
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 24 July 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1154984
This article is part of the Research TopicMolecular Characterization of Thyroid Lesions in the Era of “Next Generation” Techniques: Volume IIView all 8 articles
Objectives: To screen out the predictors and establish a prediction model of positive fine needle aspiration biopsy (FNAB) in the Chinese Guidelines for Malignant Risk Stratification of Thyroid Nodule Ultrasound (C-TIRADS) 4 thyroid nodules, and this nomogram can help clinicians evaluate the risk of positive FNAB and determine if FNAB is necessary.
Methods: We retrospectively analyzed data from 547 patients who had C-TIRADS 4 thyroid nodules and underwent fine-needle aspiration biopsy (FNAB) at the Second Affiliated Hospital of Chongqing Medical University between November 30, 2021 and September 5, 2022. Patients who met our inclusion criteria were divided into two groups based on positive or negative FNAB results. We compared their ultrasound (US) features, BRAF V600E status, thyroid function, and other general characteristics using univariate and multivariate logistic regression analyses to identify independent predictors. These predictors were then used to construct a nomogram. The calibration plot, area under the curve (AUC), and decision curve analysis were employed to evaluate the calibration, discrimination, and clinical utility of the prediction model.
Results: Out of 547 patients, 39.3% (215/547) had a positive result on fine-needle aspiration biopsy (FNAB), while 60.7% (332/547) had a negative result. Univariate logistic regression analysis revealed no significant differences in TPOAb, TgAb, TSH, Tg, nodule location, sex, or solid status between the two groups (P>0.05). However, age, nodule size, internal or surrounding blood flow signal, microcalcifications, aspect ratio, morphology, and low echo showed significant differences (P<0.05). Multivariate logistic regression analysis was conducted to explore the correlation between potential independent predictors. The results showed that only age (OR=0.444, 95% Cl=0.296~0.666, P<0.001), low echo (OR=3.549, 95% Cl=2.319~5.432, P<0.001), microcalcifications (OR=2.531, 95% Cl=1.661~3.856, P<0.001), aspect ratio (OR=3.032, 95% Cl=1.819~5.052, P<0.001), and morphology (OR=2.437, 95% Cl=1.586~3.745, P<0.001) were independent predictors for a positive FNAB. These variables were used to construct a prediction nomogram. An ROC curve analysis was performed to assess the accuracy of the nomogram, and AUC=0.793, which indicated good discrimination and decision curve analysis demonstrated clinical significance within a threshold range of 14% to 91%.
Conclusion: In conclusion, 5 independent predictors of positive FNAB, including age (≤45 years old), low echo (yes), microcalcifications (yes), aspect ratio (>1) and morphology (irregular), were identified. A nomogram was established based on the above 5 predictors, and the nomogram can be used as a complementary basis to help clinicians make decisions on FNAB of C-TI-RADS 4 thyroid nodules.
The rapid increase in morbidity due to thyroid nodules is possibly due to the widespread application of modern imaging modalities, such as ultrasound (US) and computed tomography (CT) (1). About 60% of adults have at least 1 thyroid nodules, but only approximately 5% are confirmed as malignant tumors (2). It seems that overdiagnosis and overtreatment are inevitable, which are linked to potentially unnecessary costs and nonnegligible iatrogenic injury for patients (2, 3). Hence, it is essential to accurately diagnose and treat thyroid nodules. Fine needle aspiration biopsy (FNAB) is an effective way to identify thyroid nodules, especially when the added BRAF V600E test increases the accuracy of FNAB.
The indications for FNAB are often based on thyroid ultrasound, and thyroid image report and data system (TI-RADS) 4 thyroid nodules are often the targets of FNAB in clinical practice. TI-RADS 4 thyroid nodules can be divided into 3 subtypes (4A, 4B and 4C) based on various vital ultrasound features (4), and these types roughly represent the risk of thyroid carcinoma from 2% to 90%. Without further accurate information, FNAB is often recommended for patients to avoid missed diagnosis. Several versions of the TIRADS are used in the clinic, including the Korean Thyroid Association guideline (K-TIRADS), the American Thyroid Association guideline (ATA guideline), the American College of Radiology guideline (ACR-TIRADS), and the Chinese guideline (C-TIRADS), and different versions are slightly different. In the detection of thyroid carcinoma, C-TIRADS exhibited superior diagnostic performance and relatively lower rates of unnecessary biopsy, as demonstrated by a study (5). Additionally, ultrasound doctors usually give reports relying on the C-TIRADS in China. The ultrasound features that may relate to raising concern for thyroid malignancy include low echo, solid, microcalcifications, aspect ratio, internal or surrounding blood flow signal, and morphology (regular or irregular) (6). A study showed that 3 ultrasound features of solid thyroid nodules, including microcalcifications, marked low echo, and aspect ratio >1, are valuable in the diagnosis of thyroid malignancy (7). Another study indicated that the proportion of malignancies was 16.0% in 4A, 43.2% in 4B and 72.7% in 4C (8). In fact, quite a few TI-RADS 4 thyroid nodules are benign, and FNAB is unnecessary for them. Moreover, ultrasound greatly depends on the the experience of the inspectors and quality of equipment (3, 9, 10). Therefore, it is controversial to solely rely on TI-RADS to make the decision of FNAB.
Age is a vital factor in thyroid carcinoma staging, and age >45 years indicates an advanced stage. However, a study showed that among the patients who received FNAB and were finally confirmed as having a malignant nodule, the number of patients aged ≤45 years old was twice as high as that of patients aged >45 years old. Another study showed that the risk of thyroid nodules increased with age, while the possibility of malignancy decreased (11). Moreover, sex is significantly linked to the morbidity of thyroid carcinoma, and females have a higher morbidity. Nevertheless, a study confirmed that male patients who received FNAB may be more likely to obtain malignant or determinate results (12). In most studies, the authors believe that the size of thyroid nodules is uncorrelated with their oncologic properties (13), but a study showed that a nodule with size of 1-1.9 cm was more likely to be benign (14). Thyroid function is also a group of important potential predictors. Anti-thyroperoxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) are significant predictors in many studies, and free thyroxine (T4), triiodothyronine (T3), thyroglobulin (Tg), and thyroid-stimulating hormone (TSH) are controversial (15–20). In addition, a study has demonstrated that the location of thyroid nodules, particularly in the isthmus region, is a significant independent predictor for malignancy (21). Hence, more potential predictors, such as age, sex, nodule size, thyroid function, and nodule location, need to be included to predict malignancy.
As mentioned, ultrasound-guided FNAB is more important in the diagnosis of thyroid nodules (22). The majority of complications following a fine-needle biopsy, such as pain and small hematomas, have a low occurrence rate and typically resolve on their own. Severe complications, including significant hematomas, infections, and tumor dissemination, are exceedingly rare (23). Moreover, the patients may have dizziness, palpitation, shortness of breath, hypotension and other discomfort during FNAB. Accordingly, FNAB should be cautiously recommended. Even for C-TIRADS 4 thyroid nodules, there are still many negative results after FNAB. To avoid or reduce the waste of medical resources and invasive operations, it is crucial to predict FNAB results in C-TIRADS 4 thyroid nodules.
Nomograms, as a common prediction model, have widespread use for physicians’ clinical decisions (24). It has many advantages, including high accuracy, simple calculation, and easy understanding. The objective of this study was to identify significant predictors among multiple medical information sources and construct a prediction model (nomogram) for positive fine-needle aspiration biopsy results.
All patients were examined with color Doppler ultrasound and underwent ultrasound-guided FNAB at the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China). The inclusion criteria were as follows: (1) patients whose ultrasound reports indicated C-TIRADS 4 thyroid nodules; (2) the patient had never been diagnosed with thyroid malignancy; (3) the maximum diameter of the thyroid nodule was more than or equal to 5 mm; (4) cytological examination and BRAF V600E mutation analysis were performed on the FNAB specimen; and (5) the patient had complete medical information. The exclusion criteria were as follows: (1) history of thyroid malignancy; (2) history of other malignancies, including breast cancer and lung cancer; (3) history of thyroid surgery or iodine-131 treatment; (4) maximum diameter of the thyroid nodule less than 5 mm; and (5) incomplete medical information. 547 patients were included in this study between December 2021 and September 2022 based on the inclusion and exclusion criteria. In this study, positive FNAB results met the following criteria: positive cytology results including all kinds of malignant tumors and suspicious malignant tumors with or without mutational BRAF V600E genes. In contrast, negative FNAB results met the following criteria: negative cytology results including all kinds of benign cells, such as inflammatory cells and follicular epithelial cells.
