- 1Department of Medicine, Weill Cornell Medicine–Qatar, Doha, Qatar
- 2Biostatistics Core, Weill Cornell Medicine–Qatar, Doha, Qatar
- 3Heart Hospital, Hamad Medical Corporation, Doha, Qatar
- 4Department of Endocrinology, Hamad Medical Corporation, Doha, Qatar
- 5Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, United States
Aims: Primary hyperaldosteronism (PA) is a common cause of hypertension. It is more prevalent in patients with diabetes. We assessed the cardiovascular impact of PA in patients with established hypertension and diabetes.
Methods: Data from the National Inpatient Sample (2008-2016) was used to identify adults with PA with hypertension and diabetes comorbidities and then compared to non-PA patients. The primary outcome was in-hospital death. Secondary outcomes included ischemic stroke, hemorrhagic stroke, acute renal failure, atrial fibrillation, and acute heart failure.
Results: A total of 48,434,503 patients with hypertension and diabetes were included in the analysis, of whom 12,850 (0.03%) were diagnosed with primary hyperaldosteronism (PA). Compared to patients with hypertension and diabetes but no PA, those with PA were more likely to be younger [63(13) vs. 67 (14), male (57.1% vs. 48.3%), and African-Americans (32% vs. 18.5%) (p<0.001 for all). PA was associated with a higher risk of mortality (adjusted OR 1.076 [1.076-1.077]), ischemic stroke [adjusted OR 1.049 (1.049-1.05)], hemorrhagic stroke [adjusted OR 1.05 (1.05-1.051)], acute renal failure [adjusted OR 1.058 (1.058-1.058)], acute heart failure [OR 1.104 (1.104-1.104)], and atrial fibrillation [adjusted OR 1.034 (1.033-1.034)]. As expected, older age and underlying cardiovascular disease were the strongest predictors of mortality. However, the female gender conferred protection [OR 0.889 (0.886-0.892].
Conclusion: Primary hyperaldosteronism in patients with hypertension and diabetes is associated with increased mortality and morbidity.
1 Introduction
The autonomous production of aldosterone by an aldosterone-producing adenoma in a single adrenal gland or by bilateral adrenal lesions in hyperaldosteronism. Primary hyperaldosteronism (PA) commonly presents with hypertension and hypokalemia attributed to the effect of aldosterone on the mineralocorticoid receptors resulting in renal reabsorption of sodium and excretion of potassium (1). PA was previously considered a rare cause of hypertension and was estimated to be prevalent in 0.5-2% of hypertensive patients (2, 3). However, with increased use of plasma aldosterone concentration to plasma renin activity ratio or aldosterone/renin ratio as screening tests.
PA is estimated to be present in 5-10% of hypertensive patients (4–8). Hypertension in PA is associated with left ventricular hypertrophy (LVH), stroke, renal dysfunction (9), and impaired glucose metabolism (10). Further, those complications correlate with plasma aldosterone levels. However, there is limited evidence on the co-existence of PA and hypertension in patients with established diabetes. Our study aims to characterize the outcomes of PA in hypertension patients with diabetes using a large database, the National (Nationwide) Inpatient Database (NIS).
2 Methods
2.1 Data source
We analyzed data from the National Inpatient Sample (NIS) from 2008 to 2016. The NIS is an administrative and de-identified database designed by the Healthcare Cost and Utilization Project (HCUP) to produce US regional and national estimates of inpatient utilization, access, charges, quality, and outcomes, excluding outpatients or readmissions. It accounts for 20% of all US community hospitals. Each entry in the database entails demographic details such as age, gender, race, etiology of admissions, and outcomes while using safeguards to protect the privacy of patients, physicians, and hospitals (11). The study did not require institutional review board approval but an exempt determination (number 18-00017).
2.2 Study population and outcomes
We included in our analysis hospitalized patients with both hypertension and diabetes. We then stratified them into two groups according to the presence of PA. The primary outcome was in-hospital death. Secondary outcomes included ischemic stroke, hemorrhagic stroke, acute renal failure, atrial fibrillation, and acute heart failure. Diagnosis, comorbidities, and outcomes were extracted using the ICD-9-CM and ICD-10-CM codes.
