- 1Department of Critical Care Medicine, The Second Affiliated Hospital, Kunming Medical University, Kunming, China
- 2Department of Anesthesiology, The First Affiliated Hospital, Kunming Medical University, Kunming, China
Objective: To determine the preventive effect of melatonin on delirium in the intensive care units.
Methods: We conducted a systematic search of the PubMed, Cochrane Library, Science, Embase, and CNKI databases, with retrieval dates ranging from the databases’ inception to September 2022. Controlled trials on melatonin and placebo for preventing delirium in the intensive care units were included. The meta-analysis was performed using Review Manager software (version 5.3) and Stata software (version 14.0).
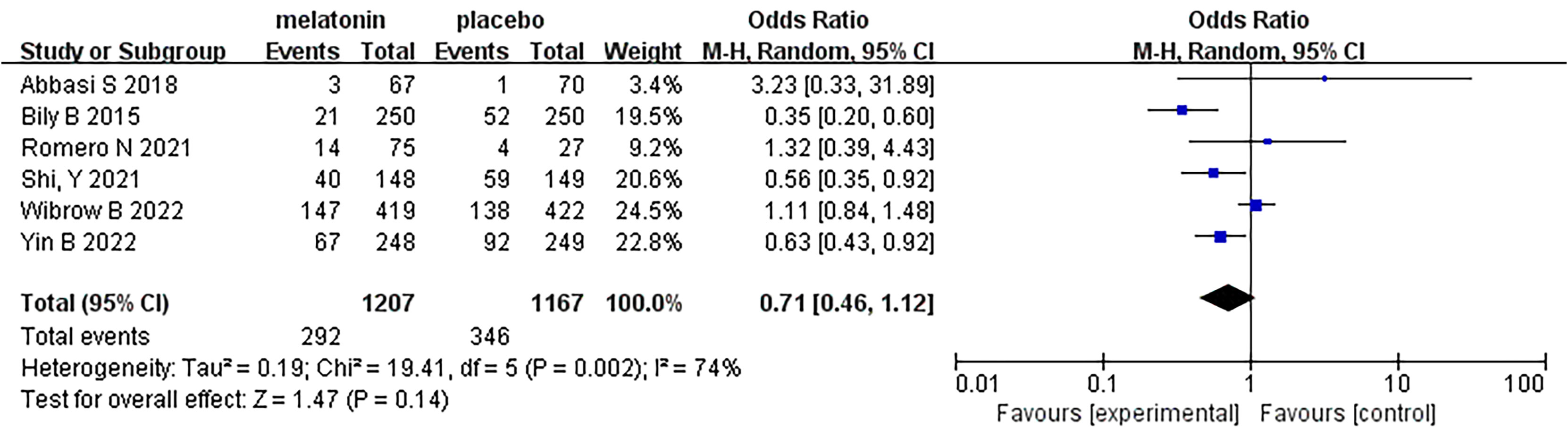
Results: Six studies involving 2374 patients were included in the meta-analysis. The results of the meta-analysis showed that melatonin did not reduce the incidence of delirium in ICU patients (odds ratio [OR]: 0.71; 95% confidence interval [CI]: 0.46 to 1.12; p = 0.14). There was a strong hetero-geneity between the selected studies (I2 = 74%). Subgroup analysis results showed that melatonin reduced the incidence of delirium in cardiovascular care unit (CCU) patients (OR: 0.52; 95% CI: 0.37 to 0.73; p=0.0001), but did not in general intensive care unit (GICU) patients (OR: 1.14; 95% CI: 0.86 to 1.50; p=0.35). In terms of the secondary outcomes, there were no significant differences in all-cause mortality (OR: 0.85; 95% CI: 0.66 to 1.09; p=0.20), length of ICU stay (mean difference [MD]: 0.33; 95% CI: -0.53 to 1.18; p=0.45), or length of hospital stay (MD: 0.51; 95% CI: -1.17 to 2.19; p=0.55).
Conclusion: Melatonin reduced the incidence of delirium in CCU patients, but did not significantly reduce the incidence of delirium in GICU patients.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022367665.
1 Introduction
Delirium has been defined as an acute impairment of attention and overall cognitive function in hospitalized patients (1). In critically ill patients, delirium is associated with increased morbidity and mortality, and may also increase the length of stay in critically ill patients (2–7). Delirium is especially common in intensive care unit (ICU) patients, with such a wide range of occurrence reported in the literature (11%-80%) (6, 8–10). Importantly, most medications currently available clinically are not effective for delirium and there is a lack of evidence-based medicine in the treatment of this disorder.
Melatonin, a serotonin-derived hormone secreted by the pineal gland, is a powerful free radical scavenger and antioxidant with a broad spectrum (11) that easily crosses the blood-brain barrier in large, non-toxic doses (12). It plays a major role in sleep regulation and circadian rhythm management (13, 14). Melatonin has anti-inflammatory and immunomodulatory properties and may have neuroprotective effects in brain injury, encephalopathy and neurodegenerative diseases (15–19).
Melatonin supplementation may prevent delirium. To date, there were many clinical trials of melatonin or melatonin agonists to prevent delirium (20–29). Due to the heterogeneity of experimental methods and patients, results between clinical trials are conflicting and it is difficult to draw firm conclusions. In light of recently published large randomized clinical trials (30), we conducted a comprehensive systematic review and meta-analysis to summarize the available evidence and provide more reliable quantitative results as a basis for delirium prevention and clinical treatment in critically ill patients.
2 Materials and methods
2.1 Search strategy
We conducted a systematic search of the PubMed, Cochrane Library, Science, Embase, and CNKI databases, with retrieval dates ranging from the databases’ inception to September 2022. We used the following search terms: “Delirium”, “Subacute Delirium”, “Delirium, Subacute”, “Deliriums, Subacute”, “Subacute Deliriums”, “Delirium of Mixed Origin”, “Mixed Origin Delirium”, “Mixed Origin Deliriums”, “Melatonin”[Mesh] and “Melatonin”. Search strategies were adapted for the various search engines. Manual retrieval from the references of subject-related articles was performed to broaden the search. We did not restrict our search by region or language. Two re-viewers (Y.D. and Y.Y.) independently assessed each included study, and any discrepancies were resolved through consensus.
2.2 Inclusion/exclusion criteria
Following the PICOS principle, inclusion criteria: (1) adult ICU inpatients; (2) delirium diagnosis criteria determined by CAM or CAM-ICU; (3) intervention: intervention group received melatonin enteral administration; control group received placebo enteral administration; (4) no statistically significant pre-experimental differences between experimental and control groups; (5) full paper containing at least one outcome parameter: incidence of delirium (primary outcome) and secondary outcomes: all-cause mortality, length of ICU stay, and length of hospital stay; (6) study type was a randomized controlled trial, or cohort study. Exclusion Criteria: reviews, case reports, letters, low-quality researches and researches with no detailed data.
2.3 Data extraction
We extracted the following data from the studies in the meta-analysis: author, publication year, study design, basic information, incidence of delirium, all-cause mortality, length of ICU stay, and length of hospital stay. If a continuous variable was present, the mean value and standard deviation were calculated.
