- 1Division of Pediatrics, Department of Health Sciences, University of Piemonte Orientale, Novara, Italy
- 2Endocrinology, Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy
Introduction: The coronavirus disease 19 (COVID-19) pandemic has prompted the development of new vaccines to reduce the morbidity and mortality associated with this disease. Recognition and report of potential adverse effects of these novel vaccines (especially the urgent and life-threatening ones) is therefore essential.
Case presentation: A 16-year-old boy presented to the Paediatric Emergency Department with polyuria, polydipsia and weight loss over the last four months. His past medical history was unremarkable. Onset of symptoms was referred to be few days after first dose of anti-COVID-19 BNT162b2 Comirnaty vaccine and then worsened after the second dose. The physical exam was normal, without neurological abnormalities. Auxological parameters were within normal limits. Daily fluid balance monitoring confirmed polyuria and polydipsia. Biochemistry laboratory analysis and urine culture were normal. Serum osmolality was 297 mOsm/Kg H2O (285-305), whereas urine osmolality was 80 mOsm/Kg H2O (100-1100), suggesting diabetes insipidus. Anterior pituitary function was preserved. Since parents refused to give consent to water deprivation test, treatment with Desmopressin was administered and confirmed ex juvantibus diagnosis of AVP deficiency (or central diabetes insipidus). Brain MRI revealed pituitary stalk thickening (4 mm) with contrast enhancement, and loss of posterior pituitary bright spot on T1 weighted imaging. Those signs were consistent with neuroinfundibulohypophysitis. Immunoglobulin levels were normal. Low doses of oral Desmopressin were sufficient to control patient’s symptoms, normalizing serum and urinary osmolality values and daily fluid balance at discharge. Brain MRI after 2 months showed stable thicken pituitary stalk and still undetectable posterior pituitary. Due to persistence of polyuria and polydipsia, therapy with Desmopressin was adjusted by increasing dosage and number of daily administrations. Clinical and neuroradiological follow-up is still ongoing.
Conclusion: Hypophysitis is a rare disorder characterized by lymphocytic, granulomatous, plasmacytic, or xanthomatous infiltration of the pituitary gland and stalk. Common manifestations are headache, hypopituitarism, and diabetes insipidus. To date, only time correlation between SARS-CoV-2 infection and development of hypophysitis and subsequent hypopituitarism has been reported. Further studies will be needed to deepen a possible causal link between anti-COVID-19 vaccine and AVP deficiency.
Introduction
At the end of 2019 severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection, named COVID-19, outbroke and plagued our healthcare systems, causing more than 6 million deaths worldwide over the last three years (1). We have learned that COVID-19 is a primarily respiratory disease, however it can affect nearly every organ system, including endocrine system (2, 3). Here, the expression of angiotensin-converting enzyme 2 (ACE2) receptors and transmembrane protease serine 2 (TMPRSS2) on many endocrine cells seems to play a crucial role in the direct pathogenetic mechanism by which the virus infects these organs (4).
Since no specific treatment was available, the pandemic urged scientists to develop targeted vaccines to face the high mortality rate of the disease. SARS‐CoV‐2 vaccines are generally safe and effective in preventing COVID‐19 severe symptoms. Injection site reactions, fever, headache, myalgia, and skin rash are the most common vaccine side effects (5). Surprisingly, some cases of endocrinopathies have occurred even after anti-COVID-19 vaccines in adults (6–8), suggesting cytokine release syndrome exacerbated by the vaccine as the possible underlying mechanism for the disease, as a result of endocrine cells susceptibility to elevation of pro-inflammatory molecules (9). To the best of our knowledge, only a few cases of AVP deficiency, also known as central diabetes insipidus (CDI) occurring after anti-COVID-19 vaccines have been cited in literature until now (10–12) and still no one in children. Here we report the first case of new onset CDI in a pediatric patient after BNT162b2 mRNA COVID-19 vaccine.
