
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol., 20 March 2023
Sec. Translational and Clinical Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1150360
This article is part of the Research TopicImmune Cells in Non-Alcoholic Fatty Liver Disease and Alcoholic Liver DiseaseView all 7 articles
The progression of non-alcoholic fatty liver disease (NAFLD), the most common liver disease, leads to non-alcoholic steatohepatitis and hepatocellular carcinoma. Despite the increasing incidence and prevalence of NAFLD, its therapeutic and preventive strategies to lower the disease burden is limited. In recent years, immunotherapy, including anti-programmed cell death 1/programmed cell death 1 ligand 1 treatment, has emerged as a potential approach to reach satisfactory modulation for the progression of NAFLD and treatment of NAFLD-related hepatocellular carcinoma. However, the effectiveness of immunotherapy against NAFLD and NAFLD-related hepatocellular carcinoma is in the early phase and it is yet not advanced. In addition, conflicting results are being reported regarding the prognosis of patients with NAFLD-related hepatocellular carcinoma and high expression of programmed cell death 1/programmed cell death 1 ligand 1. Herein, this review will discuss and elucidate the attempts and underlying mechanisms of immunotherapy against NAFLD and NAFLD-related hepatocellular carcinoma.
Non-alcoholic fatty liver disease (NAFLD) has reached a global prevalence of 30% and the increasing trend is ongoing (1). It involves a spectrum of diseases, including hepatic steatosis and non-alcoholic steatohepatitis (NASH) (2). NAFLD may lead to life-threatening hepatic diseases, such as liver cirrhosis and hepatocellular carcinoma (HCC), and extrahepatic diseases, such as cardiovascular disease and dementia (3–6). Along with advances in retrospective operational definitions, such as fatty liver index and Korea National Health and Nutrition Examination Survey-NAFLD score, and non-invasive diagnostic approaches for NAFLD, prevention, management, and treatment of NAFLD are being actively investigated (7–9).
In recent years, NAFLD-related HCC (NAFLD-HCC) has emerged as a major factor contributing to the disease’s burden (10). In addition, NAFLD-HCC in non-cirrhotic patients is increasingly identified (11). The major risk factors of NAFLD-HCC include liver cirrhosis, old age, male sex, patatin-like phospholipase domain-containing 3 variants, diabetes, and obesity (12). Epigenetic factors, including transcriptional factors and post-transcriptional modifications, individual-level characteristics, and environmental factors have been reported to be associated with the development and progression of NAFLD-HCC (13).
Up to date, the identified potential therapeutic options for NAFLD include herbal medicine, a low-calorie diet, physical activity, polyphenol, bile acid, anti-inflammatory agents, hormones, and pre and probiotics, as confirmed in a clinical trial (14–19). However, recent findings are suggesting that immunotherapy is promising against NAFLD, NASH, or NAFLD-HCC. In this study, we review the recent findings regarding the effects of immunotherapy, especially programmed cell death 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) treatment against NAFLD, NASH, or NAFLD-HCC.
Considering the close relationship between diabetes and NAFLD, a number of antidiabetic medications were testified as a therapeutic approach for NASH in an experimental setting (10). Glucagon-like peptide 1 (GLP1) is a hormone that stimulates the release of insulin, indirectly suppresses the secretion of glucagon, and lowers food intake (20). Currently, a number of G protein-coupled GLP1 receptor (GLP1R) agonists, including dulaglutide, exenatide, liraglutide, and semaglutide, are approved as therapeutics for diabetes (20). GLP1R agonist ameliorated liver damage and hepatic steatosis in diet-induced NASH mice (21). In addition, treatments with exenatide, liraglutide, and semaglutide have indicated their efficacy in the reduction of hepatic lipid contents and the level of liver enzymes (22–24). Since the expression level of GLP1R is not high, its effect may be due to systemic modification in metabolism rather than direct amelioration of the liver (25). Dipeptidyl peptidase 4 (DPP4), which inactivates GLP1, inhibitors have also been reported to reduce liver fibrosis and the development of liver tumors in the mouse NASH model (26). However, sitagliptin was found not effective in improving NAFLD activity score or fibrosis score, suggesting that DPP4 inhibitors may not be promising in the treatment of NAFLD and NASH (27). In addition, a murine liver cancer model study has identified that liraglutide has the potential to promote the anti-tumor effects of PD-1 inhibition through the reduction of neutrophil extracellular traps in liver cancer (27).
