- 1Department of Nephrology, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Department of Rheumatology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 3Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Introduction: Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is characterized by systemic small-vessel vasculitis and may rarely present as central diabetes insipidus (CDI). In this study, we aimed to determine the clinical characteristics and prognosis of patients with AAV-associated CDI.
Methods: This was a nested case-control study where AAV patients with CDI at the Peking Union Medical College Hospital were followed from January 2012 to April 2022. Case-control matching with AAV patients without CDI was performed (1:5), and participants were matched by age, sex, and AAV classification. We collected clinical data every 3–6 months and conducted a literature review using PubMed to identify relevant articles published from 1983–2022.
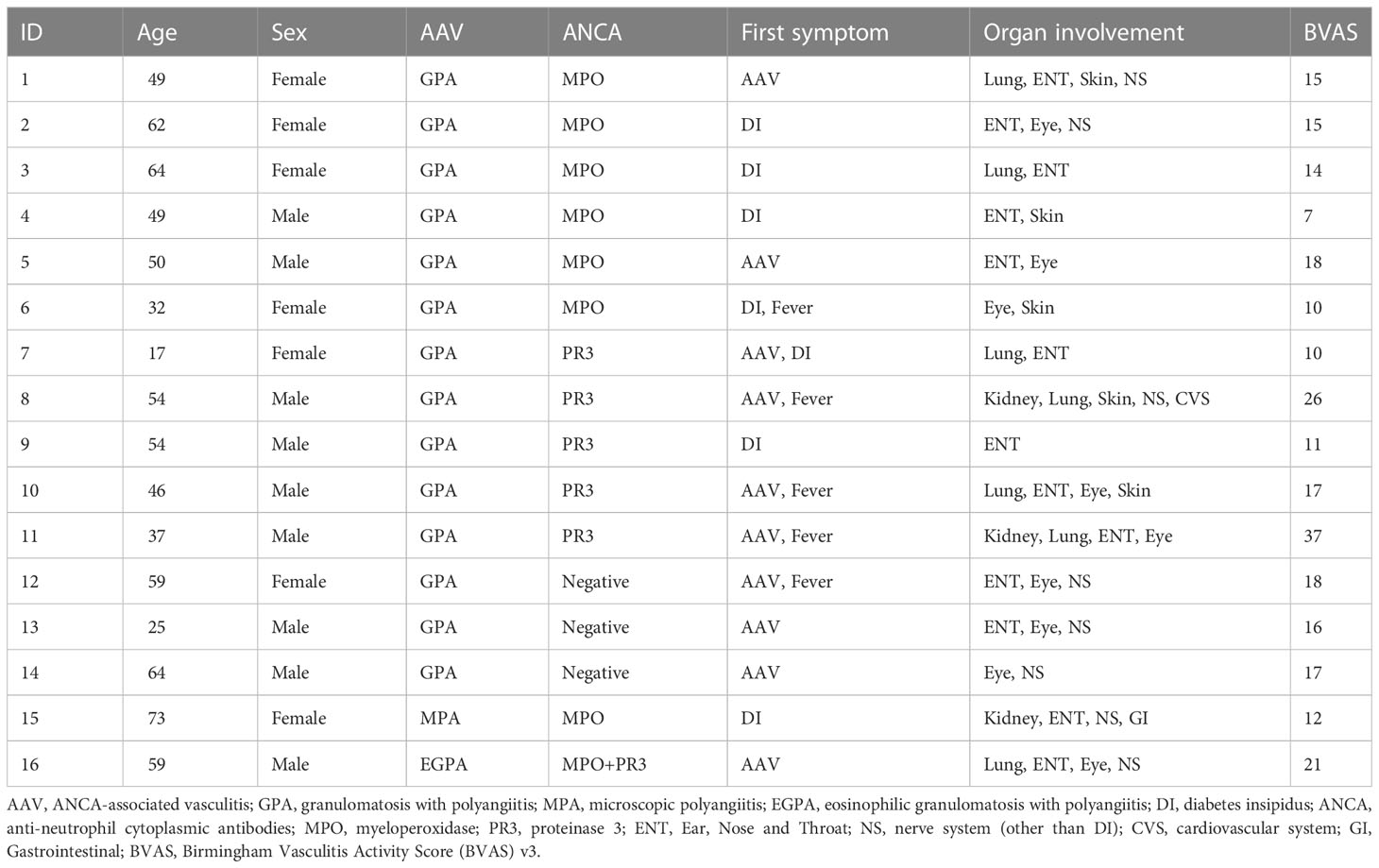
Results: Among 1203 hospitalized AAV patients, 16 patients with CDI were included (1.3%). The average age was 49 years, and men accounted for 56.3%. Granulomatosis with polyangiitis (GPA) accounted for 87.5% of patients. AAV patients with CDI had more ear, nose, and throat (ENT) (81.3%) involvement and less renal impairment than those in the control group (P<0.05). After a mean follow-up of four years, 50% of patients were in remission from AAV, 37.5% relapsed, and 12.5% died. Our literature review suggested that patients in Asian countries tend to be older men and have higher myeloperoxidase (MPO-ANCA) positivity than those in Western countries. Furthermore, proteinase 3 (PR3-ANCA) positivity may predict disease recurrence.
Discussion: AAV patients with CDI had more ENT involvement and a higher eGFR. MPO-ANCA positivity is more commonly observed in Asian countries than Western countries, and PR3-ANCA positivity may predict recurrence.
Introduction
Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) is a group of disorders characterized by severe systemic small-vessel vasculitis, marked by alveolar hemorrhage and rapidly progressive pulmonary glomerulonephritis (1). AAV includes three subgroups, namely microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA).
Central diabetes insipidus (CDI) is a rare condition associated with AAV. CDI may precede or follow the diagnosis of AAV (2, 3). The incidence rate of CDI in AAV patients is reported to be approximately 1.3–3.9% by case series and retrospective studies (3–5). The occurrence of DI in patients with AAV might be explained by vasculitis affecting the pituitary vasculature, involvement of the adjacent pituitary gland by granulomatous masses originating from the ear, nose and throat (ENT); and primary granulomatous inflammation (6). Symptoms of CDI can vary from multiple cranial nerve palsies (7) to anterior pituitary dysfunction (8). AAV-associated CDI should be recognized to avoid unnecessary biopsies of the pituitary gland and minimize the risk of irreversible loss of pituitary function.
Previous case reports and series on AAV-associated CDI have no prolonged follow-up and have not delineated the risk factors to predict prognosis. Furthermore, population differences between Asian and Western countries have not been fully elucidated. In this retrospective nested case-control study, we investigated the association between CDI and AAV, considering the disease burden, disease control situation, long-term follow-up, and prognosis. In addition, we analyzed the genetic and clinical characteristics of these patients in China and Western countries by integrating clinical data and conducting a comparative analysis.