Case presentation
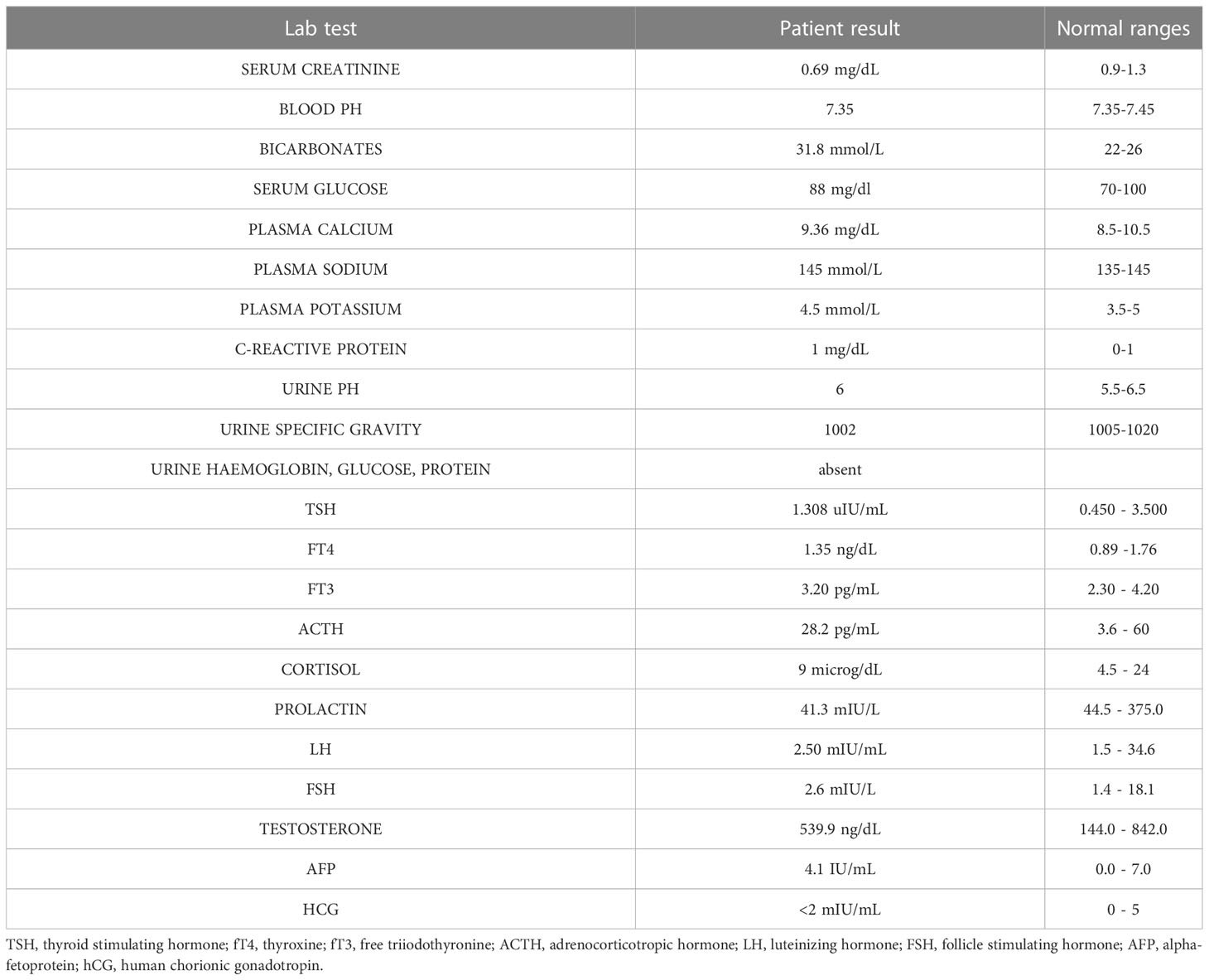
B.AR., a 16-year-old boy, presented to the Paediatric Emergency Department with polyuria, polydipsia and concomitant weight loss, reporting a urine output of 9 liters in the last 24 hours and a weight loss of nearly 6 kilograms in the previous four months. Symptoms seemed to start a few days after inoculation of the first dose of BNT162b2 mRNA COVID-19 vaccine (at the end of August 2021) and worsened after the second dose, which had been administered 28 days later. Due to the persistence of intense thirst and polyuria the patient had already undergone some medical investigations: no abnormalities were found at urologic assessment and renal ultrasound (Figure 1). Moreover, no significant features emerged from his past medical history, and familial history was unremarkable too. On admission to the Paediatric Emergency Department, the patient’s vital parameters included heart rate 110 bpm, temperature 36°C, oxygen saturation 99% on room air. Physical examination revealed a well-being adolescent boy, with auxological parameters within normal limits (height -0.12 standard deviations, weight +0.35 standard deviations), cardiac and pulmonary auscultation without pathological findings and no neurological abnormalities (including intact sense of smell and taste and cognition). Again, head and neck/gastrointestinal/musculoskeletal examinations were all grossly unremarkable. Furthermore, biochemistry laboratory analysis, venous blood gas analysis, and urine analysis were performed (Table 1) and no significant alterations were found. Diabetes mellitus was excluded by detection of normal hematic glucose levels and by the absence of glycosuria. In contrast, diabetes insipidus could be suspected because of plasma sodium level at the upper limit of normal and low urine specific gravity. Given this hypothesis, the patient was tested for SARS-CoV-2 ongoing infection by nasopharyngeal swab – which resulted negative – and was then admitted to the pediatric ward to confirm the diagnosis.
