
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 June 2023
Sec. Molecular and Structural Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1199429
This article is part of the Research Topic Interaction of Endocrine Disorders and Metabolic Associated Fatty Liver Disease: The Genetic and Epigenetic Basis View all 6 articles
 Xiaoyi Xu1,2†
Xiaoyi Xu1,2† Hangfei Xu1,2†
Hangfei Xu1,2† Xiaohui Liu1†
Xiaohui Liu1† Shuang Zhang1,3
Shuang Zhang1,3 Zhenhuan Cao1
Zhenhuan Cao1 Lixia Qiu1
Lixia Qiu1 Xiaofei Du1
Xiaofei Du1 Yali Liu1
Yali Liu1 Gang Wang3
Gang Wang3 Li Zhang3
Li Zhang3 Yang Zhang1,2*
Yang Zhang1,2* Jing Zhang1*
Jing Zhang1*Background and aim: The MBOAT7 rs641738 (C>T) variant has demonstrated an association with non-alcoholic fatty liver disease (NAFLD) in both adult and pediatric patients, while few studies have been conducted in elderly populations. Hence, a case–control study was undertaken to assess their correlation in elderly residents in a Beijing community.
Materials and methods: A total of 1,287 participants were included. Medical history, abdominal ultrasound, and laboratory tests were recorded. Liver fat content and fibrosis stage were detected by Fibroscan. Genotyping of genomic DNA was performed using the 96.96 genotyping integrated fluidics circuit.
Results: Of the recruited subjects, 638 subjects (56.60%) had NAFLD, and 398 subjects (35.28%) had atherosclerotic cardiovascular disease (ASCVD). T allele carriage was associated with higher ALT (p=0.005) and significant fibrosis in male NAFLD patients (p=0.005) compared to CC genotype. TT genotype was associated with reduced risk of metabolic syndrome (OR=0.589, 95%CI: 0.114–0.683, p=0.005) and type 2 diabetes (OR=0.804, 95%CI: 0.277–0.296, p=0.048) in NAFLD population when compared to the CC genotype. In addition, TT genotype was also associated with reduced risk of ASCVD (OR=0.570, 95%CI:0.340–0.953, p=0.032) and less obesity (OR=0.545, 95%CI: 0.346–0.856, p=0.008) in the whole population.
Conclusion: MBOAT7 rs641738 (C>T) variant was associated with fibrosis in male NAFLD patients. The variant also reduced risk of metabolic traits and type 2 diabetes in NAFLD and ASCVD risk in Chinese elders.
Given that the prevalence of non-alcoholic fatty liver disease (NAFLD) covers higher than 25% of the population, it has become the most common chronic liver disease worldwide (1, 2). The onset of NAFLD is characterized by an imbalance in lipid metabolism, leading to the accumulation of lipid droplets in hepatocytes, a condition that progresses to non-alcoholic steatohepatitis, fibrosis, cirrhosis, and ultimately hepatocellular carcinoma (HCC) (3–5). Obesity, type 2 diabetes (T2DM), dyslipidemia, hypertension, and cardiovascular disorders, etc. were linked strongly to NAFLD (6, 7). Over the last decade, there has been growing interest on the role of single nucleotide polymorphisms (SNPs) in the progression of NAFLD. Multiple SNPs, including patatin-like phospholipase domain-containing protein 3 (PNPLA3, rs738409), transmembrane 6 superfamily member 2 (TM6SF2, rs58542926), etc., were found to be closely related to the incidence and progression of NAFLD (8, 9). Furthermore, they were also associated with reduced risk of atherosclerotic cardiovascular disease (ASCVD), which is the major cause of death among NAFLD patients (10–13). The effect of SNPs deepened our understanding of NAFLD and the relationship between NAFLD and cardio-metabolic disease.
Membrane bound O-acyltransferase domain containing 7 (MBOAT7) is a multi-transmembrane protein and an important member of the “Lands’ Cycle” of membrane phosphatidyl chain remodeling (14). In 2015, a genome-wide association study identified that the genetic variant near MBOAT7 (rs641738 C>T) was a risk factor for alcohol-related cirrhosis (15). Subsequently, studies conducted on NAFLD/NASH revealed that MBOAT7 rs641738 C> T was positively related to hepatic fat content and histological severity (16) only in European descent (17). Loss or variant of MBOAT7 reduced liver mRNA and protein expression and consequently led to changes in phosphorylated inositol species, which potentially contributed to liver injury (16, 18).
The clinical significance of MBOAT7 rs641738 C>T variant on NAFLD has been evaluated in various races and age groups; however, its impact on the elderly population remained unclear. In the current study, the relationship between MBOAT7 rs641738 variant with the risk of NAFLD and hepatic fibrosis in an elderly population. Meanwhile, the correlation of the rs641738 T allele with metabolic traits and ASCVD was also analyzed. Furthermore, we explored the effect of rs641738 variant on serum ANGPTL3 level in order to explain the link between MBOAT7 with ASCVD.
Elder citizens in Beijing Mentougou community who participated in annual free physical examination was recruited from 1/11/2020 to 30/9/2021. The study protocol was approved by the individual Ethics Review Committee of Beijing Youan Hospital (IRB approval number [2020]-233). All study subjects provided written informed consent.
The study was carried out in Menkuang Hospital, Beijing Jingmei Group General Hospital. The inclusion criteria were (1) patients with fatty liver diagnosed by ultrasound and (2) who signed informed consent. The exclusion criteria were (1) excessive alcohol consumption (≥30g/day in men or 20 g/day in women); (2) lack of medical history or lab results; (3) inability to obtain reliable abdominal ultrasound results due to specific reasons, such as intestinal gas interference; (4) malignant tumors, HIV, and other serious diseases that may affect the nutritional status or organ function; and (5) comorbidity with other liver diseases, such as viral hepatitis and autoimmune hepatitis. The controlled groups were residents without fatty liver examined by ultrasound.
Usually, 10–20 residents took part in the routine examination every morning. If a patient is diagnosed with fatty liver by ultrasound, he/she is transferred to an isolated room to be recruited and further examined. The next resident without fatty liver following the previous NAFLD patients was recruited as control. Eventually, 1,287 residents were examined, and 1,128 residents were recruited in the study (Figure 1).
The routine free examination included abdomen ultrasound examination, routine blood test, routine urine test, liver function, renal function, fasting blood sugar (FBS), glycosylated hemoglobin (HbA1c), and lipid profile. Extra blood sample was obtained and tested for hypersensitive C-reactive protein (hs-CRP) and fasting insulin (FINS) in addition to the routine examination. All laboratory tests were carried out in the central lab of Menkuang Hospital or Beijing Jingmei Group General Hospital.
Two trained investigators carried out the whole study. They were responsible for recruiting patients, performing anthropometric examinations, and determining liver fat content and liver stiffness (LSM) by FibroScan 502 touch device (Echosens, Paris, France). The demographic indicators and medical histories were extracted from the community health records and checked again on site.
