
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 12 May 2023
Sec. Systems Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1167317
This article is part of the Research Topic Environmental Exposomics and Metabolic Disorders View all 5 articles
 Xun Pei1†
Xun Pei1† Junjie Yao2†
Junjie Yao2† Simiao Ran3,4†
Simiao Ran3,4† Haifei Lu4
Haifei Lu4 Shuo Yang3
Shuo Yang3 Yini Zhang4
Yini Zhang4 Miyuan Wang5
Miyuan Wang5 Heyuan Shi4*
Heyuan Shi4* Aihua Tan3,4,6*
Aihua Tan3,4,6*Introduction: Existing evidence suggests an association between certain vitamins and metabolic syndrome (MetS), but few epidemiological studies have focused on the effects of multivitamin co-exposure on MetS. This study aims to investigate the associations of the individual or multiple water-soluble vitamins (i.e., vitamin C (VC), vitamin B9 (VB9), and vitamin B12 (VB12)) with co-exposure to MetS, as well as the dose-response relationships among them.
Methods: A cross-sectional study was conducted by employing the National Health and Examination Surveys (NHANESs) 2003-2006. Multivariate-adjusted logistic regression models were used to explore the association between individual serum water-soluble vitamins and the risk of MetS and its components, including waist circumference, triglyceride, high-density lipoprotein, blood pressure, and fasting plasma glucose. Restricted cubic splines were performed to explore the dose-response relationships among them. The quantile g-computation method was adopted to explore the associations of multiple water-soluble vitamins co-exposure with MetS risk and MetS components.
Results: A total of 8983 subjects were involved in the study, of whom 1443 were diagnosed with MetS. The MetS groups had a higher proportion of participants with age ≥60 years, BMI ≥30 kg/m2, and insufficient physical activity. Compared with the lowest quartile, the third (OR=0.67, 95% CI: 0.48, 0.94) and highest quartiles (OR=0.52, 95%CI: 0.35, 0.76) of VC were associated with lower MetS risk. Restricted cubic splines showed negative dose-response relationships among VC, VB9 and VB12, and MetS. Regarding MetS components, higher VC quartiles were associated with lower waist circumference, triglyceride, blood pressure, and fasting plasma glucose, while higher VC and VB9 quartiles were associated with higher high-density lipoprotein (HDL). Co-exposure to VC, VB9, and VB12 was significantly inversely associated with MetS, with ORs (95% CI) of 0.81 (0.74, 0.89) and 0.84 (0.78, 0.90) in the conditional and marginal structural models, respectively. Furthermore, we found that VC, VB9, and VB12 co-exposure were negatively associated with waist circumference and blood pressure, while VC, VB9, and VB12 co-exposure were positively associated with HDL.
Conclusion: This study revealed negative associations of VC, VB9, and VB12 with MetS, while the high water-soluble vitamin co-exposure was associated with a lower MetS risk.
Metabolic syndrome (MetS) is a major health problem faced by sub-healthy populations. With the development of the global economy and the general improvement of living standards, more than one billion people are affected by MetS, becoming a global public health challenge (1). The definition of MetS varies slightly among healthcare organizations, but it can be summarized as a pathology characterized by obesity, insulin resistance, a pro-inflammatory state, hypertension, and hyperlipidemia. It may also be a high-risk metabolic factor that promotes the development of type 2 diabetes and cardiovascular disease (2, 3). Studies have shown that the pathogenesis of MetS involves a variety of factors, among which oxidative stress and inflammatory responses play major roles. In a state of nutrient overload, the normal metabolic balance is disrupted, and the excessive production of active substances causes changes in the regulation of redox metabolism, forming a vicious circle (4, 5). Most patients with MetS have subclinical symptoms that may not be well controlled with medication but can be addressed with dietary changes such as increasing fiber, vitamin-rich grains, vegetables, and fruits while reducing refined, high-sugar, and high-fat foods intake (6).
Vitamins are divided into water-soluble and fat-soluble according to their solubility. Both types are essential trace compounds involved in complex biochemical reactions and metabolic regulation of the human body. B vitamins and vitamin C (VC), as water-soluble vitamins, are generally considered to be cofactors in preventing metabolic disorders and in the functioning of various enzymes (7, 8). Recent studies have indicated the importance of water-soluble vitamins in regulating metabolism and maintaining physiological homeostasis. VC is a widely used free radical scavenging reductant that cannot be synthesized by the human body, but in the case of a normal diet, dietary intake can provide the daily required VC level of the human body (9). Thomas-Valdés S et al. demonstrated that the body of obese individuals produces low-grade inflammation and enhanced oxidative stress due to excessive nutrient intake, a state that inhibits VC absorption and promotes excessive consumption of VC (10). Abdominal obesity-induced adipocyte increase and dyslipidemia promote systemic oxidative stress, forming a vicious cycle of obesity, inflammation, and oxidative stress (11). B9 (folic acid) and B12 are important B vitamins that play critical roles in homocysteine metabolism and neurodevelopment. These two vitamins are often studied together because of their similar functions (12). Several recent studies have shown that B9 and B12 have equally profound effects on different pathological features of MetS, particularly affecting lipid metabolism and high risk of cardiovascular disease (13–16). A study based on 341 healthy Saudi women showed that low serum vitamin B12 levels were independently associated with dyslipidemia (17). This imbalance during pregnancy predisposes women to diabetes and also predisposes their children to metabolic diseases such as insulin resistance and obesity (18, 19).
