- 1Department of Obstetrics and Gynecology, The Second Hospital of Jilin University, Changchun, China
- 2Department of Hepato-Biliary-Pancreatic Surgery, The Second Hospital of Jilin University, Changchun, China
Endometriosis is a gynecological condition that significantly impacting women’s daily lives. In recent years, the incidence of endometriosis has been rising yearly and is now an essential contributor to female infertility. Exosomes are extracellular vesicles (EVs) that carry long noncoding RNA (lncRNA) and shield lncRNA from the outside environment thanks to their vesicle-like structure. The role of exosome-derived lncRNAs in endometriosis is also receiving more study as high-throughput sequencing technology develops. Several lncRNAs with variable expression may be crucial to the emergence and growth of endometriosis. The early diagnosis of endometriosis will be considerably improved by further high specificity and sensitivity Exosome lncRNA screening. Exosomes assist lncRNAs in carrying out their roles, offering a new target for creating endometriosis-specific medications. In order to serve as a reference for clinical research on the pathogenesis, diagnosis, and treatment options of endometriosis, this paper covers the role of exosome lncRNAs in endometriosis and related molecular mechanisms.
1 Introduction
Exosomes are microsomal vesicles with a diameter of 30–160 nm produced by cells and control the transmission of information from cells to the extracellular matrix by carrying proteins, lncRNA, DNA, and other molecules (1–3). LncRNAs are RNAs longer than 200 nucleotides without the ability to code for proteins (4). lncRNAs have a significant role in several crucial regulatory processes, including nuclear transport, chromatin silencing, genomic imprinting, chromatin remodeling, transcriptional activation, and transcriptional interference (5, 6). LncRNAs play an essential role in the development of endometriosis, an estrogen-dependent condition in which endothelial cells in ectopic lesions are controlled by estrogen, as well as cancers, cardiovascular disorders, and hematologic diseases (7–9). It is a common gynecological, endocrine condition that harms women’s physical and emotional health (10–12). Exosome lncRNAs have been demonstrated to involved in the genesis of endometriosis, govern the activity of endothelium cells, and serve as a clinical marker for endometriosis (13, 14). They have also been linked to infertility in endometriosis patients. Compared to patients with stage I/II endometriosis and non-endometriosis, patients with stage III/IV endometriosis had significantly higher levels of TC101441 expression in their serum EVs. Because TC101441 can travel through EVs and control ESC migration and invasion, its presence in serum EVs may serve as a biomarker for endometriosis. Laparoscopy is the only current confirmation available and that many women may not be able to afford the procedure and this could be a cheap screen.
2 Overview of exosome
2.1 Biogenesis of exosome
Exosomes, which can be seen as cup-shaped objects under electron microscopy, are vesicles actively produced extracellularly by various live cells (15–17). The cell membrane invaginates to generate endosomes, multiple endosomes merge to form Early-Sorting Endosomes (ESE), and then ESE invaginates once more to wrap intracellular material and form numerous vesicles, which further convert into Late-Sorting Endosomes (LSE) (18, 19). Several tightly controlled mechanisms are involved in producing, sorting, and releasing the exosome’s contents. Several molecules, including the Endosomal Sorting Complex Needed for Transport (ESCRT), four transmembrane proteins (CD9, CD63, and CD81), the apoptosis-linked gene 2-interacting protein X (Alix), and the tumor susceptibility gene 101(TSG101), are involved in the intracellular transport of the exosome (20–22). The soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein complex and the family of synaptic binding proteins are necessary for the production of exosomes (Figure 1) (23).
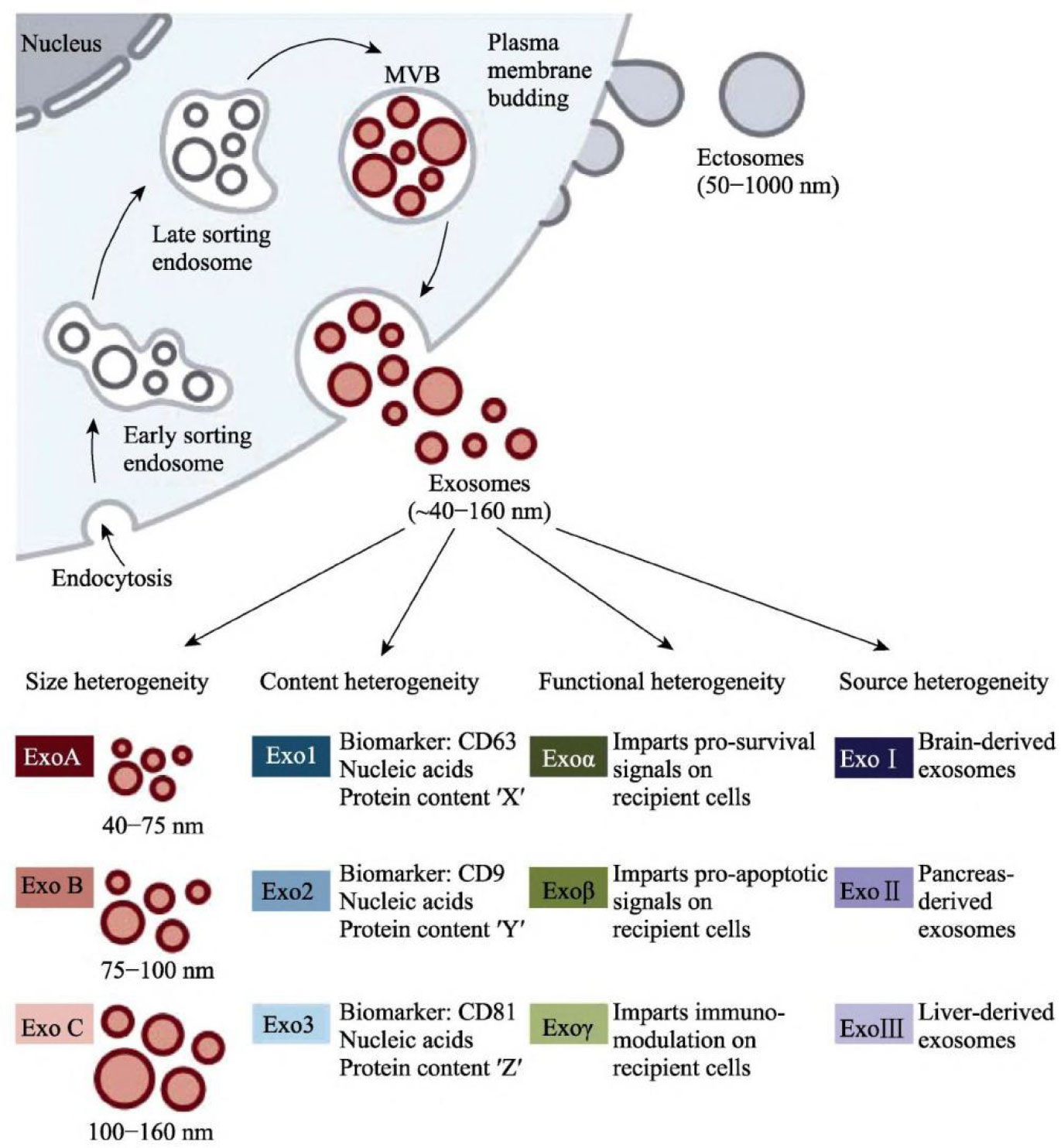
Figure 1 Production and secretion of exosomes (23).
2.2 Exosome features
Exosomes are vital in transmitting materials and information between cells (24). They can reach the appropriate target cells or organs by direct fusion, endocytosis, receptor-ligand interaction, and other mechanisms (25, 26). Intercellular communication and biological processes like antigen presentation, immunological response, and cell differentiation are regulated by endometriosis in the body (27, 28). Endometriosis are involved in intercellular communication activities and govern biological processes, including antigen presentation, immunological response, and cell differentiation, thereby partaking in the initiation and progression of diseases (2, 29, 30). Proteomics analyses have shown exosomes from different cellular origins are heterogeneous (31, 32). Exosomes can physiologically affect many cells and tissues because of their various contents (33, 34). Exosomes from a healthy microenvironment may help preserve the target cells’ function. Still, exosomes from a stressful microenvironment might cause damage by sending oxidative and inflammatory signals to the target tissues (35). So, it is suggested that exosomes serve as significant intercellular communication carriers and can transport bioactive chemicals from their source cells, which may be crucial for intercellular communication (36–38).
2.3 Exosome extraction
While exosomes represent a potential tool for endometriosis diagnosis, their efficient purification without cellular contamination is a limiting factor (39). The primary techniques for isolating exosomes are gradient density centrifugation, differential ultracentrifugation, polymer immunoprecipitation, gel exclusion separation, and membrane affinity kits. One of the most popular separation techniques nowadays is ultracentrifugation, and the purest separation can be achieved using ultracentrifugation in combination with sucrose gradient density centrifugation (40). Exosomes have a low level of toxicity, are highly bioavailable, and are biologically stable (41). Consequently, it is anticipated that the identification and isolation of disease-specific exosomes free of cellular exosomes that are typically contaminated will shed new light on the advancement of precision medicine (42). Due to the size and physicochemical characteristics of exosomes, which differ from those of lipoproteins and protein complexes, as well as the fact that a variety of cells secretes exosomes, there is still much to learn about how to isolate and purify exosomes, which is a bottleneck problem in basic research and clinical applications related to exosomes.
3 Overview of lncRNA
3.1 Occurrence of lncRNA
LncRNAs are a family of RNAs with transcripts longer than 200 nucleotides frequently present in eukaryotic genomes but do not code for proteins (43, 44). Most lncRNAs can be found in the cytoplasm or nucleus of a cell and are produced from a single strand inside a protein-coding gene sequence (45). Antisense Bidirectional lncRNAs share promoters with protein-coding genes but are transcribed in the opposite direction to protein-coding genes; long intergenic ncRNAs (lincRNAs) are produced from the complementary DNA strand of the protein-coding gene, which is transcribed in the opposite direction and overlaps with at least one exon of the forward gene. Intronic lncRNAs are found in the protein-coding gene’s intron region and do not overlap with its exons (46, 47).