Materials and methods
This was a nested case-control study where AAV patients with CDI at Peking Union Medical College Hospital were followed every 3–6 months from January 2012 to April 2022. This group was matched to a control group of AAV patients without CDI according to a 1:5 ratio. AAV relapse was defined as new evidence of systemic activity, increased inflammatory markers, or re-increase in ANCA titers. Survival analysis was used to investigate the risk factors of disease recurrence in AAV patients with CDI.
Patients
We enrolled hospitalized AAV patients with CDI at Peking Union Medical College Hospital and followed them from January 2012 to April 2022. The inclusion criteria included a diagnosis of AAV according to the definition by the 2012 revised International Chapel Hill Consensus Conference (9) and a diagnosis of CDI confirmed by the fluid deprivation-vasopressin test. Cases of CDI associated with causes other than AAV, such as tumors or infection, were excluded. Case-control matching with AAV patients without CDI was performed (1:5). Patients were matched according to age and sex by Model 1 and age, sex and AAV classification by Model 2. The study was a retrospective study, and the ethics committee agreed to waive the informed consent. All procedures and laboratory tests were performed according to the national ethical guidelines and approved by the Standing Committee for Clinical Studies at Peking Union Medical College Hospital (No. JS 3527)
Definitions
The definitions in the inclusion criteria for AAV patients with CDI were as follows: 1) AAV was defined and classified as granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), or eosinophilic granulomatosis with polyangiitis (EGPA) according to the 2012 revised International Chapel Hill Consensus Conference (9); 2) Polyuria was defined as, assessed by a 24 h urine volume of more than 2.5 L; 3) CDI was defined as polyuria with a positive fluid deprivation test (10).
Hypopituitarism refers to a partial or complete failure of secretion of anterior pituitary hormones and includes the following entities: hypogonadotropic hypogonadism (low levels of serum testosterone or estradiol and low or inappropriately normal luteinizing and follicle-stimulating hormone levels), secondary hypothyroidism (low serum thyroxine level and low or inappropriately normal thyroid stimulating hormone deficiency) and secondary hypocortisolism (low morning (0800 h) cortisol and adrenocorticotropic hormone (ACTH) levels).
Laboratory analyses and radiological assessments
Baseline clinical characteristics such as age, sex, symptoms, system involvement, AAV classification, laboratory results, and magnetic resonance imaging (MRI) were recorded as part of the data collection. Laboratory tests, including blood and urine tests, were performed on the day of admission. ANCA titers were assessed using an indirect immunofluorescence assay and an antigen-specific enzyme-linked immunosorbent assay (ELISA). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (11). Pituitary enhanced MRI was performed by radiologists, and any abnormalities were reported. The images were evaluated for posterior hyperintense signals, pituitary microadenomas, space-occupying lesions in the posterior lobe, pituitary stalk thickening, and hypertrophic cranial pachymeningitis.
Treatment and prognosis
