.011
41. Wahl M, Levy T, Manor R, Aflalo ED, Sagi A, Aizen J. Genes encoding the glycoprotein hormone GPA2/GPB5 and the receptor LGR1 in a female prawn. Front Endocrinol (Lausanne) (2022) 13:823818. doi: 10.3389/fendo.2022.823818
42. Lindemans M, Liu F, Janssen T, Husson SJ, Mertens I, Gade G, et al. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc Natl Acad Sci USA (2009) 106(5):1642–7. doi: 10.1073/pnas.0809881106
43. Van Sinay E, Mirabeau O, Depuydt G, Van Hiel MB, Peymen K, Watteyne J, et al. Evolutionarily conserved TRH neuropeptide pathway regulates growth in Caenorhabditis elegans. Proc Natl Acad Sci USA (2017) 114(20):E4065–74. doi: 10.1073/pnas.1617392114
44. Sun PD, Davies DR. The cystine-knot growth factor superfamily. Annu Rev Biophys Biomol Struct (1995) 24:269–91. doi: 10.1146/annurev.bb.24.060195.001413
45. Vitt UA, Hsu SY, Hsueh AJW. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol (2001) 15(5):681–94. doi: 10.1210/mend.15.5.0639
46. Gong Z, Wang W, el Omari K, Lebedev AA, Clarke OB, Hendrickson WA. Crystal structure of LGR ligand α2/β5 from Caenorhabditis elegans with implications for the evolution of glycoprotein hormones. Proc Natl Acad Sci USA (2023) 120(1):e2218630120. doi: 10.1073/pnas.2218630120
47. Katsura I, Kondo K, Arnano T, Ishihara T, Kawakami M. Isolation, characterization and epistasis of fluoride-resistant mutants of Caenorhabditis elegans. Genetics (1994) 136(1):145–54. doi: 10.1093/genetics/136.1.145
48. Kudo M, Chen T, Nakabayashi K, Yu Hsu S, Hsueh AJW. The nematode leucine-rich repeat-containing, G protein-coupled receptor (LGR) protein homologous to vertebrate gonadotropin and thyrotropin receptors is constitutively activated in mammalian cells. Mol Endocrinol (2000) 14(2):272–84. doi: 10.1210/mend.14.2.0422
49. Taylor SR, Santpere G, Weinreb A, Barrett A, Reilly MB, Xu C, et al. Molecular topography of an entire nervous system. Cell (2021) 184(16):4329–4347.e23. doi: 10.1016/j.cell.2021.06.023
50. Vidal B, Gulez B, Cao WX, Leyva-Diaz E, Reilly MB, Tekieli T, et al. The enteric nervous system of the C. elegans pharynx is specified by the sine oculis-like homeobox gene ceh-34. Elife (2022) 11:76003. doi: 10.7554/elife.76003
51. Jiang J, Su Y, Zhang R, Li H, Tao L, Liu Q. C. elegans enteric motor neurons fire synchronized action potentials underlying the defecation motor program. Nat Commun (2022) 13:1. doi: 10.1038/s41467-022-30452-y
52. Rhoades JL, Nelson JC, Nwabudike I, Yu SK, McLachlan IG, Madan GK, et al. ASICs mediate food responses in an enteric serotonergic neuron that controls foraging behaviors. Cell (2019) 176(1-2):85–97.e14. doi: 10.1016/j.cell.2018.11.023
53. Liu DWC, Thomas JH. Regulation of a periodic motor program in C. elegans. J Neurosci (1994) 14(4):1953. doi: 10.1523/jneurosci.14-04-01953.1994
54. Defecation motor program - C. elegans II - NCBI bookshelf (1997). Available at: https://www.ncbi.nlm.nih.gov/books/NBK20047/.