3.2 Biological functions of lncRNA
Recent research has shown that lncRNAs are crucial for maintaining intracellular homeostasis when cells or tissues mature (48). This dispels the myth that noncoding RNAs (ncRNAs) are just “transcriptional noise” with no biological purpose (49, 50). It has been demonstrated that LncRNAs have a role in a variety of physiological and pathological processes (51, 52). Via epistasis, transcription, post-transcription, and acting as mediators of biological processes, lncRNAs are implicated in the regulation of apoptosis: epistasis lncRNAs can bind to Pol II to repress DNA expression, bind to DNA to form a triple helix structure to regulate, and can bind to transcription factors or recruit transcription-related factors to target genes to regulate the transcription of target genes (53–55). These lncRNA-specific structures or sequences can be specific sites on genomic DNA and can recruit the chromatin reconstruction complex (56, 57). The post-transcriptional regulation impacts the binding shear body and regulates the shearing process of mRNA by binding to antisense lncRNA at the target region of mRNA (Figure 2) (58, 59).
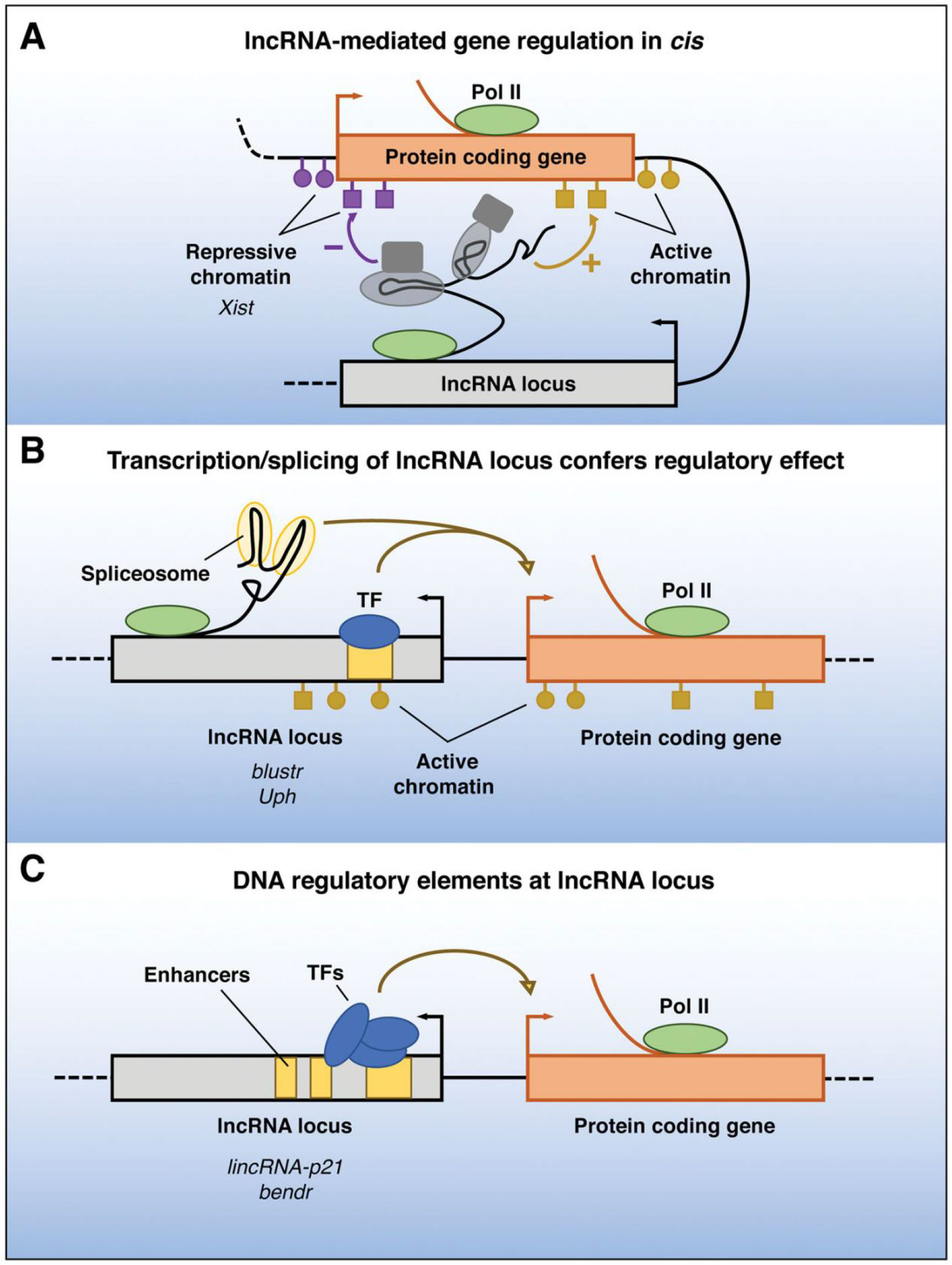
Figure 2 Functions of lncRNA loci in local gene regulation. The ability of a lncRNA locus to control the expression of nearby genes in cis may be due to DNA elements within the lncRNA promoter or gene body that function independently of the transcribed RNA (A), may require transcription or splicing of an RNA but the lncRNA itself is not functional (B), or may be due to sequence-specific functions of the mature lncRNA transcript (C)—Pol II, RNA polymerase II; TF, transcription factor (59).
3.3 LncRNA regulates mRNA transport and translation
Before being translated into protein, pre-mRNA is specially spliced in the nucleus to generate mature mRNA, which is subsequently translocated from the heart to the ribosome in the cytoplasm. Only when the structural gene ultimately succeeds in producing an active protein is the gene considered functional (60). Several studies have demonstrated that lncRNAs can control mRNA translation in various ways (61). mRNA transport can be impacted by lncRNAs, which in turn control adipogenesis. In addition to acting as an insulin-sensitizing hormone to lower blood sugar and encourage adipogenesis, lncRNAs are an endogenous bioactive peptide released by adipocytes during lipogenesis. AdipoQ ASlncRNA joins forces with AdipoQ mRNA to create a double-strand in the nucleus, preventing AdipoQ mRNA from moving from the core to the cytoplasm suppressing adipogenesis. In order to play a part in controlling the stability of the target genes, lncRNA often attaches to the relevant mRNA or particular protein to form an RNA-RNA or RNA-protein complex (62). Antisense lncRNAs’ cytoplasmic binding to sense mRNAs is a typical example of this regulation. It has been demonstrated that lncRNAs can associate with RNA-binding proteins (RBPs) to create RNA-protein complexes that subtly control the stability of mRNA (63).
3.4 Competing endogenous RNA functions
LncRNAs can indirectly regulate the expression of target genes by competing for binding one or more miRNAs with the same response element, thereby promoting or inhibiting the onset or progression of disease (64, 65). This is a critical distinction between lncRNAs and miRNAs: the latter cannot interfere with or activate gene expression. LncRNAs can interact with miRNAs as a competitive endogenous RNA (ceRNA), functioning as a “sponge” for the miRNA molecule and easing its stress (66). This is one of the many ways lncRNAs and miRNAs function in cells. The ceRNA mechanism is a large and delicate method of gene expression control whose expression level is affected by conditions (67). LncRNAs, circRNAs, pseudogenes, synthetic miRNA inhibitors, and viral miRNA inhibitors are currently ceRNAs (68).
3.5 LncRNA controls the expression of inflammatory, immunological factors
LncRNAs can regulate gene expression and immune system responses, demonstrating biological processes’ tissue specificity and complexity (69). Involved in the body’s intrinsic and adaptive immunity, lncRNAs are crucial to many physical and immunological methods. The etiology of autoimmune and inflammatory illnesses is influenced by lncRNAs, such as lincRNA-Cox2 and lncRNA-dendritic cells (DC) (70). Numerous significant pathways connected to endometriosis are enriched in lncRNAs. Transient Receptor Potential (Trp) ion channels and thyroid hormone production were discovered to produce large amounts of inflammatory mediators, which suggests that lncRNAs may regulate the expression of inflammatory and immunological components linked to endometriosis (71). Inflammatory reactions may develop and be controlled in part by lncRNAs. According to immunological research, epigenetic dysregulation of B cells, which includes aberrant lncRNA expression, histone changes, and altered DNA methylation, might result in the body producing pathogenic autoantibodies. Endometriosis and autoimmune disorders share a biological mechanism with lncRNAs. Current hormonal and surgical treatments for endometriosis may be improved if the concept of immunomodulatory therapy for autoimmune illnesses is extended to the treatment of endometriosis (72, 73).
3.6 Correlation between lncRNA, miRNA, and endometriosis
Reverse regulatory RNA, siRNA competitive binding, and target gene stability regulation are techniques that LncRNAs can use to control target genes (74). LncRNAs can work as ceRNAs to control the expression of target genes, altering how well those genes function and becoming a key player in the emergence of disease (75, 76). MiR-199a can compete with vascular endothelial growth factor (VEGF)-A for the binding site on the lncRNA ENST00000465368, which reduces miR-199a’s ability to bind to its target gene VEGF-A and weakens miR-199a (77). Inducing downstream signaling pathways, endothelial cell proliferation and migration, increased microvascular permeability, promotion of neovascularization, and successful ectopic endothelial implantation are all made possible by the angiogenesis-specific regulator VEGF-A, which binds to its receptor (78, 79). A decrease in the expression of Syndecan-1 (SDC1), a cell adhesion molecule, could result from downregulating the expression of lncRNANR 033688. This could enhance the repressive effect of miR-10b on its target gene SDC1, which would reduce cell-cell and cell-matrix adhesion, facilitating the invasion of ectopic endometrial glandular epithelial cells (80). The growth of endometriosis and the interactions between miRNAs and lncRNAs are tightly connected, and endometriosis proliferation, invasion, and metastasis are all significantly impacted by these factors (81, 82).
4 Exosome lncRNA has excellent potential for clinical applications
Exosome-derived lncRNAs have potential uses. lncRNAs are RNAs with strong regulatory abilities (83). lncRNAs can control the expression of mRNA through ceRNA processes, bind directly to proteins to alter or increase their functions, form complexes with DNA to affect gene transcription, and more (84). One of the critical pathways in the development of endometriosis is lncRNA expression imbalance (85). The neural CHL1 gene family includes the close homologue of the L1 (CHL1) gene, and CHL1-AS1 and CHL1-AS2 are two antisense lncRNA molecules of the CHL1 mRNA (86). Compared to in situ endometrial tissues, CHL1-AS1, CHL1-AS2, and CHL1 mRNA expression was considerably higher in ectopic endometrial tissues. CHL1 participates in the formation of endometriosis through interactions with CHL1-AS1 and CHL1-AS2. CHL1-AS1 or CHL1 can bind to MiR-6076, which controls the expression of CHL1. Reduced expression of the CHL1-AS1 or increased miR-6076 have a protective effect on cell migration and proliferation (87).
