
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 12 May 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1187935
This article is part of the Research TopicThyroid Nodule Evaluation: Current, Evolving, and Emerging ToolsView all 16 articles
Purpose: Ultrasound (US) is the first choice in the detection of thyroid nodules in pediatric and adult patients. The purpose of this study was to evaluate the diagnostic performance of adult-based US risk stratification systems (RSSs) when applied to the pediatric population.
Methods: Medline, Embase, and Cochrane Library (CENTRAL) were searched up to 5 March 2023 for studies about the diagnostic performance of adult-based US RSS in pediatric patients. The pooled sensitivity, specificity, positive likelihood ratio (LR), negative LR, and diagnostic odds ratio (DOR) were calculated. The summary receiver operating characteristic (SROC) curves and area under the curve (AUC) were also analyzed.
Results: The sensitivity was highest in American College of Radiology-Thyroid Imaging Reporting and Data System (ACR-TIRADS) category 4–5 and American Thyroid Association RSS high-intermediate risk (ATA), which was 0.84 [0.79, 0.88] and 0.84 [0.75, 0.90], respectively. The specificity was highest in ACR-TIRADS category 5 and Europe-TIRADS (EU-TIRADS) category 5, which was 0.93 [0.83, 0.97] and 0.93 [0.88, 0.98], respectively. The ACR-TIRADS, ATA, and EU-TIRADS showed moderate diagnostic performance in pediatric thyroid nodule patients. For Korea-TIRADS (K-TRADS) category 5, the summary sensitivity and specificity with a 95% CI were 0.64 [0.40, 0.83] and 0.84 [0.38, 0.99], respectively.
Conclusions: In conclusion, the ACR-TIRADS, ATA, and EU-TIRADS have moderate diagnostic performance in pediatric thyroid nodule patients. The diagnostic efficacy of the K-TIRADS was not as high as expected. However, the diagnostic performance of Kwak-TIRADS was uncertain because of the small sample size and small number of studies included. More studies are needed to evaluate these adult-based RSSs in pediatric patients with thyroid nodules. RSSs specific for pediatric thyroid nodules and thyroid malignancies were necessary.
Thyroid cancer is the most common pediatric endocrine cancer and presents a diagnostic challenge in pediatric populations. The reported prevalence of thyroid nodules is 3.1% in adolescents (1). However, the malignancy rate is estimated to be 22–26% in children with thyroid nodules and 5–10% in adults (2–5). Furthermore, pediatric patients are more likely to present with cervical lymph node metastases (40–80%) and distant metastases (20–30%) such as pulmonary metastases than adults (6, 7). Therefore, early and accurate diagnosis in children is extremely important.
Neck ultrasound (US) is the first choice in the detection of thyroid nodules in pediatric and adult patients (8–10). Adult-based neck US risk stratification systems (RSSs) have been developed in recent years to integrate US features and improve diagnostic accuracy as an aid in the stratification of the risk of malignancy, such as the American College of Radiology–Thyroid Imaging Reporting And Data System (ACR-TIRADS), American Thyroid Association Ultrasound Risk Stratification Systems (ATA RSS), European Thyroid Imaging and Reporting Data System (EU-TIRADS), Korean Thyroid Imaging Reporting and Data System (K-TIRADS), and Kwak Thyroid Imaging Reporting and Data System (Kwak-TIRADS) (11–15).
Korean Professor Jin Young Kwak was the first in the world to propose a practical TIRADS to categorize thyroid nodules and stratifying their risk of malignancy in 2011, which we called Kwak-TIRADS now (15). He suggested that the following US features showed a significant association with malignancy: solid component, hypoechogenicity, marked hypoechogenicity, microlobulated or irregular margins, microcalcifications, and taller-than-wide shape. Risk stratification of thyroid malignancy by using the number of suspicious US features allows for a practical and convenient Kwak-TIRADS.
However, we do not have any formalized, US-based RSS in pediatrics. Recently, a few studies have reported the utility of these adult-based RSSs in pediatric patients. However, pediatric thyroid cancers are different in clinical, molecular, and pathologic characteristics from those in adults. These RSSs depend significantly on nodule size, while thyroid volume increases with age, and nodule size is not predictive of malignancy in pediatric patients. Therefore, the appropriateness of these RSSs remains to be explored when applied to pediatric patients.
Therefore, the purpose of this study was to evaluate the diagnostic performance of the adult-based RSS when applied to the pediatric population and provide information to guide future clinical practice.
The meta-analysis was reported in accordance with the instructions of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) extension statement incorporating network meta-analyses (16, 17).
The Medline, Embase, and Cochrane Controlled Register of Trials (CENTRAL) and Web of Science databases were searched up to March 5, 2023. The search terms to retrieve related studies were as follows: [(thyroid) AND (thyroid imaging reporting and data system)] OR [(thyroid image reporting and data system) OR (TIRADS) OR (TI-RADS) OR (RSS) OR (guideline)] AND [(pediatric) OR (adolescent) OR (child) OR (children)]. Two investigators independently checked retrieved articles blinded to the journal, author, and so on. All abstracts to obtain possible applicable articles and the full text were screened to determine the final eligible articles. Relevant reviews and their reference list were also checked. Discrepancies were resolved by discussion with another investigator.
(a) The study was based on the diagnostic performance of adult-based ultrasound RSS, such as ACR-TIRADS, ATA, EU-TIRADS, K-TIRADS, and Kwak-TIRADS. (b) The patients were pediatric with thyroid nodules. (c) The reference standard was based on pathological diagnosis or imaging follow-up. (d) Data available for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy. (e) The language was limited to English.
(a) Letters, editorials, conference abstracts, and review articles. (b) The topics of articles were not about the diagnostic performance of adult-based ultrasound RSS. (c) The patients were not pediatric. (d) If studies had an overlapping population, we included the study with the largest population and excluded others.
