
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 19 May 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1159055
This article is part of the Research Topic Multi-organ Linkage Pathophysiology and Therapy for NAFLD and NASH View all 12 articles
Background: The negative effects of obesity on hepatic steatosis and fibrosis have received considerable attention in recent years. The weight-adjusted-waist index (WWI) reflects weight-independent centripetal obesity. Herein, we provide the first investigation of a link between WWI, hepatic steatosis, and liver fibrosis.
Methods: We used data from the National Health and Nutrition Examination Survey 2017-2020 to conduct a cross-sectional study. The linear relationship between WWI, controlled attenuation parameters, and liver stiffness measurements (LSM) was investigated using multivariate linear regression models. The nonlinear relationship was described using fitted smoothed curves and threshold effect analyses. Subgroup analyses were performed based on gender, age, body mass index, diabetes, hypertension, drinking, and smoking.
Results: This population-based study included 7,594 people, 50.74% of whom were men and 49.26% of whom were women. Multivariate linear regression analysis revealed a significant positive relationship between WWI and hepatic steatosis [CAP, β=7.60, 95% confidence interval (CI) (4.42, 10.78), P<0.0001]. This positive association was stronger when excessive alcohol intake was present compared to when it was absent (P for interaction = 0.031), and when hypertension was present compared to when it was not (P for interaction = 0.014). The linear relationship between WWI and liver fibrosis was not statistically significant on multiple regression analysis [LSM, β=0.03, 95% CI (-0.26, 0.32), P=0.84]. However, a U-shaped association was seen between WWI and LSM, with a negative correlation when WWI< 10.92 and a positive correlation when WWI > 10.92.
Conclusion: We report a strong association between WWI and hepatic steatosis, and suggest that it may potentially be used as a simple anthropometric index to predict hepatic steatosis.
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, which affects up to 40% of adults and children (1, 2). NAFLD can progress from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) and then to liver fibrosis, cirrhosis, and hepatocellular carcinoma (3, 4). NAFLD has been linked to obesity and obesity-related metabolic disorders such as glucose intolerance, type 2 diabetes (T2D), and dyslipidemia (5).
Excessive hepatic fat accumulation not only leads to local changes, such as hepatocyte dysfunction, proinflammatory immune response activation, and fibrogenesis, but also triggers a series of extrahepatic metabolic disorders, including cardiovascular events and T2D. Furthermore, observational studies have highlighted that both hepatic steatosis and fibrosis are associated with a higher risk of all-cause mortality. Therefore, determining the level of liver steatosis is critical in the evaluation and clinical prognosis of patients with NAFLD (6). Although pathological biopsy remains the gold standard for evaluating the severity of hepatic steatosis and liver fibrosis, vibration controlled transient elastography (VCTE) is a non-invasive alternative that is increasingly being used. Recent observational studies have suggested that VCTE has robust accuracy in estimating the grade of hepatic steatosis and the stage of liver fibrosis (7, 8). However, the use of VCTE is limited by its popularity and high learning curve (9).
Weight-adjusted-waist index (WWI) was first postulated in 2018 as an anthropometric measure of central obesity that reflects both fat and muscle mass components, regardless of the body mass index (BMI) (10, 11). The links between WWI and various cardiovascular events have since been well established (11–14). However, the relationship between WWI and these hepatic indicators has not been defined.
In the present study, we used the National Health and Nutrition Examination Survey (NHANES) to investigate the relationship between WWI and hepatic steatosis and liver fibrosis in the US population.
The NHANES is a cross-sectional survey conducted every two years to assess the nutritional and physical health of the general public in the United States (15, 16). Through interviews and related tests, demographics, dietary, and health-related information are collected (12, 13). The survey is approved by the Center for Disease Control and Prevention Research Ethics Review Board, and all survey participants provide written informed consent to participate (17).
In the present study, we used the 2017–2020 pre-coronavirus-19 pandemic data from the NHANES database. Out of the 15,560 individuals who participated, we excluded those with hepatitis B or C infection (n=215), missing VCTE data (n=434), unreliable VCTE estimation (liver stiffness interquartile range/median≥30%, n = 201), those younger than 18 years old (n=4,123), and incomplete data on weight and waist circumference (WC) (n=2993). The final analyses included 7,594 participants (Figure 1).
