
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 23 May 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1158153
This article is part of the Research TopicThe Underlying Mechanisms and Novel Approaches for Diabetes and its Related ComplicationsView all 36 articles
Objective: To assess the relationship between use of sodium-glucose cotransporter-2 inhibitors (SGLT2i) and the risk of gout among patients with type 2 diabetes mellitus (T2DM).
Methods: A systemic review and meta-analysis were designed by reviewing articles published between 2000 January 1 and 2022 December 31 using PubMed system and Web of Science system based on the PRISMA 2020 guidelines. The end point of interest was gout (including gout flares, gout events, starting uric-acid lowering therapy and starting anti-gout drugs use) among patients with T2DM using SGLT2i versus not using SGLT2i. A random-effects model was utilized to measure the pooled hazard ratio (HR) with 95% confidence interval (CI) for the risk of gout associated with SGLT2i use.
Results: Two prospective post-hoc analyses of randomized controlled trials and 5 retrospective electronic medical record-linkage cohort studies met the inclusion criteria. The meta-analysis demonstrated that there was a decreased risk of developing gout for SGLT2i use as comparing with non-use of SGLT2i among patients with T2DM (pooled HR=0.66 and 95%CI=0.57-0.76).
Conclusions: This meta-analysis demonstrates that SGLT2i use is associated with a 34% decreased risk of developing gout among patients with T2DM. SGLT2i may be the treatment options for patients with T2DM who are at high risk of gout. More randomized controlled trials and real-world data are needed to confirm whether there is a class effect of SGLT2i for the risk reduction of gout among patients with T2DM.
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) belong to a new development of oral anti-diabetic drug to treat persons with type 2 diabetes mellitus (T2DM). SGLT2i also demonstrates other beneficial effects on cardiorenal protection including the risk reduction for atherosclerotic events, hospitalization and progression of heart failure, and progression of chronic kidney disease (1–4). In addition to these pleiotropic effects mentioned above, recent studies demonstrated that SGLT2i has a uric-acid lowering effect and then such an effect seems to be a class effect for SGLT2i (5–11).
Although the academic community has not yet established a consensus, it is generally agreed that when the serum uric acid value is greater than or equal to 6.8 mg/dL, it is called hyperuricemia (12, 13). Hyperuricemia is linked to the development of gout (14). Theoretically, SGLT2i use can decrease the risk of developing gout based on the uric-acid lowering effect. However, clinical data demonstrated conflicting results about the relation between SGLT2i use and the risk of gout. Some demonstrated benefit (15–20), but some demonstrated no benefit (21). For example, a prospective post-hoc analysis of randomized controlled trials by Li et al. demonstrated that canagliflozin use (one SGLT2i) correlated with a reduced risk of gout in persons with T2DM (hazard ratio=0.53, 95% confidence interval=0.40-0.71) (15). A retrospective electronic medical record-linkage cohort study by Subramanian et al. demonstrated that no risk difference for gout was noted among patients with T2DM receiving SGLT2i compared with those receiving dipeptidyl peptidate-4 inhibitors (hazard ratio=1.10, 95% confidence interval=0.71-1.68) (21).
These conflicting results raise concern and also inspire efforts to find solution. In view of the conflicting evidence, a systematic review and meta-analysis was designed to check the relationship between SGLT2i use and the risk of gout among patients with T2DM.
A systemic review and meta-analysis was designed by reviewing articles published between 2000 January 1 and 2022 December 31 using PubMed system and Web of Science system based on the PRISMA 2020 guidelines (22). The following keywords were selected to find articles of interest:”sodium-glucose cotransporter”, “sodium-glucose transport”, “canagliflozin”, “dapagliflozin”, “empagliflozin”, “ertugliflozin”, “ipragliflozin”, “luseogliflozin”, “tofogliflozin”, “gout” and “uric acid”. These keywords were used in combination as following strategies: sodium-glucose cotransporter [title] AND gout [title], sodium-glucose transport [title] AND gout [title], canagliflozin [title] AND gout [title], dapagliflozin [title] AND gout [title], empagliflozin [title] AND gout [title], ertugliflozin [title] AND gout [title], ipragliflozin [title] AND gout [title], luseogliflozin [title] AND gout [title], tofogliflozin [title] AND gout [title], sodium-glucose cotransporter [title] AND uric acid [title], sodium-glucose transport [title] AND uric acid [title], canagliflozin [title] AND uric acid [title], dapagliflozin [title] AND uric acid [title], empagliflozin [title] AND uric acid [title], ertugliflozin [title] AND uric acid [title], ipragliflozin [title] AND uric acid [title], luseogliflozin [title] AND uric acid [title], as well as tofogliflozin [title] AND uric acid [title].
