
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 06 July 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1149328
This article is part of the Research TopicAllosteric Modulation of GPCRs involved in Hormone Signalling - An Alternative Approach to GPCR TherapeuticsView all 4 articles
A correction has been applied to this article in:
Corrigendum: GLP-1RAs caused gastrointestinal adverse reactions of drug withdrawal: a system review and network meta-analysis
Background: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) significantly reduce postprandial blood glucose, inhibit appetite, and delay gastrointestinal emptying. However, it is controversial that some patients are intolerant to GLP-1RAs.
Methods: PubMed, Embase, Web of Science, and Cochrane Library were searched for randomized controlled trials (RCTs) using GLP-1RAs with documented withdrawal due to gastrointestinal adverse reactions (GI AEs) from their inception to September 28, 2022. After extracting the information incorporated into the studies, a random-effects network meta-analysis was performed within a frequentist framework.
Results: 64 RCTs were finally enrolled, which included six major categories of the GLP-1RA. The sample size of the GLP-1RAs treatment group was 16,783 cases. The risk of intolerable gastrointestinal adverse reactions of Liraglutide and Semaglutide was higher than that of Dulaglutide. Meanwhile, the higher the dose of the same GLP-1RA preparation, the more likely to cause these adverse reactions. These intolerable GI AEs were not significantly related to drug homology or formulations and may be related to the degree of suppression of the appetite center.
Conclusion: Dulaglutide caused the lowest intolerable GI AEs, while Liraglutide and Semaglutide were the highest. For Semaglutide, the higher the dose, the more likely it is to drive GI AEs. Meanwhile, the risk of these GI AEs is independent of the different formulations of the drug. All these findings can effectively guide individualized treatment.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022359346, identifier CRD42022359346.
1. This Meta-Analysis selected people with intolerable GI AEs to withdraw from the Research.
2. GLP-1RAs are more likely to cause intolerable GI AEs than other hypoglycemic agents.
3. Among the six major categories of GLP-1RA, dulaglutide has the least risk of causing intolerable GI adverse reactions, and conversely, semaglutide has the highest chance of causing intolerable GI adverse reactions. Liraglutide has a similar risk of causing intolerable GI adverse reactions to Semaglutide.
4. The occurrences of intolerable GI AEs are related to the dose and type of drug and are not significantly related to drug homology or type of formulation but may be associated with the degree of suppression of the appetite center.
In 1993, exendin-4, which showed the same primary effect as endogenous GLP-1, was isolated from Heloderma suspectum venom (1). In 2005, Exenatide was approved for marketing by the U.S. Food and Drug Administration (FDA), indicating a new tool in the fight against diabetes. These individual GLP-1RAs have their strengths and shortcomings following their different physiochemical characteristics. Although the clinical efficacy of GLP1-RAs is unquestionable, the previous study (2) showed that the rate of HbA1c control in real-world patients deviated from the results of randomized controlled trials, which may be attributed to poor medication adherence. Many factors affect medication compliance, and adverse reactions are a common reason for poor patient compliance (3).
Juris et al. suggested that GLP-1RAs only mildly increased the risk of pancreatitis while this trend was not significant (4); however, no study has determined which GLP-1RAs are more tolerable due to these unknown intolerable adverse reactions. Monami et al. found that GLP-1RAs significantly increase the risk of gallstone disease (5). A meta-analysis of GI AEs, mainly based on the HARMONY series of trials, showed that the GI tolerability of albiglutide was lower than that of liraglutide, while the numbers of included literature were small, and the results were too single (6); Lin Xia et al. only compared the results of drop-out due to AEs of GLP-1RAs with placebo (7); Htike et al. showed higher risk for GI adverse effects with GLP-1RA versus placebo, with the lowest risk for nausea and diarrhea with Albiglutide and lowest risk for vomiting with the weekly Exenatide formulation (8). Still, these findings are of little clinical significance. Nausea, vomiting, or diarrhea could have led to discontinuation of the drug, so there is not necessary to compare these adverse reactions separately.
According to the current research findings, the mechanism of action of GLP-1RAs is similar, but due to their structural differences, the clinical-specific efficacy or adverse effects are biased. Researchers have found that the most effective GLP-1RA in lowering glucose and reducing weight is Semaglutide, the most effective GLP-1RAs in lowering postprandial glucose is Lixisenatide or Exenatide b.i.d, and the most convenient GLP-1RAs is Dulaglutide (9).
Meta-Analysis serves as the top level of evidence to guide clinical medication. This Network Meta-Analysis aimed to compare the risk of shedding different GLP-1RAs due to GI adverse reactions for providing evidence-based medical evidence for clinicians, policymakers, and guidelines deciding to choose one kind of GLP-1RAs.
This Network Meta-Analysis was guided and performed by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, and a prospective protocol was developed and registered with PROSPERO (https://www.crd.york.ac.uk) under (ID: CRD42022359346). We searched PubMed, Embase, Web of Science, and the Cochrane Library for relevant English literature, limited to randomized clinical trials (RCTs). The following search keywords were as follows: (glucagon-like peptide-1 receptor agonist) or (GLP-1RA) or (Exenatide) or (Dulaglutide) or (Semaglutide) or (Liraglutide) or (Lixisenatide) or (Benaglutide) or (Albiglutide) or (Loxenatide). The retrieval time was from the establishment date of the database to September 28, 2022. The primary outcome indicator included intolerable GI AEs, including but not limited to nausea, vomiting, abdominal pain, diarrhea, pancreatitis, and cholelithiasis.
The inclusion criteria were as follows:
(1) RCTs
(2) at least one GLP-1RA,
(3) the primary outcome included intolerable gastrointestinal adverse effects and specified the number of cases dropped due to a specific gastrointestinal negative impact,
(4) In addition, studies in which GI AEs were tolerated without discontinuation;
The exclusion criteria were as follows:
(1) Animal studies; Self-control research; Dissertations and conference reports;
(2) Non-English literature;
(3) Other articles whose main text could not be retrieved;
(4) Retracted articles.
