
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 16 October 2023
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1167508
This article is part of the Research Topic Current Perspectives in the Diagnosis of Parathyroid Disease View all 8 articles
Background: Complete resection of the tumor and the ipsilateral thyroid lobe at the primary surgery is the “gold standard” for the treatment of parathyroid carcinoma (PC). However, differences in the overall survival (OS) of patients with PC who underwent partial and total surgical resection remain to be determined.
Methods: Data on patients with PC who underwent partial and total surgical resection were extracted from the Surveillance, Epidemiology and End Results (SEER) database (2000–2018). The X-tile software (https://medicine.yale.edu/lab/rimm/research/software/) was used to define the optimal cut-off values for continuous variables. The inverse probability of treatment weighting (IPTW) method was used to reduce the selection bias. IPTW-adjusted Kaplan–Meier curves and Cox proportional hazards models were used to compare the OS of patients with PC in the partial and total surgical resection groups.
Results: A total of 334 patients with PC were included in this study (183 and 151 in the partial and total surgical resection groups, respectively). The optimal cut-off values for age at diagnosis were 53 and 73 years, respectively, while that for tumor size was 34 mm. In both the Kaplan–Meier analysis and univariable Cox proportional hazards regression analysis before IPTW, the difference in OS between the partial and total surgical resection groups was not statistically significant (p>0.05). These findings were confirmed in the IPTW-adjusted Kaplan–Meier analysis and multivariate Cox proportional hazards regression analysis (p>0.05). Subgroup analysis revealed that total surgical resection was beneficial for OS only in the subgroup with unknown tumor size.
Conclusion: There was no significant difference in the prognosis of patients who underwent partial and total surgical resection. This finding may provide a useful reference for the treatment of PC.
Parathyroid carcinoma (PC) is one of the rarest malignancies of the endocrine system. It accounts for 0.005% of all malignancies in this system, and only approximately 5% of all cases of primary hyperparathyroidism. Since 2001, the incidence of PC has remained relatively stable (i.e., 10–13 cases per 10 million individuals) (1). PC exhibits a similar frequency in both men and women, mostly developing between 45 and 59 years of age (2, 3). Germline heterozygous inactivating mutations of the CDC 73 oncogene account for approximately 50-75% of familial PCs; more than 75% of disseminated PCs have a double allele somatic inactivation/loss of CDC 73 (4). The cause of PC is unknown and may be related to genetic factors or hyperparathyroidism (5). Moreover, the diagnosis of PC is difficult owing to its clinical, radiological, and histological similarity to parathyroid adenoma (6). The rate of PC progression is slow; the rates of lymph node and distant metastasis are approximately 5% and 2%, respectively (7, 8). In previous reports, the 5- and 10-year survival rates of patients with PC were approximately 80% and 65%, respectively (9). Most deaths among those patients are attributed to uncontrolled hypercalcemia rather than target organ damage due to tumor load (10). Thus far, a prognostic staging system for patients with PC (similar to the TNM staging system) has not been developed, and there is no consensus regarding the treatment of PC (11).
Surgery is an important predictor of PC prognosis and the only curative treatment for this disease (12). Previous studies on PC recommended radical surgery with microscopic negative margins (13). Total resection should include the tumor, as well as the ipsilateral well-defined thyroid lobes and adjacent involved structures. Lymph nodes in the tracheoesophagus, paratracheal, and upper mediastinum should also be removed (14). Limited parathyroidectomy is associated with a significantly worse prognosis than extensive tumor resection (15–17). However, a lack of significant correlation between radical resection and improved survival has been observed in other studies (13, 18).
Owing to the rarity of PC and the lack of prospective studies, investigations based on small samples produce conflicting results. The prognostic impact of the extent of surgery on patients with early-stage PC is unclear. Therefore, our study was designed to assess the difference in the long-term prognosis of patients with early-stage PC who underwent partial and total surgical resection. For this purpose, we used data from the Surveillance, Epidemiology, and End Results (SEER) database. Inverse probability of treatment weighting (IPTW) was used to reduce the selection bias.
This study cohort was retrieved from the SEER program; this database records patient data from 18 population-based cancer registries, covering approximately 27.8% of the population of the United States of America (19). The SEER registries collect data on the demographics, tumor characteristics, stage at diagnosis, primary tumor site, initial course of treatment, and follow-up status of patients with cancer. The data used in this study are publicly available, plus version data from April 2021, containing information on treatment for the period 2000–2018. All data were downloaded through the SEER*Stat software (Version 8.4.0, https://seer.cancer.gov/seerstat/). SEER is an open-access public database, and private patient information has been officially withheld from this database. Hence, approval from an institutional review board was not required.
The criteria for data extraction were as follows (1): date from 2000 to 2018; (2) primary tumor site was C75.0 - parathyroid gland; and (3) diagnosis of parathyroid cancer by experienced pathologists. We extracted patient demographic variables (age at diagnosis, sex, race, and marital status), tumor characteristics (tumor size, number of malignant tumors, lymph node status, and distant metastases), treatment status (regional lymph node dissection, radiotherapy, and surgical modality), months of survival, and survival status. The exclusion criteria were: (1) unknown duration of survival; (2) surgical procedures other than partial and total resection; (3) presence of lymph node metastases; (4) lymph nodes and distant metastases at the time of diagnosis; and (5) incomplete information (Figure 1).
