- 1Reproductive Medicine Center, Foshan Women and Children Hospital, Foshan, Guangdong, China
- 2Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, Anhui, China
- 3Department of Library, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
- 4Reproductive Medical Center, Zhaoqing Westriver Hospital, Zhaoqing, Guangdong, China
- 5Department of Library, Foshan Women and Children Hospital, Foshan, Guangdong, China
- 6Department of Obstetrics, Foshan Women and Children Hospital, Foshan, Guangdong, China
- 7Foshan Institute of Fetal Medicine, Foshan Women and Children Hospital, Foshan, Guangdong, China
Background: Studies have revealed that the transplantation of mesenchymal stem cells (MSCs) might be a potential star candidate for premature ovarian failure (POF) in animal experiments. However, individual studies with a small sample size cannot be used to draw a clear conclusion. Therefore, we conducted a systematic review and meta-analysis to explore the potential of using MSCs in the treatment of POF in animals.
Methods: Seven databases were searched for studies exploring the effect of the transplantation of MSCs on POF in animal models. The PRISMA guideline was followed, and the methodological quality was ensured using SYRCLE’s risk of bias tool. RevMan 5.4 and STATA 12.0 software was performed to meta-analysis.
Results: In total, 37 studies involving 1,079 animals were included. Significant associations were found for MSCs with the levels of E2 (SMD 2.69 [95% CI 1.97, 3.41]), FSH (-2.02, [-2.74, -1.30]), primary follicles (2.04, [1.17, 2.92]), secondary follicles (1.93, [1.05, 2.81]), and primordial follicles (2.38, [1.19, 3.57]. Other outcomes, such as AMH, LH, INHB, antral follicles, growing follicles, mature follicles, and early antral were also found to be significant. There was no difference in FSH/LH, corpus leteum, follicles, and estruc cycle.
Conclusions: Our meta-analysis result indicated that the transplantation of MSCs might exert therapeutic effects on animal models of POF, and these effects might be associated with improving the disorder of the sexual cycle, modulating serum hormone expressions to a better state, and restoring ovarian function.
Introduction
Premature ovarian failure (POF) is one of the most common causes of female infertility (1). The main feature is ovarian degeneration before the age of 40, with a possibility of ovarian function fluctuating before complete cessation (2). It usually manifests hypoestrogenism, hypergonadotropism, and amenorrhea (1). POF can arise from a range of conditions, including genetic disorders, iatrogenic factors, chemotherapy injury, autoimmunity, and environmental and infectious causes (3). It has an important social and psychological impact, in addition to physiological consequences such as autoimmune disorders, heart disease, and osteoporosis (4). To date, hormone replacement therapy (HRT) is strongly recommended for women with POF, and it can improve hot flushes and vaginal and urinary symptoms for women with POF (5). In addition to HRT, new methods, such as ovarian transplantation, gene therapy, and stem cell therapy, are also urgently needed to improve the treatment efficacy of POF (6).
Mesenchymal stem cells (MSCs) are characterized by multidirectional differentiation potential. They can be isolated from various tissues, such as the peripheral blood, placenta, umbilical cord (UC), adipose tissue (AD), bone marrow (BM), and menstrual fluid (7). Studies reveal that MSC transplantation is a potential star candidate for POF. Due to their safety and manufacturing, the transplantation of MSCs has mainly focused on animal experiments for POF. Researchers found ovarian function could improve after the transplantation of human umbilical cord MSCs (hUC-MSCs) in a POF rat model (8, 9). The transplantation of BM-MSCs can also regain fertility in radiation-associated POF animal models (10). Deng T et al. (11) found that ovarian injury was reduced and ovarian function was improved after UC-MSC transplantation in a chemotherapy-induced POF mice model. In addition, studies have also revealed that Human menstrual blood MSC (HuMenSC) and chorionic plate MSC (CP-MSC) transplantation can be considered suitable treatments for POF (12, 13). It is worth noting that these studies were based on small sample sizes and were highly volatile. Because of the small sample size of each study, the treatment may overstate the efficacy of the individual animal experiments.
Studies need to be systematically combined to reach more robust conclusions. Meta-analysis can obtain stable results by increasing the sample size. There is a phenomenon of inconsistency in recent preclinical studies regarding the treatment of POF using MSC transplantation. Therefore, we conducted a systematic review and meta-analysis of preclinical animal data to evaluate the available evidence for MSCs in the treatment of POF.
Methods
We followed the PRISMA guideline for this study (Supplementary Table 1). Supplementary Data Sheet 1 shows the protocol. The study was exempt from research ethics board approval.
Search strategy
We searched three English (PubMed, Embase, and Web of Science) and four Chinese (VIP, Wanfang, CBM, and CNKI) databases from inception to August 2022. Searches included terms relating to mesenchymal stem cells, premature ovarian failure, and animal models (Supplementary Data Sheet 2). Study language was not a restriction. With the help of two medical librarians (LL and LS), two authors (CG and TS) independently searched and screened the databases.
Study selection
We considered studies if they explored the effect of MSC transplantation on POF in animal models. The animal consisted of rats, mice, rabbits, and other animals. The exposure was MSC transplantation, including both human and animal MSCs, from umbilical cord MSCs (UC-MSCs), bone marrow MSCs (BMMSCs), menstrual blood MSCs (MenSCs), placenta MSCs (PMSCs), chorionic plate MSCs (CPMSCs), adipose MSCs (ADMSCs), amniotic fluid MSCs (AFMSCs), and other tissues-derived MSCs. The control was placebo transplantation, such as 0.9% saline and phosphate-buffered saline.
The primary outcomes were estradiol (E2), follicle-stimulating hormone (FSH), primary follicles, and secondary follicles. The secondary outcomes were other sex hormone outcomes, such as luteinizing hormone (LH), anti-Mullerian hormone (AMH), FSH/LH, inhibin B (INHB), and other follicle outcomes, such as primordial follicles, growing follicles, antral follicles, mature follicles, atretic follicles, corpus leteum, follicles, early antral, and estruc cycle. The type of study included was randomized controlled studies. We excluded case studies, observational studies, reviews, and human studies. If they were duplicates, studies with the most complete information were included in the analysis.
Data extraction
Using pretested forms, two authors (CG and TS) independently screened articles for eligibility, and extracted data. Study author, publication year, author country, sample size, type and dose of MSCs, route of transplantation, type of animals, and assessment of outcomes were extracted. The closest to 4-week outcomes were extracted when results for more than one time point appeared. Conflicts were resolved through consensus with a third author (DF).
Quality assessment
The methodological quality of each included study was assessed with the use of SYRCLE’s risk of bias tool (14). The contents of the evaluation covered 10 entries in six aspects (Supplementary Table 2). Each entry was categorized as “Yes”, “No”, or “Unclear” regarding bias. Two reviewers (CG and TS) independently assessed the quality of each included study and were mediated by the third reviewer (DF).