Two authors performed the literature selection, data extraction, and quality evaluation independently. Two authors screened each paper according to inclusion and exclusion criteria to exclude the possibility of errors(Zhang Ziqi, Zhang Qiling, Chen yu, Tan ying). If there is a dispute, the decision will be made by the third author with seniority(Liu su). Excel software was used to extract data, including the title, author, publication year, trial period, experimental and control groups’ intervention measures, the number of dropped cases, sample size, average age, average duration, Region, and other information. The Risk of bias table tool in Revman5.4.1(Review Manager (RevMan) [Computer program]. Version 5.4.1, The Cochrane Collaboration, 2020) was used to evaluate the methodological quality of each included study according to the Cochrane Collaboration Risk of bias tool (version 5.1.0). The methodological quality of each included study was assessed in terms of 7 aspects: generation of randomized sequences, allocation concealment, blinding of investigators and subjects, blinding of outcome indicators, completeness of outcome data, reporting bias, and other preferences. Literature quality evaluation of the included literature was performed using GRADE profiler 3.6.1. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (10) was applied to assess the quality of each study in six primary areas: inter-study bias, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency. All trials were considered to be of “low risk,” “some concern,” or “high risk.”
After data extraction and quality evaluation, network meta-analysis and mapping were performed using the network package and mvmeta package in Stata 16.0 and netmeta package in R 4.1.2 based on the frequentist framework (11, 12). Our study was divided into four main steps. STEP1, a Network Meta-Analysis was conducted to compare GLP-1RAs as a whole versus non-GLP-1RAs hypoglycemic agents. STEP2, GLP-1RAs specific drugs and non-GLP-1RAs hypoglycemic drugs were analyzed. In STEP2, we unified five preparations of Exenatide, Loxenatide (PEX168), ITCA650, Lixisenatide, and Efpeglenatide modified based on Exenatide-4 as Exenatide for analysis. STEP3, we selected Exenatide and Semaglutide, which exist in different dosage forms, for the network meta-analysis by dosage form classification. STEP4, a network meta-analysis of Semaglutide weekly preparations, was conducted based on dose to determine whether the incidence of intolerable gastrointestinal adverse reactions is related to the dose.
Stata 16.0 software was used to pre-process the data, plot the network relationship, perform Network Meta-Analysis, calculate relative effects, and draw a Ranking of risks. The publication bias was identified by drawing corrected comparison funnel plots. The inconsistency of the Network Meta-Analysis results was tested using the node-splitting method and the loop inconsistency test. P>0.05 for the difference between direct and indirect comparison results was considered insignificant. Thus, the consistency model was used. If the prediction interval crossed the null line, the random-effect model was applied due to the existing heterogeneity. If there was no significant heterogeneity, the fixed-effect model was used. The incidence of GI AEs was a dichotomous outcome, so the relative effect value of the result was expressed as OR and 95% CI. Risk ranking using surface under the cumulative ranking curve(SUCRA) (13) assessment, a greater SUCRA represents a higher risk of occurrence of the drug class in this outcome. P<0.05 was considered a statistically significant difference.
A total of 13805 articles were searched in PubMed, Embase, Web of Science, and Cochrane Library, and 64 papers were finally included, all of which were RCTs. The screening procedure is shown in Figure 1.
The essential characteristics and relevant information of the 64 included studies are detailed in Table 1. Of these, 55 were two-armed, 7 were three-armed, and 2 were four-armed. GLP-1RAs consisting of Exenatide, Liraglutide, Dulaglutide, Semaglutide, Tirzepatide, and Taspoglutide were included in the study. Among them, Exenatide includes six different dosage forms: Exenatide b.i.d, Exenatide q.w, PEX168, ITCA650, Lixisenatide, Efpeglenatide q.w, Efpeglenatide q.m. Semaglutide includes weekly formulation and oral formulation. Other hypoglycemic agents included metformin (Met), Insulin, sulfonylureas (SU), sodium-dependent glucose cotransporter 2 inhibitors (SGLT2i), dipeptidyl peptidase-IV inhibitor (DPP4i), thiazolidinedione (TZD). A total of 37740 patients were included in this study, and 16783 patients received GLP-1RAs. The shortest duration of the included studies was ten days, and the longest was 104 weeks. The mean age of the participants ranged from 14.5 to 74.2 years. Articles with missing data were excluded without contacting the authors for additional data.
All 64 included documents were RCTs, of which 21 studies did not specify the randomization method, and 1 study had both randomized and non-randomized parts. Based on inclusion and exclusion criteria, this study was classified as low risk; we ranked the 20 open-label papers as high risk because they have a problem with blinding participants and personnel. Blinding of outcome assessment, incomplete outcome data, and selective reporting were all satisfactory in all studies. After a comprehensive analysis, we concluded that the risk of bias in this included literature was low. According to the Cochrane Risk of Bias Assessment Tool, the result of discrimination was shown in Figure 2. GRADE ratings of the included literature showed that the vast majority of comparisons were low risk. This study was considered moderate quality evidence overall. The detailed results were established in the Supplementary Materials.
