
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 02 June 2023
Sec. Neuroendocrine Science
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1173449
This article is part of the Research Topic Endocrine Disruptors and Diseases of Brain and Mind: Past and Prelude View all 7 articles
 Feng-Shun Chen1
Feng-Shun Chen1 Chih-Cheng Chen1,2
Chih-Cheng Chen1,2 Ching-Chang Tsai3
Ching-Chang Tsai3 Jian-He Lu4
Jian-He Lu4 Huey-Ling You5
Huey-Ling You5 Ching-Mei Chen5
Ching-Mei Chen5 Wan-Ting Huang5
Wan-Ting Huang5 Kai-Fan Tsai6
Kai-Fan Tsai6 Fu-Jen Cheng7
Fu-Jen Cheng7 Chia-Te Kung7
Chia-Te Kung7 Shau-Hsuan Li8
Shau-Hsuan Li8 Chin-Chou Wang9
Chin-Chou Wang9 Yu-Che Ou3
Yu-Che Ou3 Wen-Chin Lee6
Wen-Chin Lee6 Yu-Ting Chang3
Yu-Ting Chang3 Fahimah Hashim10
Fahimah Hashim10 How-Ran Chao4,11*
How-Ran Chao4,11* Liang-Jen Wang12*
Liang-Jen Wang12*Background: Organophosphate flame retardants (OPFRs) are widely distributed in the environment and their metabolites are observed in urine, but little is known regarding OPFRs in a broad-spectrum young population from newborns to those aged 18 years.
Objectives: Investigate urinary levels of OPFRs and OPFR metabolites in Taiwanese infants, young children, schoolchildren, and adolescents within the general population.
Methods: Different age groups of subjects (n=136) were recruited from southern Taiwan to detect 10 OPFR metabolites in urine samples. Associations between urinary OPFRs and their corresponding metabolites and potential health status were also examined.
Results: The mean level of urinary Σ10 OPFR in this broad-spectrum young population is 2.25 μg/L (standard deviation (SD) of 1.91 μg/L). Σ10 OPFR metabolites in urine are 3.25 ± 2.84, 3.06 ± 2.21, 1.75 ± 1.10, and 2.32 ± 2.29 μg/L in the age groups comprising of newborns, 1-5 year-olds, 6-10 year-olds, and 11-18 year-olds, respectively, and borderline significant differences were found in the different age groups (p=0.125). The OPFR metabolites of TCEP, BCEP, DPHP, TBEP, DBEP, and BDCPP predominate in urine and comprise more than 90% of the total. TBEP was highly correlated with DBEP in this population (r=0.845, p<0.001). The estimated daily intake (EDI) of Σ5OPFRs (TDCPP, TCEP, TBEP, TNBP, and TPHP) was 2,230, 461, 130, and 184 ng/kg bw/day for newborns, 1-5 yr children, 6-10 yr children, and 11-17 yr adolescents, respectively. The EDI of Σ5OPFRs for newborns was 4.83-17.2 times higher than the other age groups. Urinary OPFR metabolites are significantly correlated with birth length and chest circumference in newborns.
Conclusion: To our knowledge, this is the first investigation of urinary OPFR metabolite levels in a broad-spectrum young population. There tended to be higher exposure rates in both newborns and pre-schoolers, though little is known about their exposure levels or factors leading to exposure in the young population. Further studies should clarify the exposure levels and factor relationships.
Organophosphate flame retardants (OPFRs), which are a group of semi-volatile organic compounds, are a class of endocrine-disrupting chemicals (EDCs). OPFRs have gained increasing attention due to their high usage following the phaseout and strict regulations on brominated flame retardants (BFRs), such as polybrominated diphenyl ethers (PBDEs). Following the banning or voluntary phaseout of BFRs in the European and American markets, OPFRs are now the primary flame retardant (FR) products or alternatives to BFRs (1, 2). OPFRs are thermally stable organic compounds with organic esters of phosphoric acid, including alkyl-chain, aryl-group, halogenated, or non-halogenated OPFRs (1). Since OPFRs are used in many housing materials and consumer products, their presence in indoor dust or air has been widely studied. Linked to the ubiquitous presence of these compounds in indoor environments and their potential for adverse health effects, there is increasing concern about human exposure to OPFRs, particularly for infants, toddlers, and young children. One of the major human exposure pathways for OPFRs is house dust ingestion, especially for infants, toddlers, and young children, who are at home for longer periods of time. Compared with adults, the young population is more sensitive to OPFRs.
The observed adverse effects of human exposure to OPFRs include disruption of normal endocrine and reproductive functions, neurodevelopment, and cancer (1, 3–5). Compared with BFRs, most scientists recognize that OPFRs have fewer adverse health effects, including cardiovascular, neurological, and reproductive toxicity, disruption of endocrine hormones, and carcinogenicity (6). OPFRs induce damaging changes in immunity, metabolism, genetics, and endocrine activity (1, 7). Prolonged exposure and the accumulation of organophosphate esters in the human body may elicit various adverse effects, including kidney toxicity, neurotoxicity, reproductive toxicity, carcinogenicity, and endocrine disruption (8–10). Chlorinated OPFRs, such as tris(1,3-dichloro-2-propyl) phosphate (TDCPP), tris(2-chloro-1-methylethyl) phosphate (TCPP), and tris(2-chloroethyl) phosphate (TCEP) have been shown to be neurotoxic and carcinogenic (11, 12). TDCIPP can easily enter the bloodstream, liver, kidneys, and testicles and can induce tumors (11). TDCIPP levels in house dust were associated with lower levels of male thyroid hormone and elevated levels of prolactin (13). In women with higher levels of urinary OPFR metabolites, there were significant decreases in fertilization and implantation rates, successful pregnancy, and live birth rates (14).
OPFRs are readily metabolized, and studies have found ubiquitous detection of OPFR metabolites in child and adult urine, suggesting that these metabolites are convenient biomarkers of OPFR exposure (15, 16). Urine as a non-invasive specimen may thus be a good indicator for estimating the human burden of OPFRs, especially since cord blood, blood, or breast milk are invasive and more difficult to obtain than urine. The half-lives of OPFRs and their metabolites were estimated in animal models to be relatively short (hours to days) due to the easy conversion of OPFRs to OPFR metabolites (3, 17–19). OPFR metabolites, including mono- or diesters, are primarily excreted in urine and other metabolic products via hydroxylation and conjugation were possibly excreted by the excretion pathway such as feces or expired air (7, 19). Most studies detect urinary OPFRs and OPFR metabolites because urinary OPFRs and OPFR metabolites are frequently used in biomonitoring surveys from various countries, including China, the US, Germany, Norway, Australia, Taiwan, and Japan (20–34).
Currently, there are few reports on OPFRs in indoor dust or PM2.5 and OPFR metabolites in children’s urine in Taiwan. This study seeks to survey urinary levels of OPFRs and OPFR metabolites in the young population from newborns to adolescents under 18 years old. The distribution of OPFRs and OPFR metabolites and their correlations between OPFRs and OPFR metabolites are also examined. Health risks are evaluated for the different age groups.
This report is part of a cohort study examining the adverse health effects for the young population after exposure to OPFRs. It establishes background levels of urinary OPFRs and OPFR metabolites for a broad-spectrum young population including newborns, toddlers, young children, school children, and adolescents under 18 years old in southern Taiwan. The studied subjects participated in a multidisciplinary pediatric care program between November 2020 and April 2022, with participants recruited from the pediatric outpatient clinics of Kaohsiung Chang Gung Memorial Hospital (KCGMH). This cohort study was approved by the Institutional Review Boards (IRB) of the KCGMH Human Ethical Committee and all ethical standards were followed as outlined in the Helsinki Declarations (IRB number: 202001028A3). All subjects, subjects’ parents, or subjects’ guardians read a detailed explanation of the possible consequences and then signed informed consent forms before the enrolment. The subjects were selected based on the following criteria: the children were age-matched to this study group and had no diagnosis of neurological or physiological disease.