The eligible articles were reviewed, and the relevant data were extracted using a standardized form. (a) Study characteristics: first author, year of publication, country or region, study period, study design, sample size, and reference standard; (b) Patient characteristics: number of patients, mean age, and male-to-female ratio; (c) Diagnostic performance: numbers of total thyroid nodules, numbers of true positive (TP), true negative (TN), false positive (FP), false negative (FN) thyroid nodules, sensitivity, specificity, PPV, NPV, and diagnostic accuracy; (d) Standard reference: biopsy pathology, surgery pathology, and follow-up; (e) US examinations: US model and vendor, number of readers, and experience.
Two reviewers assessed the quality of the included articles independently using Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) (18), and disagreement was resolved by discussion. This tool is composed of four domains: patient selection, index test, reference standard, flow, and timing. Each domain is assessed according to bias. Risk of bias was judged as “low,” “high,” or “unclear.” The first three domains are assessed in terms of concerns regarding applicability.
Statistical analysis was mainly performed using Stata version 15.0 software (StataCorp, LLC; College Station, TX). A value of p < 0.05 was taken to indicate statistical significance.
The pooled sensitivity, specificity, positive likelihood ratio (LR), negative LR, and diagnosis odds ratio (DOR), each has 95% confidence intervals (95% CI), were calculated using a bivariate random-effects model, and a coupled forest plot was constructed. In addition, a hierarchical summary receiver operating characteristics (HSROC) curve with 95% confidence and prediction regions was plotted and area under the curve (AUC) was also analyzed. The criteria for the positive test results were set to be (a) RSS category 5 or (b) RSS category 4 or 5. For example, if we set category 5 as a cutoff value, TP nodules indicated the nodules classified as category 5 on US and turned out to be malignant. We followed the reference standard set in each study.
Heterogeneity was assessed using the Higgins inconsistency index (I2) test with a value > 50%, indicating the presence of heterogeneity, and a coupled forest plot was used to graphically assess the presence of a threshold effect (a positive correlation between sensitivity and false-positive rate among the selected studies). We regarded I2 > 50% or P-value of Q-test < 0.05 as high heterogeneity. Among the potential covariates such as sample size, region, standard reference of malignant nodules, and standard reference of benign nodules, we compared “sample size more than median” vs. “sample size less than median,” “America studies” vs. “Europe studies,” “surgery and/or biopsy pathology” vs. “surgery pathology” for malignant nodules, “surgery and/or biopsy pathology and follow-up” vs. “surgery and/or biopsy pathology” for benign nodules.
The details of article screening procedures were as Figure 1. A total of 940 articles were generated using search terms mentioned above, and 524 were removed because of duplications. We excluded 387 that did not meet the topic of our study, and four letters, editorials, conference abstracts, review articles after reviewing the titles and abstracts. The remaining 25 articles were screened for eligibility seriously, and five were abandoned because the assessment of diagnosis performence is based on adult patient and one study had an overlapping population. Finally, the remaining 19 studies were included in our meta-analysis.
The characteristics of included studies were detailed in Table 1 (19–37). All the studies were retrospective. The overall study period was from 1996 to 2021. A total of 1,927 pediatric thyroid cancer patients and 2,263 modules were included. Ages ranged from 0.9 to 22 years. Male patients accounted for 23.1% and female for 76.9%. This study included 660 malignant nodules and 1,603 benign nodules. All malignant nodules and most of benign nodules in the included studies have been diagnosed by surgical pathology or biopsy. Benign nodules included only in six studies were diagnosed by biopsy pathology, surgery pathology, or at least 1 year of follow-up (19–21, 27, 31, 35).
The overall quality of the included studies assessed by QUADAS-2 was moderate. Five articles satisfied six of the seven items, and nine articles satisfied five items. The details are shown in Figure 2. Thirteen studies had an unclear risk of bias in patient selection. Consecutive enrollment was not clarified in 10 studies (20, 22, 24, 25, 27, 31, 32, 35–37). Martinez-Rios et al. included thyroid nodules measuring more than 10 mm (21). Tuli et al. included thyroid nodules measuring more than 5 mm (33). Piccardo et al. included patients treated with radiotherapy for nonthyroidal cancers (29). No study had an unclear risk of bias in the index test domain because of blinding to the reference standard during the US examinations. All studies had an unclear risk of bias in the reference standard domain because of no or unclear blinding to the index test during pathologic evaluation. Six studies had an unclear risk of bias in the flow and timing domain because of inconsistency or unclear consistency on the reference standard for diagnosing benign nodules across the study population (19–21, 24, 27, 35). There were no concerns regarding the applicability of the patient selection, index test and reference standard.