We recorded the type of therapy each participant received, including hormone replacement therapy, induction therapy, and maintenance treatment for vasculitis. Clinical characteristics and laboratory indicators were followed up every 3–6 months, and structural and functional assessments of the pituitary gland were recorded. In terms of prognosis, the state of improvement for patients with AAV-associated CDI was defined as a Birmingham Vasculitis Activity Score (BVAS) ≤ 2 after treatment. Recurrence was defined as new evidence of systemic activity, increased inflammatory markers, or re-increase in ANCA levels. The resolved CDI was defined as not requiring the use of desmopressin acetate tablets (DDAVP), improved CDI as reducing the amount of DDAVP and persistent CDI as reliance on DDAVP.
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows (ver. 22, IBM Corp.). Continuous variables were expressed as means with standard deviations or medians with interquartile ranges, as appropriate. Categorical variables were expressed as proportions. One-way analysis of variance (ANOVA) was used to compare the means of more than two groups. Survival analysis was performed to investigate the risk factors for recurrence in patients with AAV-associated CDI. A two-tailed test was used, and statistical significance was set at P < 0.05.
Literature review
We conducted a systematic literature review using PubMed throughout November, 2022. We used medical subject headings and free-text search terms for our search, which included a manual search for references to existing reviews in the field. The inclusion criteria were as follows: 1) a diagnosis of GPA according to the 2012 revised International Chapel Hill Consensus Conference; 2) a diagnosis of CDI assessed by the 24 h urine output and confirmed by a positive fluid deprivation-vasopressin test; and 3) cases with well-documented clinical information. Patients with secondary vasculitis or other autoimmune diseases were excluded from this study.
Results
Baseline clinical characteristics
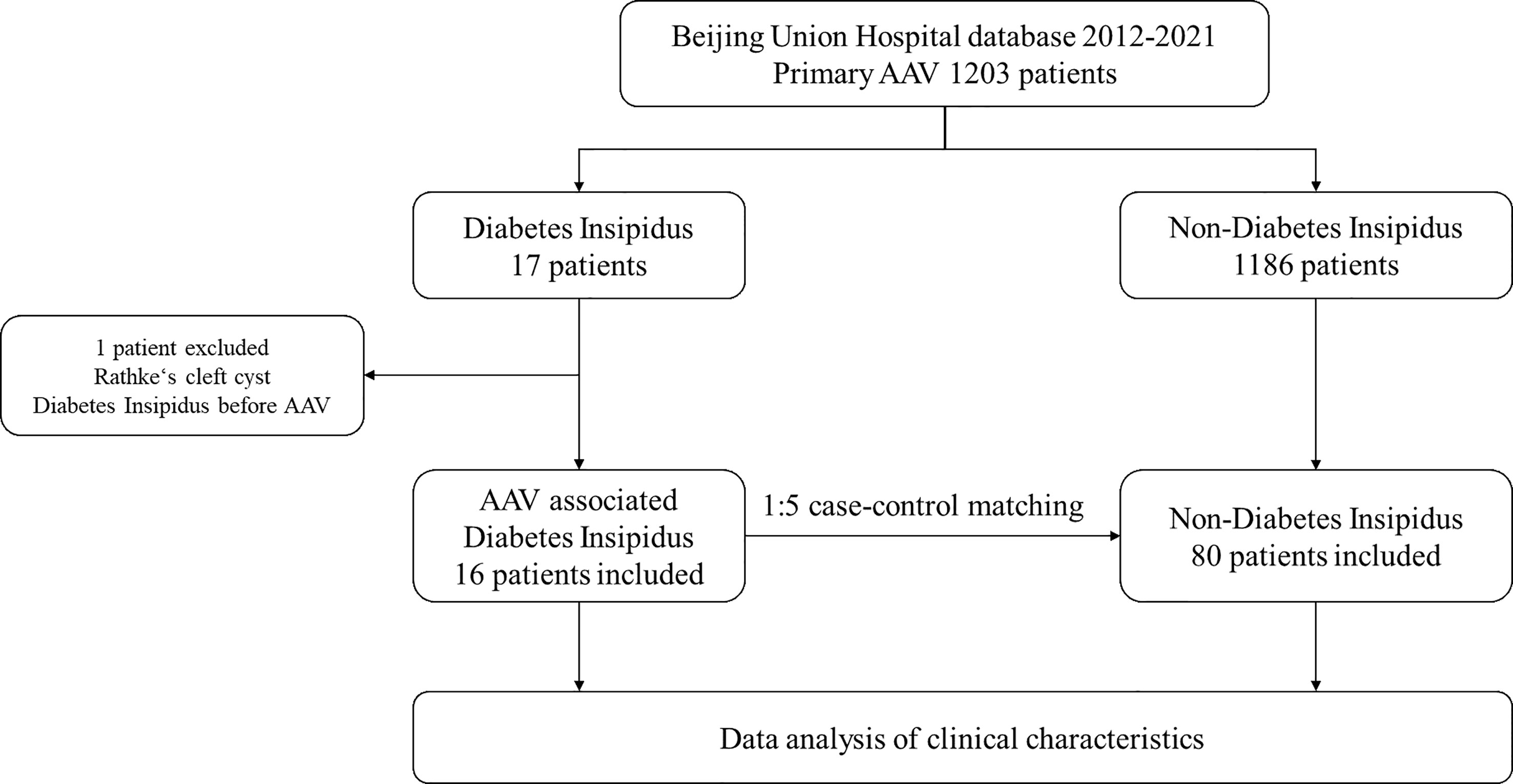
A total of 1203 patients were included in the study, among which 16 (1.3%) had AAV-associated CDI (Figure 1). The average age was 49 years, and men accounted for 56.3%. The average urine output was 7 ± 2 L/24h. GPA accounted for 87.5% of patients, while MPA and EGPA accounted for 6.25% each. Diabetes insipidus was the initial presentation in 50% of patients that sought medical attention. Organ involvement included the ENT (81.3%), eye (62.5%), nervous system (other than CDI) (50%), lung (43.8%), skin (31.3%), and kidney (18.8%) (Table 1).
Endocrinological assessment revealed hypopituitarism in 56.3% of patients, including hypogonadotropic hypogonadism in 25.0% and secondary hypothyroidism in 50.0%. Hypocortisolism, growth hormone deficiency, and hyperprolactinemia were present in 28.6%, 9%, and 44.4% of patients, respectively. MRI abnormalities were observed in 93.7% of patients. Almost all patients lacked posterior hyperintense signals; approximately one-third of them had space-occupying lesions (posterior pituitary occupancy), and one-fifth had pituitary stalk thickening. One patient had done pituitary biopsy with granulomatous inflammation. Follow-up pituitary MRI revealed that the pituitary lesions in seven patients had shrunk after treatment. (Table S1–S3).
Treatment and follow-up
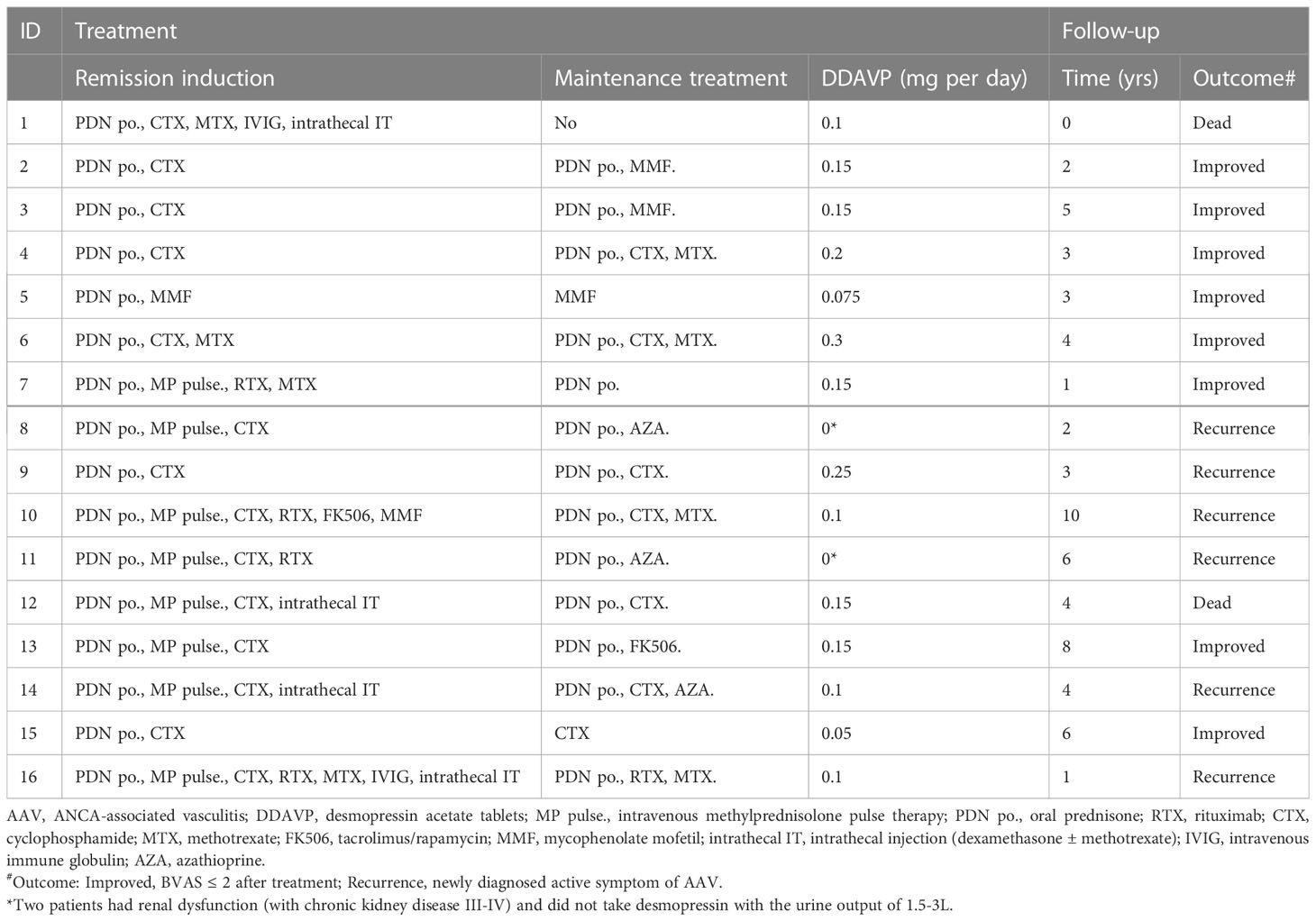
All patients were treated with oral prednisone (0.8–1 mg/kg/day), and 50% were administered intravenous methylprednisolone pulse therapy. Immunosuppressants used for inducing remission included cyclophosphamide (CTX, 87.5%), rituximab (RTX, 25%), methotrexate (MTX, 25%), and mycophenolate mofetil (MMF, 12.5%). Maintenance treatment included oral prednisone (81.3%), CTX (43.8%), MTX (25%), MMF (18.75%), azathioprine (AZA) (18.75%), and RTX (6.25%). Over the following 4.14 ± 2.63 years, eight patients showed improvement (50%), six suffered from recurrence (37.5%), and two died (12.5%) from chronic heart failure and infection (Table 2).