The analytical methods to determine OPFRs and their metabolites in urine are described in a previous study (35). First-void morning urine samples were gathered in polypropylene plastic cups or urine collection bags (Deltalab, Barcelona, Spain) and after enrollment, the samples were transferred to 1.5 mL amber microcentrifuge tubes (ExtraGene, Taichung, Taiwan) stored at -80°C before chemical analysis. The 200-μL urine samples were mixed with 20 μL β-glucuronidase enzyme (Sigma-Aldrich, USA) at a PH value of 6.5 and spiked internal standards of isotope-labeled TDCPP and DNBP (Sigma-Aldrich, USA). The urinary solution was passed by solid-phase extraction (SPE) cartridge after a 2-hour incubation at 37 °C, and the SPE cartridge was conditioned with methanol (1 mL) and ultrapure water (1 mL). A mixture of 0.5% formic acid and 95% acetonitrile in 250 μL was added to the extract. Five OPFRs (TDCPP, TCEP, tris(2-butoxyethyl)phosphate (TBEP), tri-n-butyl phosphate (TNBP), and triphenyl phosphate (TPHP)) and five OPFR metabolites (Bis(1,3-dichloro-2-propyl) phosphate (BDCPP), Bis(chloroethyl) phosphate (BCEP), Di-(2-butoxyethyl) phosphate (DBEP), DNBP (Di-n-butyl phosphate), and Diphenyl phosphate (DPHP)) in urine were determined by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The elute was then analyzed by a Waters Acquity Ultra-Performance Liquid Chromatography coupled with a Waters Xevo TQ-XS mass spectrometer (Milford, USA) using the positive or negative electrospray ionization mode with the series columns of Waters Acquity UPLC BEH Phenyl column (2.1 mm × 50 mm, 1.7 μm particle size) and Waters XBridge BEH C18 Direct Connect HP isolated column (2.1 mm × 30 mm, 10 µm particle size) in KCGMH. The injection volume and flow rate of the column were 2 μL and 0.3 mL/min, respectively. The two mobile phases were solvent A (0.5% formic acid in water) and B (0.5% formic acid in methanol) and the gradient in UPLC-MS/MS was linearly decreased from 95% to 50% of solvent A within 0.75 minutes. Then, solvent A was gradient decreased from 50% to 0% in 3 minutes to maintain 100% solvent B for another 4.5 minutes. Finally, the gradient was distinctly increased to 95% solvent A. Mass spectrometry was equipped with an electrospray ionization probe interface with 3 and 2.6 kV for electrospray mode (ES) with positive (ES+) and negative (ES-) mode, respectively. Quality assurance and control (QA/QC) of this analytical method includes blanks (field and lab blanks), recovery tests, precision, limits of detection (LODs), and limits of quantification (LOQs). Recoveries of spiked internal labeled standards, surrogate, blank, and precision and recovery of the relevant standards were between 90.7 and 11.964% within the acceptable criteria (70-130%). LODs and LOQs were defined as the ratios of signal to noise (S/N) higher than 3 and 10, respectively. LOQs for TDCPP, TCEP, TBEP, TnBP, TPHP, BDCPP, BBOEP, DnBP, and DPHP were 0.02 μg/L, and LOQ of BCEP was 0.05 μg/L.
Estimated daily intake (EDI) of TDCPP, TCEP, TBEP, TNBP, and TPHP from BDCPP, BCEP, DBEP, DNBP, and DPHP excretion, the molar fraction is calculated by a fraction of OPFRs converted to OPFR metabolites and excreted in urine per day (36). EDIs of our subjects are estimated by the following equation with minor modification (26, 27, 33, 34):
where urinary Copfr metabolite (μg/L) is the mean value of an individual OPFR metabolite; UVexcre is defined as the daily excretion volume of urine (newborn: 0.3 L, 1-5 yr: 0.45 L, 6-10 yr: 0.7 L, and 11-18 yr: 1.2 L) (37); Fue is recognized as the molar fraction of OPFR metabolites in urinary excretion with the corresponding parent OPFRs (26, 27, 34); MWs are molecular weights of TDCPP, TCEP, TBEP, TNBP, and TPHP or their corresponding metabolites of BDCPP, BCEP, DBEP, DNBP, and DPHP. Children’s weights (kg) were 3.32, 15.2, 30.0, and 55.9 kg for newborns, 1-5 yr children, 6-10 yr school children, and 11-17 adolescents, respectively, according to statistics from the Taiwan Ministry of Health and Welfare.
Measurements of OPFRs and OPFR metabolites lower than LODs were set as zero for statistical analysis. The descriptive analysis determined the mean and standard deviations (SD) of urinary OPFRs and OPFR metabolites. Urinary OPFRs and OPFR metabolites did not fulfill the normal distribution to examine the bivariate correlations among OPFR and OPFR metabolite species by Spearman’s rho correlation coefficients tests. Mann-Whitney U and Kruskill-Wallis H non-parametric methods determined differences among age groups. All statistical analyses were performed using Statistical Product and Service Solutions, version 12.0.
This study is a urinary OPFRs survey research to measure urinary levels of OPFRs (TDCPP, TCEP, TBEP, TNBP, and TPHP) and OPFR metabolites (BDCPP, BCEP, DBEP, DNBP, and DPHP) in a broad-spectrum young population from southern Taiwan. The descriptive analysis of sex, height, weight, and BMI or Quetelet’s index is shown in Table 1. Table 1 also presents a descriptive analysis of the OPFRs and their metabolites in a sample of 135 participants from four different broad-spectrum young populations. The mean Σ10OPFR level for the sample was 2.25 μg/L, with a standard deviation (SD) of 1.91 μg/L, indicating that the data was widely dispersed as SD. The sample was diverse in terms of sex, with 71 male and 65 female participants. Newborns (3.25 ± 2.84 μg/L) did not have a significantly higher Σ10 OPFRs level in urine than young children aged 1-5 years (yrs) (3.06 ± 2.21), school children aged 6-11 yrs (1.75 ± 1.10 μg/L), and adolescents aged 12-17 yrs (2.32 ± 2.29 μg/L). Results showed that the highest levels of OPFRs were found in the newborns for the compounds TCEP, BCEP, TBEP, DBEP, and DPHP, with mean ± SD of 1.13 ± 1.51, 0.705 ± 1.00, 0.424 ± 0.933, 0.406 ± 1.32, and 0.395 ± 0.592 μg/L, respectively. Similarly, young children in the 1-5 yrs category may also be particularly vulnerable to OPFR exposure, with the highest levels in BCEP (0.680 ± 0.834), TBEP (0.634 ± 0.866), DPHP (0.628 ± 0.871), and DBEP (0.469 ± 0.752). Among the OPFRs measured in the 1-5 yrs children, the compounds DPHP, TBEP, BCEP, and TCEP had the highest levels of OPFRs, with mean values ranging from 0.290 to 0.461 μg/L (SD from 0.380 to 0.773 μg/L). Finally, for the 11-18 yrs adolescents, the compounds DPHP, BCEP, and BDCPP had the highest levels of OPFRs, with a mean between 0.514 to 0.585 μg/L (SD: 0.551-1.20 μg/L). TBEP and its metabolite, DBEP, showed significant and margin-significant differences between newborns (TBEP: 0.424 ± 0.933 μg/L; DBEP: 3.25 ± 2.84 μg/L) and adolescents (TBEP: 0.217 ± 0.292 μg/L, p=0.026; DBEP: 0.149 ± 0.214 μg/L, p=0.054), respectively. The study found that the group’s average height was 86.3 cm with a range of ± 43.2 cm, the average weight was 15.9 kg with a range of ± 17.7 kg, and the average BMI was 14.5 with a range of ± 3.56.
Figures 1A, B illustrate the percentage of OPFRs and OPFR metabolites found in children’s urine samples in Southern Taiwan. Overall, there were similar patterns for the age groups 1-5, 6-10, and 11-18 years, except for the newborns, which differed significantly. Studies indicated that the percentage of BDCPP in newborns was the lowest at only 1.64%, in contrast to 9.13% for young children aged 1-5 yrs, 9.42% for school children aged 6-10 yrs, and 22.2% for adolescents aged 11-18 yrs. Similarly, the percentage of DPHP was also the lowest in newborns at 12.1%, while the other categories ranged from 20.6 to 26.3%. However, for TCEP, newborns had the highest percentage at 34.7%, compared with the other groups, which were below 20% of the composition. Figure 1C shows the urinary levels of OPFRs and OPFR metabolites between male and female subjects. The concentrations of certain compositions, such as DBEP, DPHP, TDCPP, TCEP, TBEP, and TPHP, tend to be found more often in male subjects than female subjects. The graph shows that BCEP, TCEP, and TBEP had levels over 0.5 μg/L; whereas concentrations of DNBP, TDCPP, TNBP, and TPHP were lower, all being under 0.1 μg/L.
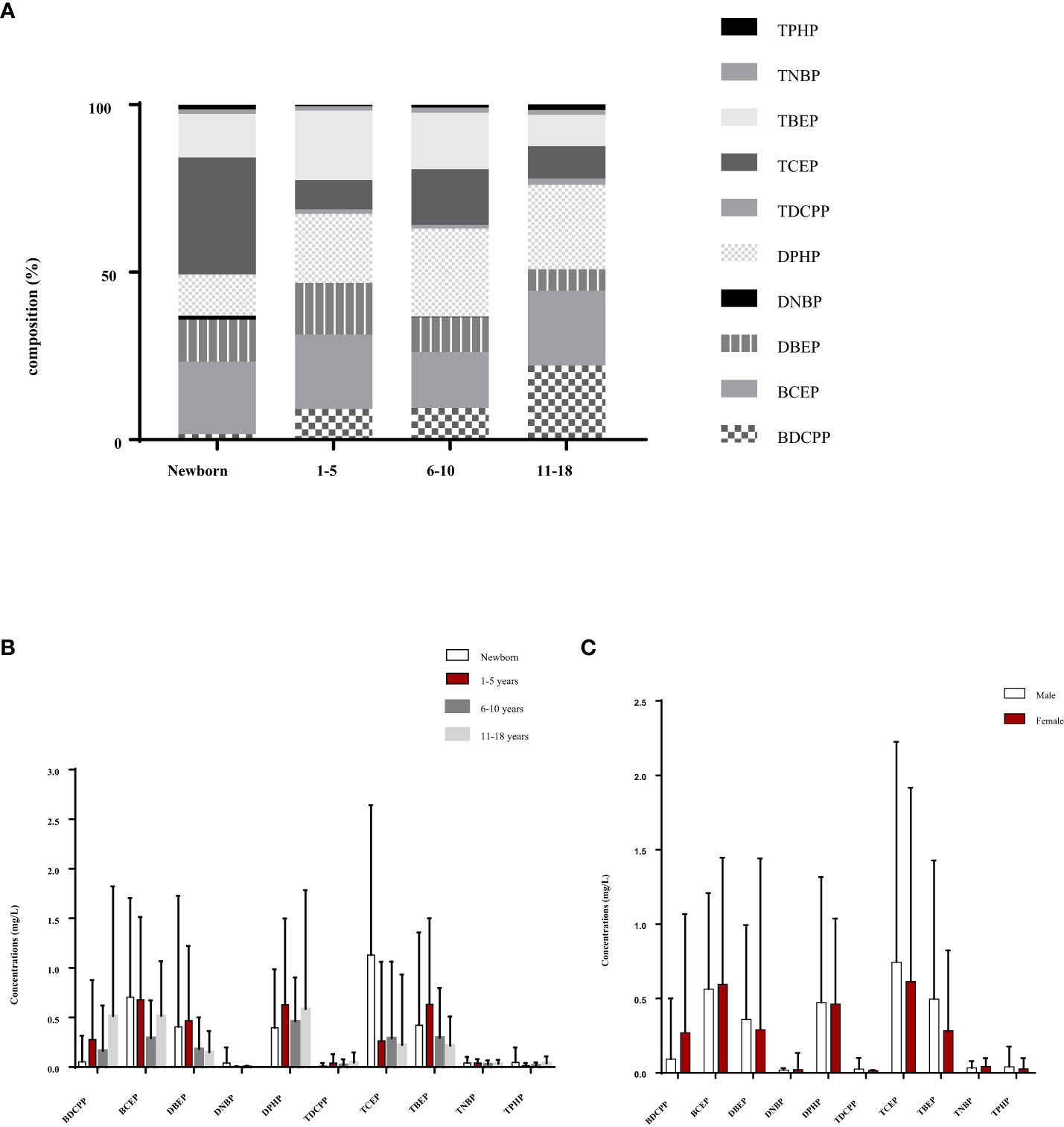
Figure 1 Urinary levels of OPFRs and OPFR metabolites for the young population in southern Taiwan are shown as (A) the percentage of OPFRs and OPFR metabolites in the young population’s urine, (B) distribution of urinary OPFR and OPFR metabolite levels in the different age groups and (C) urinary levels of OPFRs and OPFR metabolites for male and female subjects in the young population.