The following inclusion criteria were addressed to find articles of interest for meta-analysis: (1) randomized controlled trials (RCTs) and/or post-hoc analysis of RCTs which selected subjects with T2DM investigating individual SGLT2i and/or all SGLT2i; (2) observational studies (including cohort and case-control studies) which selected subjects with T2DM investigating individual SGLT2i and/or all SGLT2i; (3) the end point of interest was gout (including gout flares, gout events, starting uric-acid lowering therapy and starting anti-gout drugs use) among subjects with T2DM using SGLT2i versus not using SGLT2i; (4) the hazard ratio or odds ratio of gout was shown.
The exclusion criteria were applied as follows: (1) meeting abstract, case report, case series, study protocol, review article, comment article, editorials and a letter to the editor; (2) data were not fully demonstrated; (3) research without peer review (Figure 1).
Two authors (KFL and YHK) assessed the eligibility of all found articles according to the above inclusion and exclusion criteria. The following terms were extracted: the surname of first author, study population, baseline characteristics of study subjects (including mean age and male percentage), number of SGLT2i use, number of comparator use, treatment/follow-up duration, and adjusted hazard ratio (HR) with 95% confidence interval (CI). Two authors (BFH and CSL) discussed together to resolve the conflicting opinions.
The Newcastle-Ottawa Scale system was utilized to check the quality and the risk of bias of the observational studies included (23). The Cochrane Collaboration’s tool was utilized to check the quality and the risk of bias of RCTs and/or post-hoc analysis of RCTs (24).
A random-effects model was done to estimate the pooled HR with 95%CI for the risk of gout associated with SGLT2i use versus non-use of SGLT2i. Three sub-analyses were performed to measure the subtotal HR with 95%CI for the risk of gout associated with SGLT2i use versus placebo, SGLT2i use versus glucagon-like peptide-1 receptor agonists (GLP1RA), and SGLT2i use versus dipeptidyl peptidate-4 inhibitors (DPP4i), respectively. The I2 statistics were used to check the heterogeneity between included studies. The I2 value > 50% indicates that there could be a significant heterogeneity between included studies (25). The statistical analyses were performed by the aid of RStudio and the meta package (26, 27). The P value < 0.05 indicates statistically significant.
Table 1 lists the characteristic information of the 7 eligible studies. There were 2 prospective post-hoc analyses of RCTs and 5 retrospective electronic medical record-linkage cohort studies.
The 2 prospective post-hoc analyses of RCTs by Li et al. and by Ferreira et al. demonstrated a lower HR for gout associated with SGLT2i use as comparing with placebo, with reaching statistical significance (HR=0.53 and HR=0.67, respectively) (15, 16). The 4 retrospective electronic medical record-linkage cohort studies demonstrated a lower HR for gout associated with SGLT2i use as comparing with GLP1RA use or DPP4i use, with reaching statistical significance (17–20). But one retrospective electronic medical record-linkage cohort study by Subramanian et al. demonstrated an elevated HR for gout associated with SGLT2i use as comparing with DPP4i use, but not achieving statistical significance (HR=1.10 and 95%CI=0.71-1.68) (21).
The 2 prospective post-hoc analyses of RCTs had a low risk of bias from the Cochrane Collaboration’s tool. The 5 retrospective electronic medical record-linkage cohort studies had high-quality with a low risk of bias based on the Newcastle-Ottawa Scale system.