Fatty liver was diagnosed by ultrasound, and participants were divided into two groups, NAFLD and non NAFLD. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m). Waist-to-hip ratio (WHR) was calculated as waist circumference divided by hip circumference. Homeostasis model assessment insulin resistant (HOMA-IR) was calculated as (FINS (pmol/L) × FBS (mmol/L))/22.5. Additionally, non-invasive liver fibrosis score formulas fibrosis-4 index (FIB-4) (19) and AST-to-platelet ratio index (APRI) (20) were calculated according to the following formulas:
The comorbid diseases were diagnosed according to international guidelines, including hypertension, ASCVD, T2DM, and metabolic syndrome (MetS). In brief, hypertension is diagnosed when systolic blood pressure ≥130mmHg or diastolic blood pressure ≥85mmHg or when taking antihypertensive drugs. T2DM is diagnosed when FBS ≥7.0 mmol/L, HbA1c ≥ 6.5%, OGTT 2 h blood sugar ≥ 11.1mmo/L, or taking hypoglycemic drugs. MetS was defined in accordance with the criteria, which was based on the presence of at least three of the following components: (1) elevated waist circumference (≥90cm for men or ≥80 cm for women); (2) elevated triglycerides (≥1.70 mmol/L) or drug treatment for elevated triglycerides; (3) reduced HDL-C (<1.0 mmol/L for men and<1.3 mmol/L for women) or drug treatment to reduce HDL-C; (4) elevated blood pressure (≥ 130/85 mmHg) or drug treatment for hypertension; and (5) elevated FBS (≥5.6 mmol/L) or drug treatment for elevated glucose(21). ASCVD was diagnosed when coronary angiography (CAG) showed that the left main artery, left anterior descending artery, left circumflex artery, right coronary artery, or any one or more of its main branches had stenosis of ≥50% (22) or history of myocardial infarction. In this study, LSM ≥8.2 kPa and APRI ≥0.5 were used to predict significant hepatic fibrosis (23, 20). Obesity was defined as BMI≥25 kg/m2 (24).
Genomic DNA was extracted from the patient’s blood specimens (BGI-Shenzhen, China). DNA concentration and quality were determined by spectrophotometry (Nanodrop 2000, Thermo Scientific, Wilmington, DE) and standardized to approximately 50 ng/ml before genotyping. Next, genomic DNA has genotyped with the usage of a 96.96 genotyping integrated fluidics circuit (IFC) with customized SNP-type assays on the Juno™ system (Fluidigm, South San Francisco, CA, USA) and quantification on the Biomark™ (Fluidigm, South San Francisco, USA) in accordance with the manufacturers’ instructions. All genotyping was blinded to clinical variables. The Fluidigm SNP Genotyping Analysis program, version 4.5.1, was used to analyze the data (South San Francisco, CA, USA).
Serum ANGPTL3 levels were determined in 120 patients who were randomly selected from subjects with the three genotypes, each group containing 40 patients. ANGPTL3 was quantified by ELISA according to the instructions (Cusabio, Wuhan, China). The intra- and inter-assay coefficients of variation of ANPTL3 were<8% and 10%, respectively.
Clinical characteristics were compared between the rs641738 T allele carriage and non-T carriage or among the three genotypes. Descriptive values were expressed as mean ± standard deviation (SD) or medians and interquartile ranges (IQRs) depending on the distribution of the data. Student’s t-test or Mann–Whitney–Wilcoxon test was used to assess continuous variables according to value distributions. Frequencies and percentages were used to summarize categorical variables, and data were compared by using Pearson’s chi-square when appropriate. The χ2 test was also used if indicated. All comparison tests between the two groups were two-tailed with a 95% confidence interval. Univariate and multivariate logistic regression analyses were performed to assess the independent predictors for NAFLD, ASCVD, hypertension, and MetS. The statistical significance was set at p-value<0.05. The Statistical Package for Social Science for Windows, version 19.0 (SPSS Inc., IBM, New York), GraphPad Prism (version 2.0), and R language (version 3.4.3) were used for data analyses.
A total of 1,128 subjects were enrolled in the study. Characteristics of the NAFLD and non-NAFLD populations are provided in Table 1. Among them, 638 subjects (56.60%) had NAFLD, 469 subjects (41.58%) had T2DM, 869 subjects (77.04%) had hypertension, 948 subjects (84.93%) had MetS, and 398 subjects (35.28%) had ASCVD. When compared to the non-NAFLD subjects, NAFLD patients were likely to be elderly, female, with higher weight, BMI, waist circumference, and WHR. In addition, these patients exhibited significantly higher CAP, LSM, ALT, AST, TG, FINS, FBS, HOMA-IR, FIB-4, and APRI, and lower HDL (Table 1). However, the serum levels of TC and LDL and the prevalence of ASCVD were comparable between the NAFLD and non-NAFLD groups. The distribution of MBOAT7 rs641738 polymorphism in NAFLD and non-NAFLD groups was consistent with Hardy–Weinberg balance and was representative of the population (p>0.05).
Among the NAFLD patients, carriage of the rs641738 C >T polymorphism accounted for 47.81% (305/638). These patients presented significantly higher TC, HDL, LDL, and FINS than non-T carriage (p<0.05, Table 2). After adjusting usage of lipid-lowering agents, TC and LDL levels were comparable between T carriage and non-T carriage groups, while HDL is still statistically significant (OR: 2.476, 95% CI:1.218–4.998, p=0.012). T allele carriage was also significantly associated with advanced fibrosis (5.4% versus 1.8%, OR: 3.053, 95% CI: 1.179–7.908, p=0.022, Figure 2). Even after adjusting for confounders, such as age, sex, and BMI, the risk of significant liver fibrosis remained statistically significant (OR: 3.024, 95% CI: 1.165–7.848, p=0.023).
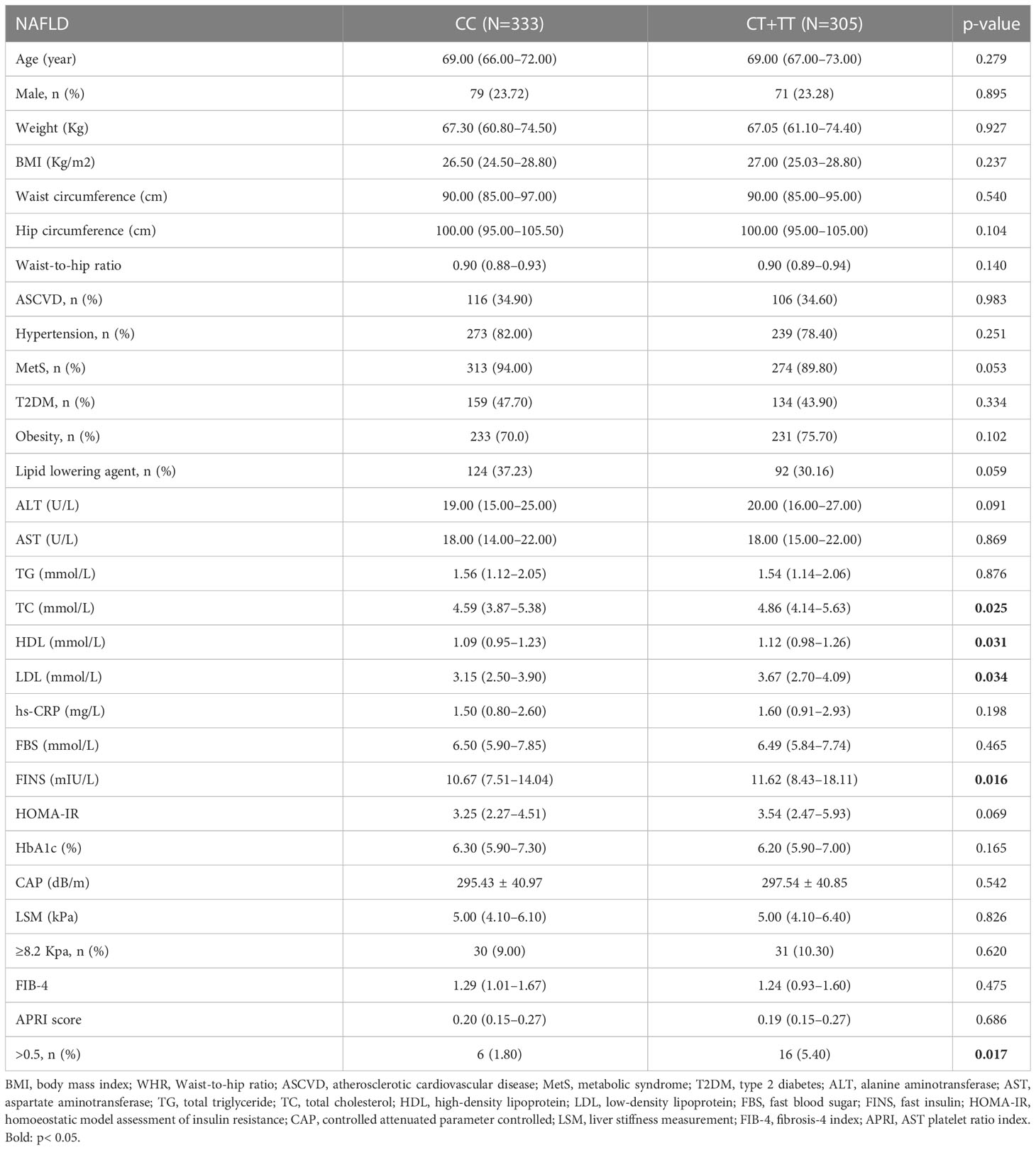
Table 2 Comparison of clinical characteristics according to MBOAT7 rs641738 genotypes within NAFLD cohorts.
