- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Republic of Korea
- 2Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 3Center for Obesity and Metabolic Diseases, Korea University Anam Hospital, Seoul, Republic of Korea
- 4Division of Foregut Surgery, Korea University College of Medicine, Seoul, Republic of Korea
Background: Beneficial role of fibroblast growth factor 1 (FGF1) in the regulation of glucose metabolism and adipose tissue remodeling was suggested in rodents. This study aimed to investigate the association between serum FGF1 levels and metabolic parameters in adults with glucose intolerance.
Methods: Serum FGF1 levels were examined using an enzyme-linked immunosorbent assay in 153 individuals with glucose intolerance. Associations between serum FGF1 levels and metabolic parameters, including body mass index (BMI), glycated hemoglobin (HbA1c), and 75 g oral glucose tolerance test-derived parameters, including insulinogenic index (IGI), Matsuda insulin sensitivity index (ISI), and disposition index (DI), were examined.
Results: Serum FGF1 was detected in 35 individuals (22.9%), possibly due to the autocrine/paracrine nature of the peptide. IGI and DI levels were significantly lower in individuals with higher FGF1 levels than in those with lower FGF1 levels or undetectable FGF1 (p=0.006 and 0.005 for IGI and DI, respectively, after adjustment for age, sex, and BMI). Univariable and multivariable analyses using the Tobit regression model also revealed a negative association between FGF1 levels and IGI and DI. The regression coefficients per 1-SD of log-transformed IGI and DI were −0.461 (p=0.013) and −0.467 (p=0.012), respectively, after adjustment for age, sex, and BMI. In contrast, serum FGF1 levels were not significantly associated with ISI, BMI, or HbA1c.
Conclusions: The serum concentration of FGF1 was significantly elevated in individuals with low insulin secretion, suggesting a possible interaction between FGF1 and beta cell function in humans.
Introduction
Fibroblast growth factor 1 (FGF1) is an autocrine/paracrine peptide that participates in adipose tissue remodeling and regulates glucose metabolism (1). FGF1 was induced in adipose tissue by a high-fat diet, and FGF1-lacking mice developed an aggressive diabetic phenotype with aberrant adipose expansion, suggesting a role of FGF1 in adipose remodeling (2). A single intracerebroventricular injection of FGF1 induced sustained diabetes remission for 18 weeks in mouse models of type 2 diabetes (3). The exact mechanism of the glucose-lowering effect of central FGF1 administration is not fully understood; hypothalamic neurons and glial cells, such as tanycytes and astrocytes, have been suggested as targets of the central action of FGF1 (4). Peripheral injection of FGF1 also reverses hyperglycemia partly by suppressing adipose lipolysis and reducing hepatic glucose production (5). Evidence suggests that FGF1 also regulates pancreatic beta cell function (6–8). Peripheral injection of FGF1 increases beta cell density and ex vivo insulin secretion in diabetic mouse islets (6).
However, the metabolic effects of FGF1, including insulin secretion and sensitivity, have not been well elucidated in humans. This study aimed to investigate the association between serum FGF1 levels and metabolic parameters in adults with glucose intolerance.
Materials and methods
This study included participants from two separate cohorts from November 2016 to January 2022. The first cohort comprised participants aged ≥19 years who were diagnosed with glucose intolerance at a single tertiary hospital, Korea University Anam Hospital. Glucose intolerance was defined as fasting plasma glucose ≥100 mg/dL or 2-h glucose ≥140 mg/dL on the 75 g oral glucose tolerance test (OGTT) or HbA1c ≥5.7% (39 mmol/mol). The second cohort comprised adults with obesity, with a BMI ≥25 kg/m2, who were planning to undergo bariatric surgery at four tertiary medical centers in South Korea. The participants in both cohorts underwent blood tests after overnight fasting. Serum samples were stored at −70°C before FGF1 analysis. Serum FGF1 levels were examined using an enzyme-linked immunosorbent assay (catalog no. DFA00B; R&D Systems, Inc., Minneapolis, MN USA). Samples were analyzed in duplicates.
All participants underwent a 75 g OGTT at enrollment. Serum glucose and insulin levels were measured at fasting (0), 30, and 120 min after 75 g glucose loading. Insulin secretion was estimated using the insulinogenic index (IGI), which was calculated as (insulin30 min – insulin0 min [μIU/mL])/(glucose30 min – glucose0 min [mg/dL]) (9). Insulin sensitivity was measured using the Matsuda insulin sensitivity index (ISI), calculated as 10000/(fasting glucose [mg/dL] × fasting insulin [μIU/mL] × mean glucose [mg/dL] × mean insulin [μIU/mL])1/2 (10). The disposition index (DI), reflecting beta cell function adjusted for insulin sensitivity, was calculated as IGI × ISI (11).