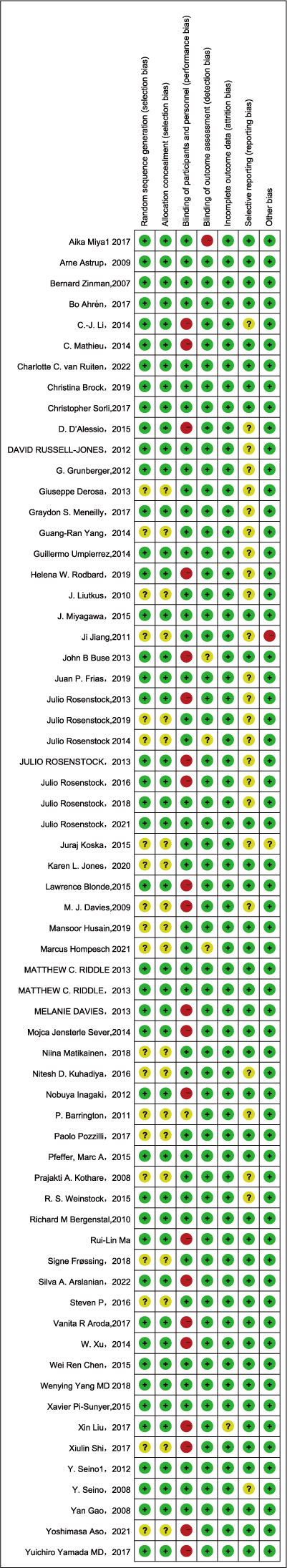
Figure 2 Risk of bias summary for each item and the included studies. (+) green circle, favorable; yellow circle, moderate (-).
In the reticulated body of evidence for STEP 1-4 (Figure 3) and the GLP-1RAs, eight control interventions, including Met, Insulin, SU, SGLT2i, DPP4i, TZD, Placebo, and Blank, were involved. The most significant direct comparison study with the GLP-1RAs was Placebo. The drug with the largest sample size among GLP-1RAs was Semaglutide. In this study, the four Network maps of comparisons included in the analyses were tested using the loop inconsistency test and the node-splitting method. No significant inconsistency or heterogeneity was found. We performed the Network Meta-Analysis in a random-effects model with a consistency model. The results of each Network league table in STEP1-4 are shown in Table 2. A funnel plot assessing the risk of publication bias showed symmetric distribution, indicating a low risk of publication bias(Figure 4). SUCRAs for all results are available in Figure 5. Prediction interval plots also showed low heterogeneity among studies (Figure 6).

Figure 6 The interval plot for STEP1-4 (In STEP 4, A: Semaglutide 0.5mg qw; B: Semaglutide 1.0mg qw; C: Insulin; D: Placebo; E: DPP4i.).
Sixty-one studies were included in STEP 1. The network evidence map is shown in Figure 3. STEP 1 results showed that GLP-1RA preparations differed from other hypoglycemic agents or placebo, except for Met and SU. GI AEs analysis found that GLP-1RAs showed a higher risk compared to Insulin (OR=7.54, 95%CI 3.39, 16.78), SGLT2i(OR=11.78, 95%CI 2.90, 47.90), DPP4i(OR=4.20, 95%CI 1.76, 10.05), TZD(OR=6.89, 95%CI 1.19, 39.80), Placebo(OR=4.84, 95%CI 3.45, 6.79) and Blank(OR=4.41, 95%CI 1.34, 14.58). Also, we observed that Met caused a higher risk of GI AEs than Insulin (OR=4.64. 95%CI 1.20, 17.99) and SGLT2i (OR=7.25, 95%CI 1.22, 43.02). Risk ranking and SUCRA analysis showed that GLP-1RAs ranked first (91.8), followed by SU (79.3), Met (78.0), DPP4i (48), Blank (45.3), Placebo (41.9), TZD (29.8), Insulin (22.6), and SGLT2i(13.4) had the lowest rate of intolerable GI AEs. The high incidence of adverse reactions with SU may be related to the fact that only one study intervention included SU. Therefore, we do not consider this result to be clinically meaningful. Overall, the results of STEP1 showed that GLP-1RAs were more prone to higher intolerable GI AEs than Insulin, DPP4i, TZD, Placebo, and especially SGLT2i.
Sixty-four studies were included in STEP 2. The network evidence map was shown in Figure 3. The results of STEP 2 indicate that Exenatide has a lower risk of causing GI AEs than Liraglutide and Semaglutide (OR=0.50 95%CI 0.29,0.86), (OR=0.52 95%CI 0.28,1.00); Liraglutide has a higher risk of causing intolerable GI adverse reactions compared to Dulaglutide (OR=3.36 95%CI 1.31,8.57); Dulaglutide has a lower risk of GI AEs compared to Semaglutide, Taspoglutide (OR=0.31 95%CI 0.12,0.86), (OR=0.32 95%CI 0.10,1.00). Meanwhile, the odds ratios and confidence intervals of Exenatide, Liraglutide, Semaglutide, and Taspoglutide all had statistically significant risks of GI AEs compared to placebo (OR=3.45 95%CI 2.27,5.24), (OR=6.95 95%CI 4.07,11.86), (OR=6.58 95%CI 3.95,10.96), and (OR=6.50 95%CI 2.87,14.76). Risk ranking showed that Liraglutide (87.7) ranked first, followed by Semaglutide (85.8), Taspoglutide (85.4), Tirzepatide (76.4), Exenatide (66.3), Dulaglutide (52.7), Placebo (30.9). The results of STEP2 showed that Dulaglutide had the lowest risk of intolerable GI AEs. Significantly, Liraglutide and Semaglutide have similar SUCRAs.
Forty studies were included in STEP 3. The network evidence diagram is shown in Figure 3. STEP 3 results showed no significant difference between various preparations of Exenatide and Semaglutide. The risk ranking showed that Semaglutide q.w (87.1) ranked first, followed by ITCA650 (83.8), Lixisenatide (83.2), Liraglutide (78.1), Taspoglutide (74.1), Efpeglenatide q.m (68.4), Exenatide b.i.d (57.3), oral Semaglutide (55.3), Exenatide q.w (54.9), Efpeglenatide q.w (39.4), and PEX168 (35.8), Placebo (16.5), while PEX168(Loxenatide) had the lowest rate of GI adverse reactions. Although there was a sequential ranking between the different dosage forms of Exenatide and Semaglutide, we could not confirm the difference between the injectable and oral dosage forms of Semaglutide or the modified dosage form or method of use of Exenatide. The STEP3 results showed no significant difference in the incidence of intolerable GI AEs between disparate dosage forms of the same GLP-1RA and disparate modifications. In addition, we found that the incidence of intolerable GI AEs remained at the top for Semaglutide versus Liraglutide, thus confirming the results in STEP 2.