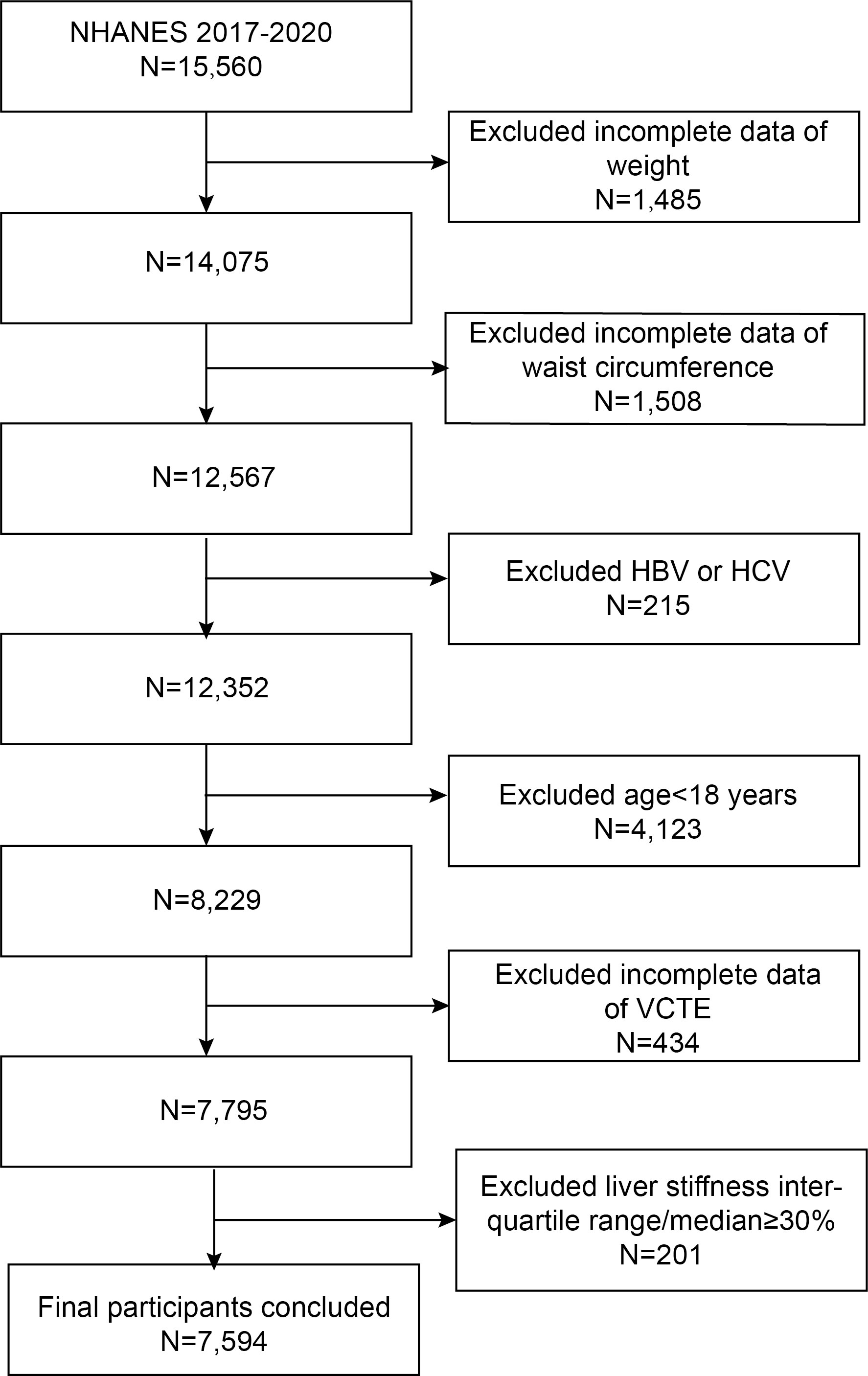
Figure 1 Flowchart of the sample selection from NHANES 2017–2020. NHANES, National Health and Nutrition Examination Survey; HBV/HCV, participants with infection of hepatitis B virus and hepatitis C virus; VCTE, vibration controlled transient elastography.
WWI is a novel index that estimates central obesity based on WC and weight. WC and weight were measured in a mobile examination center (MEC), where laboratory tests were carried out under controlled conditions (14). WWI was included as an exposure variable in our study and calculated as follows:
Hepatic steatosis and liver fibrosis was detected with VCTE. Specifically, controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) represented the levels of steatosis and fibrosis, respectively. We only included participants with a reliable VCTE estimation (interquartile range/median of LSM ≤ 30%).
Based on a review of the literature, we summarized potential confounding covariates between WWI and hepatic steatosis and liver fibrosis in our multivariable-adjusted model (10, 11, 18, 19). Gender, age, race, family income-to-poverty ratio, and education level were all demographic covariates in our study. Anthropometric and laboratory covariates included BMI, direct high-density lipoprotein cholesterol (HDL, mmol/L), low-density lipoprotein cholesterol (LDL, mmol/L), triglycerides (TG, mmol/L), total cholesterol (TC, mmol/L), serum uric acid (μmol/L), alanine aminotransferase (ALT, IU/L), alkaline phosphatase (ALP, IU/L), and aspartate aminotransferase (AST, IU/L). Medical history covariates included the presence or absence of T2D, excess alcohol consumption (≥ 4 drinks per day), smoking, or cardiovascular disease (CVD, defined as history of coronary artery disease, congestive heart failure, heart attack, stroke, or angina pectoris).
