
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 17 February 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1140111
This article is part of the Research Topic Advanced Approaches in the Diagnosis and Treatment of Diabetes Mellitus and Secondary Complications View all 46 articles
Objective: To investigate the application value of 3T MRI qDixon-WIP technique in the quantitative measurement of pancreatic fat content in patients with type 2 diabetes mellitus (T2DM).
Methods: The 3T MRI qDixon-WIP sequence was used to scan the livers and the pancreas of 47 T2DM patients (experimental group) and 48 healthy volunteers (control group). Pancreatic fat fraction (PFF), hepatic fat fraction (HFF), Body mass index (BMI) ratio of pancreatic volume to body surface area (PVI) were measured. Total cholesterol (TC), subcutaneous fat area (SA), triglyceride (TG), abdominal visceral fat area (VA), high density lipoprotein (HDL-c), fasting blood glucose (FPC) and low-density lipoprotein (LDL-c) were collected. The relationship between the experimental group and the control group and between PFF and other indicators was compared. The differences of PFF between the control group and different disease course subgroups were also explored.
Results: There was no significant difference in BMI between the experimental group and the control group (P=0.231). PVI, SA, VA, PFF and HFF had statistical differences (P<0.05). In the experimental group, PFF was highly positively correlated with HFF (r=0.964, P<0.001), it was moderately positively correlated with TG and abdominal fat area (r=0.676, 0.591, P<0.001), and it was weakly positively correlated with subcutaneous fat area (r=0.321, P=0.033). And it had no correlation with FPC, PVI, HDL-c, TC and LDL-c (P>0.05). There were statistical differences in PFF between the control group and the patients with different course of T2DM (P<0.05). There was no significant difference in PFF between T2DM patients with a disease course ≤1 year and those with a disease course <5 years (P>0.05). There were significant differences in PFF between the groups with a disease course of 1-5 years and those with a disease course of more than 5 years (P<0.001).
Conclusion: PVI of T2DM patients is lower than normal, but SA, VA, PFF, HFF are higher than normal. The degree of pancreatic fat accumulation in T2DM patients with long disease course was higher than that in patients with short disease course. The qDixon-WIP sequence can provide an important reference for clinical quantitative evaluation of fat content in T2DM patients.
Type 2 diabetes mellitus(T2DM) is the most common type of diabetes mellitus (1, 2). Pancreatic fat infiltration may play an important role in the occurrence and development of T2DM (3, 4). The degree of lipid infiltration in the pancreas is closely related to abnormal lipid metabolism. With β-cell dysfunction and defective insulin secretion, lipid oxidation and lipolysis are inhibited, which leads to the increase of lipid deposition in the pancreas. The increased degree of pancreatic fat infiltration promotes the development of T2DM (5–7). Therefore, monitoring pancreatic fat content in T2DM patients may provide a certain reference for clinical evaluation of efficacy and disease progression.
Although pancreatic biopsy is the “golden standard” for the quantitative determination of pancreatic fat content, due to the fact that this method only provides small tissue samples, the final measured pancreatic fat content may vary with the different range and degree of pancreatic fat infiltration. Moreover, its invasiveness and poor patient compliance limit the regular detection of pancreatic fat content in T2DM patients. the pancreas is a retroperitoneal organ surrounded by abundant blood vessels and intestines, which makes puncture more difficult (8, 9).
In recent years, multi-echo dixon technology based on Magnetic resonance image (MRI), which is safe, non-invasive and has good tissue resolution, has been confirmed in various organs including the pancreas in terms of tissue fat quantification (10–13). The early two-point Dixon technique could only quantify the adipogenic variation below 50% (14), which was greatly affected by the non-uniformity of the main magnetic field and the attenuation effect of T1 and T2* (15). Three-point Dixon technique can collect one more in-phase echo signal on the basis of two-point method, which can correct T2* attenuation to a certain extent. However, the obtained organ fat fraction is susceptible to various confounding factors, and its accuracy and repeatability are not enough to be a reliable index of fat quantification (16). 6 Echo Dixon (qDixon) technology, compared with the earlier Dixon technology, effectively corrects the errors caused by the magnetic field inhomogeneity and T2* attenuation, making the quantitative results more accurate. The fat distribution map can not only directly measure the fat content quantitatively, but also fully reflect the fat distribution (17).
The purpose of this study was to investigate the value of 3T qDixon technique in the quantitative determination of pancreatic fat content in T2DM patients, and to provide reference for the early diagnosis, clinical treatment, disease progression and efficacy evaluation of pancreatic changes in T2DM patients by comparing the relationship between relevant indicators.
A total of 95 volunteers were recruited from April 1, 2019 to June 30, 2022, including 36 females (17 T2DM patients, 19 normal controls) and 59 males (30 T2DM patients, 29 normal controls). T2DM Patients ranged from 32 to 71 years old (51.32 ± 10.60). The normal control group ranged from 31 to 68 years old (51.28 ± 8.91). Inclusion criteria: (1) patients diagnosed with T2DM and healthy volunteers with similar age to T2DM patients ( ± 3 years old) and no related diseases. Exclusion criteria: (1) patients unable to participate in MRI examination due to contraindications or other reasons; (2) patients with liver and pancreatic tumors; (3) patients after splenectomy; (4) patients with abnormal metabolic function or metabolic diseases excluding T2DM; (5) patients with hepatitis virus or hepatitis B, and liver iron deposition; (6) patients with liver trauma or patients receiving a liver transplant; (7) patients with pancreatic inflammation and alcoholics; (8) Patients with a history of drug therapy for the the pancreas (Sulfonamides, azathioprine, glucocorticoids, thiazide diuretics) and liver (Platinum agents, antibiotics, alkylating agents, antipsychotics, anti-tuberculosis drugs, and anti-tumor drugs) within six months. This study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the hospital Ethics Committee (NO.2022-E460-01).
MRI scans were performed on all subjects by the same operator with 10 years of extensive MRI scanning experience. Abdominal axial scan was performed at the end of breath using a 3.0T MRI scanner Siemens 3T MRI scanner (Prisma, Siemens Healthcare, Erlangen, Germany). qDdixon-WIP sequence scanning parameters: echo time (TE): 1.26, 2.60, 3.94, 5.28, 6.62, 7.96ms; repetition time (TR): 9.25ms; slice thickness: 3.5mm, matrix: 160×120; bandwidth: 1040Hz/Pixel; field of view: 380mm×313.5mm; scanning time: about 18s.
Data measurements were performed by two radiologists who were familiar with image post-processing and had more than 5 years of experience in abdominal diagnosis. Measurement process: The Region of interest (ROI) was delineated independently on the fat content (FF) diagram of qDixon-WIP sequence, and the fat fraction was directly measured (fat fraction =10%× the mean measured by software). For liver, the intrahepatic sink area was avoided as far as possible. Four ROIs (liver S2/3, S4, S5/8, S6/7) were selected (Figures 1A, B) to measure liver fat fraction, each ROI was about 0.4 ~ 0.6cm2, and the corresponding Goodness of fit was measured (Figures 2A, B). Average values were taken (<5% indicates good accuracy). For the pancreas, three ROIs (head, body and tail of the pancreas) were selected (Figures 3A–C) to measure pancreatic fat fraction, each ROI was about 0.1-0.2 cm2, and Goodness of fit was also measured (Figures 4A–C), and average values were taken. The images were uploaded to Ziostation workstation (Ziostation2 Version 2.4.0.2), and the “3D standard and Viewer” functions in the workstation were used for image processing: The whole the pancreas was manually delineated, and the pancreatic volume was automatically calculated by the software (Figure 5A), and visceral fat area (VA) and subcutaneous fat area (SA) were measured in the experimental and control groups via the umbilical plane (Figure 5B). For pancreatic volume, in order to exclude the influence of height, weight and other factors among individuals, pancreatic volume to body surface area (PVI) was obtained by conversion (male: body surface area [m2] = 0.0057 × height [cm] + 0.0121 × weight [kg] + 0.0882; Female: body surface area [m2] = 0.0073 × height [cm] + 0.0127 × weight [kg] - 0.2106; Pancreatic volume per unit body surface area: PVI [cm3/m2]= pancreatic volume cm3/body surface area m2) (18). All measurement data were taken from the mean values measured by two doctors. The patient’s clinical data was queried through HIS system of our institution; Height and weight were measured on the day of MRI scan.
