- The Reproductive Center, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Objective: To explore whether season and temperature on oocyte retrieval day affect the cumulative live birth rate and time to live birth.
Methods: This was a retrospective cohort study. A total of 14420 oocyte retrieval cycles from October 2015 to September 2019. According to the date of oocyte retrieval, the patients were divided into four groups (Spring(n=3634);Summer(n=4414); Autumn(n=3706); Winter(n=2666)). The primary outcome measures were cumulative live birth rate and time to live birth. The secondary outcome measures included the number of oocytes retrieved, number of 2PN, number of available embryos and number of high-quality embryos.
Results: The number of oocytes retrieved was similar among the groups. Other outcomes, including the number of 2PN (P=0.02), number of available embryos (p=0.04), and number of high-quality embryos (p<0.01) were different among the groups. The quality of embryos in summer was relatively poor. There were no differences between the four groups in terms of cumulative live birth rate (P=0.17) or time to live birth (P=0.08). After adjusting for confounding factors by binary logistic regression, temperature (P=0.80), season (P=0.47) and duration of sunshine(P=0.46) had no effect on cumulative live births. Only maternal age (P<0.01) and basal FSH (P<0.01) had an effect on cumulative live births. Cox regression analysis suggested no effect of season(P=0.18) and temperature(P=0.89) on time to live birth. Maternal age did have an effect on time to live birth (P<0.01).
Conclusion: Although season has an effect on the embryo, there was no evidence that season or temperature affect the cumulative live birth rate or time to live birth. It is not necessary to select a specific season when preparing for IVF.
Introduction
Climate change poses potential future risks to human health. Climate change may increase the frequency of heat stress, floods, droughts, and severe storms, with adverse effects on human health (1). Earth’s surface temperature is rising as greenhouse gas emissions increase. Earth warmed by approximately 0.85°C between 1880 and 2012 (2). The temperature of the Earth’s surface continues to rise as past emissions remain in the atmosphere and greenhouse gas emissions continue (3). The impact of rising ambient temperature on human health is well known (4). There is growing evidence that maternal heat exposure is associated with an increased risk of stillbirth, preterm birth, low birth weight, placental abruption, low amniotic fluid, and birth defects (5–11). However, little research has been done on fertility in women exposed to heat. Many animal studies have revealed the effects of temperature on fertility. Studies in South Africa on the iconic ostrich (Struthio camelus) have found that overheating adversely affects the number of oocytes females lay rather than gamete viability (12). In sows, elevated temperatures can adversely affect the farrowing rate and fertility performance (13–16). In dairy cows, high temperatures can affect embryonic development and endometrial receptivity, thereby reducing cow fecundity (17, 18). Studies on the effect of high temperature on human female fertility suggest that high temperature can adversely affect fertility, but the specific mechanism remains unclear. Barreca et al. showed that exposure to 8-10 months of days with a mean temperature above 80°F causes a significant drop in birth rates (19). A study that analyzed 55 years of data from 65 countries suggested that higher maximum temperatures can negatively affect human fertility and that these effects can persist into the next generation (20). Exposure to higher ambient temperatures is associated with lower ovarian reserve, according to a study from the Massachusetts General Hospital Fertility Center (21).
Natural conception birth rates vary by season (22, 23). Cultural behaviors and sociodemographic influences affect the relationship between fertility and seasons (24, 25). As many factors affecting human reproductive activity are relatively controllable through assisted reproductive technology, many studies have examined assisted reproductive technology as a means of elucidating the relationship between seasonality and reproductive outcomes. However, the results of these studies are controversial. Some studies have suggested that assisted reproductive outcomes are not related to season (26–29). A Belgian study found a link between weather conditions in the month prior to IVF treatment and live birth rates per cycle (30). A University of Arizona study suggested that higher temperatures are associated with higher odds of clinical pregnancy (31). Another recent study found that higher oocyte retrieval day temperatures are associated with higher odds of clinical pregnancy and live birth at the time of frozen embryo transfer cycles, independent of temperature at the time of frozen embryo transfer (32). This suggests that temperature may affect ovarian function more than uterine receptivity.
As global temperatures rise, the effects of temperature on fertility will increase. Several recent studies suggested that higher temperatures may have an impact on IVF clinical pregnancy rates and live birth rates. Should we advise patients to retrieve oocytes at specific temperatures or during certain seasons to improve IVF outcomes? This study aimed to explore whether the temperature on oocyte retrieval day affects the cumulative live birth rate (CLBR) and time to live birth.
Materials and methods
Study design and population
This was a retrospective cohort study conducted at the Reproductive Center of the Third Affiliated Hospital of Zhengzhou University. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (2022–199–01). The oocyte retrieval time of the included patients was from October 2015 to September 2019. Exclusion criteria were as follows: ① Intracytoplasmic sperm injection (ICSI) or preimplantation genetic testing (PGT) cycle; ② oocyte recipient cycle, oocyte donor cycle, and oocyte freezing recovery cycle; ③ had not obtained oocyte cycle; ④ any chromosome abnormality in either spouse; ⑤ cycle with incomplete data.