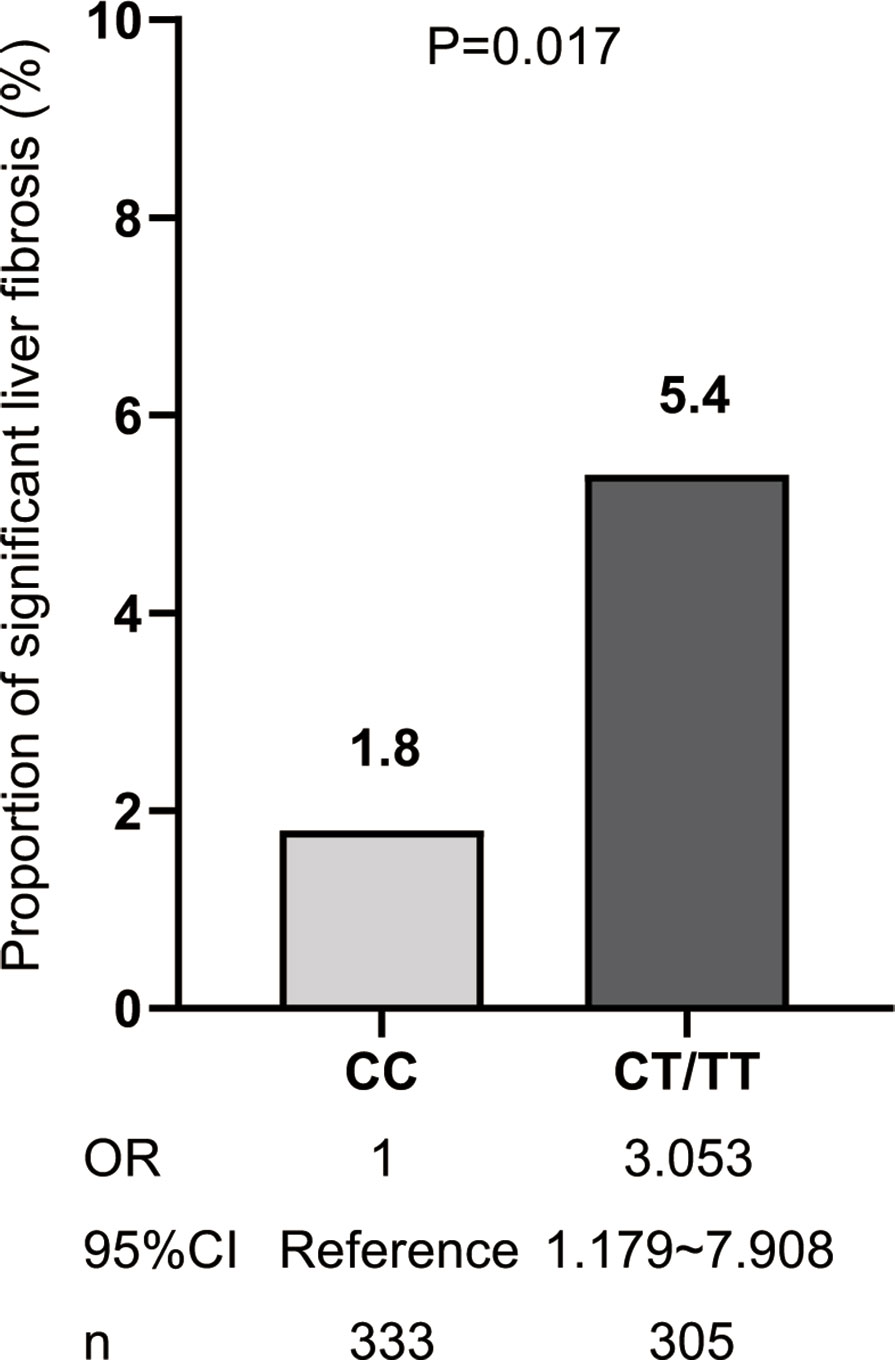
Figure 2 The risk of significant fibrosis for MBOAT7 rs641738 T allele carriage. OR, CT/TT versus CC genotype.
The demographic and clinical characteristics of NAFLD patients according to C→T genotypes are listed in Supplementary Table S1. In multivariate logistic regression analysis, adjusting for demographics and anthropometrics (age, sex, and BMI), the TT genotype had a significantly lower risk of T2DM (OR:0.804, 95%CI: 0.277–0.996, p= 0.048) and MetS (OR: 0.590, 95%CI: 0.114–0.683, p= 0.005) when compared to the CC genotype (Figure 3A).