Weighted Student’s t-tests for continuous variables and weighted chi-squared tests for categorical variables were used to assess differences between WWI quartiles. The NHANES used an inferential statistics method to represent a large, nationally representative sample due to its complex multistage probability sampling design. Thus, using linear regression analyses, we summarized continuous variables as means with standard errors (SE) and categorical parameters as proportions using logistic regression analyses. Weighted multivariable regression models were used in three different models to investigate the relationship between WWI and hepatic steatosis and liver fibrosis. No covariates were adjusted in Model 1. Model 2 was adjusted for gender, age, and race. Model 3 was adjusted for gender, age, race, education level, BMI, excess alcohol consumption, HDL, LDL, TG, total cholesterol, serum uric acid, ALT, ALP, AST, CVD, and T2D status.
We performed a sensitivity analysis after categorizing the WWI into quartiles to assess robustness. A generalized additive model (GAM) and smooth curve fitting were used to address non-linearity. When a non-linear correlation was observed, a two-piecewise linear regression model (segmented regression model) was used to fit each interval and calculate the threshold effect. Subgroup analyses were then performed based on gender, age, BMI, T2D, hypertension, excess alcohol consumption, smoking, and CVD. A log-likelihood ratio test was used to determine whether a threshold existed by comparing a one-line model (non-segmented) to a two-piecewise linear regression model. The inflection point (K) was determined using a two-step recursive method (14). Furthermore, a subgroup analysis of the correlations between WWI and hepatic steatosis and liver fibrosis was carried out using stratified multivariable logistic regression models with stratified covariates such as gender, age, BMI, and T2D. A two-sided P value ≤ 0.05 was considered statistically significant. R (version 4.1.3) and EmpowerStats, two statistical computing and graphical programs, were used to conduct the statistical studies (version 2.0).
Table 1 summarizes the demographic profiles of the 7,594 participants. These participants had a mean ± SD age of 42.59 ± 20.99 years; 50.74% were men, and 49.26% were women. The WWI ranges for the first, second, third, and fourth quartiles were 8.04-10.45, 10.45-11.05, 11.05-11.64, and 11.64-14.14, respectively. Compared with participants in the lowest WWI quartile, those in the highest quartile were more likely to be male, older, excess alcohol consumers, or have CVD, lower education level, lower socioeconomic status, higher TG levels, higher BMI, higher total cholesterol, higher LDL, higher ALT, higher ALP, and higher serum uric acid.
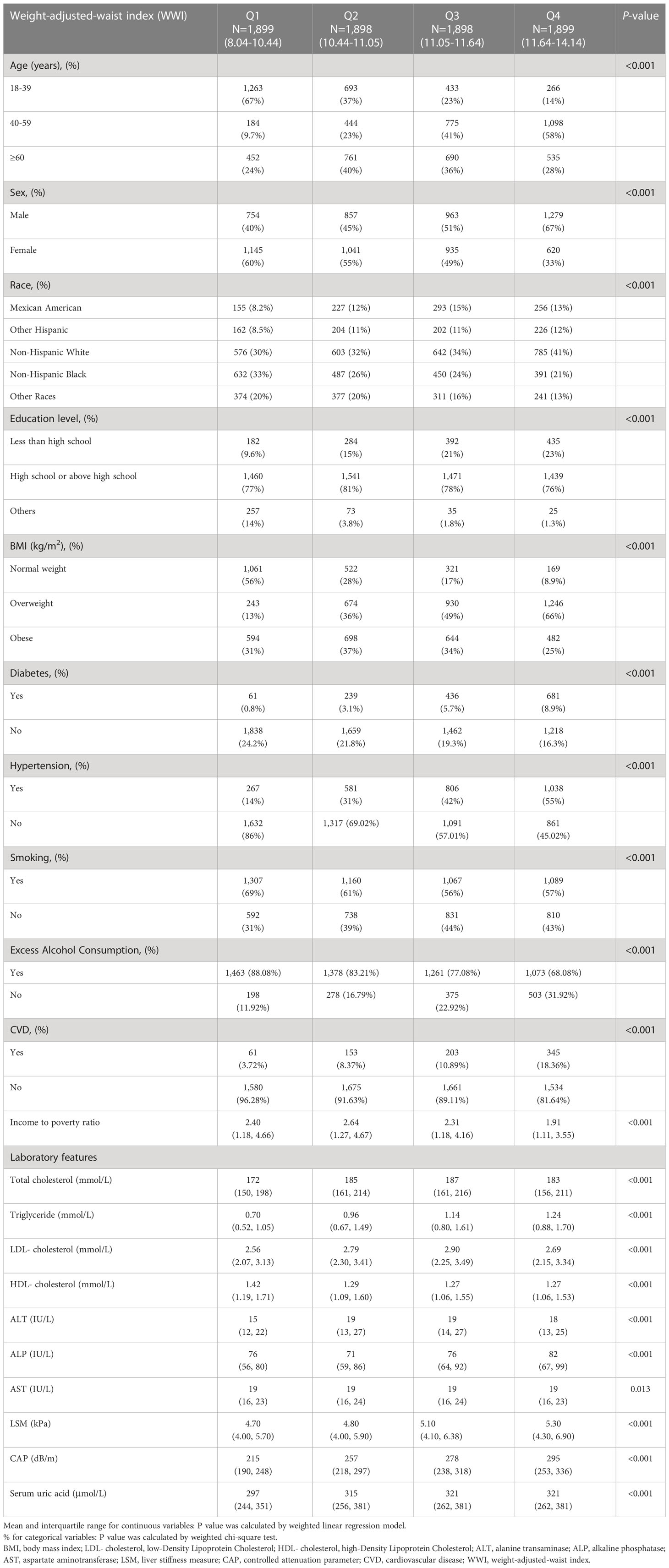
Table 1 Weighted characteristics of the study population based on controlled attenuated parameter (CAP) and median liver stiffness measurement (LSM).