Almost all patients were administered desmopressin acetate tablets (87.5%), except for those with chronic renal disease who are at risk of substantially decreased urine output with desmopressin. Among the patients treated with desmopressin, 70% showed improvement in polyuria and reduced reliance on hormonal replacement therapy. Imaging follow up was performed in seven out of the sixteen patients. Patient 9 demonstrated complete recovery with improvement in imaging and no longer required desmopressin (Figure 2). Imaging revealed improvement with shrinkage of the lesions in two patients. The time span between the start of AAV treatment and improvement of CDI was 28 ± 15 days. Approximately one-fifth of patients with hypogonadotropic hypogonadism showed improvement without the need for hormonal replacement therapy.
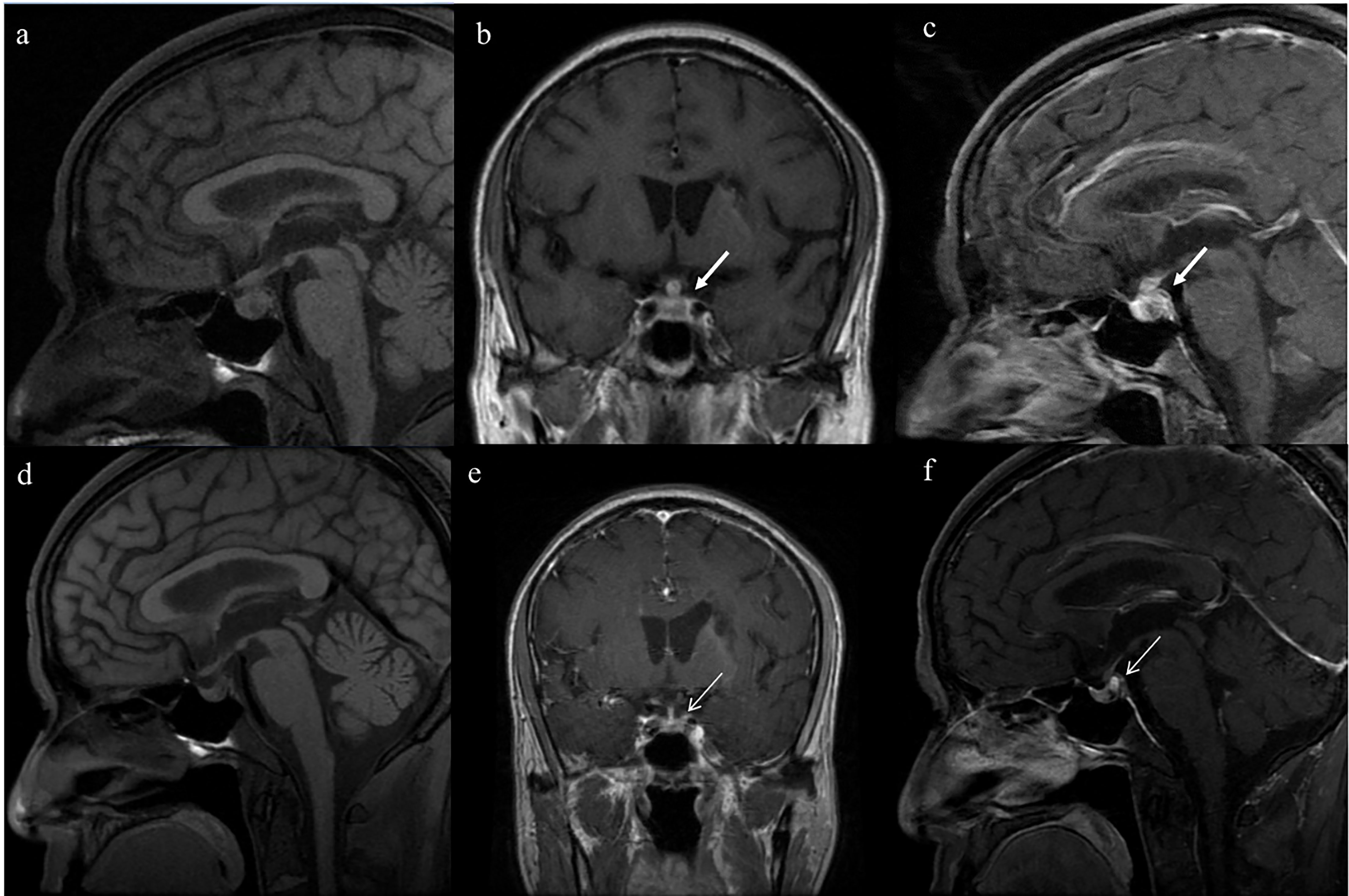
Figure 2 Serial magnetic resonance imaging (MRI) of the pituitary in patient No.9, who fully recovered from diabetes insipidus. Enlargement of the posterior pituitary lobe (5.7mm×7.8mm) and thickening pituitary stalk (transverse diameter 5.3mm) on T1-weighted images (Panels A–C; thick white arrow). After the treatment, the enlargement of the posterior pituitary shrank(3.8mm×4.5mm) and the normalization of the pituitary stalk (transverse diameter 2.3mm) (Panels D–F thin white arrow).
Comparison of patients with GPA-associated CDI between our cohort and the literature review
Among the 14 patients with GPA-associated CDI in our cohort, 42.9% had positive myeloperoxidase (MPO-ANCA), 35.7% had positive proteinase 3 (PR3-ANCA), and 21.4% had negative serologies (Table S4). The BVAS score was higher in patients with positive PR3-ANCA who received more aggressive treatment, including intravenous methylprednisolone pulse therapy (80%, P=0.005), CTX (80%, P=0.719), and RTX (60%, P=0.024). However, the outcome was not optimistic for patients with positive PR3-ANCA; these patients had a higher recurrence rate than patients with positive MPO-ANCA or negative serologies (80% vs. 0% vs. 33.3%, P=0.022).