55. Branicky R, Hekimi S. What keeps C. elegans regular: the genetics of defecation. Trends Genet (2006) 22(10):571–9. doi: 10.1016/j.tig.2006.08.006
56. Tilleman L, de Henau S, Pauwels M, Nagy N, Pintelon I, Braeckman BP, et al. An n-myristoylated globin with a redox-sensing function that regulates the defecation cycle in Caenorhabditis elegans. PloS One (2012) 7(12):e48768. doi: 10.1371/journal.pone.0048768
57. Choi U, Hu M, Sieburth D. Hmc, a cell with previously unknown function couples neuropeptide transmitters with muscle contraction during a rhythmic behavior in C. elegans. Research Square - preprint (2022). doi: 10.21203/rs.3.rs-2289832/v1
58. Kim S, Sieburth D. FSHR-1/GPCR regulates the mitochondrial unfolded protein response in Caenorhabditis elegans. Genetics (2020) 214(2):409–18. doi: 10.1534/genetics.119.302947
59. Powell JR, Kim DH, Ausubel FM. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc Natl Acad Sci USA (2009) 106(8):2782–7. doi: 10.1073/pnas.0813048106
60. Miller Ev, Grandi LN, Giannini JA, Robinson JD, Powell JR. The conserved G-protein coupled receptor FSHR-1 regulates protective host responses to infection and oxidative stress. PloS One (2015) 10(9):e0137403. doi: 10.1371/journal.pone.0137403
61. Kennedy BP, Aamodt EJ, Allen FL, Chung MA, Heschl MFP, McGhee JD. The gut esterase gene (ges-1) from the nematodes Caenorhabditis elegans and Caenorhabditis briggsae. J Mol Biol (1993) 229(4):890–908. doi: 10.1006/jmbi.1993.1094
62. Wang H, Girskis K, Janssen T, Chan JP, Dasgupta K, Knowles JA, et al. A neuropeptide secreted from a pacemaker activates neurons to control a rhythmic behavior. Curr Biol (2013) 23:746–54. doi: 10.1016/j.cub.2013.03.049
63. Edgar LG, McGhee JD. Embryonic expression of a gut-specific esterase in Caenorhabditis elegans. Dev Biol (1986) 114(1):109–18. doi: 10.1016/0012-1606(86)90387-8
64. Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, Soukup GA. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev (2008) 10(1):106–13. doi: 10.1111/J.1525-142X.2007.00217.X
65. Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz RH, et al. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci (1997) 17(21):8061–73. doi: 10.1523/jneurosci.17-21-08061.1997
66. Stefanakis N, Carrera I, Hobert O. Regulatory logic of pan-neuronal gene expression in C. elegans. Neuron (2015) 87(4):733–50. doi: 10.1016/j.neuron.2015.07.031
67. Wang H, Liu J, Gharib S, Chai CM, Schwarz EM, Pokala N, et al. cGAL, a temperature-robust GAL4-UAS system for C. elegans. Nat Methods (2017) 14(2):145. doi: 10.1038/nmeth.4109
68. Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, et al. Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PloS Genet (2016) 12(7):e1006135. doi: 10.1371/journal.pgen.1006135
69. Chen L, Fu Y, Ren M, Xiao B, Rubin CS. A RasGRP, C. elegans RGEF-1b, couples external stimuli to behavior by activating LET-60 (Ras) in sensory neurons. Neuron (2011) 70(1):51–65. doi: 10.1016/j.neuron.2011.02.039
70. Beg AA, Jorgensen EM. EXP-1 is an excitatory GABA-gated cation channel. Nat Neurosci (2003) 6:11. doi: 10.1038/nn1136
71. Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci (1999) 19(2):539–48. doi: 10.1523/jneurosci.19-02-00539.1999
72. Mclntire SL, Jorgensen E, Kaplan J, Horvitz HR. The GABAergic nervous system of Caenorhabditis elegans. Nature (1993) 364:6435. doi: 10.1038/364337a0
73. Joseph-Bravo P, Jaimes-Hoy L, Uribe RM, Charli JL. 60 years of neuroendocrinology: TRH, the first hypophysiotropic releasing hormone isolated: control of the pituitary-thyroid axis. J Endocrinol (2015) 226(2):85–100. doi: 10.1530/joe-15-0124
74. Galas L, Raoult E, Tonon MC, Okada R, Jenks BG, Castaño JP, et al. TRH acts as a multifunctional hypophysiotropic factor in vertebrates. Gen Comp Endocrinol (2009) 164(1):40–50. doi: 10.1016/j.ygcen.2009.05.003
75. Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res (2006) 153:209–35. doi: 10.1016/S0079-6123(06)53012-2