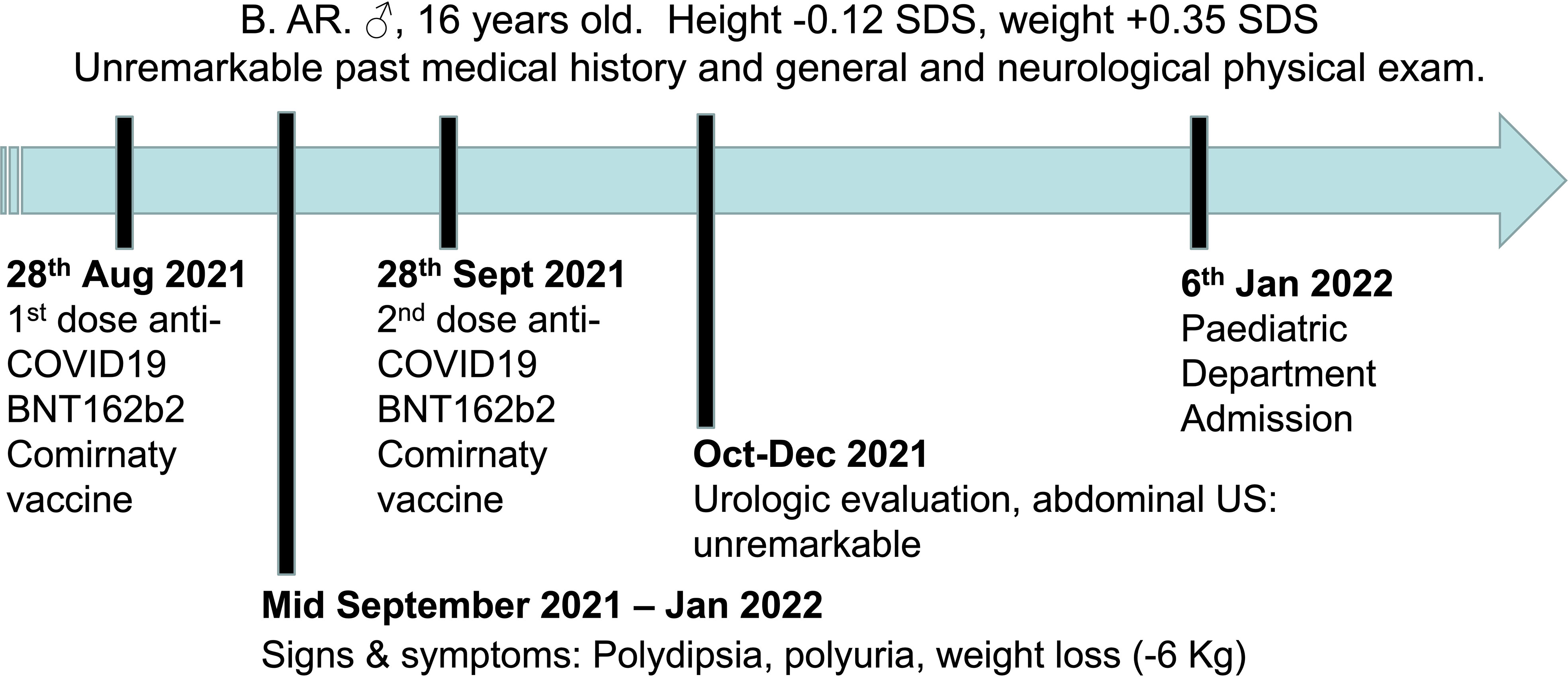
Figure 1 Timeline depicting the clinical course of the disease, from the onset of signs and symptoms until admission to the Paediatric Department.
Management and outcome
Primarily, daily fluid balance monitoring confirmed polyuria and polydipsia (IN 6.250 L/OUT 7.100 L). Secondly, serum osmolality (p-Osm) levels were 287 mOsm/Kg H2O (normal values 285-305), urine osmolality (u-Osm) was 68 mOsm/Kg H2O (normal values 100-1100) and u-Osm/p-Osm ratio was < 1, so that diabetes insipidus could be suspected. However, parents did not give consent to water deprivation test. A test with desmopressin (the synthetic analog of antidiuretic hormone) was then performed to discern between central and nephrogenic DI. After administration of low dose oral desmopressin (sublingual 60 micrograms) an immediate response was evident in view of both normalization of daily fluid balance (IN 0.520 L/OUT 0.600 L) and rapid increase of u-Osm (119 mOsm/Kg H2O). Consistent with those findings, ex juvantibus diagnosis of complete CDI could be made. To complete investigations, hormonal tests showed no significant impairment of anterior pituitary function (Table 1), immunological assessment revealed normal immunoglobulin levels (IgA, IgM, and IgG subclasses, including IgG4) and urine culture and Quantiferon were negative as well. Brain contrast-enhanced magnetic resonance imaging (MRI) focused on the study of the pituitary region was carried out. It revealed pituitary stalk thickening (PST) (maximum diameter of 4 mm) with contrast enhancement and loss of posterior pituitary bright spot on T1 weighted imaging (Figure 2). Those signs were consistent with neuroinfundibulohypophysitis. In the view of strict time correlation between COVID-19 vaccine and onset of symptoms and granted that no other differential diagnosis had fit the clinical picture, the case was signaled as an adverse drug reaction to the Italian Pharmacological Agency (AIFA). During hospitalization, B.AR. maintained good general conditions. He was discharged after 7 days with prescription of low doses of oral desmopressin (60 mcg twice daily), as the same dose had been sufficient to control signs and symptoms during hospitalization. After 2 months, follow-up MRI showed stable PST and still undetectable posterior pituitary, consistent with the persistence of inflammation of this cerebral area (Figure 3). An endocrinological follow-up was also planned at our center: three months after discharge, due to persisting polyuria and polydipsia, therapy with desmopressin was adjusted by increasing dosage and number of daily administrations (60 mcg three times a day). Clinical and neuroradiological follow-up is still ongoing.