Exosome lncRNA plays a significant role in the entire biological process of endometriosis compared to conventional endometriosis diagnosis and treatment. It exhibits the following features: Exosome lncRNA research helps ESCs proliferate, metastasize, and evolve while providing the molecular underpinnings for their targeted therapy. It is also more accessible for clinical diagnosis and relatively non-invasive (available in plasma, urine, and vaginal fluids) (88, 89). The exosome can influence the immune response in targeted therapy and release chemotherapeutic medicines and nucleic acids locally with low toxicity and high efficacy. Its contents are proteins and different nucleic acids uniquely expressed in distinct diseases. Exosome lncRNA research can investigate the pathophysiology of endometriosis, screen endometriosis for biomarkers, and offer a new fundamental framework for illness diagnosis and treatment (90).
5 Antifibrotic effects of exosome lncRNAs
The uterine cavity is invaded by endometrial cells, which then migrate to locations outside of it and undergo cyclic damage repair (91). The ability of stromal tissue to infiltrate and migrate causes a change from endometrial stromal fibrosis to fibrosis, which causes cell adhesion, collagen aggregation, and finally, fibrosis, resulting in the frequent presence of fibrous connective tissue around endometriosis lesions (92, 93). Endometrial stromal cells’ ability to transition from stromal fibrosis to fibrosis and the invasion and migration of endometrial cells can be aggravated by prolonged high estrogen stimulation levels in the body (94, 95). This can cause several clinical symptoms, including chronic inflammation, progressive pelvic pain, and infertility (96). Infertility is more difficult by the fibrosis forming the walls and surrounding ovarian endometriotic cysts (97). Under estrogenic control, highly expressed H19 can enhance actin alpha 2 (ACTA2) expression by decreasing miR-216a-5p, further boosting the invasive and migratory abilities of stromal cells and ultimately leading to the development of endometriosis lesions and the formation of fibrosis at the lesions (98, 99). A lncRNA called Homeobox Gene Transcript Antisense Intergenic RNA (HOTAIR) has been linked to several fibrotic disorders. By increasing miR-326, which decreases cell proliferation, promotes fibrosis, and promotes apoptosis, HOTAIR silencing may inhibit NUS1 expression. Exosome lncRNAs may develop into novel endometriosis inhibitory vectors and present novel therapeutic approaches for managing endometriosis. To treat fibrosis and aid in the prevention and treatment of endometriosis, antifibrotic lncRNAs carried by exosomes may be exploited as therapeutic targets.
6 Exosomal lncRNAs and endometriosis
6.1 Endometrial cells with tumorigenic properties
Endometrial glands and stromal implants, known as endometriosis, are found inside the uterine cavity but develop outside (100). Even though endometriosis is a benign condition, it is frequently described as “benign cancer” due to its local infiltration, implantation, metastasis, and recurrence (101–105). Surgery is an invasive procedure with some dangers, and pathological examinations take too long to diagnose endometriosis. More significant and of theoretical relevance are future investigations into the pathogenesis of endometriosis and the hunt for novel biomarkers and molecular targets for diagnosis and therapy (106). An essential characteristic of endometriosis is the enhanced capacity of endometrial cells to infiltrate and migrate (107). Endometriosis lesions adhere extensively or densely to the tissues around them, and many different lesions exist. lncRNAs play a significant role in controlling human disease, physiology, and cancer (108, 109). As elaborated in section 6.3, more researchers are discovering that lncRNAs are abnormally expressed in endometriosis patients and play a role in controlling the progression of the illness due to the in-depth study of lncRNAs in recent years. Exosome extracellular nucleotidase was discovered to be a diagnostic sign for endometriosis in ovarian endometriosis cysts.
6.2 Immunomodulatory imbalance is closely associated with the occurrence of endometriosis
The pathogenesis and pathophysiology of endometriosis, classified as chronic inflammatory illnesses, are significantly influenced by immunological variables. However, only a tiny portion of the population has endometrial fragments that spread, grow, and eventually develop into endometriosis. This is likely because of changes in the internal environment of endometriosis patients, such as immune imbalance, and the ectopic lesions are typically accompanied by inflammatory congestion and inflammatory infiltration (110). Many women experience the “menstrual reflux” phenomenon multiple times. One of the clinical classification indicators of endometriosis is the degree of inflammation. The abnormal immune function in endometriosis patients is primarily exhibited in two ways: on the one hand, it is a compromised immunosurveillance and immunocidal cytotoxic effect, which prevents the body from effectively clearing the endothelium; and on the other hand, it is a compromised immune environment in the abdominal cavity, which interferes with pregnancy and causes spots. Infertility, repeated implantation failure, early miscarriage, and aberrant histogenesis are related to endometriosis worsened by the peripheral circulating immune system and female endometrial autoimmune imbalance (Figures 3, 4) (111).
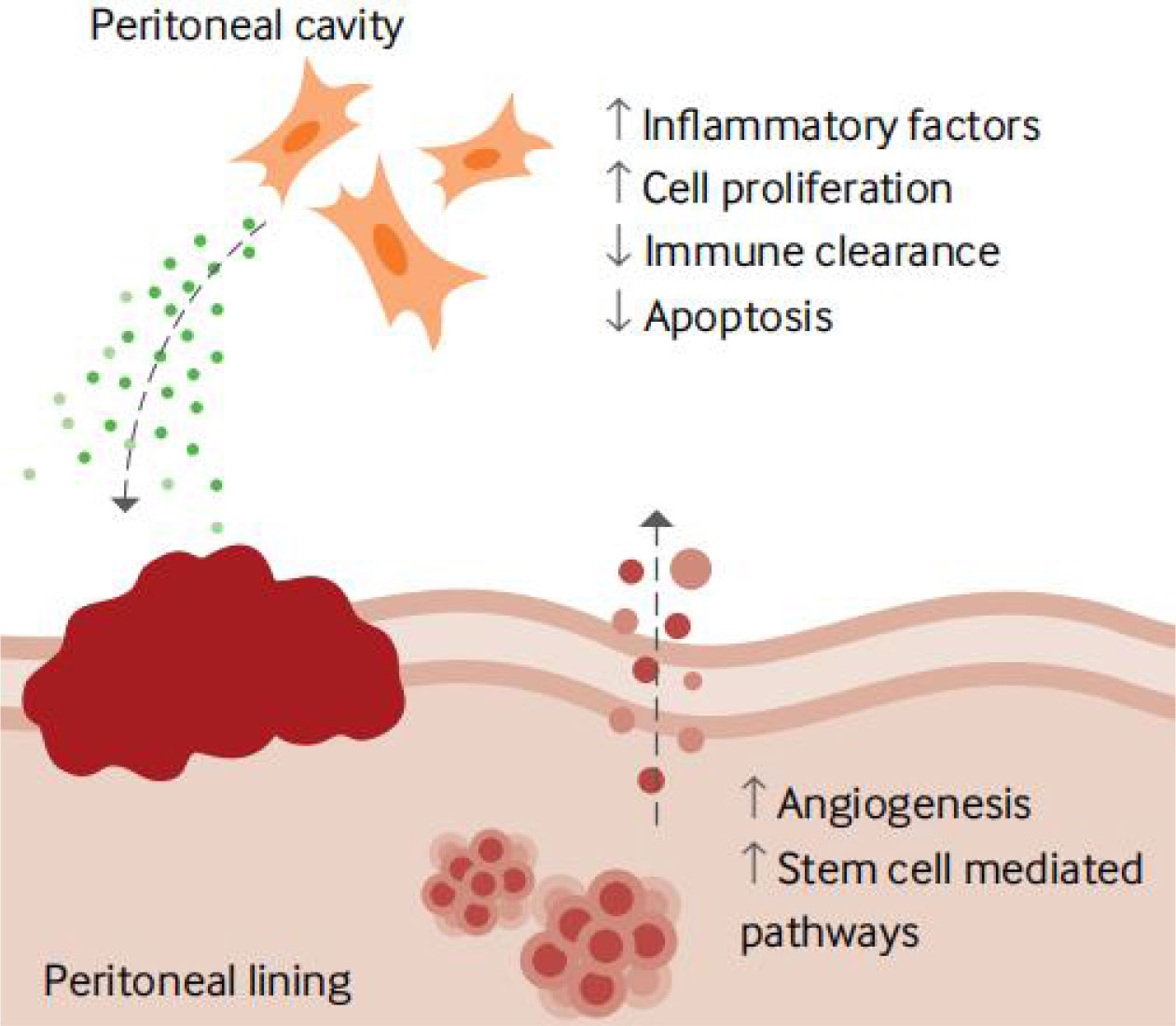
Figure 3 Local factors involved in developing an endometriosis lesion (111).