Individuals were divided into three groups—undetected FGF1, low FGF1, and high FGF1—and their clinical characteristics were compared. We also used a Tobit regression model with a lower bound of half of the observed minimum value to assess the association between FGF1 and metabolic parameters, since there was censoring below 0.056 pg/mL at the observed FGF1 value. Information on age, sex, hypertension, dyslipidemia, and cardiovascular disease was collected using a questionnaire. BMI, waist circumference, systolic blood pressure, total cholesterol, HbA1c, fasting glucose, alanine aminotransferase, and creatinine levels were measured. Variables with right-skewed distributions were transformed logarithmically. The Tobit regression model included log-transformed FGF1 as a dependent variable and each metabolic parameter, log-transformed IGI, ISI, and DI as independent variables. The model was adjusted for age, sex, and BMI. The results are presented as the regression coefficient per 1-SD of the independent variable and the standard error of the coefficient. Additionally, we evaluated the difference in mean serum FGF1 levels according to the quartile of each variable, adjusted for age and sex. All statistical analyses were performed using the SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Statistical significance was set at a two-sided p-value of <0.05.
This study was approved by the Institutional Review Board of Korea University Anam Hospital (IRB number 2017AN0050). Written informed consent was obtained from all participants.
Results
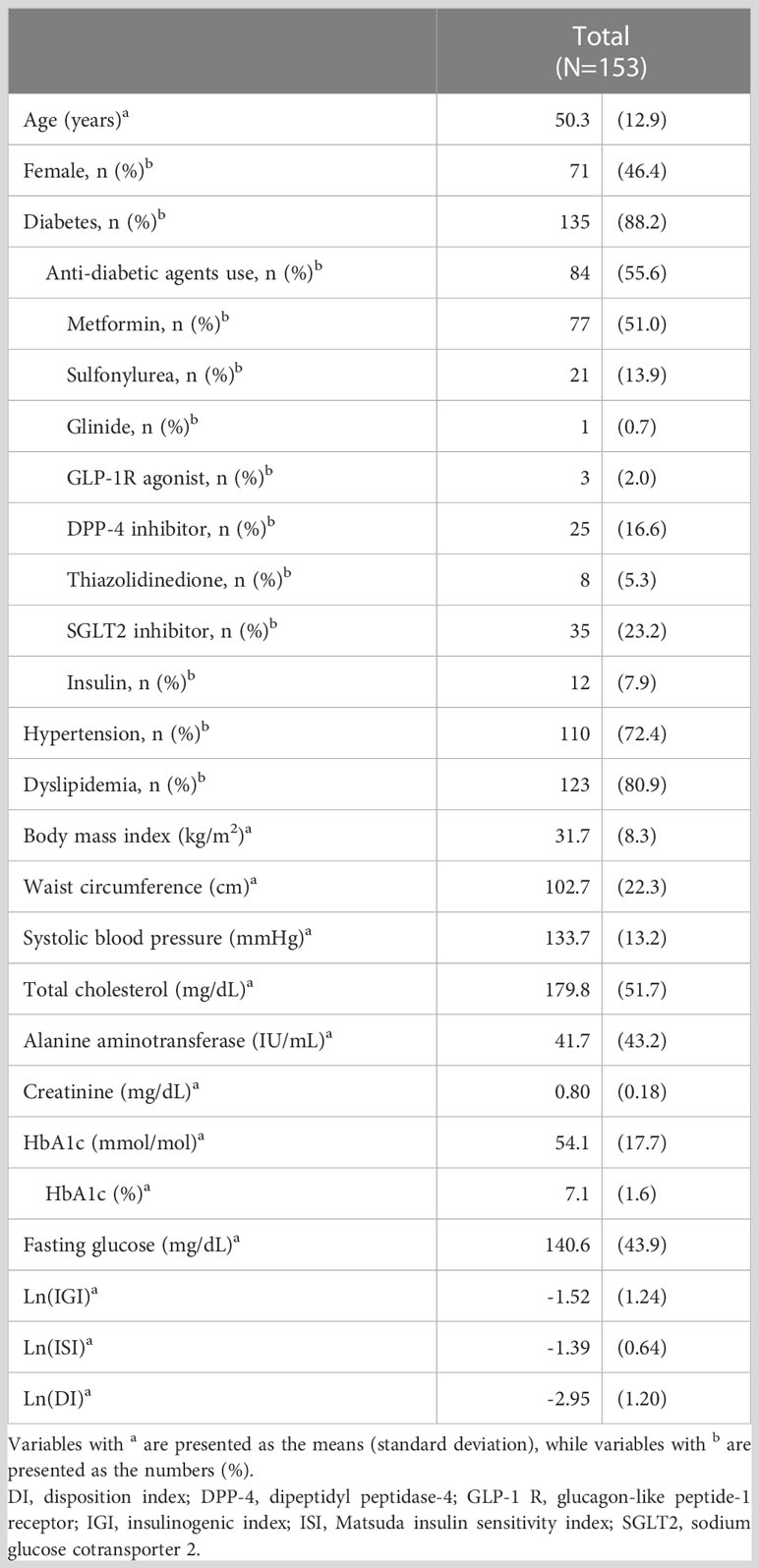
The characteristics of the 153 participants are presented in Table 1. The mean age of the patients was 50 years, and 46.4% were female. The mean BMI was 31.7 kg/m2 and the mean HbA1c level was 54 mmol/mol (7.1%). Type 2 diabetes was present in 88.2% of the participants, and 55.6% of the participants received anti-diabetic agents.