Past epidemiological studies indicated the relationship between vitamin D and other fat-soluble vitamins and MetS (20). However, relatively few studies have been conducted on water-soluble vitamins. In addition, the effects of individual vitamins have often been studied and few studies have focused on the effects of multivitamin co-exposure on MetS. An earlier observational study demonstrated an association between individual concentrations of water-soluble vitamins and MetS. However, controlled trials only examined results for B1, B3, B6, and VC with small sample sizes, but did not evaluate their co-exposure effects (21). The intake of fresh vegetables and fruits has been shown to be significantly and inversely associated with the prevalence of MetS. Because these foods are rich in vitamins and phytochemicals, they can reduce oxidative stress, regulate endothelial function, and reduce insulin resistance (22). However, there is currently insufficient evidence to prove that the use of multivitamins has any effect on reducing the prevalence of metabolic syndrome.
Therefore, in this study, the relationship between circulating levels of three common water-soluble vitamins (VC, VB9, and VB12) and MetS was analyzed by using data from U.S. adults who participated in the 2003−2006 National Health and Nutrition Examination Survey (NHANES) to investigate the specific effects of individual or multi-exposure of water-soluble vitamins in serum on MetS.
This study conducted a cross-sectional study utilizing data from the NHANES 2003-2004 and 2005-2006. The NHANES was conducted using a complex, multistage probability sampling design, aiming to assess the health and nutritional status of the noninstitutionalized household population. The National Center for Health Statistics Research Ethics Review Board approved the protocol of the NHANES. Each participant signed the consent form.
All respondents that participated in the NHANES were eligible for inclusion (n=20 470). We excluded participants who only received an interview without physical examination (n=877), whose age <20 years (n=10 078), and who missed data on serum Vitamin C (VC), Vitamin B9 (VB9), Vitamin B12 (VB12), and MetS (n=532). Finally, a total of 8 983 U.S. adults were included in the present study (See Figure 1).
Exposure in this study included 3 kinds of water-soluble vitamins, namely VC, VB9 (folic acid), and VB12. Serum concentrations of VC were measured by using high-performance liquid chromatography (HPLC) and quantified by spectrophotometry. Serum concentrations of VB9 and VB12 were measured by using the radioassay kit from Bio-Rad Laboratories. Details could be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/).
The main outcome of this study was MetS. According to the ATP III guidelines (23), clinical identification of the Mets was diagnosed when any 3 of the following occurred: (1) male waist circumference >102 cm or female waist circumference >88cm; (2) triglyceride ≥150 mg/dL; (3) high-density lipoprotein <40 mg/dL for men or <50 mg/dL for women; (4) blood pressure ≥130/≥85 mmHg; and (5) fasting glucose ≥110 mg/dL. Each component of MetS was categorized into two groups according to ATP III guidelines and was used as the secondary outcome. Waist circumference was categorized into low (<102 cm in men or <88 cm in women) and high (≥102 cm in men or ≥88 cm in women) groups; triglyceride was categorized into low (<150 mg/dL) and high (≥150 mg/dL) groups; high-density lipoprotein was categorized into low (<40 mg/dL in men or <50 mg/dL in women) and high (≥40 mg/dL in men or ≥50 mg/dL in women) groups; blood pressure was categorized into low (systolic blood pressure <130 mmHg and diastolic blood pressure<85 mmHg) and high (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg) groups; fasting plasma glucose was categorized into low (<110 mg/dL) and high (≥110 mg/dL) groups.
Covariates included demographics and lifestyle factors. Demographic information was collected through means such as questionnaires or physical examinations, including age (20-39, 40-59, and ≥ 60 years), ethnicity (Mexican American, Hispanic, non-Hispanic White, non-Hispanic Black, and others), educational background (below high school, high school or equivalent and above high school), marital status (married/living with a partner, widowed/separated/devoiced, never married), the ratio of family income to poverty (Family PIR) and body mass index (BMI, <18.5 kg/m2, 18.5-24.9 kg/m2, 25-29.9 kg/m2, ≥30 kg/m2). Lifestyle information was collected by questionnaires or dietary interviews, including leisure-time physical activity (sedentary, insufficient, moderate, and high), drinking status (never, ever, and current), smoking status (never, ever, and current), and dietary total energy.
BMI was calculated by using weight (kg) divided by the square of height (m2). Leisure-time physical activity was categorized into 4 groups based on metabolic equivalent (MET)-minutes per week: sedentary (MET=0), insufficient (0<MET ≤ 500), moderate (500<MET ≤ 1000), and high (MET>1000).
Categorical variables were presented as numbers (percentage), and a chi-square test was adopted to compare differences between the two groups. Continuous variables that did not satisfy the normal distribution were presented as medians (interquartile ranges), and the Wilcoxon rank-sum test was used to compare differences between the two groups. Given the complex sampling design of NHANES, we employed appropriate sampling weights in our analysis.