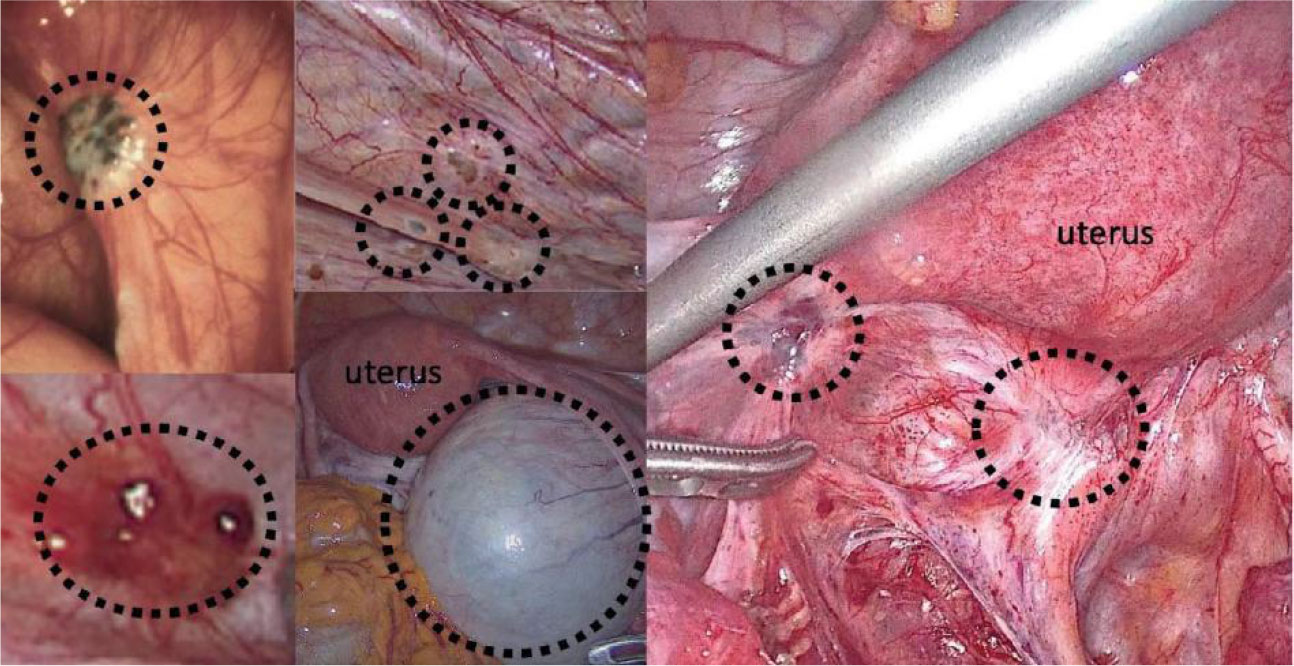
Figure 4 Surgical images of endometriosis sub-phenotypes (111).
6.3 Exosome lncRNA regulates the development of endometriosis
6.3.1 Promotes neovascularization in endometriosis
Research has shown that neuro angiogenesis is crucial for the growth of endometriosis and that ESC-derived exosomes promote angiogenesis in vitro (112). Local angiogenesis and neurogenesis are encouraged, macrophages are activated, endometriosis lesions are made more extensive, and endometriosis advance when in situ ESC-derived exosomes are present (113). Angiogenesis-related lncRNA antisense hypoxia-inducible factor (a-HIF) is widely known (114). In the ectopic endometrium and serum of endometriosis patients, aHIF is abundantly expressed. Hence, exosome lncRNA-aHIFs are being investigated as potential molecular markers for the detection of endometriosis (115, 116). a-HIF plays a role in increasing angiogenesis in endometriosis and is strongly expressed in the endometriotic stromal cells (ECSCs) of patients with endometriosis. Another hypothesis for a molecular marker for the diagnosis of endometriosis is a-HIF. By activating VEGF-A, VEGF-D, and primary fibroblast growth factor (bFGF) to induce angiogenesis in human umbilical vein endothelial cells(HUVECs), exosomeaHIF is transported from ECSCs to HUVECs, which may have diagnostic relevance by stimulating angiogenesis in endometriosis (117, 118). The basic idea for the pathogenesis of endometriosis is the transcatheter blood flow reversal theory, and it is plausible that exosomes generated by healthy endometrium might travel through the blood to the pelvis or other places to advance endometriosis (119).
6.3.2 Regulating the biological behavior of endometriosis cells
The emphasis of the research is on how exosome lncRNA affects the biological function of endometriosis. LINC00261 directly binds to miR-132-3p to regulate BCL2L11 expression, which inhibits the proliferation and invasion of endometriosis cells, suggesting that LINC00261 plays an inhibitory role in the occurrence and development of endometriosis (120, 121). Expression of LINC00261 was significantly downregulated in ectopic endothelial tissues. Cell proliferation and migration decreased considerably after LINC00261 was overexpressed. Since LINC01279 expression in endometriosis tissues was markedly increased and connected with cyclin-dependent kinase 14 (CDK14) expression, LINC01279 may control endometriosis’ cell cycle (122). In patients with endometriosis, lncRNACCDC144NL-AS1 showed differential expression in matched in situ and ectopic endothelial tissues. ESC migration and invasion were hindered by CCDC144NL-AS1 deletion, and CCDC144NL-AS1 knock-down reduced wave protein and matrix metalloprotein (MMP-9) expression, indicating that CCDC-144NL-AS1 may play a role in the pathogenesis of endometriosis (123). Application of BRAF-activated noncoding RNA (BANCR) inhibitors in a rat model of endometriosis resulted in a significant reduction in lesion size, accompanied by a decrease in mesenchymal cells and blood supply, and the BANCR inhibitors may inhibit endometriosis by inhibiting the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling pathway to reduce the expression of molecules associated with angiogenesis (124).
6.3.3 Correlation of cellular autophagy with the occurrence of endometriosis
Cellular autophagy is induced by hypoxia, a crucial microenvironmental element in endometriosis growth of endometriosis (125). Hypoxia caused a time-dependent induction of autophagy and the lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in cultured human ESCs (126). nuclear factor kappa-B/induciblenitricoxidesynthase (NF-κB/iNOS) co-stimulation was linked to an inflammatory response, and increased expression of iNOS promotes angiogenesis (127). MALTA1 expression was considerably raised in ectopic tissues of patients with endometriosis. MALAT1 regulated the proliferation, invasion, and death of ESCs via NF-B/iNOS. On the one hand, the hypoxic environment in the pelvis boosted MALAT1 expression, while on the other, it promoted autophagy in ESCs, allowing endothelium debris to survive ectopically. And by inhibiting the autophagy brought on by hypoxia, MALAT1 expression suppression in ESCs may enhance apoptosis (126). Actinfilament-associatedprotein1-antisenseRNA1 (AFAP1-AS1) was significantly up-regulated in endometriosis tissues, and lncRNA-AFAP1-AS1 significantly downregulated the expression of pGL3-P886, the promoter of the transcription factor zinc-fingerE-boxbindinghomeobox1(ZTB1), promoted epithelial-mesenchymal transition (EMT), and increased ectopic endometrial invasion and implantation (128). By encouraging EMT, LINC01541 can be silenced to improve ESC invasion, whereas LINC01541 overexpression can inhibit EMT, ESC metastasis, and VEGF-A production, as well as cause apoptosis through controlling the Wnt/-catenin pathway (129). Exosome lncRNA encourages endometriosisESC invasion and proliferation, and it can act as a diagnostic marker for endometriosis to aid in the early detection of endometriosis.
6.4 Exosome lncRNA mediates infertility linked to endometriosis
Patients with endometriosis frequently experience infertility, and aberrant changes in endometrial status and uterine artery flow resistance can impact endometrial tolerance and, in turn, affect pregnancy and prognosis (130–132). Studies have revealed that the in situ endometrium of endometriosis exhibits a marked reduction in H19 expression (133). H19 usually is abundantly expressed in late-proliferating ESCs, whereas in the situ endometrium of endometriosis, its expression is downregulated. Low levels of H19 raise the activity of miRNALet-7, which in turn suppresses in situ ESC proliferation and survival and inhibits the downstream target insulin-like growth factor 1 receptor (IGF1R) at the post-transcriptional level. This results in situ endometrial defects, which affect endometrial tolerance and obstruct embryo implantation. By interfering with the H19/Let-7/IGF1R regulation system, endometriosis patients may experience spontaneous miscarriage or infertility due to diminished endometrial readiness and pregnancy receptivity. Through controlling miR-124-3p and Integrin beta-3 (ITGB3), lncRNA-H19 downregulation may prevent the proliferation and invasion of ectopic endometrial cells. This gives the treatment of endometriosis a new target (134).
Endometriosis can hinder oocyte growth and maturation, and as endometriosis levels rise, so does the disruption of ovarian reserve function. The expression of lncRNMALAT1 is increased in ectopic endometrial tissue (135). Through increasing the EMT-related transcription factors ZEB1 and ZEB2, MALAT1, a ceRNA of miR-200C, can support ESCs, boosting proliferation, invasion, and ectopic pregnancy. In ovarian granulosa cells of endometriosis, the expression of lncRNA-MALAT1 was markedly downregulated and positively associated with the number of sinus follicles (136). Further in vitro research supported the hypothesis that MALAT1 knock-down could affect follicular granulosa cell proliferation by activating the ERK/MAPK signaling pathway and up-regulating the expression of cell cycle regulatory proteins and that up-regulating the lncRNAENST00000433673 could affect embryo implantation and impair female fertility (137–139).
6.5 A potential diagnostic sign for endometriosis is exosome lncRNA
Exosome lncRNAs have proven to offer promise as non-invasive biomarkers for determining the presence of illness. In endometriosis, lncRNA AC002454.1 is considerably overexpressed, and the expression level is closely associated with cyclin-dependent kinase6(CDK6) levels (140). The biological behavior of endometrial cells may be cooperatively influenced by AC002454.1 and CDK6, which may work in concert to encourage the growth of endometriosis (141). In the endometrial tissues of endometriosis patients and healthy individuals, 86 lncRNAs showed differential expression. KEGG pathway analysis revealed that these lncRNAs were connected to various biological functions and signaling pathways in endometriosis (142). The lncRNA ENST00000482343 in the serum of endometriosis patients could be used as a potential molecular marker, according to earlier studies that examined the differential expression profiles of lncRNAs in 110 serum samples and 24 tissue samples and assessed their diagnostic value using the receiver operating characteristic curve. In ovarian endometriotic tissues, Urothelial Cancer Associated 1 (UCA1) expression was markedly downregulated, and the extent of UCA1 downregulation was positively connected with the severity of ovarian endometriosis (143). UCA1 might be used as a biomarker to evaluate the prognosis and staging of ovarian endometriosis (143). HOXA11-AS1 and HOXA gene mRNA fragments Patients with endometriosis had considerably lower expression levels in their in situ endometrium than in their ectopic endometrium, which raises the possibility that HOXA11-AS1 may be involved in the growth of peritoneal endometriosis (144).
