- 1Department of Urology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 2Department of Endocrinology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 3Department of Urology, Hainan General Hospital, Haikou, China
Background: Adult pure androgen–secreting adrenal tumors (PASATs) are extremely rare, and their characteristics are largely unknown.
Methods: A rare case of adult bilateral PASATs was reported, and a systematic literature review of adult PASATs was conducted to summarize the characteristics of PASATs.
Results: In total, 48 studies, including 40 case reports and 8 articles, were identified in this review. Analysis based on data of 42 patients (including current case and 41 patients from 40 case reports) showed that average age was 40.48 ± 15.80 years (range of 18-76). The incidence of adult PASAT peaked at 21-30 years old, while that of malignant PASAT peaked at 41-50 years old. Most PASAT patients were female (40/42, 95.23%), and hirsutism was the most common symptom (37/39, 94.87%). Testosterone (T) was the most commonly elevated androgen (36/42, 85.71%), and 26 of 32 tested patients presented increased dehydroepiandrosterone sulfate (DS) levels. In malignancy cases, disease duration was significantly decreased (1.96 vs. 4.51 years, P=0.025), and tumor diameter was significantly increased (8.9 vs. 4.9 cm, p=0.011). Moreover, the androgen levels, namely, T/upper normal range limit (UNRL) (11.94 vs. 4.943, P=0.770) and DS/UNRL (16.5 vs. 5.28, P=0.625), were higher in patients with malignancy. In total, 5 out of 7 patients showed an increase in DS or T in the human chorionic gonadotropin (HCG) stimulation test. Overall, 41 out of 42 patients (including current case) underwent adrenal surgery, and recurrence, metastasis, or death was reported in 5 out of 11 malignant patients even with adjuvant or rescue mitotane chemotherapy.
Conclusion: Adult PASAT, which is predominant in women, is characterized by virilism and menstrual dysfunction, especially hirsutism. Elevated T and DS may contribute to the diagnosis of adult PASAT, and HCG stimulation test might also be of help in diagnosis. Patients with malignant PASAT have a shorter disease duration, larger tumor sizes and relatively higher androgen levels. Surgery is recommended for all local PASATs, and Malignancy of PASAT should be fully considered due to the high risk of malignancy, poor prognosis and limited effective approaches.
Introduction
The adrenal gland is a complex structure consisting of an inner adrenal medulla and an outer adrenal cortex. Adrenal steroid hormones are synthesized in the following three zones: aldosterone in the zona glomerulosa (ZG); glucocorticoids (particularly cortisol) in the zona fasciculata (ZF); and androgens and estrogens in the zona reticularis (ZR). Aldosterone production is regulated by the renin–angiotensin–aldosterone system, while the synthesis of cortisol and androgens is modulated by the hypothalamic−pituitary−adrenal (HPA) axis (1). The primary function of adrenocorticotropic hormone (ACTH) is to stimulate cortisol and adrenal androgen secretion by increasing their synthesis. The negative feedback from circulating cortisol suppresses the release of corticotropin-releasing hormone (CRH) and ACTH, but adrenal androgens do not have similar suppressive effects on the HPA axis. Compared to cortisol- and aldosterone-secreting tumors, androgen-secreting adrenocortical tumors are less common. Adrenocortical tumors, including androgen-secreting tumors, in children have been investigated in previous studies (2–4), but androgen-secreting adrenal tumors in adults, especially pure androgen–secreting adrenal tumors (PASATs), which are usually reported as case reports or case series, are rare. In the present study, we report a rare case of adult bilateral PASATs and conduct a systematic review of adult PASATs.