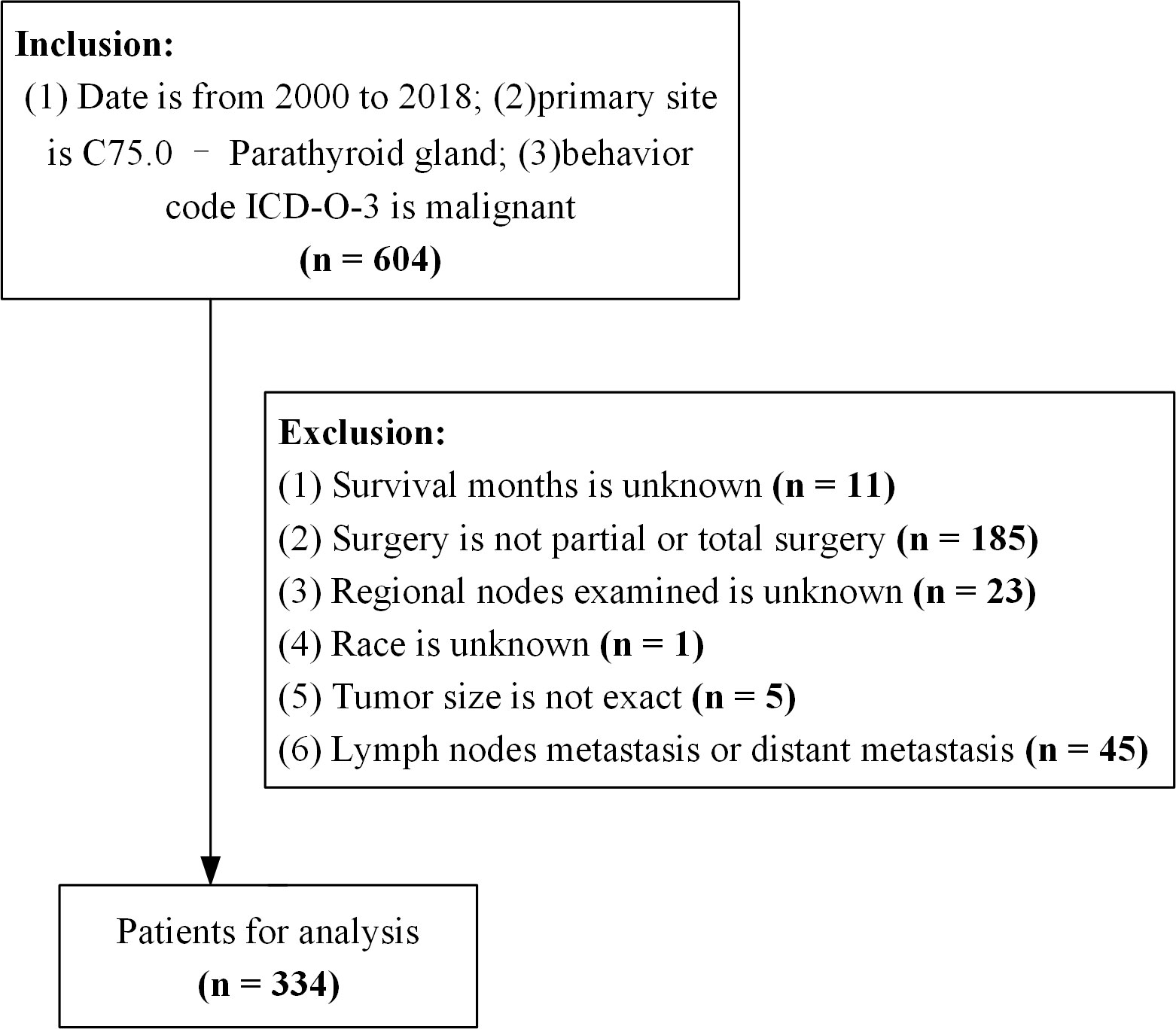
Figure 1 Flowchart of the data process. ICD-O-3, International Classification of Disease for Oncology version 3.
Variable reassignment was not performed for sex and race in the extracted data. Age at diagnosis and tumor size were converted to categorical variables after determining the cut-off values using the X-tile software (https://medicine.yale.edu/lab/rimm/research/software/). Marital status was categorized as “married at diagnosis” and “other”, the number of malignancies was categorized as “single” and “multiple”, radiotherapy was categorized as “yes” and “no/unknown”, and regional nodes examined were categorized as “yes” or “no”; the above variables were binary processed. According to the SEER Program Surgery Codes Manual, surgical procedure codes 30 and 40 denote partial and total surgical resection. In addition, the patient profiles were screened for lymph nodes and distant metastases based on variables, such as the extent of disease for regional nodes. According to the explanation of variables in the SEER database, overall survival (OS) was the endpoint of interest in this study. OS was defined as the period from the date of PC diagnosis to that of death from any cause.