Table 2 shows Spearman’s rho correlation coefficient (r) between urinary OPFRs and OPFR metabolites in the newborn group of 0.816 (p < 0.001) between TBEP and DBEP, indicating a strong positive relationship between the two variables. There was also a strong positive correlation between DNBP and TNBP (r= 0.509, p <0.001), as well as between TDCPP and TPHP (r = 0.458, p <0.001) and between DBEP and DPHP (r=0.384, p=0.001). TNBP and TPHP showed a strong positive correlation (r=0.244, p=0.048), while TCEP and TPHP exhibited a strong negative correlation (r=-0.279, p=0.0230). Similarly, the relationship between BCEP and DNBP was positively strong (r=0.305, p=0.013), whereas the relationship between BCEP and TBEP was negatively strong (r=-0.365, p=0.003).
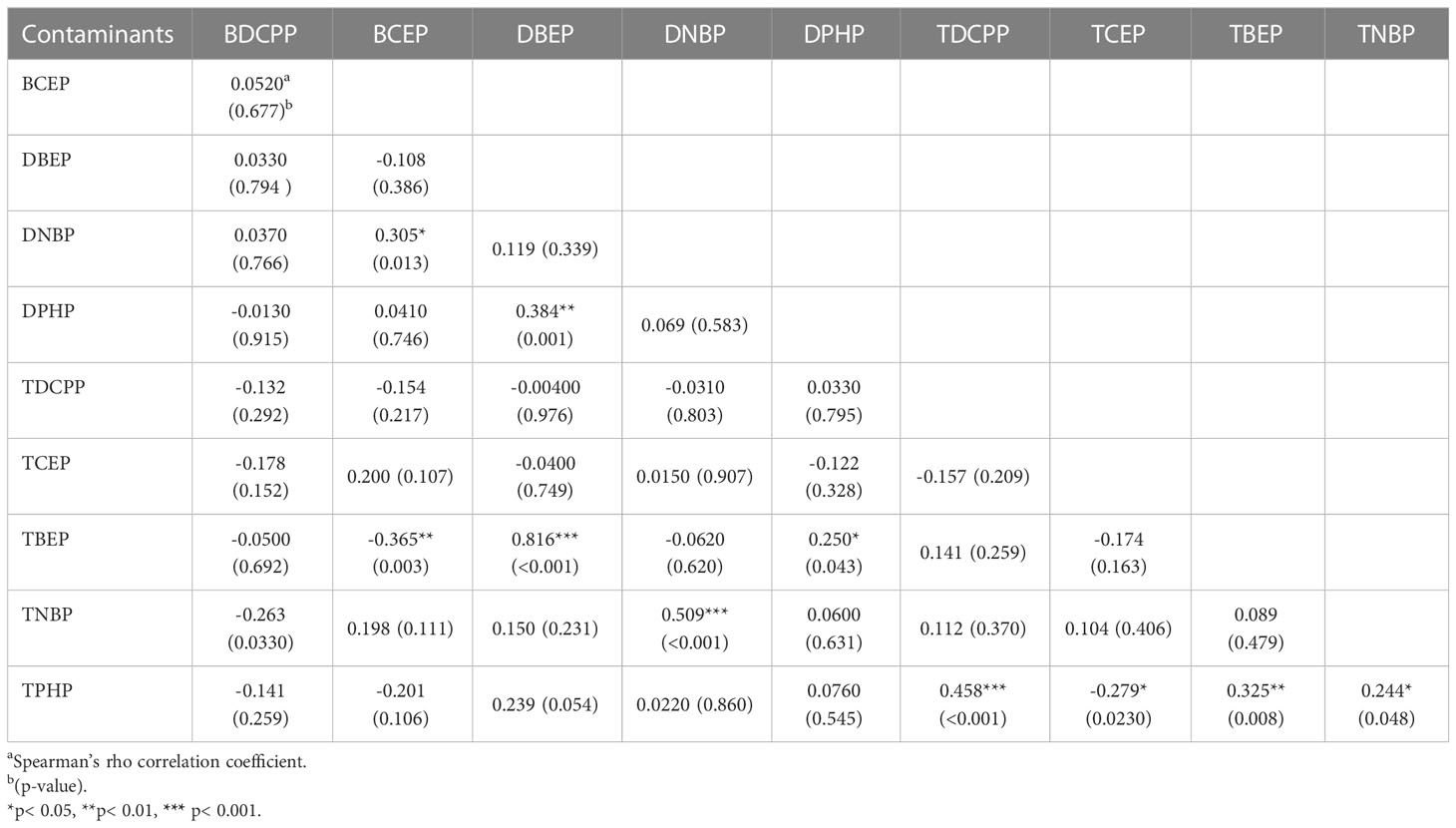
Table 2 Spearman’s rho correlation coefficients between urinary OPFRs and OPFR metabolites in the newborn group (n=66).
Table 3 presents the correlation coefficient between OPFR metabolites and urinary OPFRs for the 69 participants of the 1-17 yrs group. Similar to the newborn group, correlation analysis for the DBEP and TBEP relationship revealed a strong correlation, with the highest coefficient of r=0.845 and p <0.001. The second highest correlation was between TDCPP and TNBP, with a coefficient of r=0.374 and a p-value of 0.002, followed by the correlation between DBEP and TNBP (r=0.344, p-value=0.004). There was a significant positive relationship between BCEP and DPHP and between TBEP and TNBP, with r=0.244, p=0.0440, and r=0.247, p=0.041, respectively. In contrast, the relationships between DBEP and TCEP, as well as TCEP and TBEP, were both negative. The correlation coefficient for DBEP and TCEP was -0.260 with a p-value of 0.031, and for TCEP and TBEP, it was -0.296 with a p-value of 0.014. The age group in Figure 2A had a high correlation with certain OPFR metabolites in urine, such as BDCPP, DNBP, TDCPP, TCEP, and TNBP; while BCEP and TPHP were moderately related to this age group.