We first estimated the association between WWI and the severity of liver fibrosis without adjusting for any covariates. Higher WWI was associated with a higher grade of hepatic steatosis. After full adjustment (see Methods), each unit with a higher WWI score was found to be associated with 7.60 dB/m increased units of CAP [β=7.60, 95% CI (4.42, 10.78), P<0.001]. Sensitivity analysis was conducted after treating the WWI as a categorical variable (quartile). In the fully adjusted model, compared with the lowest WWI quartile (first quartile), the adjusted β for participants in the second quartile, third quartile, and fourth quartile were 6.51, 11.03, and 14.06, respectively (Table 2).
As shown in Table 2, in the unadjusted model, each unit of higher WWI score was found to be associated with 0.72 kPa increased units of LSM [β=0.72, 95% CI (0.61, 0.84), P<0.0001]. However, after adjusting for all covariates, the relationship between WWI and LSM was not significant in Model 3 [β=0.03, 95% CI (-0.26, 0.32), P=0.84].
We used stratified weighted multivariate regression analysis to investigate the association between WWI and CAP and LSM in different population settings, stratified by gender, age, BMI, T2D, hypertension, excess alcohol consumption, smoking, and CVD.
As displayed in Figure 2, a stronger positive association between WWI and CAP was observed in participants with excess alcohol consumption and hypertension (P< 0.05). However, the correlation between WWI and CAP was similar in the population with different subgroups of gender, age, smoking, BMI, T2D, and CVD. Furthermore, A significant correlation between WWI and LSM was observed in participants with BMI>30 and experience CVD (Figure 3).
After adjusting for all variables, a non-linear association between WWI and LSM levels was found (Figure 4). We observed a U-shaped relationship between the WWI and LSM (inflection point: 10.92) (Table 3). Specifically, LSM was negatively associated with WWI<10.92 [β=-0.57, 95% CI (-1.06, -0.08), P=0.022], and positively association with WWI >10.92 [β=0.44, 95% CI (0.05, 0.84), P=0.028].
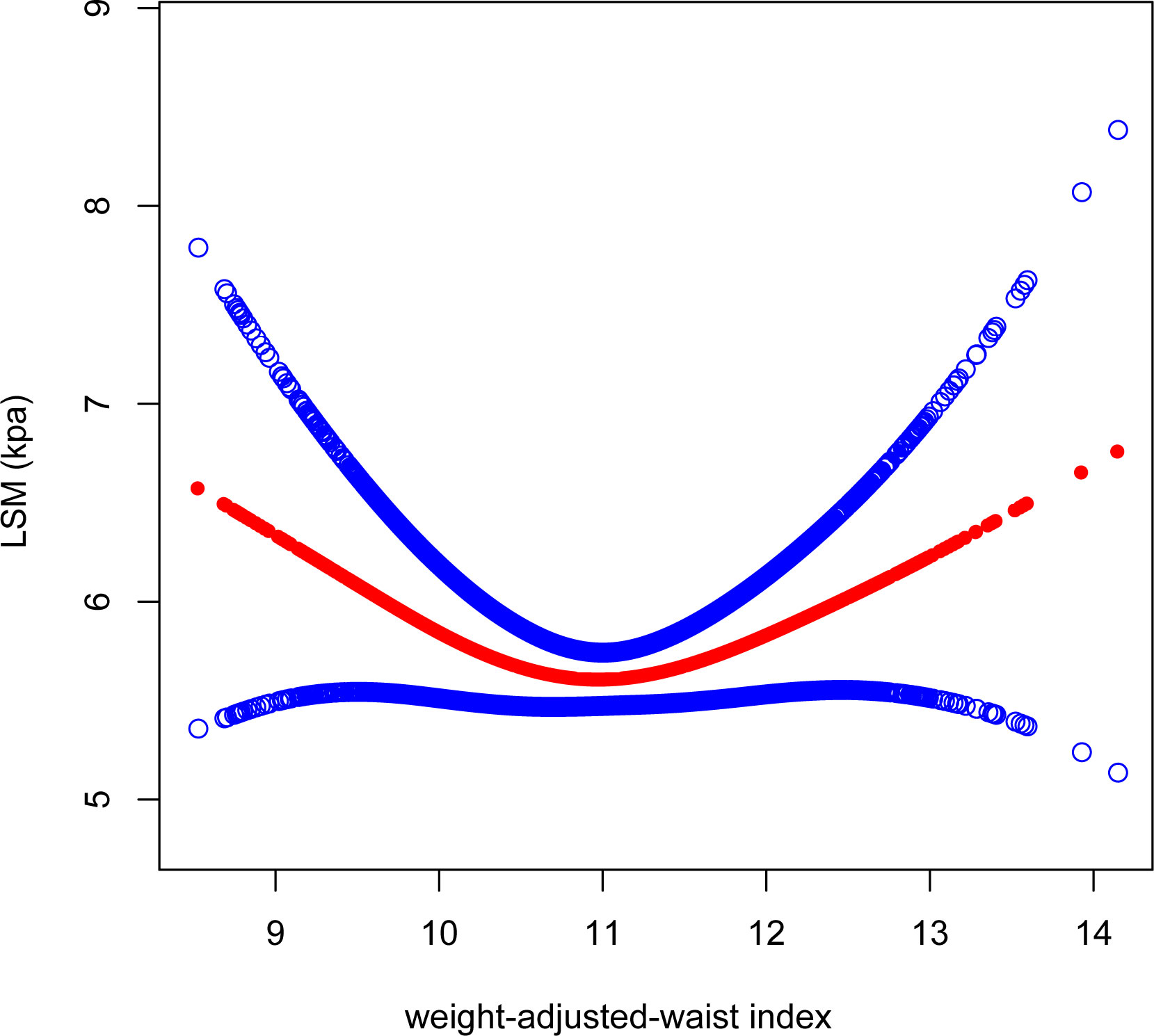
Figure 4 Smooth curve fitting for WWI and LSM. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. WWI, weight-adjusted-waist index; LSM, liver stiffness measurement.
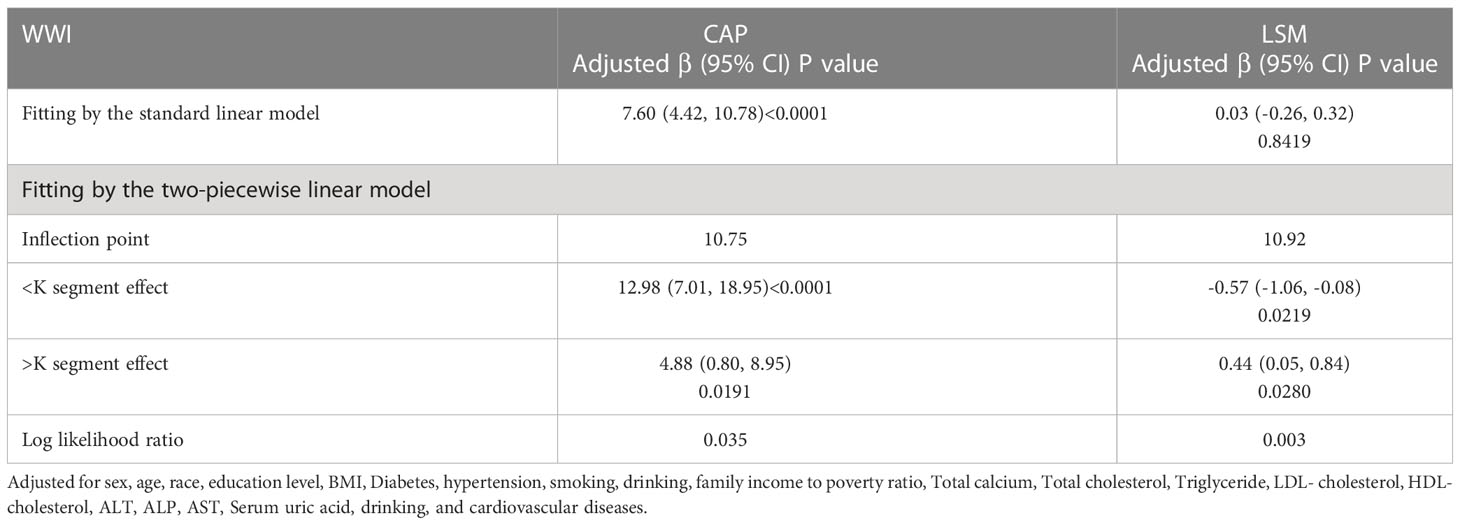
Table 3 Threshold effect analysis of WWI on LSM and CAP using a two-piecewise linear regression model.
As with the linear association, we also observed a positive correlation between WWI and CAP when conducting the non-linear model. We found consistent positive association between WWI and CAP. Notably, the association was much stronger when WWI>10.75 [WWI>10.75: β=12.98, 95% CI (7.01, 18.95), P<0.0001]; WWI<10.75: [β=4.88, 95% CI (0.80, 8.95), P=0.02] (Figure 5).