Thiazolidinedione, including rosiglitazone and pioglitazone, has been suggested to modify the sensitivity of peripheral insulin through stimulation of the adipokine release, enhancing the inhibitory effects of insulin on lipolysis and leading to a reduced plasma level of free fatty acids, as well as lowered accretion of hepatic lipids (28). A previous study also reported that thiazolidinedione reduces the activation of hepatic stellate cells and liver fibrosis in rats (29). Rosiglitazone was previously reported to be effective in enhancing insulin sensitivity and reducing hepatic steatosis, but there were cases of weight gain and edema in the lower extremity (30, 31). In addition, the efficacy of sodium-glucose cotransporter 2 (SGLT2) has been testified in NAFLD, and found that dapagliflozin and empagliflozin treatment modifies liver fat and liver enzyme levels (32, 33). Apart from antidiabetic treatment, nuclear receptor modulators, de novo lipogenesis inhibitors, and fibroblast growth factors were also suggested as potential therapeutic approaches (10).
Recently, obeticholic acid, an agonist of farnesoid X receptor (FXR) has emerged a potential therapeutic approach for adult NASH patients with at least 4 NAFLD activity score and fibrosis stages of F2 to F3 or F1 with comorbidity (34). The improvement in fibrosis was significantly detected in the obeticholic acid 25 mg group (n=71; 23%) compared to the placebo group (n=37; 12%) in a phase 3 trial. In addition, the efficacy and safety of another FXR agonist, tropifexor, has more recently been tested for NASH in a phase 2 trial (35). Dose-related pruritus was detected, and decreases in hepatic fat fraction and alanine aminotransferase were sustained until 48th week. Therefore, FXR agonists may be the closest treatment option against NAFLD and NASH.
The immune system is expected to play an important role in the development, modulation, and progression of NAFLD. To date, potential targets to prevent the progression of NAFLD have been selected, including molecules that express on the immune system cell surface, and the PD-1/PD-L1 complex is receiving attention (36). Within the liver, PD-1, which is a membrane protein that is exposed by all T cells, responds against lymphocyte activation through PD-L1, of which its upregulation is supported by interferon-γ (37).
Cenicriviroc first showed its targeting ability for proinflammatory monocytes through the dual chemokine 2 and chemokine 5 receptor antagonists in a murine model, then it was found to improve liver fibrosis in patients with biopsy-proven NAFLD in a phase 2b clinical trial (38). However, the subsequent phase 3 clinical trial could not reach the primary endpoint, which is an improvement of liver fibrosis in a condition of not worsening NASH. Another phase 2b trial is evaluating the efficacy of cenicriviroc with Tropifexor, which is a farnesoid X receptor agonist that is involved in lipid metabolism and bile acid synthesis, for NASH (NCT03517540) (39). However, the study results are yet unpublished in the literature.
Peroxisome proliferator-activated receptor (PPAR) agonists-mediated and exerted anti-inflammatory effects via targeting macrophages were also tested in trials (40). The activation of PPARs regulates lipid metabolism and inflammation via the modulation of macrophage in tissues, including the liver. However, elafibranor, which is a PPAR agonist, revealed no significant benefits for liver histology in the phase 3 trial (40). Another phase 2 clinical trial evaluated the efficacy of a murine monoclonal antibody targeting T cell receptor-associated CD3 (41). In addition, a human anti-CD3 antibody (foralumab) was developed and tested in a phase 2 clinical trial (NCT03291249) for patients with NASH and diabetes. However, this trial was withdrawn due to a request from the Ministry of Health.