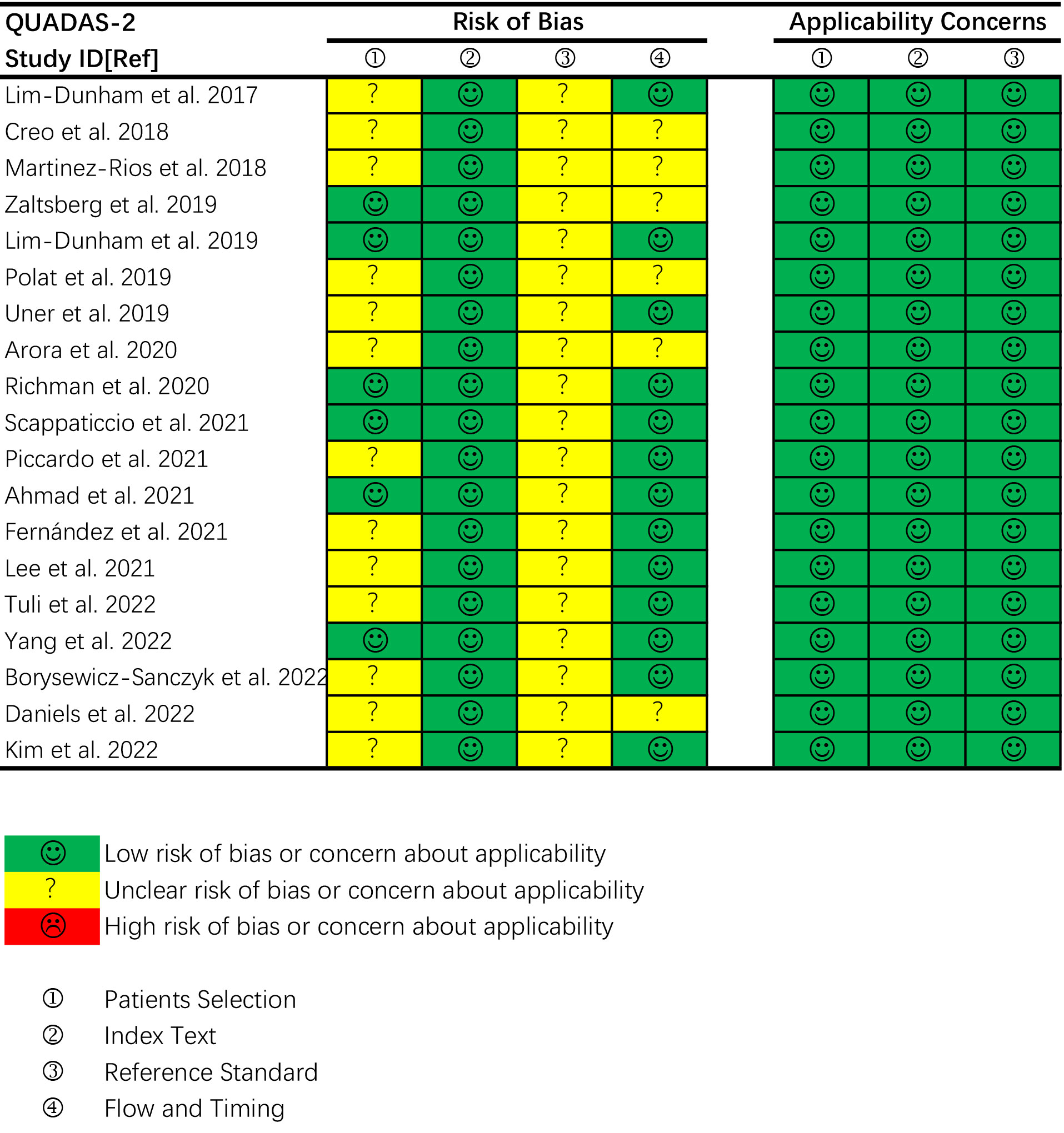
Figure 2 Quality assessment of the included studies according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria.
The diagnostic performance and AUC of ACR-TIRADS, ATA system, EU-TIRADS, K-TIRADS and Kwak-TIRADS was synthesized in Table 2.
Diagnostic performance of ACR-TIRADS
Thirteen studies including 1,868 nodules were pooled to analyze the diagnostic performance of ACR-TIRADS category 5 (ACR 5). As shown in Figure 3, the summary sensitivity and specificity with a 95% CI were 0.57 [0.41,0.71] and 0.93 [0.83, 0.97], respectively. For ACR-TIRADS category 4 or 5 (ACR 4-5), 10 studies including 1,486 nodules were pooled and analyzed, and the sensitivity and specificity were 0.84 [0.79, 0.88] and 0.61 [0.49, 0.72], respectively. The AUC was 0.82 [0.79, 0.85] for ACR 5 and 0.85 [0.81, 0.87] for ACR 4-5, shown in Figure 4.
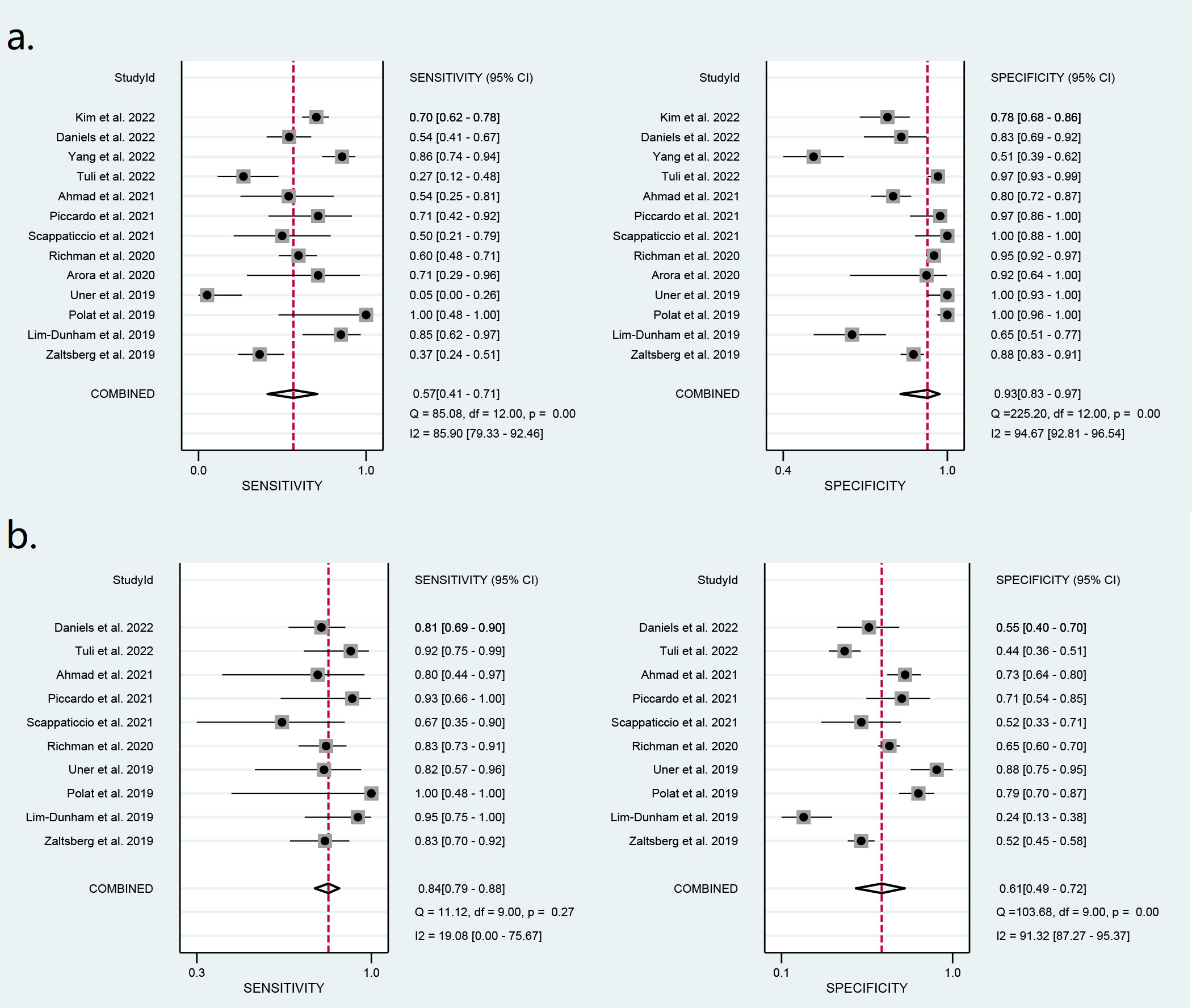
Figure 3 Forest plots of the pooled sensitivity and specificity for the diagnosis of malignant thyroid nodules: (A) ACR 5 and (B) ACR 4-5.