In comparison to in situ and normal endometrium, ectopic endometrium expressed TC0101441 at a greater level. High expression of TC0101441 in ECSCs produced EVs, which were subsequently internalized and absorbed by low-expression ECSCs to achieve TC0101441 transfer in ECSCs and eventually encourage endometriosis migration and invasion (145). Women with endometriosis had less maternally expressed gene 3 (MEG3)-210 in their ectopic endometrium (146). ESC migration and invasion are encouraged by the downregulation of MEG3-210. Through interacting with Galectin-1 via p38MAPK and cyclic AMP-dependent protein kinase A/sarco-endoplasmic reticulum ATPase (PKA/SERCA) signaling, mEG3-210 promotes ESC migration and invasion (147). In endometriosis, miR-145 reduces tumor growth, invasiveness, and stem cell characteristics. Prostate cancer-associated transcript-1 (PCAT-1) lncRNA and siRNA dramatically boosted miR-145 expression, reducing endometriotic stem cell proliferation and invasiveness (148). Normal endometrial tissues had a lower ratio of lncRNA steroid receptor activator (SRA) to steroid receptor activating protein (SRAP) than endometriosis tissues. In contrast to normal endometrial tissue, the expression of SRA and ER-lncRNA in endometriotic ovarian tissue was lower than that of SRAP and ER-lncRNA. Through controlling ER, SRA in ovarian endometriosis may play a significant regulatory function in the development of ESCs (149).
7 Conclusion
Exosome lncRNA can be a valuable biomarker for endometriosis, a prevalent gynecological, endocrine disorder that harms women’s reproductive health and significantly burdens society. The present diagnostic indicators for endometriosis have low specificity and sensitivity. Hence, alternative diagnostic indicators must be discovered to increase the diagnostic effectiveness of endometriosis. lncRNAs are crucial for epigenetic control, transcription, translation, RNA metabolism regulation, cell autophagy, and death, among other processes. LncRNAs are quickly disturbed by other components in the circulation; however, because of the protection provided by the EVs structure, lncRNAs encapsulated in exosomes are not as easily destroyed once they have entered the circulation. Exosome lncRNA is stably expressed in serum or bodily fluids and can be employed as a disease biomarker to create a diagnostic model that will direct early diagnosis and postoperative follow-up of endometriosis, enable early disease treatment, and avoid recurrence. Exosome lncRNA research in endometriosis is still in its infancy, and there are still a lot of unexplored frontiers. The issues mentioned above can be resolved piecemeal with the rapid advancement of proteomics, transcriptomics, and bioinformatics analysis. Researchers will have a better knowledge of the role of exosome lncRNA in the genesis of endometriosis and its therapeutic utility.
Author contributions
MW, LZ, RL, SM, JL, and SY performed literature searches and selected the studies and reviews discussed in the manuscript. The first draft of the manuscript was prepared by MW. LZ, RL, SM, and JL made subsequent amendments. SY revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was provided by Project of Jilin Provincial Education Department and Jilin Provincial Health and Family Planning Commission Project (No.JJKH20201049KJ and No.2018J059).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Nojima T, Proudfoot NJ. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat Rev Mol Cell Biol (2022) 23(12):853–. doi: 10.1038/s41580-022-00551-1
2. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol (2018) 19(4):213–28. doi: 10.1038/nrm.2017.125
3. Wang Y, Liu Q, Wang F. Potential roles of exosome noncoding RNAs in cancer chemoresistance. Oncol Rep (2021) 45(2):439–47. doi: 10.3892/or.2020.7887
4. Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng X, et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer (2020) 19(1):22. doi: 10.1186/s12943-020-1147-3
5. Tsagakis I, Douka K, Birds I, Aspden JL. Long noncoding RNAs in development and disease: conservation to mechanisms. J Pathol (2020) 250(5):480–95. doi: 10.1002/path.5405
6. Zubrzycka A, Zubrzycki M, Perdas E, Zubrzycka M. Genetic, epigenetic, and steroidogenic modulation mechanisms in endometriosis. J Clin Med (2020) 9(5):1309. doi: 10.3390/jcm9051309
7. Lan X, Wu N, Wu LT, Qu K, Osoro EK, Guan DX, et al. The human novel gene LNC-HC inhibits hepatocellular carcinoma cell proliferation by sequestering has-miR-183-5p. Mol Therapy-Nucleic Acids (2020) 20:468–79. doi: 10.1016/j.omtn.2020.03.008
8. Zhao JL, Chen FD, Ma W, Zhang P. Suppression of long noncoding RNA NEAT1 attenuates hypoxia-induced cardiomyocytes injury by targeting miR-378a-3p. Gene (2020) 731:144324. doi: 10.1016/j.gene.2019.144324
9. Kar R, Dhar R, Mukherjee S, Nag S, Gorai S, Mukerjee N, et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Engineering (2023) 9(2):577–94. doi: 10.1021/acsbiomaterials.2c01329
10. Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell (2021) 184(11):2807–24. doi: 10.1016/j.cell.2021.04.041
11. Xiang DF, Zhao M, Cai XF, Wang YX, Zhang L, Yao HL, et al. Betulinic acid inhibits endometriosis through suppression of estrogen receptor beta signaling pathway. Front Endocrinol (2020) 11. doi: 10.3389/fendo.2020.604648
12. Ceccaroni M, Bounous VE, Clarizia R, Mautone D, Mabrouk M. Recurrent endometriosis: a battle against an unknown enemy. Eur J Contraception Reprod Health Care (2019) 24(6):464–74. doi: 10.1080/13625187.2019.1662391
13. Hudson QJ, Proestling K, Perricos A, Kuessel L, Husslein H, Wenzl R, et al. The role of long noncoding RNAs in endometriosis. Int J Mol Sci (2021) 22(21):11425. doi: 10.3390/ijms222111425
14. Ahmed W, Liu ZF. Long noncoding RNAs: novel players in regulation of immune response upon herpesvirus infection. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.00761
15. Han J, Zhang Y, Ge P, Dakal TC, Wen H, Tang S, et al. Exosome-derived CIRP: an amplifier of inflammatory diseases. Front Immunol (2023) 14. doi: 10.3389/fimmu.2023.1066721
16. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell (1983) 33(3):967–78. doi: 10.1016/0092-8674(83)90040-5
17. Khazaei F, Rezakhani L, Alizadeh M, Mahdavian E, Khazaei M. Exosomes and exosome-loaded scaffolds: characterization and application in modern regenerative medicine. Tissue Cell (2023) 80:102007. doi: 10.1016/j.tice.2022.102007
18. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367(6478):640. doi: 10.1126/science.aau6977
19. Nafar S, Nouri N, Alipour M, Fallahi J, Zare F, Tabei SMB. Exosome as a target for cancer treatment. J Invest Med (2022) 70(5):1212–8. doi: 10.1136/jim-2021-002194
20. Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harbor Perspect Biol (2013) 5(9):a016766. doi: 10.1101/cshperspect.a016766
21. Gall AR, Amoah S, Kitase Y, Jantzie LL. Placental mediated mechanisms of perinatal brain injury: evolving inflammation and exosomes. Exp Neurol (2022) 347:113914. doi: 10.1016/j.expneurol.2021.113914
22. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem (2019) 88(1):487–514. doi: 10.1146/annurev-biochem-013118-111902
23. Tang YF, Long JQ. Placenta-derived exosomes and their role in the pathogenesis of adverse pregnancies. Chin J Birth Health Heredity (2023) 31(03):649–54. doi: 10.13404/j.cnki.cjbhh.2023.03.030
24. Xia X, Wang Y, Qin Y, Zhao S, Zheng JC. Exosome: a novel neurotransmission modulator or non-canonical neurotransmitter? Ageing Res Rev (2022) 74:101558. doi: 10.1016/j.arr.2021.101558
25. Hannafon BN, Gin AL, Xu YF, Bruns M, Calloway CL, Ding WQ. Metastasis-associated protein 1 (MTA1) is transferred by exosomes and contributes to the regulation of hypoxia and estrogen signaling in breast cancer cells. Cell Communication Signaling: CCS (2019) 17(1):13. doi: 10.1186/s12964-019-0325-7
26. Saheera S, Potnuri AG, Krishnamurthy P. Nano-vesicle (Mis)Communication in senescence-related pathologies. Cells (2020) 9(9):1974. doi: 10.3390/cells9091974
27. Guo M, Hao Y, Feng Y, Li H, Mao Y, Dong Q, et al. Microglial exosomes in neurodegenerative disease. Front Mol Neurosci (2021) 14. doi: 10.3389/fnmol.2021.630808
28. Sun X, Ding T, Wang B, Chang Z, Fei H, Geng L, et al. Identification of lncRNA-miRNA-mRNA networks in circulating exosomes as potential biomarkers for systemic sclerosis. Front Med (2023) 10. doi: 10.3389/fmed.2023.1111812
29. Negahdaripour M, Vakili B, Nezafat N. Exosome-based vaccines and their position in next-generation vaccines. Int Immunopharmacol (2022) 113(Pt A):109265. doi: 10.1016/j.intimp.2022.109265
30. Roy A, Girija As S, Sankar Ganesh P, Saravanan M, Sunny B. Exosome mediated cancer therapeutic approach: present status and future prospectives. Asian Pacific J Cancer prevention: APJCP (2023) 24(2):363–73. doi: 10.31557/APJCP.2023.24.2.363
31. Abak A, Abhari A, Rahimzadeh S. Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. Peerj (2018) 6:e4763. doi: 10.7717/peerj.4763
32. Wen SW, Lima LG, Lobb RJ, Norris EL, Hastie ML, Krumeich S, et al. Breast cancer-derived exosomes reflect the cell-of-Origin phenotype. Proteomics (2019) 19(8):e1800180. doi: 10.1002/pmic.201800180
33. Simona F, Laura S, Simona T, Riccardo A. Contribution of proteomics to understanding the role of tumor-derived exosomes in cancer progression: state of the art and new perspectives. Proteomics (2013) 13(10-11):1581–94. doi: 10.1002/pmic.201200398