The upregulation of PD-1, which is a membrane protein that is exposed by all T cells, is modulated by interferon-γ, and the inhibition of PD-1/PD-L1 T cells is modulated by interfering with the T-cell receptor/CD28 signal, leading to reduced production of pro-inflammatory cytokines (36). PD-1 was upregulated after the interleukin-15 treatment-medicated downregulation of forkhead box protein O1 in mice CD8+ T cells, and the level of interleukin-15 was found to be associated with high C-X-C chemokine receptor type 6+PD-1-high CD8+ T cells and low expression of forkhead box protein O1 (36, 42). In addition, PD-1/PD-L1 may express in dendritic cells, which are exposed to intestinal pathogen-associated molecular patterns to decelerate immune responses, to prevent inflammation (43). However, despite a number of attempts and efforts in controlling the progression and pathogenesis of NAFLD by modulating the immune system, no conclusive evidence has been provided.
PD-1 interferes with protective immune responses and contributes to the expansion of malignant cells (44). PD-L1, which can prevent the proliferation of tumor-specific T cells via suppressive signals, leading to impaired anti-tumor immunity, is commonly expressed in malignant cells (Figure 1) (45). Polymorphisms in PD-1 have been found associated with a higher risk of cancers (44). The rs10204525 and rs7421861 variants boosted the expression of PD-1 and were found associated with a higher risk of esophageal cancer in the Asian population (46). A multi-European cohort of 391 NAFLD-HCC patients found that the PD-1 rs7421861 variant is associated with NAFLD-HCC in the United Kingdom population only, suggesting that there may be a difference in PD-1-related risk of NAFLD-HCC according to ethnicity (47). In an Egyptian cohort of 134 NASH and NASH-related HCC patients, the PD-L1 rs2282055 variant was associated with the risk of cancer (48). In another study of 167 patients with HCC, the level of PD-L1 was increased within the liver and was positively correlated with interferon-γ (49). Notably, patients with a higher expression of PD-L1 and CD8+ had better survival compared to those with a lower expression of PD-L1 and CD8+, indicating that PD-L1 and CD8+ cytotoxic T cells may promote the eradication of HCC. Another study also confirmed that a lower expression of PD-L1 and CD8+ tumor-infiltrating lymphocytes predicted poor HCC-specific survival in patients after liver resection (50). However, there were a few studies that demonstrated a higher expression of PD-L1 as an unfavorable factor for the prognosis of patients with HCC, including the recurrence of HCC after resection (51, 52).
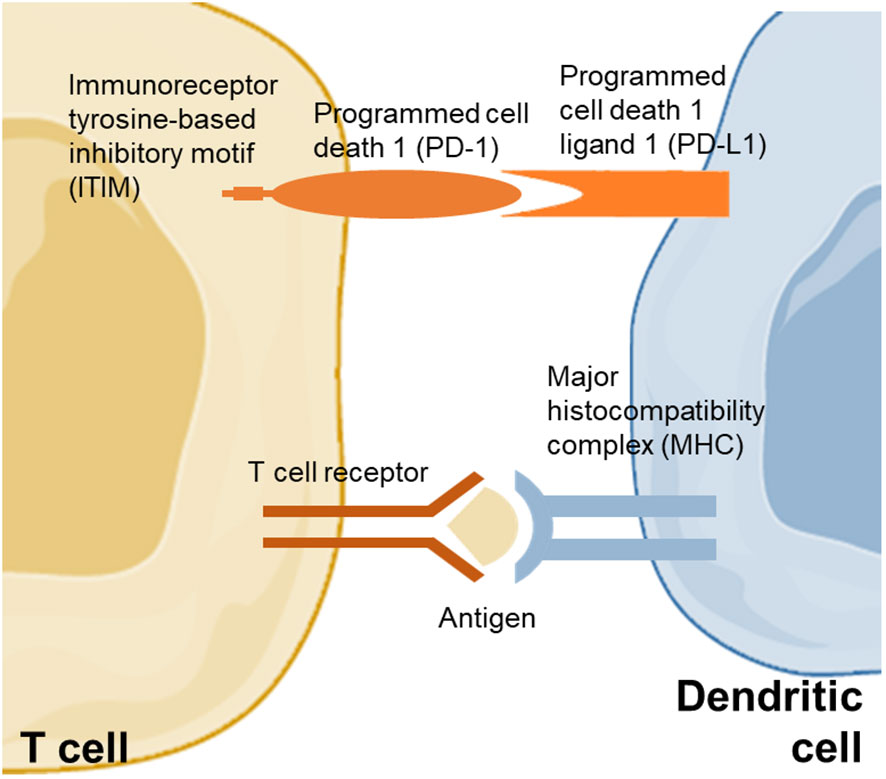
Figure 1 PD-1 and PD-L1 between dendritic cell and T cell. PD-1 pathway activation is modulated by the binding of Src homology domain-containing protein tyrosine phosphatase 1 and 2 to immunoreceptor tyrosine-based inhibitory motif that repress the proliferation of T cell. The pathway modulates inhibitory signals for the activation of T cell.