Case presentation
A 28-year-old woman was admitted to our hospital in 2022 due to oligomenorrhea and then amenorrhea. The patient (menarche at 13 years) had regular menstruation with a menstrual cycle of 30-40 days and a menstrual period of 6-7 days. The patient’s menstrual cycle then shortened, and her menstrual bleeding gradually decreased from 2012. At that time, her testosterone (T) level was within the normal range according to her statement. Finally, amenorrhea occurred despite the administration of estrogen and progesterone drugs. In 2015, the T level increased to 0.91 ng/ml (normal: 0.10-0.75) despite dydrogesterone administration, and ultrasound indicated polycystic morphology in bilateral ovaries. Polycystic ovary syndrome (PCOS) was considered by physicians of other hospitals, and contraceptive drugs were prescribed. During 2019-2020, the patient presented with clitoromegaly, mild hirsutism (Ferriman–Gallwey score of 9) and amenorrhea. At the same time, bilateral adrenal tumors were reported incidentally by computed tomography (CT) in a health examination.
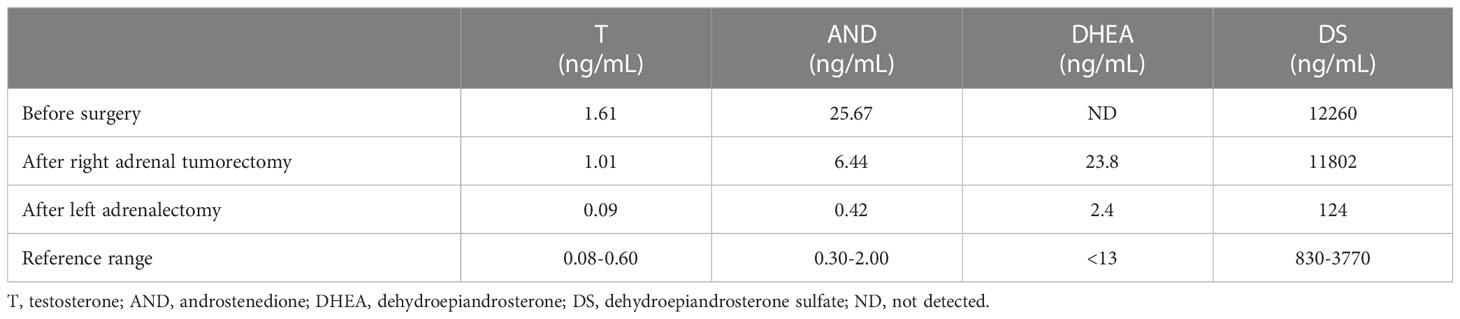
An endocrine investigation in 2021 showed that the patient’s T levels continued to increase to 1.61 ng/ml (Table 1). In addition, androstenedione (AND), dehydroepiandrosterone sulfate (DS) and 17α-hydroxypregnenolone (17OHP) also increased to 25.67 ng/mL (normal: 0.30-2.00 ng/mL), 12,260 ng/mL (normal: 830-3,770 ng/mL) and 2.49 ng/ml (normal: 0.10-2.30 ng/ml), respectively, in the patient. The ACTH level (8 am) of this patient was 28.9 pg/ml (normal: 7.2-63.3). Both the cortisol level (10.0 µg/dl, normal: 4-22) and 24-h urinary free cortisol level (52.0 µg/24 hr, normal: 12.3-103) were normal. The cortisol level was suppressed (<1.8 µg/dl) by the 48-h low-dose dexamethasone-suppression test (0.5 mg orally every 6 hours for a total of eight doses). In addition, the levels of plasma aldosterone, catecholamine and sexual hormones, including follicle-stimulating hormone (FSH), luteinizing hormone (LH) and oestradiol, were normal. The patient underwent ACTH stimulation test, and the 17OHP level was 3.89 ng/ml at 60 minutes after administering cosyntropin (0.25 mg), which basically excluded classic and nonclassic congenital adrenal hyperplasia (CAH) according to the endocrine society clinical practice guidelines (17OHP <10 ng/ml) (5, 6). The patient’s bilateral adrenal tumors were gradually enlarged (Figure 1A) and were considered to be responsible for hyperandrogenism. The patient’s right adrenal tumor was removed by laparoscopic partial adrenalectomy at another hospital, and adrenocortical adenoma was identified according to pathological findings.