Eight studies including 773 ATA high-risk nodules were pooled and analyzed, and the summary sensitivity and specificity were 0.73 [0.65, 0.79] and 0.73 [0.43, 0.91], respectively. For ATA high-intermediate risk, six studies including 410 nodules were pooled and analyzed. The sensitivity and specificity were 0.84 [0.75, 0.90] and 0.55 [0.40, 0.70], respectively. The details are shown in Figure 5. The AUC was 0.76 [0.72, 0.79] for ATA high risk and 0.82 [0.78, 0.85] for ATA high-intermediate risk, shown in Figure 6.
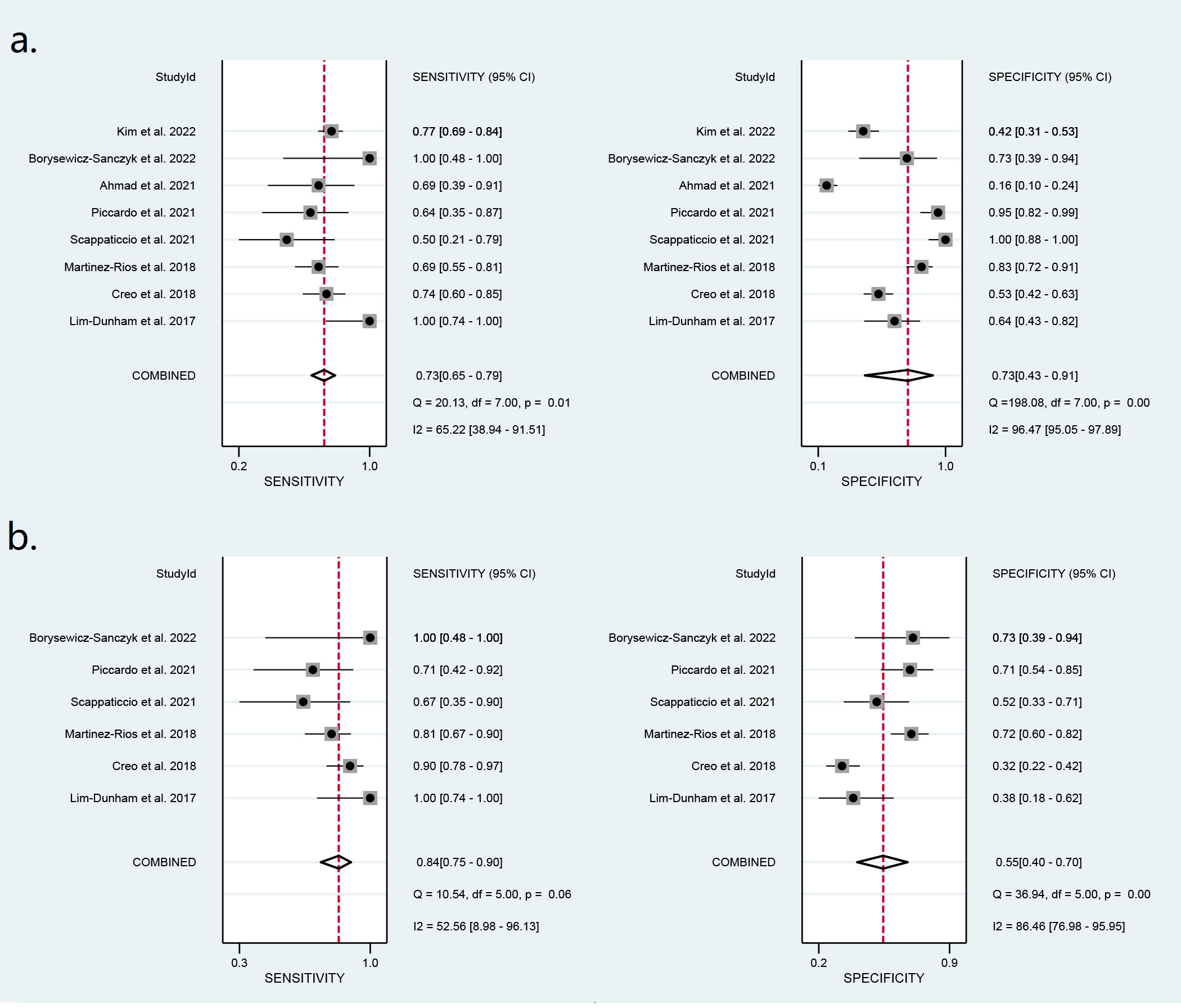
Figure 5 Forest plots of the pooled sensitivity and specificity for the diagnosis of malignant thyroid nodules: (A) ATA high risk and (B) ATA high-intermediate risk.