Four articles were included in STEP 4. Figure 3 showed the network evidence plot result. STEP 4 results showed that Semaglutide 0.5 mg q.w caused a lower risk of intolerable GI AEs than Semaglutide (OR=0.66, 95%CI 0.47,0.91). The risk ranking results also showed that Semaglutide 1mg q.w (99.6) was associated with a higher risk than 0.5mg q.w (74.8). Meanwhile, the STEP 4 results showed that intolerable GI AEs were more pronounced with higher doses.
Research on GLP-1RAs over the past 30 years has been sufficient to confirm their place in glucose-lowering and weight loss (78, 79). Given the broad range of indications for GLP-1RAs, we included both diabetic and obese patients. Due to the remarkable efficacy of GLP-1RAs, it has been widely used in the clinic, and many adverse effects have occurred. GLP-1 receptors are expressed in several organs throughout the body, such as the intestine, heart, brain, kidneys, and even the peripheral nervous system (80), and activation of GLP-1 receptors in other organs or systems may result in unpredictable responses (81). Notably, the most common adverse reaction was digestive system adverse reactions (82). Most GI AEs are mild or moderate and can be resolved independently without intervention within a few weeks or months (83, 84). However, some patients may not tolerate them. Some studies have shown that this variation may be due to genetic variation (85). Considering that the intolerable GI adverse reaction will occur relatively shortly after the injection, the present study did not restrict the dosing duration.
Researchers have been concerned about the gastrointestinal adverse effects caused by GLP-1RAs. The influence of these adverse reactions effects on efficacy is still controversial. Lean et al. concluded that patients with GI AEs have greater weight loss (86), while other studies pointed out that nausea caused by GLP-1RAs is not significantly correlated with weight loss (87). Due to the remarkable efficacy of GLP-1RAs, it is increasingly used in clinical practice. Since we cannot prove a positive effect of gastrointestinal adverse effects on efficacy, should we consider sparing patients such suffering?
This is the first Network Meta-Analysis based on the number of sample withdrawals due to intolerable GI AEs by GLP-1RAs. The present study found that GLP-1RAs had a higher risk of intolerance than Insulin, SGLT2i, DPP4i, TZD, and Placebo. Among the GLP-1RA drugs, Liraglutide or Semaglutide had a higher risk of intolerant GI AE, and Dulaglutide had the lowest chance. Our findings are consistent with Alatorre et al. (88), who found a lower rate of adverse discontinuation with Dulaglutide. In a real-world study (89) using the disproportionality analysis model, it was also shown that Semaglutide and Liraglutide had the highest rate of adverse reactions among several GLP-1RAs. Still, in this literature, the authors also concluded that the incidence of GI adverse reactions with Dulaglutide was also higher. This differs from our findings, and we consider that this may be related to the fact that the studies we included were RCTs and not real-world studies and that the investigation by Li Chen et al. was based on the FDA adverse event reporting database, which differs from the data we included. Therefore, we believe that under both computational models, it can be assumed that Semaglutide and Liraglutide are more likely to cause intolerable GI adverse reactions in users. Dulaglutide causes GI adverse reactions but has a lower probability of being unbearable. In our study, we also found that there did not appear to be a statistically significant difference in intolerable GI AEs caused by Semaglutide weekly versus oral formulations. At the same time, the incidence of intolerable GI AEs caused by Semaglutide was positively correlated with the dose. In short, the higher the dose, the more the side effects.
Semaglutide, developed based on Liraglutide (90), showed similar results in this study. Semaglutide shares 94% identity with human GLP-1 (91), while Exenatide showed 53% homology to human-derived GLP-1 (92). However, Dulaglutide is also a human-derived GLP-1RA agent, indicating that Dulaglutide shares more than 90% identity with human GLP-1, so the intolerable GI AEs do not appear to be associated with an autoimmune response. Meanwhile, the GI AEs responsible for the intolerability of Semaglutide were unrelated to weekly or oral formulations. Drucker et al. (93) found that compared with the daily formula, the weekly recipe of exenatide has a lower risk of gastrointestinal adverse events, which is consistent with our research results. They also found no association between two different dosage forms of Exenatide and the production of corresponding anti-exenatide antibodies. So what accounts for the difference in intolerable GI AEs between Semaglutide, Liraglutide, and Dulaglutide? We hypothesized that the incidence of intolerable GI adverse effects caused by GLP-1RA might be related to the degree of central appetite suppression because endogenous GLP-1 has a very short half-life in the body (94). It mainly acts in a paracrine form, while exogenous GLP-1 can cross the blood-brain barrier (95), bind to GLP-1 receptors in the brain, and stimulate continuously, unlike endogenous GLP-1. The sensation of satiety is caused by the stimulation of GLP-1 receptors in the brain by GLP-1 RAs entering the blood-brain barrier. Currently, the brain-gut axis is thought to be associated with several functional gastrointestinal disorders (96). The physiological responses produced by the brain in response to GLP-1RA stimulation affect the gastrointestinal tract via the brain-gut axis. The gastrointestinal tract may attenuate this feeling of satiety through specific reactions. We hypothesized that the stronger the appetite suppression of GLP-1RA preparations, the more likely it is to lead to intolerable gastrointestinal adverse effects. However, this is only our conjecture and needs to be verified by further experiments.
Several methods were suggested to reduce the risk of GI AEs caused by GLP-1RAs. Choosing a small dose for the initial injection may reduce the risk of GI AEs (97), so pharmaceutical companies have developed a combination of GLP-1RA and insulin as a way to reduce the amount of GLP-1RA used (98, 99). This can both reduce the risk of hypoglycemic response caused by insulin and allow for better efficacy of GLP-1RAs. There are also different methods of injection to use Exenatide to reduce the incidence of intolerable GI AEs. However, this difference was not observed in our study.
