
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 August 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1192108
This article is part of the Research Topic Diabetes and Mental Health: from understanding biomedical and social determinants, to promoting wellness in diabetes View all 14 articles
Aim: The objective of this study was to translate the Barriers to Insulin Treatment Questionnaire (BIT) into Chinese and test its psychometric properties in middle-aged and elderly type 2 diabetes mellitus (T2D) patients using insulin in the Han people of urban China.
Methods: We established the Barriers to Insulin Treatment Questionnaire in Chinese (BIT-C). We selected 296 patients with T2D for testing BIT-C's the reliability and validity, of which 120 patients were retested four weeks later. Another 200 patients with T2D were selected to perform the confirmatory factor analysis (CFA).
Results: The final BIT-C consisted of 11 items (BIT-C-11) and four factors. The explained variances of the BIT-C-11 and its four factors were 90.153%, 51.308%, 18.810%, 10.863%, and 9.173%. CFA validated that the four-factor model fit with the data of the BIT-C-11. Standardized factor loadings ranged between 0.77 and 0.90. The Cronbach’s α coefficients of the BIT-C-11 and its four factors were 0.903, 0.952, 0.927, 0.938, and 0.917. Correlation analysis was performed between the BIT-C-11 and General Adherence Scale in Chinese (GAS-C) to calculate the criterion-related validity (r = 0.598, p < 0.001). The correlation coefficient r of the BIT-C-11’s test–retest reliability was 0.810 (p < 0.001).
Conclusion: The BIT-C-11 has good reliability and validity. It can be used for psychological resistance to insulin therapy studies of middle-aged and elderly patients with T2D using insulin in the Han people of Chinese cities.
If non-insulin medication has failed, type 2 diabetes mellitus (T2D) patients may need insulin injections to control hyperglycemia to recommended levels (1, 2). However, studies in recent years have found poor adherence in patients with T2D who use insulin to treat their diabetes (3, 4).
A 2017 study found that the insulin adherence and persistence of patients with T2D in China are generally poor. Only 53% of patients with T2D persisted with insulin therapy until 12 months. After 1 year of insulin injections, only 30.9% of patients with T2D had a medication possession rate (MPR) ≥0.8 (5). Psychological factors such as negative beliefs about insulin therapy are the most common reasons for these patients’ poor adherence to insulin therapy (6, 7). Psychological insulin resistance (PIR) is a barrier for providers and patients in starting and maintaining insulin therapy (8). The patient’s psychological resistance to insulin therapy can result in poor glycemic control, damaging their health and burdening their families and society (9, 10). China has many patients with T2D, and many need insulin to control their blood sugar (11, 12). Improving the adherence of these patients with T2D who require long-term insulin therapy is an urgent challenge for the prevention and control of T2D in China. Regarding population health, it may be more effective to focus efforts on those who are least likely to adhere or those with poorly controlled diseases (13). Therefore, there is an urgent need to investigate psychological resistance to insulin therapy in patients with T2D in China. However, no standardized research tools can quantitatively assess psychological resistance to insulin therapy in patients with T2D in China. The Barriers to Insulin Treatment Questionnaire (BIT) is a valuable tool for studying psychological resistance to insulin therapy in patients with T2D, which Petrak et al. (6) developed. This scale has been widely used (14, 15). So, we decided to revise the Barriers to Insulin Treatment Questionnaire in Chinese (BIT-C) and select middle-aged and elderly Chinese patients with T2D who were on insulin therapy as the research objects to evaluate the reliability and validity of the BIT-C.
This study has two parts:
Study I: translating the BIT into Chinese and conducting an exploratory factor analysis (EFA) on it;
Study II: confirmatory factor analysis (CFA) of the Chinese version of the BIT.
This study was conducted following the Declaration of Helsinki and was approved by the Shanghai Pudong New Area Mental Health Center (approval number: 2017009). It was conducted from May 2018 to December 2020 in the diabetes wards of several general hospitals in Haicheng City in northeast China.
The inclusion criteria of the study subjects were as follows:
i. Patients who meet the WHO diagnostic criteria for T2D,
ii. currently on insulin therapy,
iii. aged 45–74 years,
iv. Han Chinese who had been continuously residing at the survey site for at least 5 years at the time of the survey,
v. voluntary participation.
Subjects will not be included in our study if they match the following exclusion criteria:
i. those who were seriously ill and unable to complete the study,
ii. those who had a disturbance of consciousness,
iii. those suffering from various severe mental illnesses who cannot complete the study.
Demographic and medical data (glycosylated hemoglobin level, insulin use, diabetes duration) were collected by self-report.
