
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 05 June 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1170881
This article is part of the Research TopicMulti-organ Linkage Pathophysiology and Therapy for NAFLD and NASHView all 12 articles
Objective: In the present network meta-analysis (NMA), we aimed to compare the effectiveness of daily and weekly treatment with glucagon-like peptide-1 receptor agonists for patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM).
Method: We used Stata 17.0 for the NMA. Eligible Randomized controlled trials (RCTs) were searched in PubMed, Cochrane, and Embase databases until December 2022. Two researchers independently screened the available studies. The Cochrane Risk of Bias tool was used to assess the risk of bias in the included studies. We used GRADEprofiler (version3.6) to analyze the evidence certainty. Primary outcomes such as liver fat content (LFC), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels, as well as secondary outcomes such as γ-glutamyltransferase (γGGT) and body weight, were evaluated. Then, each intervention was ranked by the surface under the cumulative ranking curve (SUCRA). As a supplement, we drew forest plots of subgroup using RevMan (version 5.4).
Results: Fourteen RCTs involving 1666 participants were included in the present study. The NMA results showed that exenatide (bid) was the best treatment for improving LFC compared with other agents, liraglutide, dulaglutide, semaglutide (qw) and placebo), and the SUCRA values were 66.8%. Among five interventions (except exenatide (bid) and semaglutide (qw)) evaluated for AST outcome, and six interventions (except exenatide (bid)) evaluated for ALT outcome, semaglutide (qd) was the most effective drug (SUCRA (AST) = 100%, SUCRA (ALT) = 95.6%). The result of LFC in daily group was MD = -3.66, 95% CI [-5.56, -1.76] and in weekly GLP-1RAs group, it was MD = -3.51, 95% CI [-4, -3.02]. As to AST and ALT, the results in daily group versus weekly group were AST: MD = -7.45, 95% CI [-14.57, -0.32] versus MD= -0.58, 95% CI [-3.18, 2.01] and ALT: MD = -11.12, 95% CI [-24.18, 1.95] versus MD = -5.62, 95% CI [-15.25, 4]. The quality of evidence was assessed as moderate or low.
Conclusion: The daily GLP-1RAs may be more effective in primary outcomes. And the daily semaglutide may be the most effective treatment for NAFLD and T2DM among the six interventions.
Nonalcoholic fatty liver disease (NAFLD) refers to the excessive accumulation of fats in the liver caused by factors other than alcohol and drug consumption (1). NAFLD is the most common chronic liver disease, ranging from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH) (2). NAFLD is often closely related to metabolic disorders such as obesity and type 2 diabetes mellitus (T2DM) (3). Moreover, NAFLD is highly likely to progress to cirrhosis and cancer without active intervention, thus reducing the quality of life of patients and leading to psychological and physical burdens. Weight loss remains the basic of treatment for NAFLD and NASH (4). Although weight loss can improve NAFLD, the effect cannot last for an extended period, thus NAFLD requires long-term and adequate treatment with some drugs (5). However, specific drugs for NAFLD are scarce.
Recently, some studies have shown the role of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in NAFLD treatment. GLP-1RAs can control energy intake and weight gain by prolonging gastric emptying and suppressing appetite (6, 7). Furthermore, GLP-1RAs can improve liver enzyme functions and liver steatosis and significantly reduce liver fat content (8–12). Many GLP-1RA preparations are available for selection, which can be divided into daily preparations and weekly preparations according to the frequency of administration. Weekly agents include semaglutide (qw), dulaglutide and exenatide (qw), whereas daily agents include liraglutide, semaglutide (qd), and exenatide (bid), which are commonly used preparations. The elimination half-life of weekly preparations is of several weeks, and their structural peculiarity results in a slow release, thus maintaining effective blood concentrations for a long time, delaying the onset. In contrast, the elimination half-life of daily preparations is shorter, thus providing active circulating concentrations, and effective blood concentrations can be reached earlier (13). Therefore, the efficacies of these two preparations differ. Although GLP-1RAs can significantly improve liver enzyme functions and liver fat content, a comparative study on the effect of weekly and daily GLP-1RAs on NAFLD with T2DM is unavailable.
Thus, in the present network meta-analysis (NMA), we aimed to compare the efficacy of the long-term use of weekly and daily GLP-1RAs for NAFLD with T2DM, hoping to provide a basis for selecting appropriate clinical drugs.
A search for all treatments in NAFLD was conducted across the PubMed databases from the date of inception until December 2022 using the following search strategy: (Liraglutide OR Dulaglutide OR Semaglutide OR Albiglutide OR Lixisenatide OR Exenatide OR glucagon-like peptide-1 agonists OR glucagon like peptide OR GLP-1 receptor agonists OR glp-1) AND (Non-Alcoholic Fatty Liver Disease OR Nonalcoholic Fatty Liver Disease OR Nonalcoholic Fatty Liver OR NAFLD OR Nonalcoholic Steatohepatitis OR NASH) AND (liver enzymes OR alanine aminotransferase OR aspartate aminotransferase OR γ-glutamyl transferase OR ALT OR AST OR γGGT OR intrahepatic fat content OR liver fat content OR intrahepatic content of lipid OR hepatic lipid content OR hepatic fat content OR LFC OR IHF OR IHCL OR HFC) in all fields without other limitations.
And search strategies for PubMed, Cochrane and Embase databases were shown in Table 1.
The paper inclusion criteria were as follows: (1) Subjects: clinically diagnosed as NAFLD or NASH with T2DM; (2) Drug interventions: patients in the experimental group were treated with GLP-1RAs; (3) Study type: randomized controlled trials (RCTs);
The paper exclusion criteria were as follows: (1)Animal models; (2)Duplicate articles; (3) Subjects were aged <18 years; (4) Study duration <12weeks. (5) The outcomes: liver fat content (LFC), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γGGT) and body weight were not clearly reported. (6)The interventions were not GLP-1RAs versus placebo or blank control; (7)Data outcomes could not be extracted.