2.4 Quality assessment
The risk of bias in Cochrane systematic reviews was used to evaluate the quality of the literature, in accordance with the guidelines for doing research in evidence-based medicine (31). Using six criteria randomization method, concealment of allocation scheme, blinding, completeness of outcome data, selective reporting of study results, and other sources of bias. The quality of the included studies was evaluated. Studies with fewer than five items were categorized as having a low risk of bias, those with three to four items as having a moderate risk of bias, and those with more than three items as having a high risk of bias. All six articles were of a high caliber, with four having a low risk of bias and the other two all having a moderate risk. In Figure 1A, the standard is “+”, and the non-compliance is “-”. Figure 1B shows the proportion of each item in the methodological assessment.
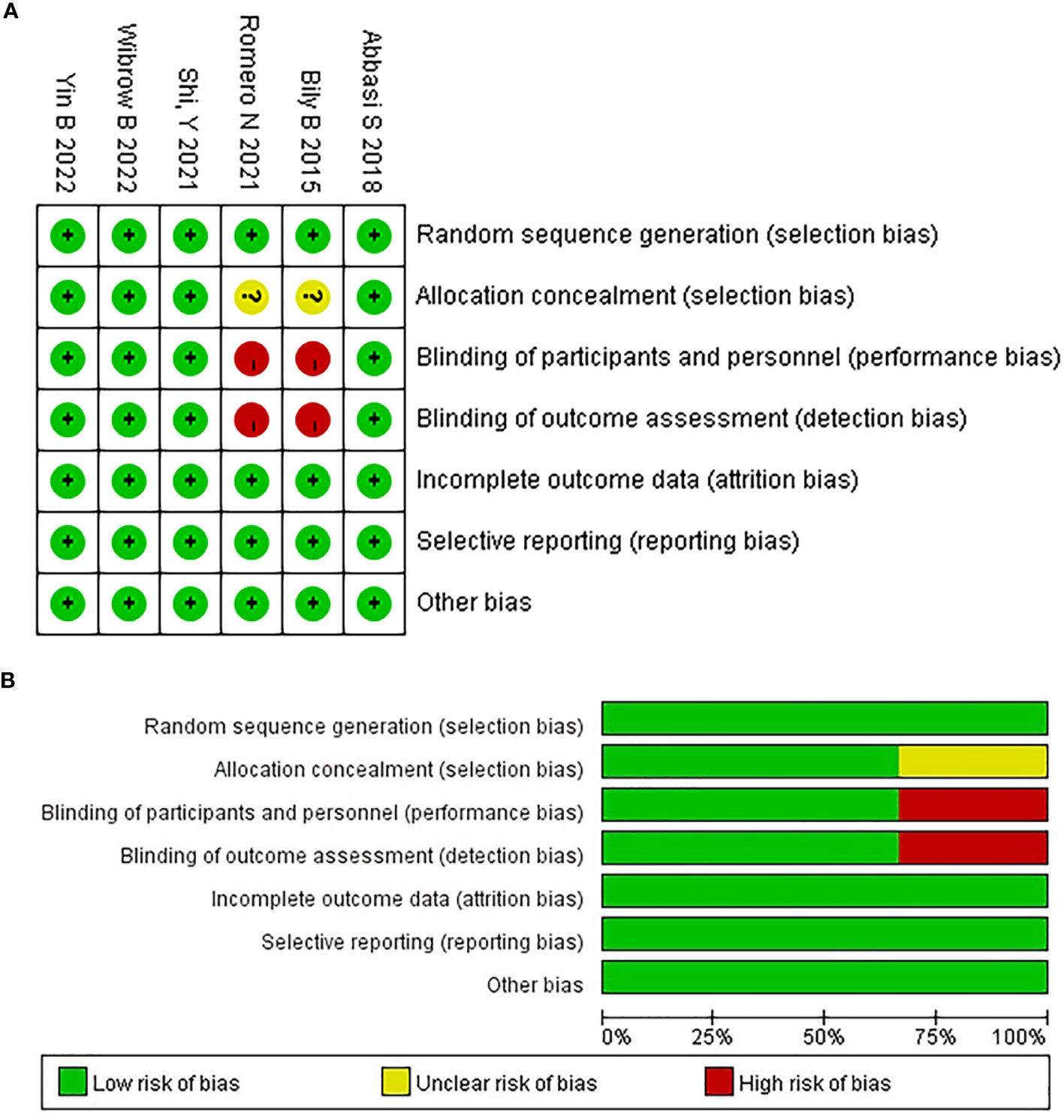
Figure 1 The risk of bias assessment for each trial using the risk of bias in Cochrane systematic reviews. (A) Risk of bias summary, (B) Risk of bias graph.
2.5 Statistical analysis
A meta-analysis of the included studies was performed using Review Manager software (version 5.3) and Stata software (version 14.0). For dichotomous variables, the odds ratio (OR) and 95% confidence interval (CI) were used as efficacy indicators for statistical analysis. Heterogeneity between studies was assessed using the Q-test and I2-test. If p< 0.05 or I2>50%, The data were combined using the random-effects model with the assumption that there was heterogeneity; however, if (p≥0.05 or I2<50%), data were integrated using a fixed-effects model with no consideration given to the existence of heterogeneity. P<0.05 indicated that differences were statistically significant. In order to examine potential publishing bias, a funnel plot was created.
2.6 Registration
The study was registered on PROSPERO (CRD42022367665).