The multivariate-adjusted logistic regression model was used to explore the associations of individual serum water-soluble vitamins with MetS risk and each component. Serum water-soluble vitamins were categorized into four groups based on corresponding quartiles and the first quartile was used as a reference. We estimated the odds ratio and corresponding 95% confidence interval in the two models. Model 1 was adjusted for general characteristics, including age, sex, ethnicity, educational background, marital status, BMI, and family PIR. Model 2 was adjusted for lifestyle factors in addition to those in model 1, including leisure time physical activity, drinking status, smoking status, and dietary total energy. Also, continuous serum water-soluble vitamins were used as exposure to performing the same analysis to test the robustness of the results.
To explore the dose-response relationships between serum water-soluble vitamins and MetS, we further performed a restricted cubic spline with three knots located at the 5th, 50th, and 95th percentiles of the vitamin concentration. Covariates adjusted were consistent with the multivariate-adjusted logistic regression model 2.
To explore the associations of water-soluble vitamins co-exposure with MetS risk and each component, we used the quantile g-computation method. Prior to the analysis, three kinds of water-soluble vitamins were log-transformed to ensure a normal distribution and then were standardized to eliminate the influence of units. We used the “qgcomp.noboot” function to obtain a positive or negative weight of each water-soluble vitamin in the contribution for qgcomp index and a conditional odds ratio. And “qgcomp.boot” function was used to assess the linearity of the total exposure effect and get a marginal odds ratio.
All data analyses were performed using SAS 9.4. All analyses were on two sides, and a P value of <0.05 was considered statistically significant.
A total of 8983 participants were involved in the present study, 16.0% (n=1 443) of them were diagnosed with MetS according to the ATP III guidelines. Apart from sex and drinking status, the distributions of the other variables were all significantly different between the two groups. We found the proportion of participants aged ≥60 years, overweight (BMI ≥25 kg/m2) and with insufficient physical activity in the MetS group, were higher than that in the non-MetS group. Besides, concentrations of serum VC and VB12 in the MetS group were lower than those in the non-MetS group. No significant difference was found in serum VB9 between the two groups (Table 1).
The results of the multivariate-adjusted logistic regression model were shown in Table 2. After adjusting for all covariates, compared with the lowest quartile, the third (OR=0.67, 95%CI: 0.48, 0.94) and highest quartiles (OR=0.52, 95%CI: 0.35, 0.76) of VC were found to be associated with 33% and 48% reduction of MetS risk, respectively. No significant associations of VB9 and VB12 with MetS risk were found.
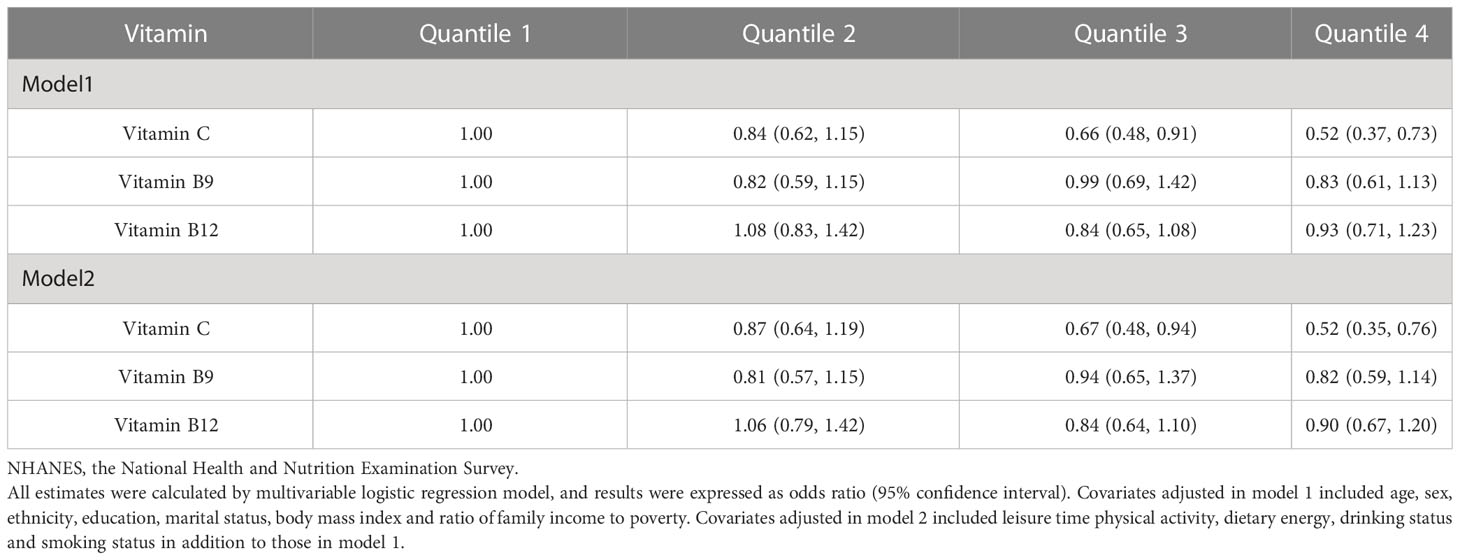
Table 2 Associations of serum water-soluble vitamins with metabolic syndromes in NHANES 2003-2006 participants.
