
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 08 May 2023
Sec. Cellular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1172750
This article is part of the Research TopicCell Death in Endocrine Diseases: From Molecular Mechanisms to Therapeutic TargetsView all 7 articles
Background: Polycystic ovary syndrome (PCOS) is one of the most common gynecological endocrine diseases for women of puberty and reproductive age. PCOS can affect women’s health for the rest of their lives since the incidence of coronary heart disease (CHD) may increase in the perimenopausal and senile periods among PCOS women compared with non-PCOS women.
Method: A literature retrieval based on the Science Citation Index Expanded (SCI-E) database. All obtained records results were downloaded in plain text format for subsequent analysis. VOSviewer v1.6.10, Citespace and Microsoft Excel 2010 software were utilized for analyzing the following terms: countries, institutions, authors, journals, references and keywords.
Results: There were 312 articles retrieved from January 1, 2000 to February 8, 2023, and the frequency of citations was 23,587. The United States, England, and Italy contributed the majority of the records. Harvard University, the University of Athens, and Monash University were the top 3 most productive institutions with publications on the relationship between PCOS and CHD. Journal of clinical endocrinology & metabolism ranked first with the highest publications (24 records), followed by Fertility and sterility (18 records). The keywords were divided into six clusters in the overlay keywords network: (1) the correlation between CHD risk factors and PCOS women; (2) the relationship between cardiovascular disease and female reproductive system hormone secretion; (3) the interaction between CHD and metabolic syndrome; (4) the relationship between c-reactive protein and endothelial function and oxidative stress in PCOS patients; (5) the potential positive effect of metformin on reducing CHD risk factors in PCOS patients; (6) the study of serum cholesterol and body-fat distribution in patients with CHD in PCOS. Oxidative stress, genome-wide association, obesity, primary prevention, and sex difference were main hotspots in this field in recent five years according to the keyword citation burst analysis.
Conclusion: The article obtained the hotspots and trends and provided a reference for subsequent research on the association between PCOS and CHD. Moreover, it is hypothesized that oxidative stress and genome-wide association were frontier hotspots in studies that explore the relationship between PCOS and CHD, and prevention research may be valued in the future.
Polycystic ovary syndrome (PCOS) is one of the most common gynecological endocrine diseases in adolescent and reproductive-age women, with an incidence of 6-10% (1, 2). Its clinical manifestations are varied and mainly characterized by irregular menstruation, anovulation or high androgen levels, infertility, and an increased risk of metabolic diseases (3). The risk of hypertension in PCOS women is twice that in non-PCOS women, which may be related to insulin resistance or hyperinsulinemia that damages vascular smooth muscle cells (VSMCs) and leads to the thickening and decreased elasticity of vascular walls (4). PCOS patients are prone to complications such as hypertension, abnormal lipid metabolism, type 2 diabetes mellitus (T2DM) and obesity, which are risk factors for coronary heart disease (CHD) (5). PCOS could enhance the overall cardiovascular risk, especially myocardial infarction, angina pectoris, and revascularization (6, 7). Increased serum triglyceride and low-density lipoprotein cholesterol (LDL-C) levels, decreased high-density lipoprotein cholesterol (HDL-C) cholesterol levels, and the altered ratio of apolipoprotein B to apolipoprotein A1 levels in PCOS patients are also associated with increased risk of CHD. In addition, high levels of C-reactive protein (CRP) and homocysteine in PCOS patients have been considered risk factors for the development of CHD (8, 9).
However, after searching various major databases, we found considerable literature on the relationship between the risk of CHD and PCOS. Although some scholars have done literature review studies (10–12), the studies’ systematization and comprehensive visual analysis still need to be improved.
Bibliometric analysis is a method of analyzing, cleaning and mining the quantitative information of literature using mathematical and statistical functions, which explore structures, characteristics and laws of science and technology. The VOSviewer software developed by Van Eck of Netherlands University can intuitively display terms in different color clusters and clearly show the connections between clusters (13). CiteSpace software is a visual tool developed by Chen Chaomei that can realize keyword measurement and literature data analysis, which integrates cluster analysis and social network analysis. Also, both are important tools for mining research hotspots in a certain field and predicting development trends (14).
Science Citation Index Expanded (SCI-E) database, as one of the three major databases in the core collection of Web of Science (WOS) databse, is an authoritative and high-impact citation index database of scientific journals. Therefore, this study intended to use VOSviewer and CiteSpace to visually analyze the literature related to the relationship between CHD and PCOS in the SCI-E database to explore the main research content and track the research hotspots. Based on the data analysis findings in the literature, we attempted to describe the significant impact of PCOS on the health integrity of patients in order to provide a reference for the further research direction of the relationship between PCOS and CHD and the preventive measures for PCOS patients with CHD.
Our study referred to the general internationally accepted method of bibliometrics. The SCI-E database (http://www.webofscience.com/), the most common database for bibliometrics, was used to retrieve literature. The retrieval method was subject-term searching. Using keywords related to polycystic ovary syndrome, including “polycystic ovary syndrome”, “polycystic ovarian syndrome”, “PCOS”, and “Stein-Leventhal Syndrome”; and coronary heart disease-related keywords, including “coronary heart disease”, “CHD”, and “Coronary Diseases”, and operation method was “AND”. All obtained records results were downloaded in plain text format for subsequent analysis. We did all the searches and data exports on the same day (February 8, 2023) since metrics constantly changed over time.
The inclusion and exclusion of studies were based on the filters of the WOS database. The studies that met the following criteria were included (1): articles published in the period from January 1, 2000 to February 8, 2023 (2), articles about PCOS and CHD, (3) original articles or reviews, and (4) published in English. Exclusion criteria were (1) repeated publications, (2) proceeding paper, editorial material, meeting abstract, book chapters, letters, early access, correction, reprint, and other types. Two researchers strictly screened the literature by detailed examining the abstract section according to the inclusion and exclusion criteria. The third researcher decided on the contents with differences and uncertainties.
The following basic information was collected for each article: countries, authors, institutions, journals, references and keywords. Keywords with the same meaning but in different styles were standardized: “syndrome pcos” was replaced by “polycystic ovary syndrome”, “coronary-artery disease” was replaced by “coronary heart disease”, “type 2 diabetes mellitus” was replaced by “type 2 diabetes”, “obese women” and “overweight” were replaced by “obesity”, “BMI” was replaced by “body mass index”.
The above plain text was imported into VOSviewer v.1.6.10 (13) and CiteSpace 5.5.R2 (14) software. VOSviewer (http://www.vosviewer.com) is software for mapping scientific knowledge, which can construct and visualize the relationship between network data. It shows the structure, evolution and cooperation of the knowledge domain with its outstanding feature of the ability of graphic display and large-scale data analysis. Compared with VOSviewer, CiteSpace software has its certain advantages in visualizing burst words and revealing the dynamic development and change of research hotspots and discipline.
In the knowledge map generated by the VOSviewer, research projects were presented, including countries, institutions, authors, co-cited references and keywords, et al. The research projects can be demonstrated as nodes, and links between nodes represent collaboration, co-occurrence or co-citations among them. The bibliographic co-citation analysis and keyword co-occurrence analysis networks were used to construct a knowledge map of studies on the association between PCOS and CHD. Reference co-citation cluster analysis can summarize the main topics in the research field. Keyword co-occurrence analysis can illustrate the theme of the literature. The distribution trend of obtained research was generated by Microsoft Excel 2010 software. The burst words were analyzed by CiteSpace.
There were a total of 312 records in the search results from January 1, 2000 to February 8, 2023 (Figure 1). More than half of the records were original articles (202/312, 64.7%), which greatly reflected the development trends and changes in the field of research on the association between CHD and PCOS. The distribution of publications by year was shown in Figure 2, demonstrating a flat trend. As of February 8, 2023, there are only two articles, but this number will continue to increase.