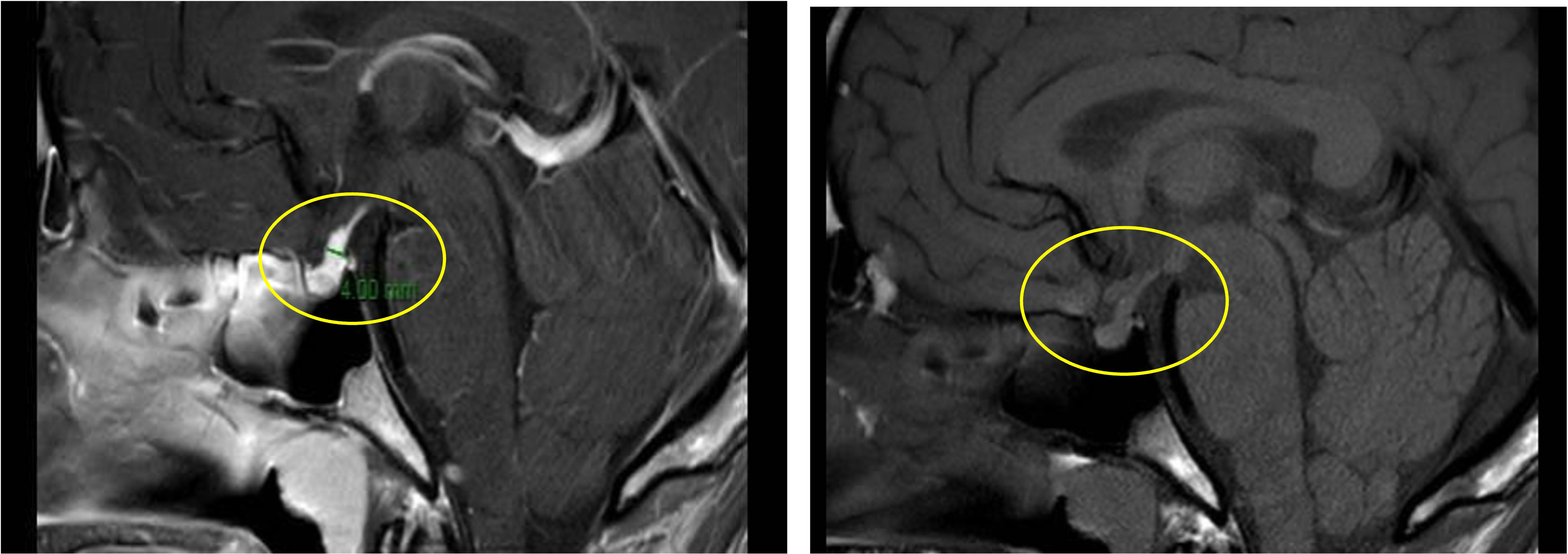
Figure 2 Pituitary stalk enlargement with contrast enhancement (left panel, yellow circle); loss of posterior pituitary bright (right panel, yellow circle).
