
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 03 April 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1150036
This article is part of the Research Topic The Association of Other Autoimmune Diseases in Patients with Thyroid Autoimmunity: Volume II View all 20 articles
Iodine is a crucial trace element for the human body and the basic raw material for the synthesis of thyroid hormones. Oral inorganic iodine includes dietary iodine and therapeutic iodine, both of which are closely associated with thyroid immunity and metabolism. Graves’ disease (GD), also known as diffuse toxic goiter, is characterized by hyperthyroidism and high iodine metabolism. Clinically, patients diagnosed with GD are often asked to limit iodine intake or even avoid iodine in their diet. The latest research has demonstrated that the interference of dietary iodine with antithyroid drugs (ATDs) treatment may be overestimated. In addition, as a medication for GD treatment, the administration of inorganic iodine has shown positive results in patients with mild hyperthyroidism, a low thyroid autoantibody concentration, a small thyroid volume, a high iodine diet and so on. Inorganic iodine may also be used as an alternative when patients experience side effects with traditional ATDs and for those who still prefer conservative treatment. Due to its low teratogenicity, blood toxicity and bone marrow toxicity, inorganic iodine plays a unique role in special populations, such as pregnant or lactating patients and patients receiving tumor radiotherapy or chemotherapy. In this review, the research progress, biological function, doses and effects, applicable populations and specific applications of dietary iodine and therapeutic iodine are summarized to provide references for the diagnosis and treatment of GD, thus improving the quality of life of GD patients.
The thyroid gland is an organ characterized by iodine enrichment, and the rate of iodine uptake is significantly increased in patients with Graves’ disease (GD). The role and importance of inorganic iodine in the pathogenesis, treatment and prevention of GD has always been an issue of concern (1). In daily life, the iodine needed to maintain the physiological state is realized through the absorption of iodine from consumed food and iodized salt. The recommended intake of iodine by the World Health Organization is 150 mcg per day for adults over 12 years of age and 250 mcg per day for pregnant and lactating women; the median urinary iodine concentration (UIC) should be controlled at 100-200 mcg per day to ensure that the population has an appropriate iodine nutritional status and is free from iodine deficiency or excess (2). In clinical practice, the oral administration of inorganic iodine is also used as a treatment for GD. Inorganic iodine drugs commonly used in the clinic include potassium iodide and sodium iodide. In China, Lugol’s solution is also approved for clinical use by the China Food and Drug Administration. Lugol’s solution is a composite iodine solution consisting of elemental iodine (5%), potassium iodide (10%) and distilled water. Since the solution tastes bitter and is irritating to the gastrointestinal tract, it is often recommended to fully dilute it with a sweet drink (3). In the clinic, inorganic iodine is generally used as an adjuvant in antithyroid drugs (ATDs); however, the effect of inorganic iodine alone on GD remains to be evaluated (4). In ATDs treatment, the oral administration of inorganic iodine may play an important role in the appearance of adverse reactions such as liver dysfunction, neutropenia and rash, yet patients may not meet the indications for surgery or radioiodine (RAI) therapy. In particular, the prospects for administration for women in the gestational and lactational periods, infants and young children, tumor patients and other special populations should be considered (5, 6). High-dose iodide results in the temporary suppression of thyroid hormone release and the escape of suppressed thyroid function, designated the Plummer effect and Wolff-Chaikoff effect, respectively (7, 8).Therefore, inorganic iodine is often administered in specific situations, such as in the preoperative stage and during thyroid storm (9), to stabilize patients’ metabolism and vital signs and to help subsequent treatment go smoothly. The applications and underlying related mechanisms remain to be further investigated and summarized to clarify the value and safety of inorganic iodine administration in patients with GD.
The key molecular structure of iodine uptake in the thyroid gland is the sodium iodide symporter (NIS), which is located in the external basement membranes of thyroid follicular cells. The NIS couples the “uphill” inward transport of I− against its electrochemical gradient under the help of the “downhill” inward translocation of Na+ down its electrochemical gradient generated by the Na+/K+ ATPase (10). Thyroid peroxidase (TPO) utilizes hydrogen peroxide, which is mostly produced under the action of dual oxidase 2 (DUOX2) under physiological conditions, to combine iodine with the tyrosine residues of thyroglobulin molecules to form monoiodotyrosine and diiodotyrosine. Monoiodotyrosine and diiodotyrosine are coupled with TPO to form thyroid hormones, consisting of triiodothyronine (T3) and thyroxine (T4), most of which are transported to the thyroid colloid for storage by the anion exchanger pendrin (PDS) located at the apical membrane of follicular cells. Thyroid hormones are endocytosed through the thyroid colloid into the cytoplasm of thyroid follicular cells and transported to the blood through monocarboxylate transporter 8 (MCT8) when needed (11). An intuitive view of the mechanism of normal/high iodine levels in the thyroid gland is shown in Figure 1. It has been reported that the immune-related pathway enrichment map of nuclear erythroid factor 2 (Nrf2) knockout mice after exposure to iodide is 65.75%, consistent with that of GD mouse models. As a transcription factor, Nrf2 attenuates the inflammatory-autoimmune-fibrotic response activated by excessive iodide, which may be related to the pathological mechanism of GD (12). Recently, Eleftheriadou et al. demonstrated a higher positive rate of NIS autoantibodies in patients with thyroid disease than in healthy controls (7.7% vs. 1.8%) and an even higher positive rate in patients with GD (12.3%) (13). Supplementation with sodium perchlorate reduces the absorption of iodide by reducing the NIS transport rate of thyroid follicular cells in a concentration-dependent manner.
