
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 06 November 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1184895
This article is part of the Research Topic Deep Learning Assisted Diagnosis and Treatment for Endocrine Tumors View all 6 articles
 Qian Xiao1,2
Qian Xiao1,2 Weixiao Zhang3
Weixiao Zhang3 Jingfeng Jing1,2
Jingfeng Jing1,2 Tingting Zhong4
Tingting Zhong4 Daxue Li1,2
Daxue Li1,2 Jing Zhou1,2
Jing Zhou1,2 Pan Liu5
Pan Liu5 Zhongxu Duan1,2
Zhongxu Duan1,2 Han Gao1,2*
Han Gao1,2* Liyuan Shen6,7*
Liyuan Shen6,7*Background: The role of age in metastatic disease, including breast cancer, remains obscure. This study was conducted to determine the role of age in patients with de novo metastatic breast cancer.
Methods: Breast cancer patients diagnosed with distant metastases between 2010 and 2019 were retrieved from the Surveillance, Epidemiology, and End Results database. Comparisons were performed between young (aged ≤ 40 years), middle-aged (41–60 years), older (61–80 years), and the oldest old (> 80 years) patients. Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were estimated using multivariate Cox proportional hazard models. Survival analysis was performed by the Kaplan–Meier method.
Results: This study included 24155 (4.4% of all patients) de novo metastatic breast cancer patients. The number of young, middle-aged, older, and the oldest old patients were 195 (8.3%), 9397 (38.9%), 10224 (42.3%), and 2539 (10.5%), respectively. The 5-year OS rate was highest in the young (42.1%), followed by middle-aged (34.8%), older (28.3%), and the oldest old patients (11.8%). Multivariable Cox regression analysis showed that middle-aged (aHR, 1.18; 95% CI, 1.10–1.27), older (aHR, 1.42; 95% CI, 1.32–1.52), and the oldest old patients (aHR, 2.15; 95% CI, 1.98–2.33) had worse OS than young patients. Consistently, middle-aged (aHR, 1.16; 95% CI, 1.08–1.25), older (aHR, 1.32; 95% CI, 1.23–1.43), and the oldest old patients (aHR, 1.86; 95% CI, 1.71–2.03) had worse BCSS than young patients.
Conclusion: This study provided clear evidence that de novo metastatic breast cancer had an age-specific pattern. Age was an independent risk factor for mortality in patients with de novo metastatic breast cancer.
Breast cancer is the most frequently diagnosed malignancy in women, with an estimated 2.3 million new cases (11.7%) in 2020 worldwide (1). After lung cancer, breast cancer is the second leading cause of cancer-associated deaths in women (2). Among the new breast cancer cases, 2.4%–6% of patients were diagnosed with advanced breast cancer with stage IV, namely, de novo metastatic breast cancer (3, 4). According to the data from 2009 to 2015 in the United States, patients with de novo metastatic breast cancer had the lowest 5-year overall survival (OS) rate (27%), which was much lower than stage I (98%), stage II (92%), and stage III (75%) (5). Identifying clinical risk factors that are closely correlated with distant metastases may help provide clues for the underlying mechanism of distant metastases and the development of treatment strategies for advanced breast cancer.
Cancer cells disseminated from the primary tumor before detection and persist at distant organs are thought to be the source of distant metastasis (6). The classic model of metastasis includes three steps: first, the primary tumor grows and infiltrates vessels; second, cancer cells enter the blood or lymphatic vessels and spread to distant sites; and third, disseminated cells colonize new sites and outgrowths of metastases (7). Only a small percentage of disseminated tumor cells emerging from step one acquire sufficient genetic or epigenetic alterations that enable them to complete the metastasis (8). The latency between tumor cell dissemination and outgrowth of metastases varies from a very short period (early metastasis) to decades, which experience a long-term democracy (9). According to epidemiologic studies, some sociodemographic and clinicopathologic features are found to be risk factors associated with metastases. For example, it is well-known that patients with triple-negative breast cancers (TNBC) have an early tendency to undergo metastasis and a higher tumor recurrence rate than other types of breast cancers (10, 11). Further studies focusing on TNBC revealed mechanisms and targets for distant metastasis that contributed to an improvement in survival outcomes among patients with TNBC (10).
Age, as a major risk factor of tumorigenesis, contributes greatly to metastasis, likely because of the age-related changes in patient homeostasis and the tumor microenvironment (12). Distant metastases occur earlier and are more synchronized in older patients than in younger patients with melanoma (13). Age-induced reprogramming of lung fibroblasts enables metastatic outgrowth of melanoma in the lungs by increasing the secretion of secreted frizzled-related protein 1 (sFRP1) (14). In breast cancer, age has been identified as a major risk factor for tumor initiation, metastasis, and disease-specific mortality (15). Young breast cancer patients usually exhibit more aggressive tumor characteristics, while older patients often have a worse prognosis (16, 17); however, whether there is a close relationship between age and metastasis in breast cancer patients remains largely elusive. In this retrospective study, we systemically evaluated the association among different age groups with de novo metastatic breast cancer. The clinicopathologic characteristics and the risk of all-cause mortality and breast cancer-specific mortality were also determined in de novo metastases based on patient age at the time of diagnosis.