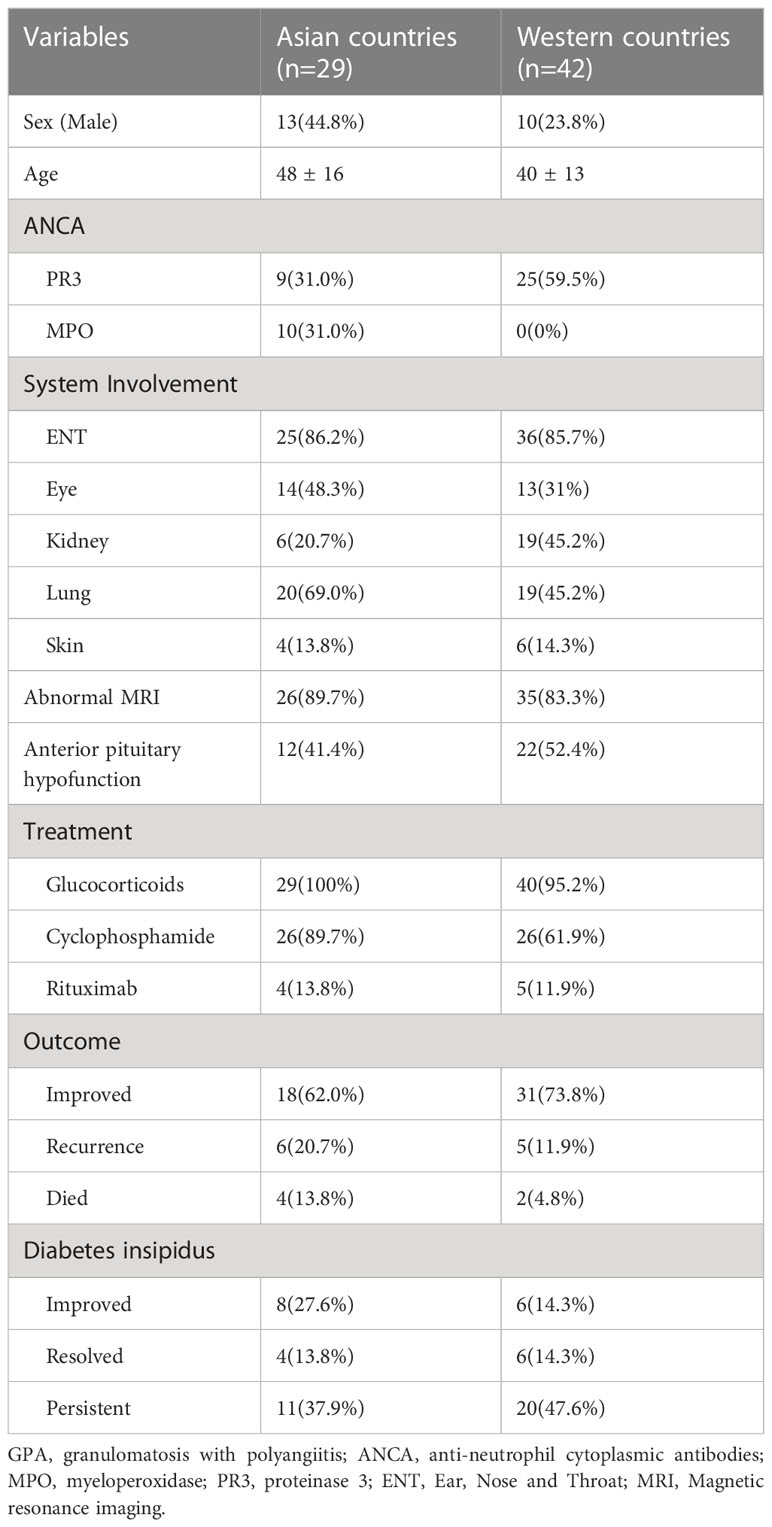
We studied 71 patients from former case reports, case series and retrospective studies (2–5, 12–38) (Table 3), 29 of whom were Asian and 42 Western. By integrating our cohort and published data on PUMCH (six patients had been included in previous studies) (3, 39), we included 20 patients from 2000 to 2022. Asian patients were older (48 ± 16 vs. 40 ± 13 years) and had a higher MPO-ANCA positivity (31% vs. 0%) than Western patients. There was no significant difference between the two groups in terms of systemic involvement. Glucocorticoids were administered to all Asian patients (100%) and most Western patients (95.2%). CTX administration was higher in Asian countries (89.7% vs. 61.9%); however, the difference was not statistically significant. Over 50% of the patients showed improvement after treatment, approximately one-sixth relapsed, and one-tenth died. Approximately half of the patients had persistent CDI, one-fifth improved with the decreased need for desmopressin replacement therapy, and one-tenth persistently required full doses.
Difference between AAV with and without CDI
The critical difference between AAV patients with and without CDI matched by sex and age by Model 1 was AAV classification (Table S5). GPA was more common in patients with AAV-associated CDI (87.6% vs. 33.8%), whereas MPA was more common in patients with AAV without CDI (6.3% vs. 38.8%, P=0.007). After adjustment for sex, age, and AAV classification by Model 2, patients with AAV-associated CDI had more ENT and eye involvement (81.3% vs. 35%, P<0.001; 62.5% vs. 11.3%, P<0.001, respectively) and less renal involvement (18.8% vs. 63.3%, P<0.001) than those in the non-CDI group. The CDI group had a lower U-RBC value (2.8 vs. 13.5 Cells/μL, P=0.014) and a higher eGFR (121.8 vs. 73.7 ml/min/1.73 m2, P=0.029) than the non-CDI group. There were no statistically significant differences in treatment or prognosis between the two groups.
Risk factors for death and recurrence
No risk factors between the death and survival groups were identified in patients with AAV-associated CDI. Among six patients who suffered from AAV recurrence, and half of them had the recurrence of CDI. Kaplan–Meier curves suggested that CDI was not associated with AAV recurrence (P=0.471), whereas PR3-ANCA positivity was a risk factor for effectively predicting recurrence in patients with AAV-associated CDI (P=0.025) (Figure 3).