2.3 Statistical analysis
Data were weighted per the recommendation of the HCUP to generate national estimates of admissions each year (12). Patients were divided into two groups according to the presence of PA and compared in terms of baseline characteristics and outcomes. Finally, we assessed the predictors of mortality in hypertensive patients with diabetes and PA. Data are presented as mean (SD), median (IQR), number (%), or OR (95% CI). A Chi-squared test, student T-test, or ANOVA was used to compare groups, as appropriate. Binary logistic regression was used to calculate the unadjusted odds ratios of outcomes, which were further adjusted for all baseline characteristics that were different among both groups. Multivariate logistic regression was performed to determine predictors of mortality. The significance level was set at 5%; all analyses were done using SPSS version 26 (IBM SPSS Statistics, IBM, Armonk, New York).
3 Results
3.1 Population
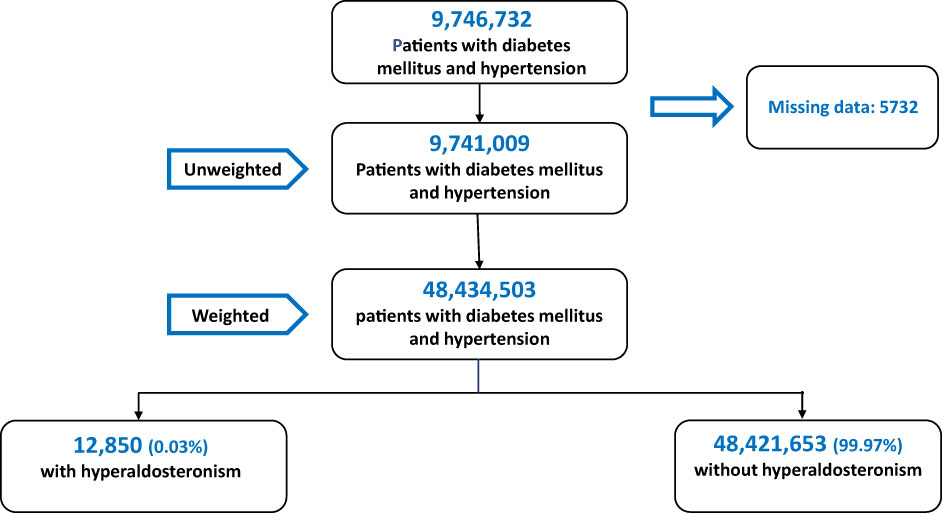
A total of 9,746,732 patients with diabetes and hypertension were hospitalized between 2008 and 2016. After excluding patients under 18 and those with incomplete or missing records, 9,741,009 patients were included in the analysis. After the application of weights, the data set included 48,434,503 patients. Of these, 12,850 (0.03%) were diagnosed with PA (Figure 1).
3.2 Baseline characteristics
Patients in the PA group were more likely to be younger [63(13) vs. 67 (14) years], male (57.1% vs. 48.3%), and African-American (32% vs. 18.5%) (p<0.001 for all) (Table 1). They were also more likely to have comorbidities such as obesity, dyslipidemia, and renal failure but less likely to smoke or have comorbidities including valvular heart disease, peripheral vascular disease, and coronary artery disease (p < 0.001). As expected, patients with PA had a significantly higher prevalence of hypokalemia than their non-PA counterparts (39.3% vs 9.2%, respectively; p<0.001).