Our study has some shortcomings. First of all, almost all the studies were lack of the dose of GLP-1RA when it was discontinued. Therefore, the current study could not conduct further Network Meta-Analysis according to the dose or the course of medication. Secondly, more than 25% of the RCTs included in this study had a sample size of fewer than 100 people, which made the OR and 95%CI of some outcomes with low accuracy; Thirdly, this study did not combine the curative effect with the comprehensive analysis of GLP-1RAs, but only analyzed from the direction of adverse reactions of the digestive tract; Fourthly, due to the lack of data, we only analyzed the correlation between the adverse reactions caused by Semaglutide and the dose. Whether other GLP-1RAs are consistent with semaglutide requires further validation in subsequent studies.
In conclusion, no single GLP-1RA has been proven to be superior across the board to the others. Each of these GLP-1RAs has its own merits (100), and physicians have the flexibility to choose the appropriate medicine based on the patient’s actual situation. In terms of our findings, we recommend Dulaglutide, which has a low risk of intolerable GI AEs.
In conclusion, we found that Dulaglutide had the lowest risk of causing intolerable GI AEs. In contrast, Semaglutide and Liraglutide were associated with a higher risk of these AEs. We ruled out the possibility that the difference was due to drug homology or dosage form. However, no single GLP-1RA has been shown to be superior to the others. Each GLP-1RA has its advantages, and physicians have the flexibility to choose the appropriate drug according to the actual situation of the patient. In the future, we hope that more researchers will pay attention to these patients with adverse reactions in clinical trials, record their detailed data, and verify the reliability of our findings.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
ZZ designed the network meta-analysis. ZZ, QZ, YC, SL, and YT selected the eligible articles. ZZ and XZ abstracted the data. ZZ and JY analyzed the data. ZZ wrote the paper. ZZ, QZ, YT, YC, XZ, SL, JY interpreted the results and all authors approved submitting the final manuscript.
The study was funded by the National Natural Science Foundation of China (Grant number: 81774117). The funding source was used for article processing charges. Beyond that, the study’s funder had no role in study design, data collection, analysis, interpretation, or report writing.
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1149328/full#supplementary-material
1. Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem (1993) 268(26):19650–5. doi: 10.1016/S0021-9258(19)36565-2
2. Carls GS, Tuttle E, Tan RD, Huynh J, Yee J, Edelman SV, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care (2017) 40(11):1469–78. doi: 10.2337/dc16-2725
3. Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab (2016) 18(4):317–32. doi: 10.1111/dom.12596
4. Meier JJ, Nauck MA. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia (2014) 57(7):1320–4. doi: 10.1007/s00125-014-3231-y
5. Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): d ata from randomized controlled trials. Diabetes Obes Metab (2017) 19(9):1233–41. doi: 10.1111/dom.12926
6. Leiter LA, Mallory JM, Wilson TH, Reinhardt RR. Gastrointestinal safety across the albiglutide development programme. Diabetes Obes Metab (2016) 18(9):930–5. doi: 10.1111/dom.12679
7. Xia L, Shen T, Dong W, Su F, Wang J, Wang Q, et al. Comparative efficacy and safety of 8 GLP-1RAs in patients with type 2 diabetes: a network meta-analysis. Diabetes Res Clin Pract (2021) 177:108904. doi: 10.1016/j.diabres.2021.108904
8. Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab (2017) 19(4):524–36. doi: 10.1111/dom.12849
9. Nauck MA, Meier JJ. Management of endocrine disease: are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol (2019) 181(6):R211–34. doi: 10.1530/EJE-19-0566
10. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64(4). doi: 10.1016/j.jclinepi.2010.04.026
11. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using stata. Epidemiol Health (2017) 39:e2017047. doi: 10.4178/epih.e2017047
12. Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using r software. Epidemiol Health (2019) 41:e2019013. doi: 10.4178/epih.e2019013
13. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS One (2013) 8(10). doi: 10.1371/journal.pone.0076654
14. Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol (2017) 5(5):341–54. doi: 10.1016/S2213-8587(17)30092-X
15. Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes endocrinol (2016) 387(10019):355–66. doi: 10.1016/S2213-8587(17)30085-2
16. Arslanian SA, Hannon T, Zeitler P, Chao L. C, Boucher-Berry C, Barrientos-Pérez M, et al. Once-weekly dulaglutide for the treatment of youths with type 2 diabetes. New Engl J Med (2022) 387(5):433–43. doi: 10.1056/NEJMoa2204601
17. Aso Y, Takada Y, Tomotsune K, Chiba Y, Matsumura M, Jojima T, et al. Comparison of insulin degludec (IDeg)/insulin aspart (IAsp) co-formulation therapy twice-daily with free combination of GLP-1 receptor agonist liraglutide plus insulin degludec in tochigi: IDEAL trial. Int J Clin practice (2021) 75(4):e13734. doi: 10.1111/ijcp.13734
18. Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet (london england) (2009) 374(9701):1606–16. doi: 10.1016/S0140-6736(09)61375-1
19. Barrington P, Chien JY, Showalter HDH, Schneck K, Cui S, Tibaldi F, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab (2011) 13(5):426–33. doi: 10.1111/j.1463-1326.2011.01364.x