34. Zhu L, Sun H-T, Wang S, Huang S-L, Zheng Y, Wang C-Q, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol (2020) 13(1):152. doi: 10.1186/s13045-020-00987-y
35. Schmitz SU, Grote P, Herrmann BG. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci (2016) 73(13):2491–509. doi: 10.1007/s00018-016-2174-5
36. Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through the miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics (2018) 8(1):169–84. doi: 10.7150/thno.21234
37. Ghafourian M, Mahdavi R, Akbari Jonoush Z, Sadeghi M, Ghadiri N, Farzaneh M, et al. The implications of exosomes in pregnancy: emerging as new diagnostic markers and therapeutics targets. Cell Communication Signaling (2022) 20(1):51. doi: 10.1186/s12964-022-00853-z
38. Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells (2021) 10(8):1959. doi: 10.3390/cells10081959
39. Shi Y, Du L, Lv D, Li Y, Zhang Z, Huang X, et al. Emerging role and therapeutic application of exosome in hepatitis virus infection and associated diseases. J Gastroenterol (2021) 56(4):336–49. doi: 10.1007/s00535-021-01765-4
40. Yang H, Zhang H, Gu H, Wang J, Zhang J, Zen K, et al. Comparative analyses of human exosome proteomes. Protein J (2023). doi: 10.1007/s10930-023-10100-0
41. Jia R, Rotenberg SA V, Mirkin M. Electrochemical resistive-pulse sensing of extracellular vesicles. Anal Chem (2022) 94(37):12614–20. doi: 10.1021/acs.analchem.2c01216
42. Ye Z, Wang S, Huang X, Chen P, Deng L, Li S, et al. Plasma exosomal miRNAs associated with metabolism as early predictor of gestational diabetes mellitus. Diabetes (2022) 71(11):2272–83. doi: 10.2337/db21-0909
43. Bonasio R, Shiekhattar R. Regulation of transcription by long noncoding RNAs. Annu Rev Genet (2014) 48:433–55. doi: 10.1146/annurev-genet-120213-092323
44. Chen A, Yu R, Jiang S, Xia Y, Chen Y. Recent advances of MicroRNAs, long noncoding RNAs, and circular RNAs in preeclampsia. Front Physiol (2021) 12. doi: 10.3389/fphys.2021.659638
45. Yang J, Yang FJ, Wang YG, Su GF, Mao X. LncRNA MIR497HG inhibits proliferation and migration of retinal endothelial cells under high-level glucose treatment via miRNA-128-3p/SIRT1 axis. Eur Rev Med Pharmacol Sci (2020) 24(11):5871–7. doi: 10.26355/eurrev_202006_21479
46. St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet (2015) 31(5):239–51. doi: 10.1016/j.tig.2015.03.007
47. Wang Y, Nicholes K, Shih IM. The origin and pathogenesis of endometriosis. Annu Rev Pathol (2020) 15:71–95. doi: 10.1146/annurev-pathmechdis-012419-032654
48. Maligianni I, Yapijakis C, Nousia K, Bacopoulou F, Chrousos GPP. Exosomes and exosomal noncoding RNAs throughout human gestation (Review). Exp Ther Med (2022) 24(3):582. doi: 10.3892/etm.2022.11518
49. Jain N, Gupta P, Sahoo S, Mallick B. Noncoding RNAs and their cross-talks impacting reproductive health of women. Wiley Interdiscip Reviews-Rna (2022) 13(3):e1695. doi: 10.1002/wrna.1695
50. Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol (2021) 220(2):e202009045. doi: 10.1083/jcb.202009045
51. Zhang T, Tang X, Zhu Y, Wang C, Jiang Z, Yang N, et al. IGF2BP2 enhances LincRNA01116 stability via m(6)A: a potential biomarker and therapeutic target for patients with preeclampsia. J Cell Biochem (2023) 124(2):239–53. doi: 10.1002/jcb.30358
52. Ghafouri-Fard S, Shoorei H, Taheri M. Role of noncoding RNAs in the pathogenesis of endometriosis. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.01370
53. Nunez-Martinez HN, Recillas-Targa F. Emerging functions of lncRNA loci beyond the transcript itself. Int J Mol Sci (2022) 23(11):6258. doi: 10.3390/ijms23116258
54. Yang L, Li L-P, Yi H-C. DeepWalk-based method to predict lncRNA-miRNA associations via lncRNA-miRNA-disease-protein-drug graph. BMC Bioinf (2022) 22(SUPPL 12):621. doi: 10.1186/s12859-022-04579-0
55. Zhao W, Liu Y, Zhang C, Duan C. Multiple roles of exosomal long noncoding RNAs in cancers. BioMed Res Int (2019) 2019:1–12. doi: 10.1155/2019/1460572
56. Huang W, Li H, Yu Q, Xiao W, Wang DO. LncRNA-mediated DNA methylation: an emerging mechanism in cancer and beyond. J Exp Clin Cancer Res (2022) 41(1):100. doi: 10.1186/s13046-022-02319-z
57. Herman AB, Tsitsipatis D, Gorospe M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell (2022) 82(12):2252–66. doi: 10.1016/j.molcel.2022.05.027
58. Sanbonmatsu K. Getting to the bottom of lncRNA mechanism: structure-function relationships. Mamm Genome (2022) 33(2):343–53. doi: 10.1007/s00335-021-09924-x
59. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell (2018) 172(3):393–407. doi: 10.1016/j.cell.2018.01.011
60. An B, Kameda T, Imamura T. The evolutionary acquisition and mode of functions of promoter-associated noncoding RNAs (pancRNAs) for mammalian development. Essays Biochem (2021) 65(4):697–708. doi: 10.1042/EBC20200143
61. Tripathi V, Ellis JD, Shen Z, Song DY, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell (2010) 39(6):925–38. doi: 10.1016/j.molcel.2010.08.011
62. Wang X, Yu Q. Endometriosis-related ceRNA network to identify predictive biomarkers of endometrial receptivity. Epigenomics (2019) 11(2):147–67. doi: 10.2217/epi-2018-0190
63. Brandenburger T, Somoza AS, Devaux Y, Lorenzen JM. Noncoding RNAs in acute kidney injury. Kidney Int (2018) 94(5):870–81. doi: 10.1016/j.kint.2018.06.033
64. Zhang D, Wang L, Guo HL, Zhang ZW, Wang C, Chian RC, et al. MicroRNA-202 inhibits endometrial stromal cell migration and invasion by suppressing the K-Ras/Raf1/MEK/ERK signaling pathway. Int J Mol Med (2020) 46(6):2078–88. doi: 10.3892/ijmm.2020.4749
65. Sabaie H, Amirinejad N, Asadi MR, Jalaiei A, Daneshmandpour Y, Rezaei O, et al. Molecular insight into the therapeutic potential of long noncoding RNA-associated competing endogenous RNA axes in alzheimer’s disease: a systematic scoping review. Front Aging Neurosci (2021) 13. doi: 10.3389/fnagi.2021.742242
66. Liu LY, Zheng B, Wang ZX. Protective effects of the knock-down of lncRNA AK139328 against oxygen-glucose deprivation/reoxygenation-induced injury in PC12 cells. Mol Med Rep (2021) 24(3):621. doi: 10.3892/mmr.2021.12260
67. Schuler-Toprak S, Skrzypczak M, Weber F, Ortmann O, Treeck O. Long non-coding RNA CCAT1 is overexpressed in endometrial cancer and regulates growth and transcriptome of endometrial adenocarcinoma cells. Geburtshilfe Und Frauenheilkunde (2020) 80(10):E206–E. doi: 10.1055/s-0040-1718183
68. Cao DW, Liu MM, Duan R, Tao YF, Zhou JS, Fang WR, et al. The lncRNA Malat1 functions as a ceRNA to contribute to berberine-mediated inhibition of HMGB1 by sponging miR-181c-5p in poststroke inflammation. Acta Pharmacologica Sinica (2020) 41(1):22–33. doi: 10.1038/s41401-019-0284-y
69. Ohkura N, Yasumizu Y, Kitagawa Y, Tanaka A, Nakamura Y, Motooka D, et al. Regulatory T cell-specific epigenomic region variants are a key determinant of susceptibility to common autoimmune diseases. Immunity (2020) 52(6):1119. doi: 10.1016/j.immuni.2020.04.006
70. Mariotti B, Servaas NH, Rossato M, Tamassia N, Cassatella MA, Cossu M, et al. The long non-coding RNA NRIR drives IFN-response in monocytes: implication for systemic sclerosis. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.00100
71. Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update (2019) 25(2):202–23. doi: 10.1093/humupd/dmy044
72. Santoso B, Sa’adi A, Dwiningsih SR, Tunjungseto A, Widyanugraha MYA, Mufid AF, et al. Soluble immune checkpoints CTLA-4, HLA-G, PD-1, and PD-L1 are associated with endometriosis-related infertility. Am J Reprod Immunol (2020) 84(4):e13296. doi: 10.1111/aji.13296
73. Oksasoglu B, Hepokur C, Misir S, Yildiz C, Sonmez G, Yanik A. Determination of PD-1 expression in peripheral blood cells in patients with endometriosis. Gynecological Endocrinol (2021) 37(2):157–61. doi: 10.1080/09513590.2020.1821358
74. Cheng D, Deng JG, Zhang B, He XY, Meng Z, Li GL, et al. LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. Ebiomedicine (2018) 36:159–70. doi: 10.1016/j.ebiom.2018.08.055
75. Goodall GJ, Wickramasinghe VO. RNA In cancer. Nat Rev Cancer (2021) 21(1):22–36. doi: 10.1038/s41568-020-00306-0
76. Zafari N, Bahramy A, Zolbin MM, Allahyari SE, Farazi E, Hassannejad Z, et al. microRNAs as novel diagnostic biomarkers in endometriosis patients: a systematic review and meta-analysis. Expert Rev Mol Diagn (2022) 22(4):479–95. doi: 10.1080/14737159.2021.1960508
77. Wang WT, Sun YM, Huang W, He B, Zhao YN, Chen YQ. Genome-wide long noncoding RNA analysis identified circulating LncRNAs as novel non-invasive diagnostic biomarkers for gynecological disease. Sci Rep (2016) 6:23343. doi: 10.1038/srep23343
78. Huang YZ, Zhang T, Chen LQ, Yu MH, Liu Q, Zhou CY, et al. Elevated expressions of SHP2 and GAB2 correlated with VEGF in eutopic and ectopic endometrium of women with ovarian endometriosis. Gynecological Endocrinol (2020) 36(9):813–8. doi: 10.1080/09513590.2020.1787378
79. Wang L, Yang JJ, Xiao X, Zheng CG, Ming D. VEGF modulates the neural dynamics of hippocampal subregions in chronic global cerebral ischemia rats. Neuromolecular Med (2021) 23(3):416–27. doi: 10.1007/s12017-020-08642-y
80. Schneider C, Kassens N, Greve B, Hassan H, Schuring AN, Starzinski-Powitz A, et al. Targeting of syndecan-1 by micro-ribonucleic acid miR-10b modulates invasiveness of endometriotic cells via dysregulation of the proteolytic milieu and interleukin-6 secretion. Fertility Sterility (2013) 99(3):871. doi: 10.1016/j.fertnstert.2012.10.051