The cohort of this study was collected from the most recent Surveillance, Epidemiology, and End Results (SEER) 17 registries’ (November 2021) database (https://seer.cancer.gov/) using SEER*Stat 8.4.0.1. The SEER is a population-based dataset that covers approximately 30% of the United States, providing demographic, clinicopathologic, diagnostic, treatment, and survival information. Due to the publicity of the SEER database, the current study was exempt from the review of the Institutional Ethics Committees of the Women and Children’s Hospital of Chongqing Medical University. Because no participants were enrolled in this study, written informed consent from patients was waived. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients diagnosed with de novo metastatic breast cancer between 2010 and 2019 were included because the human epidermal growth factor receptor 2 (HER2) status and breast cancer subtype data were available since 2010. A total of 628254 breast cancer patients were originally identified without other restrictions. We obtained demographic variables, clinicopathologic features, and treatment information from the SEER, including patient age at the time of diagnosis, sex, year of diagnosis, race, tumor size, lymph node status, distant metastases, tumor grade, histologic type, molecular subtype, surgery, radiotherapy, chemotherapy, survival in months, and cause of death. Patients were excluded if the following critical information was unknown: survival months, breast subtype (2010+), cause-specific death classification, metastases, and tumor size. The flow chart for patient selection is shown in Figure 1.
Individuals were grouped into young (aged ≤ 40 years), middle-aged (41–60 years), older (61–80 years), and the oldest old (> 80 years) based on the patient’s age at the time of diagnosis. Tumor size was categorized as <2 cm, 2–5 cm, >5 cm, or unknown. According to the 3rd edition of the International Classification of Diseases for Oncology (ICD-O-3), histopathology codes and histology types were grouped into invasive ductal carcinoma (IDC, 8500/3), invasive lobular carcinoma (ILC, 8520/3), mixed IDC and ILC (8522/3), or other types. We stratified surgery into “yes,” “no,” and “unknown” groups according to the 2021 breast surgery codes from SEER. Specifically, the chemotherapy recode no/unknown represented patients who did not receive chemotherapy because the recode indicated that no evidence of chemotherapy was identified in the medical records. Radiotherapy was categorized into either a "yes" or "no/unknown" group based on the radiation code. Patients who either did not require radiotherapy, refused it, or had an unknown treatment status were consolidated into the "no/unknown" group. Information on hormonal or target therapy was not provided. Our primary outcome was OS based on the vital status recode variation, which was defined as any cause of death. In addition, breast cancer-specific survival (BCSS), as a secondary outcome, was also determined using the SEER cause-specific death classification. BCSS was considered a death caused by breast cancer. The variable survival time months were used to extract information on the follow-up period.
Demographic and clinicopathologic characteristics of patients were examined by descriptive statistics and/or chi-square test. Kaplan-Meier survival curves were carried out to assess OS and BCSS. The differences between survival curves were detected by log-rank tests. We performed a univariable (unadjusted) analysis of the hazard ratio (HR) and 95% confidence intervals (CIs) of OS and/or BCSS using the Mantel-Haenszel statistic. Multivariable Cox proportional hazards regression models were used to assess the influence of patient age at the time of diagnosis on the HRs of OS and BCSS after adjusting for sex, race, tumor size, lymph node status, distant metastases, tumor grade, molecular subtype, histologic type, surgery, chemotherapy, and radiotherapy. The proportional hazards model assumption was confirmed for each covariate by inspecting log (-log [survival]) curves. All P-values were two-sided with a statistical significance of P < 0.05. All statistical analyses were performed using GraphPad Prism version 8.0.1 (GraphPad Software Inc, La Jolla, CA, USA) and IBM SPSS 22.0 software (IBM Corporation, Armonk, NY, USA).
A total of 24155 (4.4% of all breast cancer patients) de novo metastatic breast cancer patients (mean [SD] age, 61.5 [14.4] years [range, 15-100 years]) were included in this study (Figure 1). The median follow-up was 20 months (range, 0–119) for all patients. There were 12564 (90%) death events attributed to breast cancer and 1394 (10%) deaths due to other causes. The number of patients ≤ 40 (young), 41–60 (middle-aged), 61–80 (older), and >80 years (the oldest old) were 1995 (8.3%), 9397 (38.9%), 10224 (42.3%), and 2539 (10.5%), respectively. There were 15740 (65.2%) patients with bone, 7148 (29.6%) lung, 5796 (24.0%) liver, and 1599 (6.6%) brain metastases. A total of 7550 patients underwent surgery for primary tumors after diagnosis with metastatic breast cancer. The demographic and clinical characteristics of the patients are shown in Table 1. Among the patients with de novo metastatic breast cancer, young white patients had the lowest percentage (66.8%), followed by middle-aged (70.5%), older (78.5%), and the oldest old (85.2%), whereas young Black patients had the highest percentage (21.3%), followed by middle-aged (17.8%), older (14.0%), and the oldest old (8.7%). The oldest old patients with de novo metastatic breast cancer were most frequently associated with lymph node negativity (33%), while lymph node positivity was more frequent in young patients (70.8%). With respect to distant metastases, young patients had the highest proportion of bone (66.3%) and liver (33.6%) metastases. The proportions of patients with lung metastases gradually increased from young (21.8%) to middle (27.1%) to older (32.4%) to the oldest old (33.6%) patients. The percentage of patients with brain metastasis had higher percentages in the middle (7.2%) and older patients (7.0%) than the young (5.7%) and oldest old patients (3.5%). Surgery, chemotherapy, and radiotherapy were administered more often to young patients, followed by middle-aged, older, and the oldest old patients (Table 1).
When analyzed by age, the prevalence of de novo metastatic breast cancer in overall breast cancer patients had a “w”-like shape (Figure 2A), which was highest in patients aged ≤29 years of age (8.1%), and then decreased to patients aged 44 years old (3.6%). There was a peak in 55-year-old patients (4.8%). The prevalence of de novo metastatic breast cancer gradually decreased from 4.8% (55 years of age) to 3.7% (68 years of age), and then increased up to 5.4% in patients ≥85 years of age. Between 2010 and 2019, the prevalence of de novo metastatic breast cancer patients ≤ 40 years of age increased from 5.3% to 6.5% and from 4.4% to 5.4% in patients >80 years of age, while the prevalence of de novo metastatic breast cancer was stable among patients 41–60 and 61-80 years of age with an average prevalence of 4.3% and 4.0%, respectively (Figure 2B).