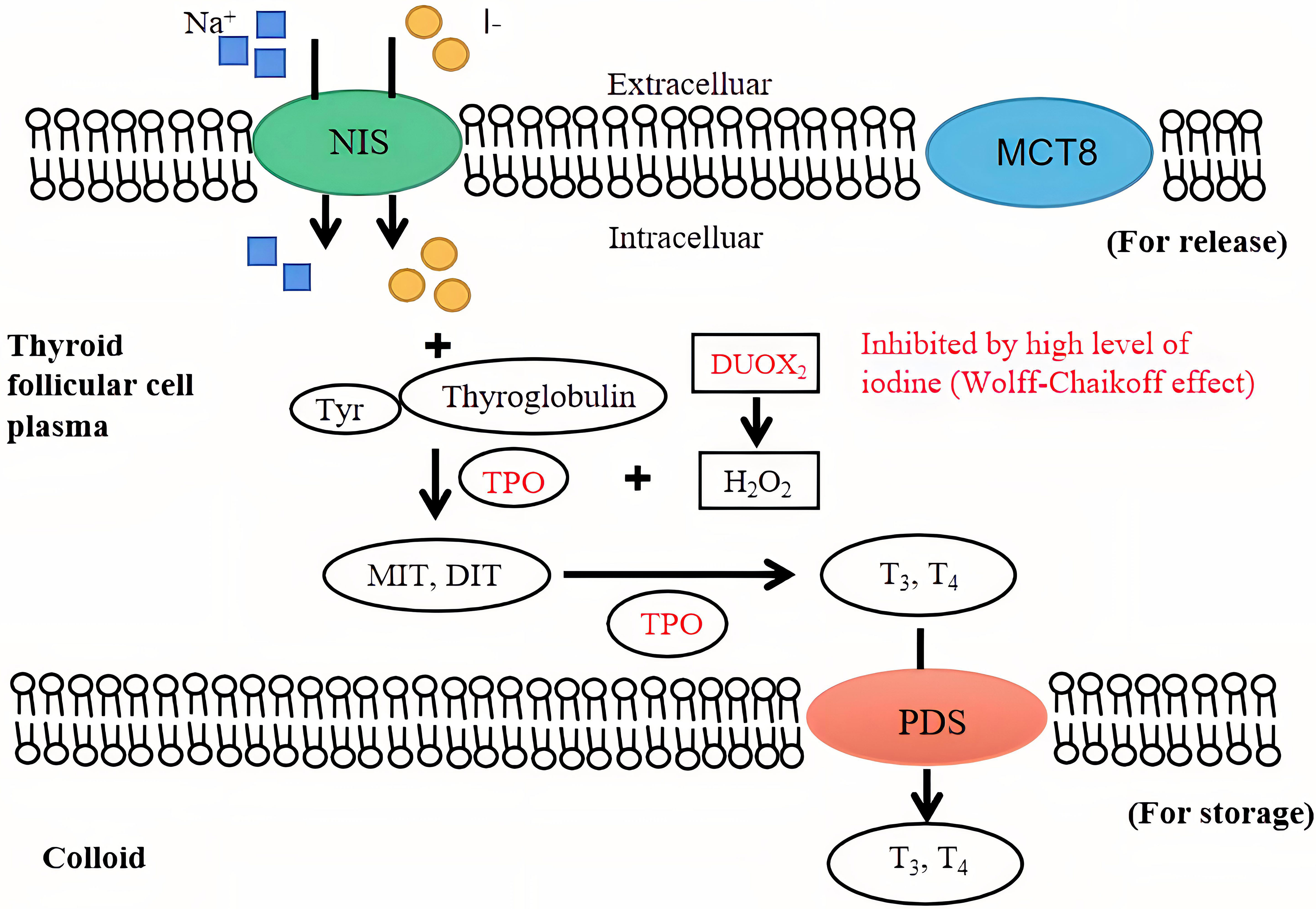
Figure 1 The mechanism of iodine in the thyroid gland. An intuitive view of the mechanism of normal and high iodine levels on the thyroid gland and thyroid hormone synthesis. The inhibition of high iodine levels on thyroid hormone synthesis (Wolff-Chaikoff effect) is shown in red. NIS, sodium iodide symporter; TPO, thyroid peroxidase; MIT, monoiodo-tyrosine; DIT, diiodo-tyrosine; T3, triiodothyronine; T4, thyroxine; PDS, anion exchanger pendrin; MCT8, monocarboxylate transporter 8.
According to the latest global dietary iodine nutrition assessment released by the World Health Organization in 2020, the number of countries with sufficient iodine intake nearly doubled from 67 in 2003 to 118 in 2020 under the benefit of the implementation of the global salt iodization programme (14). A number of epidemiological surveys have shown that the prevalence of GD is higher in iodine-deficient areas (15). Wang et al. found that the proportion of GD in individuals with predominant and subclinical hyperthyroidism was different in areas with various iodine nutritional statuses in China and that the prevalence of GD reached the highest in the group with UICs less than 50 mcg/L. Multivariate regression analysis indicated that iodine deficiency was related to the prevalence of GD (OR = 1.67, 95% CI 1.30-2.15) (16). Subsequently, the gradual introduction of iodine replacement therapy to individuals in iodine-deficient areas greatly reduced the incidence of GD. For example, the risk of hyperthyroidism was halved in the Danish population through salt iodization (17). Li et al. conducted two repeated surveys on iodine intake and thyroid status in the Chinese population in 2009 and 2015. Comparing the survey results from 2009 and 2015, the prevalence rates of dominant hyperthyroidism, subclinical hyperthyroidism and GD were 0.7% and 0.5% (P<0.05), 0.5% and 0.3% (P<0.05), and 0.5% and 0.3% (P<0.01), respectively, leading to a decrease in the prevalence of hyperthyroidism and GD in the overall population (18). Notably, a compulsory universal salt iodization (CUSI)program was carried out in 1996 in China. Afterwards, the average iodine intake value in the Chinese population was excessive from 1996 to 2001, high-normal from 2002 to 2011, and intermediate from 2012 to 2016 (19). With these changes in iodine status, the prevalence of hyperthyroidism also changed. In the fourth year after the implementation of the CUSI program, the prevalence of GD was 1.25% (20), which decreased to 0.61% in the ninth year (21). At present, the CUSI program has been implemented for more than 20 years. The latest research shows that the current prevalence of GD in China is 0.53% (16). These results suggest that the effect of iodine status on hyperthyroidism is bidirectional. As iodine is an important raw material for the synthesis of thyroid hormones, deficient or excess iodine will both increase the risk of thyroid autoimmunity in adults. There is a U-shaped relationship between iodine status and thyroid autoimmunity risk in adults. The underlying mechanisms involve the activation of the immunogenicity of thyroid globulin, inducing an immune attack on the thyroid tissue itself (22). Selenium is also essential for thyroid hormone synthesis and function. Epidemiological studies have linked an increased risk of autoimmune thyroiditis, GD and goiter to low selenium status (23) and revealed that the cause of goiter after salt iodization may be related to the insufficient intake of selenium (24). There is evidence from observational studies and randomized controlled trials that selenium/selenoproteins reduce thyroid peroxidase antibody titres, hypothyroidism, and postpartum thyroiditis. Adequate selenium intake is vital in areas of iodine deficiency/excess. In areas with low selenium intake, it may be appropriate to supplement 50-100 micrograms of selenium every day (25), which can effectively reduce autoimmune thyroid disease. However, in a randomized double-blind, placebo-controlled, supplementary trial (26), no significant effect of selenium supplementation on GD remission and recurrence rates was found. In addition, although selenium supplementation can reduce circulating thyroid antibody titres, this change is also meaningless in the pathogenesis of GD (4). Therefore, the effect of selenium supplementation on GD pathogenesis and the efficacy of GD treatment needs further study.