Compared with the lowest quartile, the third quartile of VC was associated with lower waist circumference (OR=0.63, 95%CI: 0.43, 0.93), blood pressure (OR=0.63, 95%CI: 0.49, 0.80), and fasting plasma glucose (OR=0.70, 95%CI: 0.52, 0.94); the highest quartile of VC was associated with lower triglyceride (OR=0.73, 95%CI: 0.55, 0.96) and blood pressure (OR=0.60, 95%CI: 0.43, 0.83), and higher high-density lipoprotein (OR=0.47, 95%CI: 0.32, 0.71); and the highest quartile of VB9 was associated with higher high-density lipoprotein (OR=0.68, 95%CI: 0.47, 0.97) (model S1).
Dose-response relationships of VC, VB9, and VB12 with Mets were shown in Figures 2–4. We found a non-linear dose-response relationship between VC and MetS (Figure 2) and negative non-linear dose-response relationships between VB9 (Figure 3), VB12 (Figure 4), and MetS, suggesting MetS risk increased with increasing concentrations of serum VC, VB9, and VB12.
After adjusting for all covariates, the water-soluble vitamin as whole was significantly associated with Mets, with OR=0.81 (95%CI: 0.74, 0.89) in the conditional model and OR=0.84 (95%CI: 0.78, 0.90) in the marginal structural model for every quartile increase in fat-soluble vitamin concentrations (Table 3, Figure 5B). Figure 5A and Table 4 provided the estimated weight of each water-soluble vitamin’s contribution to the qgcomp index.
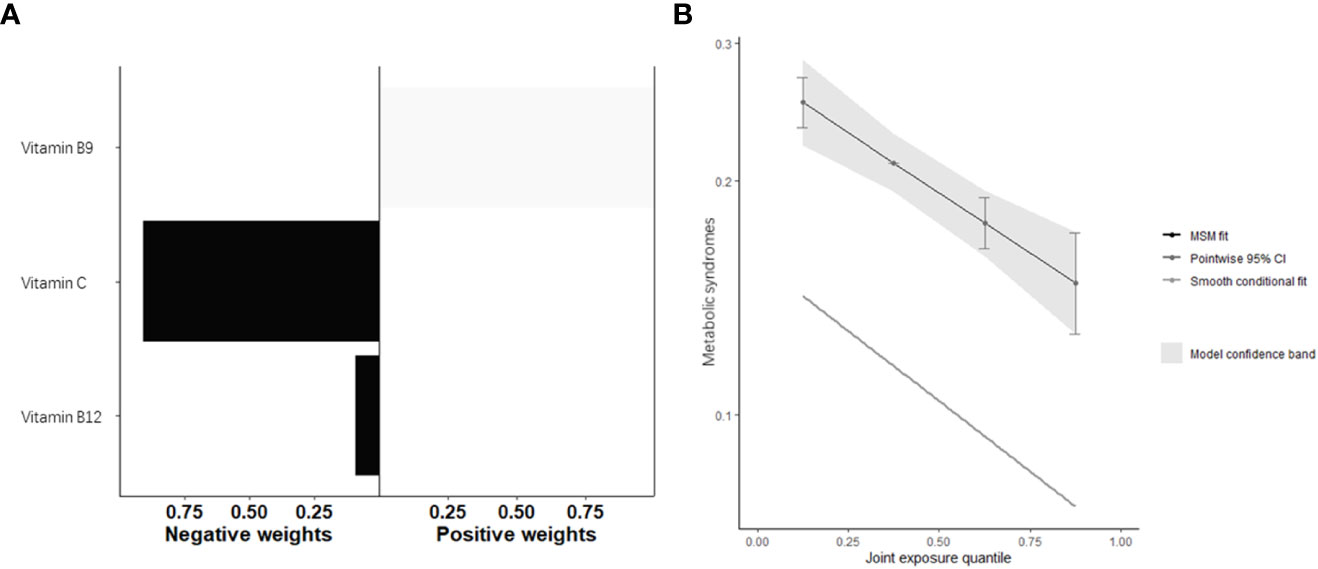
Figure 5 Quantile g-computation model regression index weighs (A) and joint effect (B) (95% confidence interval) of fat-soluble vitamins (i.e., Vitamin C, Vitamin B9, and Vitamin B12) on metabolic syndrome.

Table 4 Weights of each serum vitamin in the association of water-soluble vitamins co-exposure with metabolic syndromes.
The relationship between circulating levels of three water-soluble vitamins and MetS was evaluated using data from two consecutive NHANES periods. The researchers considered the specific individual and co-exposure effects of VC, B9, and B12 on MetS separately. The risk of MetS decreased with increased circulating levels of water-soluble vitamins. Also, the third and highest quartiles of VC were associated with a 33% and 48% reduced risk of MetS compared with the lowest quartile, respectively. A nonlinear dose-response relationship between VC and MetS and a linear dose-response relationship between B9, B12, and MetS were also noted. The findings clearly suggest the effects of co-exposure to water-soluble vitamins on MetS.