Study selection and data extraction were conducted separately by two individuals. Two reviewers initially selected the relevant studies by reading the title and abstract and then selected the studies for NMA based on the inclusion and exclusion criteria and after reading the full text. Next, any disagreements were resolved by discussion or by a third researcher.
The extracted data included: 1) the baseline information: the last name of the first author, publication year, intervention and control, sample size (female/male), dose (frequency of application), duration, baseline age (mean ± standard deviation [SD]), T2DM, with or without NASH, and the countries of study population, the characteristics of included studies were listed in Table 2; 2) the data used for analysis: mean and SD changes from the baseline to the end of each outcome, and sample size (n); 3) the information for quality assessment; 4) the items of evidence certainty assessment.
The Cochrane Risk of Bias tool (26) was used to assess the risk of bias of the included studies. The following seven items were included: 1) “random sequence generation”: describes how the sequence was generated, such as by using a random table of numbers or a computer for generating a random sequence of numbers; 2) “allocation concealment”: whether the subjects and researchers were aware of group assignments, such as through assignment hiding via telephone and Internet; 3) “blinding of the participants and personnel”: whether subjects, researchers, and all participants were blinded; 4) “blinding of outcome assessment”: describe whether an outcome assessor was blinded, but objective outcomes, such as serological outcomes, were unlikely to be affected by the lack of blinding; 5) “incomplete outcome data”: whether there was any missing data, such as loss to follow-up and exclusion of data from analysis; 6) “selective reporting”: whether all outcomes were reported; 7) “other bias”: each study was considered to have a “high”, “low”, or “unclear” risk of bias. The judgment of risk of bias was conducted by two authors separately in Review Manager (Version 5.4).
And then, we used GRADE (Grades of Recommendation, Assessment, Development and Evaluation) model to assess the evidence certainty (27). Since all the included studies were RCTs, we evaluated the following five items: 1) risk of bias: such as allocation concealment, blinding and loss to follow-up, and so on; 2) inconsistency: the results heterogeneity, and whether the authors give a reasonable explanation for its high heterogeneity; 3) indirectness; 4) imprecision: whether the confidence interval (CI) was wide and the sample size was large; 5) publication bias: the number of included studies. This assessment was performed in GRADEprofiler (version 3.6).