Categorical variables are shown as frequencies and their proportions. The baseline characteristics of patients before and after matching were assessed using standard mean difference (SMD); a value >0.1 indicated that the information was unbalanced between the groups (20). Adjustment of observed differences in baseline covariates between the two intervention groups was performed using the IPTW method to eliminate selection bias (21). Univariate analysis of the impact of patient characteristics on OS was performed using the Kaplan–Meier method; the log rank method (Mantel–Cox) was utilized to assess statistical significance. Variables with a p-value <0.2 in the univariate analysis were included in a multivariate Cox proportional hazards regression model to investigate the effect of both surgical procedures on OS. Statistical analyses and data visualization were performed using R (https://www.r-project.org/, version 4.2.1) in the RStudio environment (https://posit.co/download/rstudio-desktop/, version 2022.12.0 + 353). All statistical analyses involved two-sided testing, and p-values <0.05 denoted statistically significant differences.
A total of 334 PC patients met the inclusion criteria with a median follow-up time of 205 months. Of those, 183 and 151 patients underwent partial and total surgical resection, respectively. After processing, 53 and 73 years were identified as the best cut-off values for age at diagnosis, while 34 mm was the best cut-off value for tumor size. Of the patients, 53.6% were male, 36% were aged <53 years, 51% were aged 53–73 years, and 62% were married at the time of diagnosis. Moreover, 50% of patients had a tumor size <34 mm, and 74% had a single primary malignancy. Radiotherapy and regional lymph node testing were performed in 10% and 34% of the patients, respectively. In the unweighted data, the SMDs of age at diagnosis, sex, race, tumor size, and radiotherapy were >0.1, thereby denoting bias in the original data. After IPTW adjustment, the SMDs of all variables were <0.1, indicating that the partial and total surgical resection groups were comparable (Table 1).
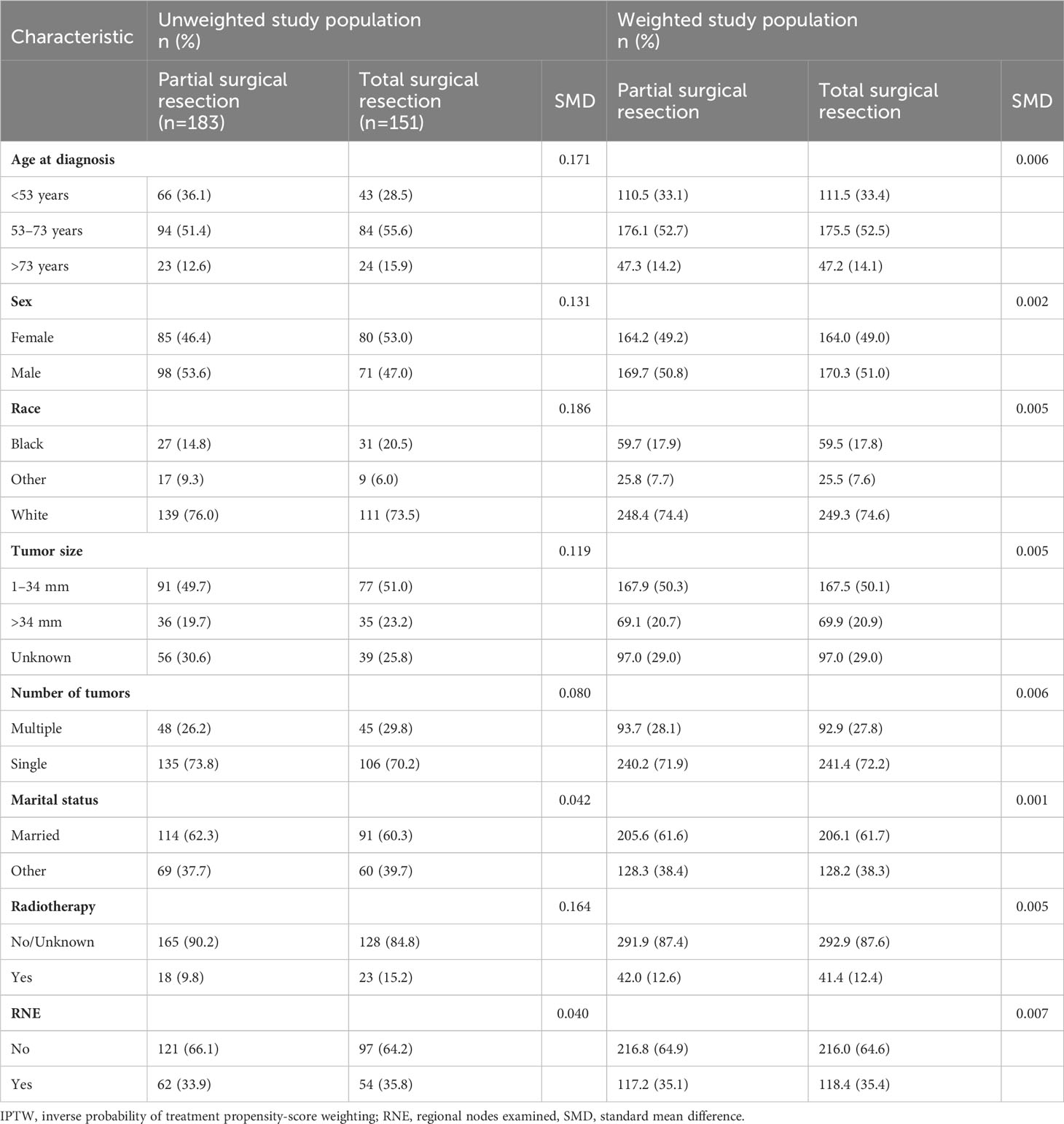
Table 1 Selected baseline characteristics of patients who underwent partial and total surgical resection before and after IPTW.