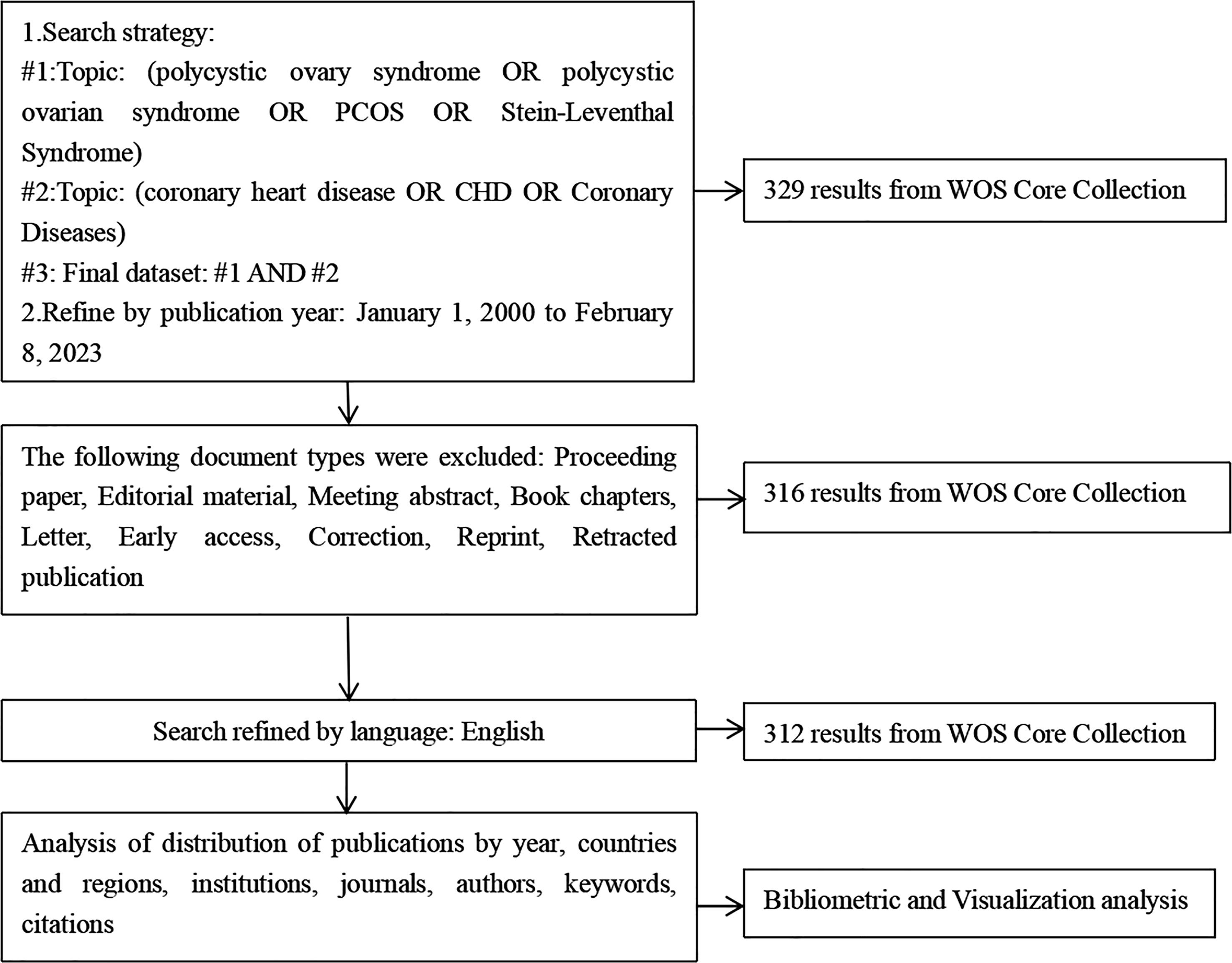
Figure 1 A frame flow diagram. The diagram shows detailed selection criteria for POI therapy publications from the WOS database and the steps of the bibliometric analysis.
According to the search results, 312 articles were from 46 countries, and the top 35 countries by the number of publications were presented in Figure 3. The top 10 countries engaged in studies on the association between PCOS and CHD were shown in Table 1. The United States of America (USA) had the largest number of publications (n=105), followed by England (n = 38) and Italy (n = 34). According to the citation analysis, there were 10,198 citations in the United States, followed by England (4,373 citations) and Australia (2,959 citations).

Figure 3 A visualization map of countries. There are nodes (circles) in the figure, representing countries. The larger the circle, the more documents were produced by that country. A connection between 2 nodes means that 2 countries appear in a document simultaneously; that is, 2 countries have a cooperative relationship in this document. (A) Network diagram of the top 35 countries. (B) Dynamics and trends of the top 35.
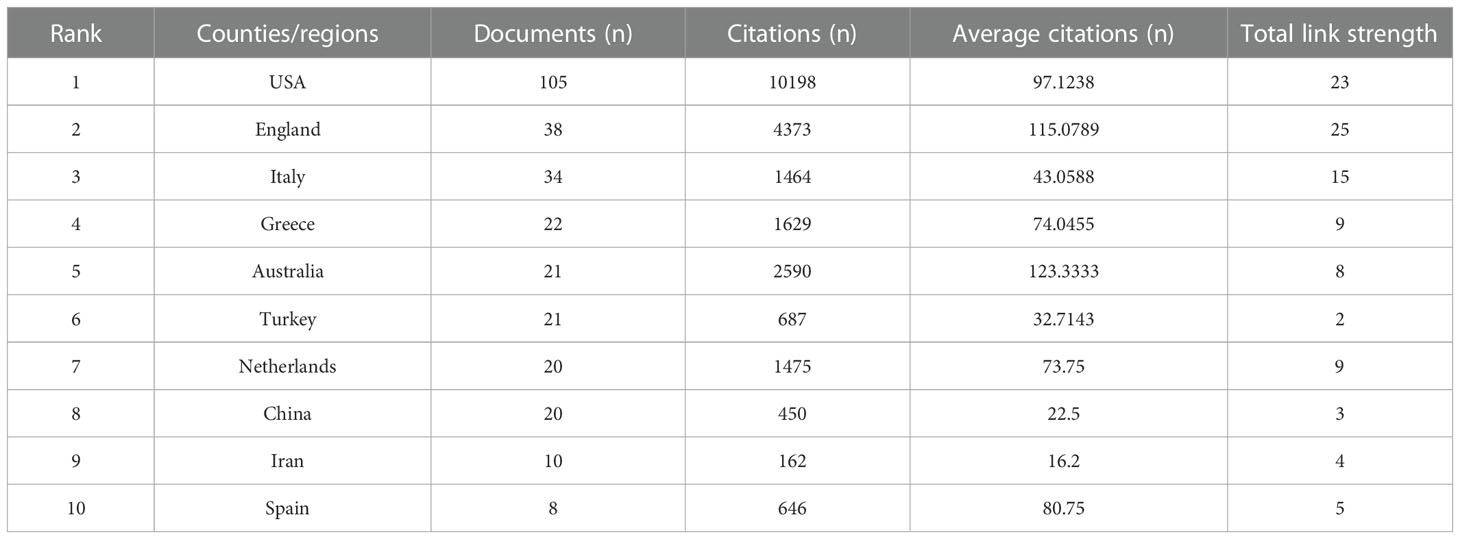
Table 1 Top 10 most productive countries/regions with publications on the relationship of PCOS and CHD from 2000 to 2023.
A total of 513 institutions were involved in these articles. Table 2 showed the top 15 institutions with more than five related publications. Among them, 8/15 were from the USA, 3/15 were from Greece, and 2/15 were from England. Harvard University was the top with the most publications, followed by the University of Athens, Monash University, Aristotle University of Thessaloniki, and Brigham & Women’s Hospital. The top 15 institutions published 100 articles, accounting for 32.15% of the total. The University of Glasgow achieved the highest citations.