Petrak et al. (6) developed the BIT, which measures psychological resistance to insulin treatment in patients with T2D. The BIT includes 14 items, a total sum score, and the following five factors: Factor 1: fear of injection and self-testing (items 1–3); Factor 2: expectations regarding positive insulin-related outcomes (items 4–6; they were reverse coded); Factor 3: expected hardship from insulin treatment (items 7–9); Factor 4: stigmatization by insulin injections (items 10–12); Factor 5: fear of hypoglycemia (items 13 and 14) (Table 1). The response format of the BIT is a 10-point Likert scale, ranging from “totally disagree” [1] to “totally agree” [10]. The BIT’s Cronbach’s α for the five subscales ranged from 0.62 to 0.85, and the BIT’s α for the total sum score was 0.78 (6). It will be revised in Chinese in this study.
The General Adherence Scale (GAS) was developed by DiMatteo and Hays and is used to assess the general tendency of patients with chronic diseases to adhere to their physicians’ recommendations during the past 4 weeks (16, 17). Shi revised the General Adherence Scale in Chinese (GAS-C), which can be applied to the general adherence study of middle-aged and elderly patients with T2D in China. Consistent with the GAS, the GAS-C has five items and is one-dimensional. The Cronbach’s α reliability coefficient of the GAS-C was 0.942 (18). In this study, the GAS-C was used to assess the criterion-related validity of the BIT-C.
After obtaining the developer’s permission, we integrated the cross-cultural approach to translate and adapt the BIT into Chinese (19, 20). The translation and adaptation stages of the BIT are as follows:
Two bilingual translators translated the BIT into Chinese separately. One translator is a teacher in the Department of English, and the other is a research group member.
Team members and two translators analyzed and compared the two drafts resulting from StageI, producing one common translation draft of the BIT.
Two other translators with no medical background translated the Chinese BIT draft into English separately to produce two back-translation English BIT scales.
The expert committees involved two linguists, one epidemiologist, four translators (forward and back translators), and research team members. They analyzed and compared the BIT, two forward translation versions, one common translation draft, and two back-translation BIT to finalize the initial Chinese version of the BIT. After discussion, the expert committee concluded that this initial Chinese version of the BIT is equivalent to the original version of the BIT in terms of semantics, idiomatic expression, experience, and concepts.
Fifteen patients with T2D who met the research criteria were asked to fill in the initial Chinese version of the BIT. Patients all filled out the questionnaire without any problems. Next, we conducted a cognitive interview with these patients to examine the comprehensibility of the questionnaire (21). All patients reported that they could understand each questionnaire item without ambiguity.
After discussion, we decided to use the 14-item initial Chinese version of the BIT as the final Chinese version of the BIT (BIT-C-14).
Data were collected the day before the study subjects were discharged from the hospital. Patients who met the subject criteria and agreed to participate in this study were included. Before filling out the questionnaire, subjects were asked to sign an informed consent form. If the subjects have poor eyesight or cannot read or write, the investigator will read the questionnaire aloud and fill out the items according to their true feelings. After the subjects completed the questionnaire, investigators asked if they would like to participate in the retest. We randomly selected 120 subjects who agreed to be retested. They would be investigated again when they returned to the outpatient clinic for physician follow-up at week 4 after discharge.
We conducted the statistical analysis using SPSS 23.0. The sociodemographic information of the subjects was described by mean and standard deviation, frequency, and percentage. Continuous variables were expressed by mean ± standard deviation (SD). Counts and percentages are used to indicate categorical variables. p < 0.05 means a statistically significant difference. AMOS 23.0 was used in the CFA.
Using the homogeneity test to analyze items, those items with a low correlation with the total score on the BIT-C-14 were removed. The removal criteria are the value of the Pearson correlation coefficient r < 0.4 or the significant difference test p ≥ 0.05 (22). If deleting an item may significantly increase the Cronbach’s α value of the scale, it means that the item is not homogeneous with the rest of the items, and the item will be removed from the scale (21). For the EFA, those items with communalities <0.2 would be removed (23).
We analyzed the scale’s content validity, construct validity, and criterion-related validity.
Six diabetologists evaluated the scale’s content validity based on the item analysis results. They rated the degree of correlation between the content of each item and the evaluation purpose for that item. Content validity was judged by the item-level content validity index (I-CVI) and content validity index (S-CVI/Ave). We would retain those items with I-CVI ≥0.78; if the S-CVI/Ave ≥0.9, the scale-level content validity is acceptable (24, 25). Otherwise, the unqualified items should be deleted or modified and reevaluated until they meet the criteria.