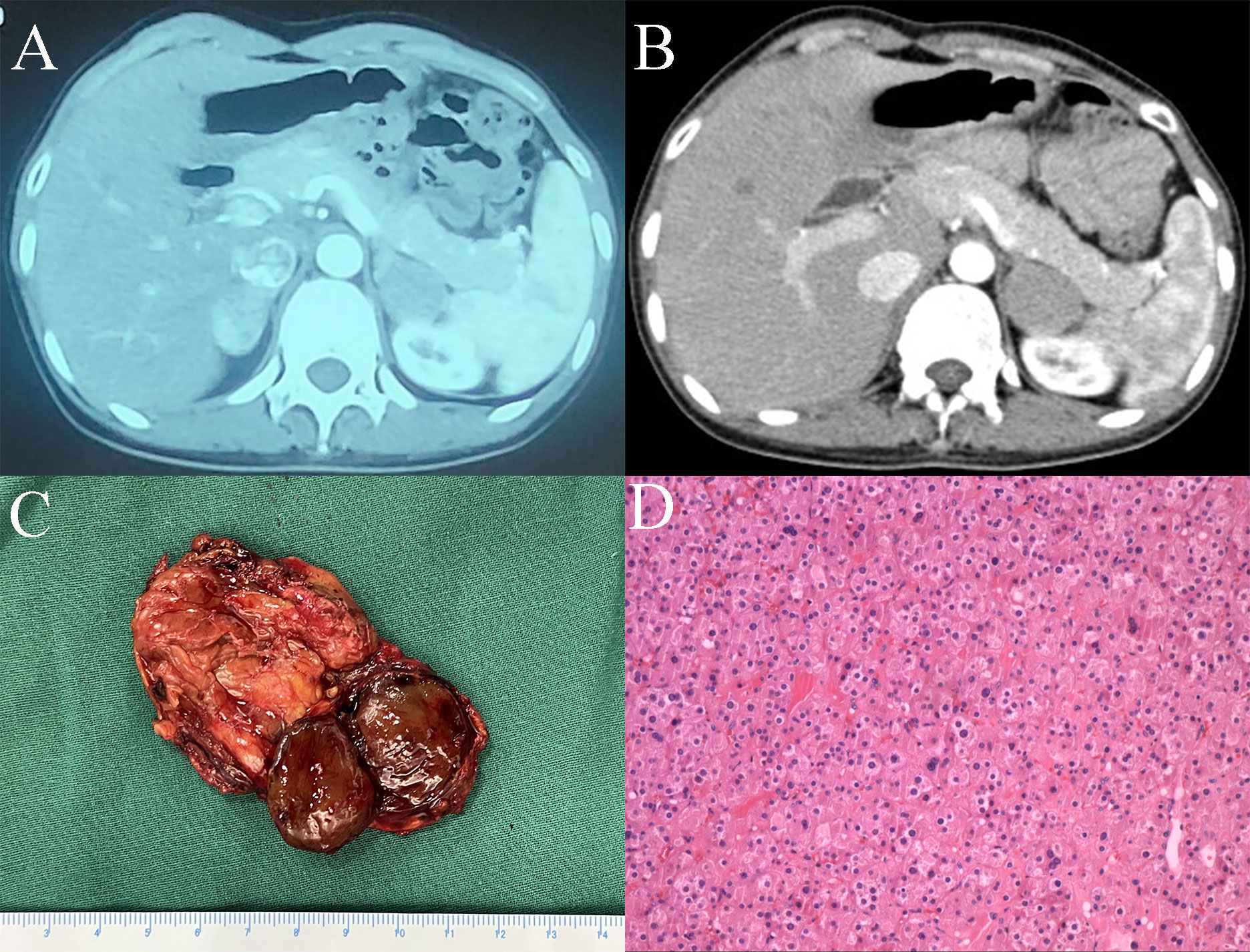
Figure 1 (A) CT image of bilateral adrenal tumors. (B) CT image of the left adrenal tumor after right adrenal tumorectomy. (C) Surgical specimen of the left adrenal tumor. (D) Haematoxylin and eosin staining of the left adrenal tumor (magnification×20).