Kaplan–Meier analysis did not show a statistical difference in the OS of patients with PC between the partial and total surgical resection groups before IPTW (p=1.000) (Figure 2A). This result was confirmed in the IPTW-adjusted Kaplan–Meier analysis (p=1.000 in the IPTW-adjusted log-rank test) (Figure 2B). In the univariate Cox proportional hazards regression model without IPTW, there was no difference in OS between the two groups (p=0.997). The multivariate Cox proportional hazards regression model with raw data confirmed that age, tumor size, and marital status were independent prognostic factors for OS in patients with PC. Consistent with the results of the pre-IPTW model, the IPTW-adjusted univariate Cox proportional hazards regression model showed that the mode of surgery remained statistically non-significant (p=0.662), whereas age at diagnosis, tumor size, and marital status were independent prognostic factors in the corrected data (Table 2).
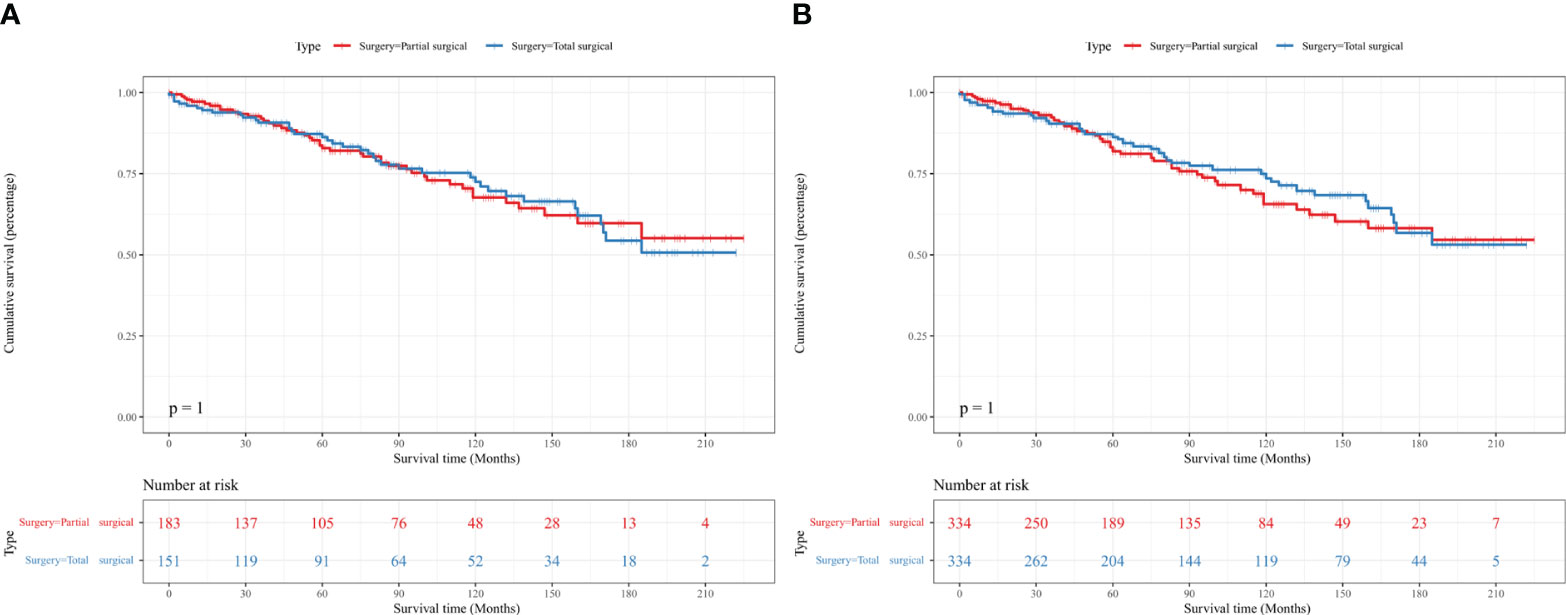
Figure 2 Kaplan–Meier survival curves showed no difference in the OS of patients with PC between the partial and total surgical resection groups before (A) and after (B) IPTW. IPTW, inverse probability of treatment propensity-score weighting; OS, overall survival; PC, parathyroid carcinoma.