76. Nishi S, Hsu SY, Zell K, Hsueh AJW. Characterization of two fly LGR (leucine-rich repeat-containing, G protein-coupled receptor) proteins homologous to vertebrate glycoprotein hormone receptors: constitutive activation of wild-type fly LGR1 but not LGR2 in transfected mammalian cells. Endocrinology (2000) 141(11):4081–90. doi: 10.1210/endo.141.11.7744
77. Kleinau G, Jaeschke H, Mueller S, Worth CL, Paschke R, Krause G. Molecular and structural effects of inverse agonistic mutations on signaling of the thyrotropin receptor - a basally active GPCR. Cell Mol Life Sci (2008) 65(22):3664–76. doi: 10.1007/S00018-008-8450-2
78. Feng X, Müller T, Mizrachi D, Fanelli F, Segaloff DL. An intracellular loop (IL2) residue confers different basal constitutive activities to the human lutropin receptor and human thyrotropin receptor through structural communication between IL2 and helix 6, via helix 3. Endocrinology (2008) 149(4):1705. doi: 10.1210/en.2007-1341
79. Xing Y, Myers RV, Cao D, Lin W, Jiang M, Bernard MP, et al. Glycoprotein hormone assembly in the endoplasmic reticulum: III. the seatbelt and its latch site determine the assembly pathway. J Biol Chem (2004) 279(34):35449–57. doi: 10.1074/jbc.M403054200
80. Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, et al. Crystal structure of human chorionic gonadotropin. Nature (1994) 369:6480. doi: 10.1038/369455a0
81. Alvarez E, Cahoreau C, Combarnous Y. Comparative structure analyses of cystine knot-containing molecules with eight aminoacyl ring including glycoprotein hormones (GPH) alpha and beta subunits and GPH-related A2 (GPA2) and B5 (GPB5) molecules. Reprod Biol Endocrinol (2009) 7(1):90. doi: 10.1186/1477-7827-7-90
82. Nagasaki H, Wang Z, Jackson VR, Lin S, Nothacker HP, Civelli O. Differential expression of the thyrostimulin subunits, glycoprotein α2 and β5 in the rat pituitary. J Mol Endocrinol (2006) 37(1):39–50. doi: 10.1677/jme.1.01932
83. Wang C, Long Y, Wang B, Zhang C, Ma DK. GPCR signaling regulates severe stress-induced organismic death in Caenorhabditis elegans. Aging Cell (2023) 22(1):e13735. doi: 10.1111/acel.13735
84. Cho S, Rogers KW, Fay DS. The C. elegans glycopeptide hormone receptor ortholog, FSHR-1, regulates germline differentiation and survival. Curr Biol (2007) 17(3):203–12. doi: 10.1016/j.cub.2006.12.027
85. Crisanti P, Omri B, Hughes EJ, Meduri G, Hery C, Clauser E, et al. The expression of thyrotropin receptor in the brain. Endocrinology (2001) 142(2):812–22. doi: 10.1210/ENDO.142.2.7943
86. Liu Y, Yang H, Liang C, Huang X, Deng X, Luo Z. Expression of functional thyroid-stimulating hormone receptor in microglia. Ann Endocrinol (Paris) (2022) 83(1):40–5. doi: 10.1016/J.ANDO.2021.11.009
87. Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron (2002) 36(6):1091–102. doi: 10.1016/S0896-6273(02)01093-0
88. Singh J, Aballay A. Intestinal infection regulates behavior and learning via neuroendocrine signaling. Elife (2019) 8:e50033. doi: 10.7554/elife.50033
89. Pukkila-Worley R, Ausubel FM, Mylonakis E. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PloS Pathog (2011) 7(6):e1002074. doi: 10.1371/journal.ppat.1002074
90. McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA, et al. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell (2011) 10(4):699–710. doi: 10.1111/J.1474-9726.2011.00713.X
91. Geisler F, Coch RA, Richardson C, Goldberg M, Bevilacqua C, Prevedel R, et al. Intestinal intermediate filament polypeptides in C. elegans: common and isotype-specific contributions to intestinal ultrastructure and function. Sci Rep (2020) 10(1):3142. doi: 10.1038/S41598-020-59791-W
92. Sheng M, Hosseinzadeh A, Muralidharan SV, Gaur R, Selstam E, Tuck S. Aberrant fat metabolism in Caenorhabditis elegans mutants with defects in the defecation motor program. PloS One (2015) 10(4):e0124515. doi: 10.1371/journal.pone.0124515
93. Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics (1990) 124(4):855. doi: 10.1093/genetics/124.4.855