3 Results
3.1 Study characteristics
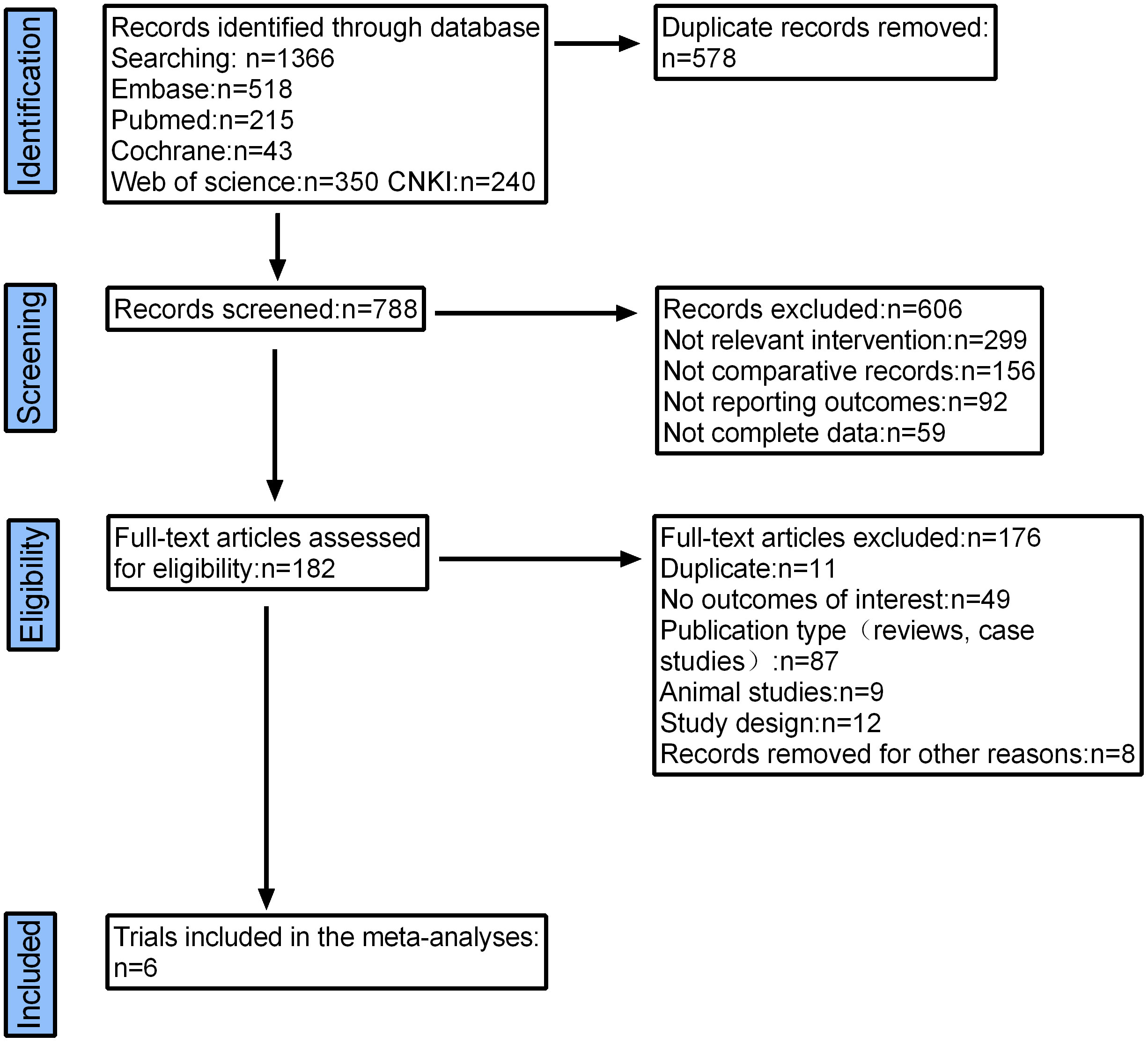
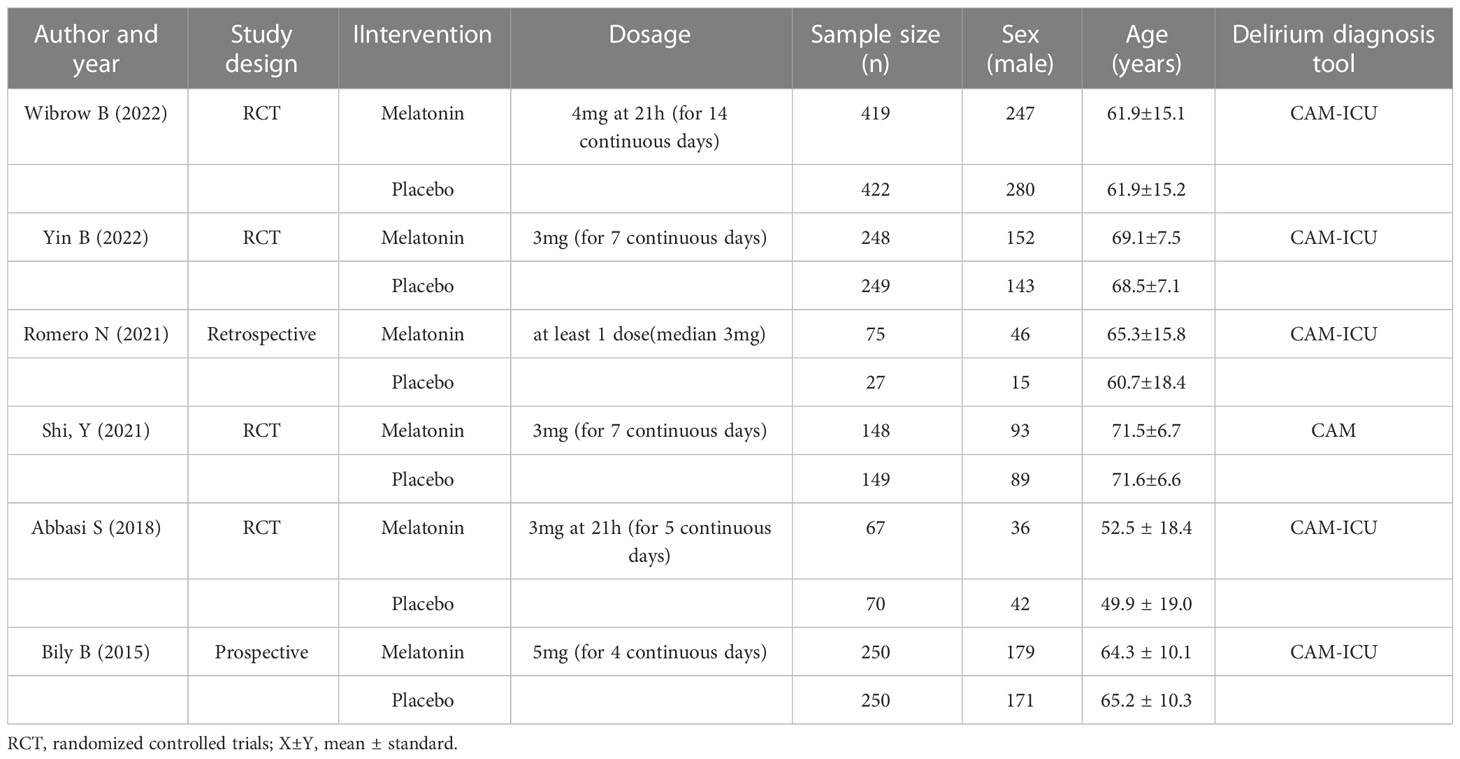
A total of 1366 articles were initially retrieved. But 1184 articles were excluded because there were duplicates or irrelevant to our study. After reading the full text, another 176 articles were excluded. Finally, six studies with 2374 patients were included in our meta-analysis (26, 30, 32–35) (Figure 2). The characteristics of the included studies are shown in Table 1. The risk of bias in the Cochrane systematic reviews was used to evaluate the quality of the studies. All studies had a risk of bias, but most were low, and the average quality of each study was good. The results are shown in Figure 1.
4 Incidence of delirium
Six articles were analyzed, and a total of 2374 patients participated in the study, with 1207 patients receiving melatonin and 1167 patients receiving placebo. After the heterogeneity test, I2 = 74%>50%, and the P=0.002<0.1 of the Q test, suggested a strong heterogeneity between the selected studies, selecting random effects for meta-analysis, while continuing to investigate the reasons of the heterogeneity. Based on the data of this study, the source of heterogeneity was highly suspected to be inconsistent reasons for patients’ admission to the ICU, and subgroup analysis will be conducted according to the type of ICU. In terms of the type of ICU, patients were divided into two subgroups: general intensive care unit (GICU) group and cardiovascular care unit (CCU) group according to the reasons for their admission to the ICU. For the overall six articles, random effects were selected for me-ta-analysis and the results were shown in Figure 3. The results of the meta-analysis showed that melatonin did not reduce the incidence of delirium in ICU patients (Random-effects model: OR, 0.71; 95% CI, 0.46 to 1.12; p=0.14; I2 = 74%).