Before FNAB, thyroid nodules were examined using color Doppler ultrasound (Philips IU22, L12-5 linear array probe, 5-12 MHz, Philips Medical). Reports showed internal or surrounding blood flow signal (poor/rich), solid (yes/no), nodule size (maximum diameter ≥1.0 cm/<1.0 cm), microcalcifications (yes/no), aspect ratio (≤1/>1), morphology (regular/irregular), low echo (yes/no).
The expert committee established the C-TIRADS by counting the score of suspicious ultrasound features (solid, microcalcification, low echo, blurry borderline, irregularity boundary, extrathyroid invasion, and vertical position are suspicious malignant ultrasound features, while the comet tail sign is a benign feature). Each suspicious malignant sign is 1 point, while the comet tail sign is -1 point. The final score of the thyroid nodules is calculated by adding the scores of all individual features. A score of 1 corresponds to C-TIRADS 4A, a score of 2 corresponds to C-TIRADS 4B, and a score of 3-4 corresponds to C-TIRADS 4C.
The patients were placed in the supine position. Before the procedure, a brief preliminary ultrasound of the thyroid was performed to determine the location of the thyroid nodule. The skin was marked at the puncture site according to the determined nodule position, disinfected with iodophor and covered with a sterile surgical towel conventionally. Similarly, the ultrasound probe was disinfected with an iodophor, and the iodophor could also be used as a coupling agent. Lidocaine (2%, 1 ml) was subcutaneously injected for local anesthesia. The probe was placed over the target nodule in the transverse position. A fine needle (GALLINI S. R. L, Italy) was used to puncture the thyroid nodule from the marked site along the long axis of the probe under the guidance of ultrasound (Figure 1). After removing the core, the fine needle was repeatedly extracted and inserted into the nodule several times and then removed quickly. One part of the specimen was fixed onto a designated slide and delivered to the Department of Pathology of the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China) for cytological examination; the other part of the specimen was placed into 1 ml of saline and delivered to the Molecular Diagnostic Laboratory of the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China) for BRAF V600E mutation analysis.
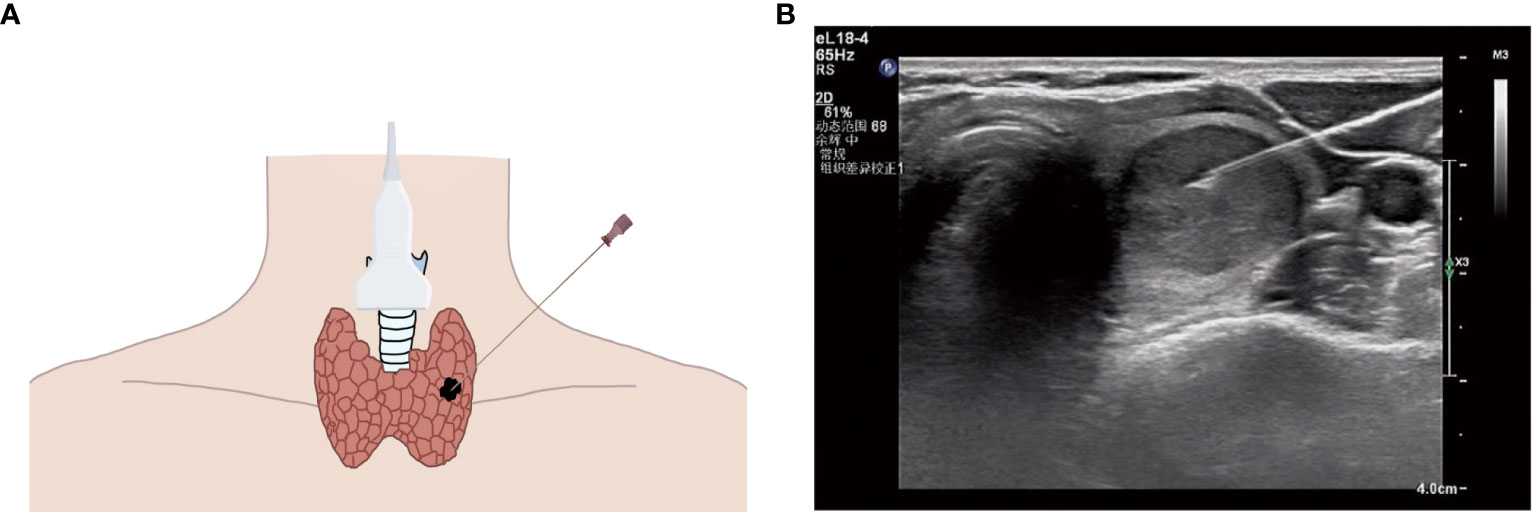
Figure 1 (A) Schematic diagram of ultrasound (US)-guided fine needle aspiration biopsy (thyroid nodule). (B) US image of fine needle aspiration (thyroid nodule).
The reaction mixture was thawed at room temperature and agitated with a vortex for 15 seconds. It was then centrifuged at 2,000 × g for 15 seconds at room temperature. The reaction mixture (35 µl) was combined with 0.4 µl of Taq enzyme and then added to a PCR tube, which was kept in an ice bath. 5 µl of DNA sample (2-5 ng), 5 µl of positive control, and 5 µl of negative control were added to each PCR tube separately. The PCR tubes were spun at 2,000 × g for 1 minute at room temperature and subjected to thermocycling using a CFX96 thermocycler (Bio-Rad Laboratories, Inc.). The following thermocycling protocol was used for quantitative PCR: an initial denaturation step at 95°C for 5 minutes; 15 cycles of 95°C for 25 seconds, 64°C for 20 seconds, and 72°C for 20 seconds; and an additional 31 cycles of 93°C for 25 seconds, 60°C for 35 seconds, and 72°C for 20 seconds. The carboxyfluorescein (FAM) and hexachlorofluorescein (HEX) signals were detected at 60°C during the final set of cycling conditions. The relative amount of DNA was quantified using the 2−DDCq method (25). If the sample’s FAM signal Cq value was ≥28, it was considered negative for the BRAF V600E mutation. If the sample’s FAM signal Cq value was <28, it was considered positive for the BRAF V600E mutation, as per the manufacturer’s instructions (26, 27).
Statistical analyses were conducted using SPSS version 26.0. Univariate and multivariate logistic regression analyses were performed to screen out the independent predictors of positive FNAB. The prediction nomogram based on independent predictors was developed by using R software (Version 4.2.1). A calibration curve was plotted to evaluate the calibration of the nomogram. Discrimination ability was measured by area under the curve (AUC) in the training cohort and validated through two internal validation cohorts, which were randomly divided from the training cohort: validation cohort 1 (n=274) and validation cohort 2 (n=273). Furthermore, a decision curve analysis was employed to determine the clinical utility of the nomogram by measuring the net benefit at the different thresholds.
A total of 547 patients were finally confirmed to have C-TIRADS 4 thyroid nodules and underwent FNAB, among which 438 (80.07%) were women, and 109 (19.93%) were men. Of the 547 patients, 226 (41.31%) were ≤45 years old, and 321 (58.69%) were >45 years old (Table 1). After cytological examination and BRAF V600E mutation analysis, positive FNAB was confirmed. Of all patients, 215 (39.31%) had a positive FNAB (Figures 2, 3, 4A, C), while 332 (60.69%) did not (Figures 2, 3, 4B, D). Among the positive FANB, there were 125 patients were confirmed as thyroid cancer after surgery, only 9 patients were confirmed as benign tumor, and 81 patients selected follow-up. It can be roughly concluded that the accuracy of FNAB for C-TIRADS 4 thyroid nodules in our center was 93.28% (125/134), and the follow-up rate of positive FNAB was 37.67% (81/215).