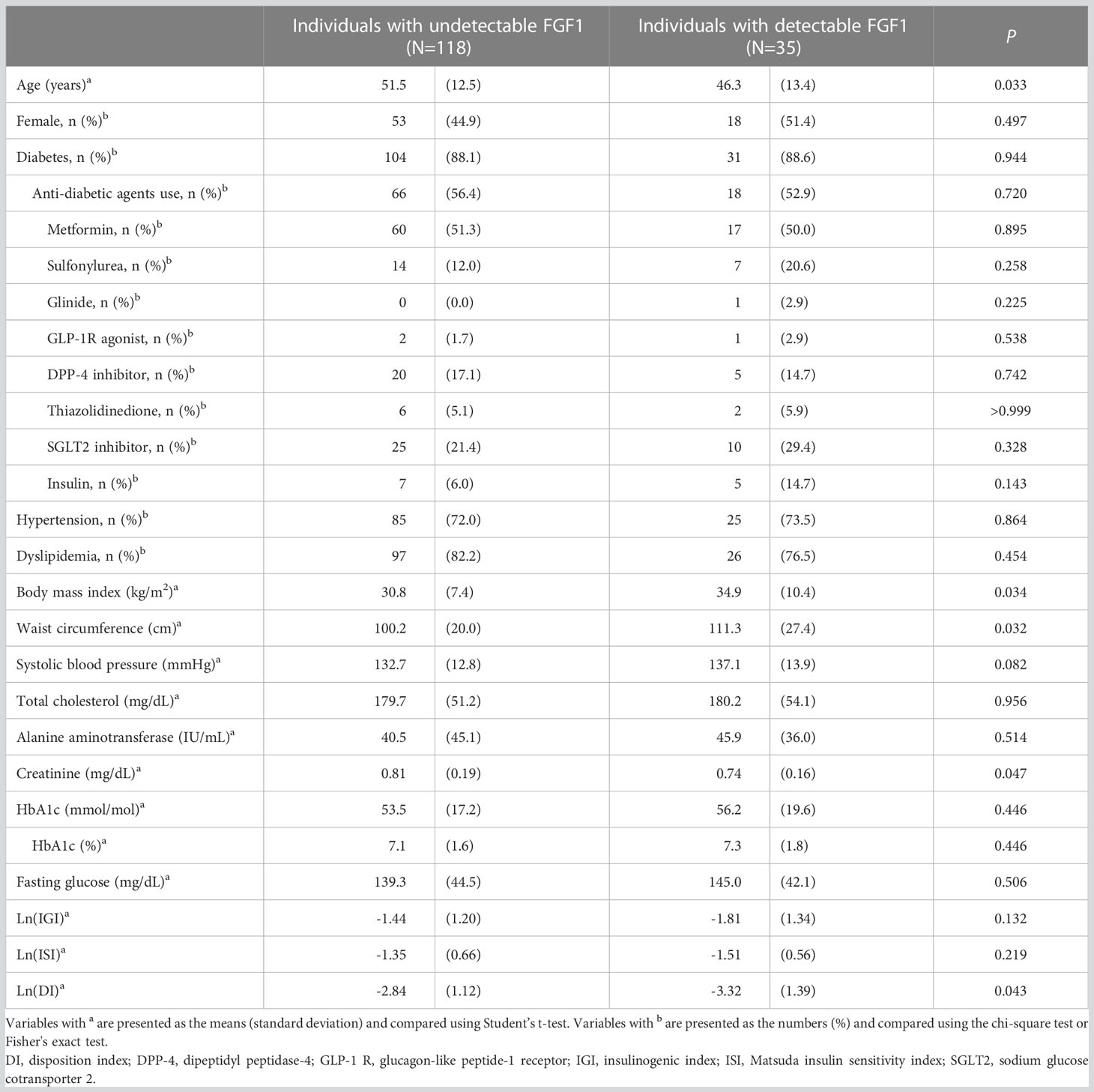
Serum FGF1 was detected in 35 participants (22.9%), and their mean serum FGF1 level was 5.07 pg/mL. Individuals with detectable FGF1 were younger and had a higher BMI than those without. While HbA1c, IGI, and ISI were not significantly different between the groups, DI was significantly lower in individuals with detectable FGF1 than in others (Ln(DI); -3.32 vs. -2.84, p=0.043) (Table 2).