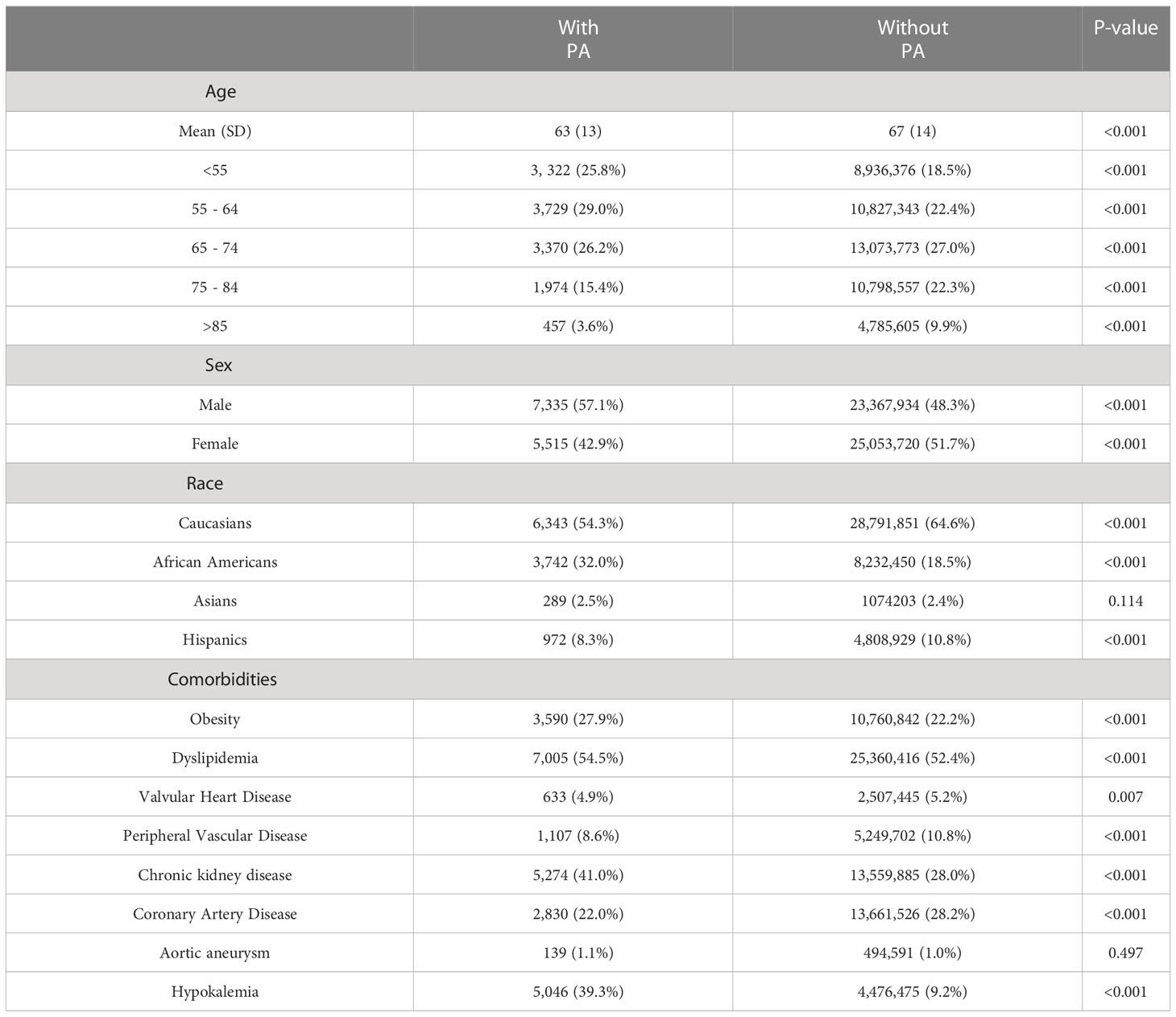
Table 1 Comparison of baseline characteristics of patients with diabetes and hypertension, with and without primary hyperaldosteronism (PA).
3.3 Comparison of PA and non-PA Groups
Patients with PA were more likely to die in hospital [adjusted OR = 1.076 (1.076-1.077)] and experience adverse outcomes, including atrial fibrillation [adjusted OR = 1.034 (1.033-1.034)], ischemic stroke [adjusted OR = 1.049 (1.049-1.05)], hemorrhagic stroke [adjusted OR = 1.05 (1.05-1.051)], acute renal failure [adjusted OR = 1.058 (1.058-1.058)], and acute heart failure [adjusted OR = 1.104 (1.104-1.104) (Table 2).