Data analysis
Data analysis and forest plots were combined for meta-analysis using Review Manager 5.4.1 and Stata version 12. Standardized mean difference (SMD) with a 95% confidence interval (95% CI) was approved for each of the included studies. I2 statistics (> 50% indicating substantial heterogeneity) assessed the variation study-to-study. If substantial heterogeneity existed, a random effects model was used. Otherwise, a fixed effects model was applied. A Funnel plot and Begg’s and Egger’s tests were provided to assess the potential publication biases if more than 10 studies for one outcome were included. We performed subgroup analyses for animal species (rats, mice, rabbits, or mice and rats), induced (chemotherapy, autoimmune, or radiation), source of MSCs (human or animal), types of MSCs (UCMSCs, BMMSCs, AFMSCs, CPMSCs, MenSCs, ADMSCs, or PMSCs), the transplantation dose (105, 106, or 107), and route of transplantation (intravenously injected or intra ovary injected). We considered p-values of less than 0.05 to be statistically significant.
Results
Search results and characteristics
Of 1,406 citations identified, we assessed 97 full articles. Eventually, 37 studies, which included 1,079 animals (540 in the MSC group and 539 in the control group), proved eligible (Figure 1). The animal species included rats (n = 23) (8–10, 12, 15–33), mice (n = 12) (11, 13, 34–43), and rabbits (n = 2) (44, 45). Most MSCs were from the umbilical cord (UCMSCs, n = 18) (8, 9, 11, 16, 18, 19, 21, 25, 27, 29, 34, 36–39, 42, 44, 45) or bone marrow (BMMSCs, n = 11) (10, 17, 22–24, 28, 30, 31, 33, 41, 43), and, other MSCs were from menstrual blood (MenMSCs, n = 2) (12, 35), placenta (PMSCs, n = 2) (20, 40), adipose (ADMSCs, n = 2) (15, 26), amniotic fluid (AFMSCs, n = 1) (32), or chorionic plate (CPMSCs, n = 1) (13). In total, 24 MSCs were from human tissues, and the other 13 MSCs were from animal tissues. Thirty-one POF models were induced by chemotherapy, five models were induced by autoimmune, and one model was induced by radiotherapy. Six studies transplanted 105 cells, 27 studies transplanted 106 cells, and four studies transplanted 107 cells. Furthermore, 25 studies used intravenous (IV) injections to transplant cells, and 12 studies used intra-ovary injections (IO). Outcomes were sex hormones, including E2 (n = 30), FSH (n = 27), AMH (n = 7), LH (n = 6), FSH/LH (n = 5), and INHB (n = 2), ovarian follicles counting, including primary follicles (n = 13), secondary follicles (n = 12), primordial follicles (n = 11), atretic follicles (n = 9), antral follicles (n = 6), growing follicles (n = 5), mature follicles (n = 5), corpus leteum (n = 5), follicles (n =3), and early antral (n = 2), and estrus cycle (n = 7) (Table 1).
Assessment of study quality and risk of bias
The quality and risk of bias for studies included in the meta-analysis are presented in Supplementary Table 2. Only six of the 37 studies mentioned sequence generation. The baseline characteristics were comparability. Allocation concealment, attrition, reporting, and other bias were not found. Performance bias and detection bias were unclear for all the included studies.
Meta-analysis results
E2
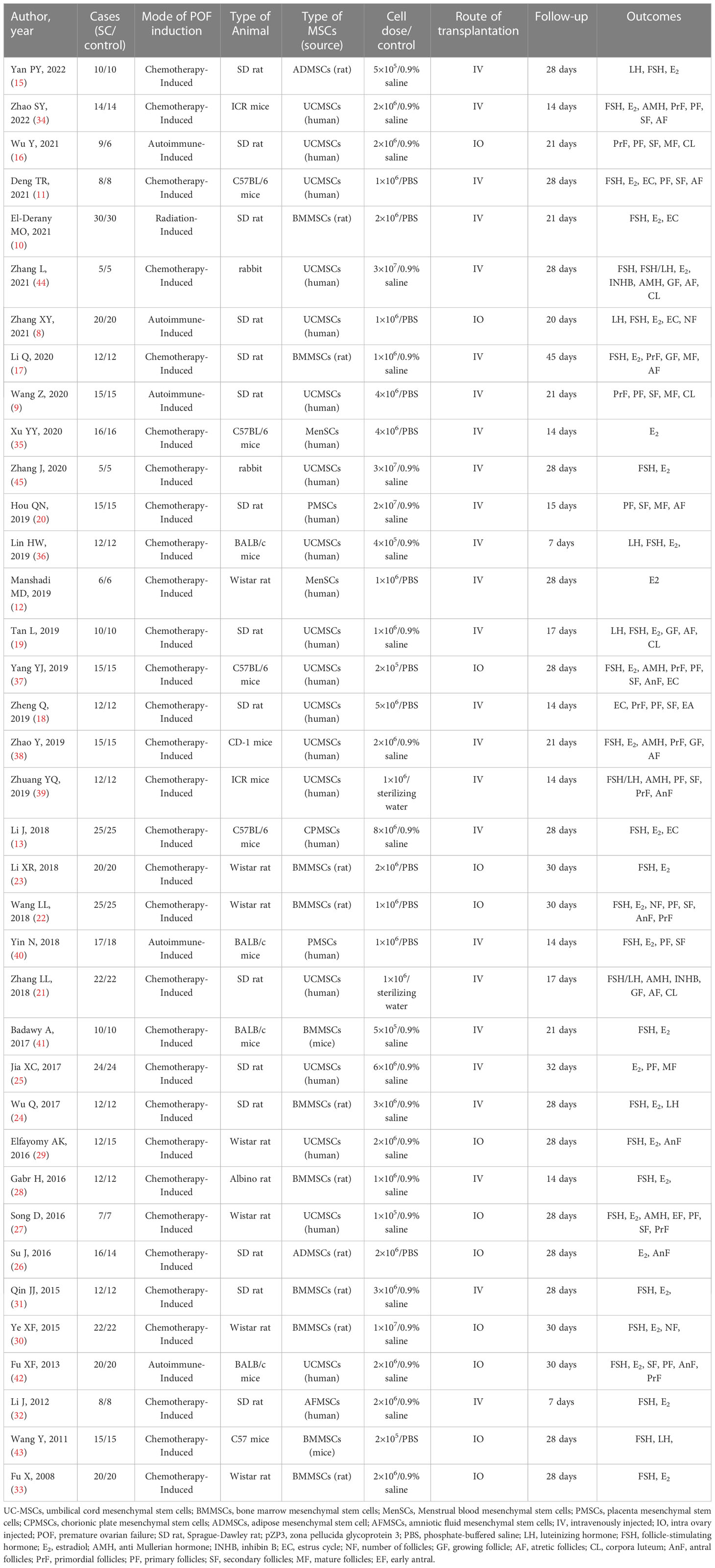
In total, 30 studies (n = 568 animals) provided data for this comparison (286 vs. 282 animals). E2 was noted to be higher in the MSC group (SMD 2.69, 95% CI 1.97 to 3.41) in the random-effect model (I2 = 88) (Figure 2). A funnel plot showed asymmetry (Supplementary Figure 1), and Begg’s (z = 4.46, p = 0.001) and Egger’s (t = 6.50, p = 0.001) tests also indicated that there was a statistical difference.