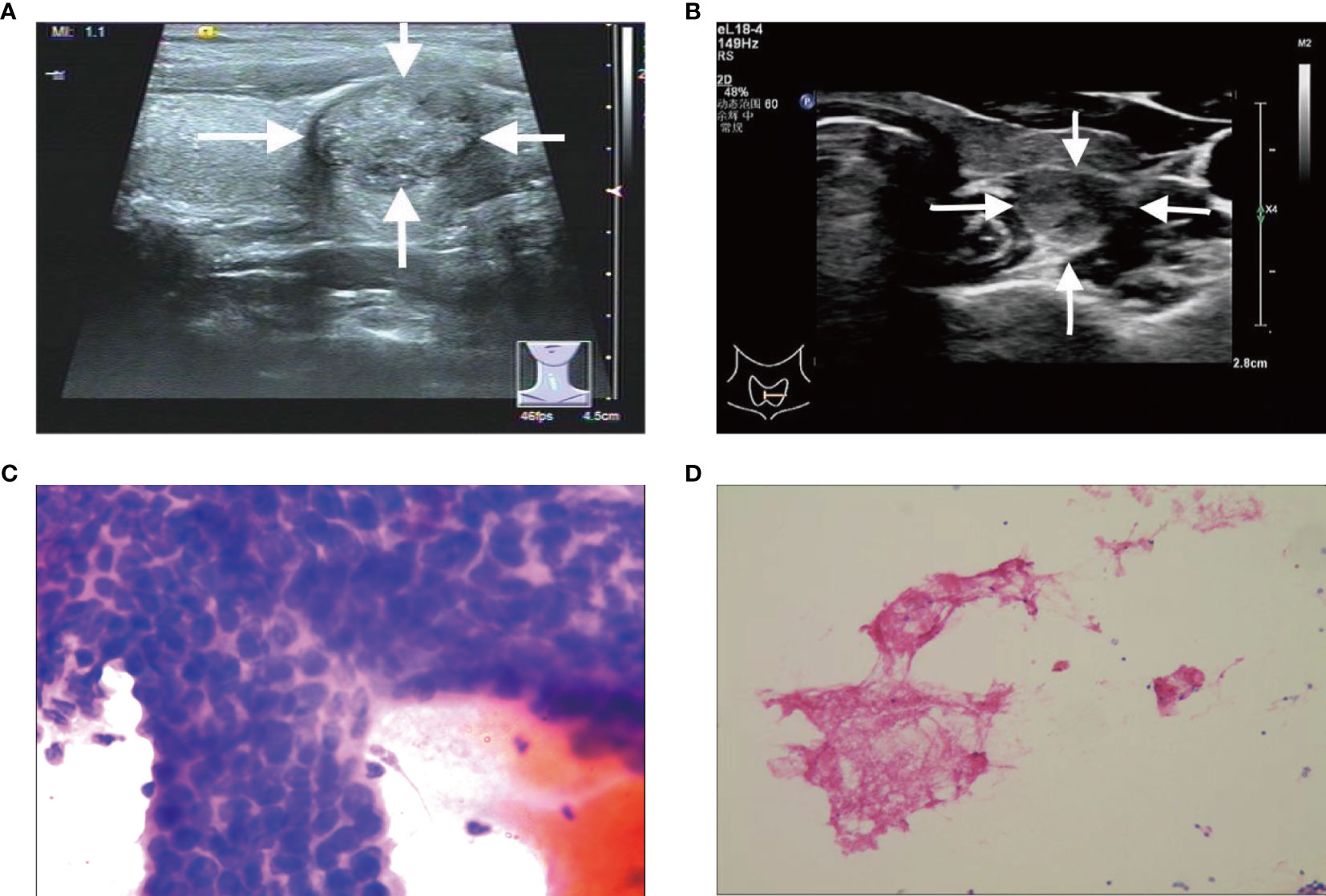
Figure 2 (A, C) A C-TIRADS 4A thyroid nodules patient (31-year-old male) with positive fine needle aspiration biopsy (FNAB). (A) The thyroid nodule had microcalcifications on US images. (C) Cytological examination indicated suspicious malignant tumor. (B, D) A C-TIRADS 4A thyroid nodules patient (40-year-old female) with negative FNAB. (B) The thyroid nodule had irregular morphology on US images. (D) Cytological examination indicated follicular epithelial cells.
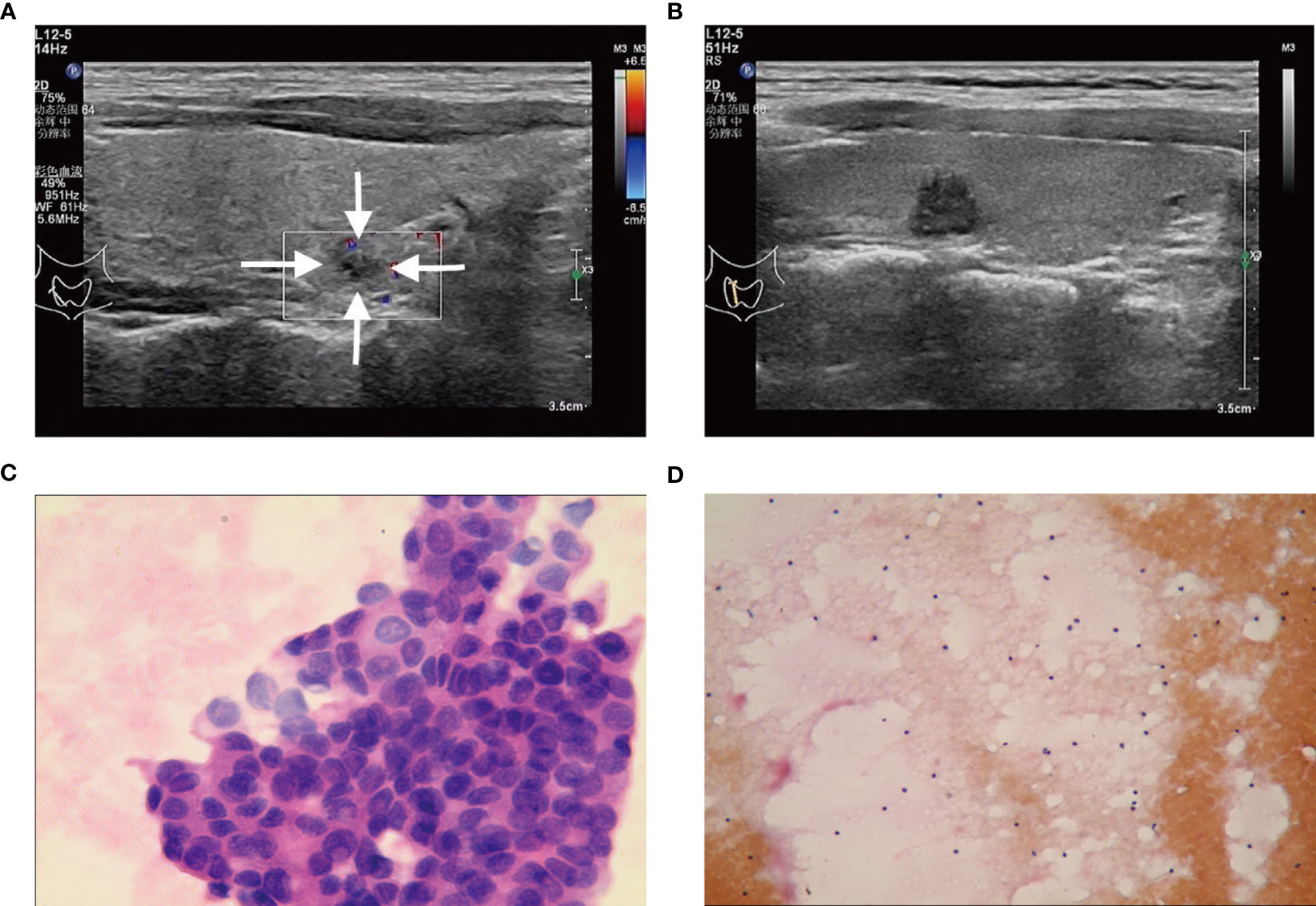
Figure 3 (A, C) A C-TIRADS 4B thyroid nodules patient (25-year-old female) with positive fine needle aspiration biopsy (FNAB). (A) The thyroid nodule had a low echo and irregular morphology on US images. (C) Cytological examination indicated suspicious malignant tumor. (B, D) A C-TIRADS 4B thyroid nodules patient (36-year-old male) with negative FNAB. (B) The thyroid nodule had a low echo and irregular morphology on US images. (D) Cytological examination indicated inflammatory.