81. Zhang L, Li HH, Yuan M, Li D, Sun C, Wang GY. Serum exosomal MicroRNAs as potential circulating biomarkers for endometriosis. Dis Markers (2020) 2020:2456340. doi: 10.1155/2020/2456340
82. Wang XQ, Wu PL, Zeng C, Zhu JW, Zhou YF, Lu Y, et al. Long intergenic non-protein coding RNA 02381 promotes the proliferation and invasion of ovarian endometrial stromal cells through the miR-27b-3p/CTNNB1 axis. Genes (2022) 13(3):433. doi: 10.3390/genes13030433
83. Zhan Y, Du L, Wang L, Jiang X, Zhang S, Li J, et al. Expression signatures of exosomal long noncoding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol Cancer (2018) 17(1):142. doi: 10.1186/s12943-018-0893-y
84. Zhang L, Li HH, Yuan M, Li D, Wang GY. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-kappa b signaling pathway. Eur Rev Med Pharmacol Sci (2020) 24(2):571–80. doi: 10.26355/eurrev_202001_20033
85. Wang Y, Hu SG, Yao GX, Zhu QL, He YQ, Lu Y, et al. A novel molecule in human cyclic endometrium: lncRNA TUNAR is involved in embryo implantation. Front Physiol (2020) 11. doi: 10.3389/fphys.2020.587448
86. Zhang C, Wu W, Ye X, Ma RQ, Luo JJ, Zhu HL, et al. Aberrant expression of CHL1 gene and long noncoding RNA CHL1-AS1, CHL1-AS2 in ovarian endometriosis. Eur J Obstetrics Gynecol Reprod Biol (2019) 236:177–82. doi: 10.1016/j.ejogrb.2019.03.020
87. Shi YM, Zha JF, Zuo MZ, Yan Q, Song HM. Long noncoding RNA CHL1-AS1 promotes cell proliferation and migration by sponging miR-6076 to regulate CHL1 expression in endometrial cancer. J Cell Biochem (2020) 121(3):2655–63. doi: 10.1002/jcb.29486
88. Farzaneh M, Najafi S, Anbiyaee O, Azizidoost S, Khoshnam SE. LncRNA MALAT1-related signaling pathways in osteosarcoma. Clin Trans Oncol (2023) 25(1):21–32. doi: 10.1007/s12094-022-02876-x
89. Martins TS, Vaz M, Henriques AG. A review on comparative studies addressing exosome isolation methods from body fluids. Analytical Bioanalytical Chem (2023) 415(7):1239–63. doi: 10.1007/s00216-022-04174-5
90. Wu JN, Huang HY, Huang W, Wang L, Xia XM, Fang XL. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics (2020) 12(14):1193–213. doi: 10.2217/epi-2020-0084
91. Filip L, Duicc F, Pradatu A, Creoiu D, Suciu N, Cretoiu SM, et al. Endometriosis associated infertility: a critical review and analysis on etiopathogenesis and therapeutic approaches. Medicina-Lithuania (2020) 56(9):460. doi: 10.3390/medicina56090460
92. Sciarrillo R, Wojtuszkiewicz A, Assaraf YG, Jansen G, Kaspers GJL, Giovannetti E, et al. The role of alternative splicing in cancer: from oncogenesis to drug resistance. Drug Resistance Updates (2020) 53:100728. doi: 10.1016/j.drup.2020.100728
93. Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstetrics Gynecol (2019) 220(4):354.e1–e12. doi: 10.1016/j.ajog.2018.12.039
94. Tarumi Y, Mori T, Shimura K, Izumi Y, Okimura H, Kataoka H, et al. Progesterone receptor status of epithelial cells as a predictive marker for postoperative recurrence of endometriosis. J Clin Endocrinol Metab (2022) 107(6):1552–9. doi: 10.1210/clinem/dgac118
95. Delbandi AA, Mahmoudi M, Shervin A, Heidari S, Kolahdouz-Mohammadi R, Zarnani AH. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health (2020) 20(1):3. doi: 10.1186/s12905-019-0865-4
96. Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JMN. Laparoscopic surgery for endometriosis. Cochrane Database Systematic Rev (2020) 10(10):CD011031. doi: 10.1002/14651858.CD011031.pub3
97. Li WN, Hsiao KY, Wang CA, Chang N, Hsu PL, Sun CH, et al. Extracellular vesicle-associated VEGF-c promotes lymphangiogenesis and immune cell infiltration in endometriosis. Proc Natl Acad Sci United States America (2020) 117(41):25859–68. doi: 10.1073/pnas.1920037117
98. Xu Z, Zhang LP, Yu Q, Zhang YN, Yan L, Chen ZJ. The estrogen-regulated lncRNA H19/miR-216a-5p axis alters stromal cell invasion and migration via ACTA2 in endometriosis. Mol Hum Reproduction (2019) 25(9):550–61. doi: 10.1093/molehr/gaz040
99. Liu SP, Xin WJ, Tang XY, Qiu JJ, Zhang YJ, Hua KQ. LncRNA H19 overexpression in endometriosis and its utility as a novel biomarker for predicting recurrence. Reprod Sci (2020) 27(9):1687–97. doi: 10.1007/s43032-019-00129-x
100. Asghari S, Valizadeh A, Aghebati-Maleki L, Nouri M, Yousefi M. Endometriosis: perspective, lights, and shadows of etiology. Biomed Pharmacother (2018) 106:163–74. doi: 10.1016/j.biopha.2018.06.109
101. Gao XY, Zhang YS, Xu XX, Lu SM, Yan L. Effects of ovarian endometrioma aspiration on in vitro fertilization-intracytoplasmic sperm injection and embryo transfer outcomes: a systematic review and meta-analysis. Arch Gynecol Obstetrics (2022) 306(1):17–28. doi: 10.1007/s00404-021-06278-2
102. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet (2021) 397(10276):839–52. doi: 10.1016/S0140-6736(21)00389-5
103. Leenen S, Hermens M, van Steenwijk PJD, Bekkers RLM, van Esch EMG. Immunologic factors involved in the malignant transformation of endometriosis to endometriosis-associated ovarian carcinoma. Cancer Immunol Immunother (2021) 70(7):1821–9. doi: 10.1007/s00262-020-02831-1
104. Murakami K, Kotani Y, Shiro R, Takaya H, Nakai H, Matsumura N. Endometriosis-associated ovarian cancer occurs early during follow-up of endometrial cysts. Int J Clin Oncol (2020) 25(1):51–8. doi: 10.1007/s10147-019-01536-5
105. Kralickova M, Lagana AS, Ghezzi F, Vetvicka V. Endometriosis and risk of ovarian cancer: what do we know? Arch Gynecol Obstetrics (2020) 301(1):1–10. doi: 10.1007/s00404-019-05358-8
106. Wang HX, Ni CX, Xiao W, Wang SL. Role of lncRNA FTX in invasion, metastasis, and epithelial-mesenchymal transition of endometrial stromal cells caused by endometriosis by regulating the PI3K/Akt signaling pathway. Ann Trans Med (2020) 8(22):1504. doi: 10.21037/atm-20-6810
107. Wuyung PE, Rahadiati FB, Tjahjadi H, Salinah S, Kusmardi K, Kodariah R, et al. Histopathology and ARID1A expression in endometriosis- associated ovarian carcinoma (EAOC) carcinogenesis model with endometrial autoimplantation and DMBA induction. Asian Pacific J Cancer Prev (2021) 22(2):553–8. doi: 10.31557/APJCP.2021.22.2.553
108. Cai Y, Yin J, Jin Y, Li Y, Pan L. Endometriosis-associated ovarian cancer is not a distinct clinical entity among young patients: a 12-year cohort study. Eur J Surg Oncol (2019) 46(5):876–82. doi: 10.1016/j.ejso.2019.11.517
109. Gadducci A, Multinu F, Cosio S, Carinelli S, Ghioni M, Aletti GD. Clear cell carcinoma of the ovary: epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecologic Oncol (2021) 162(3):741–50. doi: 10.1016/j.ygyno.2021.06.033
110. Barra F, Lagana AS, Casarin J, Ghezzi F, Desideri LF, Scala C, et al. Molecular targets for endometriosis therapy: where we are and where we are going? Int J Fertility Sterility (2019) 13(2):89–92. doi: 10.22074/ijfs.2019.5736
111. Home AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ-British Med J (2022) 379:19. doi: 10.1136/bmj-2022-070750
112. Hozain S, Hernandez A, Fuller J, Sharp G, Cottrell J. Zinc chloride affects chondrogenesis via VEGF signaling. Exp Cell Res (2021) 399(2):112436. doi: 10.1016/j.yexcr.2020.112436
113. Farahani ZK, Taherianfard M, Naderi MM, Ferrero H. Possible therapeutic effect of royal jelly on endometriotic lesion size, pain sensitivity, and neurotrophic factors in a rat model of endometriosis. Physiol Rep (2021) 9(22):e15117. doi: 10.14814/phy2.15117
114. Dong L, Zhang L, Liu H, Xie MT, Gao J, Zhou XY, et al. Circ_0007331 knock-down suppresses the progression of endometriosis via miR-200c-3p/HiF-1 alpha axis. J Cell Mol Med (2020) 24(21):12656–66. doi: 10.1111/jcmm.15833
115. Qiu JJ, Lin XJ, Zheng TT, Tang XY, Zhang Y, Hua KQ. The exosomal long noncoding RNA aHIF is up-regulated in serum from patients with endometriosis and promotes angiogenesis in endometriosis. Reprod Sci (2019) 26(12):1590–602. doi: 10.1177/1933719119831775
116. Pang CH, Wu ZJ, Xu XY, Yang WX, Wang XX, Qi YH. Paeonol alleviates migration and invasion of endometrial stromal cells by reducing HIF-1 alpha-regulated autophagy in endometriosis. Front Bioscience-Landmark (2021) 26(9):485–95. doi: 10.52586/4961
117. Razi MH, Eftekhar M, Ghasemi N, Sheikhha MH, Firoozabadi AD. Expression levels of circulatory mir-185-5p, vascular endothelial growth factor, and platelet-derived growth factor target genes in endometriosis. Int J Reprod Biomed (2020) 18(5):347–58. doi: 10.18502/ijrm.v13i5.7155
118. Galano M, Li YC, Li L, Sottas C, Papadopoulos V. Role of constitutive STAR in leydig cells. Int J Mol Sci (2021) 22(4):2021. doi: 10.3390/ijms22042021
119. Feng Y, Zhan FL, Zhong YY, Tan BZ. Effects of human umbilical cord mesenchymal stem cells derived from exosomes on migration ability of endometrial glandular epithelial cells. Mol Med Rep (2020) 22(2):715–22. doi: 10.3892/mmr.2020.11137
120. Chen YH, Liu XH, He L. The value of long noncoding RNAs for predicting the recurrence of endometriosis a protocol for meta-analysis and bioinformatics analysis. Medicine (2021) 100(21):e26036. doi: 10.1097/MD.0000000000026036
121. Wang HC, Sha LX, Huang LX, Yang SM, Zhou QY, Luo XS, et al. LINC00261 functions as a competing endogenous RNA to regulate BCL2L11 expression by sponging miR-132-3p in endometriosis. Am J Trans Res (2019) 11(4):2269–79.