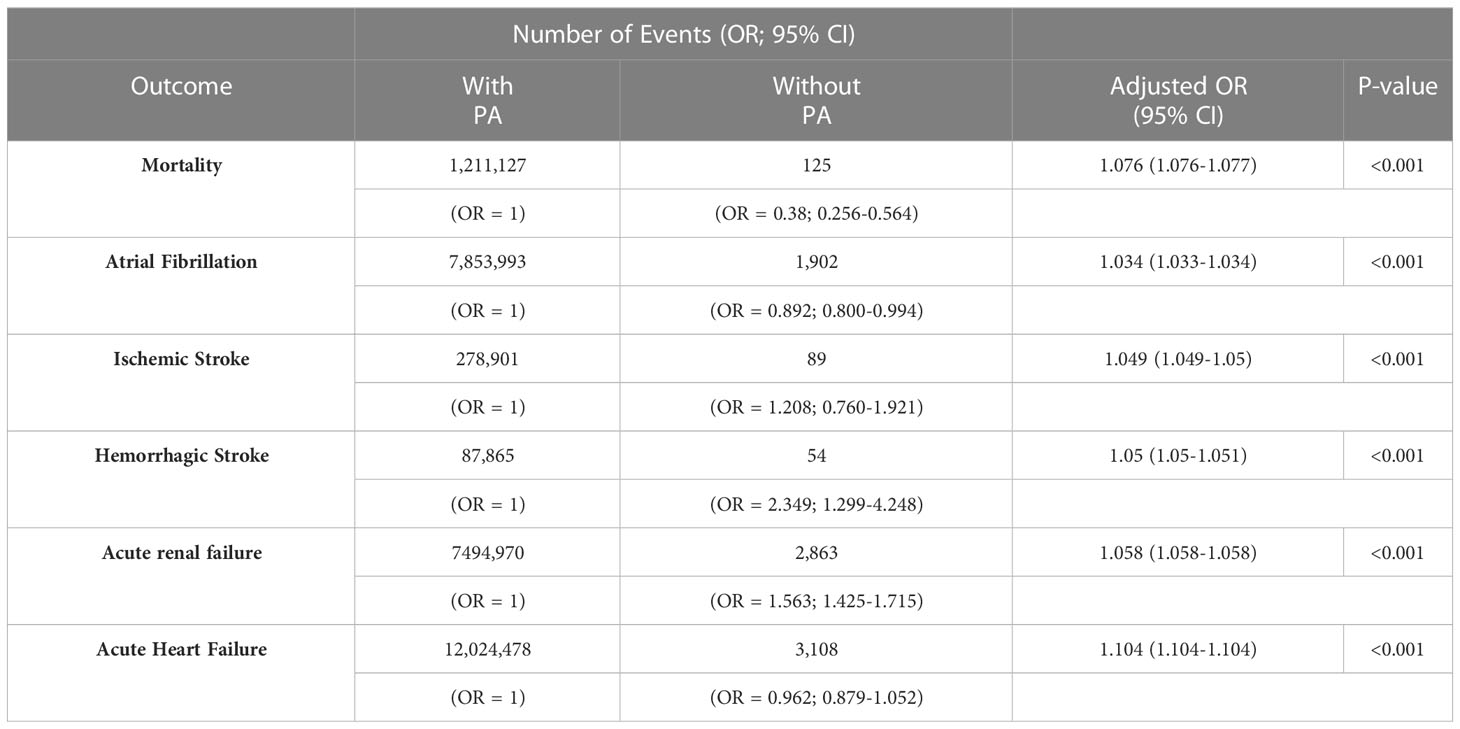
Table 2 Comparison of outcomes of patients with diabetes and hypertension with and without primary aldosteronism (PA).