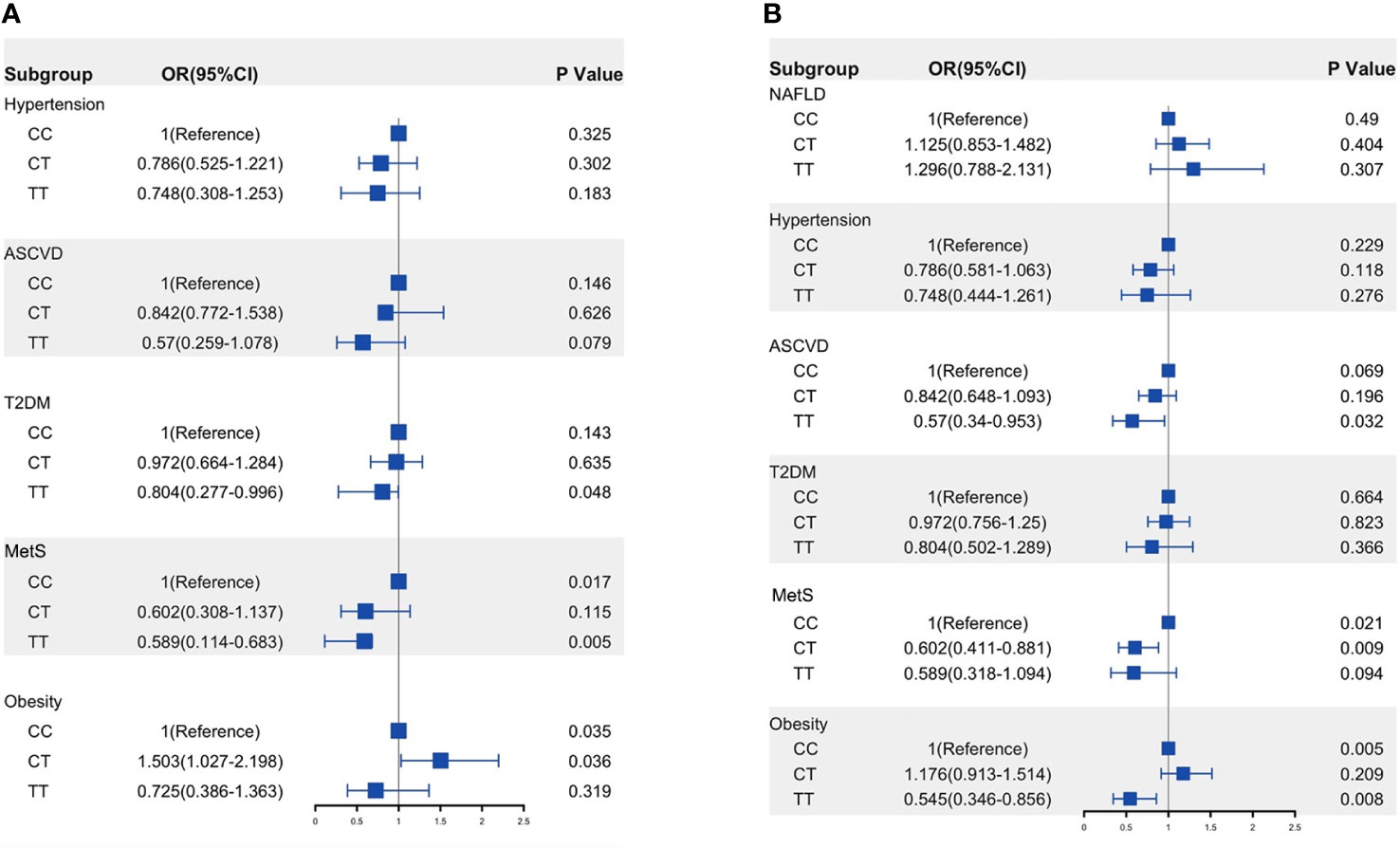
Figure 3 Risk of ASCVD and metabolic traits in the NAFLD patients (A) and whole population (B) according to MOBAT7 rs641738 C→T allele groups. OR was calculated using multivariable analysis and adjusted by age, sex, and BMI. p< 0.05 versus CC genotype. ASCVD, atherosclerotic cardiovascular disease; T2DM, type 2 diabetes; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease.
Gender is an important modified factor of NAFLD. Gender differences in clinical characteristics are listed in Supplementary Table S2. Male individuals were likely to have higher ALT and FBS levels and lower TC, HDL, and LDL levels; there were more patients with significant fibrosis and T2DM and fewer MetS patients.
To further investigate this observation, we compared the characters according to the genotypes in male and female patients separately. In male NAFLD patients, T allele carriage was associated with significantly higher levels of ALT, FINS, WHR, and significant fibrosis than non-T allele carriage (p=0.005, 0.041, 0.009, and 0.017, respectively, Table 3). However, in female NAFLD groups, the indicators were similar between the two genotypes. Although T allele carriage group demonstrated a higher level of TC (p=0.043, Supplementary Table S3) than non-T allele carriage, the difference disappeared after adjusting lipid-lowering agents usage.
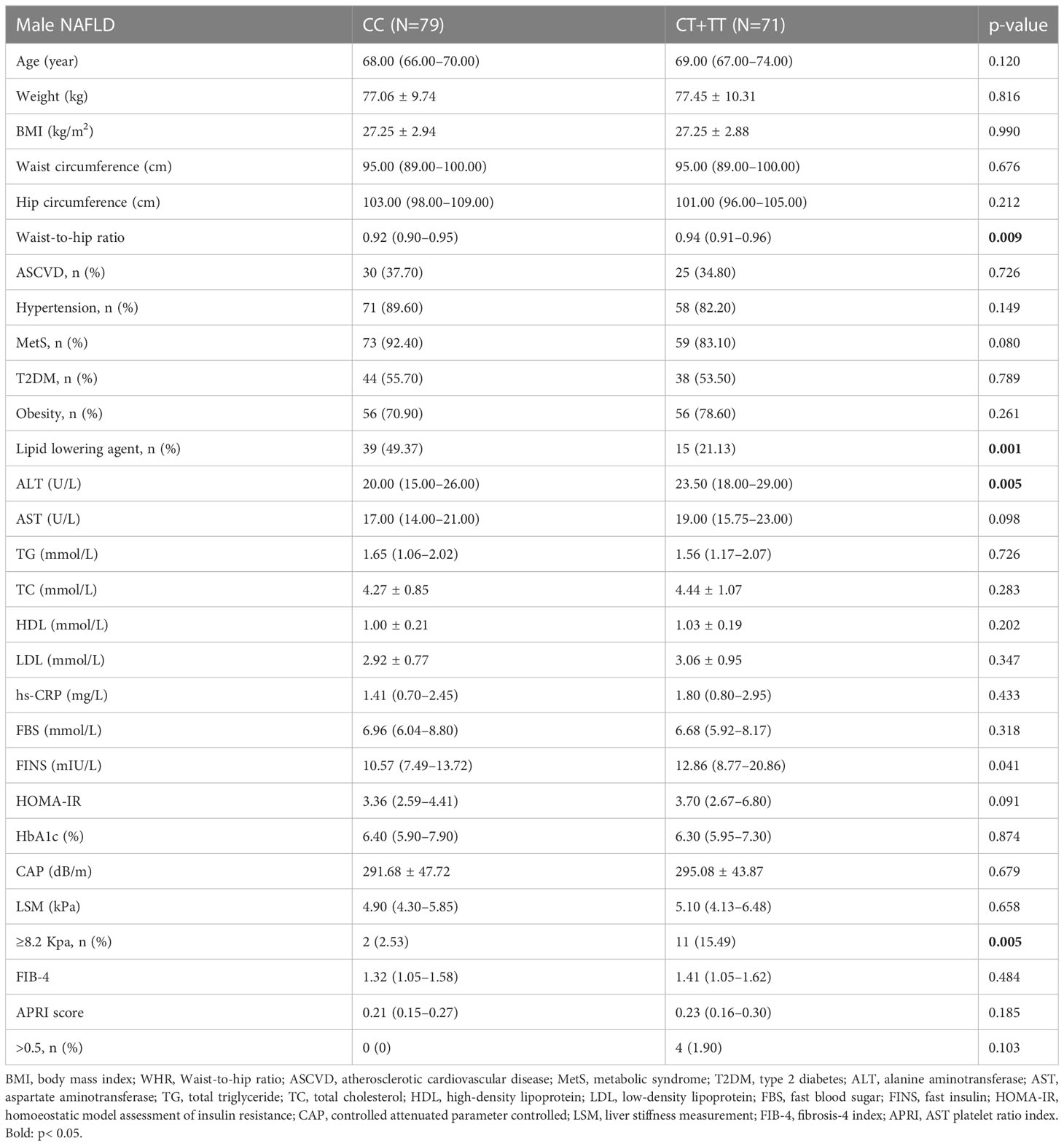
Table 3 Comparison of clinical characteristics in different MBOAT7 rs641738 genotypes in male NAFLD patients.