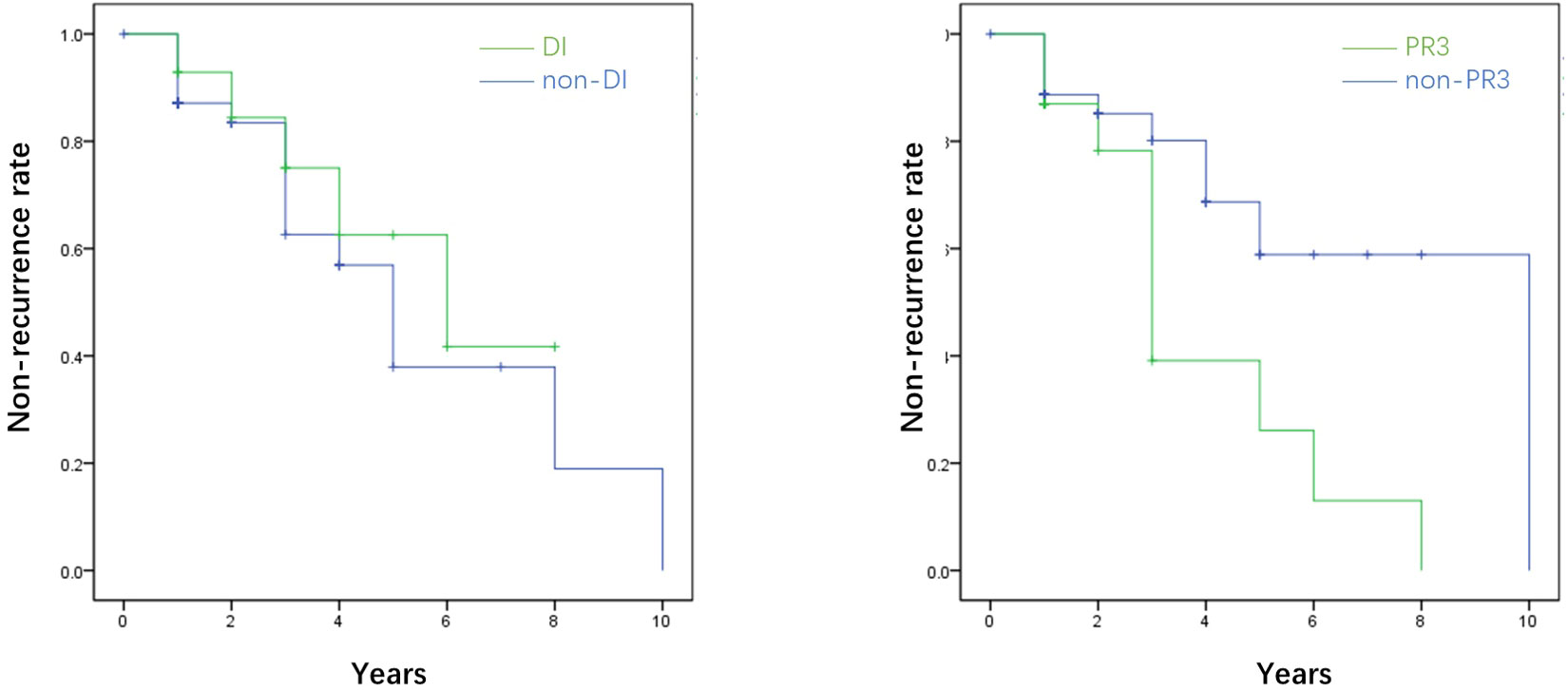
Figure 3 The survival analysis of the recurrence rate of AAV in terms of diabetes insipidus (DI) and proteinase 3 (PR3). The survival analysis showed that diabetes insipidus was not associated with the recurrence of AAV (P=0.471), while PR3 was the promising risk factor for predicting the recurrence of AAV (P=0.025). *AAV, ANCA-associated.
Discussion
We reported 16 cases of AAV-associated CDI and reviewed 71 previously reported cases. Over half of the patients had complete or partial remission with steroid treatment augmented with cyclophosphamide or rituximab. MPO-ANCA positivity was more common in Asian countries, whereas PR3-ANCA positivity prevailed in Western countries. PR3-ANCA positivity indicated a higher disease activity with a higher BVAS score, resulting in a poorer prognosis.
In our cohort, GPA accounted for 87.5% of patients with AAV-associated CDI. The incidence of pituitary involvement with GPA was 3.9% (3). There were a few cases of pituitary involvement with MPA or EGPA (12.5%). Hypertrophic pachymeningitis and CDI were associated with PR3-ANCA or MPO-ANCA positivity (7, 40); however, the detailed mechanisms of these relationships remain unclear.
CDI was present in 4% of the 357 patients with GPA in our cohort. This was higher than in the cohort of Mayo Clinic (5) (1.3%, Table S6), which were similar in terms of percentages for sex and age, therapeutic strategies, remission rates (87.5% vs. 50%, P=0.079); however, PR3-ANCA-positivity was relatively lesser (87.5% vs. 35.7%, P=0.019). Additionally, the complete resolution rate of CDI (meaning no longer requiring replacement therapy) was significantly higher in the Mayo Clinic (66.6% vs. 10%, P=0.018). The difference of remission rates between Mayo Clinic and PUMCH may be attributed to racial differences in foreign and Asian population; however, it is difficult to explain the low remission rate in Western patients (14.7%) at 10.5 months of follow-up (21). The systemic disease was alleviated in AAV patients and showed improvement on pituitary imaging; however, recovery of pituitary function was rare (3, 39), which was consistent with our cohort.
To investigate racial differences between Asian and Western countries, we summarized a literature review from previous studies on GPA-associated CDI (2–5, 12–38) (Table 3). Asian countries included China, Japan and India, and Western countries included America, Britain, the Netherlands, Canada, Turkey, France, Australia and Italy. MPO-ANCA positivity was more common in Asian countries, whereas PR3-ANCA positivity prevailed in Western countries. Even in AAV patients without CDI, MPO-ANCA is more common in southern regions of Europe and Asia, whereas PR3-ANCA is more common in northern parts of the world (41). Our data indicated that patients with GPA-associated CDI with PR3-ANCA positivity had higher BVAS scores and predicted risk of relapse even after treatment, which was also supported by the RAVE trial (42). Genome-wide association studies (GWAS) in European countries indicated that the different genetic backgrounds between patients with PR3-ANCA-positive and MPO-ANCA-positive vasculitis, which were dominant in HLA-DP and HLA-DQ variants, respectively (43). Further genomic studies on the Asian population may help elucidate the population-specific differences between Asian and Western countries.
This study has a few limitations. First, this was a single-center retrospective study which limits the external validity of the findings. Additionally, the population included in our study is hospitalized patients rather than outpatients with relatively more severe clinical condition. Moreover, AAV-associated CDI is a rare condition; therefore, prolonged follow-up periods and larger cohorts are required to illustrate the role of PR3-ANCA positivity as a risk factor for predicting recurrence.