We performed the EFA to test the scale’s construct validity. If the Kaiser-Meyer-Olkin measure of sampling adequacy (KMO) ≥0.70 and the difference of the Bartlett’s test had statistical significance (p < 0.05), the scale was suitable for factor analysis. If an item’s measure of sampling adequacy (MSA) is <0.5, the item is unsuitable for factor analysis and will be deleted (26, 27). We chose principal component analysis (PCA) combined with the Varimax orthogonal rotation method to analyze the data. The following criteria were used to determine the number of factors. 1) Kaiser’s principle of eigenvalues >1 to extract factors (28). 2) The factor contains at least two items with loadings >0.4 (29). 3) Items with cross-loading >0.75 were deleted (29). The scree test will assist us in judging the results of the PCA. Ultimately, the EFA’s results and the original BIT’s theoretical structure will guide us in determining the final version of the scale (28). We used the following criteria to assess the goodness of the CFA model: the ratio of chi-square to degrees of freedom (CMIN/df) <5; standardized root mean square residual (SRMR) <0.05; root mean square error of approximation (RMSEA) <0.08; comparative fit index (CFI), the goodness of fit index (GFI), and Tucker–Lewis index (TLI) value >0.9 (30, 31).
We used the GAS-C as a validity criterion to analyze the scale’s criterion-related validity. The criterion-related validity is acceptable if the Pearson correlation coefficient r ≥ 0.4 and is statistically significant (32).
We evaluated the scale’s reliability. Its internal consistency reliability is appropriate if Cronbach’s α ≥ 0.70 (21). The intraclass correlation coefficient (ICC) between 0.6 and 0.74 is good, and ≥0.75 is excellent (33). We assessed the test–retest reliability also. The Pearson correlation coefficient r of test–retest reliability ≥0.7 is acceptable (34).
We evaluated the ceiling and floor effects of the data. A ceiling or floor effect exists if more than 15% of respondents achieve an item’s maximum or minimum score, meaning a response bias occurred in the data (35).
Data from 496 patients with T2D were collected, from whom 296 patients with T2D were randomly selected as subjects for the item analysis, reliability analysis, and validity analysis of the BIT-C-14—the remaining 200 patients with T2D as subjects for the CFA. There were no missing data. Descriptive statistics for participants’ socioeconomic, medical, and psychological variables in Study I and Study II are provided in Table 2.
The Pearson correlation coefficients for the BIT-C-14’s items 4–6 with the BIT-C-14’s total score were all <0.4. The poor correlation means that these three items are not homogeneous with the remaining 11 items of the BIT-C-14 (22). So, we deleted them and obtained a BIT-C scale with the remaining 11 items (BIT-C-11) (Table 1). No items with communalities were <0.2 (21, 23) (Table 3). Removing an item from the BIT-C-11 would not increase its Cronbach’s α value (Table 4). All 11 items in the BIT-C-11 were retained.
Six diabetologists rated the BIT-C-11’s content validity. The I-CVI for all 11 items was 1.0, all higher than 0.78. The S-CVI/Ave was 1.0 higher than 0.9. They all meet the criterion of content validity (24, 25). The BIT-C-11 has good content validity.
We conducted the EFA on the BIT-C-11 using the PCA combined with the Varimax orthogonal rotation method. The KMO value was equal to 0.830 ≥ 0.7. The difference in the Bartlett’s test was statistically significant (p < 0.01). The chi-square value was equal to 3,131.231. The results demonstrated that the BIT-C-11 was suitable for factor analysis (24, 36).
Three items in Factor 1 are consistent with the BIT’s “fear of injection and self-testing” factor. We named Factor 1 “fear of injection and self-testing” as well. The three items in Factor 2 are consistent with those in the “expected hardship from insulin treatment” factor of the BIT, so we named Factor 2 “expected hardship from insulin treatment.” Factor 3 contains the BIT’s three items of the “stigmatization by insulin injections” factor. Therefore, we also named Factor 3 “stigmatization by insulin injections.” Factor 4 has two items corresponding to the two items in the BIT’s “fear of hypoglycemia” factor. We named Factor 4 “fear of hypoglycemia” (Table 3). The scree test also supported extracting four factors (Figure 1).
Results of the first-order CFA confirmed the structure of the BIT-C-11 with a good model fit with CMIN/DF = 1.311 < 5; GFI = 0.954, CFI = 0.961, TLI = 0.944, their values all >0.9; the SRMR = 0.032 < 0.05, and the RMSEA = 0.040 < 0.05. Standardized factor loadings ranged between 0.77 and 0.90 (Figure 2). The second-order CFA of the BIT-C-11 confirmed a good model fit with CMIN/DF = 1.104 < 5; GFI = 0.960, CFI = 0.982, TLI = 0.975; the SRMR = 0.033 < 0.05, and the RMSEA = 0.039 < 0.05. Standardized factor loadings ranged between 0.77 and 0.90 (Figure 3), so creating a total score of the BIT-C-11 is appropriate.