According to the date of oocyte retrieval, the patients were divided into four groups (spring, summer, autumn, and winter). Because the meteorological season can better reflect actual climate change, we used the meteorological season as the basis for grouping: spring (March to May); summer (June to August); autumn (September to November), and winter (December to February). Embryo evaluation standards refer to the Istanbul consensus (33). Embryos of grades I, II and III were available embryos, and embryos of grades I and II were high-quality embryos.
The primary outcome measures were CLBR and time to live birth. CLBR: In one IVF cycle (one oocyte retrieval cycle, including fresh embryo transfer and subsequent frozen embryo transfer cycle), the number of cycles of the first live birth (gestation ≥28 weeks, live birth) was used as the numerator, the entire number of oocyte retrieval cycles was used as the denominator, and the end-of-observation standard was to obtain at least one live birth or utilize all of the embryos in the ovulation induction (34). The observation period was 2 years, and single births, twins, or other multiple births were registered as one birth. We used conservative methods to calculate the cumulative live birth rate. The observation and follow-up period was 2 years. Time to live birth: Time from oocyte retrieval to delivery. The secondary outcome measures included the number of oocytes retrieved, number of 2PN, number of available embryos, and number of high-quality embryos.
Statistical analysis
Zhengzhou’s climate data were obtained from the China Meteorological Administration Meteorological Data Center (http://data.cma.cn/), China’s surface climate data daily value dataset (V3.0). This study extracted the daily average temperature and cumulative daylight duration. All data presented in this article were obtained from the electronic medical record database of The Reproductive Center, The Third Affiliated Hospital of Zhengzhou University.