Previous studies have shown evidence that GD patients in iodine-deficient areas have a higher recurrence rate after ATD treatment (27). In areas characterized by iodine deficiency, such as Sweden, Turkey and Ireland, the GD recurrence rate after ATD treatment was significantly higher than the average rate before the implementation of salt iodization policies in the last century, with recurrence rates ranging from 65% to 84% (28). The restriction of iodine intake increases the expression of thyroglobulin antibody (TgAb) and thyroid peroxidase antibody (TPOAb) under the regulation of negative feedback, resulting in a more serious autoimmune response (29). The findings of a multivariate regression analysis indicated that the increase in TPOAb had a great impact on the prevalence of GD. In contrast to TPOAb, only an increase in TgAb > 400IU/mL had a positive effect on the prevalence of GD (16). Huang et al. conducted a prospective study to compare the outcomes of ATD treatment under iodine supplementation and restriction conditions in patients with GD. Within 12 months after the cessation of ATDs, the recurrence rate in the iodine supplementation group was significantly lower than that in the iodine restriction group (35.5% vs. 45.5%) (30). Patients included in this study were all from high-iodine intake areas, and large amounts of thyroid hormones may have been stored in the thyroid gland in advance for those who were exposed to adequate iodine for a long period of time. In this case, a decrease in iodine intake may cause thyroid hormone regulation disorders. Therefore, it is unnecessary to restrict iodine intake to improve the effectiveness of ATDs and remission rate of GD in areas with sufficient or excessive iodine intake. To reduce the recurrence rate of GD following ATD treatment in iodine-deficient areas, an increase in the baseline iodine supply for the population is crucial. Therefore, in the evaluation of the long-term effect of ATDs on GD, attention should be given to the environmental iodine status.
In terms of the effect of inorganic iodine as a therapeutic drug for GD, the administration of different doses should be considered. Potassium iodide is the most commonly used inorganic iodine reagent in the clinic. According to an observational study conducted by Okamura et al., 70.8% of the patients experienced remission when treated with less than 200 mg/d of potassium iodide, compared to 35.0% of the patients who experienced remission when treated with 200 mg/d or more (31). On the other hand, S. Nagataki et al. reported that all subjects experienced clinical improvement after treatment with potassium iodide at 30 mg/d; however, half of the patients experienced recurrence 4-16 weeks after treatment (32). Considering the limited amount of literature and the controversial conclusions, further research on the specific comparison between different doses and their potential effects is warranted. Among the potential effects, special attention should be given to the confusing effect of environmental iodine on the efficacy of inorganic iodine treatment.
Here, we evaluate the oral administration of potassium iodide in the treatment of GD among various populations, including general adults, pregnant and lactating women and patients with malignant tumors. The advantages and potential adverse effects of potassium iodide administration are also emphasized (Table 1).
Suzuki et al. conducted a 3-year prospective study on newly diagnosed patients with mild GD whose free tetraiodothyronine (fT4) levels were less than 5.0 ng/dL at the time of diagnosis. Patients were treated with an initial dose of potassium iodide of 50 mg/d, and if their fT4 values did not decrease to the upper limit of normal (1.6 ng/dL) after the initiation of treatment, the dose was increased to 100 mg/d. The results showed that 58.2% of the patients responded to potassium iodide monotherapy; that is, their fT4 level could be controlled in the normal range. When the fT4 level was <2.76 ng/dL, the effective rate of potassium iodide monotherapy was more than 79% (33). The findings of the study illustrated that potassium iodide has a certain curative effect and achieves a relatively high long-term remission rate in newly treated patients with mild GD. However, the conclusions currently apply only to areas with adequate iodine intake since the participants included in the study were characterized as having a high baseline iodine intake. Yoshihara et al. noted that the drug sensitivity of potassium iodide was less than 44% when the thyrotropin receptor antibody (TRAb) level was greater than 6.8 IU/L (5). GD patients with higher TRAb levels may have worse effects from potassium iodide treatment. Moreover, the findings of studies to date have shown the possibility of painless thyroiditis in GD patients treated with potassium iodide (35). It is worth noting that 90.9% of patients diagnosed with painless thyroiditis are TgAb- or TPOAb- positive. Potassium iodide induces autoimmune changes in patients. Therefore, seroconversion or a continuous increase in thyroid autoantibodies is a situation that should be considered in the treatment of GD with potassium iodide. In addition, a larger thyroid volume is associated with a higher risk of irresponsiveness to potassium iodide, so patients with increased thyroid volume during treatment tend to have a greater risk of recurrence (33).