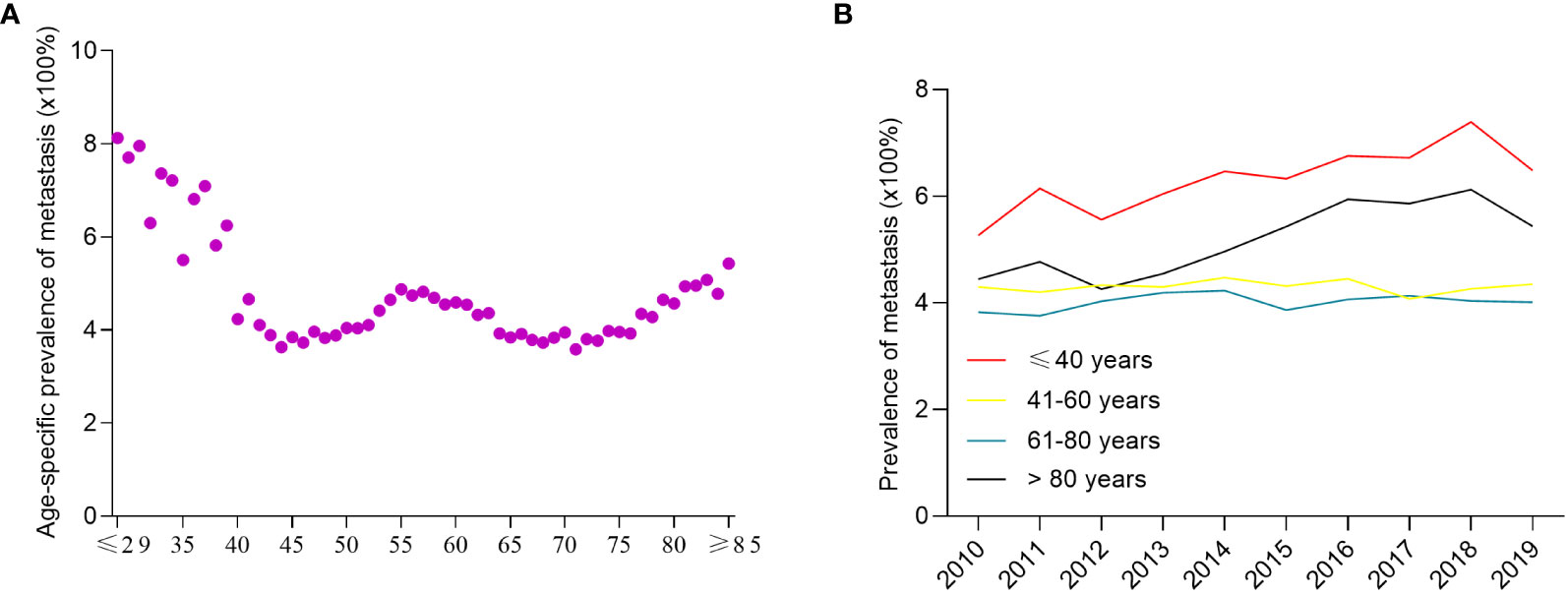
Figure 2 The prevalence of de novo metastatic breast cancer among all breast cancer patients. The age-specific prevalence of de novo metastatic breast cancer (A) and year-specific prevalence of de novo metastatic breast cancer (B).
To determine the correlation between breast cancer subtype and metastasis in different age groups, we first analyzed the percentages of four molecular subtypes in the overall cohort, including early breast cancer patients (Figure 3). Patients ≤ 40 years of age had the highest percentage of HR+/HER2+ (18.07%), HR-/HER2+ (6.71%), and HR-/HER2- (18.35%) breast cancer molecular subtypes, whereas patients >80 years of age had the highest percentage of the HR+/HER2- (80.23%) breast cancer. Subsequently, we determined the prevalence of de novo metastases in different subtypes of breast cancer. Young patients had the highest prevalence of de novo metastasis in HR+/HER2- (Figure 4A), HR+/HER2+ (Figure 4B), and HR-/HER2+ (Figure 4C) molecular subtypes, followed by the oldest old, older, and/or middle-aged patients. The oldest old patients had the highest prevalence of HR-/HER2- breast cancer, followed by older, young, and middle-aged patients (Figure 4D). To determine the correlation between primary tumor burden and de novo metastasis, we compared the tumor sizes among the four age groups. The oldest old patients had the smallest primary tumor size (mean [SD] = 45.7 [62.1] mm), followed by the older (mean = 48.1 [53.0] mm), middle-age (mean = 49.8 [51.4] mm), and young patients (mean = 50.8 [44.9] mm) (P < 0.001, Figure 5).