FSH
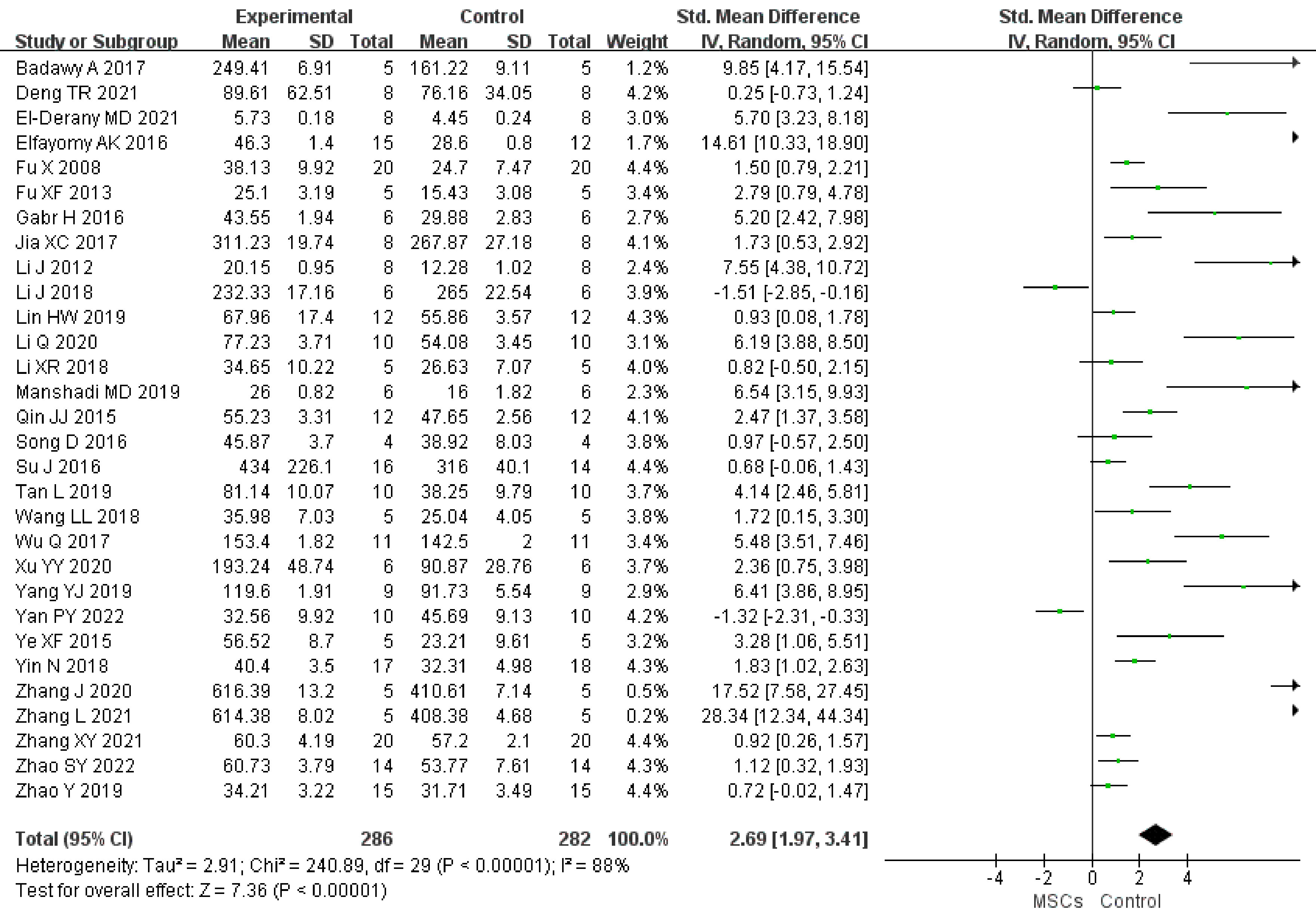
In total, 27 studies (n = 508 animals) provided data for FSH comparison (255 vs. 253 animals). FSH was noted to be lower in the MSC group (SMD -2.02, 95% CI -2.74to -1.30) in the random-effect mode (I2 = 88%) (Figure 3). A funnel plot showed asymmetry (Supplementary Figure 2), and Begg’s (z = 3.21, p = 0.001) and Egger’s (t = -2.96, p = 0.007) tests also indicated that there was a statistical difference.