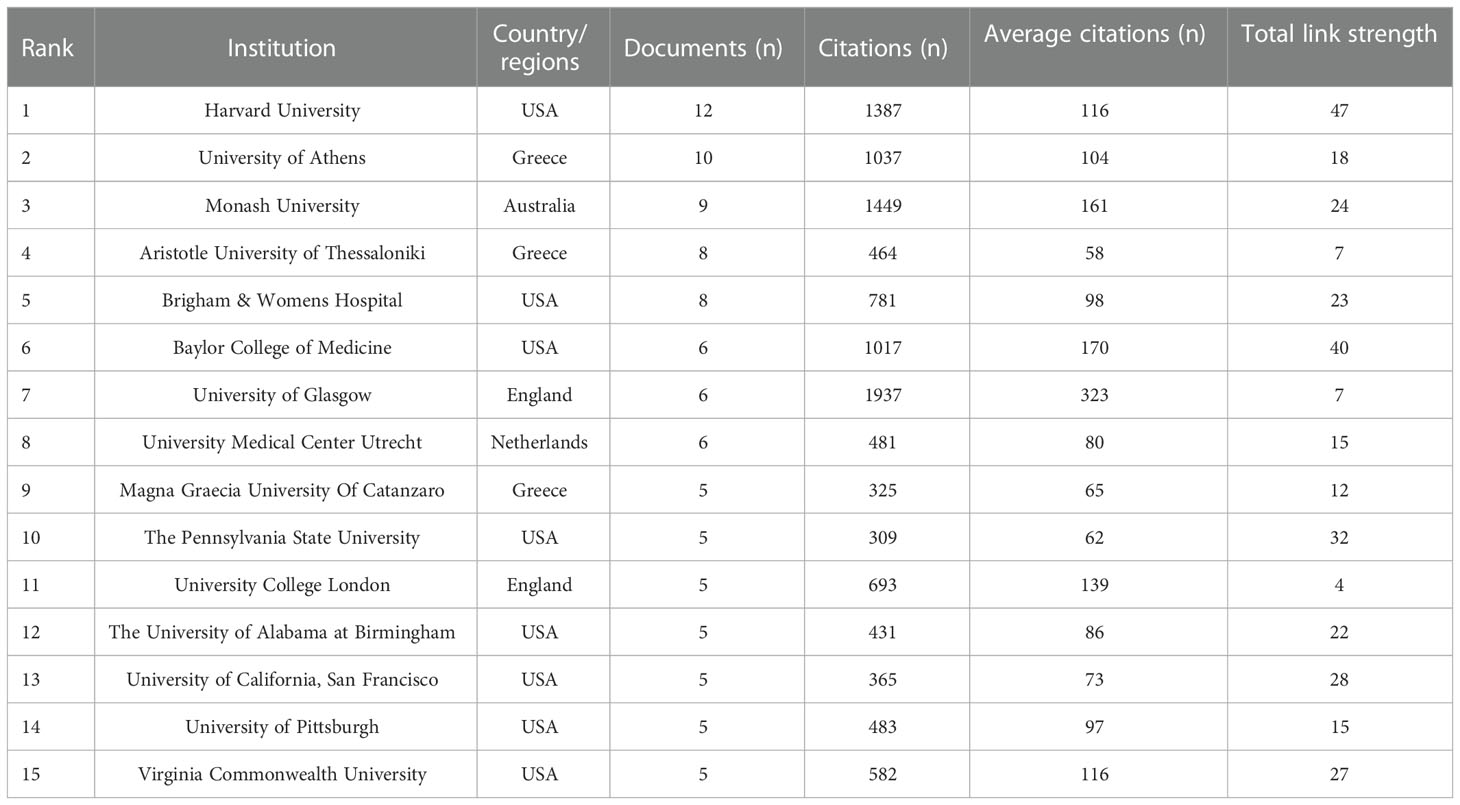
Table 2 Top 15 institutions with publications on the relationship of PCOS and CHD from 2000 to 2023.
A total of 1,518 authors were involved in related publications, authors with a frequency ≥ 2 times were incorporated in the collaboration network analysis, and 111 authors were obtained. Dr. Wang, Ping, Dr. Glueck, Charles J, Dr. Wild, Robert A, Dr. Lambrinoudaki, Irene, Dr. Azziz, and Dr. Ricardo had the highest number of publications. They were distributed in different clusters, showing close cooperative relationships with other authors (Figure 4). Although Dr. Wild, Robert A ranked non-first in the number of articles published, Dr. Wild, Robert A’s group was at the center of the network, working tightly with other groups. In addition, Dr. Lambrinoudaki Irene was not associated with the center’s collaboration network and had less collaboration with other authors.
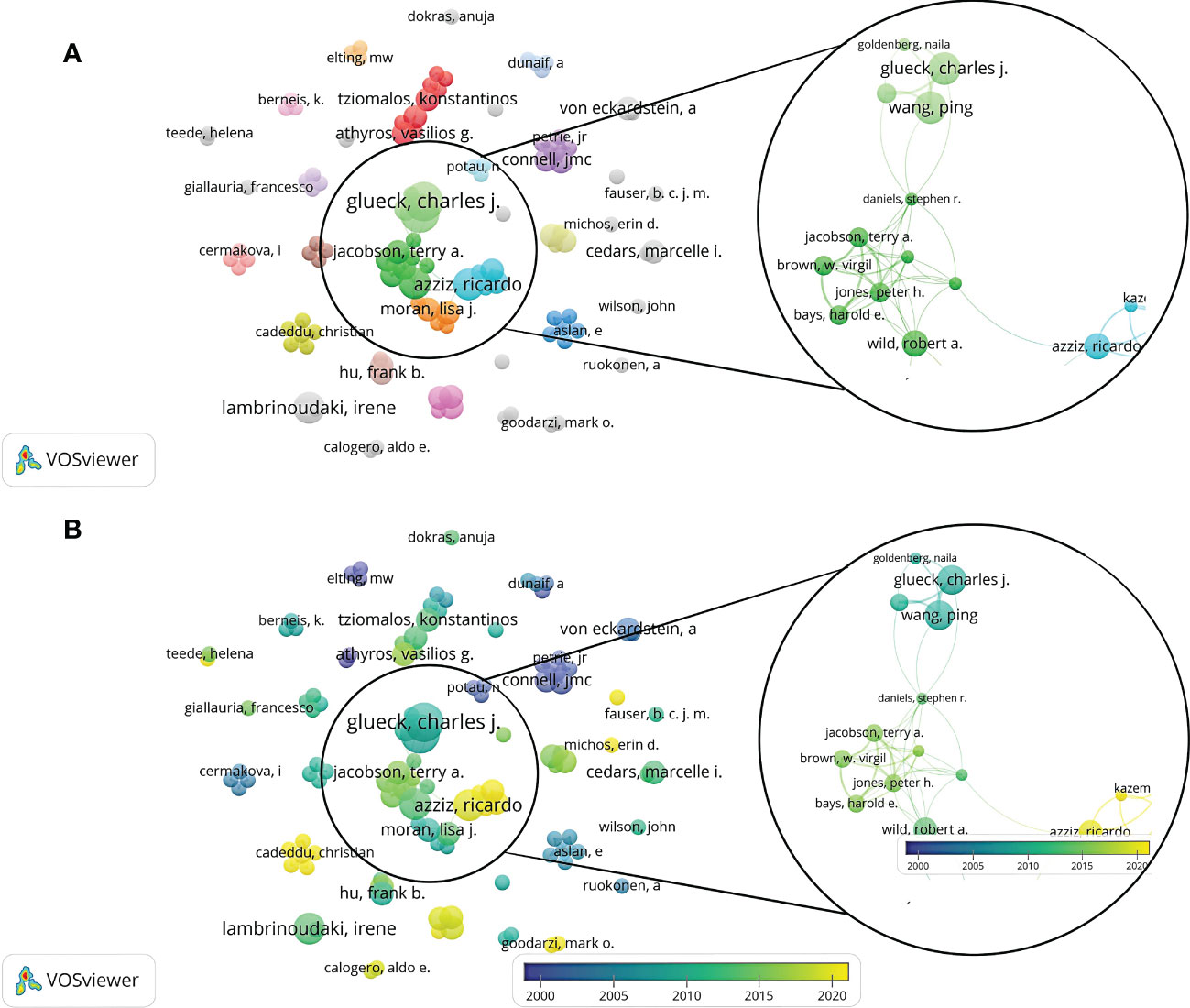
Figure 4 A visualization map of authors. There are nodes (circles) in the figure, representing authors. The larger the circle, the more documents produced by that author. A connection between 2 nodes means that 2 authors appear in a document simultaneously; that is, 2 authors have a cooperative relationship in this document. (A) Network diagram of the authors with a frequency ≥ 2 times. (B) Dynamics and trends of the authors with a frequency ≥ 2 times.