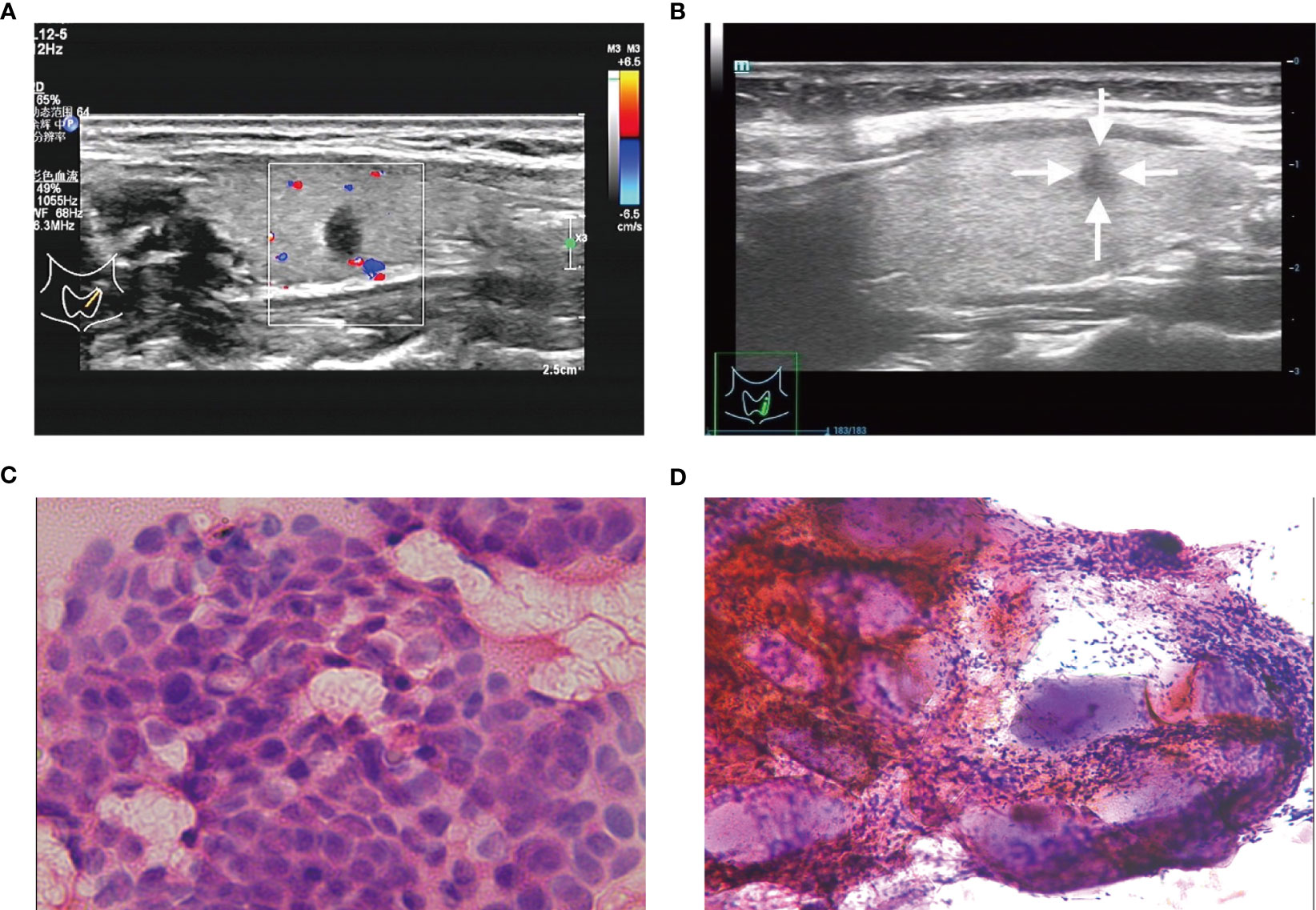
Figure 4 (A, C) A C-TIRADS 4C thyroid nodules patient (32-year-old female) with positive fine needle aspiration biopsy (FNAB). (A) The thyroid nodule had a low echo, aspect ratio >1 and irregular morphology on US images. (C) Cytological examination indicated suspicious malignant tumor. (B, D) A C-TIRADS 4C thyroid nodules patient (28-year-old female) with negative FNAB. (B) The thyroid nodule had a low echo, aspect ratio >1 and irregular morphology on US images. (D) Cytological examination indicated inflammatory cell, follicular epithelial cells and gelatine.
Table 1 displays the correlations between medical information and positive FNAB. There were significant differences in age (≤45 years old, P<0.05), nodule size (<1.0 cm, P<0.05), internal or surrounding blood flow signal (yes, P<0.05), aspect ratio (>1, P<0.05), low echo (yes, present, P<0.05), microcalcifications (yes, P<0.05) and morphology (irregular, P<0.05) between the positive-FNAB group and the negative-FNAB group. At the same time, gender, Tg, TgAb, TPOAb, TSH, solid and nodule location were not significantly different between the 2 groups (P>0.05).
After the univariate logistic regression analysis, the above predictors screened out were used for a multivariate logistic regression analysis. As a result, Table 2 demonstrates that the subsequent variables were found to have independent correlations with a favorable outcome of FNAB: age (≤45 years old) (OR=0.444, P<0.001), low echo (OR=3.549, P<0.001), microcalcifications (OR=2.531, P<0.001), aspect ratio (>1) (OR=3.032, P<0.001) and irregular morphology (OR=2.437, P<0.001).