Figure 2 demonstrates a forest plot with the pooled HR and 95%CI for the risk of gout. Overall, there was a decreased risk of developing gout for SGLT2i use as comparing with non-use of SGLT2i among patients with T2DM (pooled HR=0.66, 95%CI=0.57-0.76 and P<0.01). In sub-analysis for the 2 prospective post-hoc analyses of RCTs, there was a decreased risk of developing gout for SGLT2i use as comparing with placebo (subtotal HR=0.60 and 95%CI=0.48-0.76). In the sub-analysis for the 2 retrospective electronic medical record-linkage cohort studies (SGLT2i vs. GLP1RA), there was a decreased risk of developing gout for SGLT2i use as comparing with GLP1RA use (subtotal HR=0.63 and 95%CI=0.57-0.70). In the sub-analysis for 5 retrospective electronic medical record-linkage cohort studies (SGLT2i vs. DPP4i), there was a decreased risk of developing gout for SGLT2i use as comparing with DPP4i use (subtotal HR=0.70 and 95%CI=0.54-0.91).
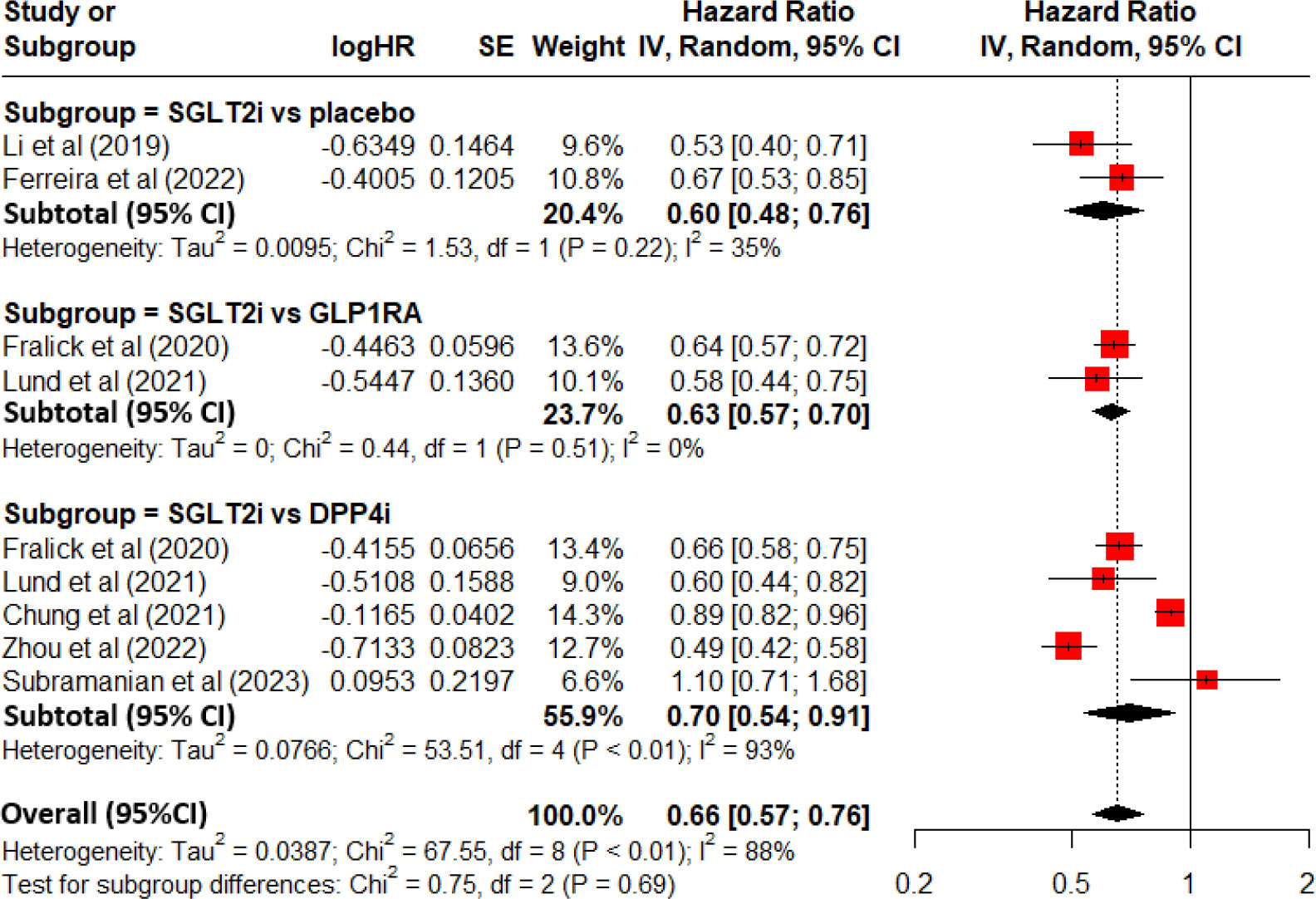
Figure 2 Forest plot for total and subgroup analyses revealing the effect of SGLT2i on the development of gout among patients with T2DM as comparing with placebo or other anti-diabetic drugs.