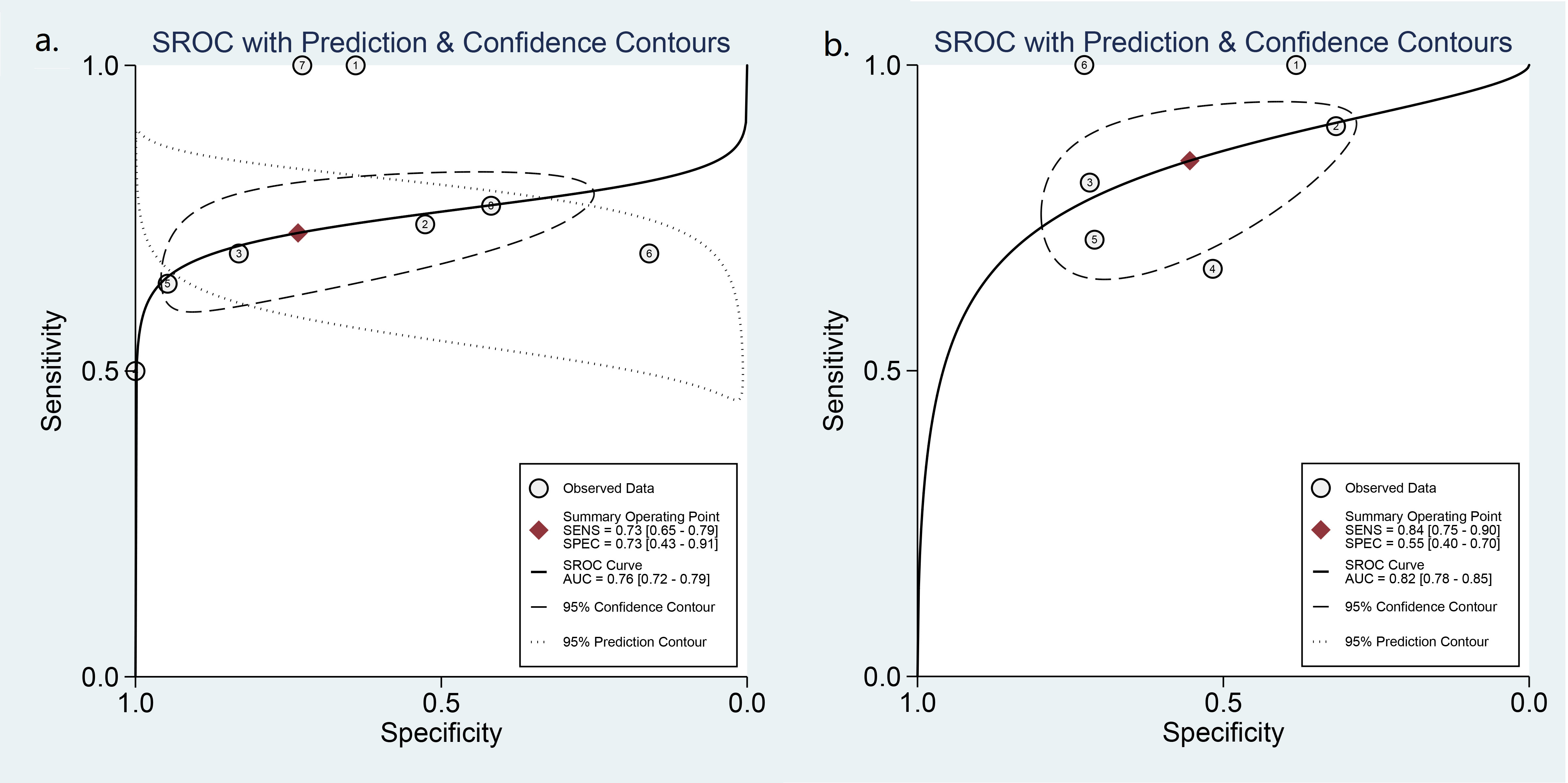
Figure 6 HSROC curve of the diagnostic performance: (A) ATA high risk and (B) ATA high-intermediate risk.
Three studies including 293 nodules of EU-TIRADS category 5 (EU 5) were pooled and analyzed. The summary sensitivity was 0.45 [0.17, 0.76], and the specificity was 0.93 [0.88, 0.98]. For EU-TIRADS category 4 or 5 (EU 4-5) shown in Figure 7, five studies including 533 nodules were pooled and analyzed. The sensitivity and specificity were 0.78 [0.68, 0.86] and 0.48 [0.36, 0.61], respectively. The AUC of EU TR5 was 0.70 [0.33, 0.94], and the AUC of EU 4-5 (Figure 8) was 0.71 [0.67, 0.75].

Figure 7 Forest plots of the pooled sensitivity and specificity for the diagnosis of malignant thyroid nodules in EU 4-5.
Only three studies including 385 nodules were pooled to analyze the diagnostic performance of the K-TIRADS category (K 5). The summary sensitivity and specificity with a 95% CI were 0.64 [0.40, 0.83] and 0.84 [0.38, 0.99], respectively. The AUC was 0.56 [0.06, 0.95].
Only two studies including 423 nodules were pooled to analyze the diagnostic performance of the Kwak-TIRADS. For Kwak 5, the pooled sensitivity and specificity were 0.10 [0.04, 0.18] and 0.99 [0.97, 0.99], respectively. For Kwak 4-5, the sensitivity and specificity were 0.99 [0.99, 1.00] and 0.33 [0.11, 0.63], respectively. The AUC was 0.09 [0.05, 0.14] for Kwak 5 and 0.48 [0.03, 0.94] for Kwak 4-5.