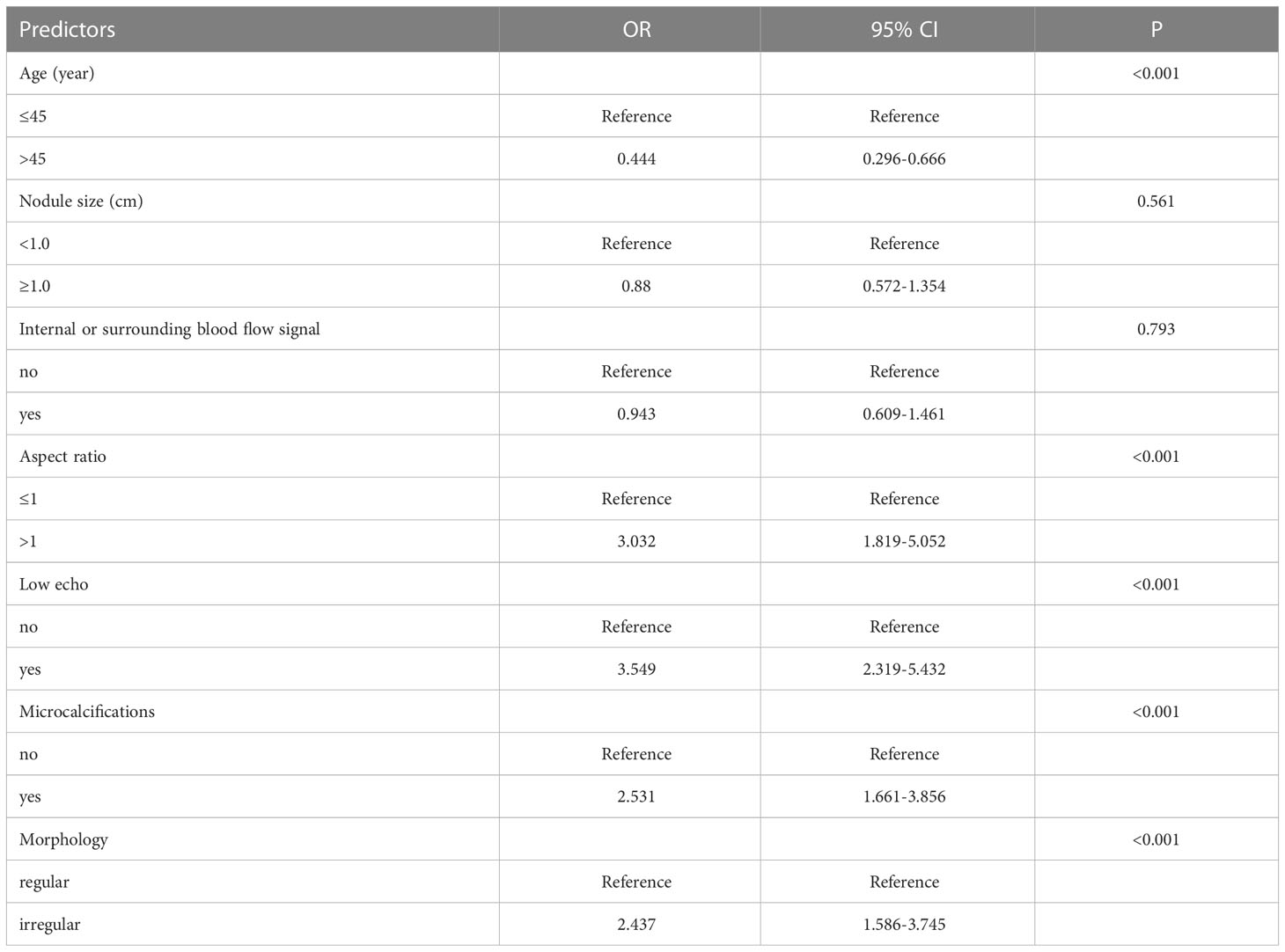
Table 2 Multivariate logistic regression analysis of the relationship between predictors of C-TIRADS 4 thyroid nodules (significant by univariate analysis) and positive FNAB.
The results of the univariate and multivariate logistic regression analyses among age (≤45 years old), low echo, microcalcifications, aspect ratio (>1) and morphology (irregular) are shown in Table 2. A nomogram (Figure 5) was developed based on the prediction model incorporating the 5 independent predictors mentioned above. Figure 5 shows that age (≤45 years old) was assigned 62 points, whereas age (>45 years old) received 0 points. A nodal aspect ratio (>1) was assigned 88 points, but a nodal aspect ratio (≤1) received 0 points. A nodule with low echo was assigned 100 points, but a nodule without low echo received 0 points. A nodule with microcalcification was assigned 70 points, and a nodule without microcalcification received 0 points. A nodule with irregular morphology was assigned 70 points, but a nodule with regular morphology received 0 points. The nomogram assigns a maximum of 390 points, and the predictive ability for positive FNAB risk ranges from about 0.05 to 0.9.
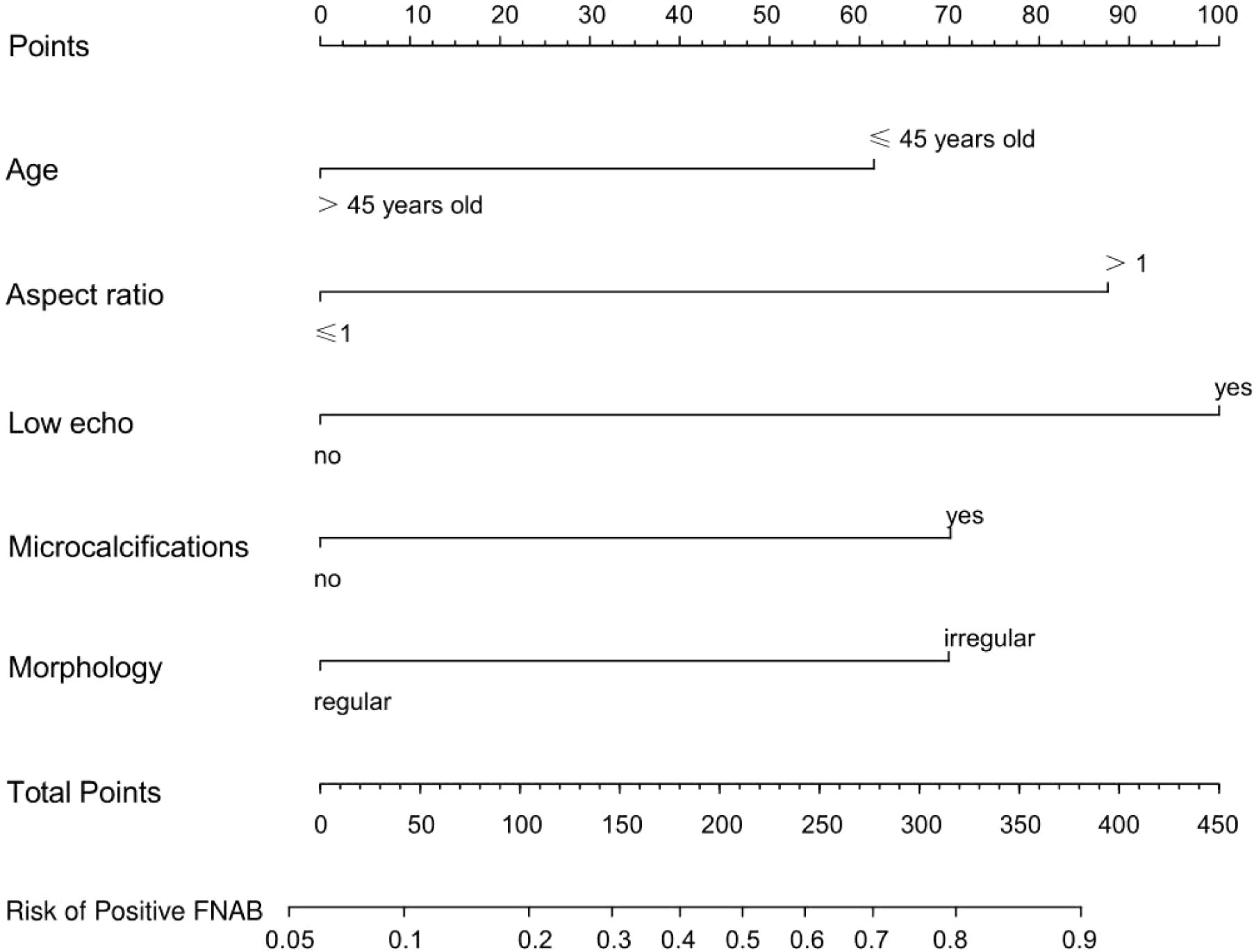
Figure 5 A prediction nomogram. The nomogram is used for the prediction of positive FNAB in C-TIRADS 4 thyroid nodules patients. The prediction nomogram was developed in the cohort, with age, aspect ratio, low echo, microcalcifications and morphology.
The calibration curve (Figure 6) of the nomogram employed for predicting the risk of positive FNAB among patients with C-TIRADS 4 thyroid nodules exhibited satisfactory consistency. The AUC was determined to be 0.793, and it was subsequently validated to be 0.814 and 0.779 through the 2 internal validations (Figure 7), which confirmed the model’s good discrimination.

Figure 6 Calibration curves of the prediction nomogram. The solid line is close to the diagonal dotted line, which represents a good prediction ability. The x-axis represents the predicted positive FNAB. The y-axis represents the actual positive FNAB. The diagonal dotted line represents the perfect prediction by the ideal model. The solid line represents the performance of the nomogram, and a closer fit to the diagonal dotted line means a better prediction.