Quantitative data are presented as the mean ± standard deviation ( ± s), quantitative data were compared using one-way analysis of variance, qualitative data are presented as the percentage (%), and multiple sets of qualitative data were compared using the chi-square test. Binary logistic regression was used to analyze the effects of season and temperature on the clinical outcomes. Factors analyzed included maternal age, basal follicle-stimulating hormone (FSH) level, infertility type, and duration of infertility. Cox regression analysis was used to assess the time to live birth(adjusted by maternal age, basal follicle-stimulating hormone (FSH) level, infertility type, and duration of infertility). When P was <0.05, the difference was considered statistically significant.
All statistical management and analyses were performed using SPSS software, version 22.0.
Results
Study population
Overall, 14420 oocyte retrieval cycles from October 2015 to September 2019 were included in the analysis. The cycles were allocated to four groups according to the date of oocyte retrieval: ① spring (March to May); ② summer (June to August); ③ autumn (September to November); ④ and winter (December to February).
Baseline characteristics
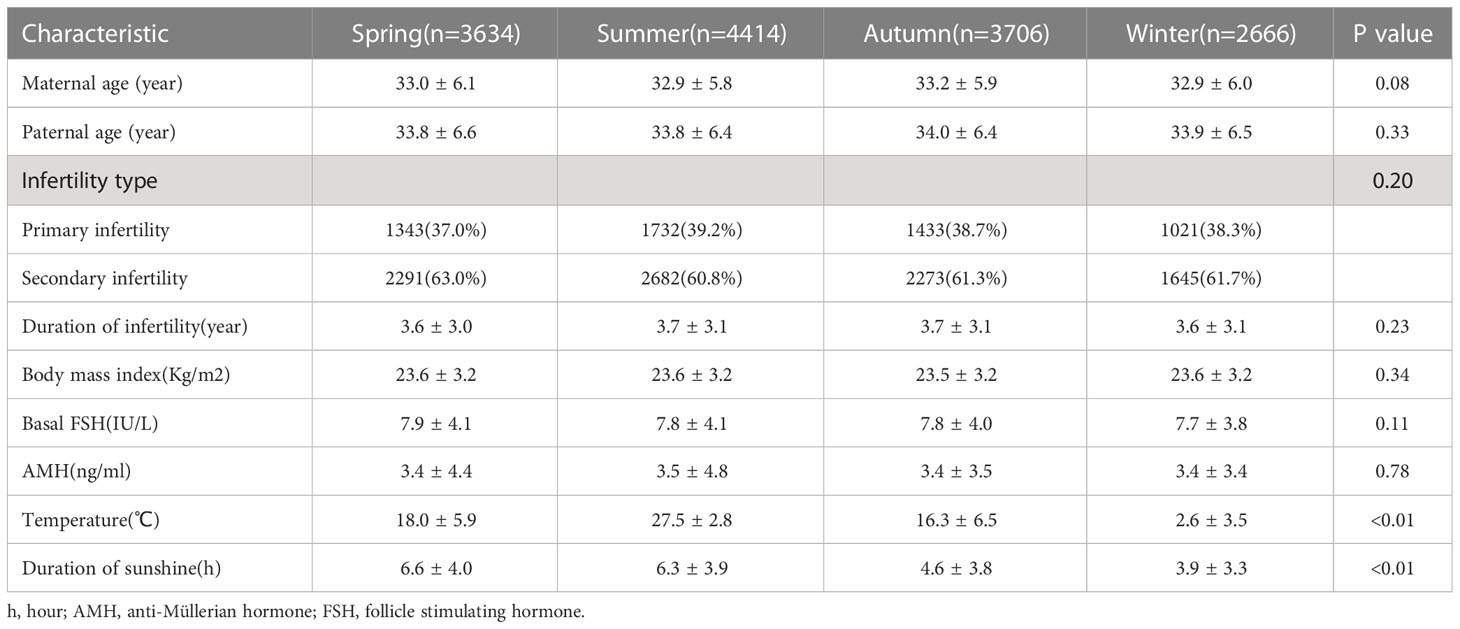
The details of the baseline and cycle characteristics among the four groups are described in Table 1. The temperature differed among the groups (spring 18.0 ± 5.9°C; summer, 27.5 ± 2.8°C; autumn, 16.3 ± 6.5°C; and winter, 2.6 ± 3.5°C, P<0.01). There were significant differences in cumulative sunshine at oocyte retrieval (spring 6.6 ± 4.0 h; summer, 6.3 ± 3.9 h; autumn, 4.6 ± 3.8 h; and winter, 3.9 ± 3.3 h, P<0.01). Other basic characteristics, including maternal age (P=0.08), paternal age (P=0.33), infertility type (P=0.20), duration of infertility (P=0.23), body mass index (P=0.34), basal FSH level (P=0.11) and anti-Müllerian hormone (AMH) level (P=0.78) were similar among the groups (Table 1).
Embryo quality by the season of oocyte retrieval
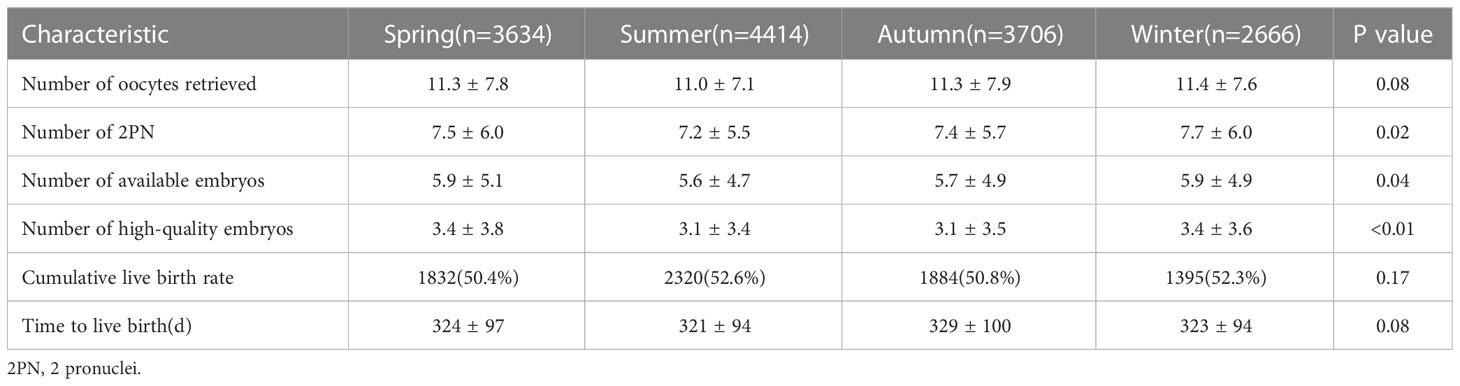
The number of oocytes retrieved was similar among the groups. Other outcomes, including the number of 2PN (P=0.02), number of available embryos (P=0.04), and number of high-quality embryos (P<0.01) differed among the groups. The quality of embryos in summer was relatively poor (Table 2).