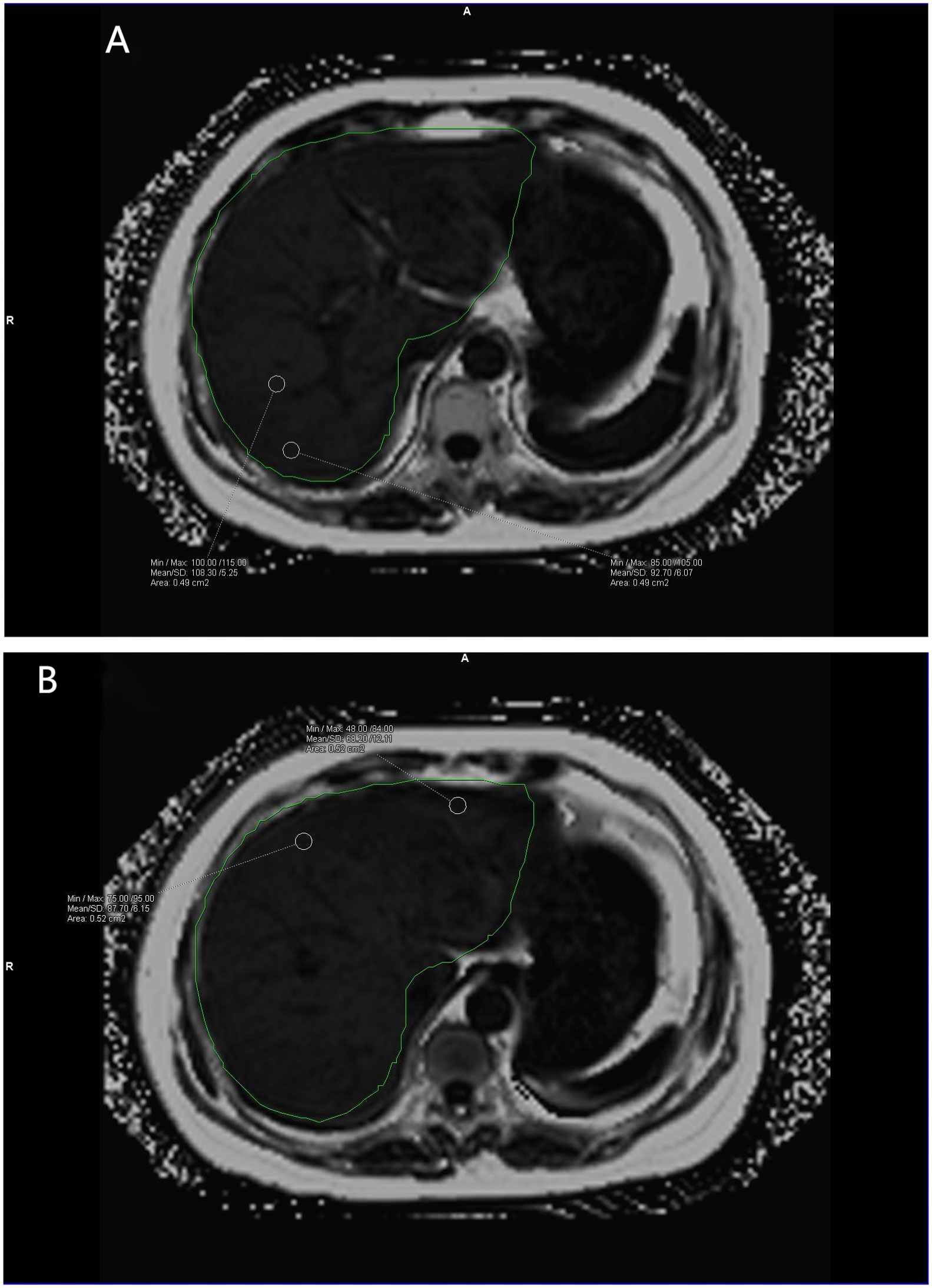
Figure 1 (A, B) show the liver fat fraction maps of volunteers. Mean fat fraction =10%× (108.30 + 92.70+68.20+87.70)/4 = 8.92 (two decimal places reserved). The green area is the liver region automatically delineated by the software.
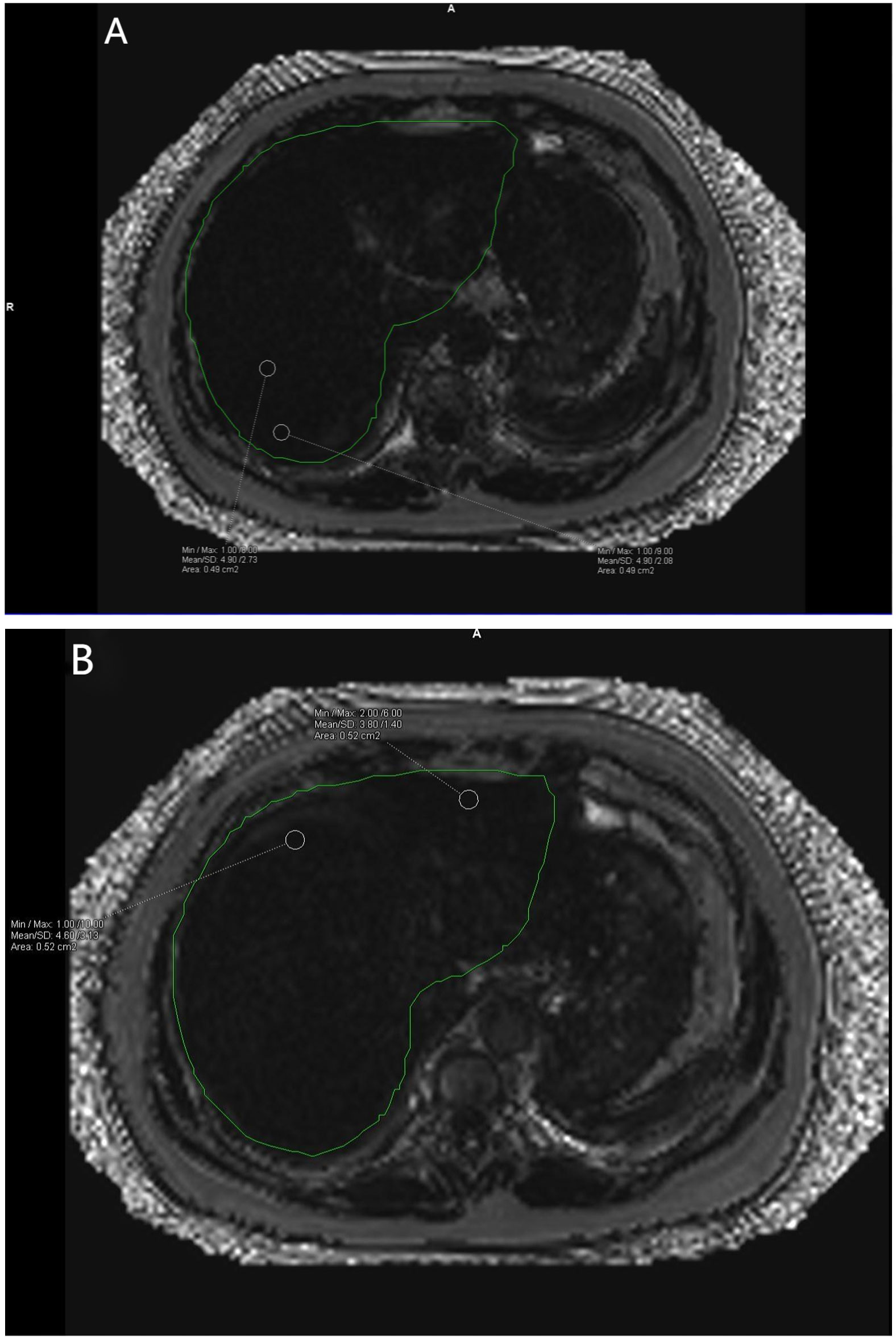
Figure 2 (A, B) show the corresponding Goodness of fit plots for liver fat fraction. A Goodness of fit average = (4.90% + 4.90% + 4.60% + 3.80%)/4 = 4.55%. The green area is the liver region automatically delineated by the software.