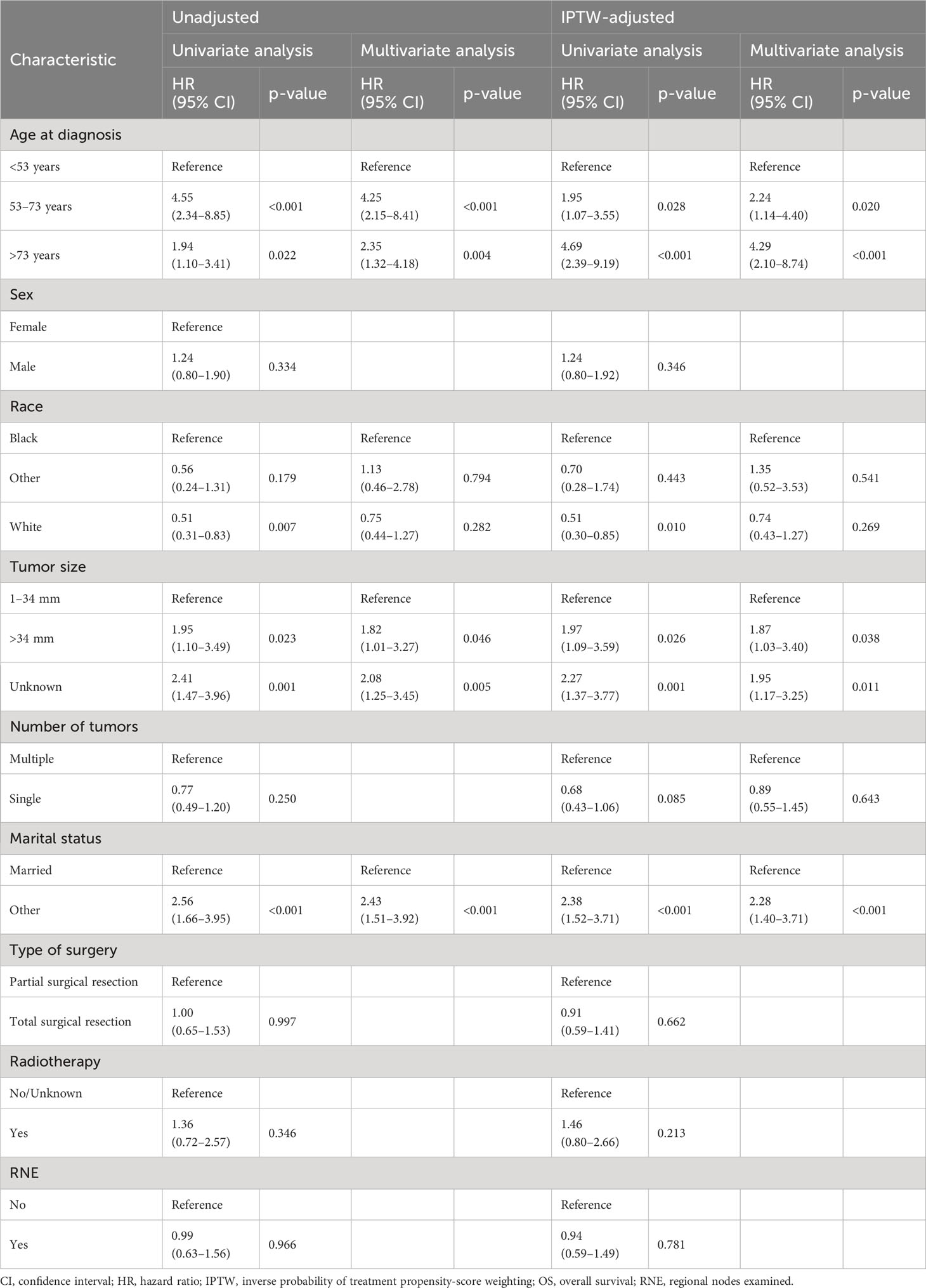
Table 2 Univariate and multivariate Cox regression analyses of OS in patients with PC before and after IPTW.
The forest plot did not show a difference in prognosis between the partial and total surgical resection groups, except for the subgroup of unknown tumor size. In the group with unknown tumor size, total surgical resection was associated with better OS than partial surgery (hazard ratio: 0.49, p=0.030). In the subgroup with tumor size >34 mm, the p-value was 0.053, almost reaching the threshold of statistical significance. In the subgroup analysis of other variables, there was no difference in the prognosis for the partial and total surgical resection groups (Figure 3).
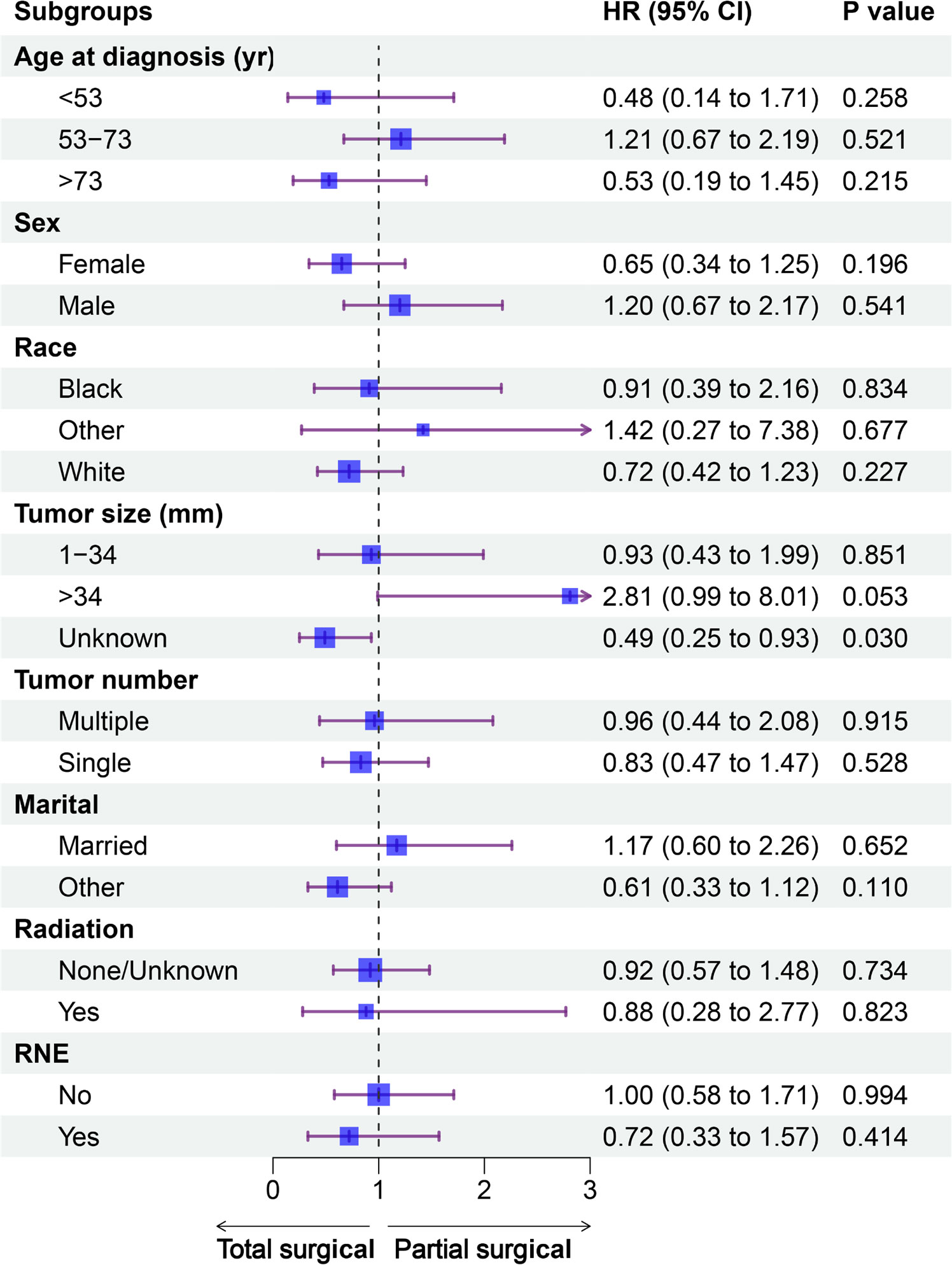
Figure 3 Forest plot of patients with PC included in the subgroup analysis (partial vs. total surgical resection). CI, confidence interval; HR, hazard ratio; PC, parathyroid carcinoma; RNE, regional nodes examined; yr, year.
In this study, we sought to identify differences in the prognostic impact of partial and total surgical resection on the OS of patients with early-stage PC by analyzing data from the representative SEER database. Owing to the wide coverage of the SEER database and the relatively complete data collection, it is possible to investigate rare types of cancer, such as PC. To the best of our knowledge, this is the first study using the IPTW method to achieve the aforementioned objective.
Due to the rarity of PC, the available literature on this disease mostly comprises reviews and case reports. Nevertheless, several studies have investigated the prognostic factors of OS and cancer-specific survival in patients with PC, providing data to support the treatment and management (11, 22–25). Most of these studies involved database analyses, such as the SEER and the American College of Surgeons National Cancer Database. Remarkably, the number of patients with lymph nodes and distant metastases in these studies was very low (typically <10), which may have led to biased results. PC is characterized by relatively lazy features and progresses slowly. Thus, it is of great practical importance to investigate the prognosis of patients with early-stage disease in studies involving relatively large sample sizes. The results of the multivariate Cox proportional hazards regression model before and after IPTW were consistent, confirming that age at diagnosis and tumor size were prognostic factors for cancer-specific survival in