3.4 Predictors of mortality
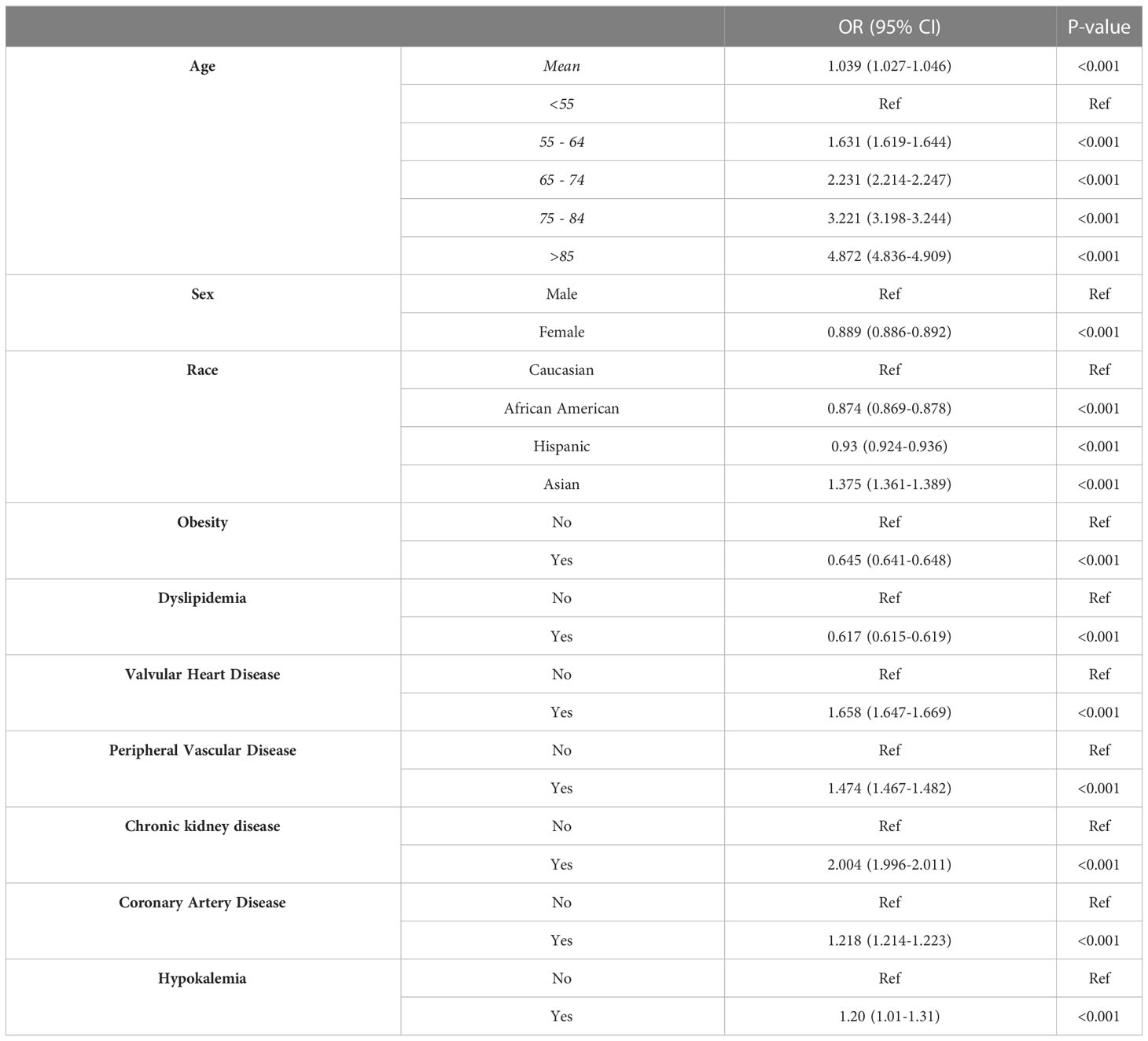
As expected, the mortality risk in patients with PA increases with age [OR = 1.039 (1.027-1.046)] (Table 3). Compared to Caucasians, Asians have a higher mortality risk [OR = 1.375 (1.361-1.389)]. Interestingly, African American and Hispanic patients had a lower risk of mortality [ORs = 0.874 (0.869-0.878) and 0.93 (0.924-0.936), respectively]. The female gender conferred protection [OR 0.889 (0.886-0.892]. Among comorbidities, valvular heart disease, peripheral vascular disease, chronic kidney disease, coronary artery disease, and hypokalemia were associated with increased mortality. Interestingly, obesity and dyslipidemia were shown to be protective.