Comparing the metabolic parameters between the three groups (undetectable, low, and high FGF1) revealed a negative correlation between Ln(IGI) and Ln(DI) and FGF1 levels (p=0.006 and 0.005, respectively, after adjustment for age, sex, and BMI). However, no significant association with BMI and HbA1c was found (Table 3; Figure 1).
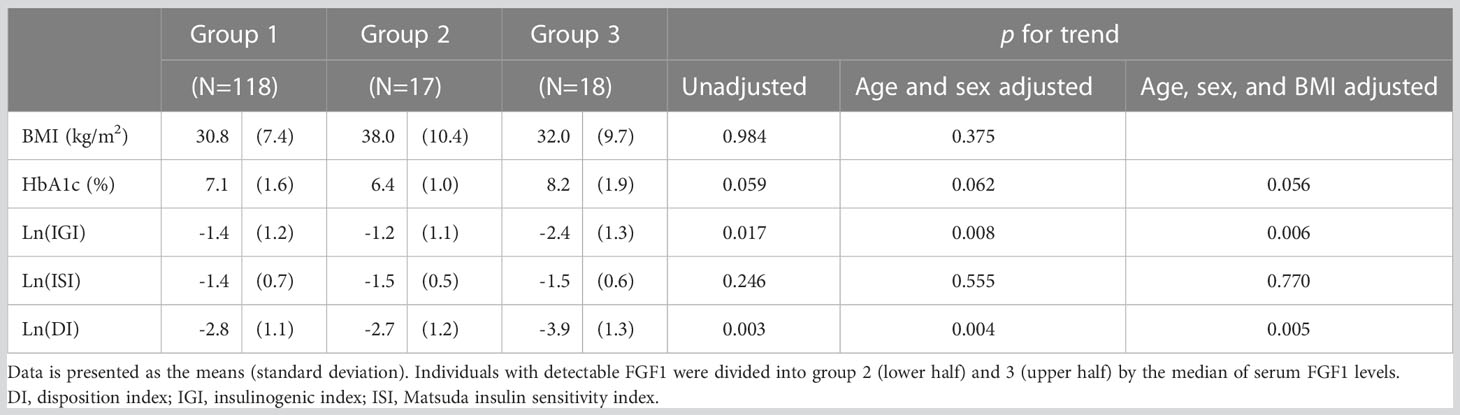
Table 3 Metabolic parameters of individuals with undetectable (group 1), low (group 2), and high (group 3) serum FGF1 levels.
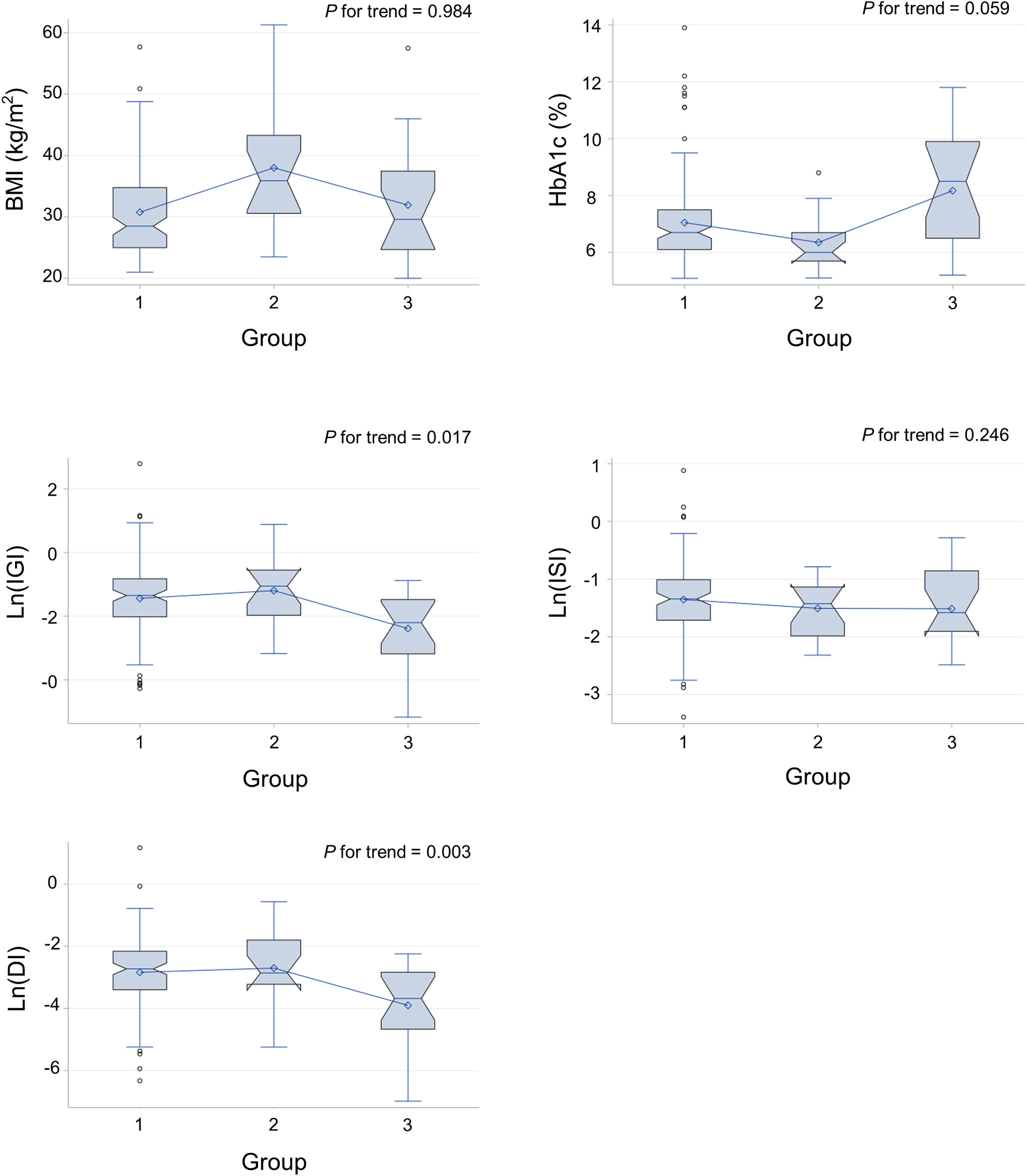
Figure 1 Metabolic parameters of individuals with undetectable (group 1), low (group 2), and high (group 3) serum FGF1 levels. DI, disposition index; IGI, insulinogenic index; ISI, Matsuda insulin sensitivity index.