Among the three water-soluble vitamins studied, a significant negative association between serum VC level and MetS risk was observed. VC, known as L-ascorbic acid, is a vitamin that relies entirely on food intake and is widely used as a common antioxidant (24). Adults with higher levels of VC in their blood generally have better metabolic health, such as body mass index, waist circumference, and blood lipids. In middle-aged and older populations, higher VC blood levels are associated with lower levels of cognitive impairment (25, 26). Previous epidemiological studies suggest that VC deficiency increases increased the risk of MetS in adults and leads to an increased incidence of type 2 diabetes (27). A meta-analysis of observational studies with 110,771 participants also concludes that both diet and circulating VC levels are negatively associated with MetS (28). These findings are consistent with our results. MetS per se has been shown to result in reduced plasma VC, and VC deficiency in turn leads to the development of MetS. Because VC deficiency will not only lead to decreased cholesterol excretion and damage hepatic lipid homeostasis, but also reduce the expression and activity of various antioxidant enzymes, increase oxidative stress markers, and thus lose the protective effect on hepatic protein and lipid oxidation (29). MetS may repeatedly produce metabolic endotoxemia due to impaired intestinal barrier function due to excess nutrition, which is a vicious cycle that decreases VC absorption while increasing inflammation and oxidative damage (30). Increasing dietary VC intake is beneficial for improving glucose metabolism and liver function, improving lipid distribution by reducing triglycerides and total cholesterol, and increasing the bioavailability of multiple antioxidant vitamins by relieving inflammation in the gut-liver axis (31–33). In addition, our study observed a nonlinear dose-response relationship between VC and MetS, although the characteristics were not significant.
A linear dose-response relationship was noted between the circulating level of vitamin B9 and MetS. There are some studies indicating the prevalence of vitamin B9 deficiency in obese children and adult populations (34, 35). Although food fortification with folic acid has been increased in most countries, levels of folic acid supplementation are still insufficient for obese individuals. Pravenec M et al. demonstrated that spontaneously hypertensive rats without folic acid intake exhibited a variety of pathological features of MetS, including hepatic ectopic fat accumulation, glucose tolerance, elevated systolic blood pressure, and significant oxidative stress (36). A study by Koo et al. based on the nutritional health survey data from 1,730 pre-menopausal women in Korea found that serum folate levels were significantly associated with MetS prevalence, abdominal obesity, triglyceride levels, and low high-density lipoprotein (37). Another cross-sectional study showed similar results in a group of older adults at higher cardiometabolic risk, with higher folic acid intake associated with lower MetS scores and other metabolic risk factors (38). There are several mechanisms that may have caused this change. Folate deficiency may lead to impaired hepatic methylation, which affects lipid metabolism in the liver (39), and may also cause epigenetic changes with potential implications for the long-term metabolic health of offspring (18, 40).
Different conclusions were made based on the previous epidemiological studies on the relationship between vitamin B12 and MetS (41, 42). Similar to that of vitamin B9, a linear dose-response relationship was found between the circulating level of vitamin B12 and MetS. Vitamin B12 is absorbed through the intestine and stored in the liver for up to a year. Therefore, the body needs very little vitamin B12, and only strict vegans or those with abnormal metabolism would suffer from a deficiency (43, 44). Although there have been animal or human trials demonstrating the association of vitamin B12 with obesity, dyslipidemia, and elevated pro-inflammatory markers (41, 45, 46), there is a clear lack of studies that can directly demonstrate the correlation between circulating levels of vitamin B12 and MetS risk.
Due to complicated life scenarios and dietary intake, there is more interest in studying the effects of multivitamin co-exposure on disease. Several studies have found that highly related or similarly acting vitamins have deeper interactions in processes involved in metabolism or disease (47, 48). In our study, we explored the effects of VC, B9, and B12 co-exposure on MetS. The exposure to three water-soluble vitamins was significantly associated with MetS, with each quartile increase in the co-exposure level of water-soluble vitamin concentration associated with a 19% reduction in MetS risk in the conditional model and 16% in the marginal structural model. More importantly, the contribution of each vitamin in reducing MetS risk was varied. In addition, the levels of water-soluble vitamin co-exposure were significantly associated with a decrease in HDL.
Studies have found that high levels of dietary antioxidant capacity, such as a vegan diet, can reduce the risk of MetS. However, a vegan diet can lead to deficiencies in some nutrients, particularly vitamin B12 (49). There is an interaction between vitamin B9 and B12, which has a significant positive effect on improving metabolic syndrome (50). Setola et al. showed that in MetS patients, increased intake of folic acid and vitamin B12 significantly improved insulin resistance and endothelial dysfunction while reducing homocysteine levels (51). In another trial on older patients with hyperhomocysteinemia, these two water-soluble vitamins did not have a positive effect on endothelial function and low inflammatory response in older adults with cardiovascular risk (52). These findings suggest that an appropriate serum vitamin level performs its specific physiological functions while interacting with other vitamins. Our study showed that increased folic acid had a very mild negative effect on reducing the risk of metabolic syndrome when exposed to all three water-soluble vitamins. A similar conclusion was made in a study by Li et al. In obese patients, especially those deficient in vitamin B12, folic acid fortification confers no additional benefit on the patient’s overall health (13).
To the best of our knowledge, this is the first nationally representative study of the association between co-exposure to water-soluble vitamins and MetS. In addition, we performed a number of covariate adjustments, including demographic characteristics and lifestyle factors. However, the study has a few limitations. First, we were unable to draw temporal conclusions between water-soluble vitamin exposure and MetS due to the cross-sectional study design. Second, the vitamin serum concentration test results might be affected by temporary dietary and nutritional supplements because VC, B9, and B12 were absorbed, stored, and metabolized in different ways. Therefore, more rigorously designed trials are needed to further validate conclusions, such as cohort studies or animal experiments to improve quality control and reduce confounding factors.