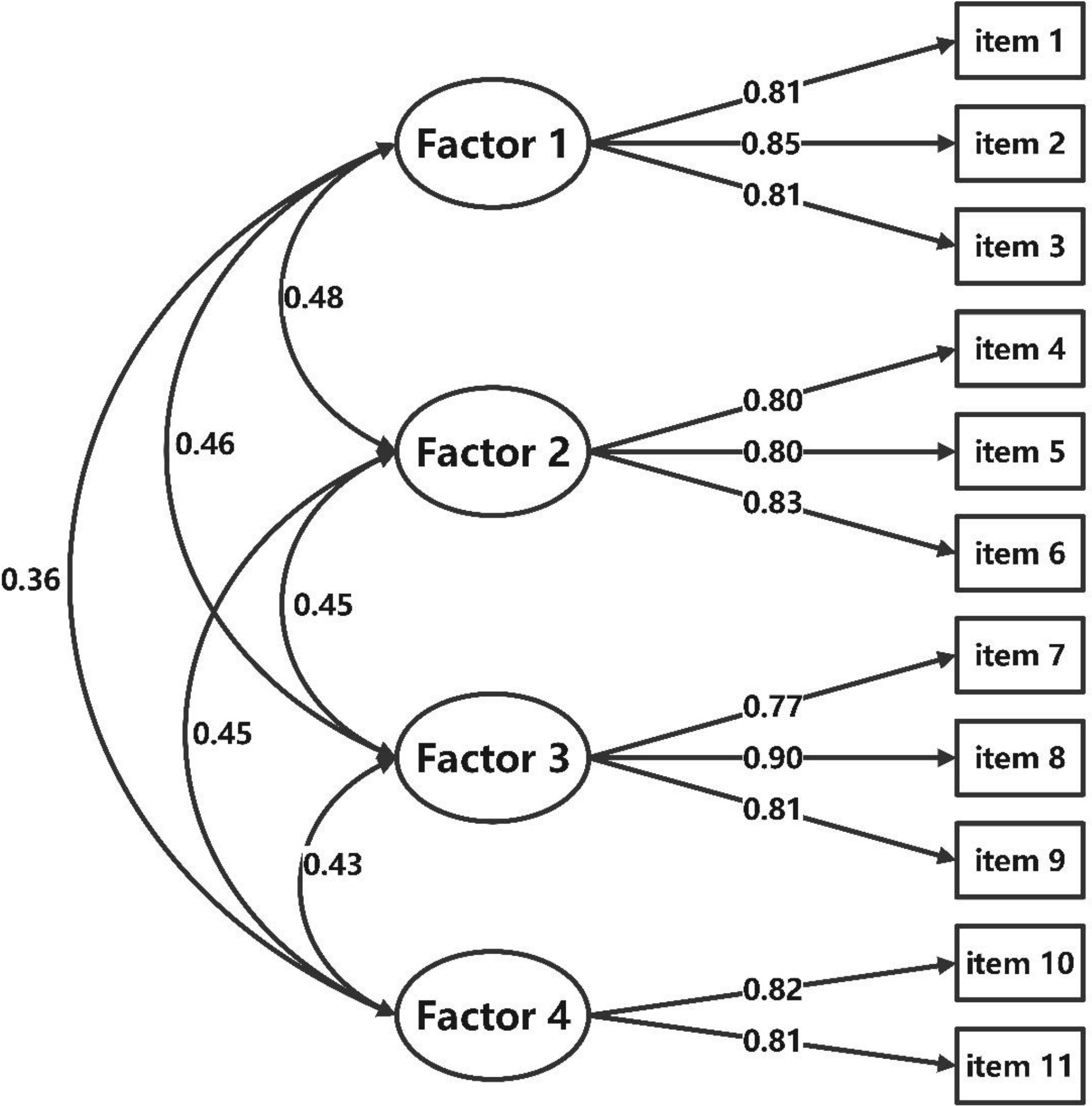
Figure 2 Path diagram for BIT-C-11’s first-order CFA. BIT-C-11, Chinese version of the BIT questionnaire with 11 items; CFA, confirmatory factor analysis; Factor 1, fear of injection and self-testing factor; Factor 2, expected hardship from insulin treatment factor; Factor 3, stigmatization by insulin injections factor; Factor 4, fear of hypoglycemia factor.