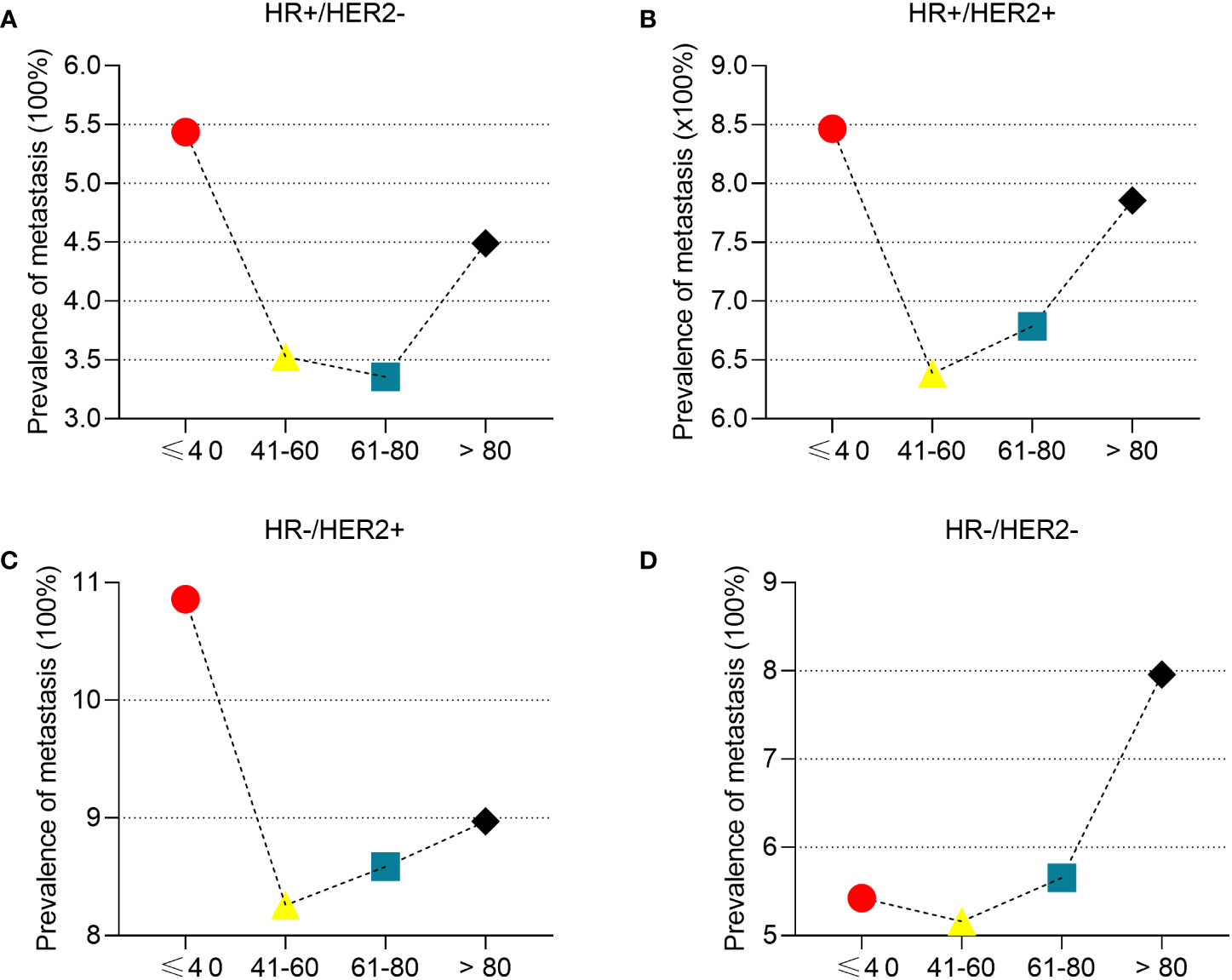
Figure 4 The prevalence of de novo metastasis in different ages stratified by subtypes of breast cancer. HR+/HER2- (A), HR+/HER2+ (B), HR-/HER2+ (C), and HR-/HER2- (D).
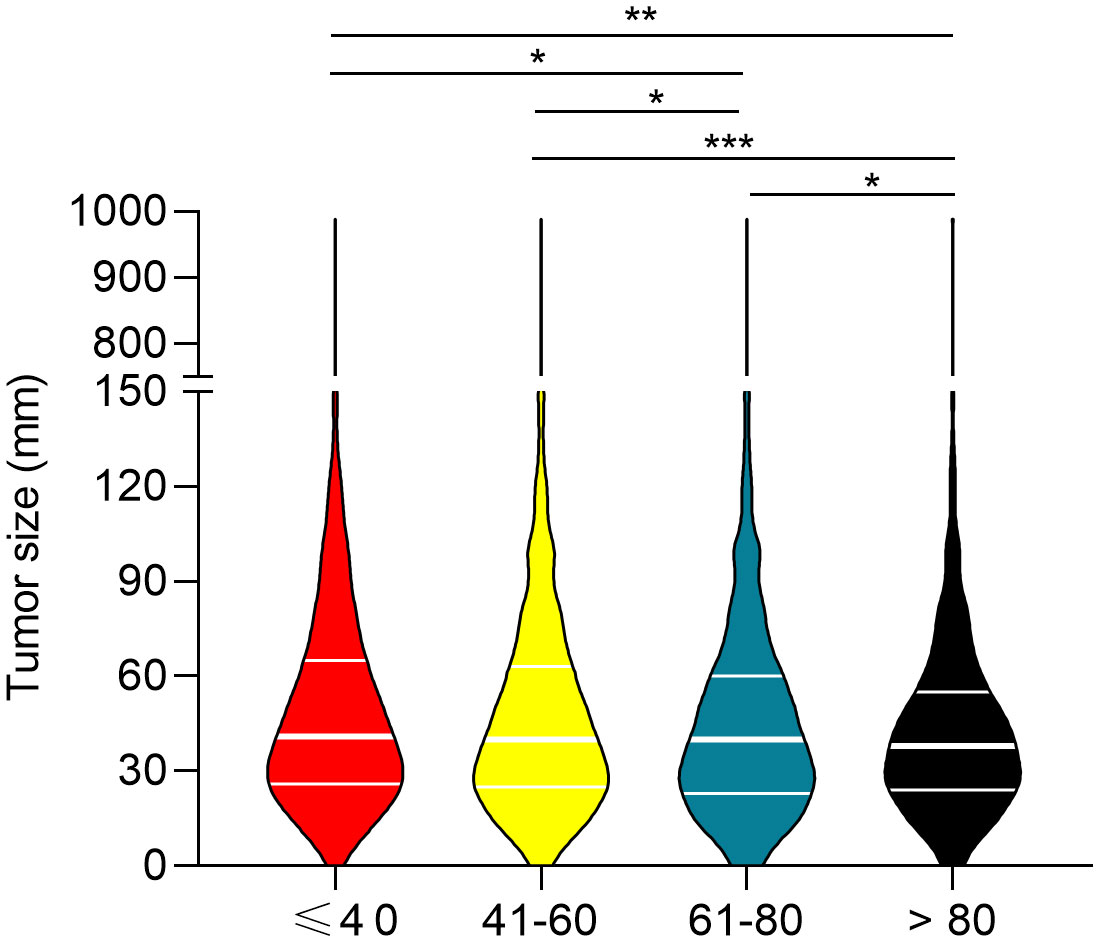
Figure 5 Comparison of primary tumor size between the four age groups. *P<0.05, **P<0.01, and ***P<0.001.