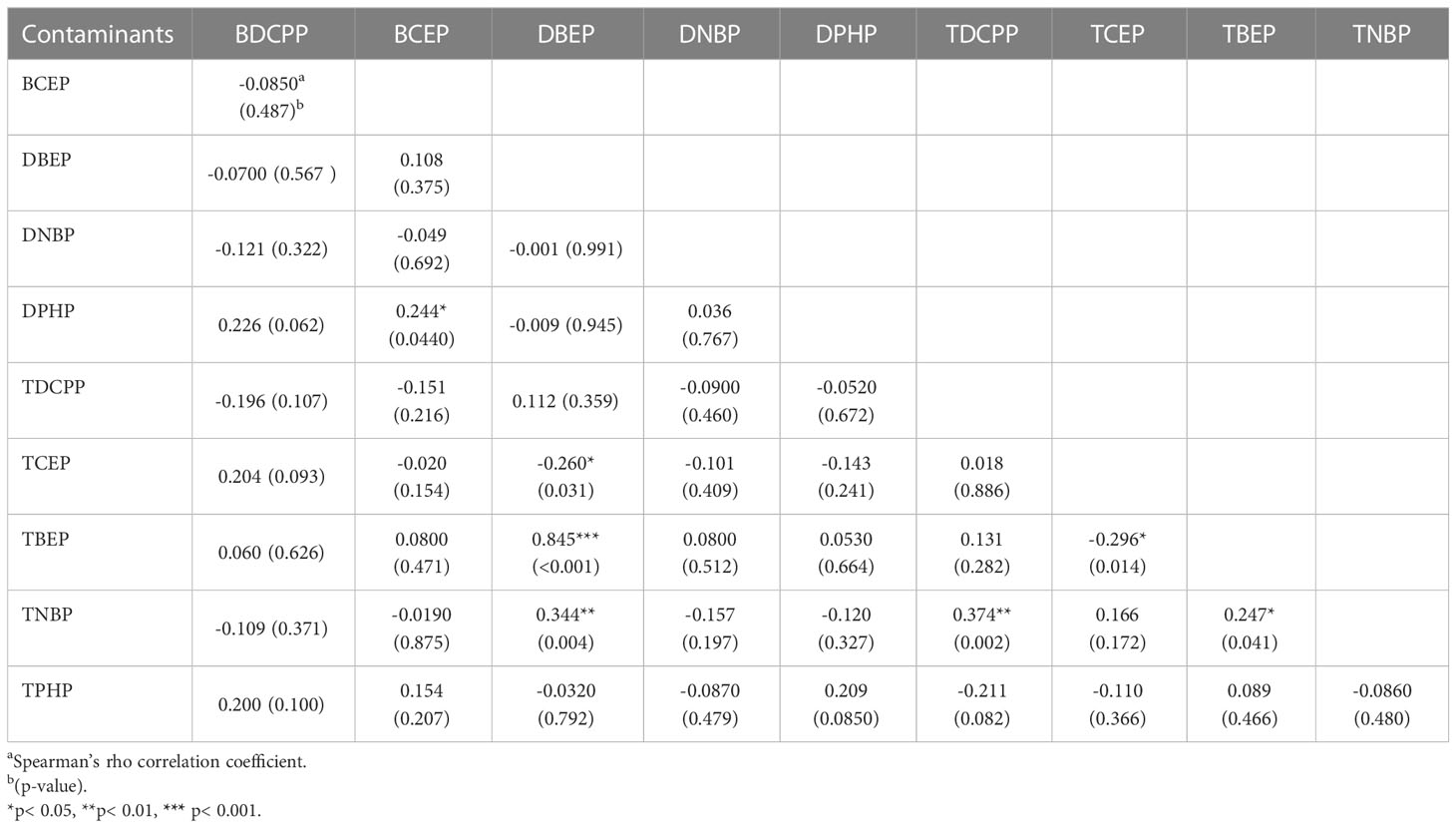
Table 3 The Spearman’s rho correlation coefficients between urinary OPFRs and OPFR metabolites in children aged 1 to 17 years (n=69).
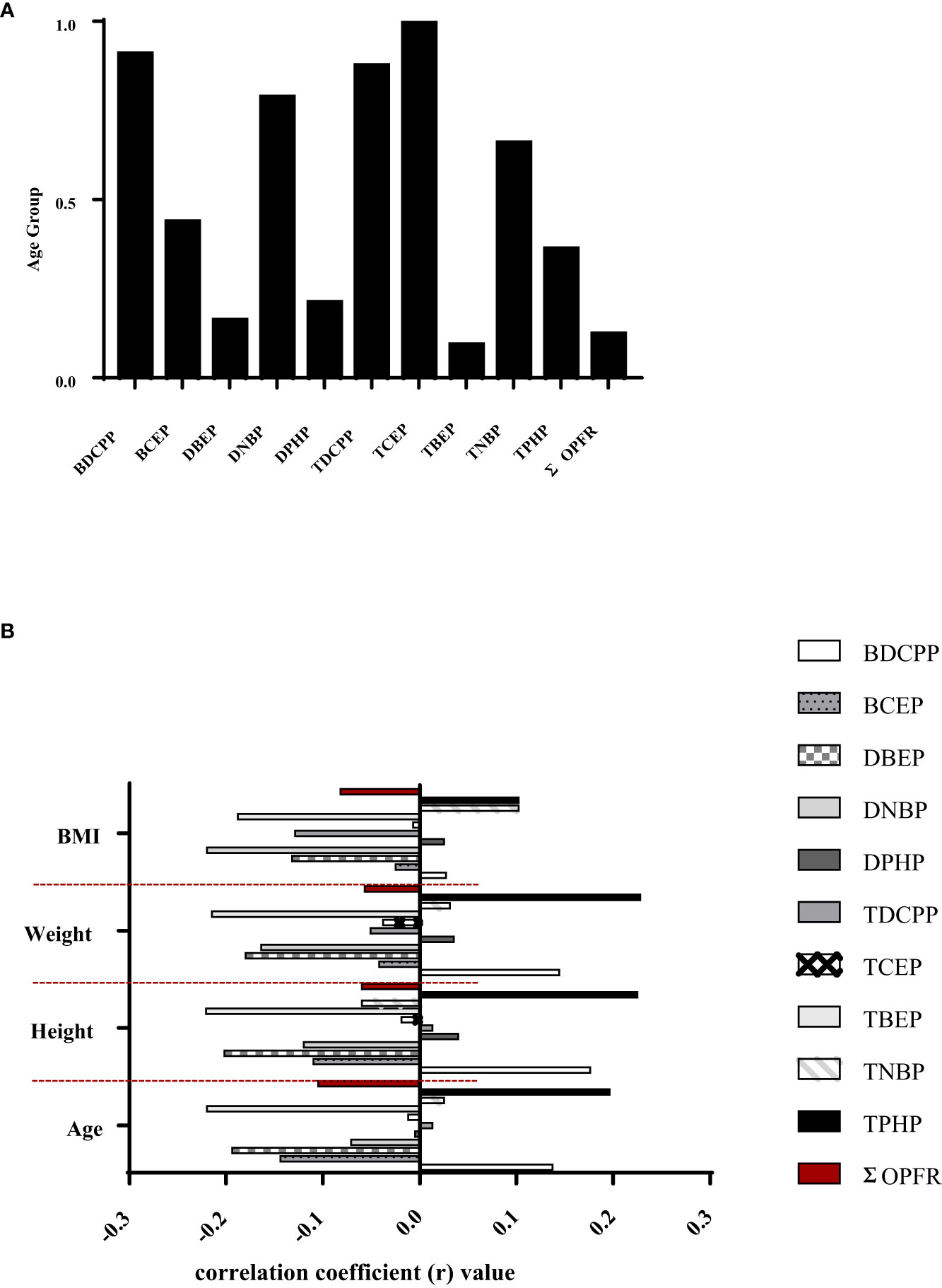
Figure 2 (A) Spearman’s rho correlation coefficients between age groups and urinary OPFRs or OPFR metabolites in the young population (n=135). (B) Spearman's rho correlations between urinary OPFRs or OPFR metabolites and height, weight, and BMI in youngster individuals (n=69).
Figure 2B depicts Spearman’s rho correlations among BMI, weight, and height for a group of young individuals. Among the OFPRs metabolites in the present study, no significant correlations with age, height, weight, and BMI are shown in Figure 2B. Table 4 displays the variability of non-neonatal questionnaire responses, including seven parameters: the frequency of eating out, hand-washing habits before eating, daily consumption of beverages from plastic cups, daily consumption of microwaved food, weekly intake of seafood and large fish, and the number of daily meat servings. Among these parameters, the frequency of eating out had the strongest correlation with TCEP (p=0.008) and TBEP (p=0.042). Additionally, the hand-washing parameter showed a significant correlation with TNBP (p=0.049). Children who frequently ate out (≥7 times/week) had significantly higher levels of TCEP (0.643 ± 1.083 μg/L) and lower magnitudes of TBEP (0.188 ± 0.273 μg/L) in urine compared with those children who did not frequently eat out, respectively (TCEP: 0.115 ± 0.513 μg/L, p=0.008; TBEP: 0.473 ± 0.695 μg/L, p=0.042). Table 5 presents Spearman’s rho correlation coefficients between urinary OPFRs and neonatal birth outcomes, based on a sample size of 66. The descriptive analyses are shown as the following: Quetelet’s index (12.0 ± 1.17 kg/m2), Apgars score 1 minute (8.97± 0.173), Apgars score 5 minutes (9.95 ± 0.210), birth length (49.3 ± 2.45 cm), birth weight (2.94 ± 0.464 kg, head circumference (49.3 ± 2.45 cm), chest circumference (31.8 ± 2.04 cm), abdominal circumference (29.4 ± 2.18 cm), and gestational age (37.9± 1.34 weeks). Significant negative relationships were observed between the urinary BDCPP chemical and birth length (r=-0.249, p=0.044) as well as between BDCPP and chest circumference (r=-0.261, p=0.034). Additionally, a negative correlation was found between DBEP and birth length, with r=-0.252 and p=0.041. In contrast, TCEP and birth weight exhibited a positive relationship, with r=0.274 and p=0.026.