A total of 170 journals were included in related research. The top 11 journals with more than five publications were shown in Table 3. Over the past 23 years, the Journal of clinical endocrinology & metabolism (24 records) showed the highest number of publications, followed by Fertility and sterility (18 records) and Human reproduction (11 records). In addition, the 2021 impact factor (IF) ranged from 2.28 (Gynecological Endocrinology) to 17.18 (Human reproduction update). The 5-year IF ranged from 2.24 (Gynecological Endocrinology) to 19.42 (Human reproduction update). Moreover, the Journal of Clinical Endocrinology and Metabolism represented the highest H-index, indicating the higher quality of studies published in this journal.
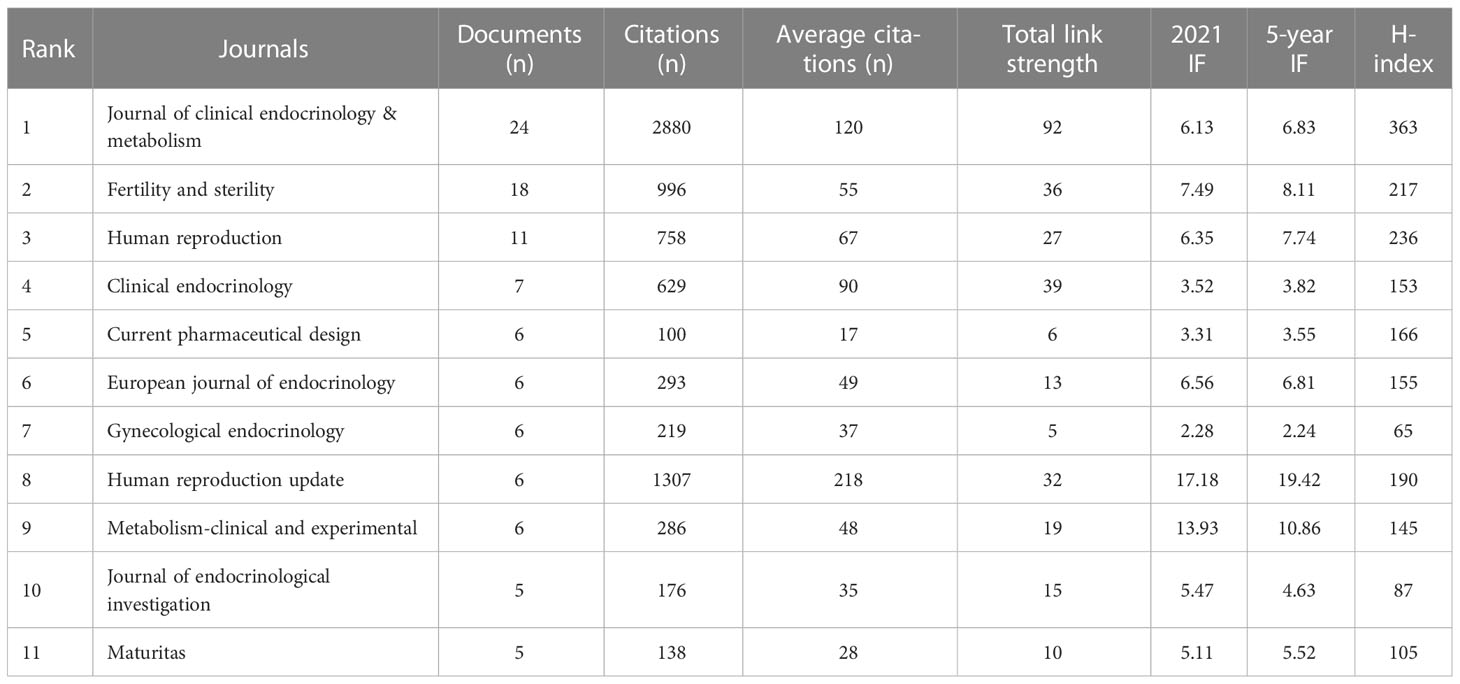
Table 3 Top 11 journals by the number of publications on the relationship of PCOS and CHD from 2000 to 2023.
Of the 312 records, the top 10 publications ranked by citation were listed in Table 4. The most cited article was published in Endocrine Reviews by Frank W Booth et al. in 2012 (15) with 1201 citations, which was higher than that of the second paper (955 citations) (16). The third (17) and fourth (18) cited papers were reviews, with 670 and 523 citations, respectively. The fifth article by Wild S, Pierpoint T et al. (19) conducted a long-term follow-up in women diagnosed with PCOS in the United Kingdom before 1979, which concluded that a history of non-fatal cerebrovascular disease and cardiovascular risk factors, including diabetes, were more common in PCOS women compared with non-PCOS women.
Through the co-citation analysis of the cited documents of related research, the research foundation in this field can be effectively constructed. Of the 23,587 cited references, the minimum number of citations for a single document was set at 20. By analyzing the citation frequency of 23,587 documents, 42 documents reached the threshold (Figure 5). The size of the node corresponds to the frequency of references. Analysis of the top 10 citation publications was represented in Table 5. Of the 3227 journals of cited references, the minimum number of citations for a single journal was set at 100. By analyzing the citation number of the journals, 51 journals reached the threshold (Figure 6). The size of the node corresponds to the number of cited references.
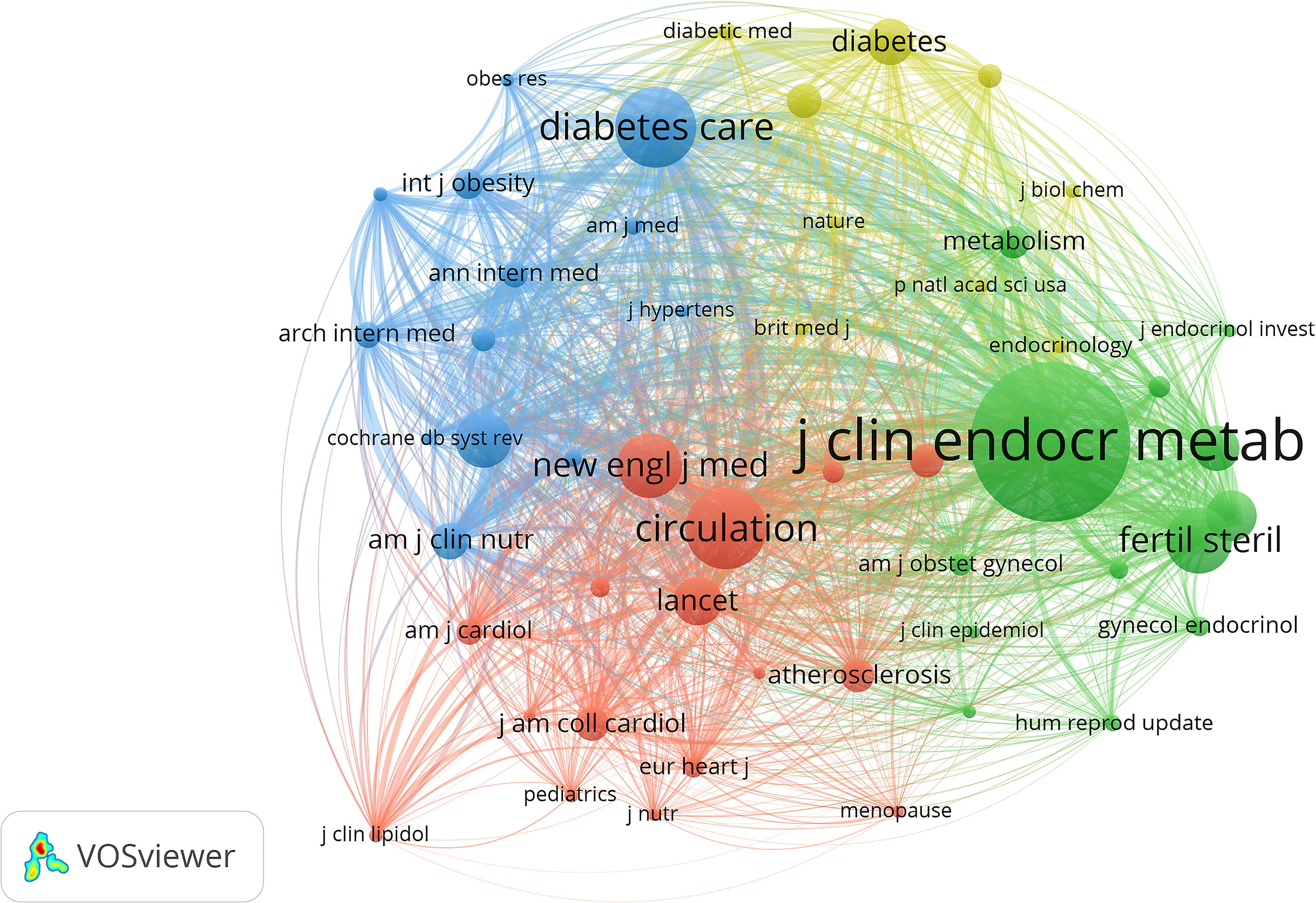
Figure 6 VOSviewer visualization map of most commonly cited journals. Of the 3,227 journals of cited references, the minimum number of citations for a single journal was set at 100; by analyzing the citation number of the journals, 51 journals reached the threshold.