The median follow-up period was 2.2 (range 0 to 9.9), 2.0 (range 0 to 9.9), 1.6 (range 0 to 9.9), and 0.9 (range 0 to 9.5) years for young-age, middle-age, older-age, and the oldest old patients, respectively. The 5-year OS rates were 42.1%, 34.8%, 28.3%, and 11.8% for young-age, middle-aged, older-age, and the oldest old patients, respectively. Patients in the oldest old group had the worst OS compared to the young-age group (HR, 2.90; 95% CI, 2.68–3.13; P < 0.001), middle-age (HR, 3.00; 95% CI, 2.97–3.20; P < 0.001), and older-age (HR, 1.97; 95% CI, 1.85–2.10; P < 0.001) based on unadjusted analysis (Figure 6A). Older-aged patients had a significantly poorer OS than middle-aged (HR, 1.29; 95% CI, 1.24–1.34; P < 0.001) and young-aged patients (HR, 2.17; 95% CI, 1.61–2.92; P <.001; Figure 6A). Middle-aged patients had a decreased OS compared to young-aged patients (HR, 1.24; 95% CI, 1.61–1.32; P < 0.001; Figure 6A). Older-aged patients had decreased OS compared to young-aged (HR, 1.51; 95% CI, 1.42–1.60; P < 0.001) and middle-aged patients (HR, 1.29; 95% CI, 1.24–1.34; P < 0.001; Figure 6A). Similar BCSS differences were noted among the four age groups of patients (Figure 6B).
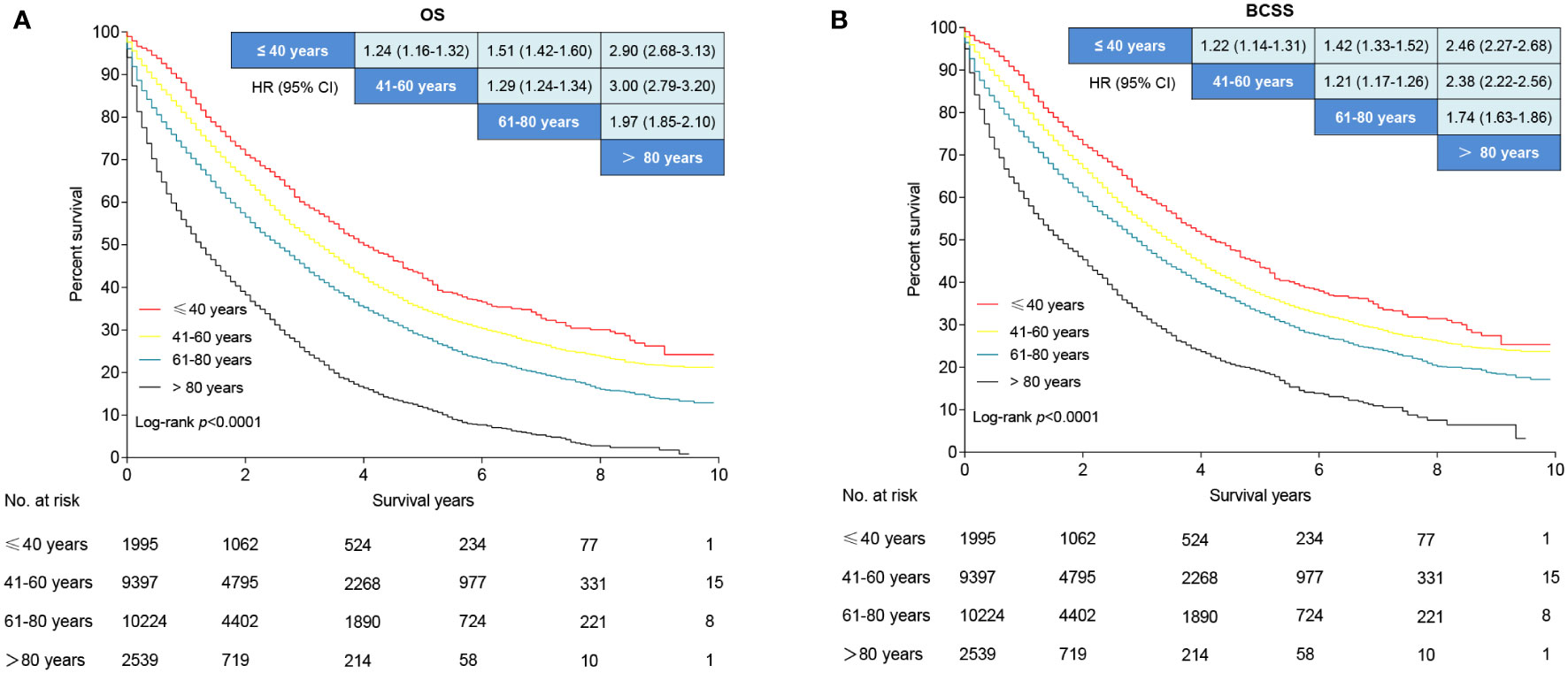
Figure 6 Survival analysis of de novo metastatic breast cancer stratified by age. (A) Overall survival curves. (B) Breast cancer-specific survival curves. Data are presented as hazard ratios and 95% confidence intervals in the league table of comparisons. An HR >1 favors the row-defining group.