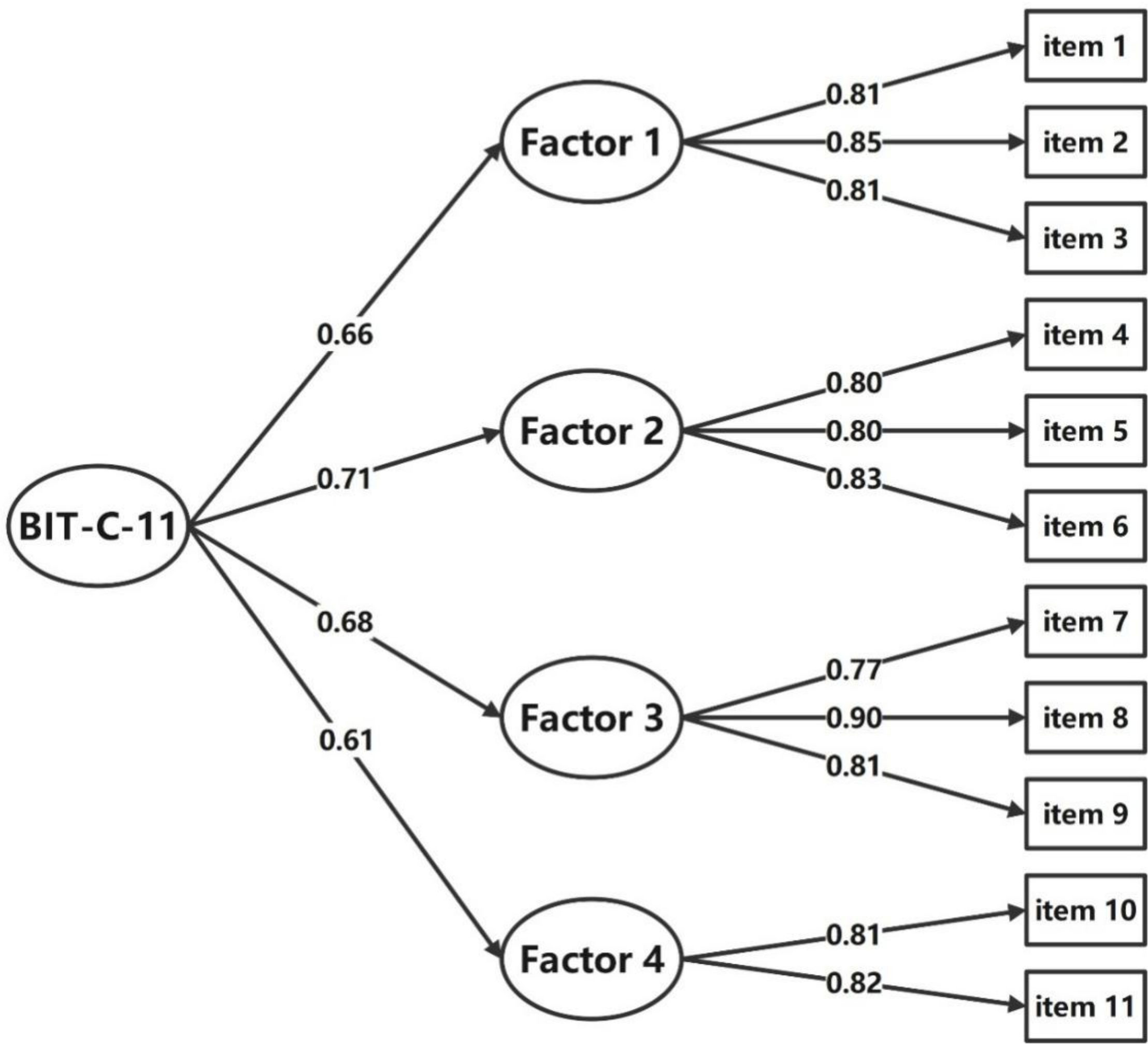
Figure 3 Path diagram for BIT-C-11’s second-order CFA. BIT-C-11, Chinese version of the BIT questionnaire with 11 items; CFA, confirmatory factor analysis; Factor 1, fear of injection and self-testing factor; Factor 2, expected hardship from insulin treatment factor; Factor 3, stigmatization by insulin injections factor; Factor 4, fear of hypoglycemia factor.
A negative correlation exists between the BIT-C-11 total score and the GAS-C total score, with a Pearson correlation coefficient of 0.598, p < 0.001. The criterion-related validity of the BIT-C-11 is acceptable (32).
The Cronbach’s α coefficients of the BIT-C-11 and its four factors were 0.903, 0.952, 0.927, 0.938, and 0.917. They are all >0.7 (p < 0.001). The Cronbach’s α values of the BIT-C-11 and its four factors are appropriate (16). The BIT-C-11’s ICC was 0.899 (95% CI 0.881–0.916). The ICC values for Factor 1, Factor 2, Factor 3, and Factor 4 were 0.951 (95% CI 0.940–0.960), 0.921 (95% CI 0.897–0.938), 0.937 (95% CI 0.923–0.949), and 0.915 (95% CI 0.893–0.933), respectively. All of which were >0.75 and met Cicchetti’s criteria for good (28). The correlation coefficient r of the test–retest reliability of the BIT-C-11, Factor 1, Factor 2, Factor 3, and Factor 4 was 0.810, 0.794, 0.756, 0.778, and 0.757, respectively. They are all >0.4. The test–retest reliability of BIT-C-11 and its four factors is acceptable (34) (Tables 4, 5).
There were no subjects who achieved the maximum total score of 110. Two subjects achieved the lowest total score of 11, 0.7% of all people. They were all below the criteria of 15% for both the ceiling and floor effects. No subject response bias was observed in the current study (35).