A total of 1,491 keywords were extracted from the retrieved records. The minimum number of keyword occurrences threshold is set to 5, and 115 reached the threshold. The network map of the 115 keywords was were showed in Figure 7A. Table 6 listed the top 10 keywords for each cluster. Table 7 listed the ranking of the top 10 keywords according to their frequency. 4 of the top 10 keywords were clustered in the red cluster. The top 3 keywords were “coronary heart disease (n=1641)”, “polycystic ovary syndrome (n=1614)”, and “insulin-resistance (n=1090)”. Figure 7B shows that the hot research directions of CHD and PCOS in recent ten years were oxidative stress, fatty liver-disease, primary prevention, estrogen plus progestin, insulin sensitivity, genome-wide association, metabolism, and dyslipidemia, et al.
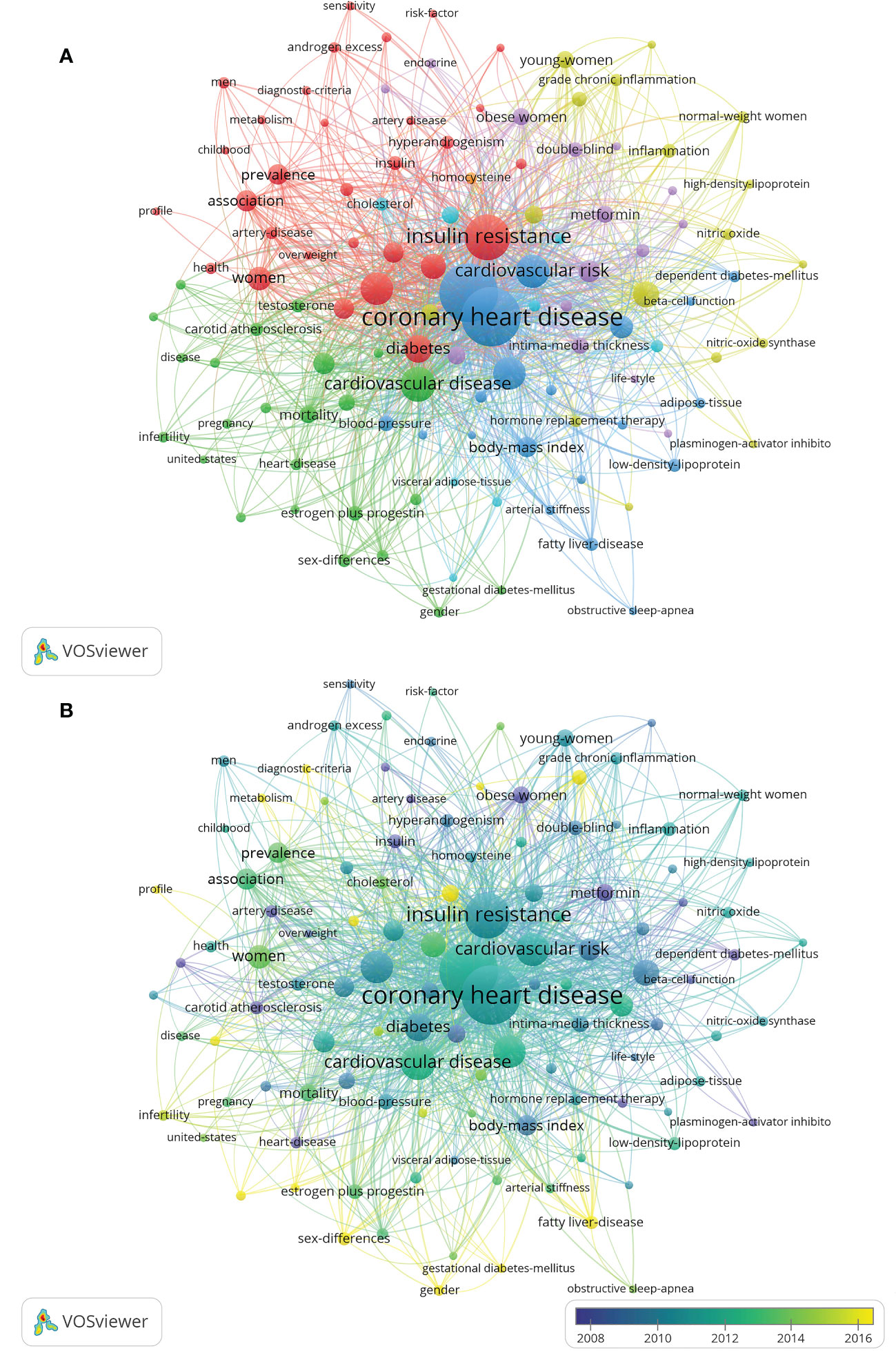
Figure 7 Visualization map of keywords. The minimum number of keyword occurrences threshold is set to 5. Of the 1,491 keywords involved in CHD in PCOS, 115 reached the threshold. (A) Network diagram of the keywords. (B) Dynamics and trends of the keywords.
The keywords were divided into six categories in different colors: (1) the interaction between CHD risk factors and PCOS women; (2) the relationship between cardiovascular disease and female reproductive system hormone secretion; (3) the interaction between CHD and metabolic syndrome; (4) the relationship between c-reactive protein and endothelial function and oxidative stress in PCOS patients; (5) the potential positive effect of metformin on reducing CHD risk factors in PCOS patients; (6) the study of serum cholesterol and body-fat distribution in patients with CHD in PCOS.
Burst words detection results revealed that popular keywords had undergone obvious annual changes (Figure 8). In recent five years, the most popular keywords included “oxidative stress”, “genome-wide association”, “obesity”, “primary prevention”, and “sex difference”.
This study conducted a statistical analysis of the literature on the WOS database to retrieve studies on the association between PCOS and CHD and obtained a total of 312 pieces of literature, generally reflecting the trend and hot spot of related research. Its research fields mainly focused on endocrinology metabolism, obstetrics and gynecology, cardiovascular diseases, and reproductive medicine, et al., indicating that the research field was relatively popular. The distribution trend result showed that the literature was on the rise from 2002 to 2008. However, it appeared to be a steady trend in recent years, which reflected that the research on the relationship between CHD and PCOS had entered a relative bottleneck period.