Clinical outcomes by season and temperature at oocyte retrieval
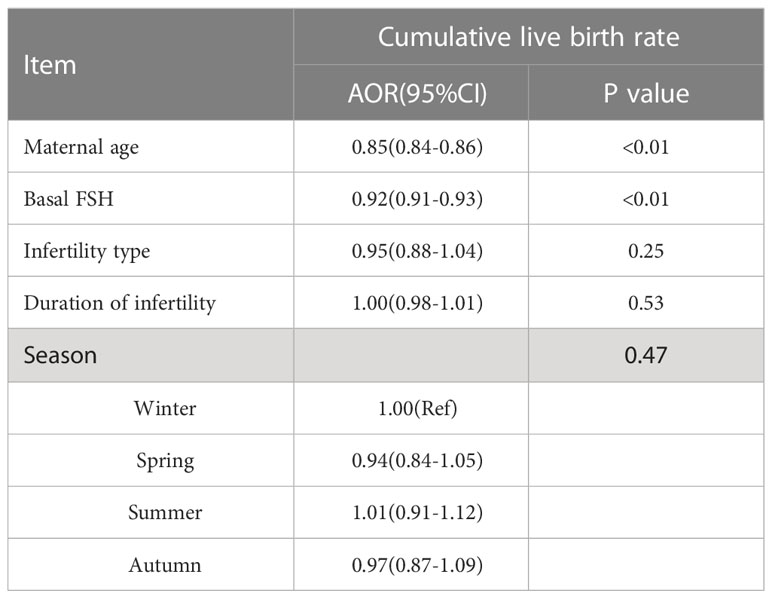
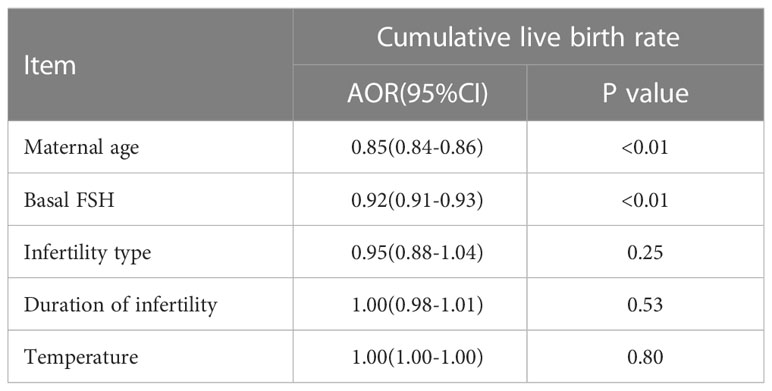
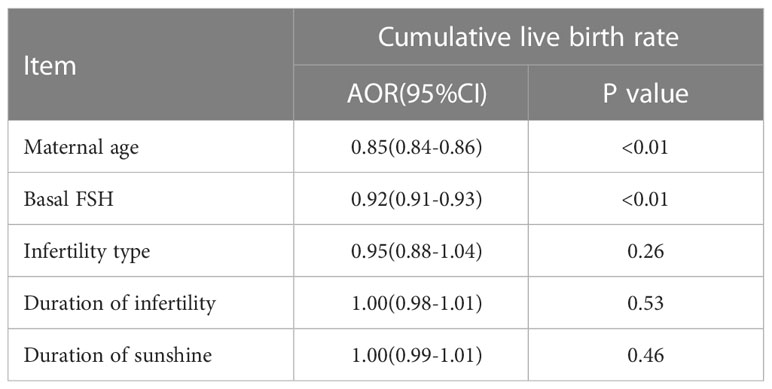
There were no differences between the four groups in terms of CLBR (P=0.17) or time to live birth (P=0.08). After adjusting for confounding factors by binary logistic regression analysis, season (P=0.47) (Table 3), temperature (P=0.80) (Table 4) and duration of sunshine (P=0.46) (Table 5) had no effect on cumulative live births. Only maternal age (P<0.01) and basal FSH level (P<0.01) had an effect on cumulative live births. Cox regression analysis suggested no effect of season (P=0.18) (Figure 1) and temperature (P=0.89) (Figure 2) on time to live birth. Maternal age did have an effect on time to live birth (P<0.01).
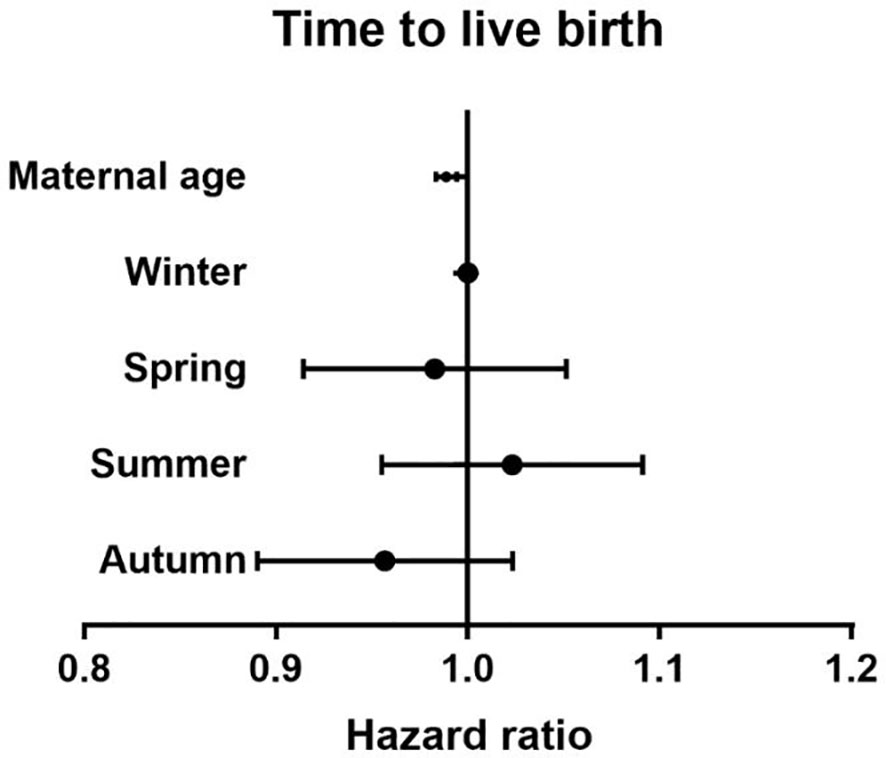
Figure 1 The effect of seasons on time to live birth. Adjust for maternal age, basal follicle-stimulating hormone (FSH), infertility type and duration of infertility.