patients with PC. These findings are consistent with those of previous studies (22, 26). Previous studies on the role of marital status as a prognostic factor affecting OS in patients with PC reported inconsistent conclusions (24, 26). This discrepancy in results may be due to differences in the criteria used in studies for the selection of patients. Hence, additional studies are warranted to validate the present result.
Surgery is an important factor in the prognosis of patients with PC. Compared with partial surgical resection, primary surgery involving total surgical resection provides the best chance of cure and is associated with a significantly better prognosis (12, 13). However, studies have shown a lack of significant correlation between total surgical resection and improved OS in patients with PC (18, 27). In this study, after adjusting for baseline data, we did not find a significant difference in the effect of partial and total surgical resection on the prognosis of OS in patients with PC. This result was consistent with previous findings (28, 29). Therefore, it is reasonable to suggest that total surgical resection is a better choice than partial surgical resection. This difference may be associated with the use of secondary and multiple surgeries. Studies have shown that extending the surgical resection and increasing the number of surgeries reduces the rates of disease recurrence and mortality in patients with PC (17, 30). Another possible explanation is that the surgical information included in the cancer registry does not adequately describe the extent of resection (3, 31, 32). In a previous study, prophylactic cervical lymph node dissection was recommended for patients with primary tumors measuring >3 cm (31). The subgroup analysis of this study found that total surgical resection did not achieve better results among patients with primary tumors measuring >34 mm. Considering the low incidence of metastasis in the PC region, prophylactic cervical lymphadenectomy should be performed with caution (33, 34). Parathyroid cancer is characterized by a high recurrence rate. Moreover, effective treatment options for this disease (beyond primary total surgical resection) are currently limited. Therefore, a high preoperative index of suspicion and regular aggressive review is essential to optimize patient care (35). In addition, multi-institutional studies with standardized surgical procedures are warranted to further assess differences between partial and total surgery.