The publication quantity and citation frequency of literature are important indicators to evaluate the scientific research strength of a certain country, region or institution. The country with the most papers was the United States, and the institutions with the most papers were Harvard University, the University of Athens, and Monash University, which showed that the difference in scientific research levels in different countries and regions was considerably large. It was related to the earlier research start, stronger scientific research ability and economic strength in a handful of countries/regions and institutions. Moreover, there were still issues regarding more cooperation among research teams and cross-regional and cross-institutional communication. It is suggested to coordinate research resources from the level of relevant national departments to facilitate cooperation opportunities and further study of different research institutions.
In terms of journals, the Journal of clinical endocrinology & metabolism, Fertility and sterility, and Human reproduction were the three most publishing journals with relevant articles. In addition, after a comprehensive analysis of the authors’ network, Dr. Wang, Ping, Dr. Glueck, Charles J, Dr. Wild, Robert A, Dr. Lambrinoudaki, Irene, and Dr. Azziz, Ricardo ranked in the top five based on the the amount of publications. The researchers and teams involved in relevant studies should pay attention to cooperation and communication with them in future research. The team of Dr. Wang, Ping and Dr. Glueck, Charles J proposed that metformin safely improved the risk factors of CHD and endocrine diseases and promoted the resumption of normal menstruation in the study of the metformin diet in women with PCOS (20). In addition, the metformin diet effectively and safely reduced body weight and LDL-C while increased HDL-C in women with PCOS and kept these outcomes stable over four years (21). Among the 312 pieces of literature cited most frequently, Booth (2012) (15), Pedersen (2015) (16), Moran (2010) (17), Liu (2003) (22), and Wild (2000) (19) et al.ranked the top 5. The most frequently co-cited pieces of literature were Chang (2004) (23), Talbott (1995) (24), Talbott (2000) (25), Dahlgren (1992) (26), and Wild (2000) (19), et al. A comprehensive analysis of co-citation frequency showed that Wild, Talbott and other representatives had higher citation frequency on the whole. Journals with high co-citation frequency were mainly in the field of reproduction and endocrine metabolism.
A keyword is a high summary and refinement of the literature content, which reflects the core substance and value of the full article and responds to hot spots and research frontiers. Six categories were obtained by cluster analysis of keywords. The first cluster emphasized the association between CHD risk factors and PCOS. CHD risk factors include insulin resistance, diabetes and obesity et al. The incidence of cardiovascular disease (including hypertension and dyslipidemia) was higher in PCOS women than in non-PCOS women (27). Young women with PCOS were at increased risk of myocardial infarction, angina and revascularization, but weight and T2DM were modifiable potential risk factors that warranted intervention (6). A study conducted on Chinese women with PCOS showed that serum lipids, glucose, insulin and homeostasis model assessment of insulin resistance (HOMA-IR) levels were higher in the hypertensives group than in the normotensive group after matching for BMI, which indicated that elevated blood pressure was a marker of metabolic risk and should be measured and monitored in PCOS women (28). In particular, a considerable number of young women with a long course of PCOS disease had a significantly increased risk of CHD and should have a long-term health management plan. Even if treatment measures have been taken for the occurrence of cardiovascular disease, continuous or even lifelong follow-up should be conducted. Although there was a strong association between CHD risk factors and PCOS, no evidence suggested that women with PCOS might be affected by an increased risk of cardiovascular death (29).
The second cluster focused on the relationship between cardiovascular disease and female reproductive system hormone secretion. Testosterone promoted visceral fat accumulation and insulin resistance by inhibiting lipolysis and promoting adipogenesis (30). Furthermore, increased visceral fat was directly related to insulin resistance and carotid intima-media thickness (31, 32). According to existing studies, androgen excess and insulin resistance may be responsible for developing all features of metabolic syndrome in PCOS (29). Future studies should investigate in detail the potential role of androgen excess in determining insulin resistance status, particularly in relation to the high risk of developing T2DM.
The third and sixth clusters demonstrated the interaction between CHD and metabolic syndromes such as diabetes-mellitus and dyslipidemia et al. in PCOS patients. Both obese and non-obese PCOS patients had greater visceral fat than non-PCOS controls (33). Also, visceral fat index (VAI) levels were higher in the PCOS group than non-PCOS control after matching for age and BMI (34). PCOS women were more likely to be obese than in non-PCOS controls and proned to have more visceral fat and a higher visceral fat index, which was strongly associated with insulin resistance (34). Furthermore, studies have shown that lipid accumulation (LAP) measurement can help identify a high cardiometabolic risk subgroup in PCOS patients (35). In summary, PCOS was associated with several endocrine and metabolic diseases, including obesity, insulin resistance and diabetes, hypertension, and dyslipidemia, which increased the risk of subclinical cardiovascular disease (1). Although the presence of lipid abnormalities, fibrinolysis abnormalities, and insulin resistance predictably placed PCOS patients at high risk for cardiovascular disease (36), further and more prospective studies should be conducted to evaluate their correlation.
The fourth cluster illustrated the relationship between CRP, endothelial function, and oxidative stress in PCOS. As previously mentioned, PCOS patients had more visceral fat, its adipocyte hypertrophy triggered an inflammatory response, and hyperandrogenemia exacerbated the inflammatory response (37). Moreover, mononuclear cells in adipose tissue were initiated to secrete pro-inflammatory cytokines in response to glucose and saturated fat uptake (38). Jena et al. studied 58 women newly diagnosed with PCOS and found that inflammatory markers were higher in patients with PCOS than in non-PCOS control after matching for age and BMI (33). In addition, insulin resistance, oxidative stress, elevated plasma homocysteine levels, and changes in lipid profiles (a risk factor for cardiovascular disease) were more frequent in PCOS compared with healthy subjects (39). Serum high-sensitive CRP (HS-CRP) levels in patients with PCOS were positively correlated with BMI, LDL, total cholesterol (TC) and triglyceride and negatively correlated with HDL (40). Previous studies had shown a strong correlation between homocysteine and CRP expression in VSMCs, and elevated homocysteine levels induced CRP expression at the transcriptional and translational levels by controlling the n-methyl-D-aspartate receptor (NMDAr) signaling pathway in VSMCs (41, 42). Elevated homocysteine enhanced platelet adhesion to endothelial cells while promoting the production of pre-thrombotic factors such as tissue plasminogen activators and beta-thromboglobulin (43, 44). Therefore, endothelial dysfunction may be one of the multiple mechanisms that may increase the risk of CHD in PCOS, especially by resulting in arterial hypertension, which was strongly associated with disordered levels of reactive oxygen species (ROS) generated in oxidative stress progress (45).
As for the fifth cluster, we concluded the potential positive effect of metformin on reducing CHD risk factors in PCOS patients. A study by Velazquez et al. (46) included 16 non-diabetic PCOS patients who took metformin (1.5 g/d) for eight weeks. The results found that metformin reduced PCOS patients’ levels of the anti-proto plasminogen activator inhibitor type 1 with a 1.3% decrease in BMI. Metformin may be preferred over combined oral contraceptives (COCs) in patients with PCOS since COCs were associated with an increased risk of thrombosis compared to metformin. What’s more, Burchall et al. (47) studied 60 overweight women with PCOS (mean BMI 37 kg/m2) who were randomly assigned to the metformin group, high-dose oral COC group, or low-dose COC plus spironolactone group. The results showed that abnormal coagulation events were observed in two COC groups but not in the metformin group. In addition, studies have shown that metformin had an advantage in reducing diastolic blood pressure and triglyceride, thereby predicting favorable metabolic and cardiovascular outcomes in women with PCOS. Moreover, metformin was more effective in reducing hyperandrogenemia indicators (48). However, there were different opinions. A previous study had shown that metformin could promote menstrual recovery in PCOS patients to a certain extent but has no regulating effect on fasting insulin, HOMA-IR, blood lipid, androstenedione and other secretory indicators (49). Carotid intima-media thickness (IMT), ambulatory blood pressure monitoring (ABPM) and other cardiovascular risk factors were not significantly affected (50). More studies are needed to explore the role of metformin in reducing the risk of CHD in PCOS patients.