In univariable analysis using the Tobit regression model, serum FGF1 levels were negatively associated with IGI (β=−0.387, p=0.039) and DI (β=−0.487, p=0.010) (Table 4). No significant association was found between serum FGF1 levels and ISI (β=−0.217, p=0.251), BMI (β=0.313, p=0.089), or HbA1c (β=0.291, p=0.117). IGI and DI remained significant after adjusting for age and sex (β=−0.438, p=0.019; β=−0.475, p=0.011, respectively) and after further adjusting for BMI (β=−0.461, p=0.013; β=−0.467, p=0.012, respectively).
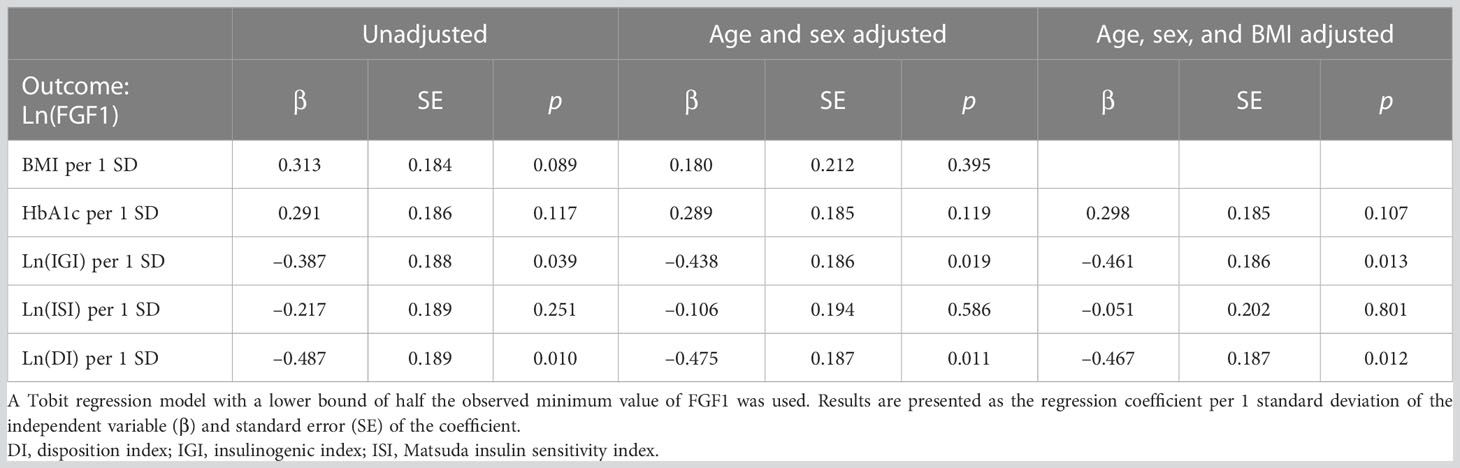
Table 4 Univariable and multivariable analysis of the association between serum FGF1 levels and metabolic parameters using a Tobit regression model.