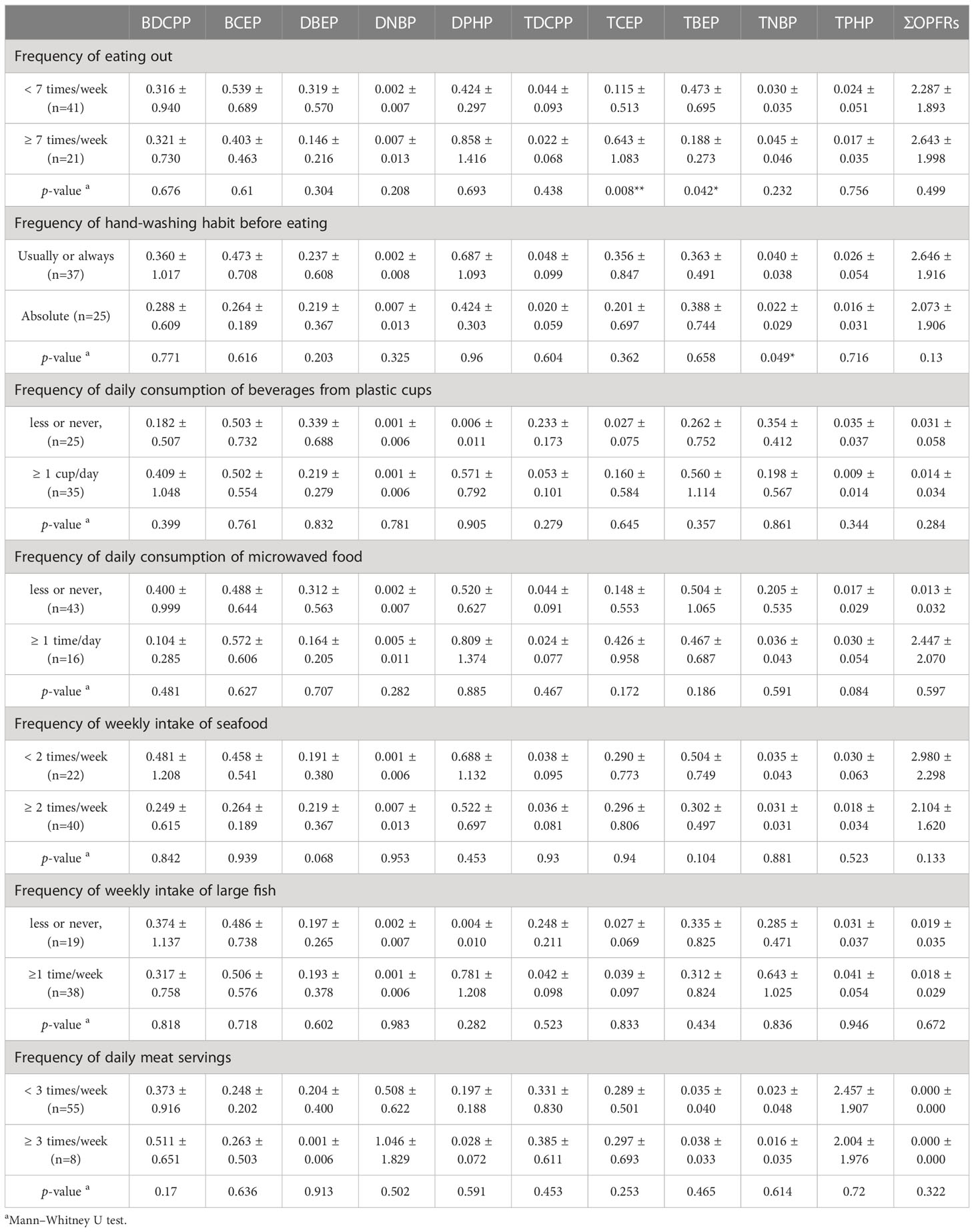
Table 4 Lifestyle and dietary habit correlated with urinary OPFRs of children aged 1-17 years (μg/L).
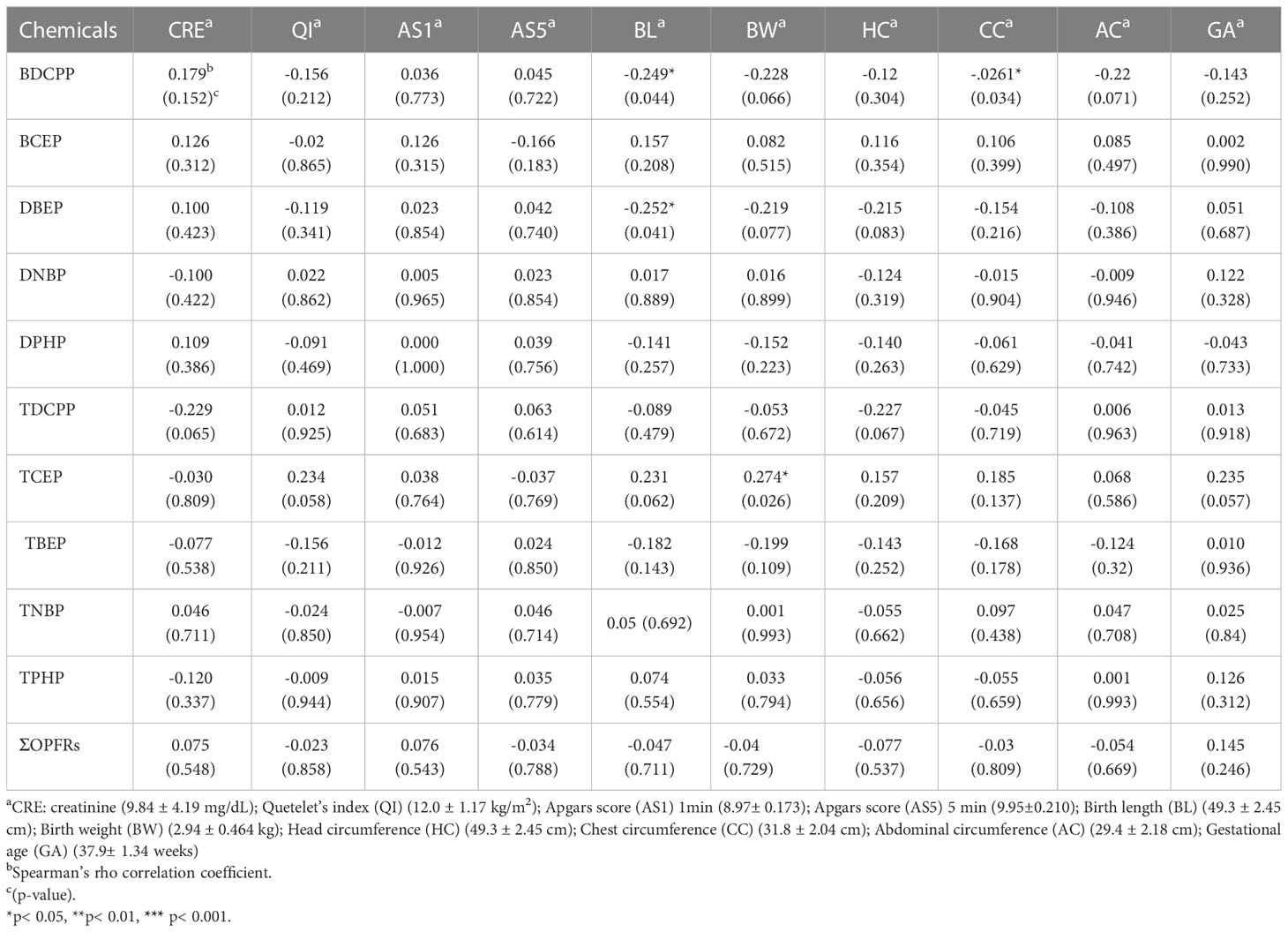
Table 5 The Spearman’s rho correlation coefficients between urinary OPFRs and neonatal birth outcomes (n=66).
EDIs of individual OPFRs, such as TDCPP, TCEP, TBEP, TNBP, and TPHP, were as follows: (1) newborns: 19.7 ± 97.0, 1170 ± 1660, 493 ± 160, and 25.4 ± 100, and 518 ± 777 ng/kg bw/day for TDCPP, TCEP, TBEP, TNBP, and TPHP, respectively; (2) young children aged 1-5 yrs: 22.5 ± 48.3, 246 ± 302, 12.4 ± 19.9, and 0.219 ± 0.955, and 180 ± 249 ng/kg bw/day for TDCPP, TCEP, TBEP, TNBP, and TPHP, respectively; (3) school children aged 6-10 yrs: 6.74 ± 18.7, 53.8 ± 69.7, 2.44 ± 4.26, and 0.234 ± 0.622, and 66.8 ± 64.3 ng/kg bw/day for TDCPP, TCEP, TBEP, TNBP, and TPHP, respectively; (4) adolescents aged 11-17 yrs: 2.09 ± 5.36, 94.9 ± 101, 2.00 ± 2.87, and <LOD, and 84.7 ± 174 ng/kg bw/day for TDCPP, TCEP, TBEP, TNBP, and TPHP, respectively.
Table 6 shows concentrations of OPFRs in the urine of individuals under 18yrs in the general population compared with previous measurements in other studies from several locations. Previous studies have shown that BDCPP and DPHP are the most dominant OPFRs (20–27, 29, 31–34, 36, 38). The concentrations of BDCIPP (0.279 μg/L) and DPHP (0.628 μg/L) measured in our study for the 1-5 yrs age group were found to be lower than those reported in previous studies from the United States and Australia (22, 23, 33). Similarly, the concentration of BDCPP (0.053 μg/L) in the newborn group measured in our study was lower than that reported by Hoffman et al. in 2015 and 2017 (29, 36). In 2018, Ospina et al. (31) found children aged 6 to 11 yrs concentrations of BDCPP and DPHP, of 2.25 μg/L and 1.69 μg/L, respectively, which was higher than the concentrations found in our study. The lower concentrations found in our study than those previously reported for newborns and young children suggest that exposure to BDCPP and DPHP in this age group may have decreased over time.