There was a significant heterogeneity between included studies (I2 = 88% and P<0.01).
The funnel plot is presented in Figure 3. A visual inspection demonstrates symmetry. It indicates that there was no publication bias. These results were confirmed by The Begg’s test (P=0.8348) and the Egger’s test (P=0.1937) (28, 29).
One study from Subramanian et al. demonstrated an elevated HR for gout associated with SGLT2i use as comparing with DPP4i use, but not achieving statistical significance (21). After excluding Subramanian et al’s study, a pooled HR was 0.63(95%CI =0.55-0.73 and P<0.001). It still achieved statistical significance.
This present meta-analysis demonstrated that there was a 34% decreased risk of developing gout for SGLT2i use as comparing with non-use of SGLT2i among patients with T2DM. In sub-analysis, no matter comparing with placebo, GLP1RA use or DPP4i use, the risk reduction of gout remained to be observed. Based on the above findings, SGLT2i may be the treatment options for patients with T2DM who are at high risk of gout, including those patients with a history of gout, high levels of serum uric acid and/or other risk factors for gout.
The potential mechanisms underlying the use of SGLT2i, and risk reduction of gout are not fully understood. We review the literature and summarize as below. It is well known that lowering serum uric acid levels is a key goal for the prevention and treatment of gout. SGLT2i works by blocking the reabsorption of glucose and sodium in the proximal tubule of the kidney, leading to increased urinary glucose and sodium excretion, and increased urinary volume, which then lowers blood glucose levels. This increased urinary volume may also enhance uric acid excretion, which could explain the reduction in blood uric acid levels (30–32). It is a rational hypothesis that SGLT2i reduces blood uric acid levels by enhancing urinary excretion of uric acid. Thus, the risk of gout is reduced after the use of SGLT2i.
Some caveats and limitations should be discussed. First, recently many real-world studies utilized the electronic medical record-linkage database for analysis. The immortal time bias is possibly encountered when using such a database (33). If the immortal time bias is not managed correctly during the stages of research design and research analysis, the outcome results can lead to the wrong direction (34–36). Some research included the immortal time into the treatment group, but some research simply excluded the immortal time from the research (34–36). All of these methods may overestimate of the benefit of the studied drug (34–36). How the immortal time bias was handled was not mentioned in the method section of the 5 retrospective electronic medical record-linkage cohort studies. Therefore, interpretation of their results should be cautious. The RCTs can avoid the immortal time bias. In order to overcome the potential immortal time bias which could be present in the 5 retrospective electronic medical record-linkage cohort studies, the sub-analysis of the 2 prospective post-hoc analyses of RCTs demonstrated that there was a risk reduction of gout associated with SGLT2i use. These findings partially support the results of the 5 retrospective electronic medical record-linkage cohort studies that SGLT2i use could be associated with a risk reduction of gout when comparing with GLP1RA use or DPP4i use. Second, there are 7 SGLT2i available in the markets, including canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, luseogliflozin and tofogliflozin. Six of these 7 drugs show a uric acid-lowering effect (5–11). It seems to be a class effect of SGLT2i for lowering uric acid. Currently, only two individual SGLT2i (canagliflozin and empagliflozin) had performed the post-hoc analyses of RCTs on the risk of gout. The other 5 SGLT2i did not demonstrate the post-hoc analyses of RCTs on the risk of gout. Further RCTs or post-hoc analyses of RCTs are needed to clarify whether there is a class effect of SGLT2i for the risk reduction of gout. Third, the heterogeneity of the 2 prospective post-hoc analyses of RCTs and the 2 retrospective electronic medical record-linkage cohort studies (SGLT2i vs. GLP1RA) was low, but the heterogeneity of all included studies seemed to be high in this meta-analysis. Some potential sources of heterogeneity should be mentioned. For example, differences in study populations, differences in study design, differences in intervention characteristics, or differences in outcome measures, might contribute to heterogeneity across studies (37, 38).