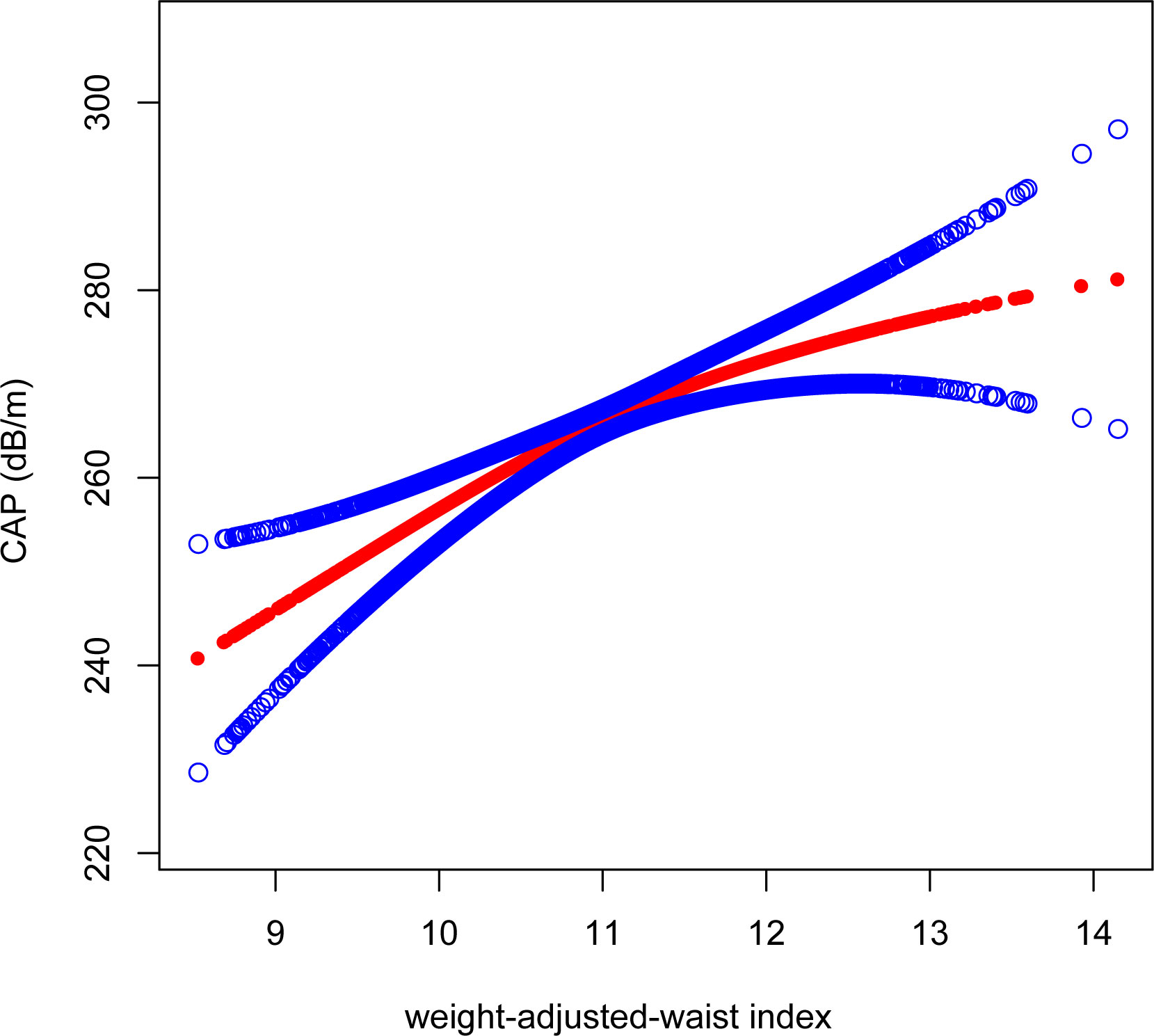
Figure 5 Smooth curve fitting for WWI and CAP. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. WWI, weight-adjusted-waist index; CAP, controlled attenuation parameter.
The present study aimed to evaluate the relationship between WWI and hepatic steatosis and liver fibrosis among civilians in the United States. In our cross-sectional study comprising 7,594 participants, we found a significant positive linear association between WWI and hepatic steatosis. Furthermore, we identified a U-shaped relationship between WWI and liver fibrosis (inflection point 10.92). By performing subgroup analyses, a stronger association between WWI and hepatic steatosis was demonstrated in participants with hypertensive disorders and excessive alcohol consumption. Additionally, we saw evidence of a strong correlation between WWI and liver fibrosis in participants with BMI>30 or CVD.
Accumulating evidence has supported the leading role of obesity in the pathogenesis of NAFLD. Given a strong link with dysregulated lipid metabolism, obesity not only contributes to the evolution of hepatic steatosis and inflammation, but also poses a threat to cardiovascular events and metabolic syndromes (20). Currently, BMI is widely used to determine the severity of obesity. However, obese patients, especially NAFLD patients, tend to demonstrate both fat accumulation and loss of skeletal muscle mass due to physical inactivity, which further increases the risk of hospitalization for NAFLD patients (21, 22). In addition, central obesity, specifically the accumulation of adipose in deep subcutaneous tissue, has been identified as the critical driver for NASH progression, insulin resistance, and cardiovascular events (23). Therefore, BMI, using total weight, may not accurately reflect the health status of obese individuals, particularly NAFLD patients. In contrast, WWI, calculated by normalizing WC with body weight, primarily reflects pure central obesity and can assess high fat mass and low muscle mass (24). For this study, we evaluated the association of WWI with estimated liver histology. We found a significant positive correlation between WWI and CAP. Additionally, we have identified a U-shaped association between WWI and LSM.