In summary, the adverse factors affecting the efficacy of potassium iodide administration in the treatment of general adults with GD include a high thyroid hormone level, thyroid autoantibody concentration and thyroid volume. Potassium iodide administration is relatively safe for disease control in general adult patients with mild GD. Attention should be given to monitoring the changes in thyroid hormone levels and thyroid autoimmune indices during long-term treatment. The use of potassium iodide should be carefully terminated when the thyroid hormone level remains abnormal. Although iodine is effective in the treatment of hyperthyroidism in patients with mild GD, it is significantly less effective than ATDs in patients with more severe diseases. At present, based on the quality of evidence, the oral administration of potassium iodide is not recommended by authoritative guidelines in the treatment of GD in general adults.
Gestational GD has always been a focus in clinical treatment and often becomes tricky, especially with the appearance of ATD side effects. Although some clinical studies have shown that there is no correlation between exposure to antithyroid drugs (such as methimazole) in early pregnancy and birth defects (44) and antithyroid drugs are the first-line treatment in pregnant women with hyperthyroidism, other studies have shown that the incidence of neonatal congenital malformations increases after treatment with methimazole, and the incidence of maternal liver toxicity increases after treatment with propylthiouracil during the first trimester of pregnancy (36, 45). In addition, recent animal studies have revealed that ATDs interfere with hypothalamus-pituitary-thyroid axis signaling in a dose-dependent manner and induce the impairment of nervous system development in offspring (46). Therefore, potassium iodide treatment for GD has become an alternative. A retrospective study showed that the incidence of neonatal malformations in a group of patients who converted from methimazole to potassium iodide treatment in the first trimester [4/260 (1.53%)] was lower than that in the group treated with methimazole [47/1134 (4.14%)], and none of the neonates exposed to potassium iodide had thyroid dysfunction or goiter (36). The findings from another retrospective cohort study demonstrated that 55.4% of patients were in remission and successfully ceased taking drugs during pregnancy after changing from methimazole to potassium iodide therapy in the first trimester of pregnancy (5). Thus, potassium iodide treatment for GD in early pregnancy can effectively prevent the occurrence of neonatal congenital malformations, and a low dose of potassium iodide can be administered as an alternative therapy in pregnant patients with new-onset mild GD when side effects appear while taking ATDs. It should be noted that in many studies on the administration of potassium iodide for the treatment of GD during pregnancy, most GD patients used ATDs as the first-line treatment before switching to potassium iodide treatment, so the interference of ATDs on the observed outcomes of clinical treatment with potassium iodide cannot be ruled out. Therefore, further prospective studies are needed to evaluate the efficacy and foetal safety of inorganic iodine in the treatment of GD during pregnancy.
Animal experiments have confirmed the negative effects of excess iodine on brain development and hippocampal metabolism in offspring (37). Clinical studies have shown that there is strong evidence of a negative correlation between the peak neonatal TSH value and placental iodine level (r=0.763, P<0.001) (47). When pregnant women take excessive iodine, their foetuses are prone to hypothyroidism, which is disadvantageous to intrauterine growth and especially the development of the nervous system (38). Considering the individual differences in GD patients during pregnancy or the improper evaluation of their condition, the therapeutic iodine intake may be excessive, so close follow-up must be carried out after iodide treatment. Drug replacement or surgical treatment in the second trimester of pregnancy is recommended if hyperthyroidism is not well controlled in mothers. It should be particularly emphasized here that the studies investigated oral potassium iodide administration during pregnancy without regulating the daily dietary iodine intake. Although the dose of dietary iodine is much smaller, studies have shown that inadequate daily iodine intake increases the risk of hypothyroidism in healthy pregnant women (48). The effects of dietary iodine on drug iodine efficacy in pregnant women with GD need to be further studied. Although iodine in supraphysiological doses has been successfully used in the treatment of pregnant women with hyperthyroidism, the research data are mainly from the Japanese population. Therefore, the guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA) recommend that additional data are needed before iodine therapy can be generally recommended for pregnant women with GD (49, 50).
It is of great significance to clarify the effect of inorganic iodine on the thyroid function of lactating mothers with GD and their infants since numerous mothers prefer breastfeeding as much as possible. Recently, Hamada et al. evaluated the thyroid function of infants breastfed by mothers with GD who were treated with potassium iodide. The average dose of maternal potassium iodide was 50 mg/d. The results among 100 breast-fed infants demonstrated that 88 had normal thyroid hormone levels and 12 had subclinical hypothyroidism, but the blood TSH levels of all infants returned to normal within 2 months after the discontinuation of potassium iodide (39). Although the frequency of breastfeeding varies from infant to infant, there is no significant effect of frequency on the degree of iodine intake, and the concentration of iodine in urine is positively correlated with that in breast milk. Infants are not easily affected by excessive iodine, and the underlying cause might be immature self-regulation mechanisms, such as iodine inhibition, which are relatively weak compared with those of adults (51). Second, serum TRAb levels in pregnant women with GD often increase in the third trimester and pass through the placenta, which assists the thyroids of infants in resisting excess iodine until 2 to 3 months after birth (52, 53). Nonetheless, the monitoring of thyroid function in infants is highly recommended during inorganic iodine therapy for lactating mothers with GD. Breastfeeding should be stopped, and levothyroxine should be administered to infants if subclinical or dominant hypothyroidism is discovered. In addition, premature infants are characterized by poor metabolic function and vulnerability to excessive iodine. The breastfeeding of premature infants should be avoided by mothers receiving inorganic iodine treatment. There is still a lack of large sample assessment data on the extent to which premature infants are affected by iodine in breast milk (40, 41). Regarding the oral administration of potassium iodide in the treatment of lactating GD patients, it is impossible to assess the benefits or risks due to insufficient evidence. At present, the international authoritative guidelines do not provide corresponding recommendations.