First, we constructed network plots of the outcomes to demonstrate all available evidence for each outcomes (Figure 1). Second, the outcomes we selected were all continuous variables, and therefore the mean and standard deviation (SD) changes from the baseline to the end and the sample size (n) were extracted for statistical analysis. The existing evidence only involved indirect comparison; therefore, the network graph had no closed loop and there was no need to examine the inconsistency of the outcomes. We employed SUCRA to evaluate the ranking of each intervention in each outcome (Figure 2). The higher the SUCRA value, the more likely the corresponding intervention to be regarded as the best treatment. “Zero” indicated that the treatment was the worst. The forest plots for each outcome were depicted in Figure 3, which shown the comparison between each intervention. The forest plots visually demonstrated the 95% confidence interval (CI) of the results of the pairwise comparison of interventions and whether they had any statistical significance. Finally, league plots were drawn based on SUCRA and the forest plots (Figure 3). The league plots ranked the effect of the intervention in each outcome from the best to the worst (Table 3). The results with statistical significance were highlighted in bold. The league plots more intuitively exhibited the effectiveness of each intervention. All of the abovementioned analyses were conducted by Stat17.0.
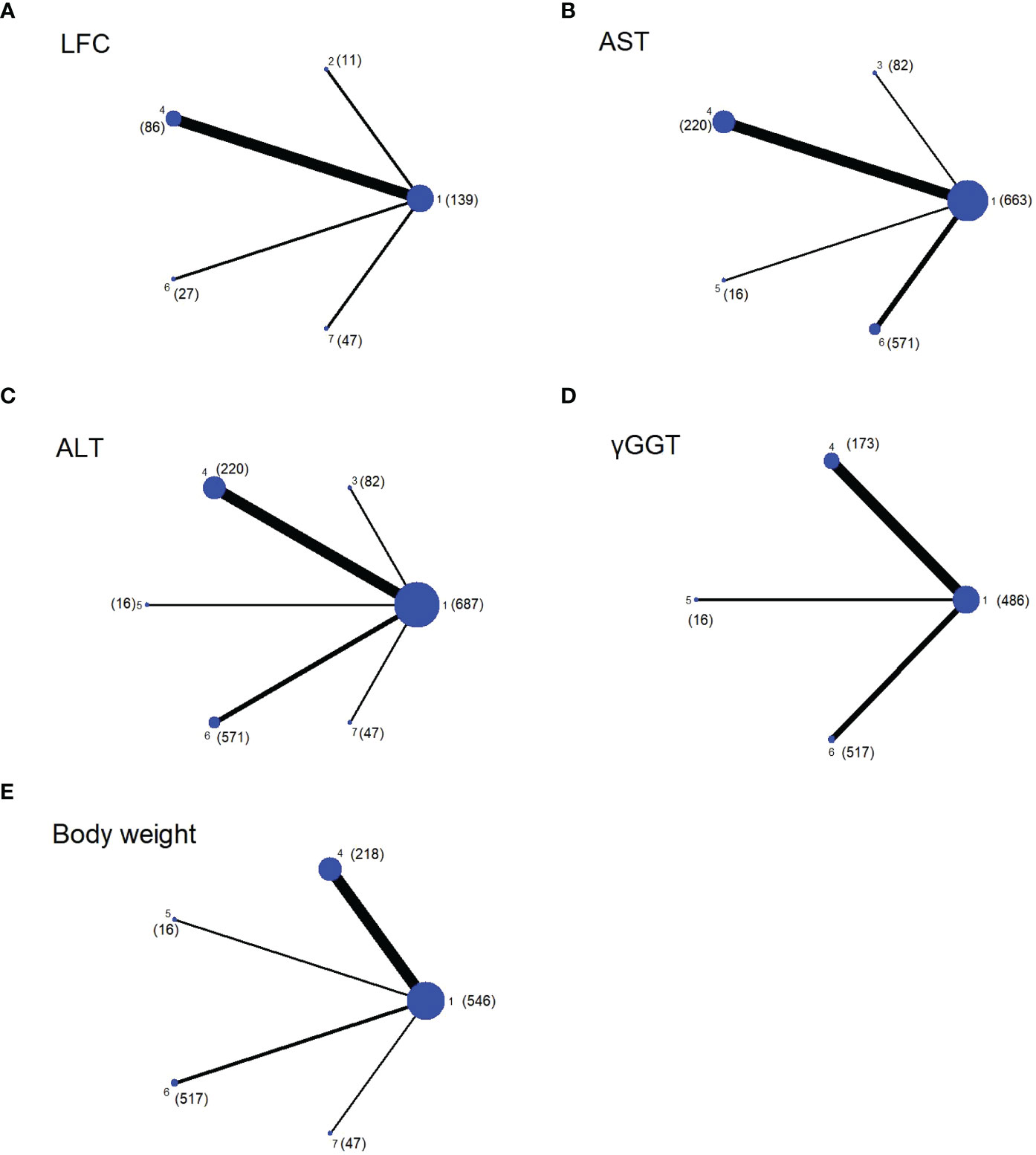
Figure 1 Network plots of evidence for each outcome, with the size of the dots representing the sample size (the specific sample size shown in brackets), and the thickness of the lines representing the number of studies comparing the two interventions. The number mean: 1. Placebo; 2. Exenatide (bid); 3. Semaglutide (qd); 4. Liraglutide; 5. Exenatide (qw); 6. Dulaglutide; 7. Semaglutide (qw). (A) Network plot of LFC; (B) Network plot of AST; (C) Network plot of ALT; (D) Network plot of γGGT; (E) Network plot of Body weight.
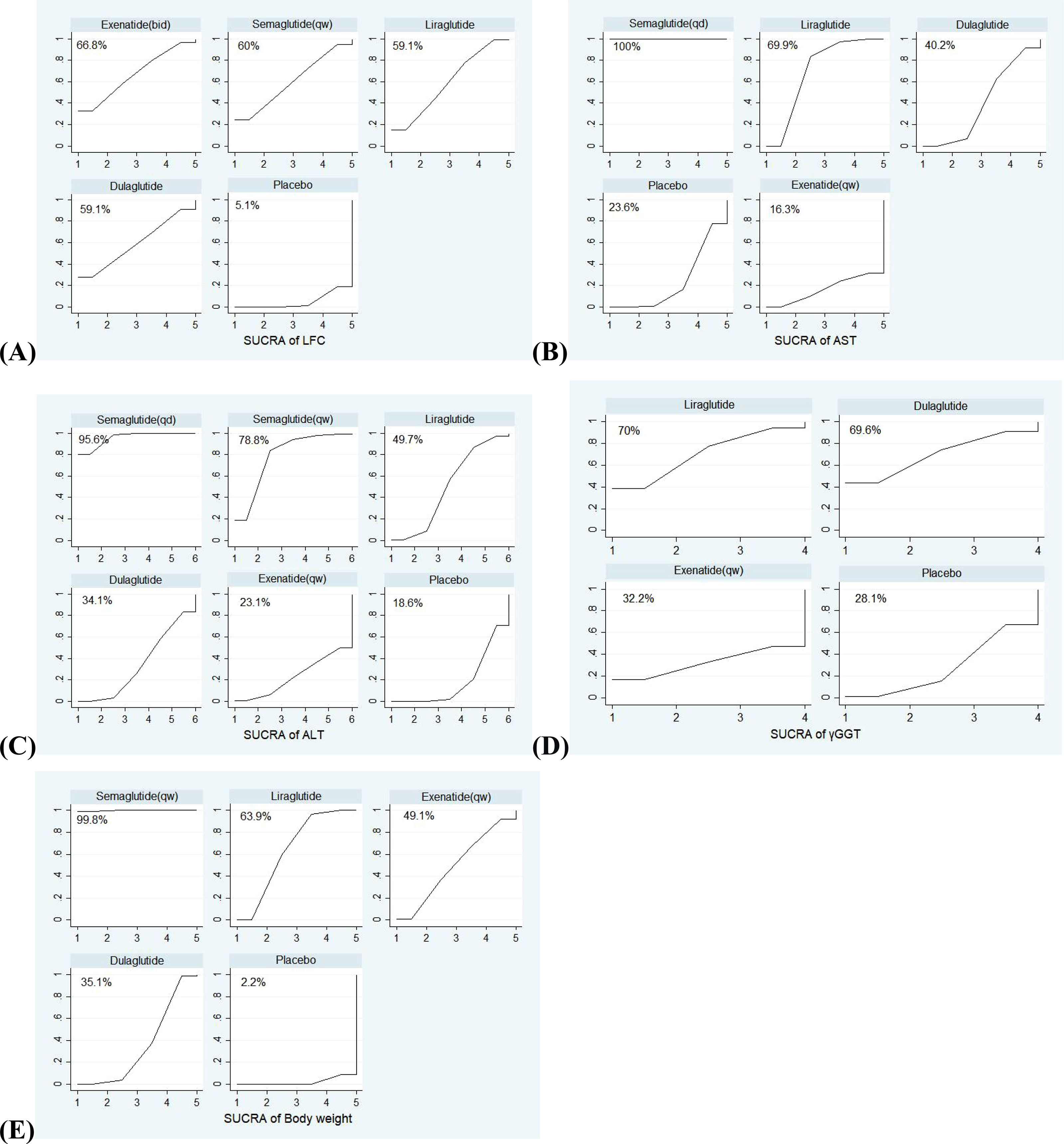
Figure 2 The SUCRA (surface under the cumulative ranking curve) of interventions for each outcome. The larger the surface under the curve, the more likely it is to be the best intervention. (A) SUCRA of LFC; (B) SUCRA of AST; (C) SUCRA of ALT; (D) SUCRA of γGGT; (E) SUCRA of Body weight.