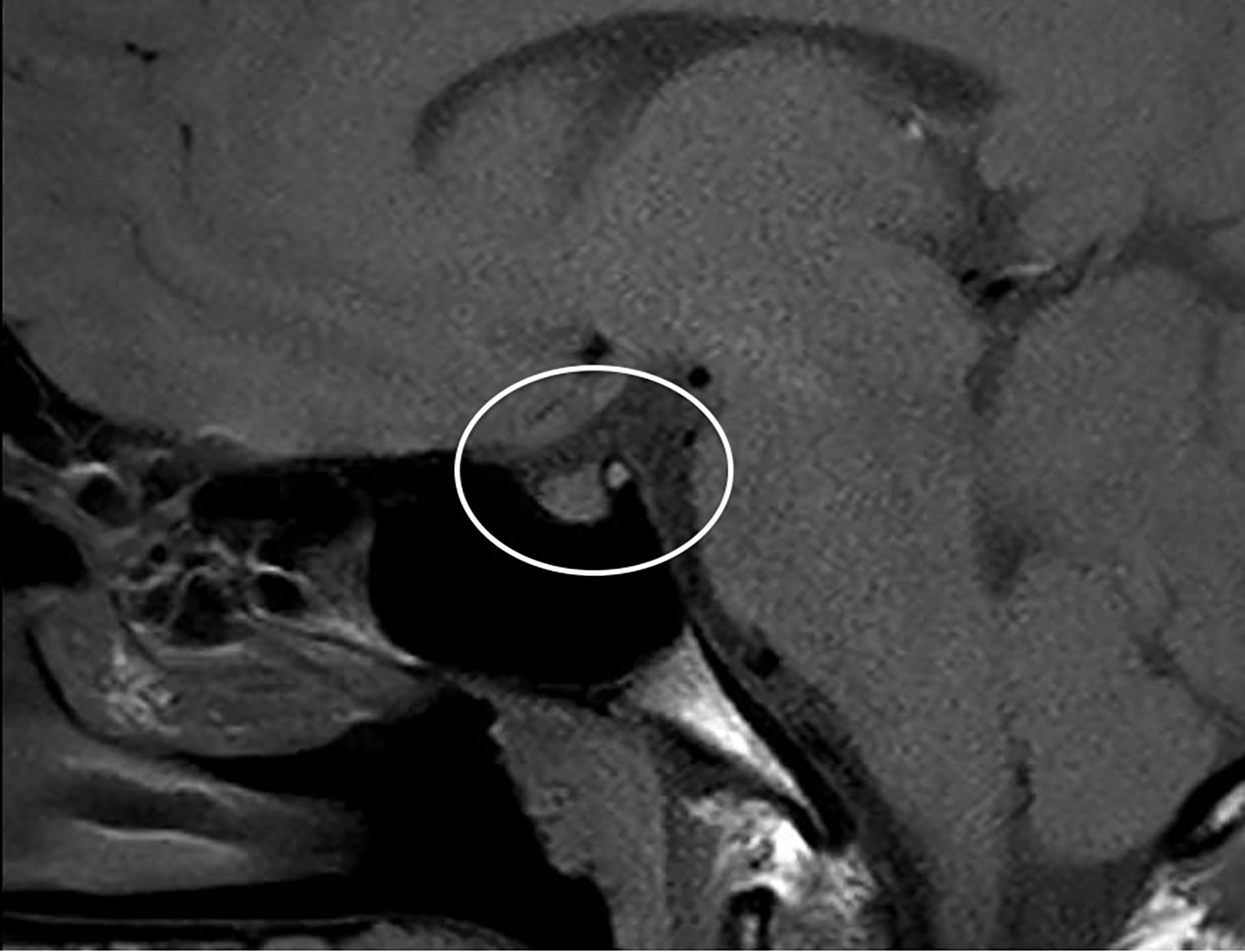
Figure 3 Brain MRI after 2 months: stable thickened pituitary stalk and undetectable posterior pituitary (circle).
Discussion
It is now widely accepted that COVID-19 is a multiorgan disease, as many tissues and organs are affected during ongoing or recent infection by SARS-CoV-2 virus, including endocrine system (13). It is becoming well established that in some cases SARS-CoV-2 infection can trigger an inflammation of the hypothalamus-pituitary axis (hypophysitis) both in adults and in children, resulting in a dysfunction that causes diabetes insipidus, either alone (14–17) or associated with anterior hypopituitarism (2, 14) with a latency of 0–16 weeks between recognition of the SARS-CoV-2 infection and development of symptoms (18). Although the exact pathogenetic mechanism has still to be defined, a direct pathway (involving ACE2-mediated hypothalamic viral infection) as well as an indirect, delayed, and immune-mediated pathway have been hypothesized. Moreover, endothelial damage in the blood–neuron interface, thrombotic microangiopathy (pituitary apoplexy), infected leukocyte‐mediated transportation and cytokine storming are other accepted theories (19).
Interestingly, hypopituitarism might have a bidirectional relationship with COVID-19 since pre-existent hypopituitarism can be per se a potential risk factor for COVID-19 due to its comorbidities (e.g. hypothalamic obesity) and can be worsened by SARS-CoV-2 infection (20). Moreover, COVID-19 vaccine can affect endocrine system, albeit infrequently and with good prognosis. Pezzaioli et al. recently collected and reviewed all published data on potential endocrine adverse effects post-COVID-19 vaccines in adult patients (6). Thyroid disorders are the most common; only eight cases of pituitary dysfunction have been described so far (10–12, 21–25), of which two presented with CDI (11, 12).