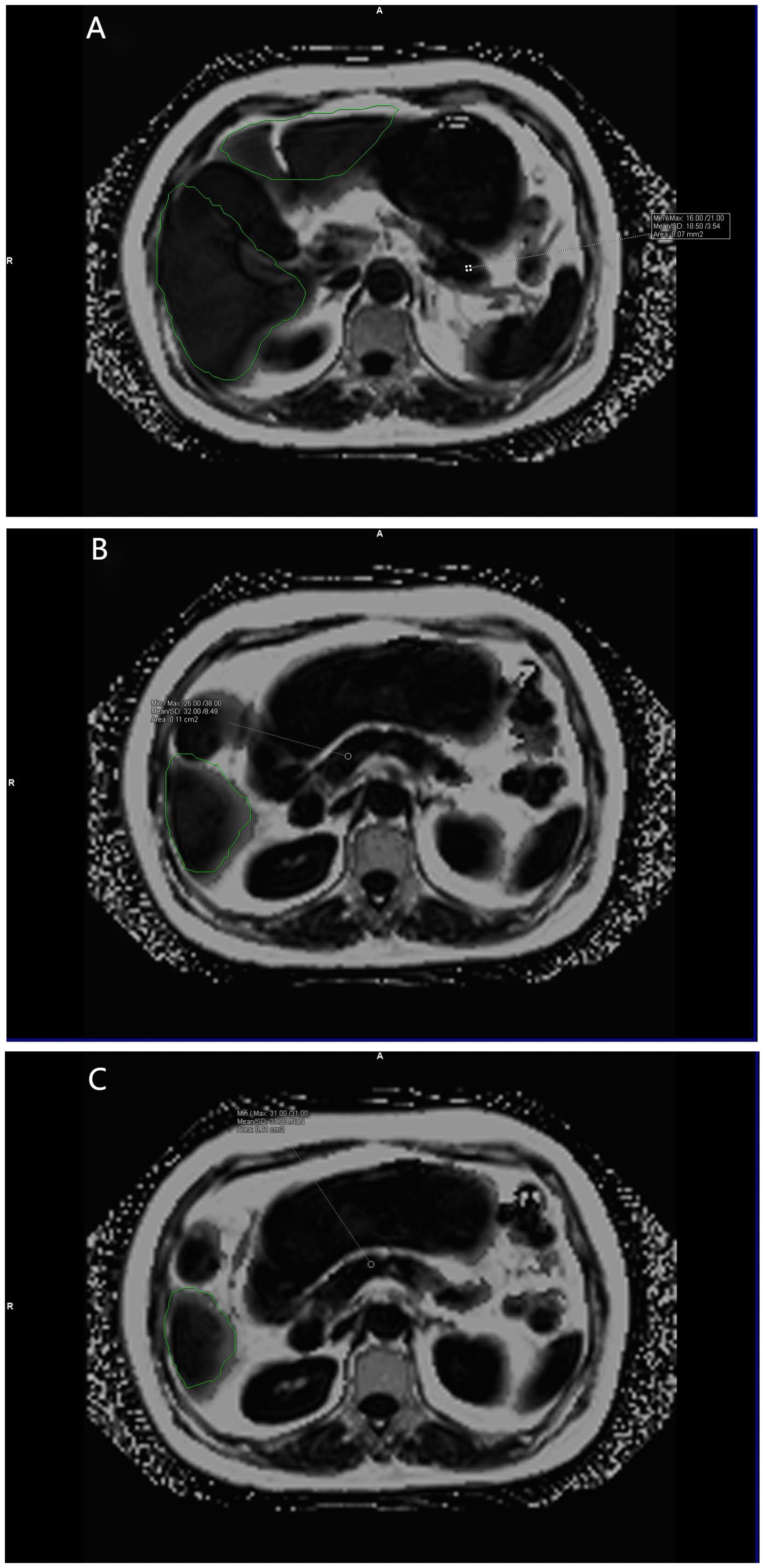
Figure 3 (A–C) show the pancreatic fat fraction maps of the volunteers. Mean pancreatic fat fraction =10%× (18.50 + 32.00+31.00)/3 = 2.72 (keep two decimal places). The green area is the liver region automatically delineated by the software.