Conclusion
Our findings from the nested case-control study and comparison between the Asian and Western populations revealed that patients with GPA-associated CDI in Asia tend to be older, have more ENT involvement, and have less renal impairment. MPO-ANCA positivity was more in Asian patients than in patients in Western countries, whereas PR3-ANCA positivity predicted a higher recurrence rate among these patients. Further genomic studies are required to illustrate the population differences between Asian and Western countries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Peking Union Medical College Hospital (No. JS 3527). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XC and SZ were responsible for the conception and design of the study. XC drafted the manuscript. XP, XS, and HW were responsible for data acquisition and analysis. YW, XT and YQ provided the patients and participated in manuscript revision. HZ and LC read and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by grants from the National Key R&D Program of China (2022ZD0116003 to LC), National Natural Scientific Foundation of China (82170709, 81970607 to LC), CAMS Innovation Fund for Medical Sciences (CIFMS 2020-I2M-C&T-A-001, CIFMS 2021-I2M-1-003 to LC), Capital’s Funds for Health Improvement and Research (CFH 2020-2-4018 to LC); Beijing Natural Science Foundation (L202035 to LC); National High Level Hospital Clinical Research Funding (2022-PUMCH-B-019 to LC); the Capital Exemplary Research Wards Project (BCRW202001 to LC); and Fundamental Research Funds for the Central Universities (3332022109 to XC). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1173903/full#supplementary-material
References
1. Kitching AR, Anders H-J, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nat Rev Dis Primers (2020) 6(1):71. doi: 10.1038/s41572-020-0204-y
2. Düzgün N, Morris Y, Güllü S, Gürsoy A, Ensari A, Kumbasar OO, et al. Diabetes insipidus presentation before renal and pulmonary features in a patient with wegener's granulomatosis. Rheumatol Int (2005) 26(1):80–2. doi: 10.1007/s00296-005-0583-0
3. Gu Y, Sun X, Peng M, Zhang T, Shi J, Mao J. Pituitary involvement in patients with granulomatosis with polyangiitis: case series and literature review. Rheumatol Int (2019) 39(8):1467–76. doi: 10.1007/s00296-019-04338-0
4. Li C, Zou Y, Lu X, Wang G, Shu X. Pituitary dysfunction in patients with ANCA associated vasculitis: prevalence, presentation, and outcomes. Ther Adv chronic disease (2020) 11:2040622320930636. doi: 10.1177/2040622320930636
5. Kapoor E, Cartin-Ceba R, Specks U, Leavitt J, Erickson B, Erickson D. Pituitary dysfunction in granulomatosis with polyangiitis: the mayo clinic experience. J Clin Endocrinol Metab (2014) 99(11):3988–94. doi: 10.1210/jc.2014-1962
6. Ohashi K, Morishita M, Watanabe H, Sada KE, Katsuyama T, Miyawaki Y, et al. Central diabetes insipidus in refractory antineutrophil cytoplasmic antibody-associated vasculitis. Internal Med (Tokyo Japan) (2017) 56(21):2943–8. doi: 10.2169/internalmedicine.8683-16
7. Takuma H, Shimada H, Inoue Y, Ishimura E, Himuro K, Miki T, et al. Hypertrophic pachymeningitis with anti-neutrophil cytoplasmic antibody (p-ANCA), and diabetes insipidus. Acta neurologica Scandinavica (2001) 104(6):397–401. doi: 10.1034/j.1600-0404.2001.00056.x
8. Garovic VD, Clarke BL, Chilson TS, Specks U. Diabetes insipidus and anterior pituitary insufficiency as presenting features of wegener's granulomatosis. Am J Kidney diseases (2001) 37(1):E5. doi: 10.1016/s0272-6386(01)90002-2
9. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis rheumatism (2012) 65(1):1–11. doi: 10.1002/art.37715
10. Garrahy A, Moran C, Thompson CJ. Diagnosis and management of central diabetes insipidus in adults. Clin endocrinology (2019) 90(1):23–30. doi: 10.1111/cen.13866
11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
12. Miesen WM, Janssens EN, van Bommel EF. Diabetes insipidus as the presenting symptom of wegener's granulomatosis. Nephrology dialysis transplantation (1999) 14(2):426–9. doi: 10.1093/ndt/14.2.426
13. Goyal M, Kucharczyk W, Keystone E. Granulomatous hypophysitis due to wegener's granulomatosis. AJNR Am J neuroradiology (2000) 21(8):1466–9.
14. Woywodt A, Knoblauch H, Kettritz R, Schneider W, Göbel U. Sudden death and wegener's granulomatosis of the pituitary. Scandinavian J Rheumatol (2000) 29(4):264–6. doi: 10.1080/030097400750041433
15. Tao J, Dong Y. Pituitary involvement in wegener's granulomatosis: a case report and review of the literature. Chin Med J (2003) 116(11):1785–8.
16. Dutta P, Hayatbhat M, Bhansali A, Bambery P, Kakar N. Wegener's granulomatosis presenting as diabetes insipidus. Exp Clin Endocrinol diabetes (2006) 114(9):533–6. doi: 10.1055/s-2006-924122
17. Seror R, Mahr A, Ramanoelina J, Pagnoux C, Cohen P, Guillevin L. Central nervous system involvement in wegener granulomatosis. Chin Med J (2006) 85(1):53–65. doi: 10.1097/01.md.0000200166.90373.41
18. Spísek R, Kolouchová E, Jensovský J, Rusina R, Fendrych P, Plas J, et al. Combined CNS and pituitary involvement as a primary manifestation of wegener granulomatosis. Clin Rheumatol (2006) 25(5):739–42. doi: 10.1007/s10067-005-0065-5
19. McIntyre EA, Perros P. Fatal inflammatory hypophysitis. Pituitary (2007) 10(1):107–11. doi: 10.1007/s11102-007-0016-z
20. Thiryayi W, Donaldson MH, Border D, Tyagi A. An enhancing pituitary lesion in a young woman: a diagnostic dilemma. J Clin Neurosci Off J Neurosurgical Soc Australasia (2007) 14(3):286–8. doi: 10.1016/j.jocn.2005.12.005
21. Yong TY, Li JY, Amato L, Mahadevan K, Phillips PJ, Coates PS, et al. Pituitary involvement in wegener's granulomatosis. Pituitary (2008) 11(1):77–84. doi: 10.1007/s11102-007-0021-2
22. Xue J, Wang H, Wu H, Jin Q. Wegener's granulomatosis complicated by central diabetes insipidus and peripheral neutrophy with normal pituitary in a patient. Rheumatol Int (2009) 29(10):1213–7. doi: 10.1007/s00296-008-0774-6