The details are shown in Table 3. Sample size and region might be the heterogeneous sources of specificity of the ACR 5 category. The region and standard reference for benign nodules might be the heterogeneous sources of specificity of the ACR 4-5 category. Sample size and standard reference for malignant nodules could lead to the heterogeneous specificity of the ATA high-risk category. Region resulted in the heterogeneous sensitivity of the ATA high-intermediate risk category. No potential heterogeneous source was found in the EU 4-5 category.
The goal of this study was to investigate the reliability and diagnostic performance of the adult-based TI-RADS in the pediatric population. We analyzed the diagnostic performance of the ACR-TIRADS, ATA RSS, EU-TIRADS, K-TIRADS, and Kwak-TIRADS in this study. Since the included studies were not paired studies, we could not directly compare diagnostic performances between different RSSs and calculate p values, which would not be statistically justified.
The sensitivity was highest in ACR category 4–5 and ATA high-intermediate risk, which was 0.84 [0.79, 0.88] and 0.84 [0.75, 0.90], respectively. The specificity was highest in ACR category 5 and EU category 5, which was 0.93 [0.83, 0.97] and 0.93 [0.88, 0.98], respectively. ACR-TIRADS, ATA RSS and EU-TIRADS showed moderate diagnostic performance in pediatric thyroid nodules with category 4-5 AUCs of 0.85 [0.81, 0.87], 0.82 [0.78, 0.85], and 0.71 [0.67, 0.75], respectively.
Although ACR-TIRADS, ATA RSS, and EU-TIRADS have moderate diagnostic performance in pediatric thyroid nodule patients. They also have some limitations.
The ACR-TIRADS subdivides features and adds points for composition, echogenicity, shape, margins, and echogenic foci, and stratifies TIRADS level based on the total points of the 5 categories of ultrasound features. This requires a high level of experience and skill, which may be difficult for primary care physicians to master and perform (38).
ATA RSS assesses the malignancy of thyroid nodules based on the performance of ultrasound features with high diagnostic weight, which improves the detection rate of malignant nodules, but has the disadvantage that the assessment of the risk of nodule malignancy is overly dependent on the stratification of suspicious ultrasound features. A small number of pediatric patients cannot be categorized according to ATA RSS because of their specific imaging presentation, and such poorly classified nodes could lead to misdiagnosis or underdiagnosis of malignant nodes (39).
ACR-TIRADS, ATA considers FNAB only for nodules greater than or equal to 10 mm, which may miss some malignant nodes in pediatric patients because thyroid volume increases with age, and nodule size is not predictive of malignancy in pediatric patients.
The EU-TIRADS concept of malignancy stratification of thyroid nodules has some similarities with the ATA guidelines. Comparatively, EU-TIRADS has a more streamlined classification of diagnostic weights for malignant nodule features, focusing on the diagnostic weights of highly specific suspicious malignant features, and has a better specificity in identifying benign and malignant nodules. However, the classification of low- and intermediate-risk nodules (EU-TIRADS 3 and 4) by EU-TIRADS explicitly requires the ultrasound features of ovoid shape and smooth margins, while some pediatric patients in the clinic do not have the above two ultrasound features meanwhile cannot be clearly classified in EU-TIRADS 5 categories. This may result in unclassifiable or subjective empirical misjudgment of risk level and is an important reason for the low sensitivity (40).The diagnostic performance of the K-TIRADS was not as expected, with an AUC of only 0.56 [0.06, 0.95]. The K-TIRADS was first proposed by the Korean Society of Thyroid Radiology and Korean Thyroid Association in 2016. Although it shows respectable diagnostic performance for thyroid nodules in adults, recent adult-based studies revealed that in comparison with ACR-TIRADS, the 2016 K-TIRADS demonstrated higher sensitivity (94.5 [92.4, 96.6] vs. 74.7 [70.7, 78.7]) but lower specificity (26.4 [24.2, 28.6] vs. 67.3 [65.0, 69.7]) (41). In this context, the modified K-TIRADS was published in 2021 (42). For pediatric populations, 2021 K-TIRADS newly recommends biopsy of nodules of 0.5–1.0 cm with high suspicion. Compared with the 2016 K-TIRADS, the 2021 K-TIRADS (biopsy cutoffs, 0.5 cm for K-TIRADS 5; 1.0–1.5 cm for K-TIRADS 4) showed higher sensitivity (34.0% vs. 67.3%; p < 0.001) while maintaining specificity (89.4% vs. 88.2%; p = 0.790) in small nodules of pediatric patients and higher specificity (5.9% vs. 25.4%; p < 0.001) while maintaining sensitivity (100% vs. 98.7%; p = 0.132) in large nodules of pediatric patients (43).
In addition, two articles investigated the diagnostic performance of the Kwak-TIRADS (19, 21). Shapira et al. reported an AUC of 0.74 [0.67–0.82] for the diagnostic performance of Kwak-TIRADS compared with 0.72 [0.61–0.82] for ACR-TIRADS. No significant difference was obtained when comparing the Kwak-TIRADS to the ACR TI-RADS (19). Martinez-Rios et al. evaluated the performance of the Kwak-TIRADS and the ATA RSS in assessing thyroid nodules in children. They showed that the test characteristics of both methods were similar to those in adults (21). However, probably because only two studies on Kwak-TIRADS were included, the results of diagnostic performance that we pooled for analysis in our study were not very meaningful.
Additionally, Borysewicz-Sanczyk et al. evaluated the ATA RSS and British Thyroid Association (BTA) ultrasound RSS in the management of thyroid nodules in pediatric patients. The sensitivity and specificity of ATA high risk were (5/5) 100% and (8/11) 72.7%, respectively, while they were (4/5) 80% and (9/11) 81.8% for BTA category 5 (37). Both RSSs showed good diagnostic performance.