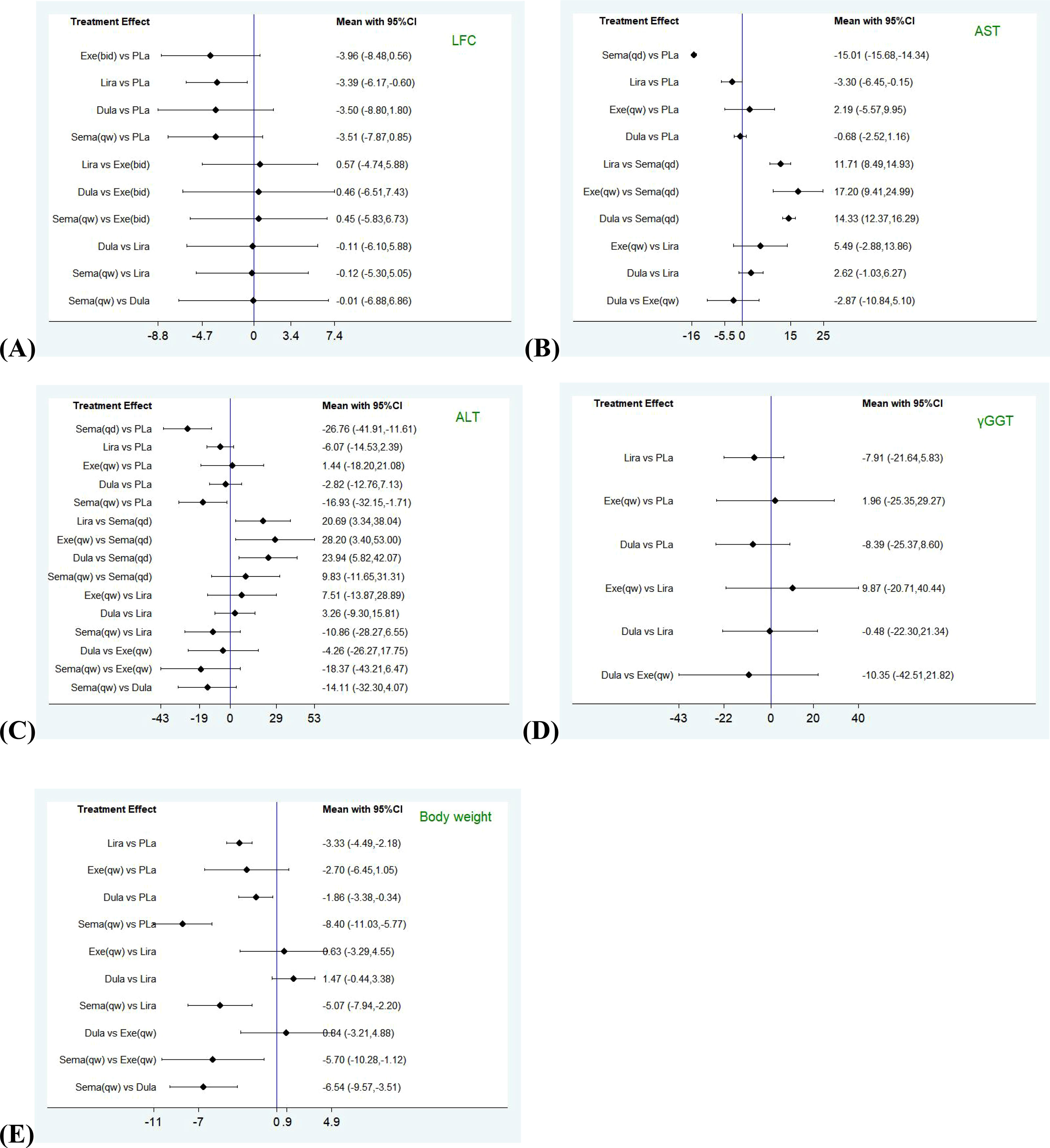
Figure 3 Forest plots comparing pairwise interventions for each outcome (LFC, AST, ALT, γGGT, Body weight). LFC, liver fat content; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGGT, γ-glutamyl transferase. (A) Forest plot comparing pairwise interventions for LFC; (B) Forest plot comparing pairwise interventions for AST; (C) Forest plot comparing pairwise interventions for ALT; (D) Forest plot comparing pairwise interventions for γGGT; (E) Forest plot comparing pairwise interventions for Body weight.
Then, we divided all studies with included outcomes into two subgroups of daily and weekly preparations, drew forest plots (Figure 4) using a random effects model to compared the mean difference (MD) between the two subgroups, and to observe which one was better in each outcome. The above analysis was performed by RevMan (version 5.4).