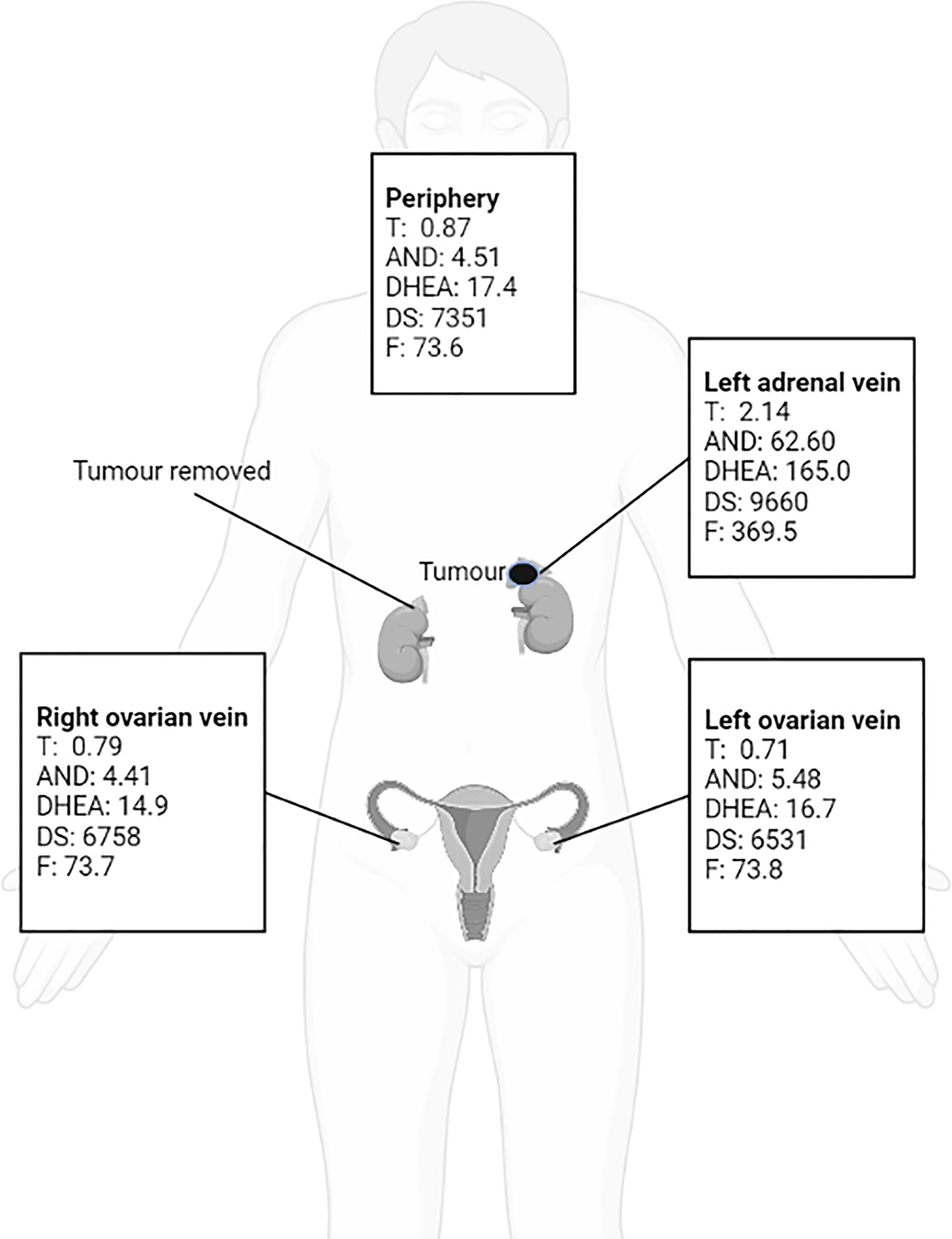
Because the patient’s amenorrhea was not relieved, she was admitted to our hospital and underwent further endocrine and imageological examination (Figure 1B). The patient’s testosterone (T), androstenedione (ADN) and dehydroepiandrosterone sulfate (DS) levels were partly decreased but still above the normal range (Table 1). Adrenal steroid hormone tests indicated that pregnenolone and dehydroepiandrosterone (DHEA) were also elevated in addition to T, DS, AND and 17OHP, while the levels of steroids located in the cortisol and aldosterone synthesis pathways were normal (Figure 2). Bilateral ovarian venous sampling (OVS) and left adrenal venous sampling (AVS) were conducted (Figure 3). The left selectivity index (SI, defined as the ratio of cortisol concentration for each adrenal vein and infra-adrenal inferior vena cava or a peripheral vein) was 5.02, which indicated successful catheterization (more than 2:1 without stimulation cosyntropin use) (7). The levels of androgens, including T, ADN, DS and DHEA, in the left adrenal vein were higher (Figure 3), which indicated that excessive androgens were produced by the left adrenal gland rather than the ovaries. The patient underwent left laparoscopic adrenalectomy in our hospital, and the surgical specimen