Primary follicles
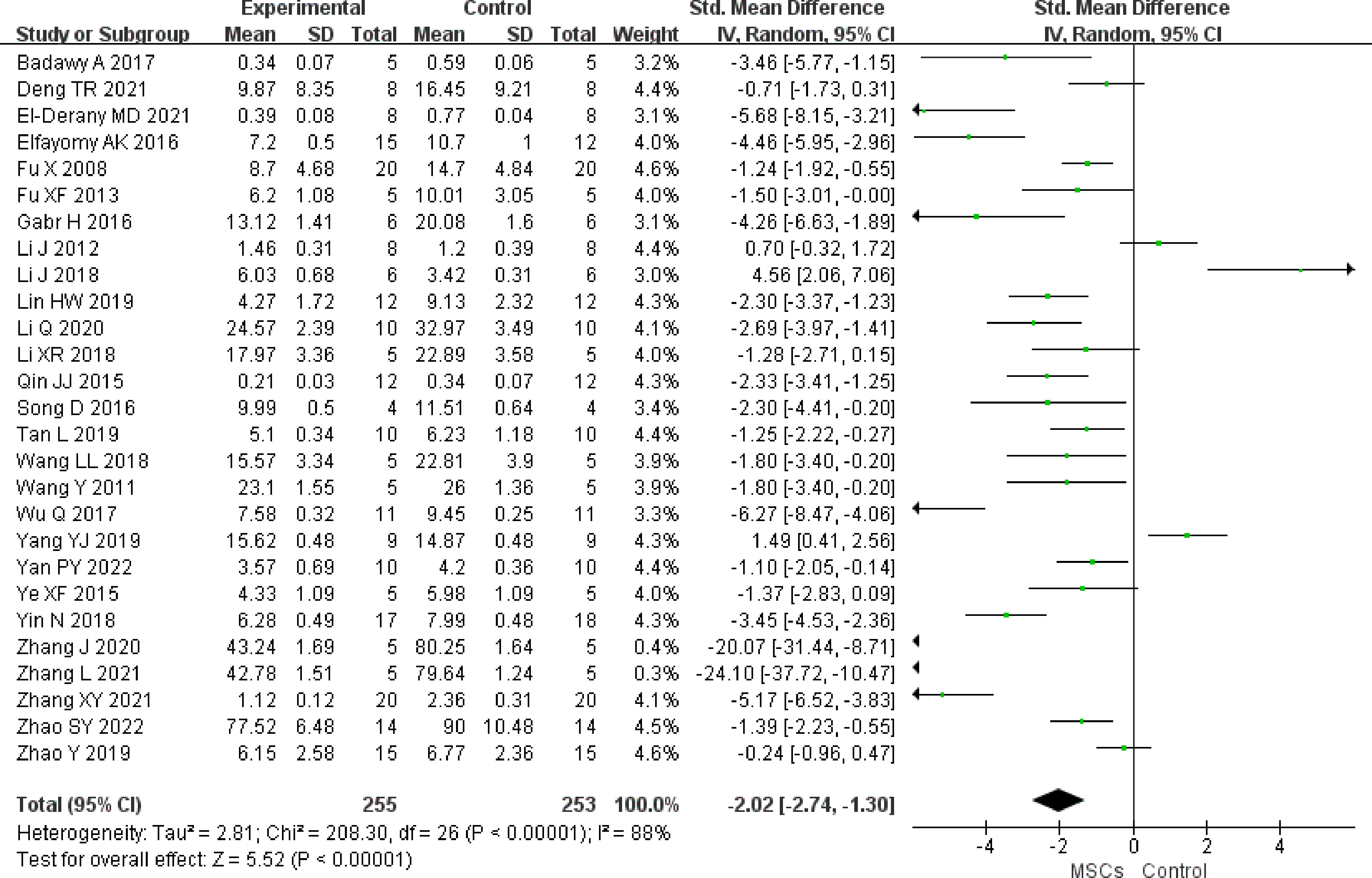
In total, 13 studies (n = 254 animals) reported primary follicles. It was noted to be higher in the MSC group (SMD 2.04, 95% CI 1.17 to 2.92) in the random-effect mode (I2 = 84%) (Figure 4). A funnel plot showed asymmetry (Supplementary Figure 3), and Begg’s (z = 1.77, p = 0.077) and Egger’s (t = 3.10, p = 0.010) tests also indicated that there was a statistical difference.
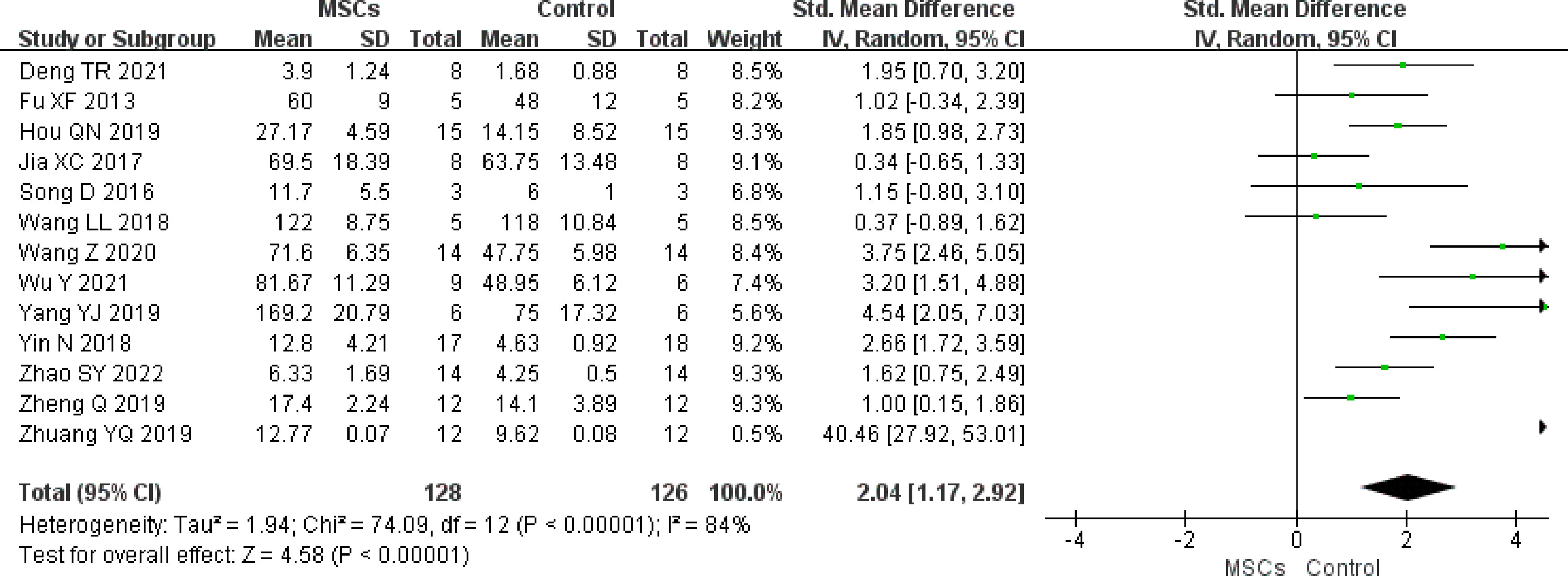
Figure 4 Forest plot summarizing the effects of MSCs on primary follicles in preclinical models of POF.
Secondary follicles
The effect size for secondary follicles was pooled from 12 studies (n = 238 animals). A significant association was found between MSC therapy and an increase in the secondary follicle counts (SMD 1.93, (95% CI 1.05 to 2.81) in the random-effect mode (I2 = 83%) (Figure 5). A funnel plot showed symmetry (Supplementary Figure 4), and Begg’s (z = 1.17, p = 0.244) and Egger’s (t = 2.72, p = 0.022) tests also indicated that there was a statistical difference.