Chinese patients with T2D generally have poor insulin adherence (5). It is urgent to assess the psychological resistance to insulin therapy in Chinese patients with T2D using a standardized scale. Our revised BIT-C-11 has relatively good psychometric characteristics and can be used to assess the psychological resistance to insulin therapy in middle-aged and elderly Chinese patients with T2D.
It is interesting to note that unlike Petrak’s original BIT, which contains five factors, the BIT-C-11 does not include the three reverse score items in the expectations regarding positive insulin-related outcomes factor of the original BIT. The reason for such differences may be due to differences in sample selection.
Petrak et al. (6) selected insulin-naive patients to develop the original BIT, who had no experience of improved health due to using insulin before.
In this study, we selected patients with T2D who were already using insulin as the study subjects. There may be differences in the content of psychological resistance to insulin therapy between patients with T2D treated with insulin and those with T2D not using insulin. Suppose we conduct an in-depth study of patients with T2D psychological resistance to insulin treatment in the future; it might be necessary to classify the study subjects in order to draw more scientific and accurate conclusions.
Another possible explanation is cultural differences. Several studies have suggested cultural differences in psychological insulin resistance, such as, for example, some studies showing ethnic differences in the causes of psychological insulin resistance (37, 38). Among Asian patients with diabetes, especially in China, there is a greater fear of injections and more incredible difficulties in using insulin than Western patients (39, 40). A 2015 study showed that psychosocial factors (rather than the presence of comorbidities) play a more critical role in determining PIR in the Chinese population (41). These studies suggest the influence of cultural differences, but the exact mechanisms are unclear, and more research needs to be done to elucidate them.
Petrak found that patients who opt for oral medications report significantly higher barriers to insulin therapy than those willing to use subcutaneous insulin. The original BIT has an apparent predictive validity for patients’ psychological resistance to insulin therapy (6). However, our subjects were patients who already used insulin injections, so we analyzed the BIT-C-11’s concurrent validity instead of its predictive validity. The GAS is a commonly used scale to assess general adherence in patients with chronic diseases (17). We found a negative correlation between the BIT-C-11 and the GAS in this study. A correlation coefficient of 0.582 > 0.4 means that the criterion-related validity of the BIT-C-11 is acceptable (32). It implies that the better the T2D patient’s general adherence to his or her physician’s recommendations, the lower is his or her psychological resistance to insulin treatment may be. General adherence encompasses many aspects of prevention, treatment, and patient health care. Adherence to insulin treatment is only a part of general adherence, which may explain why the GAS and the BIT-C-11 are related, but the correlation is not too high.
The Cronbach’s α value for the BIT-C-11 was 0.903, and the Cronbach’s α value in the original BIT was 0.78 (6). Both the original BIT and the BIT-C-11 and their subscales have relatively good internal consistency reliability. Since the subjects of these two studies differed, there was little comparability between their α values.
Petrak et al. (6) did not report the test–retest reliability of the original BIT. The correlation coefficient r of the BIT-C-11’s test–retest reliability was 0.810 after 4 weeks, which indicates that the stability of the BIT-C-11 is acceptable (34). Since patients with T2D need to use insulin for a long time, it is possible that some patients’ psychological resistance to insulin therapy decreases over time and become more receptive to insulin therapy. However, due to certain specific events, some patients already on insulin therapy may temporarily increase psychological resistance to insulin therapy and may become reluctant to receive insulin therapy for some time. Therefore, unlike personality traits that remain stable over time (42), we speculate that psychological resistance to insulin treatment is a psychological state subject to change by various factors. This changeability might be the basis for our intervention for psychological resistance to insulin treatment in patients with T2D. Further research is needed to identify the factors influencing psychological resistance to insulin therapy and develop specific and effective interventions to address these influences. However, this opinion needs to be supported by more research data in the future.
The BIT is a valuable research tool to assess patients with T2D’s psychological resistance to insulin treatment, but to our knowledge, the BIT-C-11 is the first revised Chinese version of the BIT. The number of subjects in this study met the sample size requirements for a revised scale study. The study’s objectives and the inclusion and exclusion criteria of the subjects were clear in this study. There were no missing data in the survey due to the efforts of the investigators. The BIT-C-11 has relatively good reliability and validity, and the CFA verified its structure. All of the above are the strengths of this study.
Although we have successfully revised the Chinese version of the BIT, the age and ethnic group of the subjects in this study lacked sufficient representation. On the one hand, worldwide, the trend of T2D in the younger population has surpassed that of the middle-aged and older population in recent years. We only selected patients with T2D aged 45–74 years for the study; further validation is needed if the BIT-C-11 is to be used in other age groups. We will further expand the subject’s age range to improve these shortcomings in the future.