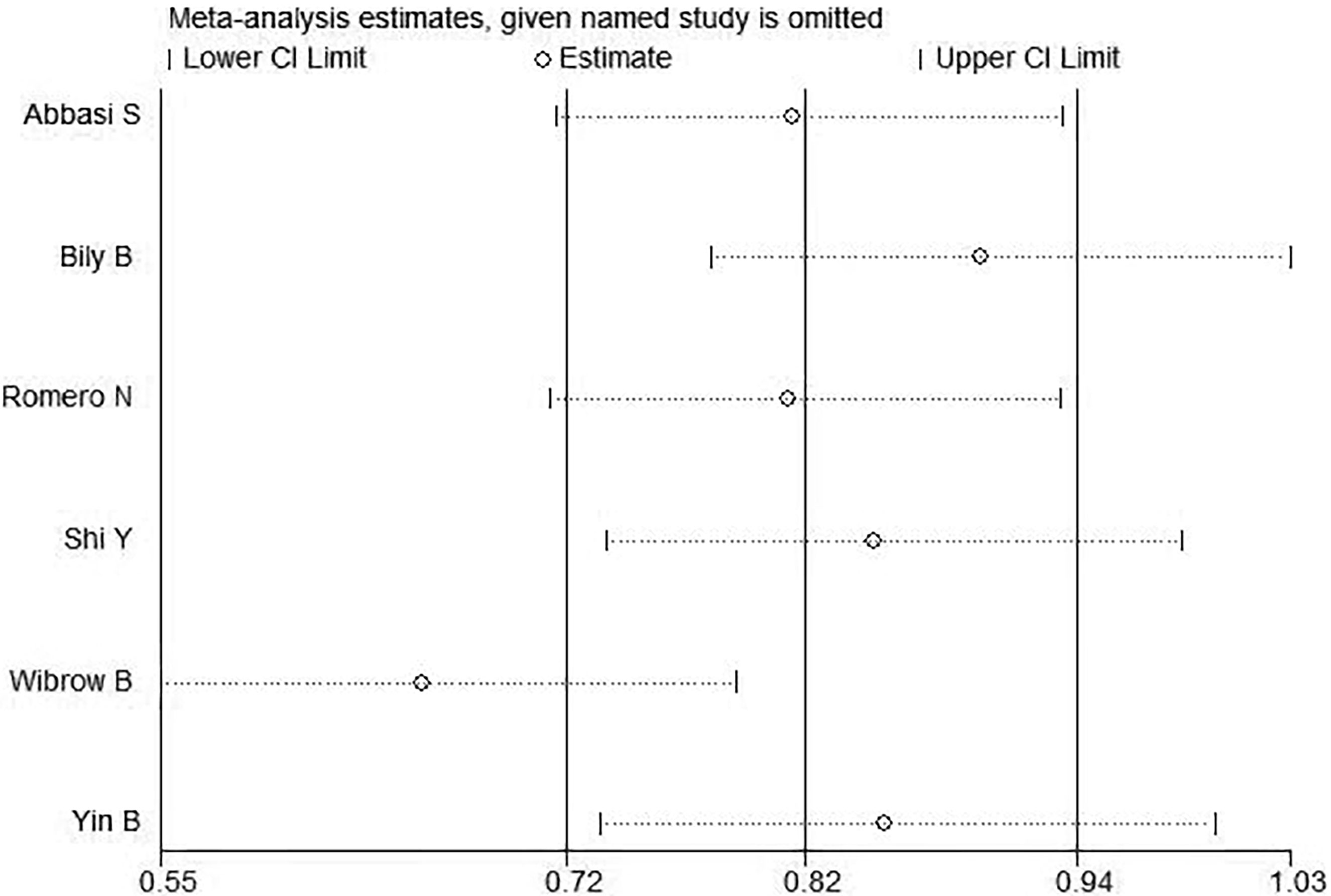
4.1 Sensitivity analysis
The results of sensitivity analysis were shown in Figure 4. As could be clearly seen from the above figure that there were two distinct groups, the studies show different sensitive situations based on different ICU types. Therefore, it was highly suspected that the different types of ICUs cause heterogeneity.
4.2 Subgroup analysis
According to the type of ICU, the six studies were divided into two groups for meta-analysis. The results were shown in Figure 5A. Based on the above subgroup analysis, the heterogeneity between the two groups was extremely high, reaching a high degree of heterogeneity, which means that inconsistency in ICU type largely influences the results of the meta-analysis. In GICU group, after heterogeneity test, I2 = 0%<50% and P=0.64>0.1 of Q test showed there was no heterogeneity in the group. Meta-analysis of three articles showed that melatonin could not reduce the incidence of delirium in GICU patients (Random-effects model: OR, 1.14; 95% CI, 0.86 to 1.50; p=0.35; I2 = 0%). Secondly, in CCU group, after heterogeneity test, I2 = 37%<50%, and P=0.21>0.1 of Q test, there was no heterogeneity in the group. Meta-analysis of three articles showed that melatonin could reduce the incidence of delirium in CCU patients (Random-effects model: OR, 0.52; 95% CI, 0.37 to 0.73; p=0.0001; I2 = 37%). Based on all of the above analyses, melatonin reduced the incidence of delirium in CCU patients, but did not significantly reduce the incidence of delirium in GICU patients.