Figure 2 shows the box plot of the estimated log-transformed FGF1 levels according to the quartile of each parameter using the Tobit regression model adjusted for age and sex. The mean FGF1 concentrations were significantly lower in the higher quartiles of DI (p for trend=0.047).
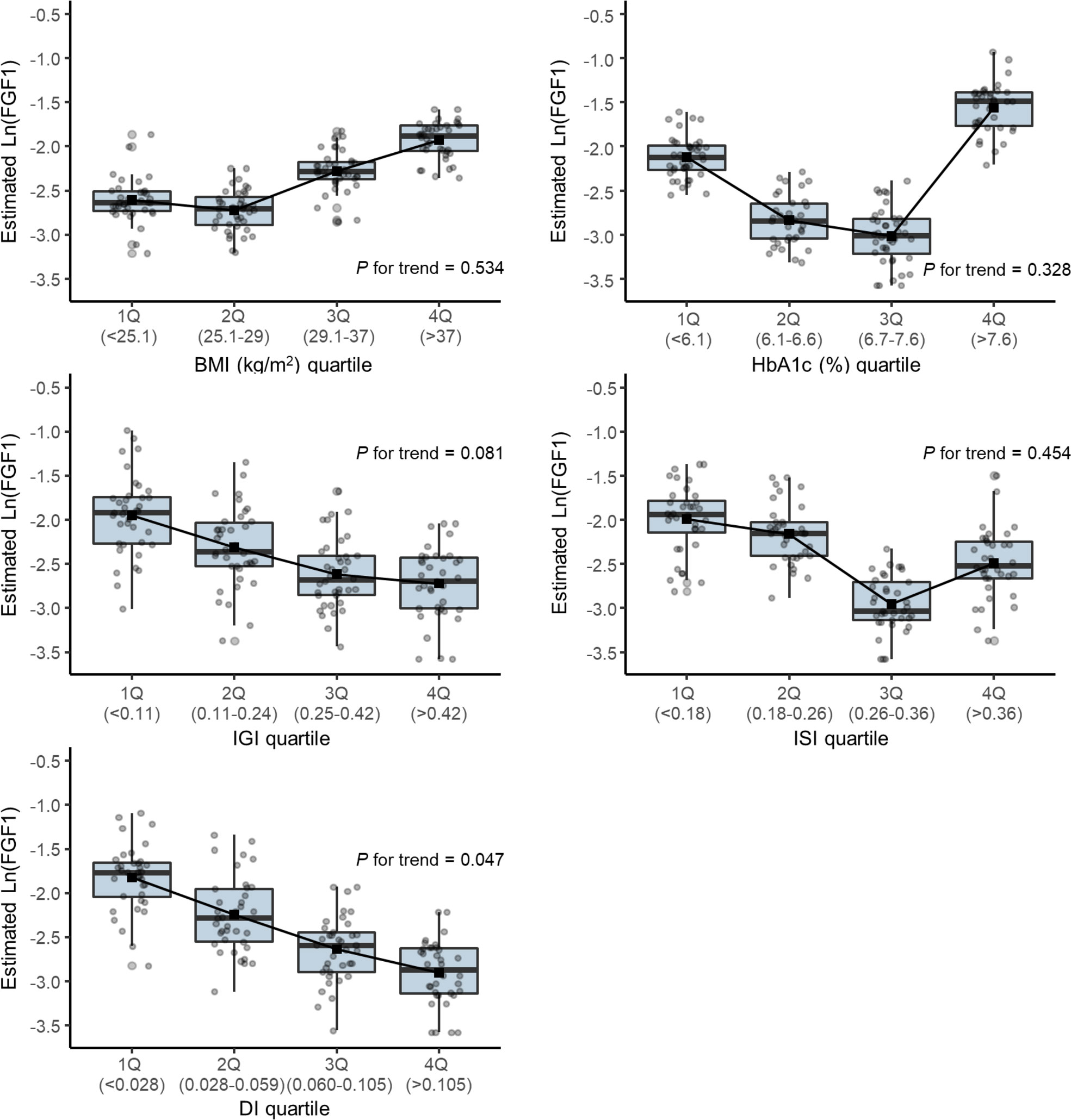
Figure 2 Box plot of the estimated log-transformed serum FGF1 level according to the quartile of each parameter using a Tobit regression model adjusted by age and sex. DI, disposition index; IGI, insulinogenic index; ISI, Matsuda insulin sensitivity index.