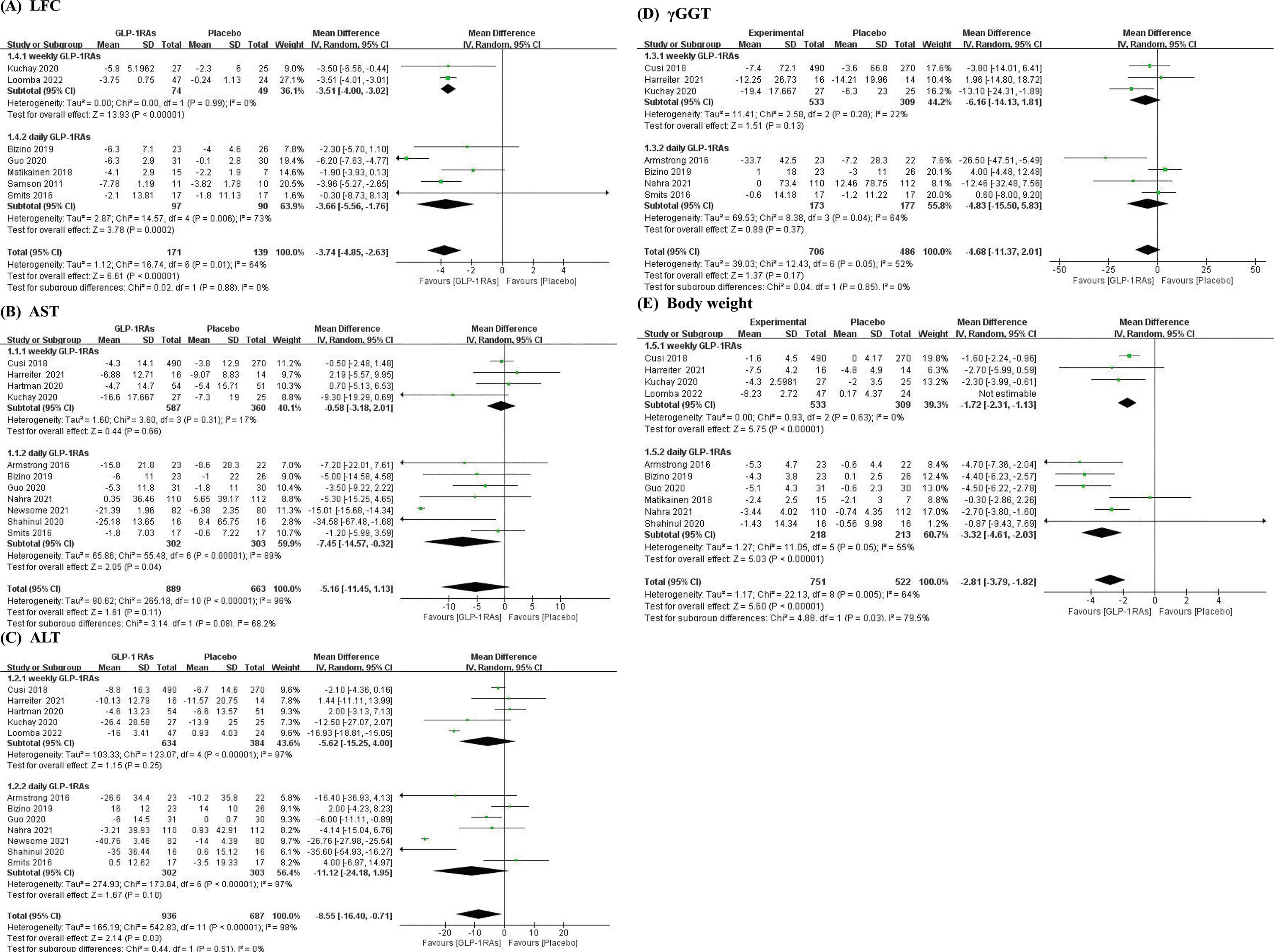
Figure 4 Forest plots of subgroup daily and weekly GLP-1RAs. (A) Subgroup forest plot of LFC; (B) Subgroup forest plot of AST; (C) Subgroup forest plot of ALT; (D) Subgroup forest plot of γGGT; (E) Subgroup forest plot of Body weight.
According to the search strategy, 1055 studies were searched from the following databases: PubMed, 224 studies; Embase, 649 studies; and Cochrane, 182 studies, and 310 duplicate references were removed. According to the inclusion and exclusion criteria, 14 RCTs were finally included in this NMA. The experimental group included five RCTs (12, 14–17) of weekly GLP-1RAs and nine RCTs (9, 18–25) of daily agents. The detailed literature selection process was shown in Figure 5.
As Table 2 shown, the female to male ratio in the study population was approximately 1.19:1. Subjects from all over the world.
The quality of the included studies was assessed by the risk assessment of Cochrane review items. The following aspects were considered during the assessment: random sequence generation, allocation hiding, the blindness of participants and personnel, the blindness of result evaluation, incomplete result data, selective reporting, and other biases. The specific evaluation results were presented in Figure 6.
Using the GRADEprofiler to assess overall quality of evidence. The evaluation results were as follows: two outcomes were assessed as “low”, three outcomes were assessed as “moderate”. The assessment results were shown in Table 4.
All experiments were included in this NMA, and the network evidence graphs of each outcome were shown in Figure 1. Among them, weekly GLP-1RA drugs in the treatment of patients with NAFLD mainly include semaglutide (qw), dulaglutide and exenatide (qw) and daily drugs include liraglutide, semaglutide (qd) and exenatide (bid). However, studies on other GLP-1RAs are scarce. The main outcomes we evaluated were LFC, ALT, and AST. Four drugs (except exenatide (bid) and semaglutide (qw)) showed the AST and five drugs (except exenatide (bid)) showed the ALT outcomes, and four drugs (except semaglutide (qd) and exenatide (qw)) showed the LFC outcome. The secondary outcomes were γGGT and body weight, whereas only three drugs (liraglutide, dulaglutide and exenatide (qw)) showed γGGT outcome, and all drugs, except exenatide (bid) and semglutide (qd), showed body weight outcome.