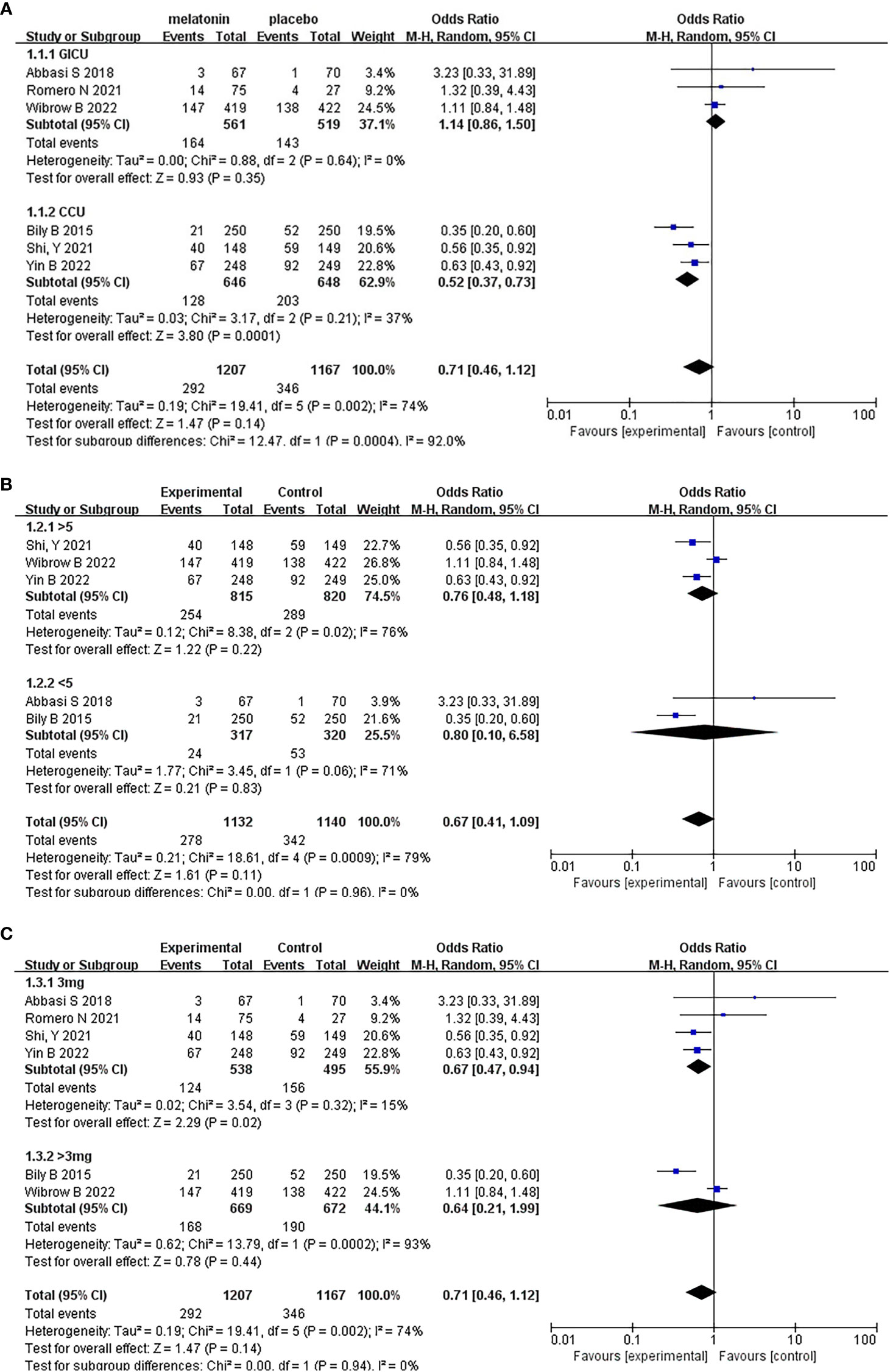
Figure 5 Subgroup analysis of incidence of delirium. (A) Type of ICU, (B) Dosing duration, (C) Daily dosage.
Based on whether the dosing duration exceeded 5 days, the five studies were divided into two groups for meta-analysis. It should be noted that Romero N (2021) was a single center, retrospective, and objective cohort study. The article did not mention the specific duration, so it cannot be included in the subgroup analysis grouped. The results were shown in Figure 5B. Based on the above subgroup analysis, the heterogeneity in the two groups and between the two groups were both high. Moreover, the results of the test for subgroup differences were not statistically significant (Random effects model: OR, 0.67; 95% CI, 0.41 to 1.09; p=0.96; I2 = 0%), which means that the duration of administration did not affect the results of the meta-analysis.
On the other hand, based on whether the daily dosage exceeded 3mg, we divided six studies into two groups, the results were shown in Figure 5C. Based on the above subgroup analysis, the results of the test for subgroup differences were not statistically significant (Random effects model: OR, 0.71; 95% CI, 0.46 to 1.12; p=0.94; I2 = 0%), Which means that different daily dosage did not affect the results of the meta-analysis.
4.3 Bias tests
Bias tests were conducted according to subgroups and funnel plots were drawn. The results were shown in Figure 6. It could be clearly seen that the funnel charts were basically symmetrical, and the bias test showed that all P values were greater than 0.05, so it could be judged that there was no publication bias of this study.
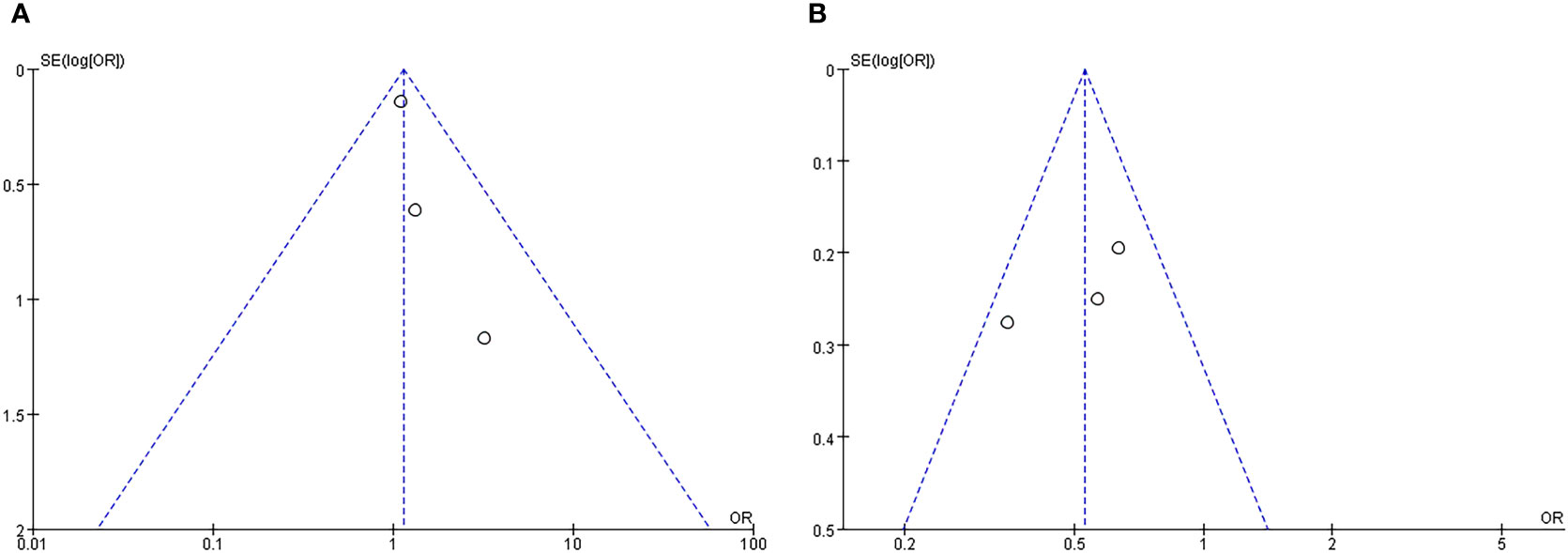
Figure 6 Funnel plot of subgroup analysis about incidence of delirium: (A) GICU group; (B) CCU group.