WWI was first used to better evaluate the morbidity and mortality of cardiovascular and metabolic diseases in the Korean population (25). Furthermore, trans-ethnic studies have revealed that WWI was positively associated with higher risks of hyperuricemia and multiple kinds of cardiovascular events, including heart failure, abdominal aortic calcification, and left ventricular hypertrophy (14, 26–29).
WWI was thought to represent the severity of central obesity rather than general obesity. As a sign of metabolic syndromes, a series of studies have suggested that central obesity is a risk factor for NAFLD, cardiovascular diseases, and other metabolic diseases for both obese and non-obese populations (30–32). Specifically, central obesity is characterized by the accumulation of abdominal adipose tissue, especially visceral adipose tissue. Mechanically, visceral adipose tissue could constantly secrete proinflammatory stimuli, which could further lead to systematic inflammation, metabolic disorders, and histological progression in the liver of obese patients. Moreover, during the expansion of visceral adipose tissue, proinflammatory macrophages in the adipose further contribute to immune-infiltration in the liver (33). In addition, some subtypes of adipose-derived ceramidases could desensitize insulin activity and contribute to insulin resistance (34). Our study has further highlighted and qualified the critical role central obesity in liver steatosis. In the future, WWI may be a liver fibrosis predictor.
This study has several limitations which should be mentioned. First, the cross-sectional study design did not allow us to determine any causal relationships. Second, the severity of hepatic steatosis and liver fibrosis in this study was determined using VCTE, which needs to be further validated in biopsy-proven cohorts. Furthermore, although the NHANES was conducted in the United States in a multi-ethnic adult population, our results may not reflect other geographic areas or ethnic groups.
In conclusion, our study found strong link between WWI and hepatic steatosis, and suggested that it may potentially be used as a simple anthropometric index to predict hepatic steatosis.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YS and YW contributed equally to the research’s conception and design. YS performed the study and wrote the draft. YS contributed to the acquisition and analysis of the data. MF and KZ conducted the statistical analysis. YS wrote the initial drafts of the manuscript. JW reviewed and revised the later drafts of the manuscript. All authors critically reviewed the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Research and Development Projects in Shanxi Province (201903D321032) and the Scientific Research Project of Shanxi Provincial Health Commission (2019142). The study was funded by the Four “Batches” Innovation Project of Invigorating Medical through Science and Technology of Shanxi Province (Key Laboratory of Esophageal Cancer Basic Research and Clinical Transformation, Heping Hospital Affiliated to Changzhi Medical College, 2020SYS22).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest (2017) 127(1):55–64. doi: 10.1172/JCI88881
2. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med (2018) 24(7):908–22. doi: 10.1038/s41591-018-0104-9
3. Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab (2012) 16(1):44–54. doi: 10.1016/j.cmet.2012.05.012
4. Meex RC, Hoy AJ, Morris A, Brown RD, Lo JC, Burke M, et al. Fetuin b is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab (2015) 22(6):1078–89. doi: 10.1016/j.cmet.2015.09.023
5. Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med (2012) 18(6):934–42. doi: 10.1038/nm.2744
6. Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology (2016) 150(3):626–37.e7. doi: 10.1053/j.gastro.2015.11.048
7. Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol (2019) 17(1):156–63.e2. doi: 10.1016/j.cgh.2018.04.043
8. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology (2019) 156(6):1717–30. doi: 10.1053/j.gastro.2019.01.042
9. Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology (2018) 67(1):134–44. doi: 10.1002/hep.29489
10. Bosserhoff A, Hellerbrand C. Obesity and fatty liver are ‘grease’ for the machinery of hepatic fibrosis. Dig Dis (2011) 29(4):377–83. doi: 10.1159/000329800
11. Tutunchi H, Naeini F, Ebrahimi-Mameghani M, Najafipour F, Mobasseri M, Ostadrahimi A. Metabolically healthy and unhealthy obesity and the progression of liver fibrosis: a cross-sectional study. Clin Res Hepatol Gastroenterol (2021) 45(6):101754. doi: 10.1016/j.clinre.2021.101754
12. Ware D, Landy DC, Rabil A, Hennekens CH, Hecht EM. Interrelationships between self reported physical health and health behaviors among healthy US adults: from the NHANES 2009-2016. Public Health Pract (Oxf) (2022) 4:100277. doi: 10.1016/j.puhip.2022.100277