20. Bergenstal RM, Wysham C, Macconell L, Malloy J, Walsh B, Yan P, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet (london england) (2010) 376(9739):431–9. doi: 10.1016/S0140-6736(10)60590-9
21. Blonde L, Jendle J, Gross J, Woo V, Jiang H, Fahrbach JL, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet (london england) (9982) 2015:2057–66:385. doi: 10.1016/S0140-6736(15)60936-9
22. Brock C, Hansen CS, Karmisholt J, Møller HJ, Juhl A, Farmer AD, et al. Liraglutide treatment reduced interleukin-6 in adults with type 1 diabetes but did not improve established autonomic or polyneuropathy. Br J Clin Pharmacol (2019) 85(11):2512–23. doi: 10.1111/bcp.14063
23. Buse JB, Nauck M, Forst T, Sheu WHH, Shenouda SK, Heilmann CR, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet (london england) (2013) 381(9861):117–24. doi: 10.1016/S0140-6736(12)61267-7
24. Chen WR, Tian F, Chen YD, Wang J, Yang JJ, Wang ZF, et al. Effects of liraglutide on no-reflow in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol (2016) 208:109–14. doi: 10.1016/j.ijcard.2015.12.009
25. D’Alessio D, Häring HU, Charbonnel B, de Pablos-Velasco P, Candelas C, Dain M-P, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab (2015) 17(2):170–8. doi: 10.1111/dom.12406
26. Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the helping evaluate exenatide in patients with diabetes compared with long-acting insulin (HEELA) study. Diabetes Obes Metab (2009) 11(12):1153–62. doi: 10.1111/j.1463-1326.2009.01154.x
27. Davies M, Heller S, Sreenan S, Sapin H, Adetunji O, Tahbaz A, et al. Once-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care (2013) 36(5):1368–76. doi: 10.2337/dc12-1333
28. Derosa G, Franzetti IG, Querci F, Carbone A, Ciccarelli L, Piccinni MN, et al. Variation in inflammatory markers and glycemic parameters after 12 months of exenatide plus metformin treatment compared with metformin alone: a randomized placebo-controlled trial. Pharmacotherapy (2013) 33(8):817–26. doi: 10.1002/phar.1301
29. Frias JP, Wynne AG, Matyjaszek-Matuszek B, Bartaskova D, Cox DA, Woodward B, et al. Efficacy and safety of an expanded dulaglutide dose range: a phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab (2019) 21(9):2048–57. doi: 10.1111/dom.13764
30. Frossing S, Nylander M, Kistorp C, Skouby S, Faber J. Effect of liraglutide on atrial natriuretic peptide, adrenomedullin, and copeptin in PCOS. Endocrine connections (2018) 7(1):115–23. doi: 10.1530/EC-17-0327
31. Gao Y, Yoon KH, Chuang LM, Mohan V, Ning G, Shah S, et al. Efficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylurea. Diabetes Res Clin Practice (2009) 83(1):69–76. doi: 10.1016/j.diabres.2008.09.037
32. Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabetic Med (2012) 29(10):1260–7. doi: 10.1111/j.1464-5491.2012.03745.x
33. Gudipaty L, Rosenfeld NK, Fuller CS, Gallop R, Schutta MH, Rickels MR. Effect of exenatide, sitagliptin, or glimepiride on beta-cell secretory capacity in early type 2 diabetes. Diabetes Care (2014) 37(9):2451–8. doi: 10.2337/dc14-0398
34. Hompesch M, Kang J, Han O, Trautmann ME, Sorli CH, Ogbaa I, et al. Effects of efpeglenatide versus liraglutide on gastric emptying, glucose metabolism and beta-cell function in people with type 2 diabetes: an exploratory, randomized phase ib study. BMJ Open Diabetes Res Care (2021) 9(1):e002208. doi: 10.1136/bmjdrc-2021-002208
35. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New Engl J Med (2019) 381(9):841–51. doi: 10.1056/NEJMoa1901118
36. Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority study. Clin Ther (2012) 34(9):1892–1908.e1. doi: 10.1016/j.clinthera.2012.07.007
37. Jensterle Sever M, Kocjan T, Pfeifer M, Kravos NA, Janez A. Short-term combined treatment with liraglutide and metformin leads to significant weight loss in obese women with polycystic ovary syndrome and previous poor response to metformin. Eur J endocrinol (2014) 170(3):451–9. doi: 10.1530/EJE-13-0797
38. Jiang J, Zhang J, Jacobsen LV, Hu P. The pharmacokinetics, pharmacodynamics, and tolerability of liraglutide, a once-daily human GLP-1 analogue, after multiple subcutaneous administration in healthy Chinese male subjects. J Clin Pharmacol (2011) 51(12):1620–7. doi: 10.1177/0091270010389468
39. Jones KL, Huynh LQ, Hatzinikolas S, Rigda RS, Phillips LK, Pham HT, et al. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentrations. Diabetes Obes Metab (2020) 22(5):788–97. doi: 10.1111/dom.13956
40. Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu J, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes (2015) 64(7):2624–35. doi: 10.2337/db14-0976
41. Kothare PA, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, et al. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with type 2 diabetes mellitus. J Clin Pharmacol (2008) 48(12):1389–99. doi: 10.1177/0091270008323750
42. Kuhadiya ND, Dhindsa S, Ghanim H, Mehta A, Makdissi A, Batra M, et al. Addition of liraglutide to insulin in patients with type 1 diabetes: a randomized placebo-controlled clinical trial of 12 weeks. Diabetes Care (2016) 39(6):1027–35. doi: 10.2337/dc15-1136
43. Li CJ, Yu Q, Yu P, Zhang Q-M, Ding M, Liu X-J, et al. Efficacy and safety comparison of add-on therapy with liraglutide, saxagliptin and vildagliptin, all in combination with current conventional oral hypoglycemic agents therapy in poorly controlled Chinese type 2 diabetes. Exp Clin Endocrinol diabetes (2014) 122(8):469–76. doi: 10.1055/s-0034-1374586
44. Liu X, Zhang Y, Zheng SY, Lin R, Xie Y-J, Chen H, et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin endocrinol (2017) 87(6):767–74. doi: 10.1111/cen.13454