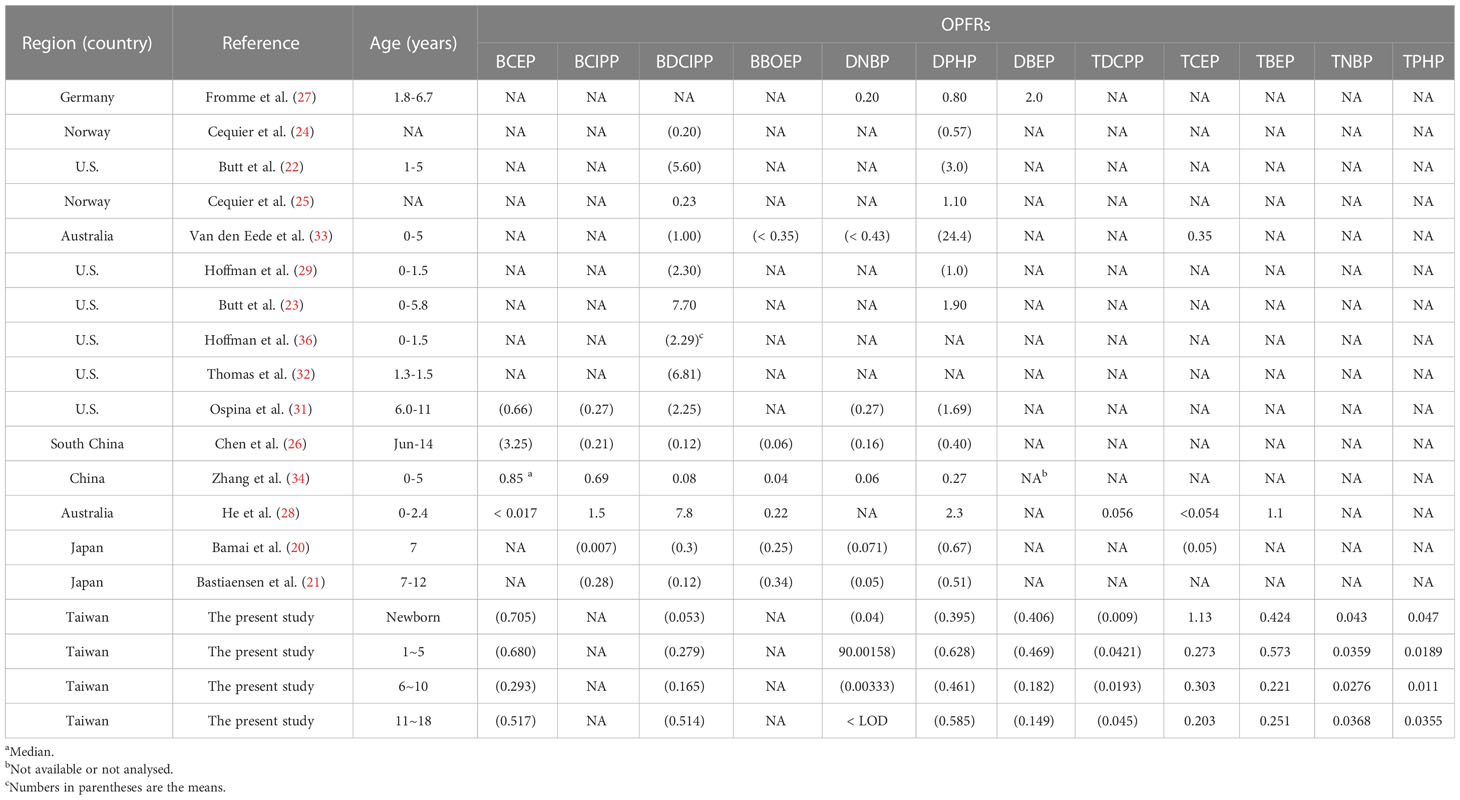
Table 6 Global data for urinary OPFRs and OPFR metabolites in the general population under 18 years old.
This is the first study to report urinary OPFRs and OPFR metabolites in the Taiwanese population under 18 years old. The findings suggest that exposure to OPFRs may be a concern across a wide range of age groups. Table 1 and Figure 1 show that the distribution of OPFRs and OPFR metabolites in newborns’ urine is different from those in the other age groups. For example, BDCPP composed 1.63% and 9.12-22.2% of Σ10 OPFRs in newborns’ and the other age groups’ urine, respectively, while TCEP comprised 34.8% and 8.67-16.9% of Σ10 OPFRs in newborns’ and the other age groups’ urine, respectively. This indicates that OPFRs and their metabolites distributed in newborns’ urine might differ from those in urine from the other age categories. These organophosphate compounds are commonly used as flame retardants in consumer products and the findings suggest that newborns may be especially vulnerable to exposure to these chemicals, particularly for breastfed infants via breast milk. Tsai et al. (2022) (35) indicated that urinary Σ10 OPFRs in patients with chronic kidney disease (CKD) (n=166 at the age of 69) in southern Taiwan were detected as the median of 2.07 μg/g creatinine (Cr), including the predominant urinary OPFRs of TBEP, BCEP, Bis(2-butoxyethyl) phosphate (BBOEP), and DPHP. Urinary OPFRs in a broad-spectrum young population in the present study differ from that of the Taiwanese patients with CKD from Tsai’s study (35). Human exposure to OPFRs is mainly from dietary and indoor dust, particularly for newborns and young children, who spend longer periods of time in the home. According to the current data by Chang, for southern Taiwan (30), TCPP contributes 39.0% to dust ΣOPFRs, followed by Triethyl phosphate (TEP) and Tri (isobutyl) phosphate (TiBP). Our results are not comparable to those of Lu (30) due to differences in the detection methods of OPFR species. Lu also assessed the hazard of residential indoor air and house dust in Taiwan, with average daily exposure doses of 152, 137, and 142 ng/kg/day for children aged 3-5 yrs, boys aged 6-12 yrs, and girls aged 6-12 yrs, respectively. Our findings, indicating that Σ10 OPFRs in urine from children aged 3-5 yrs are higher than those from children aged 6-12 yrs, are consistent with the estimated daily doses of non-dietary OPFRs reported by Lu (30). Urinary levels of DPHP and BDCPP were comparable, and urinary TCEP was higher in the present study than in the Hokkaido cohort of Japanese school children aged 7 yrs (20). DPHP is frequently detected and predominates in urine from our population, with a level comparable to previous studies from Japan (20, 21), China (26), and Europe (25, 27). Dodson et al. (2014) indicated that DPHP was not a specific biotransformation product of TPHP or the metabolites of 2-ethylhexyldiphenyl phosphate (EHDPHP) and resorcinol bis-diphenyl phosphate (RDP) (39). The Hokkaido cohort study examined associations between OPFR metabolites and urinary oxidative stress biomarkers (8-hydroxy-2′-deoxyguanosine, hexanoyl-lysine (HEL), and 4-hydroxynonenal), finding that urinary DPHP was significantly and positively correlated with HEL, which is recognized as lipid hydroperoxide-modified biomarkers oxidized from fatty acids (20).
There were no significant differences in urinary OPFRs and OPFR metabolites among the four age groups (Table 1). Most previous studies also found non-significant differences in OPFR metabolites between the age groups. Urinary levels of DPHP (6-11 yrs vs 12-18 yrs: 1.69 vs 1.41 μg/L), BCEP (0.662 vs 0.602 μg/L), and DNBP (0.272 vs 0.207 μg/L) in school children in the 6-11 yrs age group were not significantly higher than those in the 12-18 yrs adolescent group, but urinary BDCPP (2.25 vs 1.34 μg/L, p < 0.001) was statistically significant (31). According to several mother-children paired studies, urinary OPFR metabolites in children showed several folds higher than those in mothers (22, 23, 25). Based on our findings, the distribution of urinary OPFRs and OPFR metabolites in newborns significantly differed from those whose urine was from young children, school children, and adolescents (Figures 1A, B). Urinary OPFRs and their metabolites did not present significant differences between male and female subjects in a broad-spectrum young population, as shown in Figure 1C. Urinary Σ10 OPFRs in male adults were not significantly different from that in female adults from Taiwanese CKD patients (35). In a German study, urinary OPFR metabolites did not show a significant difference between boys and girls at the age of 22-80 months (n = 312) (27). For American infants, urinary BDCPP and DPHP did not show significant differences by sex (29), while data from the NHANES also showed non-significant differences by sex in urinary BDCPP, DPHP, BCEP, and DNBP (31). Table 2 presents the correlations between OPFRs and OPFR metabolites in the urine of newborns. There were high correlation coefficients (r > 0.5) in the pairs of TBEP and its metabolite of DBEP (r = 0.816, p < 0.001), as well as TNBP and its metabolite of DNBP (r = 0.509, p < 0.001). This is probably due to different metabolizing efficiencies and rates of OPFRs in newborns, indicating that TNBP and TBEP as well as their metabolites of DNBP and DBEP may have longer half-lives than the other OPFRs and OPFR metabolites. The OPFR of TPHP was significantly correlated with other OPFRs, such as TDCCP (r = 0.458, p < 0.001), TCEP (r = -0.279, p = 0.023), TBEP (r = 0.325, p = 0.008), and TNBP (r = 0.244, p = 0.048). If only OPFRs are considered in the urine of newborns, urinary OPFRs are probably predicted by TPHP. Table 3 shows the high correlation coefficient (r > 0.5) of TBEP and DBEP (r = 0.845, p < 0.001) in urine collected from a broad-spectrum young population, except for newborns. Figure 2B does not show the significant correlations between demographic parameters and urinary OPFRs in the general population of young individuals at the age 1-17 yrs (n=69). In Table 4, lifestyles and dietary habits of children are grained from the questionnaire. Among the variables listed in Table 4, frequency of eating out and frequency of hand-washing before eating are presented as significant in certain OPFRs such as TCEP, TBEP and TNBP. To our amazement, TCEP, TBEP, and TNBP were parent OPFRs and their metabolites (BCEP, DBEP, and DNBP) did not perform any associations with the questionnaires’ variables in the present study. Based on our results, for the newborn group (n=66), certain OPFR metabolites, such as BDCPP, DBEP, and TDCPP (the parent compound of BDCPP), show the negatively significant (p<0.05) or borderline-significant (p<0.1) relationship with certain birth outcomes such as birth length, birth weight, head circumference, chest circumference, or abdominal circumference. Urinary BDCPP in newborns was negatively correlated with birth length (r=-0.249, p=0.044), birth weight (r=-0.228, p=0.066), chest circumference (r=-0.261, p=0.034), and abdominal circumference (r=-0.223, p=0.071). Urinary DBEP had negative associations with birth length (r=-0.252, p=0.041), birth weight (r=-0.219, p=0.077), and head circumference (r=-0.-215, p=0.083). TDCPP levels in the urine of newborns were only shown to have a borderline-significant relationship with urinary creatinine (r=-0.229, p=0.065) and head circumference (r=-0.227, p=0.067). Inversely, urinary TCEP (the parent compound of BCEP) was positively linked to Quetelet’s index (r=0.234, p=0.058), birth length (r=0.231, p=0.062), birth weight (r=0.274, p=0.026), and gestational age (r=0.235, p=0.057). Based on our findings, the abundant compounds of BCEP and DPHP in the urine of newborns present non-significant or non-borderline-significant correlations with the variables of birth outcomes.