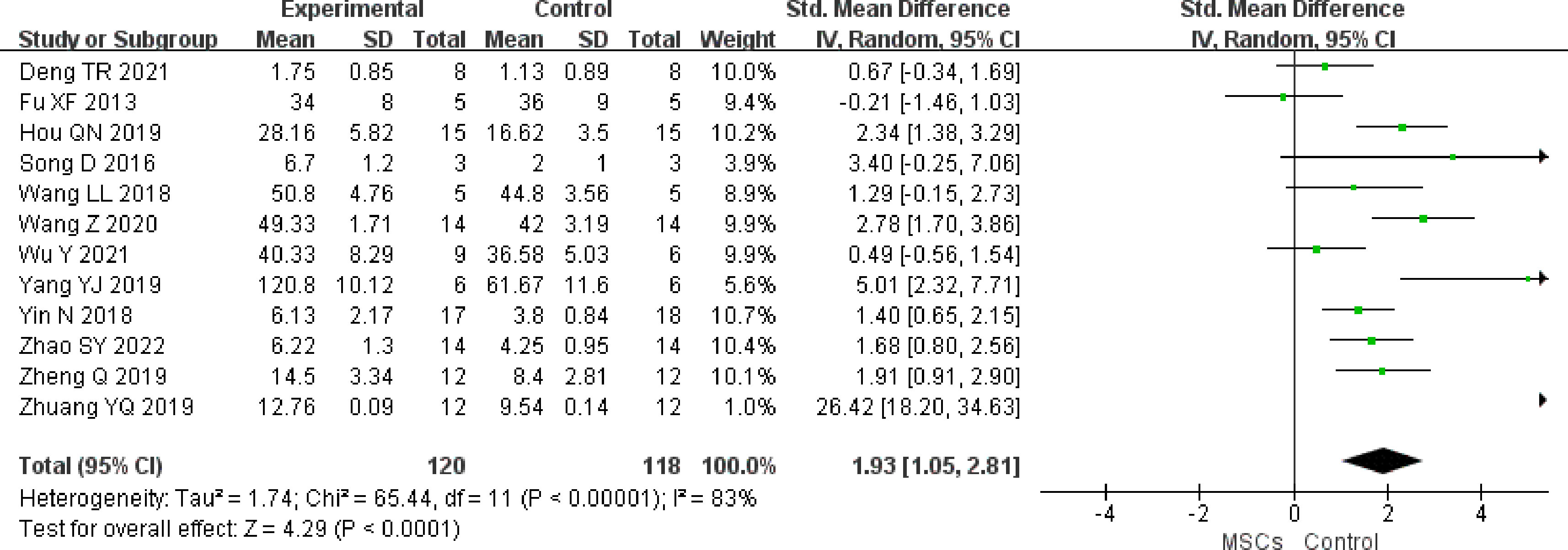
Figure 5 Forest plot summarizing the effects of MSCs on secondary follicles in preclinical models of POF.
Primordial follicles
We identified 11 studies (n = 207 animals) that compared MSC treatment with controls for primordial follicles. Higher primordial follicle counts were noted in the MSC group (SMD 2.38, 95% CI 1.19 to 3.57) in the random-effect mode (I2 = 89%) (Figure 6). A funnel plot showed asymmetry (Supplementary Figure 5), and Begg’s (z = 2.65, p = 0.008) and Egger’s (t = 3.75, p = 0.005) tests also indicated that there was a statistical difference.
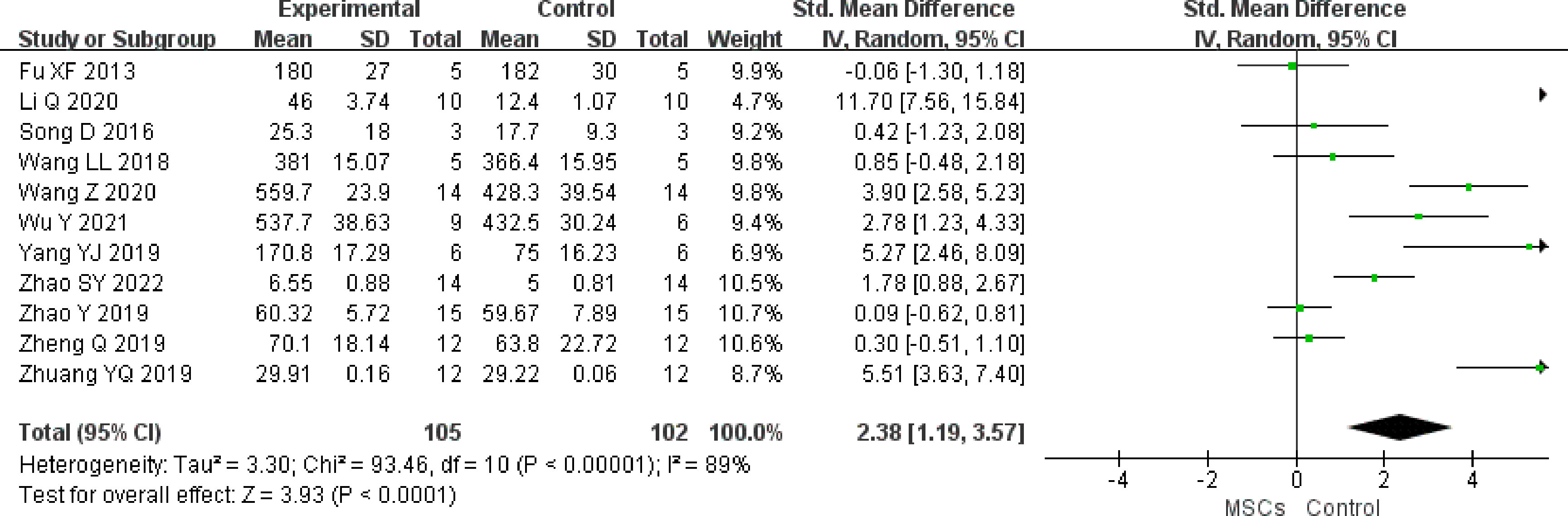
Figure 6 Forest plot summarizing the effects of MSCs on primordial follicles in preclinical models of POF.
Other outcomes
The meta-analysis showed that MSCs significantly increased AMH (seven studies, n = 130 animals, SMD 1.41 95% CI 0.67 to 2.14, I2 = 67%, random-effect mode), INHB (two studies, n = 20 animals, SMD 23.51 95% CI 14.11 to 32.91, I2 = 0, fixed-effect mode), antral follicles (six studies, n = 113 animals, SMD 2.85 95% CI 0.58 to 5.11, I2 = 93%, random-effect mode), growing follicles (five studies, n = 116 animals, SMD 2.08 95% CI 1.19 to 2.98, I2 = 68%, random-effect mode), mature follicles (five studies, n = 109 animals, SMD 3.10 95% CI 1.86 to 4.34, I2 = 76%, random-effect mode), and early antral (two studies, n = 30 animals, SMD 1.00 95% CI 0.23 to 1.78, I2 = 0, fixed-effect mode), and significantly reduced LH (six studies, n = 132 animals, SMD -2.53 95% CI -3.79 to -1.27, I2 = 85%, random-effect mode), atretic follicles (nine studies, n = 184 animals, SMD -1.71 95% CI -3.08 to -0.33, I2 = 90%, random-effect mode). However, there was no difference in FSH/LH (three studies, n = 46 animals, SMD -0.08 95% CI -0.66 to 0.49, I2 = 0, fixed-effect mode), corpus leteum (five studies, n = 85 animals, SMD 1.02 95% CI -1.47 to 3.51, I2 = 93%, random-effect mode), follicles (three studies, n = 60 animals, SMD 3.05 95% CI -0.58 to 6.69, I2 = 94%, random-effect mode), and estruc cycle (seven studies, n = 180 animals, SMD 0 95% CI -1.65 to 1.66, I2 = 94%, random-effect mode) between the MSC group and control group.