Subgroup analyses were performed based on subtypes, metastasis sites, and treatment (Supplementary Figures 1–6). The OS and BCSS were best in the young patients with HR+/HER2-, HR-/HER2+, and HR-/HER2- molecular subtypes, followed by the middle-aged, older, and the oldest old patients (Supplementary Figures 1A-C, 2A-C). There was no significant difference in OS and BCSS between the young- and middle-aged patients with TNBC; however, the oldest old patients had worse OS and BCSS than the other age groups (Supplementary Figures 1D, 2D). In patients with bone and liver metastasis, young patients had the best OS and BCSS followed by middle-aged, older, and the oldest old patients (Supplementary Figures 3A, B, 4A, B). No significant differences in OS and BCSS were noted between the young- and middle-aged patients with lung and brain metastases, whereas the two subgroups had significantly better OS and BCSS than older and the oldest old patients (Supplementary Figures 3C, D, 4C, D). Among patients who received chemotherapy or surgery, the young patients had the best OS and BCSS, followed by the middle-aged, older, and oldest old patients (Supplementary Figures 5, 6).
In multivariable analysis, middle-aged (HR, 1.18; 95% CI, 1.10–1.27; P < 0.001), older age (HR, 1.42; 95% CI, 1.32–1.52; P < 0.001), and the oldest old patients (HR, 2.15; 95% CI, 1.98-2.33; P < 0.001) had an 18%, 42%, and 115% higher risk of all-cause death rates compared with the young patients, respectively (Table 2). The oldest old patients (HR, 1.86; 95% CI, 1.71–2.03; P < 0.001) had the worst BCSS, followed by older age (HR, 1.32; 95% CI, 1.23–1.43; P < 0.001) and middle-aged patients (HR, 1.16; 95% CI, 1.08–1.25; P < 0.001) compared to the young patients (Table 2).
The incidence of breast cancer is highly associated with increasing age (18). Emerging data also suggest that age is a key factor associated with cancer metastasis (12). Nevertheless, the patterns of age-related de novo metastatic breast cancer have not been established. In the present study, we systematically determined breast cancer metastasis at the time of diagnosis focusing on patient age based on an unbiased population cohort. We found that there are three peak incidences of de novo metastatic breast cancer including young (less than 40 years old), perimenopausal period (approximately 55 years old), and the oldest old ≥ 80 years old). Potential reasons for this finding may be due to a higher proportion of aggressive molecular subtypes (TNBC [18.35%] and HER-2 positive subtypes [24.78%]; Figure 3) in young patients, dramatical changes in hormone level and homeostasis during the perimenopausal period, and a decrease in immune surveillance among the oldest old (19, 20). Moreover, we also found that young Black patients were more frequently diagnosed with stage IV breast cancer, a finding which was consistent with the concept that Black patients have poor survival outcomes (21).
The distribution of metastatic sites was distinct among the four age groups. In this study, we found that patients older than 60 years of age had a much higher percentage of lung metastases, a phenomenon that may be due to chronic inflammation of the lung, such as chronic obstructive pulmonary disease, which is usually present in old people (22). It has been shown that potential mechanisms of inflammatory cell neutrophils specifically support metastatic initiation and promote dormant cancer cell awakening in the lungs using mouse models (23, 24). In addition, we observed that young patients had a higher prevalence of liver and bone metastases. A previous study reported that the HER2-positive subtype has a high rate of metastasis to the liver and bone, but the basal-like subtype has a significantly lower rate of liver and bone metastases (25). Considering the high percentages of HER-2 positive subtypes in the young cohort, further studies focusing on potential mechanisms of liver and bone metastases in HER-2 positive breast cancer may help in developing novel strategies for metastasis prevention. Brain metastases are associated with the worst prognosis in patients with breast cancer, with median survival ranging from 2 to 25.3 months despite treatment (26). In the present study, we observed that middle- and older-aged patients had a higher frequency of brain metastases. This finding is inconsistent with previous studies that reported that younger-aged patients are more likely to develop breast cancer brain metastases after diagnosis and treatment (27, 28). Moreover, multiple risk factors for brain metastases have also been identified, such as molecular subtype, histological grade, and germline BRCA1/2 mutations (28–30). Collectively, these data indicated that there was a close correlation between breast cancer metastasis and patient age and that different ages exhibited distinct metastatic patterns.
Young patients with early breast cancer typically have more aggressive subtypes, which are more likely to develop both locoregional and distant recurrences and are usually associated with poorer survival outcomes, whereas older women more commonly have less aggressive tumors with a better prognosis (31). In contrast, our multivariable analysis revealed that older patients with de novo metastatic breast cancer were more likely to have a poorer OS and BCSS than young women because a lower percentage of older patients received therapy, including surgery, radiotherapy, and chemotherapy, than young patients, which was consistent with a previous study in which elderly patients tended to receive less chemotherapy or fewer treatment regimens than younger patients (32). It is clear that surgery, radiotherapy, and chemotherapy significantly increased the OS and BSCC in the four age groups compared with patients who did not receive surgery, radiotherapy, or chemotherapy for de novo metastatic breast cancer in the SEER cohort (33, 34). Among patients who received surgery, radiotherapy, or chemotherapy, the young patients had the best OS and BCSS, followed by the middle-aged, older, and oldest old patients. This finding indicated that older patients with de novo metastatic breast cancer did not acquire more benefits than younger patients from these treatments. Moreover, a SEER database-based retrospective study reported that older patients (i.e., >70 years) had a higher risk of death from heart disease (32). Therefore, additional strategies involving non-cancer causes of death might enhance the OS and BCSS in patients with de novo metastatic breast cancer, especially in older patients.