was showed in Figure 1C. The histopathologic examination confirmed the diagnosis of adrenocortical adenoma (Figure 1D). Two weeks after surgery, the levels of T, ADN and DHEA decreased to within the normal range, and the DS level decreased to below the normal level (Table 1). Then DS level returned to the normal range within 3 months. The patient’s menstruation recovered to normal in 2 months after surgery, and no recurrence or metastasis was detected nearly one year after surgery.
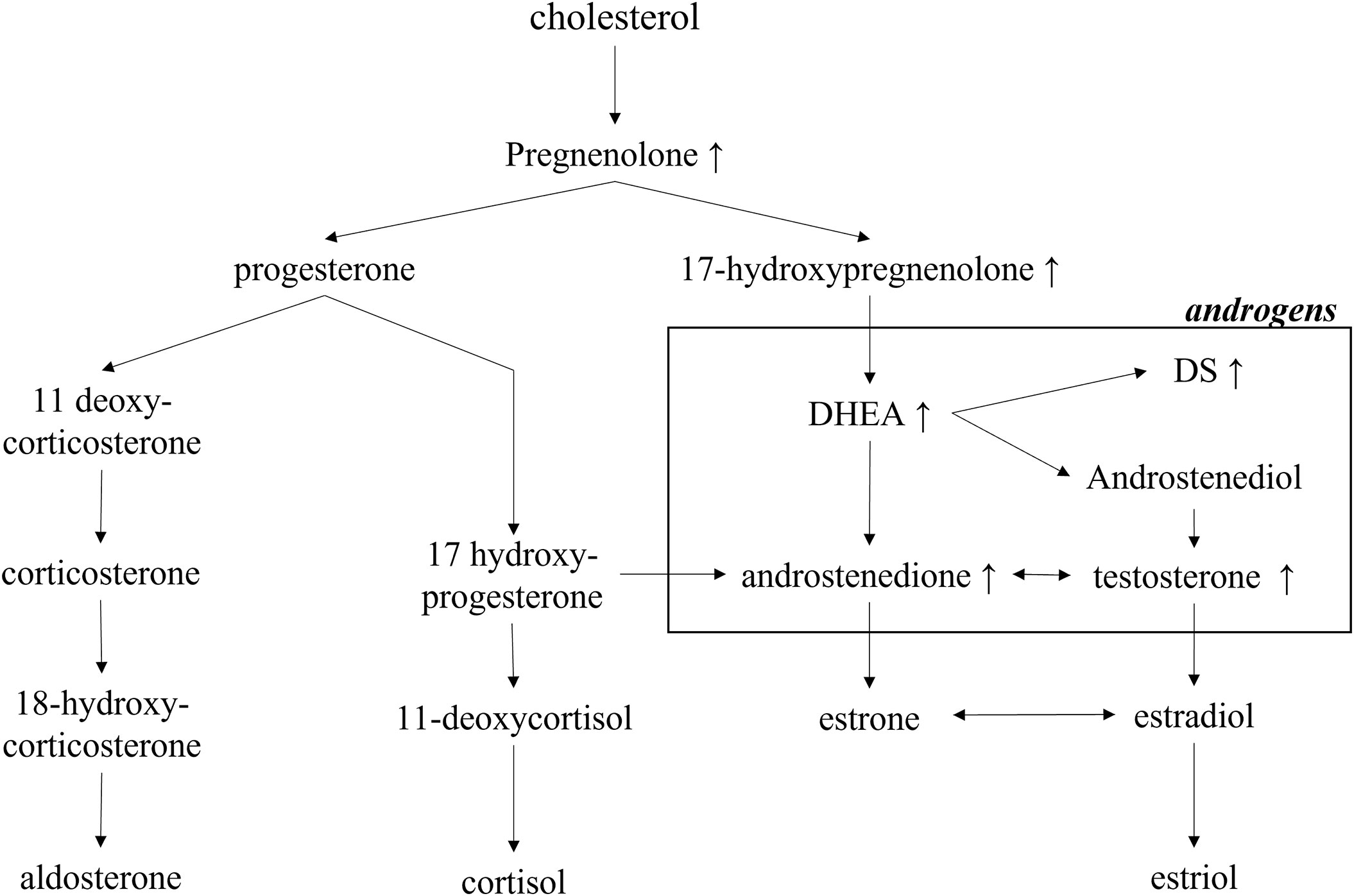
Figure 2 Exclusively increased steroid hormones in the androgen-producing pathway in the patient. DHEA, dehydroepiandrosterone; DS, dehydroepiandrosterone sulfate.
Methods
A systematic review of the literature was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 4). The following search strategy was used in the PubMed online database until December 2022: (“androgen”[Title/Abstract] OR “hyperandrogenism”[Title/Abstract] OR “virilism”[Title/Abstract]) AND (“adrenocortical”[Title/Abstract] OR “adrenal”[Title/Abstract]) AND (“tumor”[Title/Abstract] OR “carcinoma”[Title/Abstract] OR “adenoma”[Title/Abstract]) NOT (“children”[Title/Abstract] OR “child”[Title/Abstract] “childhood”[Title/Abstract] OR “paediatric”[Title/Abstract] OR “peripubertal”[Title/Abstract]). Only confirmed cases of adrenal tumors exclusively secreting androgens were included in this review. The exclusion criteria for studies were as follows: (1) age < 18 years; (2) excessive secretion of cortisol, aldosterone and catecholamine, especially cortisol, because cortisol and androgen are usually produced simultaneously in certain adrenal carcinomas or adenomas (only studies with evidence to show normal cortisol in the blood or urine were included); (3) hyperandrogenic status partly or completely caused by nonadrenal factors, including polycystic ovary syndrome (PCOS), idiopathic hyperandrogenism, hyperandrogenic insulin-resistant acanthosis nigricans syndrome (HAIRAN) and ovarian androgen-secreting neoplasms; (4) congenital adrenal hyperplasia (CAH); (5) basic science or animal experiments; (6) editorials, letters and consensus reports; (7) unavailable full text article; or (8) non-English studies.
The clinical data from the included studies were analyzed using GraphPad Prism (version 9; GraphPad Software, San Diego, California, USA).
Continuous data are presented as the mean ± standard deviation (SD), and Student’s t-test or the Mann−Whitney U test was utilized to compare the differences between the malignant and benign groups. A value of P<0.05 was considered statistically significant.