45. Liutkus J, Rosas Guzman J, Norwood P, Pop L, Northrup J, Cao D, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab (2010) 12(12):1058–65. doi: 10.1111/j.1463-1326.2010.01251.x
46. Ma RL, Deng Y, Wang YF, Zhu SY, Ding XS, Sun AJ. Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome. Chin Med J (2021) 134(23):2882–9. doi: 10.1097/CM9.0000000000001712
47. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New Engl J Med (2016) 375(19):1834–44. doi: 10.1056/NEJMoa1607141
48. Mathieu C, Rodbard HW, Cariou B, Handelsman Y, Philis-Tsimikas A, Ocampo Francisco AM, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab (2014) 16(7):636–44. doi: 10.1111/dom.12262
49. Matikainen N, Söderlund S, Björnson E, Pietiläinen K, Hakkarainen A, Lundbom N, et al. Liraglutide treatment improves postprandial lipid metabolism and cardiometabolic risk factors in humans with adequately controlled type 2 diabetes: a single-centre randomized controlled study. Diabetes Obes Metab (2019) 21(1):84–94. doi: 10.1111/dom.13487
50. Meneilly GS, Roy-Duval C, Alawi H, Dailey G, Bellido D, Trescoli C, et al. Lixisenatide therapy in older patients with type 2 diabetes inadequately controlled on their current antidiabetic treatment: the GetGoal-O randomized trial. Diabetes Care (2017) 40(4):485–93. doi: 10.2337/dc16-2143
51. Miya A, Nakamura A, Miyoshi H, Cho KY, Nagai S, Kurihara Y, et al. Satisfaction of switching to combination therapy with lixisenatide and basal insulin in patients with type 2 diabetes receiving multiple daily insulin injection therapy: a randomized controlled trial. J Diabetes Invest (2018) 9(1):119–26. doi: 10.1111/jdi.12654
52. Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab (2015) 17(10):974–83. doi: 10.1111/dom.12534
53. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. New Engl J Med (2015) 373(23):2247–57. doi: 10.1056/NEJMoa1509225
54. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New Engl J Med (2015) 373(1):11–22. doi: 10.1056/NEJMoa1411892
55. Pozzilli P, Norwood P, Jódar E, Davies MJ, Ivanyi T, Jiang H, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab (2017) 19(7):1024–31. doi: 10.1111/dom.12937
56. Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-l). Diabetes Care (2013) 36(9):2489–96. doi: 10.2337/dc12-2454
57. Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-duo 1). Diabetes Care (2013) 36(9):2497–503. doi: 10.2337/dc12-2462
58. Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care (2019) 42(12):2272–81. doi: 10.2337/dc19-0883
59. Rosenstock J, Balas B, Charbonnel B, Bolli GB, Boldrin M, Ratner R, et al. The fate of taspoglutide, a weekly GLP-1 receptor agonist, versus twice-daily exenatide for type 2 diabetes: the T-emerge 2 trial. Diabetes Care (2013) 36(3):498–504. doi: 10.2337/dc12-0709
60. Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-s). J Diabetes its complications (2014) 28(3):386–92. doi: 10.1016/j.jdiacomp.2014.01.012
61. Rosenstock J, Diamant M, Aroda VR, Silvestre L, Souhami E, Zhou T, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-Concept randomized trial. Diabetes Care (2016) 39(9):1579–86. doi: 10.2337/dc16-0046
62. Rosenstock J, Buse JB, Azeem R, Prabhakar P, Kjems L, Huang H, et al. Efficacy and safety of ITCA 650, a novel drug-device GLP-1 receptor agonist, in type 2 diabetes uncontrolled with oral antidiabetes drugs: the FREEDOM-1 trial. Diabetes Care (2018) 41(2):333–40. doi: 10.2337/dc17-1306
63. Rosenstock J, Sorli CH, Trautmann ME, Morales C, Wendisch U, Dailey G, et al. Once-weekly efpeglenatide dose-range effects on glycemic control and body weight in patients with type 2 diabetes on metformin or drug naive, referenced to liraglutide. Diabetes Care (2019) 42(9):1733–41. doi: 10.2337/dc18-2648
64. Rosenstock J, Wysham C, Frias JP, Kaneko S, Lee CJ, Lando LF, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (2021) 398(10295):143–55. doi: 10.1016/S0140-6736(21)01324-6
65. Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, Chan M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4). Diabetes Care (2012) 35(2):252–8. doi: 10.2337/dc11-1107
66. Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin practice (2008) 81(2):161–8. doi: 10.1016/j.diabres.2008.03.018
67. Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab (2012) 14(10):910–7. doi: 10.1111/j.1463-1326.2012.01618.x
68. Shi X, Shi Y, Chen N, Lin M, Su W, Zhang H, et al. Effect of exenatide after short-time intensive insulin therapy on glycaemic remission maintenance in type 2 diabetes patients: a randomized controlled trial. Sci Rep (2017) 7(1):2383. doi: 10.1038/s41598-017-02631-1
69. Sorli C, Harashima S-I, Tsoukas GM, Unger J, Karsbøl JD, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol (2017) 5(4):251–60. doi: 10.1016/S2213-8587(17)30013-X
70. Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care (2014) 37(8):2168–76. doi: 10.2337/dc13-2759
71. van Ruiten CC, Veltman DJ, Schrantee A, van Bloemendaal L, Barkhof F, Kramer M. H.H, et al. Effects of dapagliflozin and combination therapy with exenatide on food-cue induced brain activation in patients with type 2 diabetes. J Clin Endocrinol Metab (2022) 107(6):e2590–9. doi: 10.1210/clinem/dgac043
72. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab (2015) 17(9):849–58. doi: 10.1111/dom.12479
73. Xu W, Bi Y, Sun Z, Li J, Guo L, Yang T, et al. Comparison of the effects on glycaemic control and beta-cell function in newly diagnosed type 2 diabetes patients of treatment with exenatide, insulin or pioglitazone: a multicentre randomized parallel-group trial (the CONFIDENCE study). J Internal Med (2015) 277(1):137–50. doi: 10.1111/joim.12293