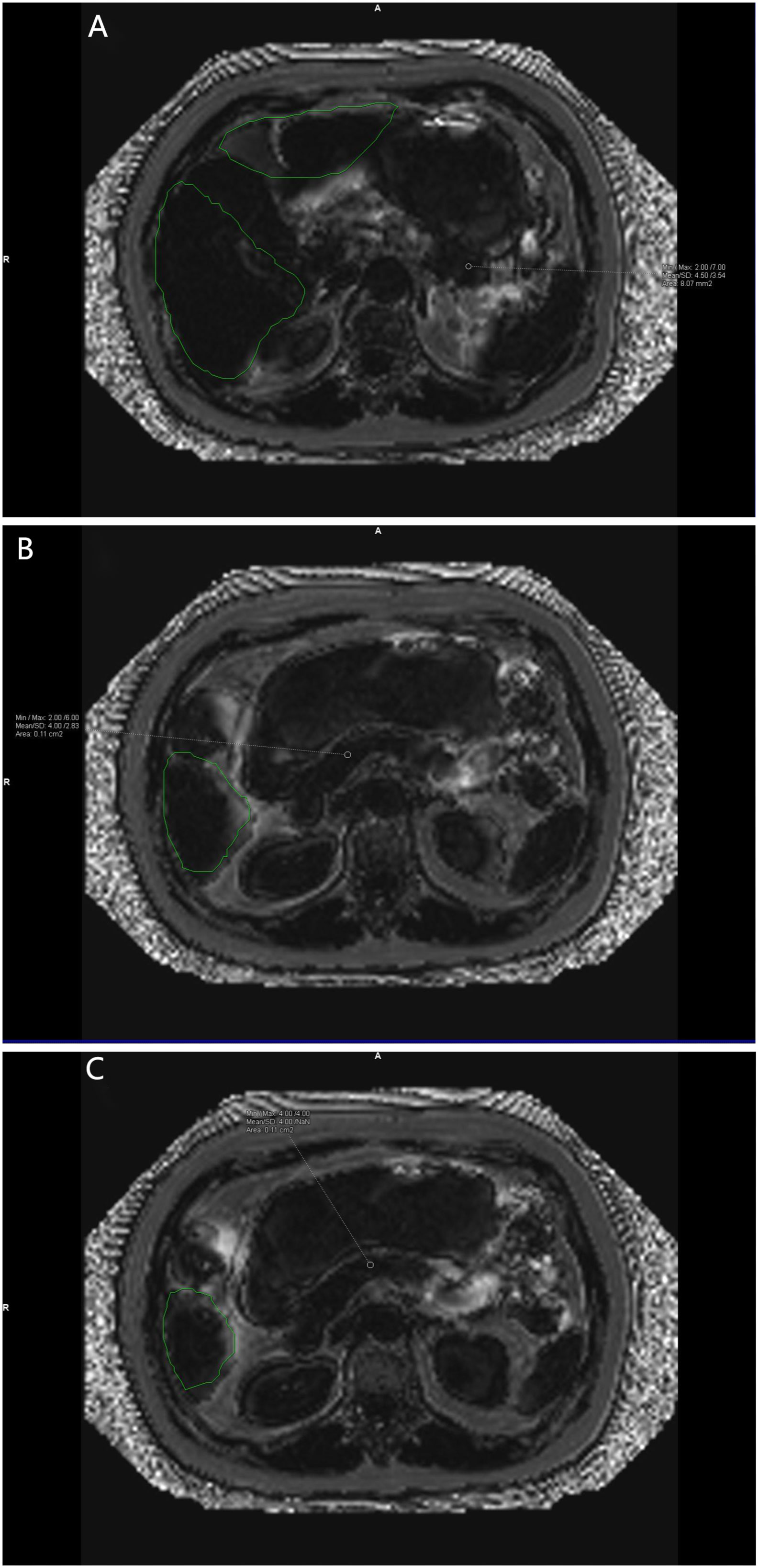
Figure 4 (A–C) show the corresponding Goodness of fit plots for pancreatic fat fraction. Goodness of fi mean = (4.5%+4.0%+4.0%)/3 = 4.17% (keep two decimal places). The green area is the area automatically delineated by the software.
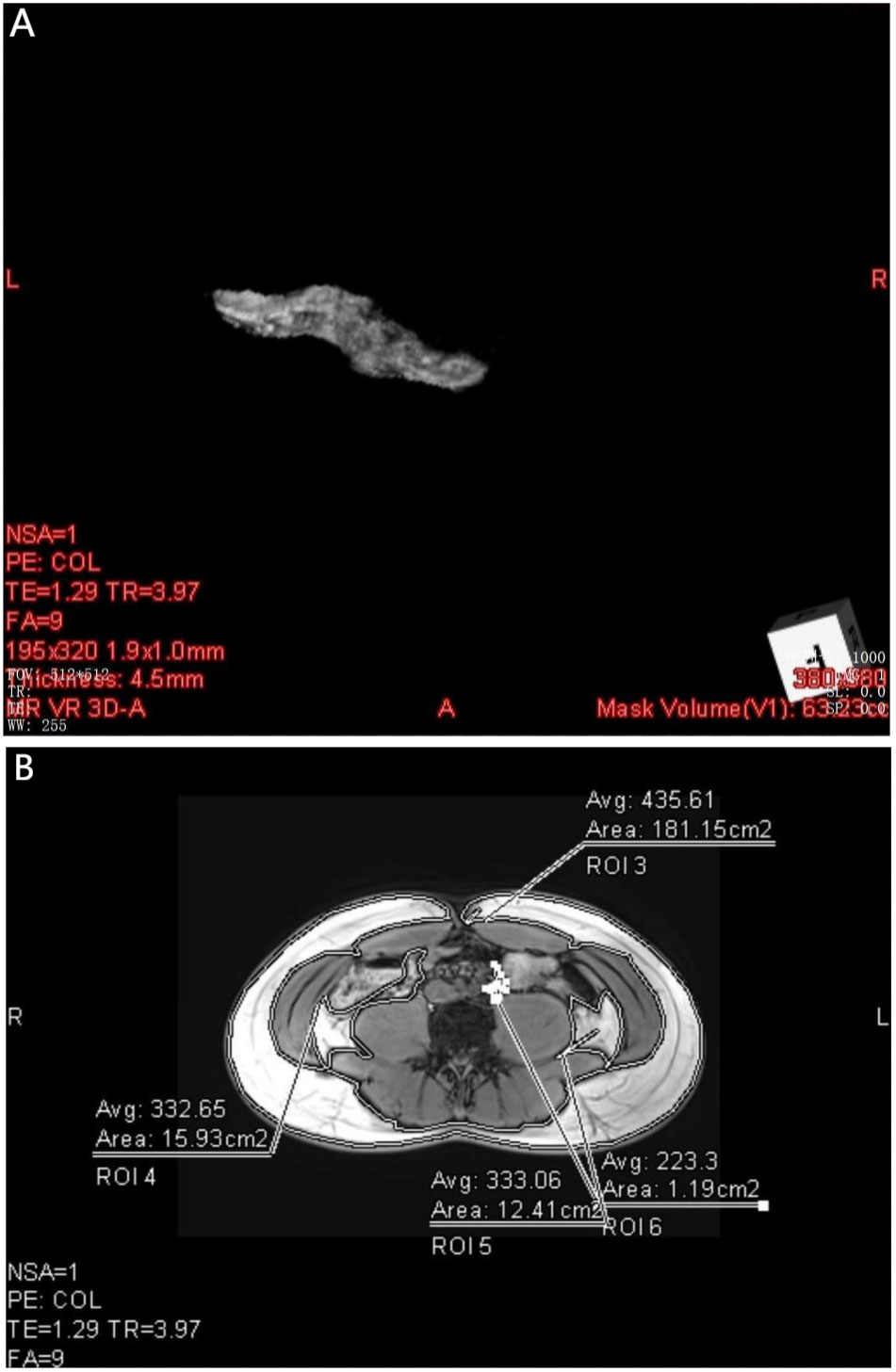
Figure 5 (A) shows the pancreatic Volume map of the volunteer the pancreas obtained by 3D standard processing, and the lower right corner of the figure shows the pancreatic volume Mask Volume(V1):63.23 cc. (B) shows the subcutaneous fat area(ROI 3 = 181.15cm2) and abdominal visceral fat area(ROI 4 = 15.93cm2, ROI 5 = 12.41cm2, ROI 6 = 1.91cm2) of volunteers after processing with Viewer.
SPSS22.0 software was used for statistical analysis. Kolmogorov-Smirnov(K) method was used to test the normal distribution of the data. Measurement data with normal distribution were represented as mean ± standard deviation (M). Measurement data with non-normal distribution were expressed as median, and quartile. Pearson chi-square test was used to compare the differences in gender composition. The independent sample t test (normal distribution) or Mann-Whitney U test (non-normal distribution) was used to compare the Pancreatic fat fraction (PFF), SA, VA and PVI between the experimental group and the control group. Pearson (normal distribution) or Spearman (non-normal distribution) correlation analysis was used to evaluate the correlation between the measured PFF and Hepatic fat component (HFF), PVI, SA, VA and clinical indicators in T2DM patients. The threshold for significance was set at 0.05.