Subgroup analysis
Most of the subgroup analyses did not change substantially, except for a few variables that resulted from fewer data (Table 2).
Discussion
This systematic review and meta-analysis examining 37 studies (1,079 animals) found that mesenchymal stem cell transplantation might effectively improve ovarian function in premature ovarian failure in animal model studies.
According to the source of the isolated tissue, MSCs can be classified into different types. Multiple previous studies indicated that MSC transplantation was effective in repairing tissue damage, including neurodegenerative diseases, skin injury, cardiovascular disease, bone damage, and diabetes (46–49). For POF, MSC therapy had promising potential for clinical use but some safety and manufacturing issues have kept it mostly in the animal model stage.
In preclinical studies, MSCs appeared to be able to improve certain functional measures of damaged ovaries, including sex hormones and the number of follicles at different stages, in POF animal models. However, MSCs from different tissues produced different effects. One study has shown (11) that primary and secondary follicles increased, atretic follicles decreased, and ovarian volume also can increase after UC-MSC treatment in POF mice. Another study found that BM-MSCs could regulate and activate ovarian function and regain fertility in a radiation-induced POF rat model (10). Meanwhile, serum hormone levels, including E2 and FSH, were restored after the transplantation of CP-MSC in a chemotherapy-induced POF mouse model (13). Overall, our results are generally consistent with previous research and showed that different MSCs from different sources are different in repairing ovarian function in this study. In addition to the properties of specialized cells, the sample size and included studies are also important and affect treatment outcomes, and small samples often produced false negative results.
It is important to note that, from the analysis of the 37 studies, it is suggested that MSC transplantation enhanced the primordial follicle formation and follicle growth up to the secondary follicle stage, but not to the antral follicle stage, thus contributing to hormonal status as far as estrogen levels are concerned while not contributing to re-establishing the estrus cycle. The reason for this may be the limitations of the original articles concerning their methodological quality or their small sample size. Meanwhile, the significant changes found in the analyses regarding different parameters should be evaluated and commented on with respect to the duration of the effect and observation period.
Although they have been shown to be safe in animal models, the risk cannot be ignored concerning MSCs being transplanted into patients. Several small-sample clinical trials indicated that ovarian function and fertility could improve in women with POF after MSC therapy. In their phase I study (1), the authors found MSCs were safe to use for more than a year, and menstruation also can resume for POF patients. Other clinical results (3, 50, 51) also showed that transplantation of MSCs could improve ovarian features, including increasing the number of follicles and improving ovarian hormone production, in POF patients. In these human studies, it seems that the improvement is somewhat transient or temporary. Thus, it is important to have a similar conclusion in animal studies and provide some evidence and guidance to the readers for the next step.
In this comprehensive, thorough, and complete systematic review and meta-analysis study, there is further evidence, obtained by increasing the sample size in POF animal models, that MSC treatment could rescue ovarian function. The results of this study will provide more powerful preclinical medical evidence support for the application of MSCs in high-quality clinical trials. Our study further demonstrates the ability of MSCs to treat POF. However, it is still a tough task for the transformation from animal to human. More detailed mechanisms of MSC treatment for POF need to be discovered in the future. In addition, continuous follow-ups are needed to evaluate the long-term effects of this new intervention.
There are still several limitations of this study that need consideration. First, we only searched the commonly used English and Chinese databases, which would lead to some language bias. Second, the included studies were generally of low methodological quality, and the sample size of each study was small. Third, some outcomes could not be subgroup analyzed because of small sample sizes. In addition, the various sources of MSCs and animal species could limit the value of MSCs in the treatment of premature ovarian failure.
Conclusions
In summary, this systematic review and meta-analysis indicated that MSCs might exert therapeutic effects on animal models of POF, and these effects might be associated with improving the disorder of the sexual cycle, modulating the serum hormone expression to a better state, and restoring ovarian function.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DF participated in the design and coordination of the study. CG conceived the study and drafted the manuscript. YM, YS, LL, GL, HL, WM, LS, and WW searched for the studies and collected and analyzed the data. WW, QW, and LW participated in the design of this study and edited the manuscript. DF, LL, and YM did the data management and analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the 2023 Foshan Health Bureau Medical Scientific Research Project (No. 20230819A010172).
Acknowledgments
We appreciate the efforts of all the researchers whose articles were included in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1165574/full#supplementary-material
Supplementary Table 1 | PRISMA Checklist.
Supplementary Table 2 | Study quality and risk of bias.
Supplementary Data Sheet 1 | Study protocol.
Supplementary Data Sheet 2 | Search strategy.
Supplementary Data Sheet 3 | Supplementary Figures 1 to 5.
Abbreviations
AD, adipose tissue; AMH, anti Mullerian hormone; BM, bone marrow; CI, confidence intervals; E2, estradiol; FSH, follicle-stimulating hormone; hUC-MSCs, human umbilical cord MSCs; INHB, inhibin B; LH, luteinizing hormone; MSCs, mesenchymal stem cells; POF, premature ovarian failure; SMD, standardized mean difference; UC, umbilical cord.