Neutropenia is an occasionally severe and potentially fatal adverse reaction to both chemotherapy and ATDs treatment (42, 54). Although the incidence is low, it may be difficult to differentiate whether neutropenia is chemotherapy-induced or ATDs-induced at the early stage of treatment in patients with GD combined with malignant tumors in other organs. Okamura et al. reported that approximately 1/3 of patients with untreated GD combined with malignant tumors in other organs were sensitive to potassium iodide therapy and achieved long-term remission, while approximately 1/3 of the patients experienced inorganic iodine escape. The inorganic iodine escape rate decreases to approximately 20% when the serum fT4 level is lower than 5 ng/dL, and the inorganic iodine escape rate decreases to approximately 10% when the patient is over 65 years old (43). The increase in the potassium iodide effect in elderly patients may be related to iodine accumulation and the decrease in renal iodine excretion function caused by age. GD patients showed varying degrees of sensitivity to potassium iodide, and nearly no side effects except hypothyroidism occurred. Therefore, it is worth attempting potassium iodide monotherapy when chemotherapy is needed in patients with both GD and malignant tumors and the underlying risk of bone marrow and blood toxicity is high, thus alleviating the possibility of neutropenia caused by thioamides or chemotherapeutic drugs.
Recent studies have provided a diverse perspective on three special circumstances of potassium iodide application: GD patients undergoing thyroidectomy, GD patients receiving RAI treatment and GD patients experiencing thyroid storm (Table 2).
Potassium iodide is often used in clinical preparation for operations in GD patients based on the fact that large doses promote the rapid recovery of thyroid function and reduce intraoperative bleeding. Recent studies indicated that the incidence of postoperative complications was not reduced by the preoperative administration of potassium iodide, and there was no solid or conclusive evidence to support the necessity of preoperative iodine use (55, 56). No significant difference was shown in the probability of postoperative complications, such as recurrent laryngeal nerve injury, hypoparathyroidism or cervical haematoma, between patients who did and did not receive potassium iodide in clinical preparation. Although the operation time was significantly prolonged without the use of potassium iodide in clinical preparation, there was no impact on the total length of stay. Ali et al. performed a retrospective analysis, and the outcomes of urgent thyroidectomy following rapid control with potassium iodide were similar to those after elective operation with ATDs alone. No significant differences in complications, such as hypoparathyroidism, vocal cord paralysis or postoperative bleeding, were observed between the groups (60). Considering the difficulty of accurately ruling out confounding factors in retrospective analyses, multicenter double-blind randomized controlled trials are needed to further evaluate the effects of the preoperative administration of inorganic iodine. Despite the low quality of evidence, according to the international guidelines, short-duration and high-dose preoperative potassium iodide administration is still recommended for the following GD patients (43, 50, 61): (1) patients who are in need of urgent or elective thyroidectomy or other types of operations; (2) patients with side effects due to intolerability of ATDs or other types of drugs; (3) women in the second trimester of pregnancy; and (4) patients in whom anaesthesia duration must be controlled due to an underlying disease or state. The specific application method is that potassium iodide can be given as 5-7 drops (0.25-0.35 mL) of LS (8 mg iodide/drop) or 1-2 drops (0.05-0.1 mL) of a saturated solution (50 mg iodide/drop) mixed in water or juice three times daily for 10 days before surgery (49).
NIS protein is expressed on the basolateral membrane of thyroid follicular cells, which is the common channel for RAI therapy and inorganic iodine intake. In the past, limited dietary iodine intake was always recommended for patients before RAI therapy and was especially suitable for patients with a relatively low radioactive iodine uptake rate to increase the proportion of trapped RAI. Iodine deficiency blocks thyroid hormone synthesis and increases TSH under feedback regulation, which stimulates an increase in NIS synthesis. However, a Japanese study conducted in 2021 reported that limiting iodine intake within 5 to 7 days before RAI therapy had no effect on GD treatment efficacy. No associations were observed between dietary iodine and therapeutic potassium iodide intake and the therapeutic effect of RAI (OR=0.974, 95% CI 0.956-0.993) (57). In addition, a clinical study from Germany confirmed that patients with GD taking 600 mg of inactive potassium iodide for three days during RAI therapy had significantly improved efficacy of RAI therapy and did not need a second RAI dose (34). Nevertheless, a restricted iodine diet has a significant positive effect on the radiotherapy of thyroid cells with a low iodine uptake rate, such as tumor cells (58), whereas NIS expression in thyroid cells is active and functional in the GD state, and the possibility of permanent hypothyroidism cannot be ignored if the iodine dose is not properly controlled. From this point of view, it is not necessary to impose strict limitations on dietary iodine and therapeutic inorganic iodine intake in patients with GD to obtain the maximum efficacy of RAI. However, the ATA pointed out that nutritional supplements that may contain excess iodine and seaweed should be avoided for at least 7 days (49).
Thyroid storm, as an endocrine emergency characterized by severe thyrotoxicosis, can be secondary to poorly controlled GD. In the clinic, potassium iodide and other medications are often administered to control thyroid storm. The 2016 ATA guidelines recommended that a saturated potassium iodide solution be used. The specific usage is to take 5 drops (0.25 mL or 250 mg) orally every 6 hours (49). Nevertheless, a series of recently published case reports have shown that thyroid storm can likewise be induced by acute or excessive iodine load. A patient suddenly suffered from respiratory and circulatory failure and cardiac arrest after taking iodine-containing medications for six months after the diagnosis of GD. The serum and urinary iodine level was approximately 1000 times the normal value, and urgent haemodialysis was needed to eliminate excess iodine. In rare cases, the administration of exogenous iodine induces hyperthyroidism in patients with abnormal thyroid function, known as Jod-Basedow syndrome (59). Dhami reported the case of a patient who was diagnosed with GD one year prior and experienced sudden cardiac arrest due to myocardial infarction caused by an iodine-containing contrast agent used during percutaneous coronary intervention (62). Transient coronary vasospasm, ischaemic ventricular fibrillation and cardiac arrest were caused by the injection of an iodine contrast agent, aggravating the symptoms and manifestations of myocardial infarction. Therefore, iodine contrast agents should be used cautiously in patients with GD complicated with cardiovascular disease. The contradictory phenomenon of the mechanisms of thyroid autoregulation and abnormal regulation during excess iodine intake is described as the Wolff-Chaikoff effect and Jod-Basedow syndrome, respectively. Jod-Basedow syndrome is a manifestation of the evasion of the physiological negative feedback mechanism of the Wolf-Chaikoff effect. The change in autoimmune mechanisms may be involved in the transition between the two effects (63).