The PFF, HFF, SA, VA and PVI of the experimental group and the control group were measured by two doctors (A and B) at different times. The Intraclass correlation coefficient (ICC) consistency test showed that the measured results were consistent between the groups (Table 1). It can be considered that the data measured by different doctors were highly consistent with intra-observer and inter-observer.
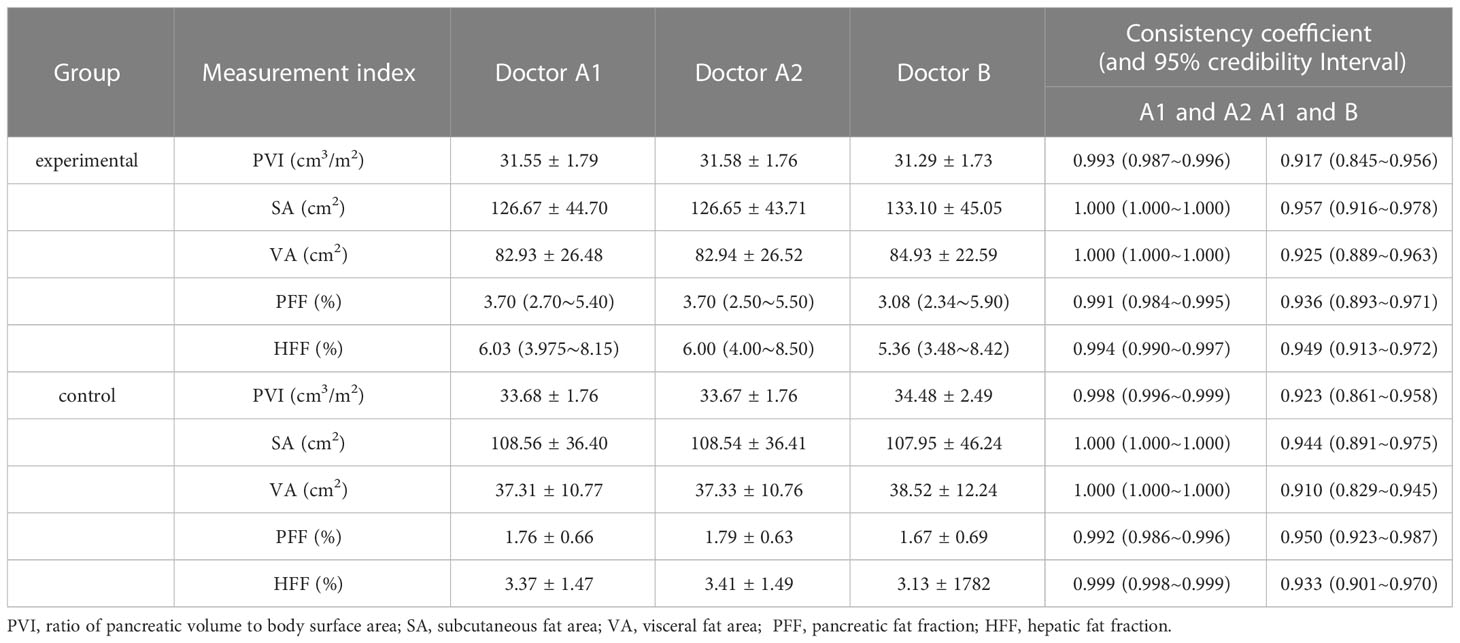
Table 1 Consistency test of PVI, subcutaneous fat area, abdominal fat area, PFF% and HFF% measured values between the experimental group and the control group by two doctors.
The normality test showed that BMI, PVI, SA, VA, TC, TG, HDL-c in the experimental group and BMI, PVI, SA, VA, PFF and HFF in the normal control group were all normal distribution (P>0.05). In the experimental group, PFF, HFF, FPC and LDL-c showed non-normal distribution (P<0.05) (Table S1).
There was no significant difference in age and gender distribution between the experimental group and the normal control group (P>0.05). The other indicators were BMI, PVI, SA, VA, PFF and HFF. There was no significant difference in BMI between the experimental group and the control group (P>0.05). There were statistical differences in PVI, SA, VA, PFF, and HFF between the two groups (P<0.05) (Table 2). PVI of T2DM patients was lower than that of control group, while SA, VA, PFF and HFF were higher than those of control group.
PFF was positively correlated with HFF in the experimental group (r=0.964, P<0.001). It was moderately positively correlated with TG, VA and Disease course (r=0.676, 0.591, 0.615, P<0.001), and weakly positively correlated with SA (r=0.321, P=0.033). There was no significant correlation with FPC, TC, PVI, HDL-c, LDL-c (r=0.385, 0.236, -0.163, -0.168, -0.002; P=0.194, 0.437, 0.292, 0.276, 0.987)(Table 3 and Figure 6). The non-standardized linear regression equation constructed with PFF as the dependent variable and the other indicators as the independent variables is: PFF=10.287+0.284HFF-0.255PVI-0.329TG+0.758Disease course(According to the inspection level of 0.05, only HFF, PVI, TG and Disease course were included in the regression equation) (Table 4). The standardization coefficients of HFF, PVI, TG and Disease course are 0.637, -0.233, -0.18 and 0.303 (Table 4).
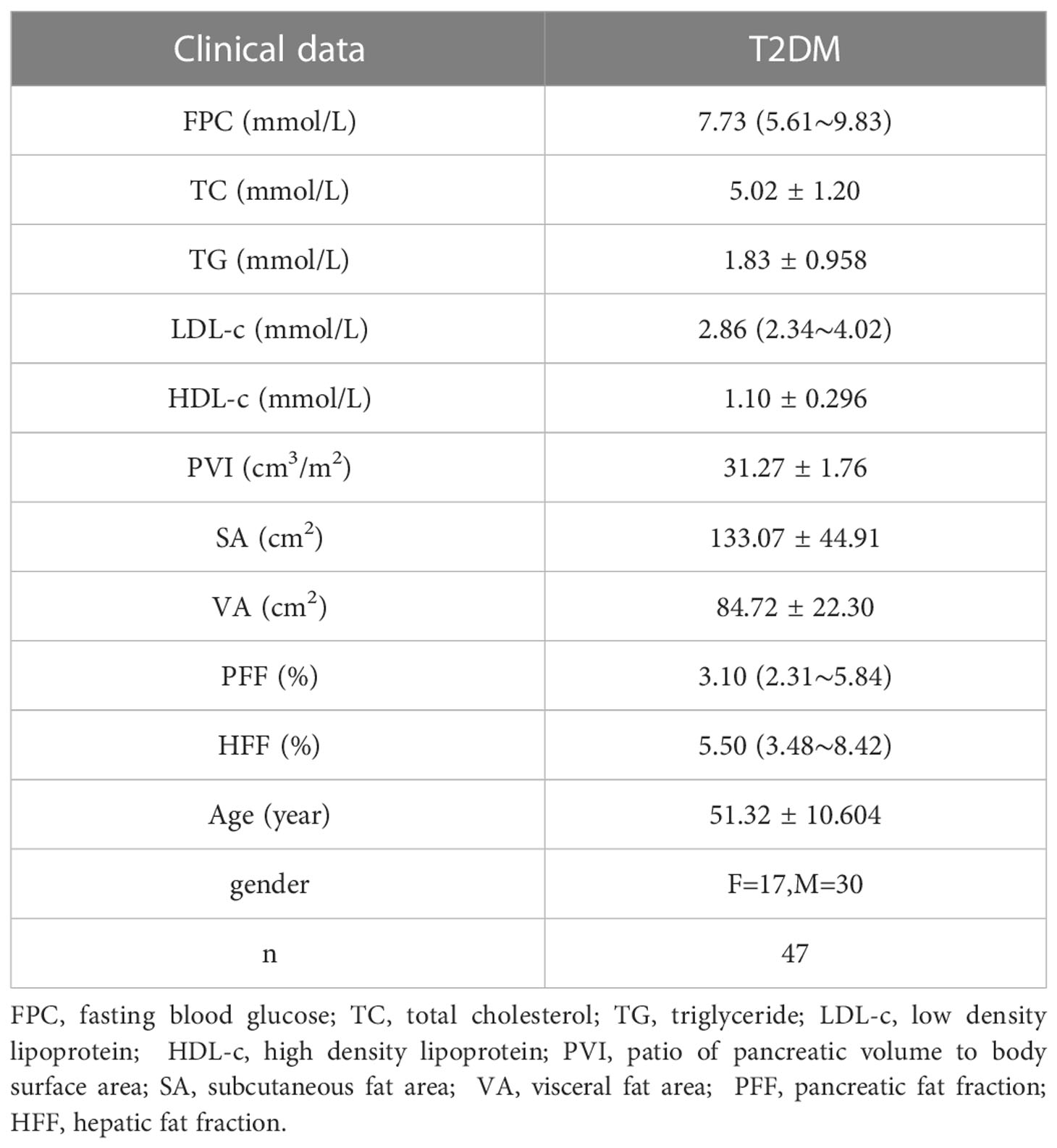
Table 3 PFF, HFF, abdominal wall, abdominal fat area and related clinical parameters in T2DM patients.