This cross-sectional study is the first to examine the association between co-exposure to three important water-soluble vitamins and MetS risk in U.S. adults. The findings suggest that high serum levels of water-soluble vitamins are associated with a reduced risk of MetS. Of the three water-soluble vitamins studied, VC plays an important role in the prevention of MetS. Our findings provide a new way to explore the effects of multiple water-soluble vitamin co-exposure on MetS. Future studies, especially on the potential mechanisms of B9 and B12 interactions with MetS and the specific effects of water-soluble vitamins on different pathological features of MetS are necessary.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving human participants were reviewed and approved by the Research Ethics Review Board of the American National Center for Health Statistics. The patients/participants provided their written informed consent to participate in this study.
HS and AT: conception and design of the study. XP, JY and SR: acquisition and interpretation of data, drafting the article. HL, SY and YZ: formal analysis and Methodology. MW: draw figures and tables, drafting the article. All authors contributed to and have approved the submitted version.
We thank the staff of the National Health and Nutrition Examination Survey for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1167317/full#supplementary-material
Supplementary Figure 1 | Quantile g-computation model regression index weighs (A) and joint effect (B) (95% confidence interval) of fat-soluble vitamins (i.e., Vitamin C, Vitamin B9, and Vitamin B12) on waist circumference. Waist circumference was categorized into low (<102 cm in men or <88 cm in women) and high (≥102 cm in men or ≥88 cm in women) groups, and the low group was used as the reference.
Supplementary Figure 2 | Quantile g-computation model regression index weighs (A) and joint effect (B) (95% confidence interval) of fat-soluble vitamins (i.e., Vitamin C, Vitamin B9, and Vitamin B12) on triglyceride. Triglyceride was categorized into low (<150 mg/dL) and high (≥150 mg/dL) groups, and the low group was used as the reference
Supplementary Figure 3 | Quantile g-computation model regression index weighs (A) and joint effect (B) (95% confidence interval) of fat-soluble vitamins (i.e., Vitamin C, Vitamin B9, and Vitamin B12) on high-density lipoprotein. High-density lipoprotein was categorized into low (<40 mg/dL in men or <50 mg/dL in women) and high (≥40 mg/dL in men or ≥50 mg/dL in women) groups, and the high group was used as the reference
Supplementary Figure 4 | Quantile g-computation model regression index weighs (A) and joint effect (B) (95% confidence interval) of fat-soluble vitamins (i.e., Vitamin C, Vitamin B9, and Vitamin B12) on blood pressure. Blood pressure was categorized into low (systolic blood pressure <130 mmHg and diastolic blood pressure<85 mmHg) and high (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg) groups, and the low group was used as the reference;
Supplementary Figure 5 | Quantile g-computation model regression index weighs (A) and joint effect (B) (95% confidence interval) of fat-soluble vitamins (i.e., Vitamin C, Vitamin B9, and Vitamin B12) on fasting plasma glucose. Fasting plasma glucose was categorized into low (<110 mg/dL) and high (≥110 mg/dL) groups, and the low group was used as the reference.
1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep (2018) 20(2):12. doi: 10.1007/s11906-018-0812-z
2. Grundy SM, Brewer HB Jr., Cleeman JI, Smith SC Jr., Lenfant C. Definition of metabolic syndrome: report of the national heart, lung, and blood Institute/American heart association conference on scientific issues related to definition. Circulation (2004) 109(3):433–8. doi: 10.1161/01.Cir.0000111245.75752.C6
3. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med (2011) 9:48. doi: 10.1186/1741-7015-9-48
4. Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci (2009) 84(21-22):705–12. doi: 10.1016/j.lfs.2009.02.026
5. Korac B, Kalezic A, Pekovic-Vaughan V, Korac A, Jankovic A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol (2021) 42:101887. doi: 10.1016/j.redox.2021.101887
6. Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr (2011) 106 Suppl 3:S5–78. doi: 10.1017/s0007114511005460
7. Chawla J, Kvarnberg D. Hydrosoluble vitamins. Handb Clin Neurol (2014) 120:891–914. doi: 10.1016/b978-0-7020-4087-0.00059-0
8. Liu Z, Farkas P, Wang K, Kohli MO, Fitzpatrick TB. B vitamin supply in plants and humans: the importance of vitamer homeostasis. Plant J (2022) 111(3):662–82. doi: 10.1111/tpj.15859
9. Padayatty SJ, Levine M. Vitamin c: the known and the unknown and goldilocks. Oral Dis (2016) 22(6):463–93. doi: 10.1111/odi.12446