4 Discussion
Our study showed that PA among hospitalized patients with hypertension and diabetes was associated with increased mortality risk, atrial fibrillation, stroke, acute heart failure, and acute renal failure. Comorbidities, including valvular heart disease, peripheral vascular disease, chronic kidney disease, and hypokalemia, independently increased mortality risk. It is already known that hypertension in the presence of PA is associated with higher cardiovascular event rates. Milliez et al. reported an increased risk of stroke, myocardial infarction, and atrial fibrillation in patients with PA compared to patients with essential hypertension (13).
HA is more frequent in females than in females. However, we observed a greater prevalence of the disease in males. One plausible explanation for this discordance is that we only assessed in-hospital patients with diabetes and PA; therefore, the male/female ratio could differ from the one observed in cohorts from outpatient clinics. Further, data pertinent to the gender difference in primary HA with diabetes are lacking. A recent Taiwanese analysis estimated the male proportion of diabetes patients with PA to be 50.8% (14). In an analysis of 256 outpatients with new-onset T2D and hypertension patients, a higher number of males with PA was reported (15).
In our study, PA patients had a higher rate of hypokalemia, increasing their mortality risk. This finding was recently reported in a 10-year follow-up of a cohort of PA patients (16). However, we did not find any difference in the prevalence of aortal aneurysms. Previous data indicated that PA patients had larger ascending aorta diameters and increased abdominal aortic calcification (17, 18), facts that, unfortunately, we can’t check in the NIS database since those data are lacking. In experimental models, excessive aldosterone levels were related to myocardial and vascular inflammation, injury, and fibrosis (19–21). Aldosterone-mediated fibrosis of the human myocardium has also been reported in cardiac imaging studies and is associated with systolic and diastolic dysfunction (22, 23). The structural changes of the heart in the setting of PA serve as a substrate for cardiac arrhythmias such as atrial fibrillation. Hypokalemia, one of the hallmarks of PA, causes prolongation of the atrioventricular conduction time and increases the likelihood of atrioventricular reentry mechanisms, increasing the risk of atrial fibrillation (24). Furthermore, aldosterone directly contributes to cardiac electrophysiologic remodeling, increasing the risk of atrial fibrillation (25). Aldosterone also induces inflammation, fibrosis, and necrosis of various other organs, including the kidneys (26), which may explain the increased risk of acute renal failure.
The pathophysiology of diabetes in PA is multifactorial. In 1964, Conn et al. reported an increased incidence of impaired glucose tolerance in a review of 145 patients with PA (1). The higher prevalence of diabetes in PA can be explained by impaired pancreatic insulin secretion and reduced insulin sensitivity in the setting of high levels of aldosterone (27). Garg et al. demonstrated that elevated aldosterone levels were associated with insulin resistance in normotensive healthy subjects (28). In a 10-year prospective study, high plasma aldosterone levels predicted the development of insulin resistance, suggesting that hyperaldosteronism may induce diabetes (29). Aldosterone also induces clonal β-cell failure through the glucocorticoid receptor (30).
The exact mechanisms by which PA increases cardiovascular mortality and morbidity are unclear. It might be possible that PA-related inflammation mediated by higher aldosterone levels accelerates atherosclerosis in diabetes since aldosterone promotes the production of inflammatory cytokines and decreases beneficial adipokines such as adiponectin (31). Further, high aldosterone levels are associated with increased reactive oxygen species (32–34), one of the hallmarks of the development of cardiovascular complications of diabetes.
The results of our study must be interpreted within its limitations. We used ICD-9-CM and ICD-10-CM codes to extract data from the NIS database; reporting and coding errors may be present. Further, the methodology and criteria used to diagnose PA are unclear. The retrospective nature of the database prevents us from establishing causality, and only correlations can be made. Due to the unavailability of patient-level data, information related to medications, biochemical parameters, echocardiography data, diabetes control and duration, and severity of comorbidities were not included. Our analysis only included hospitalized patients, which may represent a sicker cohort with an increased prevalence of adverse outcomes compared to the general population. Lastly, data following patient discharge or readmission data were unavailable, so follow-up of patients was not possible. Despite these limitations, our results are derived from a large sample representative of the US population.