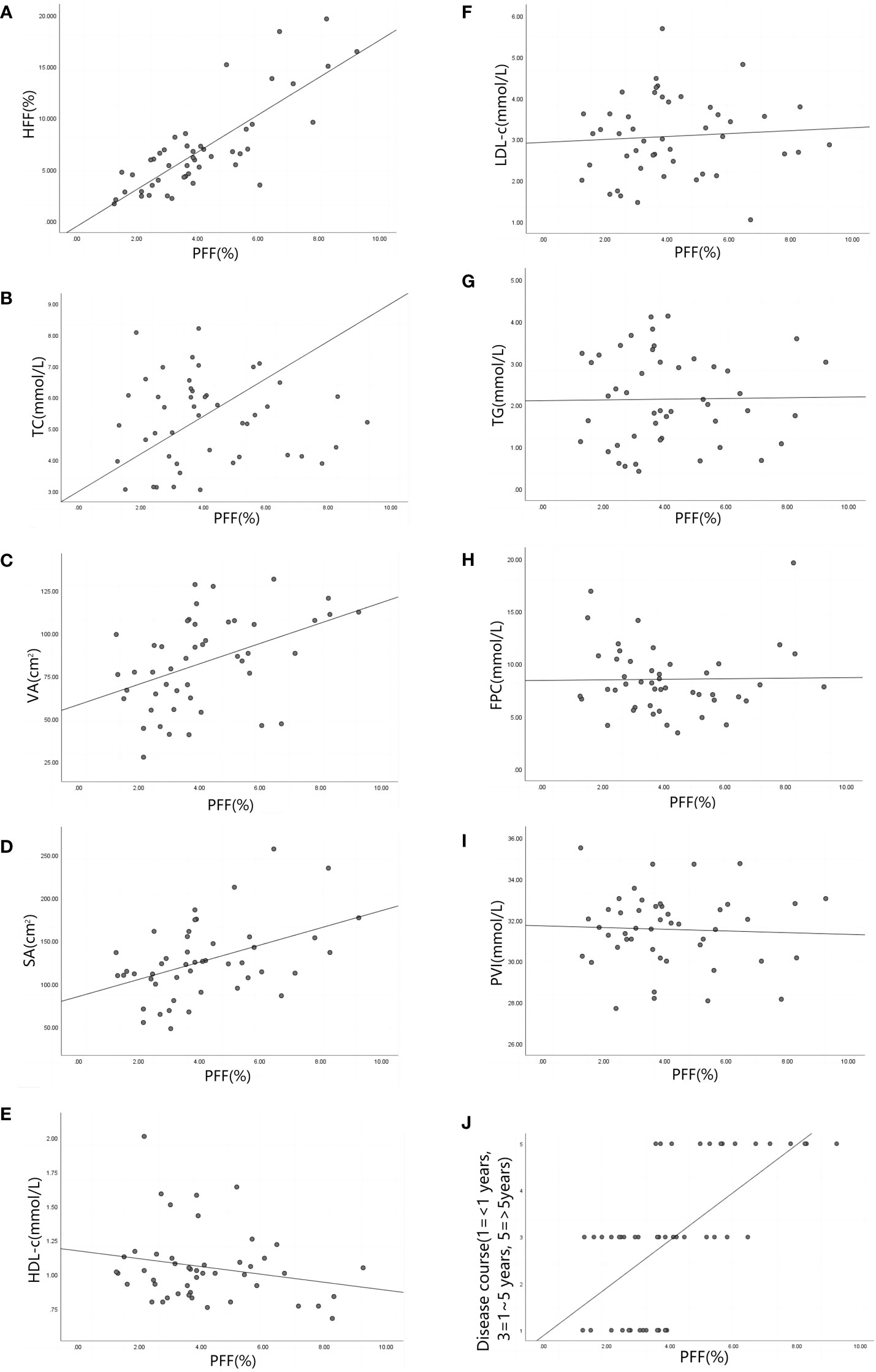
Figure 6 (A–J) are scatter plots of PFF and HFF(r=0.964, P<0.001), TC(r=0.236, P=0.437),VA(r=0.591, P<0.001), SA (r=0.321, P=0.033), HDL-c(r=-0.168, P=0.276), LDL-c(r=-0.002, P= 0.987),TG(r=0.676, P<0.001), FPC(r=0.385, P=0.194), PVI(r=-0.163, P=0.292) and Disease course(r=0.615, P<0.001) respectively. Note: pancreatic fat fraction (PFF), hepatic fat fraction (HFF), total cholesterol (TC), visceral fat area (VA), subcutaneous fat area (SA), high density lipoprotein (HDL-c), low density lipoprotein (LDL-c), triglyceride (TG), fasting blood glucose (FPC), patio of pancreatic volume to body surface area (PVI).
The measurement results of PFF values in the control group and the experimental group with different course of disease are shown in Table 5. The results of comparison between groups are shown in Table S2. The PFF of the control group and the experimental group were statistically different (P<0.05), and the pancreatic fat content of the control group was lower than that of the experimental group. There was no statistically significant difference in PFF between patients with less than one year of disease course and those with one to five years of disease course in the experimental group (P>0.05), which could not indicate that the PFF of patients with one to five years of disease course was higher than that of patients with one year of disease course. The PFF of patients with less than 1 year and 1 to 5 years of disease course was statistically different from that of patients with more than 5 years of disease course (P<0.05), which could be considered that the PFF of patients with less than 1 year and 1 to 5 years of disease course was less than that of patients with more than 5 years of disease course.