10. Thomas-Valdés S, Tostes M, Anunciação PC, da Silva BP, Sant'Ana HMP. Association between vitamin deficiency and metabolic disorders related to obesity. Crit Rev Food Sci Nutr (2017) 57(15):3332–43. doi: 10.1080/10408398.2015.1117413
11. Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci (2011) 12(5):3117–32. doi: 10.3390/ijms12053117
12. Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev (2004) 62(6 Pt 2):S3–12; discussion S3. doi: 10.1111/j.1753-4887.2004.tb00070.x
13. Li Z, Gueant-Rodriguez RM, Quilliot D, Sirveaux MA, Meyre D, Gueant JL, et al. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin Nutr (2018) 37(5):1700–6. doi: 10.1016/j.clnu.2017.07.008
14. Azzini E, Raguzzini A, Polito A. A brief review on vitamin B(12) deficiency looking at some case study reports in adults. Int J Mol Sci (2021) 22(18):9694. doi: 10.3390/ijms22189694
15. Boachie J, Adaikalakoteswari A, Samavat J, Saravanan P. Low vitamin B12 and lipid metabolism: evidence from pre-clinical and clinical studies. Nutrients (2020) 12(7)1925. doi: 10.3390/nu12071925
16. Yuan S, Mason AM, Carter P, Burgess S, Larsson SC. Homocysteine, B Vitamins, and cardiovascular disease: a mendelian randomization study. BMC Med (2021) 19(1):97. doi: 10.1186/s12916-021-01977-8
17. Al-Musharaf S, Aljuraiban GS, Danish Hussain S, Alnaami AM, Saravanan P, Al-Daghri N. Low serum vitamin B12 levels are associated with adverse lipid profiles in apparently healthy young Saudi women. Nutrients (2020) 12(8):2395. doi: 10.3390/nu12082395
18. Owen MD, Baker BC, Scott EM, Forbes K. Interaction between metformin, folate and vitamin B(12) and the potential impact on fetal growth and long-term metabolic health in diabetic pregnancies. Int J Mol Sci (2021) 22(11):5759. doi: 10.3390/ijms22115759
19. Krikke GG, Grooten IJ, Vrijkotte TG, van Eijsden M, Roseboom TJ, Painter RC. Vitamin B12 and folate status in early pregnancy and cardiometabolic risk factors in the offspring at age 5-6 years: findings from the abcd multi-ethnic birth cohort. Bjog (2016) 123(3):384–92. doi: 10.1111/1471-0528.13574
20. Wimalawansa SJ. Associations of vitamin d with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol (2018) 175:177–89. doi: 10.1016/j.jsbmb.2016.09.017
21. Odum EP, Wakwe VC. Plasma concentrations of water-soluble vitamins in metabolic syndrome subjects. Niger J Clin Pract (2012) 15(4):442–7. doi: 10.4103/1119-3077.104522
22. Kim J, Tan LJ, Jung H, Roh Y, Lim K, Shin S. The association between fruit and vegetable consumption and metabolic syndrome in Korean adults: does multivitamin use matter? Epidemiol Health (2022) 44:e2022039. doi: 10.4178/epih.e2022039
23. Executive summary of the third report of the national cholesterol education program (Ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel iii). JAMA (2001) 285(19):2486–97. doi: 10.1001/jama.285.19.2486
24. Granger M, Eck P. Dietary vitamin c in human health. Adv Food Nutr Res (2018) 83:281–310. doi: 10.1016/bs.afnr.2017.11.006
25. Sharma Y, Popescu A, Horwood C, Hakendorf P, Thompson C. Relationship between vitamin c deficiency and cognitive impairment in older hospitalised patients: a cross-sectional study. Antioxidants (Basel) (2022) 11(3):463. doi: 10.3390/antiox11030463
26. Pearson JF, Pullar JM, Wilson R, Spittlehouse JK, Vissers MCM, Skidmore PML, et al. Vitamin c status correlates with markers of metabolic and cognitive health in 50-Year-Olds: findings of the chalice cohort study. Nutrients (2017) 9(8):831. doi: 10.3390/nu9080831
27. Liu M, Park S. A causal relationship between vitamin c intake with hyperglycemia and metabolic syndrome risk: a two-sample mendelian randomization study. Antioxidants (Basel) (2022) 11(5):857. doi: 10.3390/antiox11050857
28. Guo H, Ding J, Liu Q, Li Y, Liang J, Zhang Y. Vitamin c and metabolic syndrome: a meta-analysis of observational studies. Front Nutr (2021) 8:728880. doi: 10.3389/fnut.2021.728880
29. Ipsen DH, Tveden-Nyborg P, Lykkesfeldt J. Does vitamin c deficiency promote fatty liver disease development? Nutrients (2014) 6(12):5473–99. doi: 10.3390/nu6125473
30. Vors C, Pineau G, Drai J, Meugnier E, Pesenti S, Laville M, et al. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. J Clin Endocrinol Metab (2015) 100(9):3427–35. doi: 10.1210/jc.2015-2518
31. Luo X, Zhang W, He Z, Yang H, Gao J, Wu P, et al. Dietary vitamin c intake is associated with improved liver function and glucose metabolism in Chinese adults. Front Nutr (2021) 8:779912. doi: 10.3389/fnut.2021.779912
32. Namkhah Z, Ashtary-Larky D, Naeini F, Clark CCT, Asbaghi O. Does vitamin c supplementation exert profitable effects on serum lipid profile in patients with type 2 diabetes? a systematic review and dose-response meta-analysis. Pharmacol Res (2021) 169:105665. doi: 10.1016/j.phrs.2021.105665
33. Traber MG, Buettner GR, Bruno RS. The relationship between vitamin c status, the gut-liver axis, and metabolic syndrome. Redox Biol (2019) 21:101091. doi: 10.1016/j.redox.2018.101091