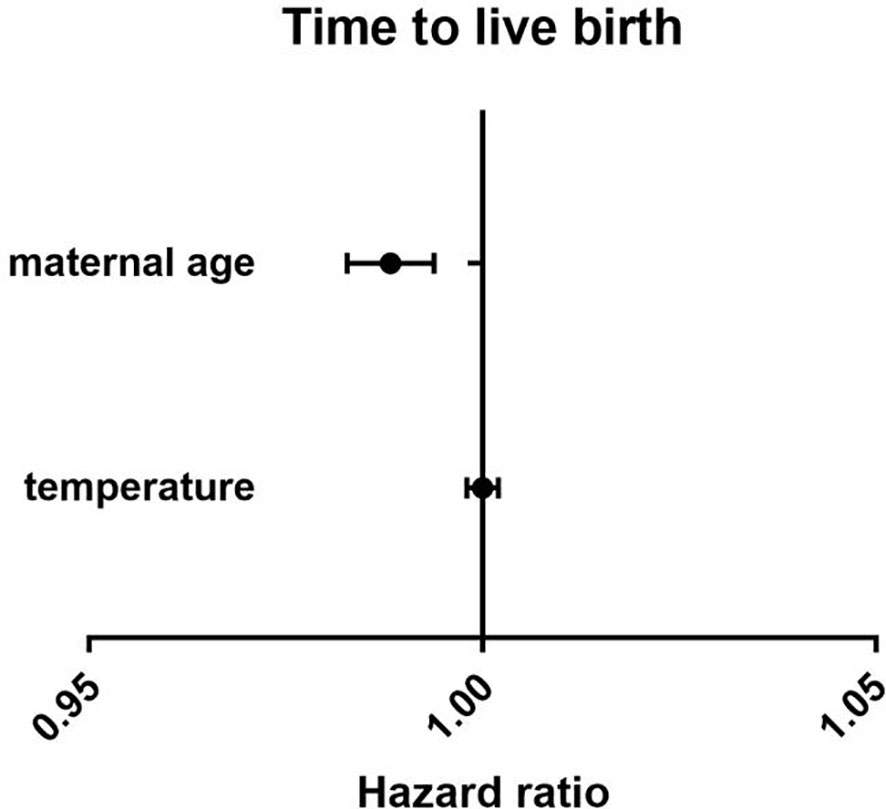
Figure 2 The effect of temperature on time to live birth. Adjust for maternal age, basal follicle-stimulating hormone (FSH), infertility type and duration of infertility.
Discussion
This study explored the effects of temperature and season on the cumulative live birth rate and time to reach live birth. We found that the CLBR and the time to live birth had no relationship with the season or temperature on the oocyte retrieval day.
The effects of season and temperature on human fertility have been studied for many years, but exploring the effects of natural pregnancy also often includes exploring cultural behaviors and sociodemographic factors (24, 25). With the maturity and development of assisted reproductive technology, it has become preferable to explore the effects of season and temperature on human fertility through assisted reproductive technology. Compared with natural pregnancy, assisted reproductive technology provides a good model to study the effects of season and temperature on the female reproductive process. In summer, the number of 2PN, number of available embryos, and number of high-quality embryos were relatively low, consistent with a previous study (35).
Regarding the clinical pregnancy rate and live birth rate, most early studies concluded that season and temperature were not independent factors (28, 36, 37). However, as research has continued, some recent studies suggested a different view. A study from the Chinese University of Hong Kong on IVF treatment suggested that changes in environmental temperature can alter the pregnancy rate (38). The authors of a recent study reported slightly higher clinical pregnancy rates in patients who had oocytes retrieved in June and July, and further analysis found that higher temperatures at the time of oocyte retrieval were associated with higher clinical pregnancy rates but not live birth rates (31). Another study examining frozen embryo transfer cycles reported the interesting finding that summer oocyte retrieval and oocyte retrieval at higher temperatures were associated with higher clinical pregnancy and live birth rates but not with seasonal parameters of frozen transfer day, suggesting that any seasonal effect on IVF success was associated with ovarian function and oocyte quality (32). In a nationwide, register-based cohort study, live birth rates were significantly higher in spring than in summer (39). These findings are controversial, however. This may be due to differences in the meteorological characteristics and geographical environments of the study populations or may be related to inconsistencies regarding time (day of oocyte retrieval/day of embryo transfer) and time length.