13. Gong R, Pu X, Cheng Z, Ding J, Chen Z, Wang Y. The association between serum cadmium and diabetes in the general population: a cross-sectional study from NHANES (1999-2020). Front Nutr (2022) 9:966500. doi: 10.3389/fnut.2022.966500
14. Xie F, Xiao Y, Li X, Wu Y. Association between the weight-adjusted-waist index and abdominal aortic calcification in united states adults: results from the national health and nutrition examination survey 2013-2014. Front Cardiovasc Med (2022) 9:948194. doi: 10.3389/fcvm.2022.948194
15. Geng R, Zhang Y, Liu M, Deng S, Ding J, Zhong H, et al. Elevated serum uric acid is associated with cognitive improvement in older American adults: a large, population-based-analysis of the NHANES database. Front Aging Neurosci (2022) 14:1024415. doi: 10.3389/fnagi.2022.1024415
16. Xie R, Liu Y, Wang J, Zhang C, Xiao M, Liu M, et al. Race and gender differences in the associations between cadmium exposure and bone mineral density in US adults. Biol Trace Elem Res (2022). doi: 10.1007/s12011-022-03521-y
17. Chen X, Tian F, Wu J, Liu L, Li Y, Yu G, et al. Associations of phthalates with NAFLD and liver fibrosis: a nationally representative cross-sectional study from NHANES 2017 to 2018. Front Nutr (2022) 9:1059675. doi: 10.3389/fnut.2022.1059675
18. Hohenester S, Christiansen S, Nagel J, Wimmer R, Artmann R, Denk G, et al. Lifestyle intervention for morbid obesity: effects on liver steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol (2018) 315(3):G329–g38. doi: 10.1152/ajpgi.00044.2018
19. Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology (2015) 149(2):379–88. doi: 10.1053/j.gastro.2015.04.014
20. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism (2019) 92:82–97. doi: 10.1016/j.metabol.2018.11.014
21. Sinn DH, Kang D, Kang M, Guallar E, Hong YS, Lee KH, et al. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: a longitudinal cohort study. Hepatology (2022) 76(6):1746–54. doi: 10.1002/hep.32578
22. Petermann-Rocha F, Gray SR, Forrest E, Welsh P, Sattar N, Celis-Morales C, et al. Associations of muscle mass and grip strength with severe NAFLD: a prospective study of 333,295 UK biobank participants. J Hepatol (2022) 76(5):1021–9. doi: 10.1016/j.jhep.2022.01.010
23. Shen W, Middleton MS, Cunha GM, Delgado TI, Wolfson T, Gamst A, et al. Changes in abdominal adipose tissue depots assessed by MRI correlate with hepatic histologic improvement in non-alcoholic steatohepatitis. J Hepatol (2023) 78(2):238–46. doi: 10.1016/j.jhep.2022.10.027
24. Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing (2021) 50(3):780–6. doi: 10.1093/ageing/afaa208
25. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep (2018) 8(1):16753. doi: 10.1038/s41598-018-35073-4
26. Zhang D, Shi W, Ding Z, Park J, Wu S, Zhang J. Association between weight-adjusted-waist index and heart failure: results from national health and nutrition examination survey 1999-2018. Front Cardiovasc Med (2022) 9:1069146. doi: 10.3389/fcvm.2022.1069146
27. Qin Z, Du D, Li Y, Chang K, Yang Q, Zhang Z, et al. The association between weight-adjusted-waist index and abdominal aortic calcification in adults aged >/= 40 years: results from NHANES 2013-2014. Sci Rep (2022) 12(1):20354. doi: 10.1038/s41598-022-24756-8
28. Cai S, Zhu T, Ding Y, Cheng B, Zhang A, Bao Q, et al. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens Res (2023) 46(1):253–60. doi: 10.1038/s41440-022-01075-z
29. Zhao P, Shi W, Shi Y, Xiong Y, Ding C, Song X, et al. Positive association between weight-adjusted-waist index and hyperuricemia in patients with hypertension: the China h-type hypertension registry study. Front Endocrinol (Lausanne) (2022) 13:1007557. doi: 10.3389/fendo.2022.1007557
30. Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci (2022) 23(2):1–38. doi: 10.3390/ijms23020786
31. Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med (2015) 163(11):827–35. doi: 10.7326/M14-2525
32. Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, et al. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology (2014) 59(2):461–70. doi: 10.1002/hep.26610
33. Bijnen M, Josefs T, Cuijpers I, Maalsen CJ, van de Gaar J, Vroomen M, et al. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut (2018) 67(7):1317–27. doi: 10.1136/gutjnl-2016-313654
Keywords: WWI, steatosis, and fibrosis weight-adjusted-waist index, hepatic steatosis, liver fibrosis, NAFLD, NHANES, VCTE
Citation: Shen Y, Wu Y, Fu M, Zhu K and Wang J (2023) Association between weight-adjusted-waist index with hepatic steatosis and liver fibrosis: a nationally representative cross-sectional study from NHANES 2017 to 2020. Front. Endocrinol. 14:1159055. doi: 10.3389/fendo.2023.1159055
Received: 05 February 2023; Accepted: 05 May 2023;
Published: 19 May 2023.
Edited by:
Takefumi Kimura, Shinshu University, JapanReviewed by:
Shun-ichi Wakabayashi, Shinshu University Hospital, JapanCopyright © 2023 Shen, Wu, Fu, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsheng Wang, anNod2FuZ0Bjem1jLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.