The SUCRA curves of interventions for outcomes were shown in Figure 2. Among five interventions (exenatide (bid), liraglutide, dulaglutide, semaglutide (qw) and placebo) evaluated for improving LFC, exenatide (bid) was the best (SUCRA = 66.8%, 59.1%, 59.1%, 60%, and 5.1%, respectively). Among five interventions (semaglutide (qd), liraglutide, dulaglutide, exenatide (qw), and placebo) evaluated for AST outcome, and six interventions (semaglutide (qd), liraglutide, dulaglutide, semaglutide (qw), exenatide (qw) and placebo) evaluated for ALT outcome, semaglutide (qd) was the most effective drug (SUCRA (AST) = 100%, SUCRA (ALT) = 95.6%). For AST, followed by liraglutide and dulaglutide (SUCRA (AST) = 69.9%, and 40.2%, respectively); For ALT, followed by semaglutide (qw) and liraglutide (SUCRA (ALT) = 78.8%, and 49.7%, respectively). Finally, the effects of four interventions (liraglutide, dulaglutide, exenatide (qw), and placebo) on γGGT were compared, and liraglutide was the most effective treatment (SUCRA (γGGT) = 70%), and the effects of five interventions (liraglutide, dulaglutide, semaglutide (qw), exenatide (qw), and placebo) on body weight were compared, semaglutide (qw) seemed better than liraglutide (SUCRA (body weight) = 99.8% vs 63.9%).
The forest plots were shown that in all outcomes except γGGT, the daily preparations seemed more effective than weekly ones. The result of LFC in daily GLP-1RAs group was MD = -3.66, 95% CI [-5.56, -1.76] and in weekly GLP-1RAs group, it was MD = -3.51, 95% CI [-4, -3.02], p=0.88. As to AST and ALT, the results in daily GLP-1RAs group versus weekly GLP-1RAs group were AST: MD = -7.45, 95% CI [-14.57, -0.32] versus MD = -0.58, 95% CI [-3.18, 2.01], p=0.08 and ALT: MD = -11.12, 95% CI [-24.18, 1.95] versus MD = -5.62, 95% CI [-15.25, 4], p=0.51. The result of Daily GLP-1RAs group also was better than weekly one in body weight (MD = -3.32, 95% CI [-4.61, -2.03] vs MD = -1.72, 95% CI [-2.31, -1.13], p=0.03). However, the result of γGGT showed contrary to other outcomes (MD daily = -4.83, 95% CI [-15.5, 5.83] vs MD weekly = -6.16, 95% CI [-14.13, 1.81], p=0.85).
In this NMA, we evaluated GLP-1RAs in the treatment of NAFLD to explore the effectiveness of the long-term use of weekly and daily preparations in improving LFC and liver enzymes involved in NAFLD. In the NMA, the subgroup results and SUCRA showed that the daily agents ranked ahead of the weekly agents with respect to primary outcomes. Though SUCRA showed that semaglutide (qw) was better than other agents on body weight, the subgroup results showed that daily group might be the most effective as a whole. Therefore, we speculate that daily agents show greater promise in NAFLD and T2DM treatment. Furthermore, the daily semaglutide seemed to improve ALT more than the weekly semaglutide, which further validated the conclusion.
Presently, NAFLD is often considered a metabolic disorder associated with liver diseases, and liver steatosis is probably closely related to insulin resistance and T2DM (28). Increased fat content and insulin resistance can lead to liver inflammation and fibrosis (29). A meta-analysis of six RCTs shows that liraglutide can improved liver steatosis (8). Moreover, liraglutide can improve liver metabolic dysfunction and insulin resistance which play a role in NASH pathogenesis (30). Therefore, we can potentially use GLP-1RAs to treat NAFLD with T2DM.
Although liver biopsy is the gold standard for NAFLD diagnosis, it is not widely used because of its invasiveness. Therefore, researchers have proposed non-invasive examinations instead to diagnose NAFLD and evaluate therapeutic effects. For example, many meta-analyses use LFC to evaluate the improvement of patients with NAFLD (31, 32). Serum biomarkers, such as ALT and AST, are the most common non-invasive tests to assess liver diseases and are commonly used as the clinical indicators of hepatocyte injuries (33). A 6-month, double-blind, and placebo-controlled study shows that lower ALT levels were associated with LFC (34). Therefore, our primary outcomes for assessing GLP-1RA efficacy were LFC and ALT and AST levels. Furthermore, a systematic review included 23 RCTs of the effects of lifestyle interventions on liver steatosis and shows that reduce LFC and lowered liver transaminase levels are strongly associated with weight loss (35). A 5%–10% weight loss resulted in a 40%–80% reduction in liver fats in patients without diabetes and with type 2 diabetes (36). Thus, we used body weight as a secondary outcome in the present NMA.