To further examine the effects of season and temperature on the day of oocyte retrieval on assisted reproductive technology, we evaluated the CLBR and time to live birth. The cumulative pregnancy rate assessment included the overall treatment outcome of both fresh embryo transfer and subsequent frozen embryo transfer cycles, reflecting the chance of a live birth throughout the course of treatment, which is of greater significance to both the patient and the clinician. CLBR is a more comprehensive and accurate indicator for evaluating the efficacy and safety of treatment. The time to live birth could be used to further evaluate the effectiveness of assisted reproductive technology, and we therefore analyzed the influence of temperature and season on live birth time using Cox regression. The association between seasonal variation and IVF outcomes has not been elucidated. Existing studies have published conflicting reports. Our study also does not support the conclusion that season or temperature affect clinical outcomes. It has been hypothesized that the mechanism by which season may affect the outcome of IVF involves serum vitamin D levels, increased vitamin D synthesis, and increased blood vitamin D levels caused by exposure to more sunlight in summer, which may influence assisted reproductive technology(ART) outcome (40). Evidence as to whether vitamin D levels are associated with IVF outcomes is conflicting, with some studies reporting that increased vitamin D levels improve the likelihood of IVF success (41, 42) and others not supporting this conclusion (43, 44). A 2018 meta-analysis of 11 published cohort studies concluded that adequate vitamin D levels are associated with higher odds of clinical pregnancy and live birth in women undergoing ART (37). However, a recent systematic review and meta-analysis showed that serum vitamin D levels are not associated with IVF/ICSI outcomes (43).
Another strength of the present study is that we selected the cumulative live birth rate and time to live birth as measures of ART outcomes, and we used Cox regression to analyze the time to live birth. Another advantage is that we could adjust for confounding factors such as maternal age, basal FSH level, infertility type, and duration of infertility. In addition, the amount of data in this study was relatively large and reliable.
Of course, there are some limitations to our study. First, we did not consider sperm parameters such as semen concentration, sperm motility, or normal morphology. However, we only included IVF cycles and did not include ICSI cycles with worse semen quality or abnormal fertilization. In addition, we used meteorological data for the day of oocyte retrieval to analyze the outcome, which may also have had an impact on the outcome. And there is a certain difference between the indoor environment and the atmospheric environment. In the future, meteorological data from other time periods may need to be considered to evaluate the outcome, such as the average temperature during oocyte development. Another limitation of the present study is that we did not consider the impact of atmospheric pollutants on the outcome and the effect of temperature on the drug. Finally, this study was a single-center, retrospective analysis, and the results therefore may not be able to be extended to regions with different climatic conditions.
Conclusion
Although season has an effect on embryos, there was no evidence in the present study that season and temperature affect the cumulative live birth rate and time to live birth. It is therefore not necessary to select a specific season when preparing for IVF. Future multicenter studies should be conducted to further explore this question.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
MD and YG designed the study and selected the population to be included and excluded. ZW, XL, ML, XW and JZ performed the data extraction and analysis. YG and LL reviewed the data. MD and JZ drafted this article. All authors have approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by project nos. LHGJ 20210441 and LHGJ 20210451.
Acknowledgments
The authors acknowledge the patients who participated in the study and thank the staff members of the reproductive center of the Third Affiliated Hospital of Zhengzhou University for their expert assistance with data collection and follow-up. We also thank American Journal Experts for their professional manuscript editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W, et al. Health and climate change: policy responses to protect public health. Lancet (London England) (2015) 386:1861–914. doi: 10.1016/s0140-6736(15)60854-6
2. Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, et al. Climate change 2013: the physical science basis. contribution of working group I to the fifth assessment report of IPCC the intergovernmental panel on climate change. Cambridge, UK and New York, NY, USA: Cambridge University Press (2013) p. 1–30.
3. Riahi K, Rao S, Krey V, Cho C, Chirkov V, Fischer G, et al. RCP 8.5–a scenario of comparatively high greenhouse gas emissions. Climatic Change (2011) 109:33. doi: 10.1007/s10584-011-0149-y
4. Kovats RS, Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health (2008) 29:41–55. doi: 10.1146/annurev.publhealth.29.020907.090843
5. He S, Kosatsky T, Smargiassi A, Bilodeau-Bertrand M, Auger N. Heat and pregnancy-related emergencies: risk of placental abruption during hot weather. Environ Int (2018) 111:295–300. doi: 10.1016/j.envint.2017.11.004
6. Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol (1987) 9:17–29.