Inorganic iodine is closely related to thyroid autoimmunity, oxidative stress and inflammation. It is of great value to explore the application prospects of inorganic iodine for patients with GD, whether as a substance in the daily diet or as a therapeutic medication. No sufficient evidence has been revealed to support the necessity of the strict limitation or compulsory use of iodine in GD patients during ATD treatment, before RAI treatment or before elective thyroidectomy. The efficacy of inorganic iodine as an oral medication in the treatment of GD is affected by thyroid function, thyroid volume and baseline iodine intake. Inorganic iodine also shows a certain degree of effectiveness and safety for special GD patients, such as those who are pregnant, lactating, have malignant tumors in other organs or are undergoing radiotherapy and chemotherapy. At present, based on the quality of evidence, the use of iodine-containing drugs in the preoperative period for hyperthyroidism and thyroid storm patients is strongly recommended by multinational guidelines. The evidence for the use of iodine-containing drugs for hyperthyroidism in other clinical settings needs to be further substantiated. It should be noted that the efficacy of inorganic iodine in the treatment of GD is closely related to environmental iodine, which needs to be considered in clinical applications. There are two sides to every coin. Inorganic iodine not only treats but also induces thyroid storm. More clinical and basic research studies are needed to verify the indications and contraindications.
YH, YX, MX, and XZ performed the literature search and data analysis. YH and YX wrote the first draft of the manuscript, and all authors commented on the previous versions of the manuscript. MC drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key R&D Program of China (2020YFC2006500, 2020YFC2006502).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Noh JY, Chino T, Ito K. Treatment with inorganic iodine for graves' hyperthyroidism. Nihon Rinsho (2006) 64(12):2269–73.
2. Zbigniew S. Iodine prophylaxis in the lights of the last recommendation of WHO on reduction of daily salt intake. Recent Pat Endocr Metab Immune Drug Discovery (2017) 11(1):39–42. doi: 10.2174/1872214811666170608120810
3. Chai J, Zhang R, Zheng W, Zhang G, Jia Q, Tan J, et al. Effect of lugol's solution on 131I therapy efficacy in graves' disease. Clin Exp Med (2022). doi: 10.1007/s10238-022-00859-4
4. Antonelli A, Fallahi P, Elia G, Ragusa F, Paparo SR, Ruffilli I, et al. Graves' disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract Res Clin Endocrinol Metab (2020) 34(1):101388. doi: 10.1016/j.beem.2020.101388
5. Yoshihara A, Noh JY, Watanabe N, Kunii Y, Suzuki M, Matsumoto M, et al. Characteristics of patients with graves' disease whose thyroid hormone levels increase after substituting potassium iodide for methimazole in the first trimester of pregnancy. Thyroid (2020) 30(3):451–6. doi: 10.1089/thy.2019.0392
6. Gaurav K, Mishra SK. Preparation of graves' disease patients for surgery - role of inorganic iodides. Am J Surg (2017) 214(5):979. doi: 10.1016/j.amjsurg.2016.11.048
7. Rump A, Eder S, Hermann C, Lamkowski A, Kinoshita M, Yamamoto T, et al. Modeling principles of protective thyroid blocking. Int J Radiat Biol (2022) 98(5):831–42. doi: 10.1080/09553002.2021.1987570
8. Saller B, Fink H, Mann K. Kinetics of acute and chronic iodine excess. Exp Clin Endocrinol Diabetes (1998) 106(Suppl 3):S34–8. doi: 10.1055/s-0029-1212044
9. Reyes-Castano JJ, Burman K. (2014) Thyrotoxic crisis: Thyroid storm. Endocrine Emergencies. Humana Press, Totowa, NJ. vol 74. 77–97. doi: 10.1007/978-1-62703-697-9_9
10. Llorente-Esteban A, Manville RW, Reyna-Neyra A, Abbott GW, Amzel LM, Carrasco N. Allosteric regulation of mammalian Na+/I- symporter activity by perchlorate. Nat Struct Mol Biol (2020) 27(6):533–9. doi: 10.1038/s41594-020-0417-5
11. van Geest FS, Gunhanlar N, Groeneweg S, Visser WE. Monocarboxylate transporter 8 deficiency: From pathophysiological understanding to therapy development. Front Endocrinol (Lausanne) (2021) 12:723750. doi: 10.3389/fendo.2021.723750
12. Chartoumpekis DV, Ziros PG, Georgakopoulos-Soares I, Smith AAT, Marques AC, Ibberson M, et al. The transcriptomic response of the murine thyroid gland to iodide overload and the role of the Nrf2 antioxidant system. Antioxid (Basel) (2020) 9(9):884. doi: 10.3390/antiox9090884
13. Eleftheriadou AM, Mehl S, Renko K, Kasim RH, Schaefer JA, Minich WB, et al. Re-visiting autoimmunity to sodium-iodide symporter and pendrin in thyroid disease. Eur J Endocrinol (2020) 183(6):571–80. doi: 10.1530/EJE-20-0566
14. Zimmermann MB, Andersson M. GLOBAL ENDOCRINOLOGY. global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur J Endocrinol (2021) 185(1):R13–21. doi: 10.1530/EJE-21-0171
15. Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol (2018) 14(5):301–16. doi: 10.1038/nrendo.2018.18
16. Wang C, Li Y, Teng D, Shi X, Ba J, Chen B, et al. Hyperthyroidism prevalence in China after universal salt iodization. Front Endocrinol (Lausanne) (2021) 12:651534. doi: 10.3389/fendo.2021.651534