5 28-day/30-day all-cause mortality
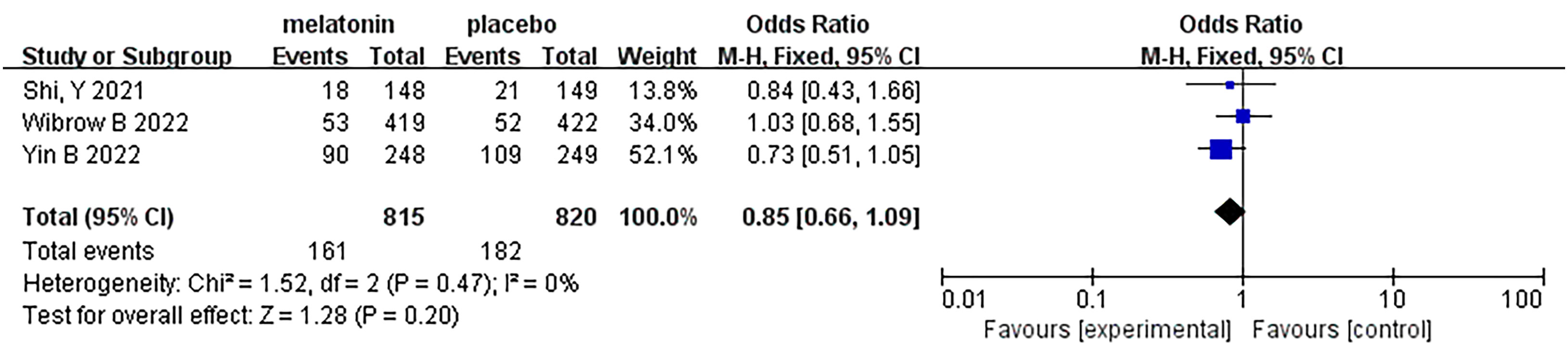
Three articles were analyzed regarding 28-day/30-day all-cause mortality. A total of 1635 patients were included in the studies, of whom 815 received melatonin treatment and 820 received placebo treatment (Figure 7). All-cause mortality was similar between the two groups, and no heterogeneity was observed (Fixed-effects model: OR, 0.85; 95% CI, 0.66 to 1.09; p=0.20; I2 = 0%).
6 Length of ICU stay
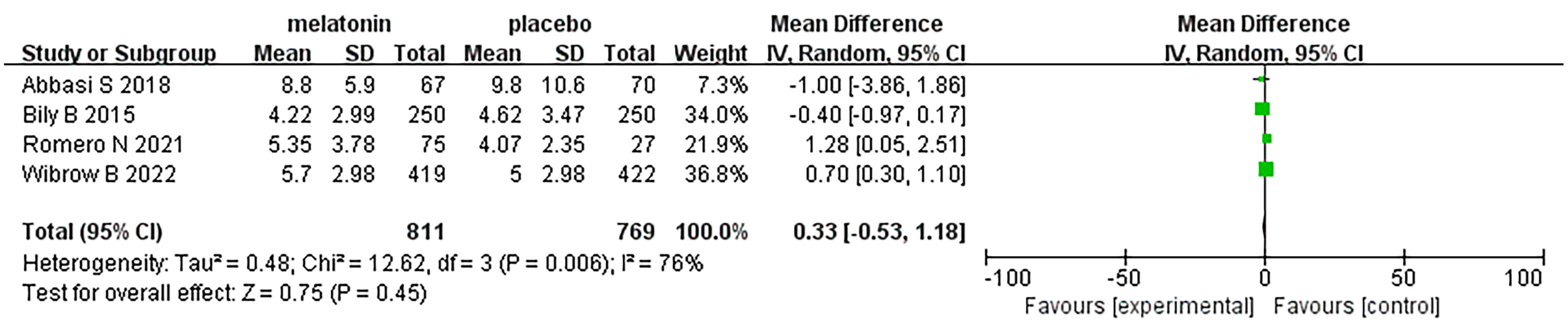
Four articles were analyzed with respect to length of ICU stay. A total of 1580 patients were included in the studies, of whom 811 received melatonin treatment and 769 received placebo treatment. Because the heterogeneity was considerable (I2 = 76%), we used a random-effects model (Figure 8). Length of ICU stay was similar between the two groups (Random-effects model: MD, 0.33; 95% CI, -0.53 to 1.18; p=0.45; I2 = 76%).
7 Length of hospital stay
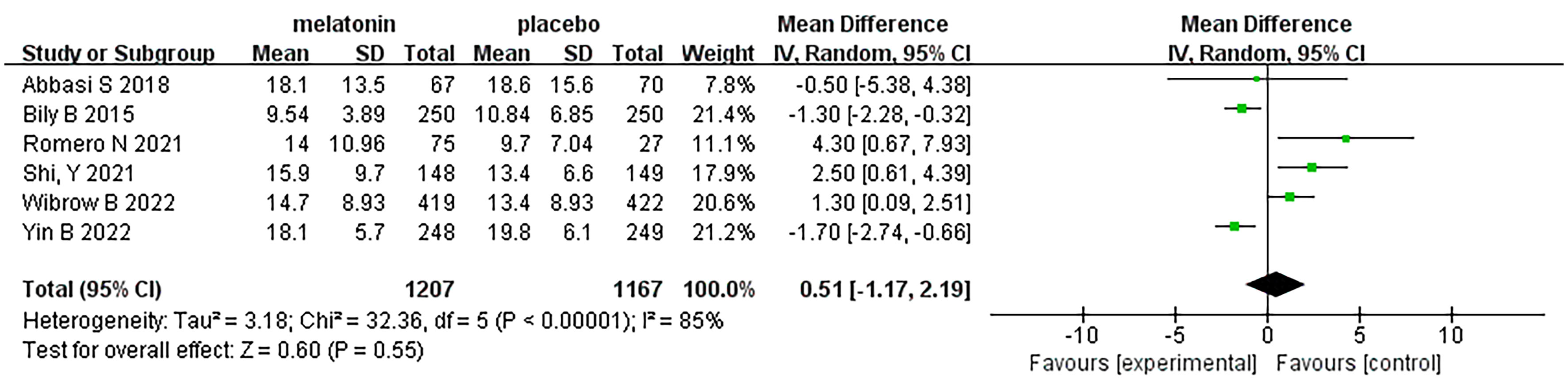
Six articles were analyzed with respect to length of ICU stay. A total of 2374 patients were included in the studies, of whom 1207 received melatonin treatment and 1167 received placebo treatment. Because the heterogeneity was considerable (I2 = 85%), we used a random-effects model (Figure 9). Length of hospital stay was similar between the two groups (Random-effects model: MD, 0.51; 95% CI, -1.17 to 2.19; p=0.55; I2 = 85%)
8 Discussion
Critical illness has been considered a risk factor for the development of delirium, which is associated with increased mortality, prolonged ICU stay, and long-term cognitive decline and poor functional recovery in patients (36, 37). Therefore, any potential benefit of melatonin may be more pronounced in critically ill patients (6, 15, 38). Melatonin is an endogenous hormone secreted by the pineal gland that regulates circadian rhythms (39), and current studies in vitro and in animal models have demonstrated the potential anti-inflammatory and antioxidant characteristics of melatonin, suggesting that its use in critically ill patients may have multiple benefits (40, 41).