NAFLD always shares multiple manifestations of MetS, such as T2DM, hyperlipidemia, obesity, and hypertension (12, 25). Genetic epidemiology may be helpful to unveil the biological pathways that relate NAFLD to MetS and ASCVD. Thus, we compared ASCVD prevalence and metabolic traits among the three MBOAT7 genotypes.
In the whole population, TT carriage had fewer MetS, obesity, and ASCVD when compared to CC carriage (Supplementary Table S4). After adjusted by age, sex, and BMI, TT carriage showed a negative association with ASCVD (OR: 0.570, 95%CI: 0.340–0.953, p=0.032) and obesity (OR: 0.545, 95%CI: 0.346–0.856, p=0.008). Unfortunately, we did not find an association between MBOAT7rs641738 and NAFLD, and an increase in the minor T allele did not increase the risk of developing NAFLD (Figure 3B).
ANGPTL3 has been identified as an important regulator of lipoprotein metabolism and is related to the risk of ASCVD (26). As shown above, the MBOAT7 rs641738 TT genotype was found to be associated with a reduced risk of ASCVD. Furthermore, the livers of MBOAT7Δhep mice showed increased expression of ANGPTL3, a protein implicated in lipoprotein metabolism (18). Based on the above, we proposed that ANGPTL3 might be one of the molecular links between MBOAT7 rs641738 driven-NAFLD and ASCVD. As shown in Figure 4, serum ANGPTL3 level decreased paralleled from CC, CT, to TT genotype. The level in the TT genotype (43.27 ± 5.00 ng/ml) was significantly lower than that in the CC genotype (60.28 ± 4.95 ng/ml, p= 0.02).
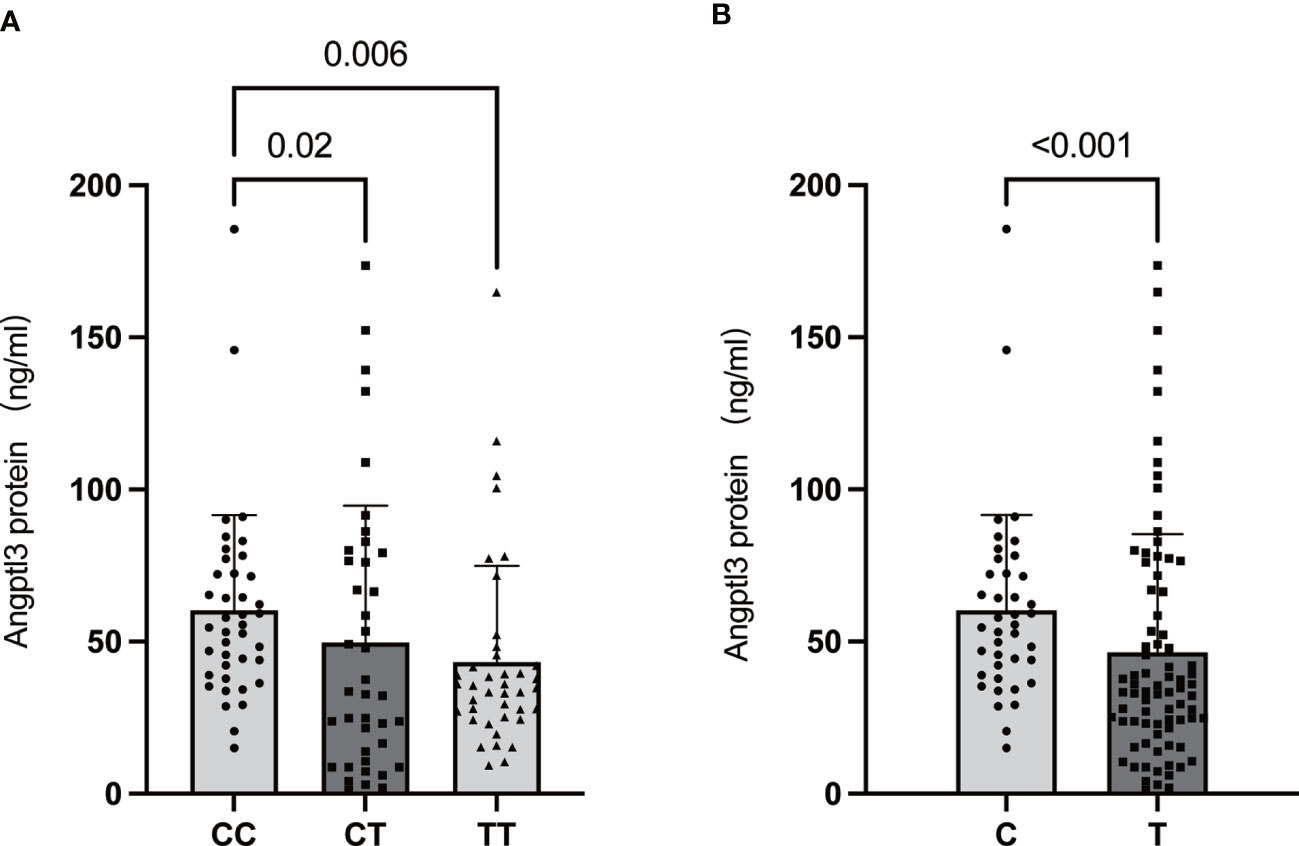
Figure 4 Association of the MBOAT7 rs641738 genotype (CC: homozygotes for the C allele; CT: heterozygotes; TT: homozygotes for the T allele) (A) and genotype carriage (C: homozygotes for the C allele; T: T allele carrier) (B) with serum ANGPTL3 levels in the total population. All data are shown as the mean ± SD. P<0.05.
This was the first study on the significance of the MBOAT7 rs641738 variant in an elderly population-based cohort. We found that the MBOAT7 rs641738 T allele was associated with higher ALT and significant fibrosis mainly in elderly male NAFLD patients. Furthermore, the TT genotype was associated with a decreased risk of T2DM and MetS in NAFLD patients and with a decreased risk of ASCVD and obesity in the total population. In addition, serum ANGPTL3 levels were significantly lower in TT allele carriers compared with CC genotype carriers, suggesting that ANGPTL3 may be the key molecular link between MBOAT7 variant-driven NAFLD and ASCVD.
Age is an important influencing factor for NAFLD. The prevalence of NAFLD peaks between 40 and 50 years old in men and 60 and 69 years old in women and then slightly decrease in people older than 70 years (27). The severity of NAFLD was also affected by age. A Hongkong study found that being older than 50 years old was one of the risk factors for NAFLD-related cirrhosis (28). Miyaaki et al. reported that being older than 60 years old was a risk factor for severe fibrosis (29). Although the subjects in our study were not a population with the highest prevalence rate of NAFLD, they might have additional advantages: (1) the severity of NAFLD seemed to peak in elders; (2) metabolic-associated diseases, such as T2DM and ASCVD, were highly prevalent in the population and facilitate the observation of their correlation with MBOAT7 rs641738 variants. Therefore, the elderly seemed to be better subjects to evaluate the association of gene variants with metabolic disease. As shown in Table 1, the prevalence rates of ASCVD and T2DM were as high as 35.28% and 41.58%.