Although we applied the IPTW method to correct the baseline features and obtained a relatively reliable conclusion. The limitations of this population-based database investigation should be acknowledged. Firstly, this study involved a retrospective analysis, which may have been biased by the removal of cases with incomplete information. Secondly, several important indicators that have been associated with PC (e.g., serum parathyroid hormone and calcium levels, and disease recurrence) are not included in the SEER database. Therefore, we were unable to examine the effects of such indicators on the prognosis of patients with PC in relation to the surgical approach. Thirdly, the SEER database does not provide PC gene information, especially the CDC73 mutation status. Again, due to the rarity of PC cases, even national databases do not include many cases (especially patients with more than 15 years of follow-up), which may lead to biased study results. Finally, clinical and oncology variables require precise coding for analysis. For example, the SEER definition of surgical modality is imprecise, and the “Surgical Codes Other Sites 2023” manual, which is available on the official website of the database, does not specifically address the coding of parathyroid surgery.
In patients with early-stage PC, there was no significant difference in OS following partial and total surgical resection. Our findings may provide insights and would assist in the selection of the appropriate surgical approach for patients with PC.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants or the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
SJ and CZ were responsible for the conception and design of the study. SJ also collected data and performed the statistical analysis. KX, WC and JY wrote and edited the manuscript. WC also made contributions to manuscript review and revision. CZ provided financial support. All authors contributed to the article and approved the submitted version.
This work was supported by the Foundation of the National Key R&D Program of China (No. 2020YFC2008900).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lo WM, Good ML, Nilubol N, Perrier ND, Patel DT. Tumor size and presence of metastatic disease at diagnosis are associated with disease-specific survival in parathyroid carcinoma. Ann Surg Oncol (2018) 25:2535–40. doi: 10.1245/s10434-018-6559-6
2. Ryhänen EM, Leijon H, Metso S, Eloranta E, Korsoff P, Ahtiainen P, et al. A nationwide study on parathyroid carcinoma. Acta Oncol (2017) 56:991–1003. doi: 10.1080/0284186X.2017.1306103
3. Sadler C, Gow KW, Beierle EA, Doski JJ, Langer M, Nuchtern JG, et al. Parathyroid carcinoma in more than 1,000 patients: A population-level analysis. Surgery (2014) 156:1622–30. doi: 10.1016/j.surg.2014.08.069
4. Marini F, Giusti F, Palmini G, Perigli G, Santoro R, Brandi ML. Genetics and epigenetics of parathyroid carcinoma. Front Endocrinol (Lausanne) (2022) 13:834362. doi: 10.3389/fendo.2022.834362
5. Torresan F, Iacobone M. Clinical features, treatment, and surveillance of hyperparathyroidism-jaw tumor syndrome: an up-to-date and review of the literature. Int J Endocrinol (2019) 2019:1761030. doi: 10.1155/2019/1761030
6. MaChado NN, Wilhelm SM. Parathyroid cancer: A review. Cancers (Basel) (2019) 11:1676. doi: 10.3390/cancers11111676
7. Wan L, Tu C, Li S, Li Z. Regional lymph node involvement is associated with poorer survivorship in patients with chondrosarcoma: A SEER analysis. Clin Orthop Relat Res (2019) 477:2508–18. doi: 10.1097/CORR.0000000000000846
8. Schulte K-M, Talat N, Miell J, Moniz C, Sinha P, Diaz-Cano S. Lymph node involvement and surgical approach in parathyroid cancer. World J Surg (2010) 34:2611–20. doi: 10.1007/s00268-010-0722-y
9. Sharretts JM, Kebebew E, Simonds WF. Parathyroid cancer. Semin Oncol (2010) 37:580–90. doi: 10.1053/j.seminoncol.2010.10.013
10. Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab (2001) 86:485–93. doi: 10.1210/jcem.86.2.7207
11. Zhang K, Su A, Wang X, Zhao W, He L, Wei T, et al. Non-linear correlation between tumor size and survival outcomes for parathyroid carcinoma: A SEER population-based cohort study. Front Endocrinol (Lausanne) (2022) 13:882579. doi: 10.3389/fendo.2022.882579
12. Schulte KM, Talat N, Galata G, Gilbert J, Miell J, Hofbauer LC, et al. Oncologic resection achieving r0 margins improves disease-free survival in parathyroid cancer. Ann Surg Oncol (2014) 21:1891–7. doi: 10.1245/s10434-014-3530-z
13. Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol (2012) 13:11–23. doi: 10.1007/s11864-011-0171-3
14. Cetani F, Pardi E, Marcocci C. Update on parathyroid carcinoma. J Endocrinol Invest (2016) 39:595–606. doi: 10.1007/s40618-016-0447-3
15. Talat N, Schulte K-M. Clinical presentation, staging and long-term evolution of parathyroid cancer. Ann Surg Oncol (2010) 17:2156–74. doi: 10.1245/s10434-010-1003-6
16. Villar-del-Moral J, Jiménez-García A, Salvador-Egea P, Martos-Martínez JM, Nuño-Vázquez-Garza JM, Serradilla-Martín M, et al. Prognostic factors and staging systems in parathyroid cancer: a multicenter cohort study. Surgery (2014) 156:1132–44. doi: 10.1016/j.surg.2014.05.014
17. Xue S, Chen H, Lv C, Shen X, Ding J, Liu J, et al. Preoperative diagnosis and prognosis in 40 Parathyroid Carcinoma Patients. Clin Endocrinol (Oxf) (2016) 85:29–36. doi: 10.1111/cen.13055
18. Silva-Figueroa AM, Hess KR, Williams MD, Clarke CN, Christakis I, Graham PH, et al. Prognostic scoring system to risk stratify parathyroid carcinoma. J Am Coll Surg (2017) S1072-7515(17):30179–5. doi: 10.1016/j.jamcollsurg.2017.01.060
19. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: incidence - SEER research plus data, 18 registries, nov 2020 sub (2000-2018) - Linked To County Attributes - Total U.S., 1969-2019 Counties. National Cancer Institute, DCCPS, Surveillance Research Program. Available at: www.seer.cancer.gov.