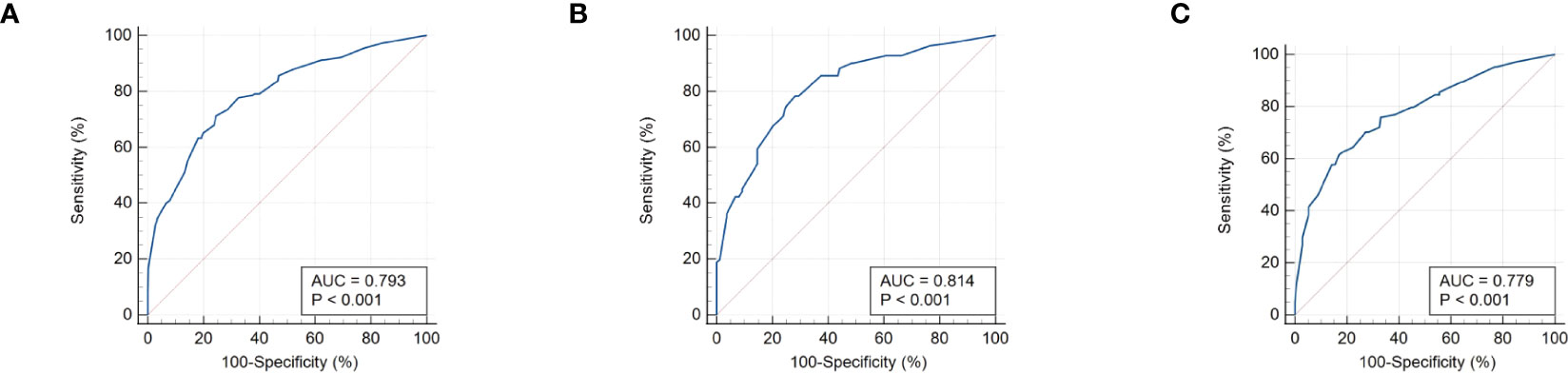
Figure 7 (A) The receiver operating characteristics (ROC) curve and area under the curve (AUC) in the training cohort. (B) ROC curve and AUC in the validating 1 cohort. (C) ROC curve and AUC in the validating 2 cohort. Validating 1 and validating 2 were performed to evaluate the accuracy of this model.
Figure 8 displays the decision curve analysis (DCA) for the risk nomogram. DCA revealed that using the nomogram to predict positive FNAB would be advantageous when the threshold probability ranges between 14% and 91%. In this range, the net benefit was comparable with several overlaps according to the risk nomogram.
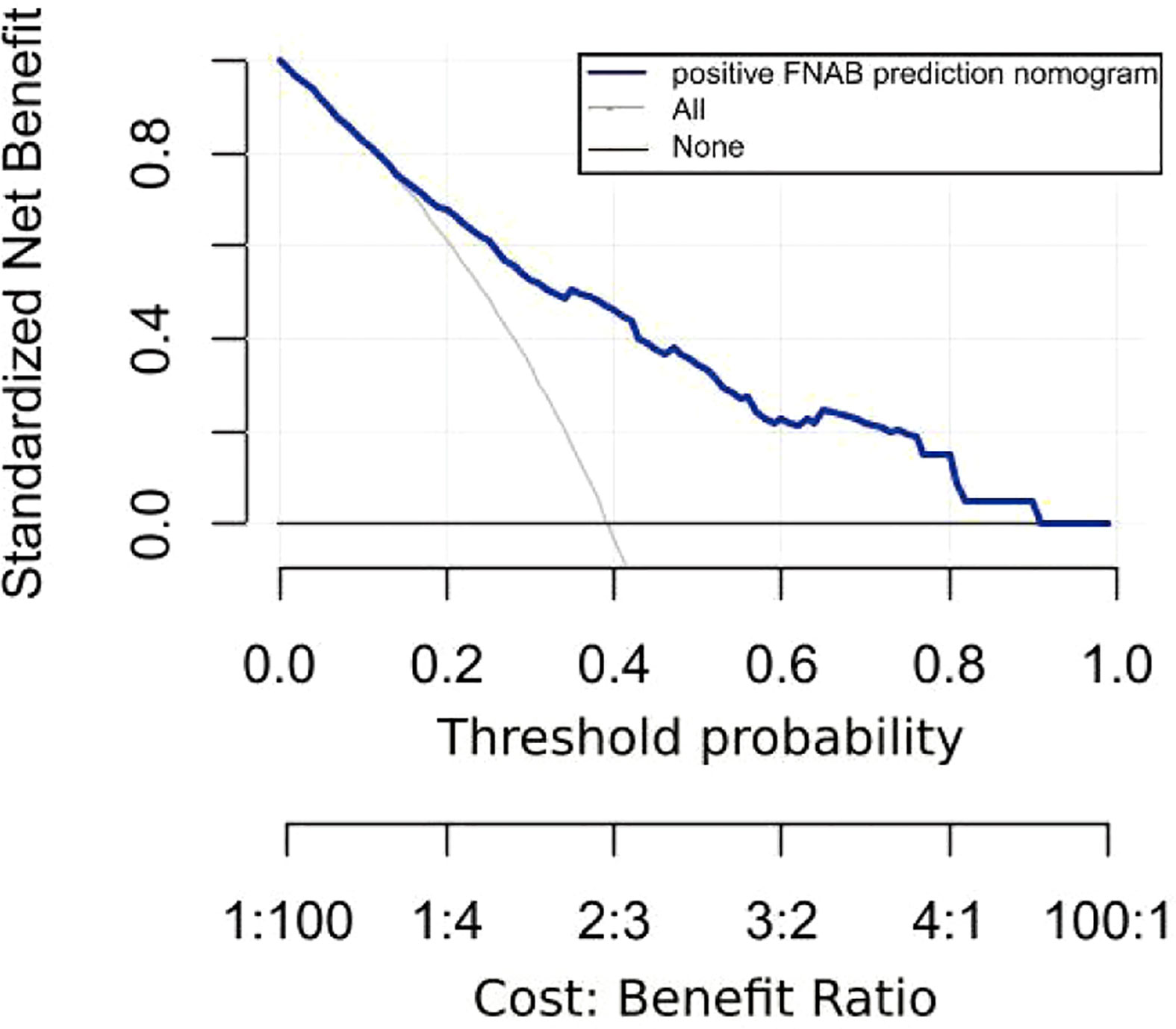
Figure 8 Decision curve analysis (DCA) for the risk nomogram. DCA shows that the model is clinically useful when intervention is decided in the threshold range of 14% to 91%.
Due to the increased use of color Doppler ultrasound, the morbidity of thyroid nodules has increased rapidly in recent decades, even up to 65% in the general population (28). In China, C-TIRADS 4 nodules are mainly considered to be further differentially diagnosed. In this study, 39.3% of the patients’ FNABs were positive, and 60.7% were negative. Similarly, a study showed that at least half of biopsied thyroid nodules are benign (29, 30), and they could be safely managed with annual follow-up (31). TI-RADS is the most effective noninvasive method for evaluating thyroid nodules, and FNAB is currently the most accurate invasive method before surgery (15, 22, 32). In fact, TI-RADS only gives a rough malignant risk (9, 10), and it is related to the ability of ultrasound doctors, which limits its application, especially in primary medical institutions. Studies have shown that ultrasound features, such as low echo, aspect ratio, irregular margins, microcalcifications, and extrathyroidal extension, are related to malignancy (31, 33). In addition, other studies have shown that thyroid function (34, 35), nodule size (15), and age (11, 12) are significantly different between malignant and benign nodules. Therefore, screening out reliable predictors from various medical information and developing a prediction model is vital for C-TIRADS 4 thyroid nodule FNAB, which can reduce at least half of invasive FNAB.