Results
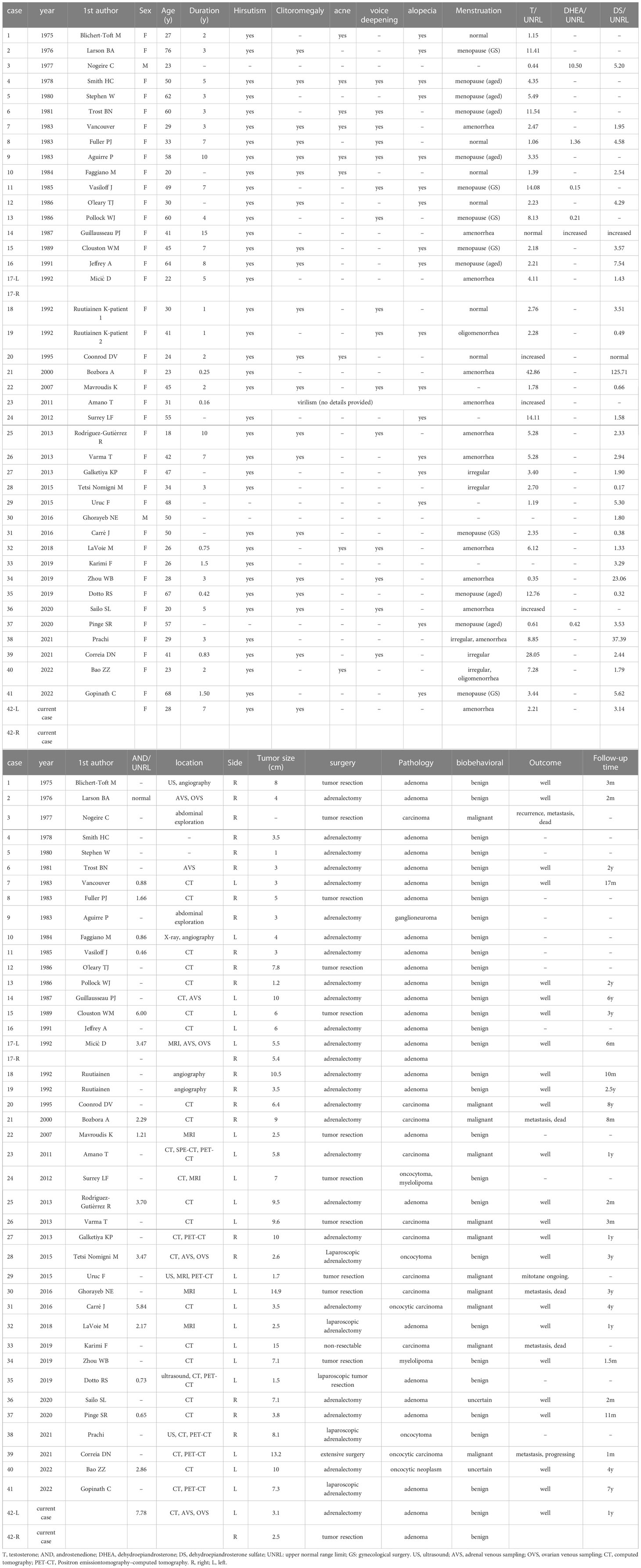
In total, 48 studies met the inclusion criteria, including 40 case reports (8–47) and 8 articles (48–55). Forty case reports including 41 adult PASAT cases are summarized in Table 2, and 8 articles containing adult PASAT patients are summarized in Table 3. Statistical analysis was conducted on the data of 42 PASAT adult patients (including the present case) (Figures 5, 6).