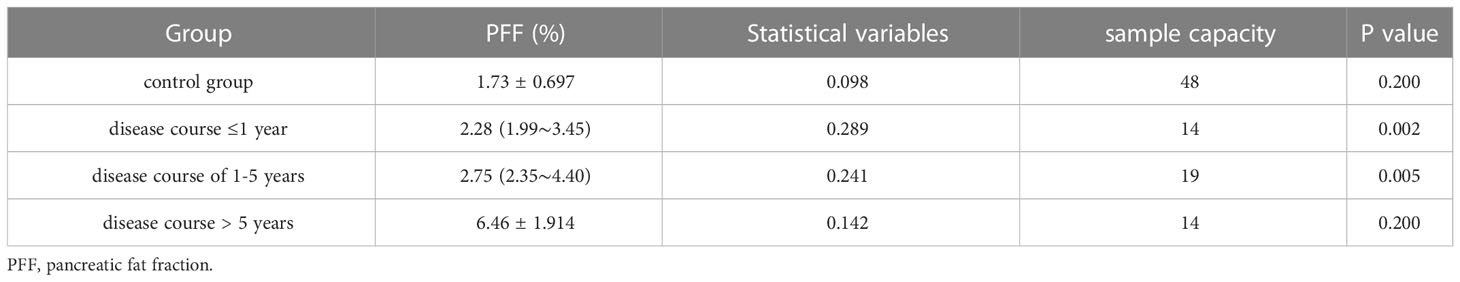
Table 5 Kolmogorov-Smirnov(K) test of PFF values of T2DM patients and control volunteers with different disease stages.
For ectopic fat accumulation in T2DM, ectopic lipid deposition can promote its development and plays an important role in its progression (19, 20). Studies have reported that pancreatic fatty infiltration is associated with insulin resistance, and the incidence of diabetes in people with pancreatic fatty infiltration is significantly higher than the other people (21, 22). At present, MRI-based fat quantification technology can identify small changes in fat content, quantify fat and monitor steatosis, making it play an increasingly important role in the assessment of pancreatic fat content (10).
In this study, 3.0T MRI qDIXon-WIP sequence was used to quantify pancreatic fat, which improved the solution to the problem of inverse calculation of water image and fat image in qDixon image. Under the condition of good consistency of gender, age and BMI matching between the experimental group and the control group, the HFF, PFF and intraperitoneal and external fat contents of the experimental group were higher than those of the control group, which reflects that there is a certain connection between abnormal fat metabolism and ectopic fat deposition. The accumulation of lipids in the the pancreas can lead to the blockage of signaling pathways and insulin resistance, thus leading to the release of inflammatory adipokines, and ultimately aggravating the deposition of fat in the abdominal organs (17). However, abnormal glucose metabolism (decreased insulin secretion or insulin resistance) will lead to weakened liver cells’ ability to metabolize fat, resulting in increased ectopic fat deposition (17, 20–22).
In this study, the PFF value of T2DM group was almost 2 times that of the normal control group (Table 2), which is similar to the study conducted by Tushuizen et al (23). However, in this study, the data in T2DM group conformed to the normal distribution and the patient sample size was sufficient. In the experimental group, HFF and PFF of patients showed a strong positive correlation, suggesting that liver fat deposition was closely related to pancreatic fat deposition, which was similar to the research results of van Geenen (24). Some of the differences in results may be related to assessment methods (ultrasound, CT, magnetic resonance), individual differences (psychological factors, diet, exercise, BMI, etc.), measurement methods (delineation of areas of interest, uneven distribution of fat deposits in the the pancreas) and other factors. T2DM patients have abnormal metabolism, which will cause the increase of TG. When the TG in the body is supersaturation in adipose tissue, lipids will be accumulated in non-fatty organs, such as the pancreas, etc., and pancreatic fat infiltration will promote the progression of T2DM and the increase of TG (5). In the experimental group, the moderate positive correlation between PFF and TG indicates that they have a close relationship. The study of Hu and Yamazaki showed that abdominal fat accumulation and abdominal fat deposition were related to diabetes and other risk factors (25, 26). The study of Yu, Van and Anderson showed that SA and VA in T2DM patients were also related to T2DM: intra-abdominal fat decreased the inhibitory effect of insulin on lipolysis by increasing gluconogenesis and insulin sensitivity (27, 28). In this study, PFF was moderately positively correlated with abdominal fat and weakly positively correlated with subcutaneous fat area, which also reflected that intra-abdominal and extra-abdominal fat deposition were related factors for pancreatic fat infiltration. Some studies also pointed out that there was a significant correlation between abdominal fat distribution and older patients (29), and the different course of T2DM patients led to certain differences in results. In addition, this study also compared the correlation between PFF and FPC, PVI, HDL-c, TC and LDL-c, and the results indicated that there was no significant correlation. The constructed linear regression equation points out that among the relevant indicators in this study, HFF, PVI, TG and Disease course have greater contribution to PFF, that is, these four factors are closely related to PFF.
The patients in the experimental group were divided into three groups according to the course of disease: course of disease ≤1 year, 1 year < course of disease < 5 years, and course of disease ≥5 years. The results suggest that pancreatic fat accumulation is higher in patients with long course of T2DM than in those with short course of T2DM. According to the linear regression analysis, the standardized regression coefficient for Disease course was 0.303, which points out the degree of fat accumulation is higher in those with long-standing diabetes. And as mentioned above, insulin resistance causes ectopic fat deposition, and pancreatic fat also accumulates in the progression of T2DM. The results of this study may partly explain that pancreatic fat infiltration is a gradual accumulation process in patients with long disease course, but the degree of fat accumulation is slower in patients with short disease course.
Limitations of this study: (1) Due to the small sample size, further sample expansion is needed to improve the reliability of the experimental results. (2) Due to the age distribution characteristics of the diabetic population, the age of T2DM patients included in this study ranged from 32 to 71 years old, and the corresponding age of normal control population was matched, and the data obtained had certain bias. (3) In this study, T2DM patients were randomly sampled, and subgroup analysis of patients with different clinical interventions was not performed. The degree of pancreatic fat infiltration is likely to be different in patients with different interventions. This study can further focus on the relationship between pancreatic fat deposition and T2DM intervention.
In this study, the qDixon-WIP sequence was used to conduct clinical experiments. The results showed that: (1) PVI decreased, while SA, VA, PFF and HFF increased in T2DM patients. (2) PFF was positively correlated with HFF, TG, abdominal fat area and subcutaneous fat area in T2DM patients. (3) The degree of pancreatic fat accumulation in patients with long course of disease was higher than that in patients with short course of disease. This sequence can be used in clinical research to quantitatively measure pancreatic fat content with good repeatability, which can provide reference for clinical assessment of pancreatic fat to achieve real-time monitoring of the occurrence and progression of diseases.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated during and/or analysed during the current study are not publicly available, but are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to LiLing Long, Y2pyLmxvbmdsaWxpbmdAdmlwLjE2My5jb20=.
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (NO.2022-E460-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Material preparation and data collection were performed by JY, FX, TL, SL and QF. Data analysis were performed by JY, FX. The first draft of the manuscript was written by JY, FX and BL and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version. LL contributed to the study conception and design.
This work was supported by grants from the Natural Science Foundation of China (81860303).
We sincerely thank all the participants of our study. We thank Cheng Tang from the Radiology Department of the First Affiliated Hospital of Guangxi Medical University for the technical support to MRI scanning.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1140111/full#supplementary-material
Supplementary Table 1 | Kolmogorov-Smirnov (K) test of normal distribution of experimental data.
Supplementary Table 2 | Test results of PFF difference between T2DM patients and control group volunteers with different disease course.