The subgroup results showed that the daily preparations might be superior to the weekly preparations with respect to primary outcome. And SUCRA showed that semaglutide (qd) might be the best GLP-1RAs among six GLP-1RAs included in our NM. The efficacy of semaglutide (qd) was markedly superior in terms of ALT and AST. A 2019 study shows that semaglutide significantly reduces ALT levels (37), and an RCT by Anne Flint et al. published in 2021 shows that semaglutide significantly improves ALT and AST levels (38). Second, the daily GLP-1RAs significantly reduced LFC and body weight compared with the weekly agents. A 24-week RCTs show that exenatide (bid) can reduce the primary outcome, LFC (10). Although semaglutide (qw) also reduced LFC, the SUCRA values showed that it was slightly less likely to be the optimal treatment than exenatide (bid). However, a NMA compared efficacy and safety of 8 GLP-1RAs show that exenatide (bid) have an increased risk of adverse events withdrawals compared to semaglutide (qw) (39). For body weight, a study including 387 participants found that weight loss with semaglutide (qw) was significantly greater than that with liraglutide (40). And two meta-analyses showed that more significant weight loss was observed after liraglutide intervention than dulaglutide and other GLP-1RA interventions (41, 42). Semaglutide and liraglutide induce weight loss by lowering energy intake (43, 44), but semaglutide can also reduce weight by reducing appetite (44), which is not obvious in liraglutide (43), this may be the reason why semaglutide is more significant in weight loss. To summarize, daily preparations may be better in the treatment of NAFLD with T2DM. Of course, due to the small number of weekly agents studies included, more weekly agents versus placebo RCTs are needed to validate our results.
GLP-1RAs have been a popular hypoglycemic drug in recent years. Apart from hypoglycemic and weight loss effects, GLP-1RAs are also of great research value in NAFLD. However, no studies have compared the efficacy of daily and weekly GLP-1RA treatments for NAFLD with T2DM yet. Therefore, we adopted the NMA method to comprehensively analyze the effect of several commonly used GLP-1RAs on the reduction of LFC, liver enzymes, and body weight in patients with NAFLD and T2DM and to obtain an optimal treatment. However, we included only five studies on the weekly agents, which was limited in number and may lead to weak evidence, thus RCTs including more studies on weekly agents vs. placebo are needed to validate the present results. Moreover, due to the lack of direct comparative studies of the two GLP-1RAs, we cannot analyze inconsistent. The league plots showed a comparison between liraglutide and the placebo, showing that the major outcome, LFC, was statistically significant; however, the rest of the results were not statistically significant, which might be because of the small sample size. And there is only one study of semaglutide(qw), thus more studies of weekly semaglutide are needed to compare with daily exenatide.to assess which is superior in LFC. In the future, more large-sample, head-to-head RCTs are required to confirm these findings.
We integrated the evidence on GLP-1RAs for NAFLD with T2DM treatment and concluded that the daily preparations were superior to the weekly preparations with respect to primary outcome. We found that the daily GLP-1RAs semaglutide among the six GLP-1RAs ((exenatide (bid), liraglutide, semaglutide (qd), dulaglutide, semaglutide (qw), exenatide (qw)) might be the most effective treatment options for NAFLD. This conclusion may provide a basis for clinicians to treat NAFLD with T2DM.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
XY and ZG contributed to the conception and design of the study. XY and CY searched the databases and screened the literature. XY and KD participated in data extraction. XY performed the statistical analysis. The first draft of this article was written by XY. All authors contributed to the article and approved the submitted version.
We would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol (2020) 73(1):202–9. doi: 10.1016/j.jhep.2020.03.039
2. Verbeek J, Cassiman D, Lannoo M, Laleman W, van der Merwe S, Verslype C, et al. Treatment of non-alcoholic fatty liver disease: can we already face the epidemic? Acta Gastroenterol Belg (2013) 76(2):200–9.
3. Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology (2020) 158(7):1851–64. doi: 10.1053/j.gastro.2020.01.052
4. Raza S, Rajak S, Upadhyay A, Tewari A, Anthony Sinha R. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front Biosci (Landmark Ed). (2021) 26(2):206–37. doi: 10.2741/4892
5. Francque S, Vonghia L. Pharmacological treatment for non-alcoholic fatty liver disease. Adv Ther (2019) 36(5):1052–74. doi: 10.1007/s12325-019-00898-6
6. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab (2018) 27(4):740–56. doi: 10.1016/j.cmet.2018.03.001
7. Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab (2017) 19(9):1242–51. doi: 10.1111/dom.12932
8. Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther (2013) 37(2):234–42. doi: 10.1111/apt.12149
9. Guo W, Tian W, Lin L, Xu X. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty-six weeks: a randomized placebo-controlled trial. Diabetes Res Clin Pract (2020) 170:108487. doi: 10.1016/j.diabres.2020.108487
10. Liu L, Yan H, Xia M, Zhao L, Lv M, Zhao N, et al. Efficacy of exenatide and insulin glargine on nonalcoholic fatty liver disease in patients with type 2 diabetes. Diabetes Metab Res Rev (2020) 36(5):e3292. doi: 10.1002/dmrr.3292
11. Yan J, Yao B, Kuang H, Yang X, Huang Q, Hong T, et al. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology (2019) 69(6):2414–26. doi: 10.1002/hep.30320
12. Kuchay MS, Krishan S, Mishra SK, Choudhary NS, Singh MK, Wasir JS, et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia (2020) 63(11):2434–45. doi: 10.1007/s00125-020-05265-7
13. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
14. Cusi K, Sattar N, García-Pérez LE, Pavo I, Yu M, Robertson KE, et al. Dulaglutide decreases plasma aminotransferases in people with type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabetes Med (2018) 35(10):1434–9. doi: 10.1111/dme.13697