Due to the study’s funding, considering that the BIT contains only 14 items, which is not a large number, we empirically selected 15 patients with T2D who met the study criteria for the pretest and cognitive interviews. Our judgment was not well grounded in theory. It should be noted that sample sizes >30 may be more scientific in pretesting and cognitive interviewing (43).
Another problem is that Factor 4 contains only two items. According to the theory of scale development, each factor should be composed of at least three variables; otherwise, the factor should be discarded or ignored (44). However, considering that “fear of hypoglycemia” is an essential aspect of psychological resistance to insulin therapy, Factor 4 of the BIT-C-11 already has good validity and reliability. We felt that the structure of the Chinese version of the BIT should be consistent with the original BIT, and the “fear of hypoglycemia” factor of the original BIT only has these two items, so we retained Factor 4.
We revised the Chinese version of the BIT, which has relatively good reliability and validity. The revised BIT-C-11 is four-dimensional and has a total of 11 items, which can be used to assess the psychological resistance to insulin therapy of middle-aged and elderly urban Han people with T2D who use insulin in China.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
MM: conceptualization, methodology, formal analysis, and writing–original draft. XM: methodology, data curation, and writing–original draft. JC: methodology and writing–original draft. FY: methodology and data curation. SM: writing–review and editing. YZ: writing–review and editing. ZS: conceptualization, methodology, writing–original draft, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was funded by a project grant from The Pudong New District Health Commission (PW2017A-29) with additional support from the Medical Discipline Construction Project of Pudong Health Committee of Shanghai (Grant No.: PWYgy2021-02).
We want to thank Ms. Hongyan Chen and the doctors from the diabetes department of the Haicheng Central Hospital, Haicheng Hospital of TCM, and Haicheng Second People’s Hospital for their help during this study and pay tribute to them.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. International, Diabetes, Federation. IDF diabetes atlas. 10th ed. Brussels, Belgium: International Diabetes Federation (2021).
2. Valadi H, Ekstrom K, Bossios A, Sjostrand M, JJ L, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol (2007) 9(6):654–9. doi: 10.1038/ncb1596
3. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care (2004) 27(5):1218–24. doi: 10.2337/diacare.27.5.1218
4. Schaper NC, Nikolajsen A, Sandberg A, Buchs S, Bogelund M. Timing of insulin injections, adherence, and glycemic control in a multinational sample of people with type 2 diabetes: A cross-sectional analysis. Diabetes Ther (2017) 8(6):1319–29. doi: 10.1007/s13300-017-0317-9
5. He X, Chen L, Wang K, Wu H, Wu J. Insulin adherence and persistence among Chinese patients with type 2 diabetes: a retrospective database analysis. Patient Prefer Adherence (2017) 11:237–45. doi: 10.2147/PPA.S123389
6. Petrak F, Stridde E, Leverkus F, Crispin AA, Forst T, Pfutzner A. Development and validation of a new measure to evaluate psychological resistance to insulin treatment. Diabetes Care (2007) 30(9):2199–204. doi: 10.2337/dc06-2042
7. Yavuz DG, Ozcan S, Deyneli O. Adherence to insulin treatment in insulin-naive type 2 diabetic patients initiated on different insulin regimens. Patient Prefer Adherence (2015) 9:1225–31. doi: 10.2147/PPA.S87935
9. Doggrell SA, Chan VJ. Adherence to insulin treatment in diabetes: can it be improved? Diabetes (2015) 7(3):315–21. doi: 10.1111/1753-0407.12212
10. Idris I, Gulati K, Perez-Nieves M, Hadjiyianni I, Cao D, Tahbaz A, et al. Associated factors that influenced persistence with basal analog insulin therapy among people with type 2 diabetes: an exploratory analysis from a UK real-world sample. Prim Care Diabetes (2019) 13:106–12. doi: 10.1016/j.pcd.2018.09.002
11. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA (2017) 317(24):2515–23. doi: 10.1001/jama.2017.7596
12. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA (2013) 310(9):948–59. doi: 10.1001/jama.2013.168118
13. Lauffenburger JC, Lewey J, Jan S, Makanji S, Ferro CA, Krumme AA, et al. Effectiveness of targeted insulin-adherence interventions for glycemic control using predictive analytics among patients with type 2 diabetes: A randomized clinical trial. JAMA Netw Open (2019) 2(3):e190657. doi: 10.1001/jamanetworkopen.2019.0657
14. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care (2010) 33(8):1747–9. doi: 10.2337/dc10-0099
15. Bahrmann A, Abel A, Zeyfang A, Petrak F, Kubiak T, Hummel J, et al. Psychological insulin resistance in geriatric patients with diabetes mellitus. Patient Educ Couns (2014) 94(3):417–22. doi: 10.1016/j.pec.2013.11.010
16. Hays RD. The medical outcomes study (mos) measures of patient adherence. Available from: https://www.rand.org/content/dam/rand/www/external/health/surveys_tools/mos/mos_adherence_survey.pdf. Accessed May 7, 2019.