7. Wolfenson D, Bartol FF, Badinga L, Barros CM, Marple DN, Cummins K, et al. Secretion of PGF2alpha and oxytocin during hyperthermia in cyclic and pregnant heifers. Theriogenology (1993) 39:1129–41. doi: 10.1016/0093-691x(93)90012-t
8. Stan C, Boulvain M, Hirsbrunner-Amagbaly P, Pfister R. Hydration for treatment of preterm labour. Cochrane Database Syst Rev (2002), Cd003096. doi: 10.1002/14651858.cd003096
9. Bekkar B, Pacheco S, Basu R, DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA network Open (2020) 3:e208243. doi: 10.1001/jamanetworkopen.2020.8243
10. Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ (Clinical Res ed.) (2020) 371:m3811. doi: 10.1136/bmj.m3811
11. Syed S, O’Sullivan TL, Phillips KP. Extreme heat and pregnancy outcomes: a scoping review of the epidemiological evidence. Int J Environ Res Public Health (2022) 19(4):2412. doi: 10.3390/ijerph19042412
12. Schou MF, Bonato M, Engelbrecht A, Brand Z, Svensson EI, Melgar J, et al. Extreme temperatures compromise male and female fertility in a large desert bird. Nat Commun (2021) 12:666. doi: 10.1038/s41467-021-20937-7
13. Sevillano CA, Mulder HA, Rashidi H, Mathur PK, Knol EF. Genetic variation for farrowing rate in pigs in response to change in photoperiod and ambient temperature. J Anim Sci (2016) 94:3185–97. doi: 10.2527/jas.2015-9915
14. Pearodwong P, Tretipskul C, Soede NM, Tummaruk P. Factors affecting estrus and ovulation time in weaned sows with induced ovulation by GnRH administration in different seasons. J veterinary Med Sci (2019) 81:1567–74. doi: 10.1292/jvms.18-0429
15. Bloemhof S, Mathur PK, Knol EF, van der Waaij EH. Effect of daily environmental temperature on farrowing rate and total born in dam line sows. J Anim Sci (2013) 91:2667–79. doi: 10.2527/jas.2012-5902
16. Iida R, Piñeiro C, Koketsu Y. Timing and temperature thresholds of heat stress effects on fertility performance of different parity sows in Spanish herds. J Anim Sci (2021) 99(7):skab173. doi: 10.1093/jas/skab173
17. Pavani K, Carvalhais I, Faheem M, Chaveiro A, Reis FV, da Silva FM. Reproductive performance of Holstein dairy cows grazing in dry-summer subtropical climatic conditions: effect of heat stress and heat shock on meiotic competence and In vitro fertilization. Asian-Australasian J Anim Sci (2015) 28:334–42. doi: 10.5713/ajas.14.0480
18. Sammad A, Umer S. Dairy cow reproduction under the influence of heat stress. J Anim Physiol Anim Nutr (Berl) (2020) 104(4):978–86. doi: 10.1111/jpn.13257
19. Barreca A, Deschenes O, Guldi M. Maybe next month? temperature shocks and dynamic adjustments in birth rates. Demography (2018) 55:1269–93. doi: 10.1007/s13524-018-0690-7
20. Jensen PM, Sørensen M, Weiner J. Human total fertility rate affected by ambient temperatures in both the present and previous generations. Int J biometeorology (2021) 65:1837–48. doi: 10.1007/s00484-021-02140-x
21. Gaskins AJ, Mínguez-Alarcón L, VoPham T, Hart JE, Chavarro JE, Schwartz J, et al. Impact of ambient temperature on ovarian reserve. Fertility sterility (2021) 116:1052–60. doi: 10.1016/j.fertnstert.2021.05.091
22. Wesselink AK, Wise LA, Hatch EE, Mikkelsen EM, Sørensen HT, Riis AH, et al. Seasonal patterns in fecundability in north America and Denmark: a preconception cohort study. Hum Reprod (Oxford England) (2020) 35:565–72. doi: 10.1093/humrep/dez265
23. Becker S. Seasonal patterns of births and conception throughout the world. Adv Exp Med Biol (1991) 286:59–72. doi: 10.1007/978-1-4684-5913-5_6
24. Dahlberg J, Andersson G. Changing seasonal variation in births by sociodemographic factors: a population-based register study. Hum Reprod Open (2018) 2018:hoy015. doi: 10.1093/hropen/hoy015
25. Odegård O. Season of birth in the population of Norway, with particular reference to the September birth maximum. Br J psychiatry: J Ment Sci (1977) 131:339–44. doi: 10.1192/bjp.131.4.339
26. Fleming C, Nice L, Hughes AO, Hull MG. Apparent lack of seasonal variation in implantation rates after in-vitro fertilization. Hum Reprod (Oxford England) (1994) 9:2164–6. doi: 10.1093/oxfordjournals.humrep.a138411
27. Dunphy BC, Anderson-Sykes S, Brant R, Pattinson HA, Greene CA. Human embryo implantation following in-vitro fertilization: is there a seasonal variation? Hum Reprod (Oxford England) (1995) 10:1825–7. doi: 10.1093/oxfordjournals.humrep.a136184
28. Wunder DM, Limoni C, Birkhäuser MH. Lack of seasonal variations in fertilization, pregnancy and implantation rates in women undergoing IVF. Hum Reprod (Oxford England) (2005) 20:3122–9. doi: 10.1093/humrep/dei177
29. Kirshenbaum M, Ben-David A, Zilberberg E, Elkan-Miller T, Haas J, Orvieto R. Influence of seasonal variation on in vitro fertilization success. PloS One (2018) 13:e0199210. doi: 10.1371/journal.pone.0199210
30. Vandekerckhove F, van der Veken H, Tilleman K, De Croo I, Van den Abbeel E, Gerris J, et al. Seasons in the sun: the impact on IVF results one month later. Facts views Vision ObGyn (2016) 8:75–83.