We acknowledge that there were certain limitations. First, all the included studies were retrospective. Second, the number of studies on K-TIRADS and Kwak-TIRADS was small, which resulted in the pooled analyzed diagnostic performance not being very informative.
In conclusion, the ACR-TIRADS, ATA, and EU-TIRADS have moderate diagnostic performance in pediatric thyroid nodule patients. The diagnostic efficacy of the K-TIRADS was not as high as expected. However, the diagnostic performance of Kwak-TIRADS was uncertain because of the small sample size and small number of studies included. More studies are needed to evaluate these adult-based RSSs in pediatric patients with thyroid nodules. RSS specific for pediatric thyroid nodules and thyroid malignancies were necessary.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
ZX conceived the meta-analysis. All authors contributed to the development of the selection criteria, the risk of bias assessment strategy, and data extraction criteria. ZX and YQ developed the search strategy, performed database search, acquired the data, analyzed the data, and drafted the manuscript. AS and WW did statistical analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin (2020) 70(6):443–59. doi: 10.3322/caac.21637
2. Al Nofal A, Gionfriddo MR, Javed A, Haydour Q, Brito JP, Prokop LJ, et al. Accuracy of thyroid nodule sonography for the detection of thyroid cancer in children: systematic review and meta-analysis. Clin Endocrinol (Oxf) (2016) 84(3):423–30. doi: 10.1111/cen.12786
3. Canfarotta M, Moote D, Finck C, Riba-Wolman R, Thaker S, Lerer TJ, et al. McGill Thyroid nodule score in differentiating benign and malignant pediatric thyroid nodules: a pilot study. Otolaryngol Head Neck Surg (2017) 157(4):589–95. doi: 10.1177/0194599817715629
4. Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab (2013) 98(8):3238–45. doi: 10.1210/jc.2013-1796
5. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid (2015) 25(7):716–59. doi: 10.1089/thy.2014.0460
6. Qaisi M, Eid I. Pediatric head and neck malignancies. Oral Maxillofac Surg Clin North Am (2016) 28(1):11–9. doi: 10.1016/j.coms.2015.07.008
7. Dinauer CA, Breuer C, Rivkees SA. Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol (2008) 20(1):59–65. doi: 10.1097/CCO.0b013e3282f30220
8. Essenmacher AC, Joyce PH, Kao SC, Epelman M, Pesce LM, D'Alessandro MP, et al. Sonographic evaluation of pediatric thyroid nodules. Radiographics (2017) 37(6):1731–52. doi: 10.1148/rg.2017170059
9. Ogle S, Merz A, Parina R, Alsayed M, Milas M. Ultrasound and the evaluation of pediatric thyroid malignancy: current recommendations for diagnosis and follow-up. J Ultrasound Med (2018) 37(10):2311–24. doi: 10.1002/jum.14593
10. Iakovou I, Giannoula E, Sachpekidis C. Imaging and imaging-based management of pediatric thyroid nodules. J Clin Med (2020) 9(2):384. doi: 10.3390/jcm9020384
11. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
12. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J (2017) 6(5):225–37. doi: 10.1159/000478927
13. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol (2017) 14(5):587–95. doi: 10.1016/j.jacr.2017.01.046
14. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol (2016) 17(3):370–95. doi: 10.3348/kjr.2016.17.3.370
15. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology (2011) 260(3):892–9. doi: 10.1148/radiol.11110206
16. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Internal Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
17. Vrabel M. Preferred reporting items for systematic reviews and meta-analyses. Oncol Nurs Forum (2015) 42(5):552–4. doi: 10.1188/15.ONF.552-554
18. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
19. Shapira-Zaltsberg G, Miller E, Martinez-Rios C, Bass J, Goldbloom EB, Tang K, et al. Comparison of the diagnostic performance of the 2017 ACR TI-RADS guideline to the kwak guideline in children with thyroid nodules. Pediatr Radiol (2019) 49(7):862–8. doi: 10.1007/s00247-019-04385-6
20. Creo A, Alahdab F, Al Nofal A, Thomas L, Kolbe A, Pittock ST. Ultrasonography and the American thyroid association ultrasound-based risk stratification tool: utility in pediatric and adolescent thyroid nodules. Horm Res Paediatr (2018) 90(2):93–101. doi: 10.1159/000490468
21. Martinez-Rios C, Daneman A, Bajno L, van der Kaay DCM, Moineddin R, Wasserman JD. Utility of adult-based ultrasound malignancy risk stratifications in pediatric thyroid nodules. Pediatr Radiol (2018) 48(1):74–84. doi: 10.1007/s00247-017-3974-y
22. Lim-Dunham JE, Erdem Toslak I, Alsabban K, Aziz A, Martin B, Okur G, et al. Ultrasound risk stratification for malignancy using the 2015 American thyroid association management guidelines for children with thyroid nodules and differentiated thyroid cancer. Pediatr Radiol (2017) 47(4):429–36. doi: 10.1007/s00247-017-3780-6
23. Lim-Dunham JE, Toslak IE, Reiter MP, Martin B. Assessment of the American college of radiology thyroid imaging reporting and data system for thyroid nodule malignancy risk stratification in a pediatric population. AJR Am J Roentgenol (2019) 212(1):188–94. doi: 10.2214/AJR.18.20099
24. Polat YD, Öztürk VS, Ersoz N, Anık A, Karaman CZ. Is thyroid imaging reporting and data system useful as an adult ultrasonographic malignancy risk stratification method ın pediatric thyroid nodules? J Med Ultrasound (2019) 27(3):141–5. doi: 10.4103/JMU.JMU_35_19