Our patient complained of exacerbation of DI symptoms after the second dose of COVID-19 vaccine. Although we were not able to conduct dose-specific analyses, previously published cases also reported onset or worsening of adverse endocrinological events following the second dose of vaccine (5, 22, 23). Mechanisms of increased reactogenicity after the second dose are largely unknown. One possibility is that the vaccine directly might cause pituitary impairment with cumulative effect. Autoimmune/inflammatory syndrome induced by vaccine adjuvants (ASIA syndrome), molecular mimicry, cross-reactivity or a pro-inflammatory state induced by vaccine components and subsequent activation of autoreactive B and T cells are speculated to be involved, as in Guillain-Barrè syndrome or optic neuritis (26–28).
COVID-19 symptoms are generally milder in the pediatric age than in the adult population, but this is not always true when the endocrine system is involved. Lizzi et al. described a pediatric case of acute onset of isolated CDI associated with recent SARS-CoV-2 infection (16), requiring 7 days of hospitalization. Here we described the case of an adolescent who developed CDI after COVID-19 vaccination, in whom posterior pituitary function has not recovered yet. Diabetes insipidus is a tricky disease whose symptoms can be underestimated by both patients and clinicians. CDI is characterized by decreased or absent secretion of antidiuretic hormone (ADH; also called arginine vasopressin or AVP), resulting in a variable degree of polyuria. Lack of AVP can be caused by disorders or lesions in the hypothalamic osmoreceptor, in the supraoptic or paraventricular nuclei, in the superior portion of the supraopticohypophyseal tract or in the pituitary sella. Recently, a panel of experts from national and international endocrinology and endocrine pediatric societies has proposed to change diabetes insipidus’ name to “arginine vasopressin deficiency (AVP-D)” for central etiologies to avoid detrimental confusion with diabetes mellitus for both patients and their caretakers (29).
Finally, pituitary stalk is a funnel-shaped structure that connects the hypothalamus to the pituitary gland. There are several etiologies that give rise to PST (30, 31), which often manifests clinically with CDI (32, 33): autoimmunity/inflammation (neuroinfundibulohypophysitis (34)) – sometimes referred as idiopathic PST, infectious diseases (e.g. tuberculosis (35)) or neoplastic lesions (33, 36). Anti-pituitary, anti-hypothalamus autoantibodies or high IgG4 levels have been detected in some patients with hypophysitis. However, their causal role remains unclear (37). Searching for these antibodies may help to diagnose an autoimmune hypophysitis, especially in cases like ours presenting with non-diagnostic pituitary MRI or hypoprolactinemia (38, 39). Unfortunately, no autoantibodies testing was performed in this case report since the lab kit was not available in our institution. The approach to the neuroradiological finding of PST is still controversial (32, 40): empirical management recommend to conduct follow-up MRI every 3-6 months and proceed with pituitary stalk biopsy only in case of stalk size ≥ 7 mm, progressive infundibular enlargement or worsening of symptoms. Moreover, all patients with pituitary stalk lesions and CDI should be routinely assessed for anterior pituitary hormonal function, which was normal in our patient.
Conclusion
To date, CDI following anti-COVID-19 vaccine remains a rare and only temporally linked occurrence, even though its pathophysiological explanation has been hypothesized. Further studies will be needed to deepen a possible causal link between the two events.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
CP and QP drafted the manuscript. AP, IR, and FP were involved in the clinical management of the patient and critically revised the manuscript. SB supervised the whole process. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barber RM, Sorensen RJD, Pigott DM, Bisignano C, Carter A, Amlag JO, et al. Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through nov 14, 2021: A statistical analysis. Lancet (2022) 399(10344):2351–80. doi: 10.1016/S0140-6736(22)00484-6
2. Mirza SA, Sheikh AAE, Barbera M, Ijaz Z, Javaid MA, Shekhar R, et al. COVID-19 and the endocrine system: A review of the current information and misinformation. Infect Dis Rep (2022) 14(2):184–97. doi: 10.3390/idr14020023