Discussion
This study found that serum FGF1 levels were significantly higher in individuals with low insulin secretion. Serum FGF1 had a negative correlation with IGI and DI in both the unadjusted and adjusted models, suggesting a possible interaction between FGF1 and insulin secretion or beta cell function in humans. To the best of our knowledge, this is the first human study to address the association between circulating FGF1 levels and insulin indices derived from 75 g OGTT.
It has been demonstrated that FGF1 partially ameliorates diabetes-induced beta cell dysfunction by improving insulin secretion at the pancreatic islet level. Central and peripheral FGF1 administration increased ex vivo islet insulin secretion in diabetic mice, and peripheral FGF1 increased islet beta cell density (6). In zebrafish, overnutrition induced compensatory beta cell differentiation via FGF1 signaling. Inactivation of fgf1 abolished compensatory beta cell differentiation. Expression of FGF1 in beta cells of fgf1-/- animals rescued the compensatory response (7). In another study, central FGF1 injection delayed the onset of progressive beta cell loss in a rat model of diabetes (8). Taken together, FGF1 increases insulin secretion and preserves beta cell function in multiple ways, partly contributing to its prolonged glucose-lowering effect in rodents. In our study, the serum FGF1 concentration was higher in individuals with impaired beta cell function. This finding implies that FGF1 has an association with beta cell function in humans. Our findings are consistent with previous findings that the HOMA-B was lower in participants with higher FGF1 levels among patients newly diagnosed with type 2 diabetes and healthy controls (12). We postulate that serum FGF1 levels may increase in individuals with low insulin secretion to compensate for beta cell dysfunction. However, our cross-sectional study design could not determine causal relationships.
FGF1 is an autocrine or paracrine hormone. FGF1 has a high affinity for heparan sulfate proteoglycans, which are components of the extracellular matrix that modulate FGF/FGF receptor signaling. This high affinity for heparan sulfate proteoglycan hinders the release of FGFs from the originating tissues into circulation (5). In our study, FGF was detected in 22.9% of the participants. To validate the assay, we used the samples from multi-centers, and the experiment was done by two different researchers. All the samples were analyzed in duplicates, and the coefficient of variation of samples was less than 15%. The low detection rate of circulating FGF1 in our study supports that FGF1 is an autocrine/paracrine hormone rather than an endocrine hormone. This is in line with previous studies showing that circulating FGF1 was detected in 5 out of 31 subjects (13) and serum FGF1 was not detected in healthy humans (14). Nonetheless, the low detection rate of serum FGF1 was a limitation of this study. Therefore, we used Tobit regression analysis to handle censored data. In contrast, circulating FGF1 was well detected in some previous studies (12, 15, 16), which may be due to the different assay methods and detection ranges, and different study populations.
Previous studies showed conflicting results on the association between FGF1 and BMI or adiposity measures. Wang et al. reported positive associations between BMI and FGF levels (12, 15) whereas Zhu et al. showed negative associations between BMI and FGF1 levels (16). Mejhert et al. reported that FGF1 expression in subcutaneous white adipose tissue was elevated in obese people, however, this did not contribute to the circulating levels (13). A preclinical study showed an insulin-sensitizing effect of FGF1 when it was pharmacologically given, suggesting a possible interaction between FGF1 and insulin sensitivity (4, 17). However, our study did not show any significant association between serum FGF1 levels and BMI or insulin sensitivity. The discrepancy between local expression, circulating level, and pharmacologic action of FGF1 suggests its complex and undiscovered nature. Further studies are required to validate the metabolic actions of FGF1.
In conclusion, serum FGF1 levels are significantly higher in individuals with lower insulin secretion. This suggests a possible interaction between FGF1 and insulin secretion in humans. Future studies are needed to reveal the exact mechanism by which FGF1 modulates beta cell function in humans.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Korea University Anam Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JK: Conceptualization, Investigation, Writing – original draft, Writing – review and editing. JC: Formal analysis, Methodology. YK: Investigation. SP: Resources. SK: Resources. NK: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Korean Endocrine Society of New Faculty Research Award 2018; and a grant (K.N.H., 2018F-1) from the Korean Diabetes Association.