25. Uner C, Aydin S, Ucan B. Thyroid image reporting and data system categorization: effectiveness in pediatric thyroid nodule assessment. Ultrasound Q (2020) 36(1):15–9. doi: 10.1097/RUQ.0000000000000476
26. Richman DM, Benson CB, Doubilet PM, Wassner AJ, Asch E, Cherella CE, et al. Assessment of American college of radiology thyroid imaging reporting and data system (TI-RADS) for pediatric thyroid nodules. Radiology (2020) 294(2):415–20. doi: 10.1148/radiol.2019191326
27. Arora S, Khoury J, Trout AT, Chuang J. Improving malignancy prediction in AUS/FLUS pediatric thyroid nodules with the aid of ultrasound. Horm Res Paediatr (2020) 93(4):239–44. doi: 10.1159/000509118
28. Scappaticcio L, Maiorino MI, Iorio S, Docimo G, Longo M, Grandone A, et al. Exploring the performance of ultrasound risk stratification systems in thyroid nodules of pediatric patients. Cancers (Basel) (2021) 13(21):5304. doi: 10.3390/cancers13215304
29. Piccardo A, Fiz F, Bottoni G, De Luca C, Massollo M, Catrambone U, et al. Facing thyroid nodules in paediatric patients previously treated with radiotherapy for non-thyroidal cancers: are adult ultrasound risk stratification systems reliable? Cancers (Basel) (2021) 13(18):4692. doi: 10.3390/cancers13184692
30. Ahmad H, Al-Hadidi A, Bobbey A, Shah S, Stanek J, Nicol K, et al. Pediatric adaptions are needed to improve the diagnostic accuracy of thyroid ultrasound using TI-RADS. J Pediatr Surg (2021) 56(6):1120–5. doi: 10.1016/j.jpedsurg.2021.02.034
31. Yeste Fernández D, Vega Amenabar E, Coma Muñoz A, Arciniegas Vallejo L, Clemente León M, Planes-Conangla M, et al. Ultrasound criteria (EU-TIRADS) to identify thyroid nodule malignancy risk in adolescents. correlation with cyto-histological findings. Endocrinol Diabetes Nutr (Engl Ed) (2021) 68(10):728–34. doi: 10.1016/j.endinu.2020.11.009
32. Lee SB, Cho YJ, Lee S, Choi YH, Cheon JE, Kim WS. Korean Society of thyroid radiology guidelines for the management of pediatric thyroid nodules: suitability and risk factors. Thyroid (2021) 31(10):1472–80. doi: 10.1089/thy.2020.0875
33. Tuli G, Munarin J, Scollo M, Quaglino F, De Sanctis L. Evaluation of the efficacy of EU-TIRADS and ACR-TIRADS in risk stratification of pediatric patients with thyroid nodules. Front Endocrinol (Lausanne) (2022) 13:1041464. doi: 10.3389/fendo.2022.1041464
34. Yang J, Page LC, Wagner L, Wildman-Tobriner B, Bisset L, Frush D, et al. Thyroid nodules on ultrasound in children and young adults: comparison of diagnostic performance of radiologists' impressions, ACR TI-RADS, and a deep learning algorithm. AJR Am J Roentgenol (2023) 220(3):408–17. doi: 10.2214/AJR.22.28231
35. Daniels KE, Shaffer AD, Garbin S, Squires JH, Vaughan KG, Viswanathan P, et al. Validity of the American college of radiology thyroid imaging reporting and data system in children. Laryngoscope (2022). doi: 10.1002/lary.30425
36. Kim PH, Yoon HM, Baek JH, Chung SR, Choi YJ, Lee JH, et al. Diagnostic performance of five adult-based US risk stratification systems in pediatric thyroid nodules. Radiology (2022) 305(1):190–8. doi: 10.1148/radiol.212762
37. Borysewicz-Sańczyk H, Sawicka B, Karny A, Bossowski F, Marcinkiewicz K, Rusak A, et al. Suspected malignant thyroid nodules in children and adolescents according to ultrasound elastography and ultrasound-based risk stratification systems-experience from one center. J Clin Med (2022) 11(7):1768. doi: 10.3390/jcm11071768
38. Alexander AA. US-Based risk stratification "guidelines" for thyroid nodules: quō vādis? J Clin Ultrasound (2020) 48(3):127–33. doi: 10.1002/jcu.22803
39. Al Maawali A, Matheson C, Baird R, Blair G. The thyroid nodules in kids study (ThyNK study): an evaluation of clinical practice variation. J Pediatr Surg (2020) 55(5):950–3. doi: 10.1016/j.jpedsurg.2020.01.046
40. Dobruch-Sobczak K, Adamczewski Z, Szczepanek-Parulska E, Migda B, Woliński K, Krauze A, et al. Histopathological verification of the diagnostic performance of the EU-TIRADS classification of thyroid nodules-results of a multicenter study performed in a previously iodine-deficient region. J Clin Med (2019) 8(11):1781. doi: 10.3390/jcm8111781
41. Ha EJ, Na DG, Baek JH, Sung JY, Kim JH, Kang SY. US Fine-needle aspiration biopsy for thyroid malignancy: diagnostic performance of seven society guidelines applied to 2000 thyroid nodules. Radiology (2018) 287(3):893–900. doi: 10.1148/radiol.2018171074
42. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol (2021) 22(12):2094–123. doi: 10.3348/kjr.2021.0713
Keywords: pediatric thyroid nodules, risk stratification systems, ultrasonography, diagnostic performance, meta-analysis
Citation: Xing Z, Qiu Y, Zhu J, Su A and Wu W (2023) Diagnostic performance of adult-based ultrasound risk stratification systems in pediatric thyroid nodules: a systematic review and meta-analysis. Front. Endocrinol. 14:1187935. doi: 10.3389/fendo.2023.1187935
Received: 16 March 2023; Accepted: 26 April 2023;
Published: 12 May 2023.
Edited by:
Andrea Frasoldati, Endocrine Unit ASMN, ItalyReviewed by:
Gerdi Tuli, Regina Margherita Hospital, ItalyCopyright © 2023 Xing, Qiu, Zhu, Su and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anping Su, c3VhbnBpbmdwaW5nQDEyNi5jb20=; Wenshuang Wu, d2Vuc2h1YW5nX3d1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.