References
1. Mashayekhi M, Mirzadeh E, Chekini Z, Ahmadi F, Eftekhari-Yazdi P, Vesali S, et al. Evaluation of safety, feasibility and efficacy of intra-ovarian transplantation of autologous adipose derived mesenchymal stromal cells in idiopathic premature ovarian failure patients: non-randomized clinical trial, phase I, first in human. J Ovarian Res (2021) 14:5. doi: 10.1186/s13048-020-00743-3
2. Grossmann B, Saur S, Rall K, Pecher AC, Hubner S, Henes J, et al. Prevalence of autoimmune disease in women with premature ovarian failure. Eur J Contracept Reprod Health Care (2020) 25:72–5. doi: 10.1080/13625187.2019.1702638
3. Yan L, Wu Y, Li L, Wu J, Zhao F, Gao Z, et al. Clinical analysis of human umbilical cord mesenchymal stem cell allotransplantation in patients with premature ovarian insufficiency. Cell Prolif (2020) 53:e12938. doi: 10.1111/cpr.12938
4. Shareghi-Oskoue O, Aghebati-Maleki L, Yousefi M. Transplantation of human umbilical cord mesenchymal stem cells to treat premature ovarian failure. Stem Cell Res Ther (2021) 12:454. doi: 10.1186/s13287-021-02529-w
5. Webber L, Anderson RA, Davies M, Janse F, Vermeulen N. HRT for women with premature ovarian insufficiency: a comprehensive review. Hum Reprod Open (2017) 2017:hox007. doi: 10.1093/hropen/hox007
6. Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: past, present, and future. Front Cell Dev Biol (2021) 9:672890. doi: 10.3389/fcell.2021.672890
7. Gao M, Yu Z, Yao D, Qian Y, Wang Q, Jia R. Mesenchymal stem cells therapy: a promising method for the treatment of uterine scars and premature ovarian failure. Tissue Cell (2022) 74:101676. doi: 10.1016/j.tice.2021.101676
8. Zhang X, Zhang L, Li Y, Yin Z, Feng Y, Ji Y. Human umbilical cord mesenchymal stem cells (hUCMSCs) promotes the recovery of ovarian function in a rat model of premature ovarian failure (POF). Gynecol Endocrinol (2021) 37:353–7. doi: 10.1080/09513590.2021.1878133
9. Wang Z, Wei Q, Wang H, Han L, Dai H, Qian X, et al. Mesenchymal stem cell therapy using human umbilical cord in a rat model of autoimmune-induced premature ovarian failure. Stem Cells Int (2020) 2020:3249495. doi: 10.1155/2020/3249495
10. El-Derany MO, Said RS, El-Demerdash E. Bone marrow-derived mesenchymal stem cells reverse radiotherapy-induced premature ovarian failure: emphasis on signal integration of TGF-beta, wnt/beta-catenin and hippo pathways. Stem Cell Rev Rep (2021) 17:1429–45. doi: 10.1007/s12015-021-10135-9
11. Deng T, He J, Yao Q, Wu L, Xue L, Wu M, et al. Human umbilical cord mesenchymal stem cells improve ovarian function in chemotherapy-induced premature ovarian failure mice through inhibiting apoptosis and inflammation via a paracrine mechanism. Reprod Sci (2021) 28:1718–32. doi: 10.1007/s43032-021-00499-1
12. Manshadi MD, Navid S, Hoshino Y, Daneshi E, Noory P, Abbasi M. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc Res Tech (2019) 82:635–42. doi: 10.1002/jemt.23120
13. Li J, Yu Q, Huang H, Deng W, Cao X, Adu-Frimpong M, et al. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res Ther (2018) 9:81. doi: 10.1186/s13287-018-0819-z
14. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol (2014) 14:43. doi: 10.1186/1471-2288-14-43
15. Yan PY, Zhang AC, Zhang H, Li Y, Zhang MM, Luo MZ, et al. Therapeutic effect of adipose-derived mesenchymal stem cells on premature ovarian failure model rats and its mechanism. J Jilin Univ (Medicine Edition) (2022) 48:648–56. doi: 10.13481/j.1671-587X.20220313
16. Wu Y, Wang H, Wang Z, Yu HL, Dai HJ, Wei QW, et al. The effect of In situ injection of human umbilical cord mesenchymal stem cells on premature ovarian failure in rats. Chin J Cell Biol (2021) 43:2334–43. doi: 10.11844/cjcb.2021.12.0007
17. Li Q, Ao HF, Li L, Luo H. Ovarian functional reconstruction in rats with premature ovarian failure through hyperbaric oxygen combined with bone marrow mesenchymal stem cell transplantation. J Chongqing Med Uni (2020) 45:1551–6. doi: 10.13406/j.cnki.cyxb.002619
18. Zheng Q, Fu X, Jiang J, Zhang N, Zou L, Wang W, et al. Umbilical cord mesenchymal stem cell transplantation prevents chemotherapy-induced ovarian failure via the NGF/TrkA pathway in rats. BioMed Res Int (2019) 2019:6539294. doi: 10.1155/2019/6539294
19. Tan L, Mao XG, Zhong Y, Liu J. Repair of premature ovarian failure in rats by transplantation of human umbilical cord mesenchymal stem cells. Chin J Comp Med (2019) 29:85–91. doi: 10.3969/j.issn.1671-7856.2019.10.015
20. Hou QN, Ma HM, Xiang L, He YT, Xu X, Chen DM, et al. Effects of human placenta mesenchymal stem cell transplantation on ovarian function in premature ovarian failure in rats. J Shandong Uni (Heal Sci) (2019) 57:52–60. doi: 10.6040/j.issn.1671-7554.0.2018.1388
21. Zhang LL, Wang T, Liu WH, Du J, Wang H. Experimental study on the feasibility and mechanism of human umbilical cord mesenchymal stem cells in the treatment of the premature ovarian failure rats. Chongqing Med (2018) 47:3968–72. doi: 10.3969/j.issn.1671-8348.2018.31.002
22. Wang LL, Fang HS, Ni MS, Gu SJ, Fu XF. Experiment of transplanting the heat shock pretreated MSCs to premature ovary caused by chemotherapy. Guangdong Med J (2018) 39:1951–4. doi: 10.13820/j.cnki.gdyx.20180717.013
23. Li XR, He YL, Wang XF, Peng DX, Cheng XY, Wang Q, et al. Therapeutic potential and mechanism of bone marrow mesenchymal stem cells with over expression of miR-21 in rat models of chemotherapy-induced premature ovarian failure. Pro Obstet Gynecol (2018) 27:924–8. doi: 10.13283/j.cnki.xdfckjz.2018.12.010
24. Wu Q, Qin JJ, Yang J, Liang JL. Effect of combined treatment of zuogui pill and mesenchymal stem cells on chemotherapy-induced premature ovarian failure in rats. CJITWM (2017) 37:1361–6. doi: 10.7661/j.cjim.20171011.238
25. Jia XC, Lu ZY, Meng WJ, Shi YH. Effects of human umbilical cord mesenchymal stem cells combined with goserelin on repairing rat ovarian damage. Tianjin Med J (2017) 45:25–9. doi: 10.11958/20160858
26. Su J, Ding L, Cheng J, Yang J, Li X, Yan G, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod (2016) 31:1075–86. doi: 10.1093/humrep/dew041
27. Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. BioMed Res Int (2016) 2016:2517514. doi: 10.1155/2016/2517514