5 Conclusion
In conclusion, we showed that primary hyperaldosteronism is associated with worse outcomes in hospitalized patients with hypertension and diabetes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study did not require institutional review board approval but an exempt determination (number 18-00017). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CAK conceived the study concept and design. KP and AF acquired data and performed statistical analyses with SD. KP, AF, JA, AJ, and CAK analyzed and interpreted data. KP and AF wrote the first draft and conducted the literature search. CAK is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Conn JW, Knopf RF, Nesbit RM. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg (1964) 107:159–72. doi: 10.1016/0002-9610(64)90252-1
2. Hiramatsu K, Yamada T, Yukimura Y, Komiya I, Ichikawa K, Ishihara M, et al. A screening test to identify aldosterone-producing adenoma by measuring plasma renin activity. results in hypertensive patients. Arch Intern Med (1981) 141:1589–93. doi: 10.1001/archinte.1981.00340130033011
3. Young WF Jr. Minireview: primary aldosteronism–changing concepts in diagnosis and treatment. Endocrinology (2003) 144:2208–13. doi: 10.1210/en.2003-0279
4. Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Rutherford JC. High incidence of primary aldosteronism in 199 patients referred with hypertension. Clin Exp Pharmacol Physiol (1994) 21:315–8. doi: 10.1111/j.1440-1681.1994.tb02519.x
5. Mosso L, Carvajal C, Gonzalez A, Barraza A, Avila F, Montero J, et al. Primary aldosteronism and hypertensive disease. Hypertension (2003) 42:161–5. doi: 10.1161/01.HYP.0000079505.25750.11
6. Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Young WF Jr., et alIncreased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab (2004) 89:1045–50. doi: 10.1210/jc.2003-031337
7. Sukor N. Endocrine hypertension–current understanding and comprehensive management review. Eur J Intern Med (2011) 22:433–40. doi: 10.1016/j.ejim.2011.05.004
8. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2016) 101:1889–916. doi: 10.1210/jc.2015-4061
9. Rocha R, Stier CT Jr. Pathophysiological effects of aldosterone in cardiovascular tissues. Trends Endocrinol Metab (2001) 12:308–14. doi: 10.1016/S1043-2760(01)00432-5
10. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab (2006) 91:454–9. doi: 10.1210/jc.2005-1733
11. Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract (2002) 5:143–51.
12. N.I.S. Healthcare cost and utilization project (HCUP) (2023). Rockville, MD: Agency for Healthcare Research and Quality (Accessed 17/01/2023).
13. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol (2005) 45:1243–8. doi: 10.1016/j.jacc.2005.01.015
14. Tsai CH, Wu XM, Liao CW, Chen ZW, Pan CT, Chang YY, et al. Diabetes mellitus is associated with worse baseline and less post-treatment recovery of arterial stiffness in patients with primary aldosteronism. Ther Adv Chronic Dis (2022) 13:20406223211066727. doi: 10.1177/20406223211066727
15. Hu Y, Zhang J, Liu W, Su X. Determining the prevalence of primary aldosteronism in patients with new-onset type 2 diabetes and hypertension. J Clin Endocrinol Metab (2020) 105(4):dgz293. doi: 10.1210/clinem/dgz293
16. Burrello J, Monticone S, Losano I, Cavaglia G, Buffolo F, Tetti M, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension (2020) 75:1025–33. doi: 10.1161/HYPERTENSIONAHA.119.14063
17. Zavatta G, Di Dalmazi G, Pizzi C, Bracchetti G, Mosconi C, Balacchi C, et al. Larger ascending aorta in primary aldosteronism: a 3-year prospective evaluation of adrenalectomy vs. medical treatment. Endocrine (2019) 63:470–5. doi: 10.1007/s12020-018-1801-3