This study detected 25 burst words that appeared from 2000 to 2023. The burst words reflected the emerging trends and abrupt changes in current research hotspots. According to Figure 8, the research hotspots in the recent five years were oxidative stress, genome-wide association, obesity, primary prevention, and sex difference. Since 2017, oxidative stress and genome-wide association have been maintained a high level of attention. Previous literature indicated a significant increase in oxidative stress in PCOS, leading to metabolic dysfunction and cardiovascular disorder features (51, 52). Therefore, the development of prevention and treatment strategies for CHD risk in these patients should involve further research on reducing oxidative stress. With the coming era of big data, genome-wide association study (GWAS) was an effective research strategy for the identification of disease susceptibility genes/loci, which pointed out the direction of PCOS research and laid a solid foundation for personalized diagnosis, prognosis and treatment of PCOS (53–55). Although GWAS provided important clues to the genetic basis of CHD in PCOS, further research is required, such as fine-mapping of susceptibility sites and functional studies of risk genes. Since 2018, burst words gradually turned to obesity and primary prevention, suggesting that the majority of scholars realize the importance of prevention. Also, previous studies suggested that after PCOS was diagnosed in obese women, they should be given primary prevention of CHD during the early stage (56, 57). Moreover, it is worth noting that sex different in the recent three years with a high strength score, and its research duration was shorter than other burst words, indicating the research results were still insufficient and there was less breakthrough research.
Similar to other bibliometrics studies, our research had some shortcomings that must be addressed. Our study’s limitations were as follows: This study only retrieved the most commonly used WOS database, lacking studies on other databases, resulting in some relevant articles may not have been covered. Also, we retrieved articles written in English; thus non-English studies with high quality were not included in our study. Moreover, part of the meta-analysis was recognized as the original article in the WOS database, which may cause discrepancies in the number of reviews and original articles.
This study explored the relationship between PCOS and CHD and displayed a deep insight into the status and trends of the research field with the bibliometric analysis method. In recent five years, the hotspots of the association between PCOS and CHD were oxidative stress, genome-wide association, obesity, primary prevention, and sex difference. Our study had a certain reference value for further research on the relationship between PCOS and CHD and future relevant research topics hunting.
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
XL and HH contributed equally to the research. XL, YW and HH retrieved articles. XL and HH wrote the first draft of the manuscript. HZ and LW performed the data collection. JY provided figures. JF and SF critically revised the paper. SF designed research. JF provided funding support. All authors contributed to the article and approved the submitted version.
The work was supported by the National Natural Science Foundation of China (No.81960464) and the Guangxi Key Research and Development Program (No. Guike AB22080045).
We thank Guangxi Medical University First Affiliated Hospital for supporting our research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gomez JMD, VanHise K, Stachenfeld N, Chan JL, Merz NB, Shufelt C. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil Steril. (2022) 117(5):912–23. doi: 10.1016/j.fertnstert.2022.02.028
2. Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, et al. Polycystic ovary syndrome: a comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci (2022) 23(2):583–614. doi: 10.3390/ijms23020583
3. Ganie MA, Vasudevan V, Wani IA, Baba MS, Arif T, Rashid A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res (2019) 150(4):333–44. doi: 10.4103/ijmr.IJMR_1937_17
4. Scicchitano P, Dentamaro I, Carbonara R, Bulzis G, Dachille A, Caputo P, et al. Cardiovascular risk in women with PCOS. Int J Endocrinol Metab (2012) 10(4):611–8. doi: 10.5812/ijem.4020
5. Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS consensus workshop group. Fertil Steril. (2012) 97(1):28–38.e25. doi: 10.1016/j.fertnstert.2011.09.024
6. Berni TR, Morgan CL, Rees DA. Women with polycystic ovary syndrome have an increased risk of major cardiovascular events: a population study. J Clin Endocrinol Metab (2021) 106(9):e3369–e80. doi: 10.1210/clinem/dgab392
7. Rajendran S, Willoughby SR, Chan WP, Liberts EA, Heresztyn T, Saha M, et al. Polycystic ovary syndrome is associated with severe platelet and endothelial dysfunction in both obese and lean subjects. Atherosclerosis. (2009) 204(2):509–14. doi: 10.1016/j.atherosclerosis.2008.09.010
8. Tosi F, Dorizzi R, Castello R, Maffeis C, Spiazzi G, Zoppini G, et al. Body fat and insulin resistance independently predict increased serum c-reactive protein in hyperandrogenic women with polycystic ovary syndrome. Eur J Endocrinol (2009) 161(5):737–45. doi: 10.1530/EJE-09-0379
9. Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C. Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecol Endocrinol (2007) 23(9):505–10. doi: 10.1080/09513590701554306
10. Wild RA. Polycystic ovary syndrome: a risk for coronary artery disease? Am J Obstet Gynecol (2002) 186(1):35–43. doi: 10.1067/mob.2002.119180
11. Anderson SA, Barry JA, Hardiman PJ. Risk of coronary heart disease and risk of stroke in women with polycystic ovary syndrome: a systematic review and meta-analysis. Int J Cardiol (2014) 176(2):486–7. doi: 10.1016/j.ijcard.2014.06.079
12. Guan C, Zahid S, Minhas AS, Ouyang P, Vaught A, Baker VL, et al. Polycystic ovary syndrome: a "risk-enhancing" factor for cardiovascular disease. Fertil Steril. (2022) 117(5):924–35. doi: 10.1016/j.fertnstert.2022.03.009
13. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3
14. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc (2005) 2005:724–8. doi: 10.2196/27434
15. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol (2012) 2(2):1143–211. doi: 10.1002/cphy.c110025
16. Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. (2015) 25 Suppl 3:1–72. doi: 10.1111/sms.12581
17. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2010) 16(4):347–63. doi: 10.1093/humupd/dmq001
18. Yeap BB. Androgens and cardiovascular disease. Curr Opin Endocrinol Diabetes Obes (2010) 17(3):269–76. doi: 10.1097/MED.0b013e3283383031
19. Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf). (2000) 52(5):595–600. doi: 10.1046/j.1365-2265.2000.01000.x
20. Morrison JA, Glueck CJ, Daniels SR, Horn PS, Wang P. Determinants of ApoB, ApoA1, and the ApoB/ApoA1 ratio in healthy schoolgirls, prospectively studied from mean ages 10 to 19 years: the Cincinnati national growth and health study. Metabolism. (2012) 61(10):1377–87. doi: 10.1016/j.metabol.2012.02.014
21. Glueck CJ, Aregawi D, Agloria M, Winiarska M, Sieve L, Wang P. Sustainability of 8% weight loss, reduction of insulin resistance, and amelioration of atherogenic-metabolic risk factors over 4 years by metformin-diet in women with polycystic ovary syndrome. Metabolism. (2006) 55(12):1582–9. doi: 10.1016/j.metabol.2006.08.001
22. Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev (2003) 24(3):313–40. doi: 10.1210/er.2003-0005
23. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004
24. Talbott E, Guzick D, Clerici A, Berga S, Detre K, Weimer K, et al. Coronary heart disease risk factors in women with polycystic ovary syndrome. Arterioscler Thromb Vasc Biol (1995) 15(7):821–6. doi: 10.1161/01.atv.15.7.821
25. Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol (2000) 20(11):2414–21. doi: 10.1161/01.atv.20.11.2414
26. Dahlgren E, Janson PO, Johansson S, Lapidus L, Oden A. Polycystic ovary syndrome and risk for myocardial infarction. evaluated from a risk factor model based on a prospective population study of women. Acta Obstet Gynecol Scand (1992) 71(8):599–604. doi: 10.3109/00016349209006227
27. Glintborg D, Rubin KH, Nybo M, Abrahamsen B, Andersen M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc Diabetol (2018) 17(1):37. doi: 10.1186/s12933-018-0680-5
28. Shi Y, Cui Y, Sun X, Ma G, Ma Z, Gao Q, et al. Hypertension in women with polycystic ovary syndrome: prevalence and associated cardiovascular risk factors. Eur J Obstet Gynecol Reprod Biol (2014) 173:66–70. doi: 10.1016/j.ejogrb.2013.11.011
29. Pasquali R. Metabolic syndrome in polycystic ovary syndrome. Front Horm Res (2018) 49:114–30. doi: 10.1159/000485995
30. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev (2016) 37(5):467–520. doi: 10.1210/er.2015-1104
31. Tripathy P, Sahu A, Sahu M, Nagy A. Ultrasonographic evaluation of intra-abdominal fat distribution and study of its influence on subclinical atherosclerosis in women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol (2017) 217:18–22. doi: 10.1016/j.ejogrb.2017.08.011
32. Cascella T, Palomba S, De Sio I, Manguso F, Giallauria F, De Simone B, et al. Visceral fat is associated with cardiovascular risk in women with polycystic ovary syndrome. Hum Reprod (2008) 23(1):153–9. doi: 10.1093/humrep/dem356
33. Jena D, Choudhury AK, Mangaraj S, Singh M, Mohanty BK, Baliarsinha AK. Study of visceral and subcutaneous abdominal fat thickness and its correlation with cardiometabolic risk factors and hormonal parameters in polycystic ovary syndrome. Indian J Endocrinol Metab (2018) 22(3):321–7. doi: 10.4103/ijem.IJEM_646_17
34. Durmus U, Duran C, Ecirli S. Visceral adiposity index levels in overweight and/or obese, and non-obese patients with polycystic ovary syndrome and its relationship with metabolic and inflammatory parameters. J Endocrinol Invest. (2017) 40(5):487–97. doi: 10.1007/s40618-016-0582-x
35. Kaluzna M, Czlapka-Matyasik M, Bykowska-Derda A, Moczko J, Ruchala M, Ziemnicka K. Indirect predictors of visceral adipose tissue in women with polycystic ovary syndrome: a comparison of methods. Nutrients. (2021) 13(8):2494–506. doi: 10.3390/nu13082494
36. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev (1997) 18(6):774–800. doi: 10.1210/edrv.18.6.0318
37. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell (2014) 156(1-2):20–44. doi: 10.1016/j.cell.2013.12.012
38. Gonzalez F. Nutrient-induced inflammation in polycystic ovary syndrome: role in the development of metabolic aberration and ovarian dysfunction. Semin Reprod Med (2015) 33(4):276–86. doi: 10.1055/s-0035-1554918
39. Yilmaz M, Bukan N, Ayvaz G, Karakoc A, Toruner F, Cakir N, et al. The effects of rosiglitazone and metformin on oxidative stress and homocysteine levels in lean patients with polycystic ovary syndrome. Hum Reprod (2005) 20(12):3333–40. doi: 10.1093/humrep/dei258
40. Verit FF. High sensitive serum c-reactive protein and its relationship with other cardiovascular risk factors in normoinsulinemic polycystic ovary patients without metabolic syndrome. Arch Gynecol Obstet. (2010) 281(6):1009–14. doi: 10.1007/s00404-009-1226-6
41. Pang X, Liu J, Zhao J, Mao J, Zhang X, Feng L, et al. Homocysteine induces the expression of c-reactive protein via NMDAr-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Atherosclerosis. (2014) 236(1):73–81. doi: 10.1016/j.atherosclerosis.2014.06.021
42. Li Y, Zhao Q, Cao Y, Si J, Li J, Cao K, et al. Probucol decreases homocysteine-stimulated CRP production in rat aortic smooth muscle cells via regulating HO-1/NADPH oxidase/ROS/p38 pathway. Acta Biochim Biophys Sin (Shanghai). (2021) 53(2):212–9. doi: 10.1093/abbs/gmaa163
43. Chen L, Wang B, Wang J, Ban Q, Wu H, Song Y, et al. Association between serum total homocysteine and arterial stiffness in adults: a community-based study. J Clin Hypertens (Greenwich). (2018) 20(4):686–93. doi: 10.1111/jch.13246
44. Faeh D, Chiolero A, Paccaud F. Homocysteine as a risk factor for cardiovascular disease: should we (still) worry about? Swiss Med Wkly (2006) 136(47-48):745–56. doi: 10.4414/smw.2006.11283
45. Duica F, Danila CA, Boboc AE, Antoniadis P, Condrat CE, Onciul S, et al. Impact of increased oxidative stress on cardiovascular diseases in women with polycystic ovary syndrome. Front Endocrinol (Lausanne) (2021) 12:614679. doi: 10.3389/fendo.2021.614679
46. Velazquez EM, Mendoza SG, Wang P, Glueck CJ. Metformin therapy is associated with a decrease in plasma plasminogen activator inhibitor-1, lipoprotein(a), and immunoreactive insulin levels in patients with the polycystic ovary syndrome. Metabolism. (1997) 46(4):454–7. doi: 10.1016/S0026-0495(97)90066-4
47. Burchall GF, Piva TJ, Ranasinha S, Teede HJ. Differential effects on haemostatic markers by metformin and the contraceptive pill: a randomized comparative trial in PCOS. Thromb Haemost. (2017) 117(11):2053–62. doi: 10.1160/TH17-04-0248
48. Soldat-Stankovic V, Popovic Pejicic S, Stankovic S, Jovanic J, Bjekic-Macut J, Livadas S, et al. The effect of myoinositol and metformin on cardiovascular risk factors in women with polycystic ovary syndrome: a randomized controlled trial. Acta Endocrinol (Buchar). (2021) 17(2):241–7. doi: 10.4183/aeb.2021.241
49. Guan Y, Wang D, Bu H, Zhao T, Wang H. The effect of metformin on polycystic ovary syndrome in overweight women: a systematic review and meta-analysis of randomized controlled trials. Int J Endocrinol (2020) 2020:5150684. doi: 10.1155/2020/5150684
50. Sahin Y, Unluhizarci K, Yilmazsoy A, Yikilmaz A, Aygen E, Kelestimur F. The effects of metformin on metabolic and cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). (2007) 67(6):904–8. doi: 10.1111/j.1365-2265.2007.02985.x
51. Rudnicka E, Duszewska AM, Kucharski M, Tyczynski P, Smolarczyk R. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: oxidative stress in polycystic ovary syndrome. Reproduction (2022) 164(6):F145–F54. doi: 10.1530/REP-22-0152
52. Papalou O, Victor VM, Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Curr Pharm Des (2016) 22(18):2709–22. doi: 10.2174/1381612822666160216151852
53. Brower MA, Hai Y, Jones MR, Guo X, Chen YI, Rotter JI, et al. Bidirectional mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod (2019) 34(1):127–36. doi: 10.1093/humrep/dey343
54. Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Large-Scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet (2018) 14(12):e1007813. doi: 10.1371/journal.pgen.1007813
55. Day FR, Hinds DA, Tung JY, Stolk L, Styrkarsdottir U, Saxena R, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun (2015) 6:8464. doi: 10.1038/ncomms9464
56. Geraghty L, Figtree GA, Schutte AE, Patel S, Woodward M, Arnott C. Cardiovascular disease in women: from pathophysiology to novel and emerging risk factors. Heart Lung Circ (2021) 30(1):9–17. doi: 10.1016/j.hlc.2020.05.108
Keywords: polycystic ovary syndrome, coronary heart disease, bibliometric analysis, risk factor, VOSviewer, citespace
Citation: Liang X, He H, Zeng H, Wei L, Yang J, Wen Y, Fan S and Fan J (2023) The relationship between polycystic ovary syndrome and coronary heart disease: a bibliometric analysis. Front. Endocrinol. 14:1172750. doi: 10.3389/fendo.2023.1172750
Received: 23 February 2023; Accepted: 14 April 2023;
Published: 08 May 2023.
Edited by:
Zhenyu Song, Shanghai Jiao Tong University, ChinaReviewed by:
Preeti Khetarpal, Central University of Punjab, IndiaCopyright © 2023 Liang, He, Zeng, Wei, Yang, Wen, Fan and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangtao Fan, anRfZmFuMjAxOEAxNjMuY29t; Siqi Fan, ZnNxMTk4NDQyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.