15. Harreiter J, Just I, Leutner M, Bastian M, Brath H, Schelkshorn C, et al. Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24-week, prospective, randomized, placebo-controlled pilot trial. Diabetes Obes Metab (2021) 23(5):1129–39. doi: 10.1111/dom.14319
16. Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care (2020) 43(6):1352–5. doi: 10.2337/dc19-1892
17. Loomba R, Abdelmalek MF, Armstrong M, Jara M, Kjaer M, Krarup N, et al. Semaglutide 2.4 mg once weekly improved liver and metabolic parameters, and was well tolerated, in patients with nonalcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. J Hepatol (2022) 77(S1):S1–S118. doi: 10.1016/S0168-8278(22)00440-8
18. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet (2016) 387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X
19. Bizino MB, Jazet IM, de Heer P, van Eyk HJ, Dekkers IA, Rensen PCN, et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia (2020) 63(1):65–74. doi: 10.1007/s00125-019-05021-6
20. Matikainen N, Söderlund S, Björnson E, Pietiläinen K, Hakkarainen A, Lundbom N, et al. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: a single-centre randomized controlled study. Diabetes Obes Metab (2019) 21(1):84–94. doi: 10.1111/dom.13487
21. Nahra R, Wang T, Gadde KM, Oscarsson J, Stumvoll M, Jermutus L, et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study [published correction appears in diabetes care. 2022 Dec 1;45(12):3112]. Diabetes Care (2021) 44(6):1433–42. doi: 10.2337/dc20-2151
22. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med (2021) 384(12):1113–24. doi: 10.1056/NEJMoa2028395
23. Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Pouwels PJ, Pieters-van den Bos IC, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia (2016) 59(12):2588–93. doi: 10.1007/s00125-016-4100-7
24. Samson SL, Sathyanarayana P, Jogi M, Gonzalez EV, Gutierrez A, Krishnamurthy R, et al. Exenatide decreases hepatic fibroblast growth factor 21 resistance in non-alcoholic fatty liver disease in a mouse model of obesity and in a randomised controlled trial. Diabetologia (2011) 54(12):3093–100. doi: 10.1007/s00125-011-2317-z
25. Shahinul A, Helen KA, Saiful I, Kamrul A. Effect of liraglutide therapy on hepatic steatosis and liver stiffness in patients with nonalcoholic fatty liver disease. Hepatol Int (2020) 14(Suppl 1):S355. doi: 10.1007/s12072-020-10030-4
26. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
27. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
28. Luyckx FH, Lefebvre PJ, Scheen AJ. Non-alcoholic steatohepatitis: association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab (2000) 26(2):98–106.
29. Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol (2011) 300(5):G697–702. doi: 10.1152/ajpgi.00426.2010
30. Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol (2016) 64(2):399–408. doi: 10.1016/j.jhep.2015.08.038
31. Wong C, Yaow CYL, Ng CH, Chin YH, Low YF, Lim AYL, et al. Sodium-glucose Co-transporter 2 inhibitors for non-alcoholic fatty liver disease in Asian patients with type 2 diabetes: a meta-analysis. Front Endocrinol (Lausanne). (2021) 11:609135. doi: 10.3389/fendo.2020.609135
32. Ghosal S, Datta D, Sinha B. A meta-analysis of the effects of glucagon-like-peptide 1 receptor agonist (GLP1-RA) in nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes (T2D). Sci Rep (2021) 11(1):22063. doi: 10.1038/s41598-021-01663-y
33. Luo Q, Wei R, Cai Y, Zhao Q, Liu WJ, Liu A, et al. Efficacy of off-label therapy for non-alcoholic fatty liver disease in improving non-invasive and invasive biomarkers: a systematic review and network meta-analysis of randomized controlled trials. Front Med (Lausanne). (2022) 9:793203. doi: 10.3389/fmed.2022.793203
34. Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A, et al. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab (2015) 100(4):1578–85. doi: 10.1210/jc.2014-3794
35. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol (2012) 56(1):255–66. doi: 10.1016/j.jhep.2011.06.010
36. Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, , Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care (2006) 29(6):1337–44. doi: 10.2337/dc05-2565
37. Newsome P, Francque S, Harrison S, Ratziu V, Van Gaal L, Calanna S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther (2019) 50(2):193–203. doi: 10.1111/apt.15316
38. Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther (2021) 54(9):1150–61. doi: 10.1111/apt.16608
39. Xia L, Shen T, Dong W, Su F, Wang J, Wang Q, et al. Comparative efficacy and safety of 8 GLP-1RAs in patients with type 2 diabetes: a network meta-analysis. Diabetes Res Clin Pract (2021) 177:108904. doi: 10.1016/j.diabres.2021.108904
40. Rubino DM, Greenway FL, Khalid U, Su F, Wang J, Wang Q, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA (2022) 327(2):138–50. doi: 10.1001/jama.2021.23619
41. Singh S, Wright EE Jr, Kwan AY, Su F, Wang, J, Wang Q, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab (2017) 19(2):228–38. doi: 10.1111/dom.12805
42. Chang KC, Shao SC, Kuo S, Yang CY, Chen HY, Chan YY, et al. Comparative effectiveness of dulaglutide versus liraglutide in Asian type 2 diabetes patients: a multi-institutional cohort study and meta-analysis. Cardiovasc Diabetol (2020) 19(1):172. doi: 10.1186/s12933-020-01148-8
43. Van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). (2014) 38(6):784–93. doi: 10.1038/ijo.2013.162
Keywords: glucagon-like peptide-1 receptor agonists, nonalcoholic fatty liver disease, type 2 diabetes, liver fat content, alanine aminotransferase, aspartate aminotransferase
Citation: Yuan X, Gao Z, Yang C, Duan K, Ren L and Song G (2023) Comparing the effectiveness of long-term use of daily and weekly glucagon-like peptide-1 receptor agonists treatments in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus: a network meta-analysis. Front. Endocrinol. 14:1170881. doi: 10.3389/fendo.2023.1170881
Received: 21 February 2023; Accepted: 22 May 2023;
Published: 05 June 2023.
Edited by:
Takefumi Kimura, Shinshu University, JapanReviewed by:
Velia Cassano, University of Magna Graecia, ItalyCopyright © 2023 Yuan, Gao, Yang, Duan, Ren and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Gao, Z3poZTIwMjBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.