To date, a number of pharmaceutical agents that produces monoclonal PD-1 receptor and prevents the escape of tumor cells via blockage of the PD-1 system are developed, including pembrolizumab and nivolumab (Table 1) (53–59). In the phase 2 clinical trial of pembrolizumab, the effectiveness and tolerance were satisfactory, suggesting that pembrolizumab may be a treatment option for patients with advanced HCC after sorafenib treatment (53). In addition, Feun et al. (54) also showed similar results and further supported that the toxicity was tolerable and reversible. In the phase 3 clinical trial of pembrolizumab, statistically significant improvement in overall survival and progression-free survival was identified but the statistical significance was insufficient for the pre-specified criteria (55). However, the phase 3 clinical trial of pembrolizumab in Asian patients demonstrated that pembrolizumab significantly improved overall survival (hazard ratio [HR], 0.79; 95% confidence interval [CI], 0.63-0.99; P=0.018), progression-free survival (HR, 0.74; 95% CI, 0.60-0.92; P=0.003), and objective response rate (P<0.001). As for nivolumab versus sorafenib, nivolumab had a higher median overall survival (HR, 0.85; 95% CI, 0.72-1.02; P=0.075) but the predefined significance level of P=0.0419 was not achieved (57).
In addition, atezolizumab, which selectively targets PD-L1 and reserves T-cell suppression, plus bevacizumab, which targets vascular endothelial growth factor and inhibits angiogenesis, was compared with sorafenib in the treatment of unresectable HCC (58). The risk of death was significantly lower in the atezolizumab plus bevacizumab group compared to sorafenib group (HR, 0.58; 95% CI, 0.42-0.79; P<0.001). Furthermore, Abou-Alfa et al. (59) testified tremelimumab (cytotoxic T lymphocyte–associated antigen 4 inhibitor) plus durvalumab (anti–PD-L1) or durvalumab monotherapy versus sorafenib in patients with unresectable HCC, which indicated significant improvement in overall survival after tremelimumab plus durvalumab compared to sorafenib and non-inferiority of durvalumab monotherapy was identified compared to sorafenib.
The immune system and related pathways play crucial roles in the progression and pathogenesis of NAFLD. Despite recent accumulating attention in immunotherapy against NAFLD, the literature remains insufficient to make a conclusive estimation on whether immunotherapy, especially targeting PD-1/PD-L1, may prevent NASH or HCC in patients with HCC. In addition, promising results are being reported and new pharmaceutical immunotherapy is being developed and tested in clinical trials. However, most of these clinical trials testified the efficacy of immunotherapy in patients with unresectable or advanced HCC, thus the results may not represent therapeutic effectiveness for all NAFLD-HCC cases. In addition, since non-viral HCC, especially NASH-HCC, may be less responsive to immunotherapy potentially due to the activation of NASH-related aberrant T cell that causes tissue damage and subsequent impaired immune surveillance, immunotherapy resistance may be one of the most critical challenges (60). Understanding the underlying mechanisms of the resistance in immunotherapy against NAFLD, NASH, or NAFLD-HCC may provide better outcomes after immunotherapy in the future (61).