74. Yamada Y, Senda M, Naito Y, Tamura M, Watanabe D, Shuto Y, et al. Reduction of postprandial glucose by lixisenatide vs sitagliptin treatment in Japanese patients with type 2 diabetes on background insulin glargine: a randomized phase IV study (NEXTAGE study). Diabetes Obes Metab (2017) 19(9):1252–9. doi: 10.1111/dom.12945
75. Yang GR, Zhao XL, Jin F, Shi LH, Yang JK. Pharmacokinetics and pharmacodynamics of a polyethylene glycol (PEG)-conjugated GLP-receptor agonist once weekly in Chinese patients with type 2 diabetes. J Clin Pharmacol (2015) 55(2):152–8. doi: 10.1002/jcph.386
76. Yang W, Min K, Zhou Z, Li L, Xu X, Zhu D, et al. Efficacy and safety of lixisenatide in a predominantly Asian population with type 2 diabetes insufficiently controlled with basal insulin: the GetGoal-L-C randomized trial. Diabetes Obes Metab (2018) 20(2):335–43. doi: 10.1111/dom.13072
77. Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes - a randomized trial. Ann Internal Med (2007) 146(7):477–85. doi: 10.7326/0003-4819-146-7-200704030-00003
78. Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med (1992) 326(20):1316–22. doi: 10.1056/NEJM199205143262003
79. Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab (2021) 46:101102. doi: 10.1016/j.molmet.2020.101102
80. Drucker DJ. The biology of incretin hormones. Cell Metab (2006) 3(3):153–65. doi: 10.1016/j.cmet.2006.01.004
81. Costa A, Ai M, Nunn N, Culotta I, Hunter J, Boudjadja MB, et al. Anorectic and aversive effects of GLP-1 receptor agonism are mediated by brainstem cholecystokinin neurons, and modulated by GIP receptor activation. Mol Metab (2021) 55:101407. doi: 10.1016/j.molmet.2021.101407
82. Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Internal Med (2014) 25(5). doi: 10.1016/j.ejim.2014.03.005
83. Wilding PH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. New Engl J Med (2021) 384(11). doi: 10.1056/NEJMoa2032183
84. Lyseng-Williamson KA. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: their use and differential features. Clin Drug Investig (2019) 39(8):805–19. doi: 10.1007/s40261-019-00826-0
85. Jakhar K, Vaishnavi S, Kaur P, Singh P, Munshi A. Pharmacogenomics of GLP-1 receptor agonists: focus on pharmacological profile. Eur J Pharmacol (2022) 936:175356. doi: 10.1016/j.ejphar.2022.175356
86. Lean MEJ, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rossner S, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (2014) 38(5):689–97. doi: 10.1038/ijo.2013.149
87. Riddle MC, Henry RR, Poon TH, Zhang B, Mac SM, Holcombe JH, et al. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes/metabolism Res Rev (2006) 22(6). doi: 10.1002/dmrr.646
88. Alatorre C, Fernández Landó L, Fernández Landó L, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab (2017) 19(7). doi: 10.1111/dom.12902
89. Liu L, Chen J, Wang L, Chen C, Chen L. Association between different GLP-1 receptor agonists and gastrointestinal adverse reactions: a real-world disproportionality study based on FDA adverse event reporting system database. Front Endocrinol (Lausanne) (2022) 13:1043789. doi: 10.3389/fendo.2022.1043789
90. Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front endocrinol (2019) 10:155. doi: 10.3389/fendo.2019.00155
91. Andersen A, Knop FK, Vilsbøll T. A pharmacological and clinical overview of oral semaglutide for the treatment of type 2 diabetes. Drugs (2021) 81(9). doi: 10.1007/s40265-021-01499-w
92. Park EJ, Lim SM, Lee KC, Na DH. Exendins and exendin analogs for diabetic therapy: a patent review (2012-2015). Expert Opin Ther patents (2016) 26(7). doi: 10.1080/13543776.2016.1192130
93. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet (2008) 372(9645):1240–50. doi: 10.1016/S0140-6736(08)61206-4
94. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev (2007) 87(4). doi: 10.1152/physrev.00034.2006
95. Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol neuroscience : MN (2002) 18(1-2). doi: 10.1385/JMN:18:1-2:07
96. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-Gut-Microbiome axis. Cell Mol Gastroenterol Hepatol (2018) 6(2):133–48. doi: 10.1016/j.jcmgh.2018.04.003
97. Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabetes Stud (2014) 11(3-4):202–30. doi: 10.1900/RDS.2014.11.202
98. Gough SC, Jain R, Woo VC. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab (2016) 11(1):7–19. doi: 10.1586/17446651.2016.1113129
99. Tabák ÁG, Anderson J, Aschner P, Liu M, Saremi A, Stella P, et al. Efficacy and safety of iGlarLixi, fixed-ratio combination of insulin glargine and lixisenatide, compared with basal-bolus regimen in patients with type 2 diabetes: propensity score matched analysis. Diabetes Ther (2020) 11(1):305–18. doi: 10.1007/s13300-019-00735-7
Keywords: Glucagon-like peptide-1 receptor agonist, intolerance, gastrointestinal adverse effects, network meta-analysis, Dulaglutide, Liraglutide, Semaglutide
Citation: Zhang Z, Zhang Q, Tan Y, Chen Y, Zhou X, Liu S and Yu J (2023) GLP-1RAs caused gastrointestinal adverse reactions of drug withdrawal: a system review and network meta-analysis. Front. Endocrinol. 14:1149328. doi: 10.3389/fendo.2023.1149328
Received: 21 January 2023; Accepted: 14 June 2023;
Published: 06 July 2023.
Edited by:
Zoltan Pataky, Hôpitaux universitaires de Genève (HUG), SwitzerlandReviewed by:
Tasma Harindhanavudhi, University of Minnesota Medical Center, United StatesCopyright © 2023 Zhang, Zhang, Tan, Chen, Zhou, Liu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangyi Yu, eWp5MjAyMTA1QG5qdWNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.