NAFLD is a disease with high heterogeneity in which genetic predisposition plays an important role (30). Some studies have found several genes to be the major genetic determinants of NAFLD, including PNPLA3, TM6SF2, HSD17B13, and GCKR(31, 32). These gene variants combined with metabolic factors, such as obesity or diabetes, were proven to promote the progression of NAFLD by aggravating liver steatosis, inflammation, fibrosis, and hepatocarcinoma (30, 33). As mentioned above, MBOAT7 rs641738 C>T was an important inheritable factor in NAFLD incidence and progression (34–38). However, the relationships were mainly found in European descendants and not in Asians(Teo et al.; 17, 39–43). Only one study discovered that rs641738 C>T increased NAFLD occurrence in Chinese with an average age of 63 years old, and the frequency of the T allele was 43.42%. However, there was no relationship between the variant and fibrosis in the population (44). Different from the results, we found that the MBOAT7 rs641738 T allele promoted NAFLD inflammation and fibrosis but not NAFLD onset. As we all know, the effect of gene variants was not comparable in different ethnic groups due to the diversity of frequencies, penetrance, and relative risk. The frequency of the T allele in our cohort was 46.36%, which was higher than other Asian cohorts. In one Japanese cohort, two Korean cohorts, and one Taiwan children cohort, the T allele frequencies were 22.00% (39), 38.20% (43), 38.50% (40), and 19.60% (41), respectively. In European descents, the frequency was as high as 58.20% 16). Obviously, the higher prevalence of the variant facilitated the discovery of the effect of the variant.
The MBOAT7 gene encodes an acyltransferase that specifically esterifies arachidonic acid-CoA to lysophosphatidylinositol (LPI), thereby producing the main molecular species of phosphatidylinositol (PI) in the cell membrane (45). Thangapandi et al. (18) discovered that MBOAT7 deletion in hepatocytes can lead to the disruption of the intracellular PI side-chain remodeling pathway, which in turn promoted the pathological progression of liver fibrosis by upregulation of ECM genes in a high-fat, methionine-low, choline-deficient diet-fed mouse model of NAFLD. In addition, imbalance in LPI 18:0 levels have also been shown to be associated with the presence of fibrosis in human MBOAT7 rs641738 TT genotype carriers and in MBOAT7Δhep mouse (18). The treatment of obese mice with exogenous LPI 18:1 promoted the expression of pro-fibrotic genes in the liver, and LPI lipids increased hepatic inflammation and fibrotic gene expression in an MBOAT7-dependent manner (46). Furthermore, circulating LPI levels have also been demonstrated to be significantly higher in patients with liver fibrosis compared to healthy individuals (46). The above-mentioned studies also demonstrated that MBOAT7-LPI-PI plays a key role in the development of fibrosis. NAFLD is an obvious sexual dimorphic disease (47, 48). The prevalence and severity of NAFLD are increasing in men more than in reproductive-age women. However, the incidence rate and severity of NAFLD were comparable or even worse than in men after menopause (49). However, NAFLD in female patients were all relatively mild compared to that in male patients in this study, characterized by lower ALT levels and less significant fibrosis. The effect of SNP is also different in male and female patients. A study showed that PNPLA3 rs738409 polymorphism was closely related to alcoholic liver disease in Chinese Han men (50). Gender differences in the MBOAT7 rs641738 variant have not been reported before. In our cohort, the associations of the T allele with ALT and fibrosis were only found in male NAFLD patients.
In our study, the association of the rs641738 variant with higher serum HDL was found but not TC and LDL-C. In a Spanish study, MBOAT7 variants were found to be associated with higher TC, LDL, and TG (51). Data from a genome-wide association study (852,409 participants) found that rs641738 variants were associated with higher TC only in Caucasian populations. A meta-analysis (17) showed that rs641738 variants were associated with lower TG and HDL only in Caucasian populations (17). We speculated that there were two reasons for the opposite results. First, the characteristics of the subjects were different in the studies. Second, there were more than 100 genes related to lipid metabolism. Thus, the relationship between a single gene and blood lipids was influenced by other factors, which were not taken into consideration collectively.
NAFLD was closely correlated with T2DM, and the relationship was recently proved to be influenced by SNPs. Moon et al. (52) discovered that a PNPLA3 polymorphism confers lower susceptibility to the incident of T2DM in NAFLD patients. In the NAFLD cohort of our study, the multivariant analysis showed that rs641738 T allele decreased the risk of T2DM. Such a correlation also resulted in the decrease in MetS in rs641738 T allele carriage. Previous research demonstrated that HDL reduce blood glucose levels through various mechanisms, such as promoting insulin secretion from pancreatic β (53) and enhancing glucose uptake into skeletal muscle by activating the AMPK signaling pathway (54). Clinical studies have also shown an association between lower levels of circulating HDL particles and T2DM, insulin resistance, and impaired glucose tolerance. (55). Femlak et al. found that low HDL-cholesterol (HDL-c) levels were an independent risk factor for type 2 diabetes and the development of microvascular complications of diabetes (56). In another study, the authors found a 50% decrease in both HDL2 cholesterol and protein levels in obese individuals by comparing HDL concentrations in 68 age-matched obese individuals with controls (57). The above study showed that HDL levels were significantly and negatively associated with the progression of metabolic diseases. In this study, we found that TT genotype carriers had a significantly lower risk of MetS in the NAFLD population after adjusting for confounding factors, which may be associated with a high HDL level being found in this population. However, as the statistical significance of the T allele and T2DM tended towards borderline values, additional mechanistic research and prospective longitudinal studies are necessary to confirm these findings.
As aforementioned, several gene loci have been found to promote NAFLD progression and prevent ASCVD, such as PNPLA3 rs738409 and TM6SF2 rs58542926. MBOAT7 variants seemed to exhibit similar effects according to our findings. A similar result was reported in a prospective follow-up study carried out in China Changfeng society. They found that the MBOAT7 T allele decreased the mortality rate of cardiovascular disease together with PNPLA3 and TM6SF2 gene variants (44). Two other findings might support such a correlation in our study. One finding was that TT variants were associated with less obesity in the whole population; the other was its association with fewer MetS and T2DM in NAFLD patients. No other clinical study or meta-analysis came to the same conclusion as us and the Changfeng study (12, 58). Whether such an association also had ethnic differences needs to be further confirmed in more studies.
A lower level of serum ANGPTL3 has been shown to decrease the risk of ASCVD by regulating serum TG. In an analysis of 13,102 patients with cardiovascular disease, carriers with loss-of-function mutations in ANGPTL3 had an approximately 40% lower risk of cardiovascular disease than non-carriers (59). In addition, Thangapandi et al. demonstrated that ANGPTL3 mRNA levels increased significantly in the liver of MBOAT7 knockout mice (18). Therefore, we supposed that ANGPTL3 may be a key regulatory molecule linked to MBOAT7 rs641738-related NAFLD and ASCVD. The hypothesis that serum levels of ANGPTL3 were significantly decreased in the TT group compared to the CC and CT groups was verified. ANGPTL3 is mainly synthesized by hepatocytes and then cleaved by PCSK9 to generate an N-terminal coiled-coil region and a C-terminal fibrinogen-like domain, which could be detected in the serum. MBOAT7 is a protein located on the hepatocyte membrane. MBOAT7 rs641738 variant decreased protein level and may interfere with syntheses or secretion of ANGPTL3 protein. The underlying mechanism needs to be further elucidated through additional in vitro and in vivo studies.