1. Jenkins A, Lee MK, Kadowaki T, IDF-WPR. Comprehensive IDF-WPR diabetes and disasters manual, 2nd edition available. Diabetes Res Clin Pract (2022), 110209. doi: 10.1016/j.diabres.2022.110209
2. Dong G, Qu L, Gong X, Pang B, Yan W, Wei J. Effect of social factors and the natural environment on the etiology and pathogenesis of diabetes mellitus. Int J Endocrinol (2019) 2019:8749291. doi: 10.1155/2019/8749291
3. Ng N, Mijares Zamuner M, Siddique N, Kim J, Burke M, Byrne MM. Genotype-phenotype correlations and response to glucose lowering therapy in subjects with HNF1β associated diabetes. Acta diabetol (2022) 59(1):83–93. doi: 10.1007/s00592-021-01794-8
4. Chan TT, Tse YK, Lui RN, Wong GL, Chim AM, Kong AP, et al. Fatty pancreas is independently associated with subsequent diabetes mellitus development: A 10-year prospective cohort study. Clin Gastroenterol Hepatol (2022) 20(9):2014–2022.e4. doi: 10.1016/j.cgh.2021.09.027
5. Kim JY, Nasr A, Tfayli H, Bacha F, Michaliszyn SF, Arslanian S. Increased lipolysis, diminished adipose tissue insulin sensitivity, and impaired β-cell function relative to adipose tissue insulin sensitivity in obese youth with impaired glucose tolerance. Diabetes (2017) 66(12):3085–90. doi: 10.2337/db17-0551
6. Chan JY, Bensellam M, Lin R, Liang C, Lee K, Jonas JC. Transcriptome analysis of islets from diabetes-resistant and diabetes-prone obese mice reveals novel gene regulatory networks involved in beta-cell compensation and failure. FASEB J (2021) 35(6):e21608. doi: 10.1096/fj.202100009R
7. Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab (2018) 28(4):547–556.e3. doi: 10.1016/j.cmet.2018.07.003
8. Suleiman M, Marselli L, Cnop M, Eizirik DL, De Luca C, Femia FR, et al. The role of beta cell recovery in type 2 diabetes remission. Int J Mol Sci (2022) 23(13):7435. doi: 10.3390/ijms23137435
9. Solimena M, Schulte AM, Marselli L, Ehehalt F, Richter D, Kleeberg M, et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia (2018) 61(3):641–57. doi: 10.1007/s00125-017-4500-3
10. Kühn JP, Hernando D, Muñoz del Rio A, Evert M, Kannengiesser S, Völzke H, et al. Effect of multipeak spectral modeling of fat for liver iron and fat quantification: Correlation of biopsy with MR imaging results. Radiology (2012) 265(1):133–42. doi: 10.1148/radiol.12112520
11. Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: Blinded comparison with MR spectroscopy. Radiology (2011) 258(3):767–75. doi: 10.1148/radiol.10100708
12. Yokoo T, Shiehmorteza M, Hamilton G, Wolfson T, Schroeder ME, Middleton MS, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology (2011) 258(3):749–59. doi: 10.1148/radiol.10100659
13. Kühn JP, Berthold F, Mayerle J, Völzke H, Reeder SB, Rathmann W, et al. Pancreatic steatosis demonstrated at MR imaging in the general population: Clinical relevance. Radiology (2015) 276(1):129–36. doi: 10.1148/radiol.15140446
14. Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol (2014) 20(23):7392–402. doi: 10.3748/wjg.v20.i23.7392
15. Yu H, Shimakawa A, Hines CD, McKenzie CA, Hamilton G, Sirlin CB, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magnetic Resonance Med (2011) 66(1):199–206. doi: 10.1002/mrm.22840
16. Hong CW, Fazeli Dehkordy S, Hooker JC, Hamilton G, Sirlin CB. Fat quantification in the abdomen. Topics magnetic resonance imaging: TMRI (2017) 26(6):221–7. doi: 10.1097/RMR.0000000000000141
17. Bawden SJ, Hoad C, Kaye P, Stephenson M, Dolman G, James MW, et al. Comparing magnetic resonance liver fat fraction measurements with histology in fibrosis: the difference between proton density fat fraction and tissue mass fat fraction. Magma (New York N.Y.) (2022). doi: 10.1007/s10334-022-01052-0
18. Hu Y, Wu X, Hu Z, Ren A, Wei X, Wang X, et al. Research on the human surface area formula in China. Acta Physiol Sin (1999) 51(1):45–8.
19. Filippatos TD, Alexakis K, Mavrikaki V, Mikhailidis DP. Nonalcoholic fatty the pancreas disease: Role in metabolic syndrome, “Prediabetes,” diabetes and atherosclerosis. Digestive Dis Sci (2022) 67(1):26–41. doi: 10.1007/s10620-021-06824-7
20. Sreedhar UL, DeSouza SV, Park B, Petrov MS. A systematic review of intra-pancreatic fat deposition and pancreatic carcinogenesis. J Gastrointestinal Surg (2020) 24(11):2560–9. doi: 10.1007/s11605-019-04417-4
21. Sanchez Caballero L, Gorgogietas V, Arroyo MN, Igoillo-Esteve M. Molecular mechanisms of β-cell dysfunction and death in monogenic forms of diabetes. Int Rev Cell Mol Biol (2021) 359:139–256. doi: 10.1016/bs.ircmb.2021.02.005
22. Miranda MA, Macias-Velasco JF, Lawson HA. Pancreatic β-cell heterogeneity in health and diabetes: Classes, sources, and subtypes. Am J Physiol Endocrinol Metab (2021) 320(4):E716–31. doi: 10.1152/ajpendo.00649.2020
23. Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care (2007) 30(11):2916–21. doi: 10.2337/dc07-0326
24. van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty the pancreas disease. Pancreas (2010) 39(8):1185–90. doi: 10.1097/MPA.0b013e3181f6fce2
25. Hu HH, Kim HW, Nayak KS, Goran MI. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obes (Silver Spring Md.) (2010) 18(4):841–7. doi: 10.1038/oby.2009.352
26. Yamazaki H, Tauchi S, Kimachi M, Dohke M, Hanawa N, Kodama Y, et al. Association between pancreatic fat and incidence of metabolic syndrome: A 5-year Japanese cohort study. J Gastroenterol Hepatol (2018) 33(12):2048–54. doi: 10.1111/jgh.14266
27. Yu TY, Wang CY. Impact of non-alcoholic fatty the pancreas disease on glucose metabolism. J Diabetes Invest (2017) 8(6):735–47. doi: 10.1111/jdi.12665
28. Anderson PJ, Chan JC, Chan YL, Tomlinson B, Young RP, Lee ZS, et al. Visceral fat and cardiovascular risk factors in Chinese NIDDM patients. Diabetes Care (1997) 20(12):1854–8. doi: 10.2337/diacare.20.12.1854
Keywords: multi-echo Dixon, magnetic resonance imaging, pancreatic fat infiltration, type 2 diabetes, quantitative study
Citation: Yi J, Xu F, Li T, Liang B, Li S, Feng Q and Long L (2023) Quantitative study of 3T MRI qDixon-WIP applied in pancreatic fat infiltration in patients with type 2 diabetes mellitus. Front. Endocrinol. 14:1140111. doi: 10.3389/fendo.2023.1140111
Received: 08 January 2023; Accepted: 02 February 2023;
Published: 17 February 2023.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Francesca Cinti, Unit of Endocrinology and Diabetology (IRCCS), ItalyCopyright © 2023 Yi, Xu, Li, Liang, Li, Feng and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liling Long, Y2pyLmxvbmdsaWxpbmdAdmlwLjE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.