3. Thakur V, Ratho RK, Kumar P, Bhatia SK, Bora I, Mohi GK, et al. Multi-organ involvement in covid-19: Beyond pulmonary manifestations. J Clin Med (2021) 10(3):1–19. doi: 10.3390/jcm10030446
4. Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinol (2020) 161(9):1–7. doi: 10.1210/endocr/bqaa108
5. Dighriri IM, Alhusayni KM, Mobarki AY, Aljerary IS, Alqurashi KA, Aljuaid FA, et al. Pfizer-BioNTech COVID-19 vaccine (BNT162b2) side effects: A systematic review. Cureus (2022) 14(3). doi: 10.7759/cureus.23526
6. Pezzaioli LC, Gatta E, Bambini F, Facondo P, Gava M, Cavadini M, et al. Endocrine system after 2 years of COVID-19 vaccines: A narrative review of the literature. Front Endocrinol (Lausanne) (2022) 13:1–12. doi: 10.3389/fendo.2022.1027047
7. Zhao Y, Wu X. Influence of COVID-19 vaccines on endocrine system. Endocrine (2022) 78(2):241–6. doi: 10.1007/s12020-022-03119-3
8. Sheikh AB, Javaid MA, Sheikh AAE, Shekhar R. Central adrenal insufficiency and diabetes insipidus as potential endocrine manifestations of covid-19 infection: A case report. Pan Afr Med J (2021) 38:222. doi: 10.11604/pamj.2021.38.222.28243
9. Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med [Internet] (2021) 27(8):1362–6. doi: 10.1038/s41591-021-01387-6
10. Murvelashvili N, Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Investig Med High Impact Case Rep (2021) 9:0–4. doi: 10.1177/23247096211043386
11. Bouça B, Roldão M, Bogalho P, Cerqueira L, Silva-Nunes J. Central diabetes insipidus following immunization with BNT162b2 mRNA COVID-19 vaccine: A case report. Front Endocrinol (Lausanne) (2022) 13:889074. doi: 10.3389/fendo.2022.889074
12. Ankireddypalli AR, Chow LS, Radulescu A, Kawakami Y, Araki T. A case of hypophysitis associated with SARS-CoV-2 vaccination. AACE Clin Case Rep [Internet] (2022) 8(5):204–9. doi: 10.1016/j.aace.2022.06.001
13. Das L, Dutta P, Walia R, Mukherjee S, Suri V, Puri GD, et al. Spectrum of endocrine dysfunction and association with disease severity in patients with COVID-19: Insights from a cross-sectional, observational study. Front Endocrinol (Lausanne) (2021) 12:1–9. doi: 10.3389/fendo.2021.645787
14. Sheikh AB, Javed N, Sheikh AAE, Upadhyay S, Shekhar R. Diabetes insipidus and concomitant myocarditis: A late sequelae of COVID-19 infection. J Investig Med High Impact Case Rep (2021) 9:0–3. doi: 10.1177/2324709621999954
15. Yavari A, Sharifan Z, Larijani B, Mosadegh Khah A. Central diabetes insipidus secondary to COVID-19 infection: a case report. BMC Endocr Disord [Internet] (2022) 22(1):4–6. doi: 10.1186/s12902-022-01048-w
16. Lizzi M, Aricò M, Carlone G, Anzellotti MT, Trotta D, Palatino V. Central diabetes insipidus: Another rare complication of SARS-CoV-2 infection in children? Pediatr Infect Dis J (2022) 41(10):E448. doi: 10.1097/INF.0000000000003632
17. Misgar RA, Rasool A, Wani AI, Bashir MI. Central diabetes insipidus (Infundibuloneuro hypophysitis): A late complication of COVID-19 infection. J Endocrinol Invest [Internet] (2021) 44(12):2855–6. doi: 10.1007/s40618-021-01627-z
18. Balmain J, Jarebi M, Al-Salameh A, Toussaint P, Timmerman M, Chenin L, et al. Pituitary apoplexy in the aftermath of a SARS-CoV-2 infection: A case series from amiens university hospital. Eur J Endocrinol (2021) 187(3):K19–25. doi: 10.1530/EJE-22-0056
19. Balcom EF, Nath A, Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain (2021) 144(12):3576–88. doi: 10.1093/brain/awab302
20. Frara S, Loli P, Allora A, Santini C, di Filippo L, Mortini P, et al. COVID-19 and hypopituitarism. Rev Endocr Metab Disord [Internet] (2022) 23(2):215–31. doi: 10.1007/s11154-021-09672-y
21. Pinar-Gutiérrez A, Remón-Ruiz P, Soto-Moreno A. Case report: Pituitary apoplexy after COVID-19 vaccination. Med Clin (Barc) (2022) 158:496–500. doi: 10.1016/j.medcle.2021.09.021
22. Roncati L, Manenti A. Pituitary apoplexy following adenoviral vector-based COVID-19 vaccination. Brain Hemorrhages (2023) 4(1):27–9. doi: 10.1016/j.hest.2022.04.002
23. Morita S, Tsuji T, Kishimoto S, Uraki S, Takeshima K, Iwakura H, et al. Isolated ACTH deficiency following immunization with the BNT162b2 SARS-CoV-2 vaccine: A case report. BMC Endocr Disord [Internet] (2022) 22(1):1–6. doi: 10.1186/s12902-022-01095-3
24. Mizuno T, Takahashi R, Kamiyama T, Suzuki A, Suzuki M. Neuroleptic malignant syndrome with adrenal insufficiency after BNT162b2 COVID-19 vaccination in a man taking valproate: A case report. Am J Case Rep (2022) 23:4–7. doi: 10.12659/AJCR.936217