122. Liu J, Wang Q, Zhang RR, Zhang C, Lin JH, Huang XJ. Identification of LINC01279 as a cell cycle-associated long noncoding RNA in endometriosis with GBA analysis. Mol Med Rep (2018) 18(4):3850–8. doi: 10.3892/mmr.2018.9387
123. Zhang C, Wu W, Zhu HL, Yu XM, Zhang YL, Ye X, et al. Knock-down of long noncoding RNA CCDC144NL-AS1 attenuates migration and invasion phenotypes in endometrial stromal cells from endometriosis. Biol Reproduction (2019) 100(4):939–49. doi: 10.1093/biolre/ioy252
124. Zhu MB, Chen LP, Hu M, Shi Z, Liu YN. Effects of lncRNA BANCR on endometriosis through ERK/MAPK pathway. Eur Rev Med Pharmacol Sci (2019) 23(16):6806–12. doi: 10.26355/eurrev_201908_18719
125. Li WN, Wu MH, Tsai SJ. HYPOXIA AND REPRODUCTIVE HEALTH: the role of hypoxia in the development and progression of endometriosis. Bioscientifica (2021) 161(1):F19–F31. doi: 10.1530/REP-20-0267
126. Liu HW, Zhang ZB, Xiong WQ, Zhang L, Du Y, Liu Y, et al. Long noncoding RNA MALAT1 mediates hypoxia-induced pro-survival autophagy of endometrial stromal cells in endometriosis. J Cell Mol Med (2019) 23(1):439–52. doi: 10.1111/jcmm.13947
127. Yu J, Chen LH, Zhang B, Zheng QM. The modulation of endometriosis by lncRNA MALAT1 via NF-kappa B/iNOS. Eur Rev Med Pharmacol Sci (2019) 23(10):4073–80. doi: 10.26355/eurrev_201905_17908
128. Lin DC, Huang QS, Wu RF, Dai SJ, Huang ZX, Ren LL, et al. Long noncoding RNA AFAP1-AS1 promoting the epithelial-mesenchymal transition of endometriosis is correlated with transcription factor ZEB1. Am J Reprod Immunol (2019) 81(1):e13074. doi: 10.1111/aji.13074
129. Mai H, Wei YP, Yin Y, Huang SJ, Lin HS, Liao Y, et al. LINC01541 overexpression attenuates the 17 beta-estradiol-induced migration and invasion capabilities of endometrial stromal cells. Syst endometriosis Biol Reprod Med (2019) 65(3):214–22. doi: 10.1080/19396368.2018.1549290
130. Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, et al. Posttraumatic stress, anxiety, and depression following a miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstetrics Gynecol (2020) 222(4):367.e1–e22. doi: 10.1016/j.ajog.2019.10.102
131. Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reproduction (2016) 31(7):1475–82. doi: 10.1093/humrep/dew085
132. Vesali S, Razavi M, Rezaeinejad M, Maleki-Hajiagha A, Maroufizadeh S, Sepidarkish M. Endometriosis fertility index for predicting non-assisted reproductive technology pregnancy after endometriosis surgery: a systematic review and meta-analysis. Bjog-an Int J Obstetrics Gynaecol (2020) 127(7):800–9. doi: 10.1111/1471-0528.16107
133. Ghazal S, McKinnon B, Zhou JC, Mueller M, Men Y, Yang LH, et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med (2015) 7(8):996–1003. doi: 10.15252/emmm.201505245
134. Liu SP, Qiu JJ, Tang XY, Cui HY, Zhang Q, Yang QH. LncRNA-H19 regulates cell proliferation and invasion of ectopic endometrium by targeting ITGB3 via modulating miR-124-3p. Exp Cell Res (2019) 381(2):215–22. doi: 10.1016/j.yexcr.2019.05.010
135. Du Y, Zhang ZB, Xiong WQ, Li N, Liu HW, He HT, et al. Estradiol promotes EMT in endometriosis via MALAT1/miR200s sponge function. Reproduction (2019) 157(2):179–88. doi: 10.1530/REP-18-0424
136. Liang ZW, Chen YJ, Zhao Y, Xu CY, Zhang AQ, Zhang Q, et al. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res Ther (2017) 8(1):251. doi: 10.1186/s13287-017-0706-z
137. Li Y, Liu YD, Chen SL, Chen X, Ye DS, Zhou XY, et al. Down-regulation of long noncoding RNA MALAT1 inhibits granulosa cell proliferation in endometriosis by up-regulating P21 via activation of the ERK/MAPK pathway. Mol Hum Reproduction (2019) 25(1):17–29. doi: 10.1093/molehr/gay045
138. Liu XJ, Zhang P, Li YM, Zhao N, Han HY. The AMPK-mTOR axis requires increased MALAT1 expression for promoting granulosa cell proliferation in endometriosis. Exp Ther Med (2021) 21(1):21. doi: 10.3892/etm.2020.9453
139. Xie QP, Chen CY, Li HY, Xu JH, Wu L, Yu Y, et al. miR-3687 overexpression promotes bladder cancer cell growth by inhibiting the negative effect of FOXP1 on cyclin E2 transcription. Mol Ther (2019) 27(5):1028–38. doi: 10.1016/j.ymthe.2019.03.006
140. Liu J, Wang Y, Chen P, Ma Y, Wang S, Tian Y, et al. AC002454.1 and CDK6 synergistically promote endometrial cell migration and invasion in endometriosis. Reproduction (2019) 157(6):535–43. doi: 10.1530/REP-19-0005
141. Wang Y, Li Y, Yang Z, Liu KR, Wang DB. Genome-wide microarray analysis of long noncoding RNAs in eutopic secretory endometrium with endometriosis. Cell Physiol Biochem (2015) 37(6):2231–45. doi: 10.1159/000438579
142. Sun PR, Jia SZ, Lin H, Leng JH, Lang JH. Genome-wide profiling of long noncoding ribonucleic acid expression patterns in ovarian endometriosis by microarray. Fertility Sterility (2014) 101(4):1038–U469. doi: 10.1016/j.fertnstert.2013.12.035
143. Huang H, Zhu ZY, Song Y. Downregulation of lncRNA uca1 as a diagnostic and prognostic biomarker for ovarian endometriosis. Rev Da Associacao Med Brasileira (2019) 65(3):336–41. doi: 10.1590/1806-9282.65.3.336
144. Wang MM, Hao CF, Huang X, Bao HC, Qu QL, Liu ZT, et al. Aberrant expression of lncRNA (HOXA11-AS1) and homeobox a (HOXA9, HOXA10, HOXA11, and HOXA13) genes in infertile women with endometriosis. Reprod Sci (2018) 25(5):654–61. doi: 10.1177/1933719117734320
145. Qiu JJ, Lin YY, Tang XY, Ding Y, Yi XF, Hua KQ. Extracellular vesicle-mediated transfer of the lncRNA-TC0101441 promotes endometriosis migration/invasion. Exp Cell Res (2020) 388(1):111815. doi: 10.1016/j.yexcr.2020.111815
146. Liu Y, Ma JY, Cui D, Fei XW, Lv YF, Lin J. LncRNA MEG3-210 regulates endometrial stromal cells migration, invasion, and apoptosis through p38 MAPK and PKA/SERCA2 signaling via interaction with galectin-1 in endometriosis. Mol Cell Endocrinol (2020) 513:110870. doi: 10.1016/j.mce.2020.110870
147. Sun KX, Wu DD, Chen S, Zhao Y, Zong ZH. LncRNA MEG3 inhibits endometrial carcinoma tumorigenesis and progression through the PI3K pathway. Apoptosis (2017) 22(12):1543–52. doi: 10.1007/s10495-017-1426-7
148. Wang LM, Xing Q, Feng TF, He M, Yu WX, Chen H. SNP rs710886A > G in long noncoding RNA PCAT1 is associated with the risk of endometriosis by modulating expression of multiple stemness-related genes via microRNA-145 signaling pathway. J Cell Biochem (2020) 121(2):1703–15. doi: 10.1002/jcb.29406
Keywords: exosome lncRNA, expression, biomarkers, therapeutics, endometriosis
Citation: Wang M, Zheng L, Lin R, Ma S, Li J and Yang S (2023) A comprehensive overview of exosome lncRNAs: emerging biomarkers and potential therapeutics in endometriosis. Front. Endocrinol. 14:1199569. doi: 10.3389/fendo.2023.1199569
Received: 03 April 2023; Accepted: 05 June 2023;
Published: 26 June 2023.
Edited by:
Achmad Kemal Harzif, University of Indonesia, IndonesiaReviewed by:
James Arthur MacLean, Washington State University, United StatesMila Maidarti, University of Indonesia, Indonesia
Copyright © 2023 Wang, Zheng, Lin, Ma, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuli Yang, eWFuZ3NsQGpsdS5lZHUuY24=
 Min Wang
Min Wang Lianwen Zheng1
Lianwen Zheng1 Ruixin Lin
Ruixin Lin Shuai Ma
Shuai Ma Jiahui Li
Jiahui Li