This study included 10 clinical and ultrasound characteristics (age, sex, nodule size, internal or surrounding blood flow signal, aspect ratio, solid, low echo, microcalcifications, morphology and nodule location) and 4 hematological indices (TSH, Tg, TgAb, and TPOAb) as potential predictors for positive FNAB in C-TIRADS 4 thyroid nodules. Previous studies reported microcalcifications, marked low echo, aspect ratio >1, age, sex, nodule size, thyroid function and nodule location as risk factors for positive FNAB (6, 7, 11–14, 21, 36). In this study, age, nodule size, internal or surrounding blood flow signal, aspect ratio, low echo, microcalcifications and morphology were associated with positive FNAB in C-TIRADS 4 thyroid nodules according to the univariate analysis. Additionally, they were not all confirmed as independent predictors through multivariate logistic analysis. Finally, age (≤45 years old), microcalcifications, low echo, aspect ratio (>1) and irregular morphology were confirmed as independent predictors for positive FNAB.
Previous studies have shown that age is associated with positive FNAB (11, 12), which was also confirmed in this study. Norra Kwong et al. reported that the incidence of thyroid carcinoma was 22.9% in the youngest cohort and 12.6% in the oldest cohort (11). Therefore, we believe that age ≤45 years is a risk factor for positive FNAB.
Ultrasound features have a close relationship with thyroid malignancy (37, 38), and this study showed that some ultrasound features, including low echo, aspect ratio >1, microcalcifications and irregular morphology, are independent predictors of positive FNAB.
The echogenicity level of solid nodules includes hyperechoic, isoechoic and hypoechoic. Hypoechoic refers to a thyroid nodule having lower echogenicity compared to the tissue surrounding thyroid (39). Studies have shown that most malignant thyroid nodules are hypoechoic, and the morbidity of malignancy in hypoechoic nodules is higher than that in isoechoic or hyperechoic nodules (40–44). Therefore, low echo is confirmed as an independent predictor in this study.
The aspect ratio is a vital ultrasound feature in determining thyroid nodules. An aspect ratio >1 implies a high possibility of malignancy. In clinical practice, we also find that an aspect ratio >1 takes up a large weight in determining thyroid nodules. This study also confirmed that an aspect ratio >1 is crucial to positive FNAB. In addition, we also concluded that an aspect ratio >1 takes up a large weight from the nomogram.
Microcalcifications are dense calcifications with a single diameter less than 1 mm (39). Under a microscope, thyroid microcalcifications appear as psammoma bodies and they are round, laminar crystalline calcific deposits which are 10-100 μm in size (45). Thyroid microcalcifications are among the most distinctive characteristics of thyroid cancer. Their occurrence is described in follicular and anaplastic thyroid carcinomas (46). In this study, microcalcifications were also included as an independent predictor to construct the nomogram.
Irregular morphology in thyroid nodules refers to characteristics such as irregular or ill-defined margins, where it is challenging to distinguish the boundary between the nodule and surrounding thyroid tissue. It can also include extrathyroidal extension, where the nodule spreads beyond the thyroid capsule, even invading the adjacent soft tissue and/or vascular structures) (39). When a thyroid nodule has a lobulated or irregular margin, its edge appears spiculated or jagged, with or without protrusions into the surrounding parenchyma (47). Irregular morphology is a common manifestation of malignancy. Irregular morphology was also included as an independent predictor of positive FNAB in this study.
In conclusion, we have developed and validated a novel prediction nomogram that utilizes the 5 independent predictors mentioned above. This nomogram allows for personalized prediction of positive FNAB in C-TIRADS 4 thyroid nodules, which can help clinicians in accurately diagnosing malignant thyroid nodules for optimal patient outcomes. The nomogram has been shown to have good discrimination and calibration through 2 internal validations in our cohort. According to DCA, using this nomogram to predict positive FNAB would be beneficial if the threshold probabilities ranged from 14% to 91%. The nomogram is user-friendly and easy to apply clinically for individualized risk assessment. For example, in Figure 4A, a 32-year-old patient with a low echo, aspect ratio >1, and irregular morphology had a total score of 320 points, corresponding to an estimated risk of positive FNAB of approximately 81%, indicating a relatively high risk, and FNAB was recommended. Conversely, in Figure 2B, a 40-year-old patient with irregular morphology had a total score of 132 points, corresponding to an estimated risk of positive FNAB of approximately 28%, and thus the decision of FNAB should be made more carefully. Finally, FNAB cytological examination indicated inflammatory cells and follicular epithelial cells.
There are certain limitations to this study that should be noted. First, it is a retrospective study, and selection bias is inevitable. Second, the sample size in our study was relatively small. In the future, we will accumulate more cases and detect more indicators to perform more validation and perfection. Third, the ultrasound results and C-TIRADS are largely dependent on the ultrasonic equipment and the examiners’ experience. Fourth, cytological examination also relies on the experience of pathologists. Therefore, subjective factors may lead to more or less bias.
In the study, the factors associated with positive FNAB were analyzed and a predictive model was constructed. However, there was some inconsistency between the positive results of FNAB and the final pathological results. Although the object of this study was positive biopsy, because of the high accuracy of FNAB, it can also be beneficial to the population of thyroid cancer confirmed by postoperative pathology, which can be used as the basis for future research on factors related to thyroid cancer.
In conclusion, this study has identified 5 independent predictors that are associated with positive FNAB results in C-TIRADS 4 thyroid nodules, which include: age (≤45 years old), low echo, microcalcifications, aspect ratio (>1), and irregular morphology. Additionally, a nomogram was developed based on these predictors, providing clinicians with a tool for quantifying risk assessment and predicting the status of C-TIRADS 4 thyroid nodules before FNAB. This can help to avoid unnecessary treatments and provide a new management strategy for C-TIRADS 4 thyroid nodules.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
LY and ZY were responsible for the study design and preparation of the manuscript. ZY and XG provided the study materials of patients and analyzed all data. LY was responsible for manuscript revisions. All authors have contributed to this article and have given their approval for the final version to be submitted.
This study was supported by Chongqing Natural Science Foundation (grant no. cstc2020jcyj-msxmX2011), Science and Technology Bureau of Yuzhong District, Chongqing (grant no. 20200145), Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University, and Senior Medical Talents Program of Chongqing for Young and Middle-aged (2022 [15]).
The authors state that there were no commercial or financial relationships that could potentially create conflicts of interest during the course of this research.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pemayun TGD. Current diagnosis and management of thyroid nodules. Acta Med Indones (2016) 48(3):247–57.