Several limitations should be noted in our study. First, the sample size was relatively small and with less opportunity to get more results. Second, the analysis was designed for the NAFLD study, so the relationship with ASCVD needs confirmation in specially designed research. Third, only one SNP was analyzed in the study; the confounders such as PNPLA3 SNP were not adjusted.
In conclusion, the rs641738 variant near MBOAT7 was found to promote inflammation and fibrosis, particularly in male patients, and decrease the risk of T2DM and metabolic traits in NAFLD patients. In addition, it was related to decreased ASCVD risk in the whole population. These results will be meaningful to better understand the pathogenesis of NAFLD and identify distinct phenotypes of the disease, which will benefit for individualized management.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://figshare.com/, https://figshare.com/articles/dataset/MBOAT7_rs641738_C_T_is_associated_with_NAFLD_progression_and_decreased_ASCVD_risk_in_elder_Chinese_population/21506427.
The study protocol was approved by the individual Ethics Review Committee of Beijing Youan Hospital (IRB approval number [2020]-233). The patients/participants provided their written informed consent to participate in this study.
XX, HX and XL participated in the acquisition, analysis, and interpretation of data; prepared the manuscript; and had equal contribution to the study. SZ, GW, and LZ reviewed the literature and built the databases. ZC, and LQ contributed to the analysis and interpretation of the data and provided important scientific input. XD and YL statistically analyzed the data and wrote the first draft of the manuscript. JZ and YZ supervised the whole study. All authors collaboratively discussed key decisions throughout the course of the review, provided critical feedback on preliminary manuscript and interpretation of results. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (82070627), Beijing Municipal Natural Science Foundation (7222090), the Beijing Municipal Institute of Public Medical Research Development and Reform Pilot Project (2021-10), the Capitals’s Funds for Health Improvement and Research (2022-2Z-2187), and the Scientific research project of Beijing Youan Hospital CCMU, 2021 (YNKTQN 2021015) and Beijing Municipal Administration of Hospitals Incubating Program (PX2023061).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1199429/full#supplementary-material
ASCVD, atherosclerotic cardiovascular disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; APRI, AST platelet ratio index; BMI, body mass index; CAP, controlled attenuated parameter controlled; FIB‐4, fibrosis‐4 index; FINS, fast insulin; HDL, high‐density lipoprotein; HOMA‐IR, homoeostatic model assessment of insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; MetS, metabolic syndrome; NAFLD, Non-alcoholic fatty liver disease; T2DM, type 2 diabetes; TG, total triglyceride; TC, total cholesterol; WHR, waist-to-hip ratio.
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol (Baltimore Md.) (2016) 64(1):73–84. doi: 10.1002/hep.28431
2. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
3. Page JM, Harrison SA. NASH and HCC. Clinics In Liver Dis (2009) 13(4):631–47. doi: 10.1016/j.cld.2009.07.007
4. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American gastroenterological association, American association for the study of liver diseases, and American college of gastroenterology. Gastroenterology (2012) 142(7):1592–609. doi: 10.1053/j.gastro.2012.04.001
5. Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet (London England) (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
6. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol (2013) 10(6):330–44. doi: 10.1038/nrgastro.2013.41
7. Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut (2017) 66(6):1138–53. doi: 10.1136/gutjnl-2017-313884
8. Severson TJ, Besur S, Bonkovsky HL. Genetic factors that affect nonalcoholic fatty liver disease: a systematic clinical review. World J Gastroenterol (2016) 22(29):6742–56. doi: 10.3748/wjg.v22.i29.6742
9. Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Alimentary Pharmacol Ther (2020) 51(12):1305–20. doi: 10.1111/apt.15738
10. Liu YL, Patman GL, Leathart JBS, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 c >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol (2014) 61(1):75–81. doi: 10.1016/j.jhep.2014.02.030
11. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol (2016) 65(3):589–600. doi: 10.1016/j.jhep.2016.05.013
12. Simons N, Isaacs A, Koek GH, Kuc S, Schaper NC, Brouwers M. PNPLA3, TM6SF2, and MBOAT7 genotypes and coronary artery disease. Gastroenterology (2017) 152(4):912–3. doi: 10.1053/j.gastro.2016.12.020
13. Gellert-Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjaerg-Hansen A, Stender S. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatol (Baltimore Md.) (2020) 72(3):845–56. doi: 10.1002/hep.31238
14. Meroni M, Longo M, Fracanzani AL, Dongiovanni P. MBOAT7 down-regulation by genetic and environmental factors predisposes to MAFLD. EBioMedicine (2020) 57:102866. doi: 10.1016/j.ebiom.2020.102866
15. Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet (2015) 47(12):1443–8. doi: 10.1038/ng.3417
16. Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology (2016) 150(5):1219–30.e6. doi: 10.1053/j.gastro.2016.01.032
17. Teo K, Abeysekera KWM, Adams L, Aigner E, Anstee QM, Banales JM, et al. rs641738C>T near MBOAT7 is associated with liver fat, ALT and fibrosis in NAFLD: a meta-analysis. J Hepatol (2021) 74(1):20–30. doi: 10.1016/j.jhep.2020.08.027
18. Thangapandi VR, Knittelfelder O, Brosch M, Patsenker E, Vvedenskaya O, Buch S, et al. Loss of hepatic Mboat7 leads to liver fibrosis. Gut (2021) 70(5):940–50. doi: 10.1136/gutjnl-2020-320853
19. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol (Baltimore Md.) (2006) 43(6):1317–25. doi: 10.1002/hep.21178
20. Kruger FC, Daniels CR, Kidd M, Swart G, Brundyn K, van Rensburg C, et al. APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. South Afr Med J = Suid-Afrikaanse Tydskrif Vir Geneeskunde (2011) 101(7):477–80.
21. Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet (London England) (2005) 366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8
22. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J (2013) 34(38):2949–3003. doi: 10.1093/eurheartj/eht296
23. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology (2019) 156(6):1717–30. doi: 10.1053/j.gastro.2019.01.042
24. WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London England) (2004) 363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3
25. Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatol (Baltimore Md.) (2020) 71(3):808–19. doi: 10.1002/hep.31014
26. Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, et al. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol (2017) 69(16):2054–63. doi: 10.1016/j.jacc.2017.02.030
27. Alqahtani SA, Schattenberg JM. NAFLD in the elderly. Clin Interv Aging (2021) 16:1633–49. doi: 10.2147/CIA.S295524
28. Zhang X, Wong GL, Yip TC, Cheung JTK, Tse YK, Hui VW, et al. Risk of liver-related events by age and diabetes duration in patients with diabetes and nonalcoholic fatty liver disease. Hepatology (2022) 76(5):1409–22. doi: 10.1002/hep.32476
29. Miyaaki H, Ichikawa T, Nakao K, Yatsuhashi H, Furukawa R, Ohba K, et al. Clinicopathological study of nonalcoholic fatty liver disease in Japan: the risk factors for fibrosis. Liver Int Off J Int Assoc For Study Liver (2008) 28(4):519–24. doi: 10.1111/j.1478-3231.2007.01614.x
30. Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology (2020) 158(7):1999–2014 e1991. doi: 10.1053/j.gastro.2019.11.312
31. Liu Z, Zhang Y, Graham S, Wang X, Cai D, Huang M, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J Hepatol (2020) 73(2):263–76. doi: 10.1016/j.jhep.2020.03.006
32. De Vincentis A, Tavaglione F, Jamialahmadi O, Picardi A, Antonelli Incalzi R, Valenti L, et al. A polygenic risk score to refine risk stratification and prediction for severe liver disease by clinical fibrosis scores. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterological Assoc (2022) 20(3):658–73. doi: 10.1016/j.cgh.2021.05.056
33. Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol (2018) 68(2):268–79. doi: 10.1016/j.jhep.2017.09.003
34. Luukkonen PK, Zhou Y, Hyötyläinen T, Leivonen M, Arola J, Orho-Melander M, et al. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol (2016) 65(6):1263–5. doi: 10.1016/j.jhep.2016.07.045
35. Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep (2017) 7(1):4492. doi: 10.1038/s41598-017-04991-0
36. Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, et al. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res (2017) 58(1):247–55. doi: 10.1194/jlr.P067454
37. Di Costanzo A, Belardinilli F, Bailetti D, Sponziello M, D'Erasmo L, Polimeni L, et al. Evaluation of polygenic determinants of non-alcoholic fatty liver disease (NAFLD) by a candidate genes resequencing strategy. Sci Rep (2018) 8(1):3702. doi: 10.1038/s41598-018-21939-0
38. Krawczyk M, Bantel H, Rau M, Schattenberg JM, Grünhage F, Pathil A, et al. ). could inherited predisposition drive non-obese fatty liver disease? results from German tertiary referral centers. J Hum Genet (2018) 63(5):621–6. doi: 10.1038/s10038-018-0420-4
39. Kawaguchi T, Shima T, Mizuno M, Mitsumoto Y, Umemura A, Kanbara Y, et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PloS One (2018) 13(1):e0185490. doi: 10.1371/journal.pone.0185490
40. Koo BK, Joo SK, Kim D, Bae JM, Park JH, Kim JH, et al. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. J Gastroenterol Hepatol (2018) 33(6):1277–85. doi: 10.1111/jgh.14056
41. Lin Y-C, Chang P-F, Chang M-H, Ni Y-H. Genetic determinants of hepatic steatosis and serum cytokeratin-18 fragment levels in Taiwanese children. Liver Int Off J Int Assoc For Study Liver (2018) 38(7):1300–7. doi: 10.1111/liv.13689
42. Xia Y, Huang C-X, Li G-Y, Chen K-H, Han L, Tang L, et al. Meta-analysis of the association between MBOAT7 rs641738, TM6SF2 rs58542926 and nonalcoholic fatty liver disease susceptibility. Clinics Res In Hepatol Gastroenterol (2019) 43(5):533–41. doi: 10.1016/j.clinre.2019.01.008
43. Koo BK, Joo SK, Kim D, Lee S, Bae JM, Park JH, et al. Development and validation of a scoring system, based on genetic and clinical factors, to determine risk of steatohepatitis in Asian patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterological Assoc (2020) 18(11):2592–9.e10. doi: 10.1016/j.cgh.2020.02.011
44. Xia M, Ma S, Huang Q, Zeng H, Ge J, Xu W, et al. NAFLD-related gene polymorphisms and all-cause and cause-specific mortality in an Asian population: the shanghai changfeng study. Alimentary Pharmacol Ther (2022) 55(6):705–21. doi: 10.1111/apt.16772
45. Lee H-C, Inoue T, Sasaki J, Kubo T, Matsuda S, Nakasaki Y, et al. LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Mol Biol Cell (2012) 23(24):4689–700. doi: 10.1091/mbc.E12-09-0673
46. Helsley RN, Varadharajan V, Brown AL, Gromovsky AD, Schugar RC, Ramachandiran I, et al. Obesity-linked suppression of membrane-bound -acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. ELife (2019) 8:e49882. doi: 10.7554/eLife.49882
47. Yang JD, Abdelmalek MF, Guy CD, Gill RM, Lavine JE, Yates K, et al. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterological Assoc (2017) 15(1):127–31.e2. doi: 10.1016/j.cgh.2016.07.034
48. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatol (Baltimore Md.) (2019) 70(4):1457–69. doi: 10.1002/hep.30626
49. Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatol (Baltimore Md.) (2014) 59(4):1406–14. doi: 10.1002/hep.26761
50. Zhang Y, Guo T, Yang F, Mao Y, Li L, Liu C, et al. Single-nucleotide rs738409 polymorphisms in the PNPLA3 gene are strongly associated with alcoholic liver disease in han Chinese males. Hepatol Int (2018) 12(5):429–37. doi: 10.1007/s12072-018-9889-3
51. Krawczyk M, Jiménez-Agüero R, Alustiza JM, Emparanza JI, Perugorria MJ, Bujanda L, et al. PNPLA3 p.I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg For Obes Related Dis Off J Am Soc For Bariatric Surg (2016) 12(10):1838–46. doi: 10.1016/j.soard.2016.06.004
52. Moon S, Chung GE, Joo SK, Park JH, Chang MS, Yoon JW, et al. A PNPLA3 polymorphism confers lower susceptibility to incident diabetes mellitus in subjects with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterological Assoc (2022) 20(3):682–91.e8. doi: 10.1016/j.cgh.2021.04.038
53. Fryirs MA, Barter PJ, Appavoo M, Tuch BE, Tabet F, Heather AK, et al. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arteriosclerosis Thrombosis Vasc Biol (2010) 30(8):1642–8. doi: 10.1161/ATVBAHA.110.207373
54. Han R, Lai R, Ding Q, Wang Z, Luo X, Zhang Y, et al. Apolipoprotein a-I stimulates AMP-activated protein kinase and improves glucose metabolism. Diabetologia (2007) 50(9):1960–8. doi: 10.1007/s00125-007-0752-7
55. Bozorgmanesh M, Hadaegh F, Ghaffari S, Harati H, Azizi F. A simple risk score effectively predicted type 2 diabetes in Iranian adult population: population-based cohort study. Eur J Public Health (2011) 21(5):554–9. doi: 10.1093/eurpub/ckq074
56. Femlak M, Gluba-Brzózka A, Ciałkowska-Rysz A, Rysz J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids In Health Dis (2017) 16(1):207. doi: 10.1186/s12944-017-0594-3
57. Rashid S, Genest J. Effect of obesity on high-density lipoprotein metabolism. Obes (Silver Spring Md.) (2007) 15(12):2875–88. doi: 10.1038/oby.2007.342
58. Brouwers MCGJ, Simons N, Stehouwer CDA, Koek GH, Schaper NC, Isaacs A. Relationship between nonalcoholic fatty liver disease susceptibility genes and coronary artery disease. Hepatol Commun (2019) 3(4):587–96. doi: 10.1002/hep4.1319
Keywords: MBOAT7, non-alcoholic fatty liver disease, fibrosis, atherosclerotic cardiovascular disease, single-nucleotide polymorphism
Citation: Xu X, Xu H, Liu X, Zhang S, Cao Z, Qiu L, Du X, Liu Y, Wang G, Zhang L, Zhang Y and Zhang J (2023) MBOAT7 rs641738 (C>T) is associated with NAFLD progression in men and decreased ASCVD risk in elder Chinese population. Front. Endocrinol. 14:1199429. doi: 10.3389/fendo.2023.1199429
Received: 03 April 2023; Accepted: 05 June 2023;
Published: 22 June 2023.
Edited by:
Qin Pan, Shanghai Jiao Tong University, ChinaReviewed by:
Yuqin Wang, Shanghai Jiao Tong University, ChinaCopyright © 2023 Xu, Xu, Liu, Zhang, Cao, Qiu, Du, Liu, Wang, Zhang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhang, emp5b3VhbkBjY211LmVkdS5jbg==; Yang Zhang, eWFuZzUxOEBtYWlsLmNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.