SJ, W-YS, and YO contributed to the conception and design of the study. All authors contributed to the article and approved the submitted version.
This research was funded by the Korean Academy of Menopause and Andropause (2023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Younossi Z, Golabi P, Paik J, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Hepatology (2023) 10.1097/HEP.0000000000000004. doi: 10.1097/HEP.0000000000000004
2. Lee KC, Wu PS, Lin HC. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin Mol Hepatol (2023) 29(1):77–98. doi: 10.3350/cmh.2022.0237
3. Chen JYS, Chua D, Lim CO, Ho WX, Tan NS. Lessons on drug development: A literature review of challenges faced in nonalcoholic fatty liver disease (NAFLD) clinical trials. Int J Mol Sci (2022) 24(1):158. doi: 10.3390/ijms24010158
4. Albhaisi S, McClish D, Kang L, Gal T, Sanyal AJ. Nonalcoholic fatty liver disease is specifically related to the risk of hepatocellular cancer but not extrahepatic malignancies. Front Endocrinol (Lausanne) (2022) 13:1037211. doi: 10.3389/fendo.2022.1037211
5. Jeong S, Oh YH, Choi S, Chang J, Kim SM, Son JS, et al. Metabolic dysfunction-associated fatty liver disease better predicts incident cardiovascular disease. Gut Liver (2022) 16(4):589–98. doi: 10.5009/gnl210256
6. Jeong S, Oh YH, Choi S, Chang J, Kim SM, Son JS, et al. Association of non-alcoholic fatty liver disease with incident dementia later in life among elder adults. Clin Mol Hepatol (2022) 28(3):510–21. doi: 10.3350/cmh.2021.0332
7. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol (2006) 6:33. doi: 10.1186/1471-230X-6-33
8. Jeong S, Kim K, Chang J, Choi S, Kim SM, Son JS, et al. Development of a simple nonalcoholic fatty liver disease scoring system indicative of metabolic risks and insulin resistance. Ann Transl Med (2020) 8(21):1414. doi: 10.21037/atm-20-2951
9. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
10. Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol (2021) 17(8):484–95. doi: 10.1038/s41574-021-00507-z
11. Singal AG, El-Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol (2022) 76(1):195–201. doi: 10.1016/j.jhep.2021.08.028
12. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2021) 18(4):223–38. doi: 10.1038/s41575-020-00381-6
13. Zhang C, Yang M. The emerging factors and treatment options for NAFLD-related hepatocellular carcinoma. Cancers (Basel) (2021) 13(15):3740. doi: 10.3390/cancers13153740
14. Kazemi S, Shidfar F, Ehsani S, Adibi P, Janani L, Eslami O. The effects of sumac (Rhus coriaria l.) powder supplementation in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement Ther Clin Pract (2020) 41:101259. doi: 10.1016/j.ctcp.2020.101259
15. Mirhafez SR, Azimi-Nezhad M, Dehabeh M, Hariri M, Naderan RD, Movahedi A, et al. The effect of curcumin phytosome on the treatment of patients with non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled trial. Adv Exp Med Biol (2021) 1308:25–35. doi: 10.1007/978-3-030-64872-5_3
16. Nadinskaia M, Maevskaya M, Ivashkin V, Kodzoeva K, Pirogova I, Chesnokov E, et al. Ursodeoxycholic acid as a means of preventing atherosclerosis, steatosis and liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol (2021) 27(10):959–75. doi: 10.3748/wjg.v27.i10.959
17. Soleimani D, Rezaie M, Rajabzadeh F, Gholizadeh Navashenaq J, Abbaspour M, Miryan M, et al. Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: A randomized clinical trial. Phytother Res (2021) 35(3):1669–79. doi: 10.1002/ptr.6937
18. Bahrami M, Cheraghpour M, Jafarirad S, Alavinejad P, Asadi F, Hekmatdoost A, et al. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: a randomized double blind clinical trial. Complement Ther Med (2020) 52:102452. doi: 10.1016/j.ctim.2020.102452
19. Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology (2020) 158(6):1597–1610.e7. doi: 10.1053/j.gastro.2020.01.031
20. Gimeno RE, Briere DA, Seeley RJ. Leveraging the gut to treat metabolic disease. Cell Metab (2020) 31(4):679–98. doi: 10.1016/j.cmet.2020.02.014
21. Tølbøl KS, Kristiansen MN, Hansen HH, Veidal SS, Rigbolt KT, Gillum MP, et al. Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J Gastroenterol (2018) 24(2):179–94. doi: 10.3748/wjg.v24.i2.179
22. Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab (2016) 18(9):882–91. doi: 10.1111/dom.12680
23. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet (2016) 387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X
24. Newsome P, Francque S, Harrison S, Ratziu V, Van Gaal L, Calanna S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther (2019) 50(2):193–203. doi: 10.1111/apt.15316
25. Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab (2019) 30:72–130. doi: 10.1016/j.molmet.2019.09.010
26. Kawakubo M, Tanaka M, Ochi K, Watanabe A, Saka-Tanaka M, Kanamori Y, et al. Dipeptidyl peptidase-4 inhibition prevents nonalcoholic steatohepatitis-associated liver fibrosis and tumor development in mice independently of its anti-diabetic effects. Sci Rep (2020) 10(1):983. doi: 10.1038/s41598-020-57935-6
27. Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S, et al. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J Gastroenterol (2017) 23(1):141–50. doi: 10.3748/wjg.v23.i1.141
28. Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes (2002) 51(3):797–802. doi: 10.2337/diabetes.51.3.797
29. Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology (2002) 122(7):1924–40. doi: 10.1053/gast.2002.33666
30. Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology (2008) 135(1):100–10. doi: 10.1053/j.gastro.2008.03.078
31. Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology (2003) 38(4):1008–17. doi: 10.1053/jhep.2003.50420
32. Latva-Rasku A, Honka MJ, Kullberg J, Mononen N, Lehtimäki T, Saltevo J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: A randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care (2019) 42(5):931–7. doi: 10.2337/dc18-1569
33. Kahl S, Gancheva S, Straßburger K, Herder C, Machann J, Katsuyama H, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: A randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care (2020) 43(2):298–305. doi: 10.2337/dc19-0641
34. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (2019) 394(10215):2184–96. doi: 10.1016/S0140-6736(19)33041-7
35. Sanyal AJ, Lopez P, Lawitz EJ, Lucas KJ, Loeffler J, Kim W, et al. Tropifexor for nonalcoholic steatohepatitis: An adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med (2023) 29(2):392–400. doi: 10.1038/s41591-022-02200-8
36. Lombardi R, Piciotti R, Dongiovanni P, Meroni M, Fargion S, Fracanzani AL. PD-1/PD-L1 immuno-mediated therapy in NAFLD: Advantages and obstacles in the treatment of advanced disease. Int J Mol Sci (2022) 23(5):2707. doi: 10.3390/ijms23052707
37. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol (2018) 18(3):153–67. doi: 10.1038/nri.2017.108
38. Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: Final analysis of the phase 2b CENTAUR study. Hepatology (2020) 72(3):892–905. doi: 10.1002/hep.31108
39. Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp Clin Trials (2020) 88:105889. doi: 10.1016/j.cct.2019.105889
40. Francque S, Szabo G, Abdelmalek MF, Byrne CD, Cusi K, Dufour JF, et al. Nonalcoholic steatohepatitis: The role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol (2021) 18(1):24–39. doi: 10.1038/s41575-020-00366-5
41. Lalazar G, Mizrahi M, Turgeman I, Adar T, Ben Ya'acov A, Shabat Y, et al. Oral administration of OKT3 MAb to patients with NASH, promotes regulatory T-cell induction, and alleviates insulin resistance: Results of a phase IIa blinded placebo-controlled trial. J Clin Immunol (2015) 35(4):399–407. doi: 10.1007/s10875-015-0160-6