EDIs of Σ5OPFRs (sum of TDCPP, TCEP, TBEP, TNBP, and TPHP) were 2230, 461, 130, and 184 ng/kg bw/day for newborns, children aged 1-5 yrs, children aged 6-10 yrs, and adolescents aged 11-17 yrs, respectively. The Σ5OPFRs EDI for newborns was 4.83-17.2 times higher than those for the other age groups. Van den Eede et al. (2011) (40) found that the reference doses (RfDs) of TDCIPP, TNBP, TCEP, and TPHP were 1500, 2400, 2200, and 7000 ng/kg bw/day, respectively, for a range of cohorts grouped by age and sex. All EDIs of TDCIPP, TNBP, TCEP, and TPHP for the young population in the present study were below the RfDs. This indicates that the high body burden of OPFRs for newborns in the present study is still lower than critical values. Lu et al. (2023) (30) assessed the Σ11OPFRs EDI via house dust and indoor air in residential houses to be 152 ng/kg bw/day for Taiwanese children aged 3-5 yrs and 137-142 ng/kg bw/day for Taiwanese children aged 6-12 yrs. Young and school-age children’s exposure to OPFRs is primarily through non-dietary (i.e., hand-mouth behavior) and dietary pathways. Our EDI for urinary metabolite excretion had a magnitude comparable to the values from Lu’s study, which were assessed by the routes of indoor matrices (30). Our EDI had the same order of magnitude as those in Chinese children (8-12 yrs and 6-14 yrs, of 474 and 172 ng/kg bw/day, respectively, n=411) (26), but lower than those in German children (TNBP: 30 ng/kg bw/day of the median, n=312) (27), and American infants at 2-18 months (110-330 ng/kg bw/day of geometric means, n=43) (36) as estimated from excreted OPFR metabolites. The EDIs of TCEP, TDCPP, and TPHP for newborns in the present study were slightly higher than those for infants in the USA (n=65) (34), considering only the assessment from urine excretion. Apart from indoor dust or indoor air, food is a major route for the young population’s exposure to OPFRs. Breastfed infants are a unique population to estimate OPFRs due to their primary consumption of breast milk. Since there is currently no data on OPFRs and their metabolites in Taiwanese breast milk, levels of OPFRs and OPFR metabolites in breast milk were referenced from previous studies. Chen et al. (2021) (41) found a mean Σ13OPFRs value of 21.7 ng/mL (587 ng/g lipid) (median=10.6 ng/mL or 157 ng/g lipid) for breastmilk OPFRs in Beijing mothers (n=105), while the predominant congeners of TEHP, TPHP, and EHDPP had medians of 1.47, 1.07, and 0.844 ng/mL, respectively. OPFRs in breast milk were also measured in US mothers, with 3.6, 1.44, 0.569, and 0.539 ng/mL for Σ14OPFRs, TBOEP, TIBP, and TNBP, respectively (42). Based on the reports by Chen (41) and Ma (42), OPFRs and their metabolites have high magnitudes in breast milk.
As seen in Figure 1A and Table 1, the newborns had higher Σ10OPFRs than the toddlers, young children, school children, and adolescents, and the profiles of urinary OPFRs in newborns were differently distributed from those in the other age groups. Newborns’ exposure to OPFRs was probably mainly from the maternal breast milk in the studies by Blum (1), Chen (41), and Ma (42) as well as in the present study. Humans, including the young population, spend more than 90% of their time indoors. For the other age groups, such as toddlers, young children, school children, and adolescents, the profiles of urinary OPFRs were similar, indicating that these age groups possibly have the same exposure sources. Recently, two published articles investigated OPFRs in the indoor environment of Taiwanese residents, including air, PM2.5, and house dust (30, 43). Liu et al. (2023) assessed children’s daily average exposure to OPFRs and found that toddlers aged 3-5 yrs had a daily average exposure of 152 ng/kg/day, boys aged 6-12 yrs had 137 ng/kg/day, and girls aged 6-12 yrs had a daily average exposure of 142 ng/kg/day, from air inhalation and dust ingestion (43). This report also indicated airborne TEP, TCPP, and TBOEP mainly from wooden materials, house dust of TBP, TDCPP, and TBOEP probably from plastic materials, TEP and TEHP in house dust possibly from foam and textile materials as well as textile and plastic materials, respectively, in the indoor environment (43). Liu et al. (2023) (30) revealed that the sources of OPFRs indoors and outdoors primarily originated from the indoor environment.
To the best of our knowledge, this research is the first study to announce urinary OPFRs and OPFR metabolites in a broad-spectrum young population. It also presents the first findings for urinary OPFRs in newborns. Table 6 shows that global data for the urine of children indicate the diverse distribution and variation of OPFR magnitudes. The different commercial formulations of OPFRs used in the different regions and different dietary habits in the different populations may have led to the geographic and population differences for OPFRs.
The distribution of OPFRs and their metabolites in the urine of newborns differs from that in urine samples from other age categories. To our knowledge, this is the first article investigating urinary OPFR metabolite levels in a broad-spectrum young population. Though there are higher exposure rates for both newborns and preschool children, little is known about their exposure levels or factors leading to exposure in the young population. Based on the present study and previous research, breastfeeding is the major pathway for OPFRs exposure for newborns while in other age groups (toddlers, young children, school children, and adolescents), the children’s exposure to OPFRs is mainly from consumer products via air inhalation and house dust ingestion. Urinary OPFRs are not significantly correlated with creatinine in the urine of newborns. Further study should clarify the exposure levels and factor relationships. There is a pressing need to improve our understanding of the potential health impacts of OPFRs, given their widespread use and persistent presence in the environment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Chang Gung Memorial Hospital (CGMH) (202001028A3). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
F-SC and C-CC are co-first authors and contributed equally to this manuscript. They participated in the study design, patient recruitment, reviewing references, and drafting the manuscript. C-CT, J-HL, H-LY, C-MC, W-TH, K-FT, F-JC, C-TK, S-HL, C-CW, Y-CO, W-CL, Y-TC, and FH participated in patient recruitment or laboratory analyses. H-RC and L-JW are co-corresponding authors and contributed equally to this manuscript. They participated in study design, laboratory analyses, as well as writing the manuscript draft and revisions. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Chang Gung Memorial Hospital Research Project (CMRPG8K1281).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blum A, Behl M, Birnbaum LS, Diamond ML, Phillips A, Singla V, et al. Organophosphate ester flame retardants: are they a regrettable substitution for polybrominated diphenyl ethers? Environ Sci Technol Lett (2019) 6(11):638–49. doi: 10.1021/acs.estlett.9b00582
2. Huang J, Ye L, Fang M, Su G. Industrial production of organophosphate flame retardants (OPFRs): big knowledge gaps need to be filled? Bull Environ Contamination Toxicol (2022) 1–10. doi: 10.1007/s00128-021-03454-7
3. Doherty BT, Hammel SC, Daniels JL, Stapleton HM, Hoffman K. Organophosphate esters: are these flame retardants and plasticizers affecting children’s health? Curr Environ Health Rep (2019) 6(4):201–13. doi: 10.1007/s40572-019-00258-0
4. Wang X, Hales BF, Robaire B. Effects of flame retardants on ovarian function. Reprod Toxicol (2021) 102:10–23. doi: 10.1016/j.reprotox.2021.03.006
5. Zhang X, Lu Z, Ren X, Chen X, Zhou X, Zhou X, et al. Genetic comprehension of organophosphate flame retardants, an emerging threat to prostate cancer. Ecotoxicol Environ Saf (2021) 223:112589. doi: 10.1016/j.ecoenv.2021.112589
6. Wang X, Zhu Q, Yan X, Wang Y, Liao C, Jiang G. A review of organophosphate flame retardants and plasticizers in the environment: analysis, occurrence and risk assessment. Sci Total Environ (2020) 731:139071. doi: 10.1016/j.scitotenv.2020.139071
7. Hou R, Xu Y, Wang Z. Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere (2016) 153:78–90. doi: 10.1016/j.chemosphere.2016.03.003
8. Kanazawa A, Saito I, Araki A, Takeda M, Ma M, Saijo Y, et al. Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air (2010) 20(1):72–84. doi: 10.1111/j.1600-0668.2009.00629.x
9. Li F, Wang L, Ji C, Wu H, Zhao J, Tang J. Toxicological effects of tris (2-chloropropyl) phosphate in human hepatic cells. Chemosphere (2017) 187:88–96. doi: 10.1016/j.chemosphere.2017.08.083