Our findings suggest that melatonin did not observe a significant advantage in preventing delirium in critically ill patients, but there was a high degree of heterogeneity between studies. In particular, the advantage of melatonin in preventing delirium was not significant in GICU, but in CCU, melatonin was shown to reduce the incidence of delirium, so based on the current findings, it cannot yet be assumed that melatonin is effective in preventing delirium in ICU patients. Although differences in disease severity may attenuate the beneficial effects of melatonin, current treatment options for delirium supported by valid evidence-based medical evidence are limited to preventive strategies with nonpharmacological interventions, such as early activity, light and noise reduction, sleep management, and involving relatives when appropriate (29, 42–44). The recent multicenter randomized controlled trial conducted by De Jonghe A in the Nether-lands for elderly patients with hip fracture also proved that melatonin treatment did not reduce the incidence of delirium (23). This is also consistent with our findings. There are still few studies on melatonin prevention of delirium in critically ill patients, and larger, multicenter randomized controlled studies are needed to support these findings, so the results of this study should be treated with caution.
There may be many reasons for the differences in the effects of melatonin due to different types of ICU, and the specific molecular mechanism of melatonin’s preventive effect on delirium in CCU patients may be closely related to its improvement of vascular endothelial cell function, anti-vasospasm, anti-neuronal apoptosis, improvement of cerebral perfusion, and reduction of microthrombosis (45). Current relevant basic studies have shown that the non-selective opening of the mitochondrial permeability transition pore in mitochondria and the overproduction of reactive oxygen species are important factors promoting heart disease and cardiac dysfunction, and melatonin can improve isoprenaline-hydrochloride-induced heart failure related to mitochondrial dysfunction (46). In addition, Wang J et al. showed that renal ischemia-reperfusion injury can attenuate cardiac diastolic function by increasing cardiomyocyte death and enhancing the inflammatory response, while also disrupting cardiomyocyte energy metabolism, causing calcium overload, and impairing mitochondrial function. In contrast, melatonin reduces cytoplasmic and mitochondrial calcium overload, while melatonin preconditioning attenuates cardiac damage mediated by renal ischemia-reperfusion injury by maintaining myocardial diastolic function and reducing myocardial cell death (47). On the other hand, in GICU, the condition of critically ill patients is relatively complex and the disease types are diverse, and the relatively complex sedative and analgesic regimens used in this group of patients as well as the use of some antipsychotics may alter neurotransmitter levels and subsequently cause direct damage to brain-related functions (48), while it is unclear whether the mixture of these drugs may affect the effectiveness of melatonin.
We completed this meta-analysis under the strict guidance of PRISMA (49), but our analysis still has some limitations. First, the different types of delirium (hyperactive, hypoactive, or mixed delirium) were not distinguished in the study. Second, the CAM-ICU score is only a screening tool and is not the gold standard for delirium diagnosis, and scores are performed by ICU nurses at different levels and may be under-estimated in some studies. Third, melatonin is not regulated in some countries, and therefore the quality of the product may vary depending on the manufacturer, which may lead to differences in the absorption of melatonin in different studies. Fourth, in some studies the authors did not directly provide means and standard deviations for continuous data, so estimated means and standard deviations were used in the analysis of relevant data. Nevertheless, our meta-analysis still had a high level of evidence, the majority of the included studies were randomized controlled trials, and most of the included studies were published in the last five years with comprehensive records of outcome indicators, which significantly increased the credibility of our results.
9 Conclusion
Our study of published trials indicates that melatonin reduced the incidence of delirium in CCU patients, but did not significantly reduce the incidence of delirium in GICU patients. The role of melatonin in reducing the incidence of delirium in ICU patients requires further study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, YY, YD, XW and JS; methodology, YY and YD; software, YD and WZ; validation, LW, GW and WZ; formal analysis, XW and YY; investigation, GW, JY and QB; resources, WZ; data curation, YY and YD; writing—original draft preparation, YD and YY; writing—review and editing, LW, XW and JS; visualization, JY and QB; supervision, XW and JS; project administration, XW; funding acquisition, JS. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Natural Science Foundation of China, grant number 81960250 and The Program of Science and Technology Agency of Yunnan Province, grant number 202101AB070114.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. European Delirium A, American Delirium S. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med (2014) 12:141. doi: 10.1186/s12916-014-0141-2
2. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA (2004) 291(14):1753–62. doi: 10.1001/jama.291.14.1753
3. Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med (2004) 32(4):955–62. doi: 10.1097/01.ccm.0000119429.16055.92
4. Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med (2010) 38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1
5. Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med (2009) 37(9):2527–34. doi: 10.1097/CCM.0b013e3181a5689f
6. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med (2007) 33(1):66–73. doi: 10.1007/s00134-006-0399-8
7. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med (2013) 41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72
8. Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med (2010) 38(2):419–27. doi: 10.1097/CCM.0b013e3181b9e302
9. Girard TD, Pandharipande PP, Carson SS, Schmidt GA, Wright PE, Canonico AE, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med (2010) 38(2):428–37. doi: 10.1097/ccm.0b013e3181c58715
10. Roberts B, Rickard CM, Rajbhandari D, Turner G, Clarke J, Dianne H, et al. Multicentre study of delirium in ICU patients using a simple screening tool. Aust Crit Care (2005) 18(1):6, 8–9, 11-4 passim. doi: 10.1016/s1036-7314(05)80019-0
11. Liu JC, Li L, Yan HC, Zhang T, Zhang P, Sun ZY, et al. Identification of oxidative stress-related Xdh gene as a di(2-ethylhexyl)phthalate (DEHP) target and the use of melatonin to alleviate the DEHP-induced impairments in newborn mouse ovaries. J Pineal Res (2019) 67(1):e12577. doi: 10.1111/jpi.12577
12. Chen J, Chen G, Li J, Qian C, Mo H, Gu C, et al. Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: a possible role for the regulation of pro-inflammatory cytokines. J Pineal Res (2014) 57(3):340–7. doi: 10.1111/jpi.12173
13. Xie Z, Chen F, Li WA, Geng X, Li C, Meng X, et al. A review of sleep disorders and melatonin. Neurol Res (2017) 39(6):559–65. doi: 10.1080/01616412.2017.1315864
14. Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, Ben-Shushan A, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev (2005) 9(1):41–50. doi: 10.1016/j.smrv.2004.06.004
15. Hardeland R. Melatonin and inflammation-Story of a double-edged blade. J Pineal Res (2018) 65(4):e12525. doi: 10.1111/jpi.12525
16. Nabavi SM, Nabavi SF, Sureda A, Xiao J, Dehpour AR, Shirooie S, et al. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit Rev Food Sci Nutr (2019) 59(sup1):S4–S16. doi: 10.1080/10408398.2018.1487927
17. Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol (2010) 8(3):228–42. doi: 10.2174/157015910792246155
18. Hendaus MA, Jomha FA, Alhammadi AH. Melatonin in the management of perinatal hypoxic-ischemic encephalopathy: light at the end of the tunnel? Neuropsychiatr Dis Treat (2016) 12:2473–9. doi: 10.2147/NDT.S115533
19. Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer's disease. J Pineal Res (2005) 38(3):145–52. doi: 10.1111/j.1600-079X.2004.00196.x
20. Ng KT, Teoh WY, Khor AJ. The effect of melatonin on delirium in hospitalised patients: A systematic review and meta-analyses with trial sequential analysis. J Clin Anesth (2020) 59:74–81. doi: 10.1016/j.jclinane.2019.06.027
21. Dianatkhah M, Ghaeli P, Hajhossein Talasaz A, Karimi A, Salehiomran A, Bina P, et al. Evaluating the potential effect of melatonin on the post-cardiac surgery sleep disorder. J Tehran Heart Cent (2015) 10(3):122–8.