Acknowledgments
The authors thank the volunteers who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gasser E, Sancar G, Downes M, Evans RM. Metabolic messengers: fibroblast growth factor 1. Nat Metab (2022) 4:663–71. doi: 10.1038/s42255-022-00580-2
2. Jonker JW, Suh JM, Atkins AR, Ahmadian M, Li P, Whyte J, et al. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature (2012) 485:391–4. doi: 10.1038/nature10998
3. Scarlett JM, Rojas JM, Matsen ME, Kaiyala KJ, Stefanovski D, Bergman RN, et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med (2016) 22:800–6. doi: 10.1038/nm.4101
4. Gasser E, Moutos CP, Downes M, Evans RM. FGF1 - a new weapon to control type 2 diabetes mellitus. Nat Rev Endocrinol (2017) 13:599–609. doi: 10.1038/nrendo.2017.78
5. Sancar G, Liu S, Gasser E, Alvarez JG, Moutos C, Kim K, et al. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab (2022) 34:171–83.e6. doi: 10.1016/j.cmet.2021.12.004
6. Tennant KG, Lindsley SR, Kirigiti MA, True C, Kievit P. Central and peripheral administration of fibroblast growth factor 1 improves pancreatic islet insulin secretion in diabetic mouse models. Diabetes (2019) 68:1462–72. doi: 10.2337/db18-1175
7. Li M, Page-McCaw P, Chen W. FGF1 mediates overnutrition-induced compensatory β-cell differentiation. Diabetes (2016) 65:96–109. doi: 10.2337/db15-0085
8. Scarlett JM, Muta K, Brown JM, Rojas JM, Matsen ME, Acharya NK, et al. Peripheral mechanisms mediating the sustained antidiabetic action of FGF1 in the brain. Diabetes (2019) 68:654–64. doi: 10.2337/db18-0498
9. Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and c-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract (2006) 72:298–301. doi: 10.1016/j.diabres.2005.10.005
10. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care (1999) 22:1462–70. doi: 10.2337/diacare.22.9.1462
11. Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, et al. 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol (2016) 4:27–34. doi: 10.1016/s2213-8587(15)00336-8
12. Wang S, Yang Q, Yu S, Pan R, Jiang D, Liu Y, et al. Fibroblast growth factor 1 levels are elevated in newly diagnosed type 2 diabetes compared to normal glucose tolerance controls. Endocr J (2016) 63:359–65. doi: 10.1507/endocrj.EJ15-0627
13. Mejhert N, Galitzky J, Pettersson AT, Bambace C, Blomqvist L, Bouloumié A, et al. Mapping of the fibroblast growth factors in human white adipose tissue. J Clin Endocrinol Metab (2010) 95:2451–7. doi: 10.1210/jc.2009-2049
14. Bradley JC, Bradley RH, McCartney DL, Mannis MJ. Serum growth factor analysis in dry eye syndrome. Clin Exp Ophthalmol (2008) 36:717–20. doi: 10.1111/j.1442-9071.2008.01895.x
15. Wang A, Yan X, Zhang C, Du C, Long W, Zhan D, et al. Characterization of fibroblast growth factor 1 in obese children and adolescents. Endocr Connect (2018) 7:932–40. doi: 10.1530/ec-18-0141
16. Zhu J, Wang Y, Zhu K, Gao J, Wan X, Pang X, et al. Serum fibroblast growth factor 1 is associated with the decreased risk of obesity in human. Exp Clin Endocrinol Diabetes (2017) 125:322–6. doi: 10.1055/s-0043-104532
Keywords: beta cell function, fibroblast growth factor 1, glucose intolerance, insulin secretion, insulin sensitivity
Citation: Kim JY, Choi J, Kwon Y, Park S, Kim SG and Kim NH (2023) Serum fibroblast growth factor 1 and its association with pancreatic beta cell function and insulin sensitivity in adults with glucose intolerance. Front. Endocrinol. 14:1198311. doi: 10.3389/fendo.2023.1198311
Received: 01 April 2023; Accepted: 02 May 2023;
Published: 22 May 2023.
Edited by:
Jun Sung Moon, Yeungnam University, Republic of KoreaReviewed by:
Sunghwan Suh, Dong-A University, Republic of KoreaJae Han Jeon, Kyungpook National University, Republic of Korea
Copyright © 2023 Kim, Choi, Kwon, Park, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nam Hoon Kim, cG91cmxpZmVAa29yZWEuYWMua3I=
 Ji Yoon Kim
Ji Yoon Kim Jimi Choi
Jimi Choi Yeongkeun Kwon
Yeongkeun Kwon Sungsoo Park
Sungsoo Park Sin Gon Kim
Sin Gon Kim Nam Hoon Kim
Nam Hoon Kim