10. Yao C, Yang H, Li Y. A review on organophosphate flame retardants in the environment: occurrence, accumulation, metabolism and toxicity. Sci Total Environ (2021) 795:148837. doi: 10.1016/j.scitotenv.2021.148837
11. Yang J, Zhao Y, Li M, Du M, Li X, Li Y. A review of a class of emerging contaminants: the classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (OPFRs). Int J Mol Sci (2019) 20(12):2874. doi: 10.3390/ijms20122874
12. Zhou X, Zhou X, Yao L, Zhang X, Cong R, Luan J, et al. Organophosphate flame retardant TDCPP: a risk factor for renal cancer? Chemosphere (2022) 305:135485. doi: 10.1016/j.chemosphere.2022.135485
13. Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect (2010) 118(3):318–23. doi: 10.1289/ehp.0901332
14. Carignan CC, Mínguez-Alarcón L, Butt CM, Williams PL, Meeker JD, Stapleton HM, et al. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ Health Perspect (2017) 125(8):087018. doi: 10.1289/EHP1021
15. Li J, Zhao L, Letcher RJ, Zhang Y, Jian K, Zhang J, et al. A review on organophosphate ester (OPE) flame retardants and plasticizers in foodstuffs: levels, distribution, human dietary exposure, and future directions. Environ Int (2019) 127:35–51. doi: 10.1016/j.envint.2019.03.009
16. Li M, Yao Y, Wang Y, Bastiaensen M, Covaci A, Sun H. Organophosphate ester flame retardants and plasticizers in a Chinese population: significance of hydroxylated metabolites and implication for human exposure. Environ Pollut (2020) 257:113633. doi: 10.1016/j.envpol.2019.113633
17. Nomeir A, Kato S, Matthews H. The metabolism and disposition of tris (1, 3-dichloro-2-propyl) phosphate (Fyrol FR-2) in the rat. Toxicol Appl Pharmacol (1981) 57(3):401–13. doi: 10.1016/0041-008X(81)90238-6
18. Sasaki K, Takeda M, Uchiyama M. Toxicity, absorption and elimination of phosphoric acid triesters by killifish and goldfish. Bull Environ Contamination Toxicol (1981) 27(1):775–82. doi: 10.1007/BF01611095
19. Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett (2013) 223(1):9–15. doi: 10.1016/j.toxlet.2013.08.012
20. Ait Bamai Y, Bastiaensen M, Araki A, Goudarzi H, Konno S, Ito S, et al. Multiple exposures to organophosphate flame retardants alter urinary oxidative stress biomarkers among children: the Hokkaido study. Environ Int (2019) 131:105003. doi: 10.1016/j.envint.2019.105003
21. Bastiaensen M, Ait Bamai Y, Araki A, Van den Eede N, Kawai T, Tsuboi T, et al. Biomonitoring of organophosphate flame retardants and plasticizers in children: associations with house dust and housing characteristics in Japan. Environ Res (2019) 172:543–51. doi: 10.1016/j.envres.2019.02.045
22. Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol (2014) 48(17):10432–8. doi: 10.1021/es5025299
23. Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM, et al. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and new Jersey. Environ Int (2016) 94:627–34. doi: 10.1016/j.envint.2016.06.029
24. Cequier E, Marcé RM, Becher G, Thomsen C. A high-throughput method for determination of metabolites of organophosphate flame retardants in urine by ultra performance liquid chromatography–high resolution mass spectrometry. Analytica Chimica Acta (2014) 845:98–104. doi: 10.1016/j.aca.2014.06.026
25. Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters {{/amp]]mdash; a biomonitoring study of mother–child pairs. Environ Int (2015) 75:159–65. doi: 10.1016/j.envint.2014.11.009
26. Chen Y, Fang J, Ren L, Fan R, Zhang J, Liu G, et al. Urinary metabolites of organophosphate esters in children in south China: concentrations, profiles and estimated daily intake. Environ Pollut (2018) 235:358–64. doi: 10.1016/j.envpol.2017.12.092
27. Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, et al. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ Int (2014) 71:158–63. doi: 10.1016/j.envint.2014.06.016
28. He C, Wang X, Thai P, Baduel C, Gallen C, Banks A, et al. Organophosphate and brominated flame retardants in Australian indoor environments: levels, sources, and preliminary assessment of human exposure. Environ Pollut (2018) 235:670–9. doi: 10.1016/j.envpol.2017.12.017
29. Hoffman K, Butt CM, Chen A, Limkakeng AT Jr, Stapleton HM. High exposure to organophosphate flame retardants in infants: associations with baby products. Environ Sci Technol (2015) 49(24):14554–9. doi: 10.1021/acs.est.5b03577
30. Lu QO, Jung CC, Chao HR, Chen PS, Lee CW, Thi Phuong Tran Q, et al. Investigating the associations between organophosphate flame retardants (OPFRs) and fine particles in paired indoor and outdoor air: a probabilistic prediction model for deriving OPFRs in indoor environments. Environ Int (2023) 174:107871. doi: 10.1016/j.envint.2023.107871
31. Ospina M, Jayatilaka NK, Wong L-Y, Restrepo P, Calafat AM. Exposure to organophosphate flame retardant chemicals in the U.S. general population: data from the 2013–2014 national health and nutrition examination survey. Environ Int (2018) 110:32–41. doi: 10.1016/j.envint.2017.10.001
32. Thomas MB, Stapleton HM, Dills RL, Violette HD, Christakis DA, Sathyanarayana S. Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere (2017) 185:918–25. doi: 10.1016/j.chemosphere.2017.07.015
33. Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, et al. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int (2015) 74:1–8. doi: 10.1016/j.envint.2014.09.005
34. Zhang B, Lu S, Huang M, Zhou M, Zhou Z, Zheng H, et al. Urinary metabolites of organophosphate flame retardants in 0–5-year-old children: potential exposure risk for inpatients and home-stay infants. Environ Pollut (2018) 243:318–25. doi: 10.1016/j.envpol.2018.08.051
35. Tsai K-F, Cheng F-J, Huang W-T, Kung C-T, Lee C-T, Cheng B-C, et al. The associations between renal disease severity and exposure to organophosphate flame retardants in patients with chronic kidney disease. Environ Int (2022) 170:107573. doi: 10.1016/j.envint.2022.107573
36. Hoffman K, Gearhart-Serna L, Lorber M, Webster TF, Stapleton HM. Estimated tris (1, 3-dichloro-2-propyl) phosphate exposure levels for US infants suggest potential health risks. Environ Sci Technol Lett (2017) 4(8):334–8. doi: 10.1021/acs.estlett.7b00196
37. ICRP A. Basic anatomical and physiological data for use in radiological protection: reference values. Ann ICRP (2002) 32(3–4):1–277. doi: 10.1016/s0146-6453(03)00002-2
38. He C, English K, Baduel C, Thai P, Jagals P, Ware RS, et al. Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ Res (2018) 164:262–70. doi: 10.1016/j.envres.2018.02.040
39. Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol (2014) 48(23):13625–33. doi: 10.1021/es503445c
40. Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ Int (2011) 37(2):454–61. doi: 10.1016/j.envint.2010.11.010
41. Chen X, Zhao X, Shi Z. Organophosphorus flame retardants in breast milk from Beijing, China: occurrence, nursing infant's exposure and risk assessment. Sci Total Environ (2021) 771:145404. doi: 10.1016/j.scitotenv.2021.145404
42. Ma J, Zhu H, Kannan K. Organophosphorus flame retardants and plasticizers in breast milk from the united states. Environ Sci Technol Lett (2019) 6(9):525–31. doi: 10.1021/acs.estlett.9b00394
43. Lu QO, Jung CC, Liu YH, Chang WH. Seasonal and source characteristics of organophosphorus flame retardants in air and house dust in Taiwan residential microenvironments: Implications for young children's exposure and risk assessment using a probabilistic approach. Environ Pollut (2023) 318:120893. doi: 10.1016/j.envpol.2022.120893
Keywords: organophosphate flame retardants (OPFRs), OPFR metabolites, urine, a broad-spectrum pediatric population, newborn, hazardous chemicals
Citation: Chen F-S, Chen C-C, Tsai C-C, Lu J-H, You H-L, Chen C-M, Huang W-T, Tsai K-F, Cheng F-J, Kung C-T, Li S-H, Wang C-C, Ou Y-C, Lee W-C, Chang Y-T, Hashim F, Chao H-R and Wang L-J (2023) Urinary levels of organophosphate flame retardants metabolites in a young population from Southern Taiwan and potential health effects. Front. Endocrinol. 14:1173449. doi: 10.3389/fendo.2023.1173449
Received: 24 February 2023; Accepted: 05 May 2023;
Published: 02 June 2023.
Edited by:
Fernando Lizcano, Universidad de La Sabana, ColombiaReviewed by:
Jordan Crago, Texas Tech University, United StatesCopyright © 2023 Chen, Chen, Tsai, Lu, You, Chen, Huang, Tsai, Cheng, Kung, Li, Wang, Ou, Lee, Chang, Hashim, Chao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: How-Ran Chao, aHJjaGFvQG1haWwubnB1c3QuZWR1LnR3; Liang-Jen Wang, d2FuZ2xpYW5namVuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.