94. Pfeiffer J, Johnson D, Nehrke K. Oscillatory transepithelial H+ flux regulates a rhythmic behavior in C. elegans. Curr Biol (2008) 18(4):297–302. doi: 10.1016/j.cub.2008.01.054
95. Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C. elegans. Cell (2008) 132(1):149–60. doi: 10.1016/j.cell.2007.10.058
96. Allman E, Johnson D, Nehrke K. Loss of the apical V-ATPase a-subunit VHA-6 prevents acidification of the intestinal lumen during a rhythmic behavior in C. elegans. Am J Physiol Cell Physiol (2009) 297(5):C1071. doi: 10.1152/ajpcell.00284.2009
97. Branicky R, Shibata Y, Feng J, Hekimi S. Phenotypic and suppressor analysis of defecation in clk-1 mutants reveals that reaction to changes in temperature is an active process in Caenorhabditis elegans. Genetics (2001) 159(3):997. doi: 10.1093/genetics/159.3.997
98. Yuen GJ, Ausubel FM. Both live and dead Enterococci activate Caenorhabditis elegans host defense via immune and stress pathways. Virulence (2018) 9(1):683–99. doi: 10.1080/21505594.2018.1438025
99. Kaulich E, Carroll T, Ackley BD, Tang YQ, Hardege I, Nehrke K, et al. Distinct roles for two Caenorhabditis elegans acid-sensing ion channels in an ultradian clock. Elife (2022) 11:75837. doi: 10.7554/elife.75837
100. Benomar S, Lansdon P, Bender AM, Peterson BR, Chandler JR, Ackley BD. The C. elegans CHP1 homolog, pbo-1, functions in innate immunity by regulating the pH of the intestinal lumen. PloS Pathog (2020) 16(1):e1008134. doi: 10.1371/journal.ppat.1008134
101. Singh J, Aballay A. Microbial colonization activates an immune fight-and-flight response via neuroendocrine signaling. Dev Cell (2019) 49(1):89. doi: 10.1016/j.devcel.2019.02.001
102. de Loof A, Lindemans M, Liu F, de Groef B, Schoofs L. Endocrine archeology: do insects retain ancestrally inherited counterparts of the vertebrate releasing hormones GnRH, GHRH, TRH, and CRF? Gen Comp Endocrinol (2012) 177(1):18–27. doi: 10.1016/j.ygcen.2012.02.002
103. Bauknecht P, Jékely G. Large-Scale combinatorial deorphanization of Platynereis neuropeptide GPCRs. Cell Rep (2015) 12(4):684–93. doi: 10.1016/j.celrep.2015.06.052
104. Jékely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc Natl Acad Sci (2013) 110(21):8702–7. doi: 10.1073/pnas.1221833110
105. Mirabeau O, Joly JS. Molecular evolution of peptidergic signaling systems in bilaterians. Proc Natl Acad Sci (2013) 110(22):E2028–37. doi: 10.1073/pnas.1219956110
106. Suzuki Y, Yandell MD, Roy PJ, Krishna S, Savage-Dunn C, Ross RM, et al. A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development (1999) 126(2):241–50. doi: 10.1242/DEV.126.2.241
107. So S, Miyahara K, Ohshima Y. Control of body size in C. elegans dependent on food and insulin/IGF-1 signal. Genes to Cells (2011) 16(6):639–51. doi: 10.1111/J.1365-2443.2011.01514.X
108. Dokshin GA, Ghanta KS, Piscopo KM, Mello CC. Robust genome editing with short single-stranded and long, partially single-stranded DNA donors in Caenorhabditis elegans. Genetics (2018) 210(3):781–7. doi: 10.1534/genetics.118.301532
Keywords: glycoprotein hormone, thyrostimulin, G protein-coupled receptor, growth regulation, C. elegans
Citation: Kenis S, Istiban MN, Van Damme S, Vandewyer E, Watteyne J, Schoofs L and Beets I (2023) Ancestral glycoprotein hormone-receptor pathway controls growth in C. elegans. Front. Endocrinol. 14:1200407. doi: 10.3389/fendo.2023.1200407
Received: 04 April 2023; Accepted: 23 May 2023;
Published: 20 June 2023.
Edited by:
James A. Carr, Texas Tech University, United StatesReviewed by:
Carlos Diaz-Balzac, University of Rochester, United StatesJean-Paul V. Paluzzi, York University, Canada
Copyright © 2023 Kenis, Istiban, Van Damme, Vandewyer, Watteyne, Schoofs and Beets. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabel Beets, aXNhYmVsLmJlZXRzQGt1bGV1dmVuLmJl
 Signe Kenis
Signe Kenis Majdulin Nabil Istiban
Majdulin Nabil Istiban Sara Van Damme
Sara Van Damme Elke Vandewyer1
Elke Vandewyer1 Isabel Beets
Isabel Beets