A recent study reported that lymph node involvement and T stage were independent risk factors for mortality in the population with de novo metastatic breast cancer (4). In the present study, we also showed that the locoregional tumor burden (tumor size and lymph node status) decreased with aging, which implied that older patients might develop distant metastases earlier than younger patients. Different distant metastases were associated with distinct survival outcomes in breast cancer, of which brain metastasis is the leading cause of death (35). Our subgroup analyses also detected the impact of age on different metastatic organs. We found that older patients had poorer OS and BCSS compared to younger patients with bone, liver, lung, and brain metastases, except that no OS and BCSS differences were observed between young- and middle-aged patients with lung and brain metastases. Moreover, older patients were associated with a poorer OS and BCSS than younger patients with different molecular subtypes of breast cancer, including HR+/HER2-, HR-/HER2+, and HR-/HER2-. Although there were no significant OS and BCSS differences between young- and middle-aged patients with TNBC, the oldest old patients had a poorer OS and BCSS compared to the other patients. The results of the subgroup analysis showed the robustness of our findings, which suggested that older patients were associated with a poorer OS and BCSS compared to younger patients with de novo metastatic breast cancer.
There were some limitations to our study. First, potential selection bias could not be avoided due to the properties of a retrospective study and the exclusion of patients because of the lack of essential information. Second, the SEER database did not provide historical information on accompanying diseases, such as cardiovascular and chronic obstructive pulmonary diseases; therefore, we could not adjust for these factors when performing the multivariable analysis. Third, because the detailed systemic treatment information, such as chemotherapy, endocrine therapy, and targeted therapy, was not available in the SEER database, we could not investigate the benefits of systemic treatment in different age groups.
In conclusion, age was a critical factor that was closely correlated with de novo metastatic breast cancer. Different ages had distinct distant metastasis patterns at the time of diagnosis. Older patients with de novo metastatic breast cancer had poorer OS and BCSS compared to young women. Future basic research and clinical studies may help to disclose novel mechanisms of how age influences distant metastasis and develop personalized treatment strategies for patients with de novo metastatic breast cancer in different ages.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The requirement of ethical approval was waived by the Institutional Ethics Committees of the Women and Children’s Hospital of Chongqing Medical University for the studies involving humans because no participants were enrolled in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because no participants were enrolled in this study.
QX: Writing - Original Draft; LS: Writing - Review & Editing; WZ, JJ, and ZD: Data Curation; DL, JZ, PL, and TZ: Visualization; HG: Writing - Review &Editing, Methodology, Supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1184895/full#supplementary-material
Supplementary Figure 1 | Overall survival of de novo metastatic breast cancer stratified by subtypes. HR+/HER2- (A), HR+/HER2+ (B), HR-/HER2+ (C), and HR-/HER2- (D).
Supplementary Figure 2 | Breast cancer-specific survival of de novo metastatic breast cancer stratified by subtypes. HR+/HER2- (A), HR+/HER2+ (B), HR-/HER2+ (C), and HR-/HER2- (D).
Supplementary Figure 3 | Overall survival of de novo metastatic breast cancer stratified by distribution of metastatic sites. Bone (A), liver (B), lung (C), and brain (D).
Supplementary Figure 4 | Breast cancer-specific survival of de novo metastatic breast cancer stratified by distribution of metastatic sites. Bone (A), liver (B), lung (C), and brain (D).
Supplementary Figure 5 | Overall survival and breast cancer-specific survival of de novo metastatic breast cancer patients who received chemotherapy. (A) Overall survival curves. (B) Breast cancer-specific survival curves.
Supplementary Figure 6 | Overall survival and breast cancer-specific survival of de novo metastatic breast cancer patients who underwent surgery. (A) Overall survival curves. (B) Breast cancer-specific survival curves.