17. Sherbourne CD, Hays RD, Ordway L, DiMatteo MR, Kravitz RL. Antecedents of adherence to medical recommendations: results from the Medical Outcomes Study. J Behav Med (1992) 15(5):447–68. doi: 10.1007/BF00844941
18. Shi Z, Chang J, Ma X, Yin F, Ma M, Li W, et al. The psychometric properties of general adherence scale in Chinese (GAS-C) in patients with type 2 diabetes using insulin. Diabetes Metab Syndr Obes (2021) 14:801–11. doi: 10.2147/DMSO.S286153
19. Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) (2000) 25(24):3186–91. doi: 10.1097/00007632-200012150-00014
20. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol (1993) 46(12):1417–32. doi: 10.1016/0895-4356(93)90142-n
21. DeVellis RF. Scale development: Theory and applications. In: Thousand oaks, 3rd ed. California: Sage publications (2016).
23. Minglong W. Questionnaire statistical analysis practice—SPSS operation and application. 1st ed. Chongqing: Chongqing University Press (2010).
24. Lynn MR. Determination and quantification of content validity. Nurs Res (1986) 35(6):382–5. doi: 10.1097/00006199-198611000-00017
25. Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health (2007) 30(4):459–67. doi: 10.1002/nur.20199
26. Bartlett MS. A further note on tests of significance in factor analysis. Br J Stat Psychol (1951) 4(1):1–2. doi: 10.1111/j.2044-8317.1951.tb00299.x
27. Kaiser HF, Rice J. Little Jiffy, Mark Iv. Educ Psychol Meas (1974) 34(1):111–7. doi: 10.1177/001316447403400115
28. Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas (1960) 20(1):141–51. doi: 10.1177/001316446002000116
30. Bentler PM. Comparative fit indexes in structural models. Psychological bulletin. (1990) 107(2):238. doi: 10.1037/0033-2909.107.2.238
31. Hoyle RH. Structural equation modeling concepts, issues, and applications. Thousand Oaks: SAGE Publications, Inc (1995).
32. Oluwatayo JA. Validity and reliability issues in educational research. J Educ Soc Res (2012) 2(2):391–400.
33. Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am J Ment Deficiency (1981) 86(2):127–37.
34. Vilagut G. Test-retest reliability. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer Netherlands (2014). p. 6622–5.
35. Everitt BS, Skrondal A. The cambridge dictionary of statistics. In: The Edinburgh building, Cambridge CB2 8RU, 4th ed. UK: Cambridge University Press (2010).
36. Bartlett MS. Tests of significance in factor analysis. Br J Stat Psychol (1950) 3(2):77–85. doi: 10.1111/j.2044-8317.1950.tb00285.x
37. Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care (2005) 28(10):2543–5. doi: 10.2337/diacare.28.10.2543
38. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes care (2010) 33(8):1747–9. doi: 10.2337/dc10-0099
39. Fitzgerald JT, Gruppen LD, Anderson RM, Funnell MM, Jacober SJ, Grunberger G, et al. The influence of treatment modality and ethnicity on attitudes in type 2 diabetes. Diabetes Care (2000) 23:313–8. doi: 10.2337/diacare.23.3.313
40. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care (2010) 33:1747–9. doi: 10.2337/dc10-0099
41. Lee KP. Psycholosocial factors associated with psychological insulin resistance in primary care patients in Hong Kong. J Clin Transl ndocrinol (2015) 2(4):157–62. doi: 10.1016/j.jcte.2015.10.001
42. Conley JJ. Longitudinal stability of personality traits: A multitrait–multimethod–multioccasion analysis. J Pers Soc Psychol (1985) 49(5):1266. doi: 10.1037/0022-3514.49.5.1266
43. Perneger TV, Courvoisier DS, Hudelson PM, Gayet-Ageron A. Sample size for pre-tests of questionnaires. Qual Life Res (2015) 24:147–51. doi: 10.1007/s11136-014-0752-2
Keywords: type 2 diabetes mellitus, barriers to insulin treatment, psychological resistance to insulin therapy, adherence, scale revision, reliability, validity
Citation: Ma M, Ma X, Chang J, Yin F, Ma S, Zhang Y and Shi Z (2023) The psychometric properties of the barriers to insulin treatment questionnaire in Chinese patients with type 2 diabetes mellitus using insulin. Front. Endocrinol. 14:1192108. doi: 10.3389/fendo.2023.1192108
Received: 22 March 2023; Accepted: 31 July 2023;
Published: 15 August 2023.
Edited by:
Mona Nasrallah, American University of Beirut, LebanonReviewed by:
Catherine Davis, Augusta University, United StatesCopyright © 2023 Ma, Ma, Chang, Yin, Ma, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidao Shi, ZHIuc3pkQG91dGxvb2suY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.