This present meta-analysis demonstrates that SGLT2i use is associated with a 34% decreased risk of developing gout among patients with T2DM. SGLT2i may be the treatment options for patients with T2DM who are at high risk of gout. More RCTs and real-world data are needed to confirm whether there is a class effect of SGLT2i for the risk reduction of gout among patients with T2DM.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
S-WL contributed to the conception of the study, initiated the draft of the study, and approved the final draft. Y-HK and K-FL conducted data analysis. B-FH and C-SL interpreted the data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. (2018) 61:2108–17. doi: 10.1007/s00125-018-4670-7
2. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int (2018) 94:26–39. doi: 10.1016/j.kint.2017.12.027
3. Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-Art review. J Am Coll Cardiol (2020) 75:422–34. doi: 10.1016/j.jacc.2019.11.031
4. Zelniker TA, Braunwald E. Clinical benefit of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-Art review. J Am Coll Cardiol (2020) 75:435–47. doi: 10.1016/j.jacc.2019.11.036
5. Davies MJ, Trujillo A, Vijapurkar U, Damaraju CV, Meininger G. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab (2015) 17:426–9. doi: 10.1111/dom.12439
6. McDowell K, Welsh P, Docherty KF, Morrow DA, Jhund PS, de Boer RA, et al. Dapagliflozin reduces uric acid concentration, an independent predictor of adverse outcomes in DAPA-HF. Eur J Heart Fail (2022) 24:1066–76. doi: 10.1002/ejhf.2433
7. Doehner W, Anker SD, Butler J, Zannad F, Filippatos G, Ferreira JP, et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: the EMPEROR-reduced trial. Eur Heart J (2022) 43:3435–46. doi: 10.1093/eurheartj/ehac320
8. Tanaka M, Yamakage H, Inoue T, Odori S, Kusakabe T, Shimatsu A, et al. Beneficial effects of ipragliflozin on the renal function and serum uric acid levels in Japanese patients with type 2 diabetes: a randomized, 12-week, open-label, active-controlled trial. Intern Med (2020) 59:601–9. doi: 10.2169/internalmedicine.3473-19
9. Chino Y, Kuwabara M, Hisatome I. Factors influencing change in serum uric acid after administration of the sodium-glucose cotransporter 2 inhibitor luseogliflozin in patients with type 2 diabetes mellitus. J Clin Pharmacol (2022) 62:366–75. doi: 10.1002/jcph.1970
10. Ouchi M, Oba K, Kaku K, Suganami H, Yoshida A, Fukunaka Y, et al. Uric acid lowering in relation to HbA1c reductions with the SGLT2 inhibitor tofogliflozin. Diabetes Obes Metab (2018) 20:1061–5. doi: 10.1111/dom.13170
11. Akbari A, Rafiee M, Sathyapalan T, Sahebkar A. Impacts of Sodium/Glucose cotransporter-2 inhibitors on circulating uric acid concentrations: a systematic review and meta-analysis. J Diabetes Res (2022) 2022:7520632. doi: 10.1155/2022/7520632
12. Hyndman D, Liu S, Miner JN. Urate handling in the human body. Curr Rheumatol Rep (2016) 18:34. doi: 10.1007/s11926-016-0587-7
13. Cutolo M, Cimmino MA, Perez-Ruiz F. Potency on lowering serum uric acid in gout patients: a pooled analysis of registrative studies comparing febuxostat vs. allopurinol. Eur Rev Med Pharmacol Sci (2017) 21:4186–95.
14. Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, et al. Gout. Nat Rev Dis Primers. (2019) 5:69. doi: 10.1038/s41572-019-0115-y
15. Li J, Badve SV, Zhou Z, Rodgers A, Day R, Oh R, et al. The effects of canagliflozin on gout in type 2 diabetes: a post-hoc analysis of the CANVAS program. Lancet Rheumatol (2019) 1:e220–e8. doi: 10.1016/S2665-9913(19)30078-5
16. Ferreira JP, Inzucchi SE, Mattheus M, Meinicke T, Steubl D, Wanner C, et al. Empagliflozin and uric acid metabolism in diabetes: a post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes Metab (2022) 24:135–41. doi: 10.1111/dom.14559
17. Fralick M, Chen SK, Patorno E, Kim SC. Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: a population-based cohort study. Ann Intern Med (2020) 172:186–94. doi: 10.7326/M19-2610
18. Lund LC, Højlund M, Henriksen DP, Hallas J, Kristensen KB. Sodium-glucose cotransporter-2 inhibitors and the risk of gout: a Danish population based cohort study and symmetry analysis. Pharmacoepidemiol Drug Saf. (2021) 30:1391–5. doi: 10.1002/pds.5252
19. Chung MC, Hung PH, Hsiao PJ, Wu LY, Chang CH, Wu MJ, et al. Association of sodium-glucose transport protein 2 inhibitor use for type 2 diabetes and incidence of gout in Taiwan. JAMA Netw Open (2021) 4:e2135353. doi: 10.1001/jamanetworkopen.2021.35353
20. Zhou J, Liu X, Chou OH, Li L, Lee S, Wong WT, et al. Lower risk of gout in sodium glucose cotransporter 2 (SGLT2) inhibitors versus dipeptidyl peptidase-4 (DPP4) inhibitors in type-2 diabetes. Rheumatol (Oxford) (2023) 62(4):1501–10. doi: 10.1093/eurheartj/ehac544.2681
21. Subramanian A, Gokhale K, Sainsbury C, Nirantharakumar K, Toulis KA. Sodium-glucose cotransporter-2 inhibitors and the risk of gout in patients with type 2 diabetes mellitus: a propensity-score-matched, new-user design study with an active comparator using the IQVIA medical research data UK database. Diabetes Obes Metab (2023) 25:156–65. doi: 10.1111/dom.14858
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
23. Wells GA SB, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
24. Higgins JP, Sterne JA, Savovic J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database systematic Rev (2016) 10:29–31.
25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
26. Posit-team. RStudio: integrated development environment for r. Posit Software,PBC,Boston,MA. Available at: http://www.posit.co.
27. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with r: a practical tutorial. Evid Based Ment Health (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
28. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
30. Scheen AJ. SGLT2 inhibitors: Benefit/Risk balance. Curr Diabetes Rep (2016) 16:92. doi: 10.1007/s11892-016-0789-4
31. Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab (2019) 21:1291–8. doi: 10.1111/dom.13670
32. Bonora BM, Avogaro A, Fadini GP. Extraglycemic effects of SGLT2 inhibitors: a review of the evidence. Diabetes Metab Syndr Obes (2020) 13:161–74. doi: 10.2147/DMSO.S233538
33. Bae JM. Statin intake and gastric cancer risk: an updated subgroup meta-analysis considering immortal time bias. J Prev Med Public Health (2022) 55:424–7. doi: 10.3961/jpmph.22.209
34. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. (2007) 16:241–9. doi: 10.1002/pds.1357
35. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. (2008) 167:492–9. doi: 10.1093/aje/kwm324
36. Targownik LE, Suissa S. Understanding and avoiding immortal-time bias in gastrointestinal observational research. Am J Gastroenterol (2015) 110:1647–50. doi: 10.1038/ajg.2015.210
37. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med (2002) 21:1559–73. doi: 10.1002/sim.1187
Keywords: diabetes mellitus, gout, meta-analysis, sodium-glucose cotransporter-2 inhibitor (SGLT2i), randomized controlled trials (RCT)
Citation: Lai S-W, Hwang B-F, Kuo Y-H, Liu C-S and Liao K-F (2023) Sodium-glucose cotransporter-2 inhibitors use and the risk of gout: a systematic review and meta-analysis. Front. Endocrinol. 14:1158153. doi: 10.3389/fendo.2023.1158153
Received: 11 February 2023; Accepted: 04 May 2023;
Published: 23 May 2023.
Edited by:
Lixin Guo, Peking University, ChinaReviewed by:
Fengshi Jing, University of North Carolina, United StatesCopyright © 2023 Lai, Hwang, Kuo, Liu and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuan-Fu Liao, a3VhbmZ1bGlhb2dAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.