23. Barlas NB, Hassan HH, Al Badr FB, Bilal A. Structural and functional involvement of pituitary gland in wegener's granulomatosis. Clin neuroradiology (2011) 21(1):31–3. doi: 10.1007/s00062-010-0037-2
24. Santoro SG, Guida AH, Furioso AE, Glikman P, Rogozinski AS. Panhypopituitarism due to wegener's granulomatosis. Arquivos brasileiros endocrinologia e metabologia (2011) 55(7):481–5. doi: 10.1590/s0004-27302011000700008
25. Tenorio Jimenez C, Montalvo Valdivieso A, López Gallardo G, McGowan B. Pituitary involvement in wegener's granulomatosis: unusual biochemical findings and severe malnutrition. BMJ Case Rep (2011) 2011:bcr0220113850. doi: 10.1136/bcr.02.2011.3850
26. Kara O, Demirel F, Acar BC, Cakar N. Wegener granulomatosis as an uncommon cause of panhypopituitarism in childhood. J Pediatr Endocrinol Metab JPEM (2013) 26(9-10):959–62. doi: 10.1515/jpem-2013-0033
27. Pereira EA, Plaha P, Hofer M, Karavitaki N, Cudlip SA. Hypophyseal wegener's granulomatosis presenting by visual field constriction without hypopituitarism. Clin Neurol neurosurgery (2013) 115(6):762–4. doi: 10.1016/j.clineuro.2012.06.041
28. Slabu H, Arnason T. Pituitary granulomatosis with polyangiitis. BMJ Case Rep (2013) 2013:bcr2013008656. doi: 10.1136/bcr-2013-008656
29. Al-Fakhouri A, Manadan A, Gan J, Sreih AG. Central diabetes insipidus as the presenting symptom of granulomatosis with polyangiitis. J Clin Rheumatol Pract Rep rheumatic musculoskeletal diseases (2014) 20(3):151–4. doi: 10.1097/rhu.0000000000000093
30. De Parisot A, Puéchal X, Langrand C, Raverot G, Gil H, Perard L, et al. Pituitary involvement in granulomatosis with polyangiitis: report of 9 patients and review of the literature. Medicine (2015) 94(16):e748. doi: 10.1097/md.0000000000000748
31. Yasuda K, Sainouchi M, Goto M, Murase N, Ohtani R, Nakamura M. [A case of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA)-associated hypertrophic pachymeningitis presenting with multiple cranial nerve palsies and diabetes insipidus]. Clin neurology (2016) 56(5):334–7. doi: 10.5692/clinicalneurol.cn-000859
32. Christou L, Thomas A, Dutta S, Shaw S, Jose B. Granulomatosis with polyangiitis presenting as a pituitary lesion. Br J Hosp Med (2017) 78(4):234. doi: 10.12968/hmed.2017.78.4.234
33. Esposito D, Trimpou P, Giugliano D, Dehlin M, Ragnarsson O. Pituitary dysfunction in granulomatosis with polyangiitis. Pituitary (2017) 20(5):594–601. doi: 10.1007/s11102-017-0811-0
34. Tsuji H, Yoshifuji H, Fujii T, Matsuo T, Nakashima R, Imura Y, et al. Visceral disseminated varicella zoster virus infection after rituximab treatment for granulomatosis with polyangiitis. Mod Rheumatol (2017) 27(1):155–61. doi: 10.3109/14397595.2014.948981
35. Peters JE, Gupta V, Saeed IT, Offiah C, Jawad ASM. Severe localised granulomatosis with polyangiitis (Wegener's granulomatosis) manifesting with extensive cranial nerve palsies and cranial diabetes insipidus: a case report and literature review. BMC neurology (2018) 18(1):59. doi: 10.1186/s12883-018-1058-8
36. Kocaer SB, Ozer E, Yilmaz E, Sari I. Hypophysis involvement in granulomatosis with polyangiitis. Arthritis Rheumatol (2019) 71(7):1124. doi: 10.1002/art.40873
37. Asakura K, Ogata H, Omatsu M, Yamamoto M, Yoshida K, Ito H. A case of nephrogenic diabetes insipidus likely caused by anti-neutrophil cytoplastic antibody-associated vasculitis. CEN Case Rep (2022). doi: 10.1007/s13730-022-00741-y
38. Koenen L, Elbelt U, Olze H, Zappe S, Dommerich S. Granulomatosis with polyangiitis in a patient with polydipsia, facial nerve paralysis, and severe otologic complaints: a case report and review of the literature. J Med Case Rep (2022) 16(1):291. doi: 10.1186/s13256-022-03492-7
39. Liu S, Xu Y, Li N, Chen S, Zhang S, Peng L, et al. Pituitary involvement in granulomatosis with polyangiitis: A retrospective analysis in a single chinese hospital and a literature review. Int J endocrinology (2019) 2019:2176878. doi: 10.1155/2019/2176878
40. Yasuda K, Sainouchi M, Goto M, Murase N, Ohtani R, Nakamura M. A case of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA)-associated hypertrophic pachymeningitis presenting with multiple cranial nerve palsies and diabetes insipidus. Clin neurology (2016) 56(5):334–7. doi: 10.5692/clinicalneurol.cn-000859
41. Hilhorst M, van Paassen P, Tervaert JW. Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol (2015) 26(10):2314–27. doi: 10.1681/asn.2014090903
42. Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol (2016) 68(7):1700–10. doi: 10.1002/art.39637
Keywords: antineutrophil cytoplasmic autoantibody-associated vasculitis, diabetes insipidus, recurrence rate, PR3-ANCA, MPO-ANCA
Citation: Chen X, Zhang S, Peng X, Shi X, Wu H, Wen Y, Qin Y, Tian X, Zhu H and Chen L (2023) Clinical characteristics and primary outcomes of patients with ANCA-associated vasculitis and central diabetes insipidus. Front. Endocrinol. 14:1173903. doi: 10.3389/fendo.2023.1173903
Received: 25 February 2023; Accepted: 13 April 2023;
Published: 12 May 2023.
Edited by:
Elena Varlamov, Oregon Health and Science University, United StatesReviewed by:
Dan Alexandru Niculescu, Carol Davila University of Medicine and Pharmacy, RomaniaAkira Shimatsu, Omi Medical Center, Japan
Stuti Fernandes, United States Department of Veterans Affairs, United States
Copyright © 2023 Chen, Zhang, Peng, Shi, Wu, Wen, Qin, Tian, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limeng Chen, Y2hlbmxpbWVuZ0BwdW1jaC5jbg==; Huijuan Zhu, c2hlbmd4aW4yMDA0QDE2My5jb20=
†These authors have contributed equally to this work
‡ORCID: Limeng Chen, orcid.org/0000-0002-8425-5742
 Xin Chen
Xin Chen Shuo Zhang
Shuo Zhang Xia Peng1
Xia Peng1 Yan Qin
Yan Qin Xinping Tian
Xinping Tian Huijuan Zhu
Huijuan Zhu Limeng Chen
Limeng Chen