18. Kantauskaite M, Bolten K, Boschheidgen M, Schmidt C, Kolb T, Eckardt KU, et al. Serum calcification propensity and calcification of the abdominal aorta in patients with primary aldosteronism. Front Cardiovasc Med (2022) 9:771096. doi: 10.3389/fcvm.2022.771096
19. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res (1990) 67:1355–64. doi: 10.1161/01.RES.67.6.1355
20. Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol (2002) 283:H1802–1810. doi: 10.1152/ajpheart.01096.2001
21. Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, et al. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res (2003) 93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5
22. Kozakova M, Buralli S, Palombo C, Bernini G, Moretti A, Favilla S, et al. Myocardial ultrasonic backscatter in hypertension: relation to aldosterone and endothelin. Hypertension (2003) 41:230–6. doi: 10.1161/01.HYP.0000052542.68896.2B
23. Freel EM, Mark PB, Weir RA, Mcquarrie EP, Allan K, Dargie HJ, et al. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging (2012) 5:740–7. doi: 10.1161/CIRCIMAGING.112.974576
24. Seccia TM, Caroccia B, Adler GK, Maiolino G, Cesari M, Rossi GP. Arterial hypertension, atrial fibrillation, and hyperaldosteronism: the triple trouble. Hypertension (2017) 69:545–50. doi: 10.1161/HYPERTENSIONAHA.116.08956
25. Rossi GP, Seccia TM, Maiolino G, Cesari M. The cardiovascular consequences of hyperaldosteronism. Ann Endocrinol (Paris) (2021) 82:174–8. doi: 10.1016/j.ando.2020.02.006
26. Mulatero P, Milan A, Williams TA, Veglio F. Mineralocorticoid receptor blockade in the protection of target organ damage. Cardiovasc Hematol Agents Med Chem (2006) 4:75–91. doi: 10.2174/187152506775268776
27. Corry DB, Tuck ML. The effect of aldosterone on glucose metabolism. Curr Hypertens Rep (2003) 5:106–9. doi: 10.1007/s11906-003-0065-2
28. Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab (2010) 95:1986–90. doi: 10.1210/jc.2009-2521
29. Kumagai E, Adachi H, Jacobs DR Jr., Hirai Y, Enomoto M, Fukami A, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension (2011) 58:1043–8. doi: 10.1161/HYPERTENSIONAHA.111.180521
30. Chen F, Liu J, Wang Y, Wu T, Shan W, Zhu Y, et al. Aldosterone induces clonal beta-cell failure through glucocorticoid receptor. Sci Rep (2015) 5:13215. doi: 10.1038/srep13215
31. Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med (2009) 150:776–83. doi: 10.7326/0003-4819-150-11-200906020-00005
32. Pierluissi J, Navas FO, Ashcroft SJ. Effect of adrenal steroids on insulin release from cultured rat islets of langerhans. Diabetologia (1986) 29:119–21. doi: 10.1007/BF00456122
33. Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, et al. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia (2011) 54:2152–63. doi: 10.1007/s00125-011-2158-9
Keywords: hypertension, cardiovascular disease, diabetes, National Inpatient Database (NIS), primary hyperaldosteronism (PA)
Citation: Pillai K, Fares A, Dargham S, Al Suwaidi J, Jayyousi A and Abi Khalil C (2023) Primary hyperaldosteronism is associated with increased mortality and morbidity in patients with hypertension and diabetes. Front. Endocrinol. 14:1147225. doi: 10.3389/fendo.2023.1147225
Received: 18 January 2023; Accepted: 15 May 2023;
Published: 26 May 2023.
Edited by:
Paola Di Pietro, University of Salerno, ItalyReviewed by:
Guido Zavatta, University of Bologna, ItalyLuigi Petramala, Sapienza University of Rome, Italy
Copyright © 2023 Pillai, Fares, Dargham, Al Suwaidi, Jayyousi and Abi Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charbel Abi Khalil, Y2hhMjAyMkBtZWQuY29ybmVsbC5lZHU=
†These authors have contributed equally to this work
 Krishnadev Pillai
Krishnadev Pillai Ahmed Fares1†
Ahmed Fares1† Soha Dargham
Soha Dargham Jassim Al Suwaidi
Jassim Al Suwaidi Amin Jayyousi
Amin Jayyousi Charbel Abi Khalil
Charbel Abi Khalil