17. Zimmermann MB. Salt iodization halves risk of thyrotoxicosis in Denmark. Nat Rev Endocrinol (2019) 15(11):632–3. doi: 10.1038/s41574-019-0261-z
18. Li Y, Shan Z, Teng W. Effect of the transition from more than adequate iodine to adequate iodine on national changes in the prevalence of thyroid disorders: Repeat national cross-sectional surveys in China. Eur J Endocrinol (2021) 186(1):115–22. doi: 10.1530/EJE-21-0975
19. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: Epidemiological evidence from 31 provinces of mainland China. Thyroid (2020) 30(4):568–79. doi: 10.1089/thy.2019.0067
20. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med (2006) 354:2783–93. doi: 10.1056/NEJMoa054022
21. Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: A cross-sectional study in 10 cities. Thyroid (2016) 26:1125–30. doi: 10.1089/thy.2015.0613
22. Wang B, He W, Li Q, Jia X, Yao Q, Song R, et al. U-Shaped relationship between iodine status and thyroid autoimmunity risk in adults. Eur J Endocrinol (2019) 181(3):255–66. doi: 10.1530/EJE-19-0212
23. Guastamacchia E, Giagulli VA, Licchelli B, Triggiani V. Selenium and iodine in autoimmune thyroiditis. Endocr Metab Immune Disord Drug Targets (2015) 15(4):288–92. doi: 10.2174/1871530315666150619094242
24. Fernando GWAR, Liyanage PLCL, Rajapaksha AU, Vithanage M. Medical geology of endemic goiter in kalutara, Sri lanka; distribution and possible causes. Environ Geochem Health (2017) 39(6):1501–11. doi: 10.1007/s10653-017-9985-7
25. Hu S, Rayman MP. Multiple nutritional factors and the risk of hashimoto's thyroiditis. Thyroid (2017) 27(5):597–610. doi: 10.1089/thy.2016.0635
26. Kahaly GJ, Riedl M, König J, Diana T, Schomburg L. Double-blind, placebo-controlled, randomized trial of selenium in graves hyperthyroidism. J Clin Endocrinol Metab (2017) 102(11):4333–41. doi: 10.1210/jc.2017-01736
27. Chung JH. Antithyroid drug treatment in graves' disease. Endocrinol Metab (Seoul) (2021) 36(3):491–9. doi: 10.3803/EnM.2021.1070
28. Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, et al. Comparative effectiveness of therapies for graves' hyperthyroidism: A systematic review and network meta-analysis. J Clin Endocrinol Metab (2013) 98(9):3671–7. doi: 10.1210/jc.2013-1954
29. Sorrenti S, Baldini E, Pironi D, Lauro A, D'Orazi V, Tartaglia F, et al. Iodine: Its role in thyroid hormone biosynthesis and beyond. Nutrients (2021) 13(12):4469. doi: 10.3390/nu13124469
30. Huang H, Shi Y, Liang B, Cai H, Cai Q, Lin R. Optimal iodine supplementation during antithyroid drug therapy for graves' disease is associated with lower recurrence rates than iodine restriction. Clin Endocrinol (Oxf) (2018) 88(3):473–8. doi: 10.1111/cen.13543
31. Okamura K, Sato K, Fujikawa M, Bandai S, Ikenoue H, Kitazono T. Remission after potassium iodide therapy in patients with graves' hyperthyroidism exhibiting thionamide-associated side effects. J Clin Endocrinol Metab (2014) 99(11):3995–4002. doi: 10.1210/jc.2013-4466
32. Nagataki S, Shizume K, Nakao K. Effect of iodide on thyroidal iodine turnover in hyperthyroid subjects. J Clin Endocrinol Metab (1970) 30:469–78. doi: 10.1210/jcem-30-4-469
33. Suzuki N, Yoshimura Noh J, Sugisawa C, Hoshiyama A, Hiruma M, Kawaguchi A, et al. Therapeutic efficacy and limitations of potassium iodide for patients newly diagnosed with graves' disease. Endocr J (2020) 67(6):631–8. doi: 10.1507/endocrj.EJ19-0379
34. Dietlein M, Moka D, Reinholz U, Schmidt M, Schomäcker K, Schicha H, et al. Administration of additional inactive iodide during radioiodine therapy for graves' disease: Who might benefit? Nuklearmedizin (2007) 46(3):77–84. doi: 10.1160/nukmed-0045
35. Kamijo K. Clinical studies on potassium iodide-induced painless thyroiditis in 11 graves' disease patients. Intern Med (2021) 60(11):1675–80. doi: 10.2169/internalmedicine.6411-20
36. Yoshihara A, Noh JY, Watanabe N, Mukasa K, Ohye H, Suzuki M, et al. Substituting potassium iodide for methimazole as the treatment for graves' disease during the first trimester may reduce the incidence of congenital anomalies: A retrospective study at a single medical institution in Japan. Thyroid (2015) 25(10):1155–61. doi: 10.1089/thy.2014.0581
37. Zhang L, Fan L, Li F, Sun Q, Chen Y, He Y, et al. Study on the effect of different iodine intake on hippocampal metabolism in offspring rats. Biol Trace Elem Res (2022) 200(10):4385–94. doi: 10.1007/s12011-021-03032-2
38. Žabková K, Krátký J, Jiskra J, Vítková H. The importance of iodine nutrition during pregnancy - clinical aspect. Cas Lek Cesk (2021) 160(6):224–8.
39. Hamada K, Mizokami T, Maruta T, Higashi K, Konishi K, Momotani N, et al. Thyroid function of infants breastfed by mothers with graves disease treated with inorganic iodine: A study of 100 cases. J Endocr Soc (2020) 5(2):bvaa187. doi: 10.1210/jendso/bvaa187
40. LaFranchi SH. Thyroid function in Preterm/Low birth weight infants: Impact on diagnosis and management of thyroid dysfunction. Front Endocrinol (Lausanne) (2021) 12:666207. doi: 10.3389/fendo.2021.666207