28. Gabr H, Rateb MA, El Sissy MH, Ahmed Seddiek H, Ali Abdelhameed Gouda S. The effect of bone marrow-derived mesenchymal stem cells on chemotherapy induced ovarian failure in albino rats. Microsc Res Tech (2016) 79:938–47. doi: 10.1002/jemt.22725
29. Elfayomy AK, Almasry SM, El-Tarhouny SA, Eldomiaty MA. Human umbilical cord blood-mesenchymal stem cells transplantation renovates the ovarian surface epithelium in a rat model of premature ovarian failure: possible direct and indirect effects. Tissue Cell (2016) 48:370–82. doi: 10.1016/j.tice.2016.05.001
30. Ye XF, He YL, Fu XF, Wang XF. Bone marrow mesenchymal stem cell transplantation for treatment of cis-platinum-induced ovarian damage. Chin J Tis Eng Res (2015) 19:1597–62. doi: 10.3969/j.issn.2095-4344.2015.10.22
31. Qin JJ, Wu Q, Yang J, Liang JL. Chemotherapy-induced premature ovarian failure rats treated by TCM therapy of tonifying kidney-yin with bone marrow mesenchymal stem cells transplantation. J Beijing Uni Trad Chin Med (2015) 38:735–9. doi: 10.3969/j.issn.1006-2157.2015.11.004
32. Li J, Zhang JF, Bai L, Chen BL. Preliminary study on the effects of human amniotic fluid-derived stem cells on rats premature ovarian failure. Pro Obstet Gynecol (2012) 21:116–9. doi: 10.13283/j.cnki.xdfckjz.2012.02.003
33. Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy (2008) 10:353–63. doi: 10.1080/14653240802035926
34. Zhao SY, Guo GL, Dong SR, Zhang C, Gong QQ, Liu CC, et al. Effects of transplantation of umbilical cord mesenchymal stem cells on ovarian function in mice with premature ovarian failure. BME Clin Med (2022) 26:15–21. doi: 10.13339/j.cnki.sgle.20211217.018
35. Xu YY, Yan B, Wang R, Wang HY. Menstrual blood mesenchymal stem cells improve premature ovarian failure in mice via IGF-1 signaling. J Shandong Uni (Heal Sci) (2020) 58:14. doi: 10.6040/j.issn.1671-7554.0.2019.1278;
36. Lin HW, Liang ZJ, Wu Z, Liang Y, Cai ZQ, Zhong ZY. Mechanism of human umbilical cord mesenchymal stem cells for premature ovarian failure mice model induced by tripterygium wilfordii polyglycosides. BME Clin Med (2019) 23:387–93. doi: 10.13339/j.cnki.sgle.20190708.003
37. Yang Y, Lei L, Wang S, Sheng X, Yan G, Xu L, et al. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In Vitro Cell Dev Biol Anim (2019) 55:302–11. doi: 10.1007/s11626-019-00337-4
38. Zhao Y, Ruan XY, Wang FC, Li YL, Cheng JJ, Mueck. AO. Therapeutic effect of hypoxia-preconditioned human umbilical cord mesenchymal stem cell on premature ovarian insufficiency mouse model. J Capital Med Uni (2019) 40:565–71. doi: 10.3969/j.issn.1006-7795.2019.04.014
39. Zhuang YQ, Yang QQ, Duan C, Zhang LD, Feng QL. Effects of human umbilical cord mesenchymal stem cells on rats with premature ovarian insufficiency. Med Innovat Chin (2019) 16:15–8. doi: 10.3969/j.issn.1674-4985.2019.23.004
40. Yin N, Zhao W, Luo Q, Yuan W, Luan X, Zhang H. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by treg cells and associated cytokines. Reprod Sci (2018) 25:1073–82. doi: 10.1177/1933719117732156
41. Badawy A, Sobh MA, Ahdy M, Abdelhafez MS. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Womens Health (2017) 9:441–7. doi: 10.2147/IJWH.S134074
42. Fu XF, He YL. The effects of umbilical cord mesenchymal stem cell transplantation on immune premature ovarian failure. Guangdong Med J (2013) 34:3535–8. doi: 10.13820/j.cnki.gdyx.2013.23.026
43. Wang Y, Yang Y, Liu B, Chen YY, Chen DB. Bone marrow mesenchymal stem cell transplantation for premature ovarian failure induced by 4-vinylcyclohexene diepoxide. Prog Mod Biomed (2011) 11:1844–6. doi: 10.13241/j.cnki.pmb.2011.10.030
44. Zhang L, Li QY, Ceng L, Yi HY, Cao JY. Therapeutic effect and mechanism of umbilical cord mesenchymal stem cells on premature ovarian failure in rabbits. Prog Mod Biomed (2021) 21:2432–6. doi: 10.13241/j.cnkipmb.2021.13.007
45. Zhang J, Wang J, Zhou L, Tou P, Wang G, Zhu CC. Effect of umbilical cord-derived mesenchymal stem cells on the expression of sex hormones CYR61 and CTGF in rabbits with premature ovarian failure. Chin J Comp Med (2020) 30:56–62. doi: 10.3969/j.issn.1671-7856.2020.03.010
46. Fan D, Zeng M, Xia Q, Wu S, Ye S, Rao J, et al. Efficacy and safety of umbilical cord mesenchymal stem cells in treatment of cesarean section skin scars: a randomized clinical trial. Stem Cell Res Ther (2020) 11:244. doi: 10.1186/s13287-020-01695-7
47. Chi H, Guan Y, Li F, Chen Z. The effect of human umbilical cord mesenchymal stromal cells in protection of dopaminergic neurons from apoptosis by reducing oxidative stress in the early stage of a 6-OHDA-Induced parkinson’s disease model. Cell Transplant (2019) 28:87S–99S. doi: 10.1177/0963689719891134
48. Yamazaki K, Kawabori M, Seki T, Takamiya S, Konno K, Watanabe M, et al. Mesenchymal stem cell sheet promotes functional recovery and palliates neuropathic pain in a subacute spinal cord injury model. Stem Cells Int (2021) 2021:9964877. doi: 10.1155/2021/9964877
49. He Q, Song J, Cui C, Wang J, Hu H, Guo X, et al. Mesenchymal stem cell-derived exosomal miR-146a reverses diabetic beta-cell dedifferentiation. Stem Cell Res Ther (2021) 12:449. doi: 10.1186/s13287-021-02371-0
50. Cheng YC, Takagi M, Milbourne A, Champlin RE, Ueno NT. Phase II study of gonadotropin-releasing hormone analog for ovarian function preservation in hematopoietic stem cell transplantation patients. Oncologist (2012) 17:233–8. doi: 10.1634/theoncologist.2011-0205
Keywords: mesenchymal stem cells, premature ovarian failure, ovarian function, fertility, meta-analysis
Citation: Guo C, Ma Y, Situ Y, Liu L, Luo G, Li H, Ma W, Sun L, Wang W, Weng Q, Wu L and Fan D (2023) Mesenchymal stem cells therapy improves ovarian function in premature ovarian failure: a systematic review and meta-analysis based on preclinical studies. Front. Endocrinol. 14:1165574. doi: 10.3389/fendo.2023.1165574
Received: 08 March 2023; Accepted: 12 June 2023;
Published: 06 July 2023.
Edited by:
Eytan R. Barnea, BioIncept, LLC, United StatesReviewed by:
Hang-soo Park, The University of Chicago, United StatesNecati Findikli, Bahçeci Fulya IVF Center, Türkiye
Bunpei Ishizuka, Rose Ladies Clinic, Japan
Copyright © 2023 Guo, Ma, Situ, Liu, Luo, Li, Ma, Sun, Wang, Weng, Wu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dazhi Fan, ZmFuZGF6aGlnd0AxNjMuY29t
 Congcong Guo1
Congcong Guo1 Yubo Ma
Yubo Ma Dazhi Fan
Dazhi Fan