25. Lindner G, Ryser B. The syndrome of inappropriate antidiuresis after vaccination against COVID-19: Case report. BMC Infect Dis [Internet] (2021) 21(1):1–3. doi: 10.1186/s12879-021-06690-8
26. Burçin Gİ, Süleyman NŞ, Uğur Ü.. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: Post- vaccination ASIA syndrome burçin. J Clin Endocrinol Metab (2021) 106(9):2600–5. doi: 10.1210/clinem/dgab373
27. Lo Sardo L, Parisi S, Ditto MC, De Giovanni R, Maletta F, Grimaldi S, et al. New onset of giant cell arteritis following ChAdOx1-s (Vaxevria®) vaccine administration. Vaccines (2023) 11(2):1–8. doi: 10.3390/vaccines11020434
28. Paras PS, Samuel G, Daniel Z, Amanda W, Rashmi V. Optic neuritis secondary to the pfizer-BioNTech-162b2 COVID-19 vaccine managed with plasmapheresis: A case report and review. Oman J Ophthalmol (2022) 15(3):397–402. doi: 10.4103/ojo.ojo_354_21
29. Arima H, Bichet DG, Cheetham T, Christ-Crain M, Drummond J, Gurnell M, et al. Changing the name of diabetes insipidus. Pituitary (2022) 0:1–3. doi: 10.1007/s11102-022-01276-2
30. Satogami N, Miki Y, Koyama T, Kataoka M, Togashi K. Normal pituitary stalk: High-resolution MR imaging at 3T. Am J Neuroradiol (2010) 31(2):355–9. doi: 10.3174/ajnr.A1836
31. Elisabetta G, Morana G, Di Iorgi N, Pistorio A, Allegri AEM, Napoli F, et al. Role of MRI T2-DRIVE in the assessment of pituitary stalk abnormalities without gadolinium in pituitary diseases. Eur J Endocrinol (2018) 178:613–6–22. doi: 10.1530/EJE-18-0094
32. Zhou X, Zhu H, Yao Y, Lian X, Feng F, Wang L, et al. Etiological spectrum and pattern of change in pituitary stalk thickening: Experience in 321 patients. J Clin Endocrinol Metab (2019) 104(8):3419–27. doi: 10.1210/jc.2018-02297
33. Moszczyńska E, Kunecka K, Baszyńska-Wilk M, Perek-Polnik M, Majak D, Grajkowska W. Pituitary stalk thickening: Causes and consequences. the children’s memorial health institute experience and literature review. Front Endocrinol (Lausanne) (2022) 13:868558. doi: 10.3389/fendo.2022.868558
34. Prodam F, Caputo M, Mele C, Marzullo P, Aimaretti G. Insights into non-classic and emerging causes of hypopituitarism. Nat Rev Endocrinol [Internet] (2021) 17(2):114–29. doi: 10.1038/s41574-020-00437-2
35. Kumar T, Nigam JS, Jamal I, Jha VC. Primary pituitary tuberculosis. Autops Case Rep (2021) 11:e2020228. doi: 10.4322/acr.2020.228
36. Partenope C, Carceller F, Pozzobon G, Albanese A, Weber G. Implications of deferred diagnosis of paediatric intracranial germ cell tumours. Pediatr Blood Cancer (2023) 70(3):e30168. doi: 10.1002/pbc.30168
37. Bellastella G, Maiorino MI, Bizzarro A, Giugliano D, Esposito K, Bellastella A, et al. Revisitation of autoimmune hypophysitis: knowledge and uncertainties on pathophysiological and clinical aspects. Pituitary (2016) 19(6):625–42. doi: 10.1007/s11102-016-0736-z
38. Iwama S, Welt CK, Romero CJ, Radovick S, Caturegli P. Isolated prolactin deficiency associated with serum autoantibodies against prolactin-secreting cells. J Clin Endocrinol Metab (2013) 98(10):3920–5. doi: 10.1210/jc.2013-2411
39. Das L, Dutta P. Approach to the patient: A case with an unusual cause of hypopituitarism. J Clin Endocrinol Metab (2022) dgac747. doi: 10.1210/clinem/dgac747
Keywords: AVP deficiency, diabetes insipidus, COVID-19, SARS-CoV-2 vaccination, vaccine, pituitary stalk thickening, BNT162b2 Comirnaty vaccine, adolescent
Citation: Partenope C, Pedranzini Q, Petri A, Rabbone I, Prodam F and Bellone S (2023) AVP deficiency (central diabetes insipidus) following immunization with anti-COVID-19 BNT162b2 Comirnaty vaccine in adolescents: A case report. Front. Endocrinol. 14:1166953. doi: 10.3389/fendo.2023.1166953
Received: 15 February 2023; Accepted: 31 March 2023;
Published: 18 April 2023.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Liza Das, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaValeria Hasenmajer, Sapienza University of Rome, Italy
Copyright © 2023 Partenope, Pedranzini, Petri, Rabbone, Prodam and Bellone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Partenope, cGFydGVub3BlLmNyaXN0aW5hQGdtYWlsLmNvbQ==
 Cristina Partenope
Cristina Partenope Quincy Pedranzini
Quincy Pedranzini Antonella Petri
Antonella Petri Ivana Rabbone
Ivana Rabbone Flavia Prodam
Flavia Prodam Simonetta Bellone
Simonetta Bellone