22. Artemiou P, Bily B, Bilecova-Rabajdova M, Sabol F, Torok P, Kolarcik P, et al. Melatonin treatment in the prevention of postoperative delirium in cardiac surgery patients. Kardiochir Torakochirurgia Pol (2015) 12(2):126–33. doi: 10.5114/kitp.2015.52853
23. de Jonghe A, van Munster BC, Goslings JC, Kloen P, Rees van C, Wolvius R, et al. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double-blind randomized controlled trial. CMAJ (2014) 186(14):E547–56. doi: 10.1503/cmaj.140495
24. Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth (2010) 4(3):169–73. doi: 10.4103/1658-354X.71132
25. Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, et al. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry (2014) 71(4):397–403. doi: 10.1001/jamapsychiatry.2013.3320
26. Abbasi S, Farsaei S, Ghasemi D, Mansourian M. Potential role of exogenous melatonin supplement in delirium prevention in critically ill patients: A double-blind randomized pilot study. Iran J Pharm Res (2018) 17(4):1571–80.
27. Jaiswal SJ, McCarthy TJ, Wineinger NE, Kang DY, Song J, Garcia S, et al. Melatonin and sleep in preventing hospitalized delirium: A randomized clinical trial. Am J Med (2018) 131(9):1110–1117.e4. doi: 10.1016/j.amjmed.2018.04.009
28. Nishikimi M, Numaguchi A, Takahashi K, Miyagawa Y, Matsui K, Higashi M, et al. Effect of administration of ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: A single-center randomized placebo-controlled trial. Crit Care Med (2018) 46(7):1099–105. doi: 10.1097/CCM.0000000000003132
29. Serafim RB, Bozza FA, Soares M, Brasil PEAA, Tura BR, Ely EW, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: A systematic review. J Crit Care (2015) 30(4):799–807. doi: 10.1016/j.jcrc.2015.04.005
30. Wibrow B, Martinez FE, Myers E, Chapman A, Litton E, Ho KM, et al. Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): a randomized controlled trial. Intensive Care Med (2022) 48(4):414–25. doi: 10.1007/s00134-022-06638-9
31. Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
32. Romero N, Dube KM, Lupi KE, DeGrado JR. Evaluation of delirium in critically ill patients prescribed melatonin or ramelteon. Ann Pharmacother (2021) 55(11):1347–54. doi: 10.1177/10600280211002054
33. Shi Y. Effects of melatonin on postoperative delirium after PCI in elderly patients: A randomized, single-center, double-blind, placebo-controlled trial. Heart Surg Forum (2021) 24(5):E893–7. doi: 10.1532/hsf.4049
34. Yin B, Ye T, Liu X, Wan R, Gu L, Zong G. Effects of melatonin for delirium in elderly acute heart failure patients: A randomized, single-center, double-blind, and placebo-controlled trial. Heart Surg Forum (2022) 25(1):E037–41. doi: 10.1532/hsf.4325
35. Bily B, Sabol F, Torok P, Artemiou P. Influence of prophylactic melatonin administration on the incidence of early postoperative delirium in cardiac surgery patients. Anest. Intenziv. Med (2015) 26:319–27:2015.
36. Pandharipande PP, Girard TD, Ely EW. Long-term cognitive impairment after critical illness. N Engl J Med (2014) 370(2):185–6. doi: 10.1056/NEJMc1313886
37. Abelha FJ, Luis C, Veiga D, Parente D, Fernandes V, Santos P, et al. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit Care (2013) 17(5):R257. doi: 10.1186/cc13084
38. Lemoine P, Zisapel N. Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia. Expert Opin Pharmacother (2012) 13(6):895–905. doi: 10.1517/14656566.2012.667076
39. Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol (2017) 15(3):434–43. doi: 10.2174/1570159X14666161228122115
40. Scott BK. Disruption of circadian rhythms and sleep in critical illness and its impact on the development of delirium. Curr Pharm Des (2015) 21(24):3443–52. doi: 10.2174/1381612821666150706110656
41. Marra A, McGrane TJ, Henson CP, Pandharipande PP. Melatonin in critical care. Crit Care Clin (2019) 35(2):329–40. doi: 10.1016/j.ccc.2018.11.008
42. Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med (2019) 47(1):3–14. doi: 10.1097/CCM.0000000000003482
43. Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing (2011) 40(1):23–9. doi: 10.1093/ageing/afq140
44. Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia (2014) 69(6):540–9. doi: 10.1111/anae.12638
45. Chen J, Wang Y, Hu X, Li M, Xiong K, Zhang Z, et al. The role of statins in the management of delirium: recent advances. CNS Neurol Disord Drug Targets (2021) 20(3):203–15. doi: 10.2174/1871527319666200720111318
46. Odinokova I, Baburina Y, Kruglov A, Fadeeva I, Zvyagina A, Sotnikova L, et al. Effect of melatonin on rat heart mitochondria in acute heart failure in aged rats. Int J Mol Sci (2018) 19(6). doi: 10.3390/ijms19061555
47. Wang J, Toan S, Li R, Zhou H. Melatonin fine-tunes intracellular calcium signals and eliminates myocardial damage through the IP3R/MCU pathways in cardiorenal syndrome type 3. Biochem Pharmacol (2020) 174:113832. doi: 10.1016/j.bcp.2020.113832
48. Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res (2008) 65(3):229–38. doi: 10.1016/j.jpsychores.2008.05.019
Keywords: melatonin, delirium, intensive care, meta-analysis, randomized controlled trials
Citation: Duan Y, Yang Y, Zhu W, Wan L, Wang G, Yue J, Bao Q, Shao J and Wan X (2023) Melatonin intervention to prevent delirium in the intensive care units: a systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 14:1191830. doi: 10.3389/fendo.2023.1191830
Received: 22 March 2023; Accepted: 07 July 2023;
Published: 26 July 2023.
Edited by:
Nils Lambrecht, United States Department of Veterans Affairs, United StatesReviewed by:
Daniel P. Cardinali, Pontifical Catholic University of Argentina, ArgentinaJesus Rico-Feijoo, Hospital Universitario Río Hortega, Spain
Copyright © 2023 Duan, Yang, Zhu, Wan, Wang, Yue, Bao, Shao and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianlin Shao, c2hhb2psQHlkeXkuY24=; Xiaohong Wan, MTM4ODg1ODYxNjJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yushan Duan
Yushan Duan Yuan Yang2†
Yuan Yang2†