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol (2014) 15(7):e279–89. doi: 10.1016/s1470-2045(13)70567-9
4. Yang SX, Hewitt SM, Yu J. Locoregional tumor burden and risk of mortality in metastatic breast cancer. NPJ Precis Oncol (2022) 6(1):22. doi: 10.1038/s41698-022-00265-9
5. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA: Cancer J Clin (2019) 69(6):438–51. doi: 10.3322/caac.21583
6. Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer (2009) 9(4):302–12. doi: 10.1038/nrc2627
7. Röcken M. Early Tumor Dissemination, but Late Metastasis: Insights into Tumor Dormancy. J Clin Invest (2010) 120(6):1800–3. doi: 10.1172/jci43424
8. Fidler IJ. The pathogenesis of cancer metastasis: the 'Seed and soil' Hypothesis revisited. Nat Rev Cancer (2003) 3(6):453–8. doi: 10.1038/nrc1098
9. Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell (2013) 155(4):750–64. doi: 10.1016/j.cell.2013.10.029
10. Bai X, Ni J, Beretov J, Graham P, Li Y. Triple-negative breast cancer therapeutic resistance: where is the achilles' Heel? Cancer Lett (2021) 497:100–11. doi: 10.1016/j.canlet.2020.10.016
11. Jain V, Kumar H, Anod HV, Chand P, Gupta NV, Dey S, et al. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J Controlled release (2020) 326:628–47. doi: 10.1016/j.jconrel.2020.07.003
12. Fane M, Weeraratna AT. Normal aging and its role in cancer metastasis. Cold Spring Harbor Perspect Med (2020). doi: 10.1101/cshperspect.a037341
13. Gassenmaier M, Keim U, Leiter U, Eigentler TK, Röcken M, Gesierich A, et al. Age as key factor for pattern, timing, and extent of distant metastasis in patients with cutaneous melanoma: A study of the German central malignant melanoma registry. J Am Acad Dermatol (2019) 80(5):1299–307.e7. doi: 10.1016/j.jaad.2019.01.044
14. Fane ME, Chhabra Y, Alicea GM, Maranto DA, Douglass SM, Webster MR, et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature (2022) 606(7913):396–405. doi: 10.1038/s41586-022-04774-2
15. van de Water W, Markopoulos C, van de Velde CJ, Seynaeve C, Hasenburg A, Rea D, et al. Association between Age at Diagnosis and Disease-Specific Mortality among Postmenopausal Women with Hormone Receptor-Positive Breast Cancer. Jama (2012) 307(6):590–7. doi: 10.1001/jama.2012.84
16. Chen HL, Zhou MQ, Tian W, Meng KX, He HF. Effect of age on breast cancer patient prognoses: A population-based study using the seer 18 database. PloS One (2016) 11(10):e0165409. doi: 10.1371/journal.pone.0165409
17. Tzikas AK, Nemes S, Linderholm BK. A comparison between young and old patients with triple-negative breast cancer: biology, survival and metastatic patterns. Breast Cancer Res Treat (2020) 182(3):643–54. doi: 10.1007/s10549-020-05727-x
18. Holmes CE, Muss HB. Diagnosis and treatment of breast cancer in the elderly. CA: Cancer J Clin (2003) 53(4):227–44. doi: 10.3322/canjclin.53.4.227
19. Azim HA Jr., Michiels S, Bedard PL, Singhal SK, Criscitiello C, Ignatiadis M, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res (2012) 18(5):1341–51. doi: 10.1158/1078-0432.ccr-11-2599
20. Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol (2017) 8:1960. doi: 10.3389/fimmu.2017.01960
21. Li Y, Yang D, Yin X, Zhang X, Huang J, Wu Y, et al. Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw Open (2020) 3(1):e1918160. doi: 10.1001/jamanetworkopen.2019.18160
22. Cosio MG, Cazzuffi R, Saetta M. Is chronic obstructive pulmonary disease a disease of aging? Respiration; Int Rev Thorac Dis (2014) 87(6):508–12. doi: 10.1159/000360770
23. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science (2018) 361(6409):eaao4227. doi: 10.1126/science.aao4227
24. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature (2015) 528(7582):413–7. doi: 10.1038/nature16140
25. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol (2010) 28(20):3271–7. doi: 10.1200/jco.2009.25.9820
26. Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol (2015) 4:33. doi: 10.1186/s40164-015-0028-8
27. Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, et al. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol (Royal Coll Radiol (Great Britain)) (2004) 16(5):345–9. doi: 10.1016/j.clon.2004.03.012
28. Koniali L, Hadjisavvas A, Constantinidou A, Christodoulou K, Christou Y, Demetriou C, et al. Risk factors for breast cancer brain metastases: A systematic review. Oncotarget (2020) 11(6):650–69. doi: 10.18632/oncotarget.27453
29. Arvold ND, Oh KS, Niemierko A, Taghian AG, Lin NU, Abi-Raad RF, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat (2012) 136(1):153–60. doi: 10.1007/s10549-012-2243-x
30. Song Y, Barry WT, Seah DS, Tung NM, Garber JE, Lin NU. Patterns of recurrence and metastasis in brca1/brca2-associated breast cancers. Cancer (2020) 126(2):271–80. doi: 10.1002/cncr.32540
31. Fredholm H, Magnusson K, Lindström LS, Garmo H, Fält SE, Lindman H, et al. Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Res Treat (2016) 160(1):131–43. doi: 10.1007/s10549-016-3983-9
32. Vetter M, Huang DJ, Bosshard G, Güth U. Breast cancer in women 80 years of age and older: A comprehensive analysis of an underreported entity. Acta Oncol (Stockholm Sweden) (2013) 52(1):57–65. doi: 10.3109/0284186x.2012.731523
33. Wang J, Yang S-P, Zhou P, Lian C-L, Lei J, Hua L, et al. Additional radiotherapy to breast-conserving surgery is an optional treatment for de novo stage iv breast cancer: A population-based analysis. Cancer Med (2021) 10(5):1634–43. doi: 10.1002/cam4.3751
34. Wu SG, Zhang WW, Sun JY, Li FY, Lin HX, Zhou J, et al. The survival benefits of local surgery in stage iv breast cancer are not affected by breast cancer subtypes: A population-based analysis. Oncotarget (2017) 8(40):67851–60. doi: 10.18632/oncotarget.18889
Keywords: de novo metastatic breast cancer, age, overall survival, breast cancer-specific survival, SEER
Citation: Xiao Q, Zhang W, Jing J, Zhong T, Li D, Zhou J, Liu P, Duan Z, Gao H and Shen L (2023) Patterns of de novo metastasis and survival outcomes by age in breast cancer patients: a SEER population-based study. Front. Endocrinol. 14:1184895. doi: 10.3389/fendo.2023.1184895
Received: 12 March 2023; Accepted: 17 October 2023;
Published: 06 November 2023.
Edited by:
Haoji Hu, Zhejiang Chinese Medical University, ChinaReviewed by:
Chang Ik Yoon, The Catholic University of Korea, Republic of KoreaCopyright © 2023 Xiao, Zhang, Jing, Zhong, Li, Zhou, Liu, Duan, Gao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyuan Shen, c2hlbmxpeXVhbjExMkBzaW5hLmNvbQ==; Han Gao, MTUyMjMzMjMxMjBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.