31. Farland LV, Correia KFB, Missmer SA, Racowsky C. Seasonal variation, temperature, day length, and IVF outcomes from fresh cycles. J assisted Reprod Genet (2020) 37:2427–33. doi: 10.1007/s10815-020-01915-2
32. Correia KFB, Farland LV, Missmer SA, Racowsky C. The association between season, day length, and temperature on clinical outcomes after cryopreserved embryo transfer. Fertility sterility (2022) 117:539–47. doi: 10.1016/j.fertnstert.2021.11.014
33. Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod (Oxford England) (2011) 26:1270–83. doi: 10.1093/humrep/der037
34. Du M, Zhang J, Li Z, Liu X, Li J, Liu W, et al. Comparison of the cumulative live birth rates of progestin-primed ovarian stimulation and flexible GnRH antagonist protocols in patients with low prognosis. Front Endocrinol (2021) 12:705264. doi: 10.3389/fendo.2021.705264
35. Chu T, Wang D, Yu T, Zhai J. Effects of seasonal variations and meteorological factors on IVF pregnancy outcomes: a cohort study from henan province, China. Reprod Biol endocrinology: RB&E (2022) 20:113. doi: 10.1186/s12958-022-00986-3
36. Liu X, Bai H, Mol BW, Shi W, Gao M, Shi J. Seasonal variability does not impact in vitro fertilization success. Sci Rep (2019) 9:17185. doi: 10.1038/s41598-019-53919-3
37. Xiao Y, Wang M, Liu K. The influence of seasonal variations on in vitro fertilization and fresh/frozen embryo transfer: a retrospective study. Arch gynecology obstetrics (2018) 298:649–54. doi: 10.1007/s00404-018-4843-0
38. Zhao M, Zhang H, Waters THB, Chung JPW, Li TC, Chan DYL. The effects of daily meteorological perturbation on pregnancy outcome: follow-up of a cohort of young women undergoing IVF treatment. Environ health: Global Access Sci Source (2019) 18:103. doi: 10.1186/s12940-019-0538-7
39. Carlsson Humla E, Bergh C, Akouri R, Tsiartas P. Summer is not associated with higher live birth rates in fresh IVF/ICSI cycles: a population-based nationwide registry study. Hum Reprod Open (2022) 2022(4):hoac036. doi: 10.1093/hropen/hoac036
40. Iliuta F, Pijoan JI, Lainz L, Exposito A, Matorras R. Women’s vitamin d levels and IVF results: a systematic review of the literature and meta-analysis, considering three categories of vitamin status (replete, insufficient and deficient). Hum fertility (Cambridge England) (2022) 25:228–46. doi: 10.1080/14647273.2020.1807618
41. Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin d stores predict reproductive success following in vitro fertilization. Fertility sterility (2010) 94:1314–9. doi: 10.1016/j.fertnstert.2009.05.019
42. Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin d levels on IVF outcomes. Hum Reprod (2012) 27:3321–7. doi: 10.1093/humrep/des280
43. Cozzolino M, Busnelli A, Pellegrini L, Riviello E, Vitagliano A. How vitamin d level influences in vitro fertilization outcomes: results of a systematic review and meta-analysis. Fertility sterility (2020) 114:1014–25. doi: 10.1016/j.fertnstert.2020.05.040
Keywords: IVF, season, temperature, cumulative live birth, time to live birth
Citation: Du M, Zhang J, Wei Z, Li L, Liu X, Liu M, Wang X and Guan Y (2023) Season and temperature do not affect cumulative live birth rate and time to live birth in in vitro fertilization. Front. Endocrinol. 14:1156299. doi: 10.3389/fendo.2023.1156299
Received: 01 February 2023; Accepted: 01 May 2023;
Published: 21 June 2023.
Edited by:
Wei Wu, Nanjing Medical University, ChinaReviewed by:
Ruma Satwik, Sir Ganga Ram Hospital, IndiaAkmal El-Mazny, Cairo University, Egypt
Zainab M. Alawad, University of Baghdad, Iraq
Copyright © 2023 Du, Zhang, Wei, Li, Liu, Liu, Wang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichun Guan, bGlzYW1heWd1YW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mingze Du
Mingze Du Junwei Zhang†
Junwei Zhang† Xinmi Liu
Xinmi Liu Xingling Wang
Xingling Wang Yichun Guan
Yichun Guan