20. Austin PC. Goodness-of-fit diagnostics for the propensity score model when estimating treatment effects using covariate adjustment with the propensity score. Pharmacoepidemiol Drug Saf (2008) 17:1202–17. doi: 10.1002/pds.1673
21. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int (2016) 113:597–603. doi: 10.3238/arztebl.2016.0597
22. Qian B, Qian Y, Hu L, Zhang S, Mei L, Qu X. Prognostic analysis for patients with parathyroid carcinoma: A population-based study. Front Neurosci (2022) 16:784599. doi: 10.3389/fnins.2022.784599
23. Ullah A, Khan J, Waheed A, Sharma N, Pryor E, Stumpe T, et al. Parathyroid carcinoma: incidence, survival analysis, and management: A study from the SEER database and insights into future therapeutic perspectives. Cancers (2022) 14:1426. doi: 10.3390/cancers14061426
24. Tao M, Luo S, Wang X, Jia M, Lu X. A nomogram predicting the overall survival and cancer-specific survival in patients with parathyroid cancer: A retrospective study. Front Endocrinol (Lausanne) (2022) 13:850457. doi: 10.3389/fendo.2022.850457
25. Limberg J, Stefanova D, Ullmann TM, Thiesmeyer JW, Bains S, Beninato T, et al. The use and benefit of adjuvant radiotherapy in parathyroid carcinoma: A national cancer database analysis. Ann Surg Oncol (2021) 28:502–11. doi: 10.1245/s10434-020-08825-8
26. Yin F, Hou C, Wang S, Wang X, Yang Z. Predictive a nomogram for overall survival in patients with parathyroid cancer: A novel web-based calculator. Asian J Surg (2022) S1015-9584(22):01387–2. doi: 10.1016/j.asjsur.2022.10.012
27. Harari A, Waring A, Fernandez-Ranvier G, Hwang J, Suh I, Mitmaker E, et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab (2011) 96:3679–86. doi: 10.1210/jc.2011-1571
28. Zhou L, Huang Y, Zeng W, Chen S, Zhou W, Wang M, et al. Surgical disparities of parathyroid carcinoma: long-term outcomes and deep excavation based on a large database. J Oncol (2021) 2021:8898926. doi: 10.1155/2021/8898926
29. Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, et al. Parathyroid carcinoma: a 22-year experience. Head Neck (2004) 26:716–26. doi: 10.1002/hed.20049
30. Rodrigo JP, Hernandez-Prera JC, Randolph GW, Zafereo ME, Hartl DM, Silver CE, et al. Parathyroid cancer: An update. Cancer Treat Rev (2020) 86:102012. doi: 10.1016/j.ctrv.2020.102012
31. Hsu K-T, Sippel RS, Chen H, Schneider DF. Is central lymph node dissection necessary for parathyroid carcinoma? Surgery (2014) 156:1336–41. doi: 10.1016/j.surg.2014.08.005
32. Asare EA, Sturgeon C, Winchester DJ, Liu L, Palis B, Perrier ND, et al. Parathyroid carcinoma: an update on treatment outcomes and prognostic factors from the national cancer data base (NCDB). Ann Surg Oncol (2015) 22:3990–5. doi: 10.1245/s10434-015-4672-3
33. Sawhney S, Vaish R, Jain S, Mittal N, Ankathi SK, Thiagarajan S, et al. Parathyroid carcinoma: a review. Indian J Surg Oncol (2022) 13:133–42. doi: 10.1007/s13193-021-01343-3
34. Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer (2007) 109:1736–41. doi: 10.1002/cncr.22599
Keywords: parathyroid carcinoma, partial surgical resection, total surgical resection, SEER database, inverse probability of treatment weighting
Citation: Jin S, Cho WC, Yang J, Xia K and Zhou C (2023) Comparison of prognosis after partial and total surgical resection for parathyroid carcinoma: an inverse probability of treatment weighting analysis of the SEER database. Front. Endocrinol. 14:1167508. doi: 10.3389/fendo.2023.1167508
Received: 16 February 2023; Accepted: 27 September 2023;
Published: 16 October 2023.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Pietro Locantore, Catholic University of the Sacred Heart, ItalyCopyright © 2023 Jin, Cho, Yang, Xia and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxi Zhou, emhvdWNoYW5neGkyMDAyQGFsaXl1bi5jb20=; Kaide Xia, eGlha2FpZGVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.