42. Leonard WJ, Lin JX, O'Shea JJ. The γc family of cytokines: Basic biology to therapeutic ramifications. Immunity (2019) 50(4):832–50. doi: 10.1016/j.immuni.2019.03.028
43. Sumpter TL, Thomson AW. The STATus of PD-L1 (B7-H1) on tolerogenic APCs. Eur J Immunol (2011) 41(2):286–90. doi: 10.1002/eji.201041353
44. Salmaninejad A, Khoramshahi V, Azani A, Soltaninejad E, Aslani S, Zamani MR, et al. PD-1 and cancer: Molecular mechanisms and polymorphisms. Immunogenetics (2018) 70(2):73–86. doi: 10.1007/s00251-017-1015-5
45. Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev (2013) 12(11):1091–100. doi: 10.1016/j.autrev.2013.05.003
46. Chihab H, Jadid FZ, Foka P, Zaidane I, El Fihry R, Georgopoulou U, et al. Programmed cell death-1 3'-untranslated region polymorphism is associated with spontaneous clearance of hepatitis b virus infection. J Med Virol (2018) 90(11):1730–8. doi: 10.1002/jmv.25265
47. Eldafashi N, Darlay R, Shukla R, McCain MV, Watson R, Liu YL, et al. A PDCD1 role in the genetic predisposition to NAFLD-HCC? Cancers (Basel) (2021) 13(6):1412. doi: 10.3390/cancers13061412
48. El-Derany MO. Polymorphisms in interleukin 13 signaling and interacting genes predict advanced fibrosis and hepatocellular carcinoma development in non-alcoholic steatohepatitis. Biol (Basel) (2020) 9(4):75. doi: 10.3390/biology9040075
49. Xie QK, Zhao YJ, Pan T, Lyu N, Mu LW, Li SL, et al. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology (2016) 5(7):e1181252. doi: 10.1080/2162402X.2016.1181252
50. Sideras K, Biermann K, Verheij J, Takkenberg BR, Mancham S, Hansen BE, et al. PD-L1, galectin-9 and CD8+ tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology (2017) 6(2):e1273309. doi: 10.1080/2162402X.2016.1273309
51. Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res (2009) 15(3):971–9. doi: 10.1158/1078-0432.CCR-08-1608
52. Chen L, Huang X, Zhang W, Liu Y, Chen B, Xiang Y, et al. Correlation of PD-L1 and SOCS3 Co-expression with the prognosis of hepatocellular carcinoma patients. J Cancer (2020) 11(18):5440–8. doi: 10.7150/jca.46158
53. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial [published correction appears in lancet oncol. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6
54. Feun LG, Li YY, Wu C, Wangpaichitr M, Jones PD, Richman SP, et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer (2019) 125(20):3603–14. doi: 10.1002/cncr.32339
55. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307
56. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from Asia with advanced hepatocellular carcinoma: A randomized, double-blind, phase III trial. J Clin Oncol (2023) 41(7):1434–43. doi: 10.1200/JCO.22.00620
57. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5
58. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
59. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid (2022) 1(8). doi: 10.1056/EVIDoa2100070
60. Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature (2021) 592(7854):450–6. doi: 10.1038/s41586-021-03362-0
Keywords: non-alcoholic fatty liver disease, immune system, immunity, liver cancer, chronic liver disease
Citation: Jeong S, Shin W-Y and Oh YH (2023) Immunotherapy for NAFLD and NAFLD-related hepatocellular carcinoma. Front. Endocrinol. 14:1150360. doi: 10.3389/fendo.2023.1150360
Received: 24 January 2023; Accepted: 06 March 2023;
Published: 20 March 2023.
Edited by:
Dongwei Xu, Shanghai Jiao Tong University, ChinaReviewed by:
Bibo Ke, University of California, Los Angeles, United StatesCopyright © 2023 Jeong, Shin and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Hwan Oh, c3dpbWF5b0BjYXVocy5vci5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.