2. Grani G, Sponziello M, Pecce V, Ramundo V, Durante C. Contemporary thyroid nodule evaluation and management. J Clin Endocrinol Metab (2020) 105(9):dgaa322. doi: 10.1210/clinem/dgaa322
3. Hawkins SP, Jamieson SG, Coomarasamy CN, Low IC. The global epidemic of thyroid cancer overdiagnosis illustrated using 18 months of consecutive nodule biopsy correlating clinical priority, ACR-TIRADS and Bethesda scoring. J Med Imag Radiat Oncol (2021) 65(3):309–16. doi: 10.1111/1754-9485.13161
4. Han Z, Huang Y, Wang H, Chu Z. Multimodal ultrasound imaging: A method to improve the accuracy of diagnosing thyroid TI-RADS 4 nodules. J Clin Ultrasound (2022) 50(9):1345–52. doi: 10.1002/jcu.23352
5. Zhu H, Yang Y, Wu S, Chen K, Luo H, Huang J. Diagnostic performance of US-based FNAB criteria of the 2020 Chinese guideline for malignant thyroid nodules: comparison with the 2017 American College of Radiology guideline, the 2015 American Thyroid Association guideline, and the 2016 Korean Thyroid Association guideline. Quant Imag Med Surg (2021) 11(8):3604–18. doi: 10.21037/qims-20-1365
6. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A user’s guide. Radiology (2018) 287(1):29–36. doi: 10.1148/radiol.2017171240
7. Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS. Positive predictive values of sonographic features of solid thyroid nodule. Clin Imag (2010) 34(2):127–33. doi: 10.1016/j.clinimag.2008.10.034
8. Rahal A, Falsarella PM, Rocha RD, Lima JPBC, Iani MJ, Vieira FAC, et al. Correlation of Thyroid Imaging Reporting and Data System [TI-RADS] and fine needle aspiration: experience in 1,000 nodules. Einstein Sao Paulo Braz (2016) 14(2):119–23. doi: 10.1590/S1679-45082016AO3640
9. Dobruch-Sobczak K, Adamczewski Z, Dedecjus M, Lewiński A, Migda B, Ruchała M, et al. Summary of meta-analyses of studies considering lesion size cut-off thresholds for the assessment of eligibility for FNAB and sonoelastography and inter- and intra-observer agreement in estimating the malignant potential of focal lesions of the thyroid gland. J Ultrason (2022) 22(89):130–5. doi: 10.15557/JoU.2022.0021
10. Chung R, Rosenkrantz AB, Bennett GL, Dane B, Jacobs JE, Slywotzky C, et al. Interreader concordance of the TI-RADS: impact of radiologist experience. Am J Roentgenol (2020) 214(5):1152–7. doi: 10.2214/AJR.19.21913
11. Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab (2015) 100(12):4434–40. doi: 10.1210/jc.2015-3100
12. Bessey LJ, Lai NBK, Coorough NE, Chen H, Sippel RS. The incidence of thyroid cancer by fine needle aspiration varies by age and gender. J Surg Res (2013) 184(2):761–5. doi: 10.1016/j.jss.2013.03.086
13. Jinih M, Faisal F, Abdalla K, Majeed M, Achakzai AA, Heffron C, et al. Association between thyroid nodule size and malignancy rate. Ann R Coll Surg Engl (2020) 102(1):43–8. doi: 10.1308/rcsann.2019.0156
14. Al-Hakami HA, Alqahtani R, Alahmadi A, Almutairi D, Algarni M, Alandejani T. Thyroid nodule size and prediction of cancer: A study at Tertiary Care Hospital in Saudi Arabia. Cureus (2020) 12(3):e7478. doi: 10.7759/cureus.7478
15. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
16. Spencer CA. Assay of thyroid hormones and related substances. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al, editors. Endotext [Internet]. South Dartmouth (MA: MDText.com, Inc (2000). Available at: http://www.ncbi.nlm.nih.gov/books/NBK279113/.
17. Spencer C, LoPresti J, Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes (2014) 21(5):394–404. doi: 10.1097/MED.0000000000000092
18. Iervasi A, Iervasi G, Carpi A, Zucchelli GC. Serum thyroglobulin measurement: clinical background and main methodological aspects with clinical impact. BioMed Pharmacother Biomed Pharmacother (2006) 60(8):414–24. doi: 10.1016/j.biopha.2006.07.007
19. Okosieme OE, Evans C, Moss L, Parkes AB, Premawardhana LDKE, Lazarus JH. Thyroglobulin antibodies in serum of patients with differentiated thyroid cancer: relationship between epitope specificities and thyroglobulin recovery. Clin Chem (2005) 51(4):729–34. doi: 10.1373/clinchem.2004.044511
20. Giovanella L, Feldt-Rasmussen U, Verburg FA, Grebe SK, Plebani M, Clark PM. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin Chem Lab Med (2015) 53(9):1301–14. doi: 10.1515/cclm-2014-0813
21. Jasim S, Baranski TJ, Teefey SA, Middleton WD. Investigating the effect of thyroid nodule location on the risk of thyroid cancer. Thyroid (2020) 30(3):401–7. doi: 10.1089/thy.2019.0478
22. Tafti D, Schultz D. Thyroid nodule biopsy. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2022). Available at: http://www.ncbi.nlm.nih.gov/books/NBK557883/.
23. Polyzos SA, Anastasilakis AD. Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol (Oxf) (2009) 71(2):157–65. doi: 10.1111/j.1365-2265.2009.03522.x
24. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology – More than Meets the Eye. Lancet Oncol (2015) 16(4):e173–80. doi: 10.1016/S1470-2045(14)71116-7
25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods (2001) 25(4):402–8. doi: 10.1006/meth.2001.1262
26. Liu Y, He L, Yin G, Cheng L, Zeng B, Cheng J, et al. Association analysis and the clinical significance of BRAF gene mutations and ultrasound features in papillary thyroid carcinoma. Oncol Lett (2019) 18(3):2995–3002. doi: 10.3892/ol.2019.10641
27. Gao X, Luo W, He L, Cheng J, Yang L. Predictors and a prediction model for central cervical lymph node metastasis in papillary thyroid carcinoma (cN0). Front Endocrinol (2022) 12:789310. doi: 10.3389/fendo.2021.789310
28. Bernet VJ, Chindris AM. Update on the evaluation of thyroid nodules. J Nucl Med (2021) 62(Supplement 2):13S–9S. doi: 10.2967/jnumed.120.246025
29. Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “Right” TIRADS. J Clin Endocrinol Metab (2019) 104(1):95–102. doi: 10.1210/jc.2018-01674
30. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol (2012) 56(4):333–9. doi: 10.1159/000339959
31. Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: A review. JAMA (2018) 319(9):914–24. doi: 10.1001/jama.2018.0898
32. Tumino D, Grani G, Di Stefano M, Di Mauro M, Scutari M, Rago T, et al. Nodular thyroid disease in the era of precision medicine. Front Endocrinol (2020) 10:907. doi: 10.3389/fendo.2019.00907
33. Trimboli P, Castellana M, Piccardo A, Romanelli F, Grani G, Giovanella L, et al. The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta-analysis. Rev Endocr Metab Disord (2021) 22(2):453–60. doi: 10.1007/s11154-020-09592-3
34. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab (2008) 93(3):809–14. doi: 10.1210/jc.2007-2215
35. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab (2006) 91(11):4295–301. doi: 10.1210/jc.2006-0527
36. Cho YA, Kong SY, Shin A, Lee J, Lee EK, Lee YJ, et al. Biomarkers of thyroid function and autoimmunity for predicting high-risk groups of thyroid cancer: a nested case-control study. BMC Cancer (2014) 14:873. doi: 10.1186/1471-2407-14-873
37. Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol (2014) 170(5):R203–211. doi: 10.1530/EJE-13-0995
38. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab (2014) 99(4):1253–63. doi: 10.1210/jc.2013-2928
39. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS - PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/32827126/.
40. Salmaslioğlu A, Erbil Y, Dural C, Işsever H, Kapran Y, Ozarmağan S, et al. Predictive value of sonographic features in preoperative evaluation of malignant thyroid nodules in a multinodular goiter. World J Surg (2008) 32(9):1948–54. doi: 10.1007/s00268-008-9600-2
41. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology (2011) 260(3):892–9. doi: 10.1148/radiol.11110206
42. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid imaging reporting and data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid Off J Am Thyroid Assoc (2016) 26(4):562–72. doi: 10.1089/thy.2015.0460
43. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation–multicenter retrospective study. Radiology (2008) 247(3):762–70. doi: 10.1148/radiol.2473070944
44. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM Mon J Assoc Physicians (2007) 100(1):29–35. doi: 10.1093/qjmed/hcl121
45. Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US features of thyroid malignancy: pearls and pitfalls. RadioGraphics (2007) 27(3):847–60. doi: 10.1148/rg.273065038
46. Taki S, Terahata S, Yamashita R, Kinuya K, Nobata K, Kakuda K, et al. Thyroid calcifications: sonographic patterns and incidence of cancer. Clin Imaging (2004) 28(5):368–71. doi: 10.1016/S0899-7071(03)00190-6
Keywords: thyroid nodules, C-TIRADS, fine needle aspiration biopsy, predictor, prediction model 444, 95% Cl=0.296~0.666, low echo (OR=3.549)
Citation: Yang Z, Gao X and Yang L (2023) Predictors and a prediction model for positive fine needle aspiration biopsy in C-TIRADS 4 thyroid nodules. Front. Endocrinol. 14:1154984. doi: 10.3389/fendo.2023.1154984
Received: 31 January 2023; Accepted: 04 July 2023;
Published: 24 July 2023.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2023 Yang, Gao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Yang, MzAyMTE4QGNxbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.