34. Kardaş F, Yücel AD, Kendirci M, Kurtoğlu S, Hatipoğlu N, Akın L, et al. Evaluation of micronutrient levels in children and adolescents with obesity and their correlation with the components of metabolic syndrome. Turk J Pediatr (2021) 63(1):48–58. doi: 10.24953/turkjped.2021.01.006
35. Köse S, Sözlü S, Bölükbaşi H, Ünsal N, Gezmen-Karadağ M. Obesity is associated with folate metabolism. Int J Vitam Nutr Res (2020) 90(3-4):353–64. doi: 10.1024/0300-9831/a000602
36. Pravenec M, Kozich V, Krijt J, Sokolová J, Zídek V, Landa V, et al. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am J Hypertens (2013) 26(1):135–40. doi: 10.1093/ajh/hps015
37. Koo YS, Lee YJ, Park JM. Inverse association of serum folate level with metabolic syndrome and its components in Korean premenopausal women: findings of the 2016-2018 Korean national health nutrition examination survey. Nutrients (2022) 14(4):880. doi: 10.3390/nu14040880
38. Navarrete-Muñoz EM, Vioque J, Toledo E, Oncina-Canovas A, Martínez-González M, Salas-Salvadó J, et al. Dietary folate intake and metabolic syndrome in participants of predimed-plus study: a cross-sectional study. Eur J Nutr (2021) 60(2):1125–36. doi: 10.1007/s00394-020-02364-4
39. da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. Biofactors (2014) 40(3):277–83. doi: 10.1002/biof.1154
40. Sie KK, Li J, Ly A, Sohn KJ, Croxford R, Kim YI. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol Nutr Food Res (2013) 57(4):677–85. doi: 10.1002/mnfr.201200186
41. Baltaci D, Kutlucan A, Turker Y, Yilmaz A, Karacam S, Deler H, et al. Association of vitamin B12 with obesity, overweight, insulin resistance and metabolic syndrome, and body fat composition; primary care-based study. Med Glas (Zenica) (2013) 10(2):203–10.
42. Villatoro-Santos CR, Ramirez-Zea M, Villamor E. B-vitamins and metabolic syndrome in mesoamerican children and their adult parents. Public Health Nutr (2021) 24(14):4537–45. doi: 10.1017/s1368980020003936
43. Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull (2008) 29(2 Suppl):S20–34; discussion S5-7. doi: 10.1177/15648265080292s105
44. Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B(12) deficiency among vegetarians? Nutr Rev (2013) 71(2):110–7. doi: 10.1111/nure.12001
45. Ghosh S, Sinha JK, Muralikrishna B, Putcha UK, Raghunath M. Chronic transgenerational vitamin B12 deficiency of severe and moderate magnitudes modulates adiposity-probable underlying mechanisms. Biofactors (2017) 43(3):400–14. doi: 10.1002/biof.1350
46. Ghosh S, Sinha JK, Putcha UK, Raghunath M. Severe but not moderate vitamin B12 deficiency impairs lipid profile, induces adiposity, and leads to adverse gestational outcome in female C57bl/6 mice. Front Nutr (2016) 3:1. doi: 10.3389/fnut.2016.00001
47. Paul L, Selhub J. Interaction between excess folate and low vitamin B12 status. Mol Aspects Med (2017) 53:43–7. doi: 10.1016/j.mam.2016.11.004
48. Cui Y, Zhou HL, Wei MH, Song WJ, Di DS, Zhang RY, et al. Multiple vitamin Co-exposure and mortality risk: a prospective study. Clin Nutr (2022) 41(2):337–47. doi: 10.1016/j.clnu.2021.12.010
49. Marrone G, Guerriero C, Palazzetti D, Lido P, Marolla A, Di Daniele F, et al. Vegan diet health benefits in metabolic syndrome. Nutrients (2021) 13(3):817. doi: 10.3390/nu13030817
50. Ashok T, Puttam H, Tarnate VCA, Jhaveri S, Avanthika C, Trejo Treviño AG, et al. Role of vitamin B12 and folate in metabolic syndrome. Cureus (2021) 13(10):e18521. doi: 10.7759/cureus.18521
51. Setola E, Monti LD, Galluccio E, Palloshi A, Fragasso G, Paroni R, et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol (2004) 151(4):483–9. doi: 10.1530/eje.0.1510483
52. van Dijk SC, Enneman AW, Swart KM, van Wijngaarden JP, Ham AC, de Jonge R, et al. Effect of vitamin B12 and folic acid supplementation on biomarkers of endothelial function and inflammation among elderly individuals with hyperhomocysteinemia. Vasc Med (2016) 21(2):91–8. doi: 10.1177/1358863x15622281
Keywords: vitamin, metabolic syndrome, weighted quantile sum regression, NHANES, co-exposure
Citation: Pei X, Yao J, Ran S, Lu H, Yang S, Zhang Y, Wang M, Shi H and Tan A (2023) Association of serum water-soluble vitamin exposures with the risk of metabolic syndrome: results from NHANES 2003-2006. Front. Endocrinol. 14:1167317. doi: 10.3389/fendo.2023.1167317
Received: 16 February 2023; Accepted: 12 April 2023;
Published: 12 May 2023.
Edited by:
Caixia Guo, Capital Medical University, ChinaReviewed by:
Shi-Nan Wu, The First Affiliated Hospital of Nanchang University, ChinaCopyright © 2023 Pei, Yao, Ran, Lu, Yang, Zhang, Wang, Shi and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heyuan Shi, U2h5NzlAaGJ0Y20uZWR1LmNu; Aihua Tan, ZXZhbjIwMThAc3RtYWlsLmhidGNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.