41. Ibrahim R, Krylová K, Neumann D. Iodine status in premature newborns. Cas Lek Cesk (2021) 160(6):230–2.
42. Watanabe N, Narimatsu H, Noh JY, Yamaguchi T, Kobayashi K, Kami M, et al. Antithyroid drug-induced hematopoietic damage: A retrospective cohort study of agranulocytosis and pancytopenia involving 50,385 patients with graves' disease. J Clin Endocrinol Metab (2012) 97(1):E49–53. doi: 10.1210/jc.2011-2221
43. Okamura K, Bandai S, Fujikawa M, Sato K, Kitazono T. Clinical experience of treating graves' hyperthyroidism complicated with malignancy-the possible role of potassium iodide for avoiding the risk of thionamide-associated neutropenia. Endocr J (2020) 67(7):751–8. doi: 10.1507/endocrj.EJ20-0016
44. Gianetti E, Russo L, Orlandi F, Chiovato L, Giusti M, Benvenga S, et al. Pregnancy outcome in women treated with methimazole or propylthiouracil during pregnancy. J Endocrinol Invest (2015) 38(9):977–85. doi: 10.1007/s40618-015-0281-z
45. Laurberg P, Andersen SL. Antithyroid drug use in pregnancy and birth defects: Why some studies find clear associations, and some studies report none. Thyroid (2015) 25(11):1185–90. doi: 10.1089/thy.2015.0182
46. Ramhøj L, Svingen T, Frädrich C, Rijntjes E, Wirth EK, Pedersen K, et al. Perinatal exposure to the thyroperoxidase inhibitors methimazole and amitrole perturbs thyroid hormone system signaling and alters motor activity in rat offspring. Toxicol Lett (2022) 354:44–55. doi: 10.1016/j.toxlet.2021.10.010
47. Karaoglan M, İşbilen E. The role of placental iodine storage in the neonatal thyroid stimulating hormone surge: Iodine as a driving force to adapt the terrestrial life. J Endocrinol Invest (2021) 44(5):1041–52. doi: 10.1007/s40618-020-01399-y
48. Du Y, Liu P, Meng F, Fan L, Jia Q, Li W, et al. What iodine intervention measures should be taken in different water iodine areas? evidence from a cross-sectional Chinese survey. Biol Trace Elem Res (2022) 200(11):4654–63. doi: 10.1007/s12011-021-03050-0
49. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid (2016) 26(10):1343–421. doi: 10.1089/thy.2016.0229
50. Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid association guideline for the management of graves' hyperthyroidism. Eur Thyroid J (2018) 7(4):167–86. doi: 10.1159/000490384
51. Andersson M, Braegger CP. The role of iodine for thyroid function in lactating women and infants. Endocr Rev (2022) 43(3):469–506. doi: 10.1210/endrev/bnab029
52. Benlarbi H, Simon D, Rosenblatt J, Dumaine C, de Roux N, Chevenne D, et al. Prevalence and course of thyroid dysfunction in neonates at high risk of graves' disease or with non-autoimmune hyperthyroidism. Eur J Endocrinol (2021) 184(3):427–36. doi: 10.1530/EJE-20-1320
53. Maximiano C, Silva MR, Carvalho F, Almeida J, Gomes MM, Martins S, et al. Follow-up of infants born to mothers with graves' disease. Endocrinol Diabetes Nutr (Engl Ed) (2021) 68(7):472–80. doi: 10.1016/j.endien.2021.11.005
54. Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer (2004) 100(2):228–37. doi: 10.1002/cncr.11882
55. Barranquero AG, Muñoz de Nova JL, Gómez-Ramírez J, Valdés de Anca Á, Porrero B, Blanco Terés L, et al. Effect of preoperative potassium iodide administration on graves' disease surgery: A propensity score analysis. Am J Surg (2021) 222(5):959–63. doi: 10.1016/j.amjsurg.2021.04.023
56. Lindner K, Kußmann J, Fendrich V. Preoperative potassium iodide treatment in patients undergoing thyroidectomy for graves' disease-perspective of a European high-volume center. World J Surg (2020) 44(10):3405–9. doi: 10.1007/s00268-020-05593-0
57. Nishio R, Uchida T, Suzuki L, Onose H, Goto H, Yamada E, et al. Influence of short-term dietary and therapeutic iodine restriction on the therapeutic effects of radioactive iodine therapy in patients with graves' disease. Thyroid (2021) 31(3):439–45. doi: 10.1089/thy.2020.0126
58. Lee H, Paeng JC, Choi H, Cho SW, Park YJ, Park DJ, et al. Effect of TSH stimulation protocols on adequacy of low-iodine diet for radioiodine administration. PloS One (2021) 16(9):e0256727. doi: 10.1371/journal.pone.0256727
59. Daffini L, Pirola I, Saccà G, Salvetti M, Cappelli C. Graves' disease treated by complementary medicine leading to thyroid storm: A case report. Caspian J Intern Med (2021) 12(Suppl 2):S371–5. doi: 10.22088/cjim.12.0.371
60. Ali A, Debono M, Balasubramanian SP. Outcomes after urgent thyroidectomy following rapid control of thyrotoxicosis in graves' disease are similar to those after elective surgery in well-controlled disease. World J Surg (2019) 43(12):3051–8. doi: 10.1007/s00268-019-05125-5
61. Chiapponi C, Schmidt M, Faust M. Potassium iodide tablets instead of a saturated solution preoperatively for reaching euthyroidism quickly in refractory graves' disease. Cureus (2021) 13(6):e15854. doi: 10.7759/cureus.15854
62. Dhami A, Iqbal A, Novodvorsky P, Grech ED. Coronary artery spasm-induced cardiac arrest precipitated by iodine contrast load in unknown pre-existing graves' disease. JACC Cardiovasc Interv (2020) 13(16):e147–9. doi: 10.1016/j.jcin.2020.05.029
Keywords: inorganic iodine, Graves’ disease, therapy, diet, special population
Citation: Huang Y, Xu Y, Xu M, Zhao X and Chen M (2023) Application of oral inorganic iodine in the treatment of Graves’ disease. Front. Endocrinol. 14:1150036. doi: 10.3389/fendo.2023.1150036
Received: 23 January 2023; Accepted: 22 March 2023;
Published: 03 April 2023.
Edited by:
Poupak Fallahi, University of Pisa, ItalyReviewed by:
Marco Centanni, Sapienza University of Rome, ItalyCopyright © 2023 Huang, Xu, Xu, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingwei Chen, Y2htdzFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.