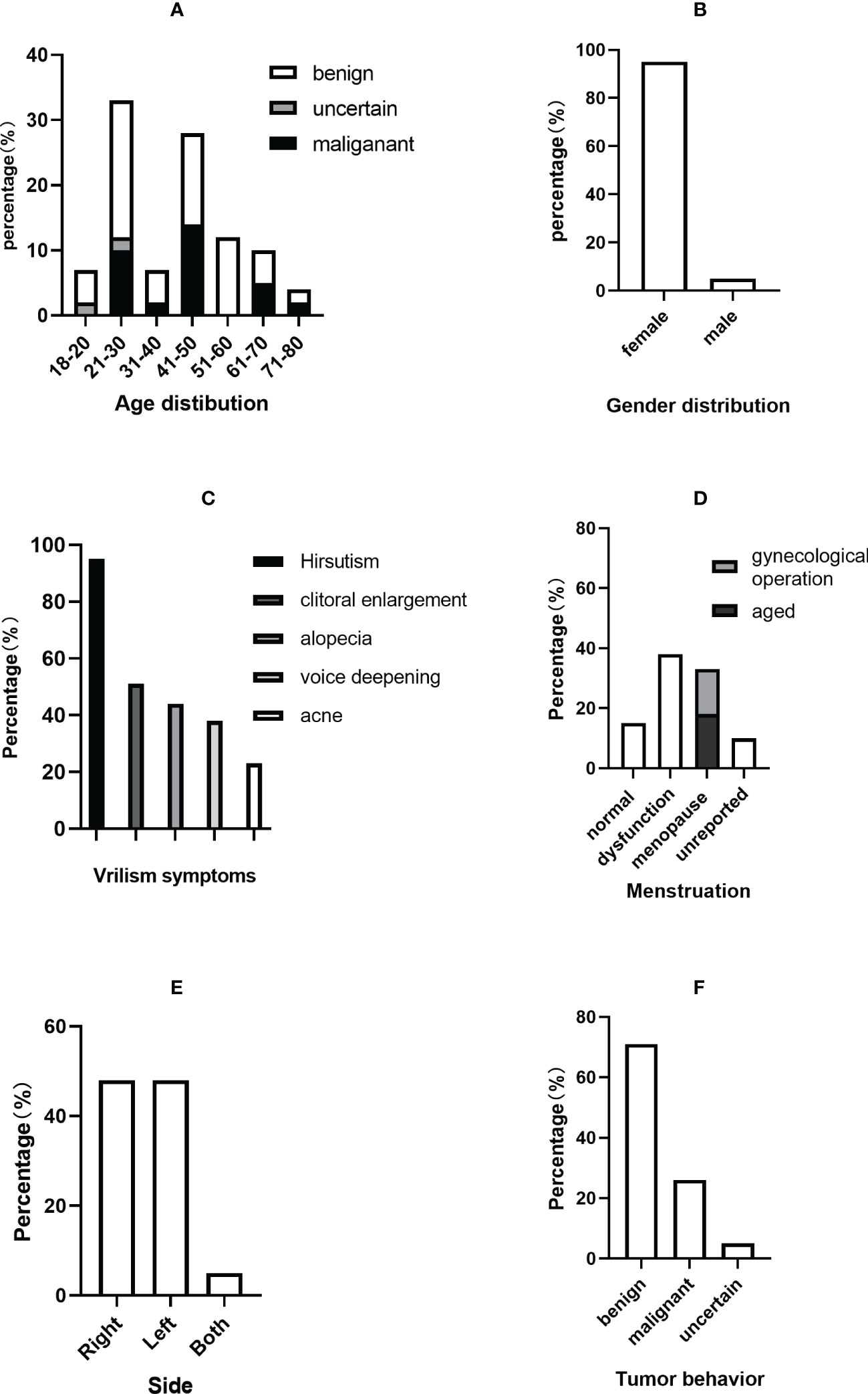
Figure 5 Clinical characteristics of PASAT cases. (A) Age distribution. (B) Sex distribution. (C) Virilism symptoms. (D) Menstruation change. (E) Tumor side. (F) Tumor behavior.
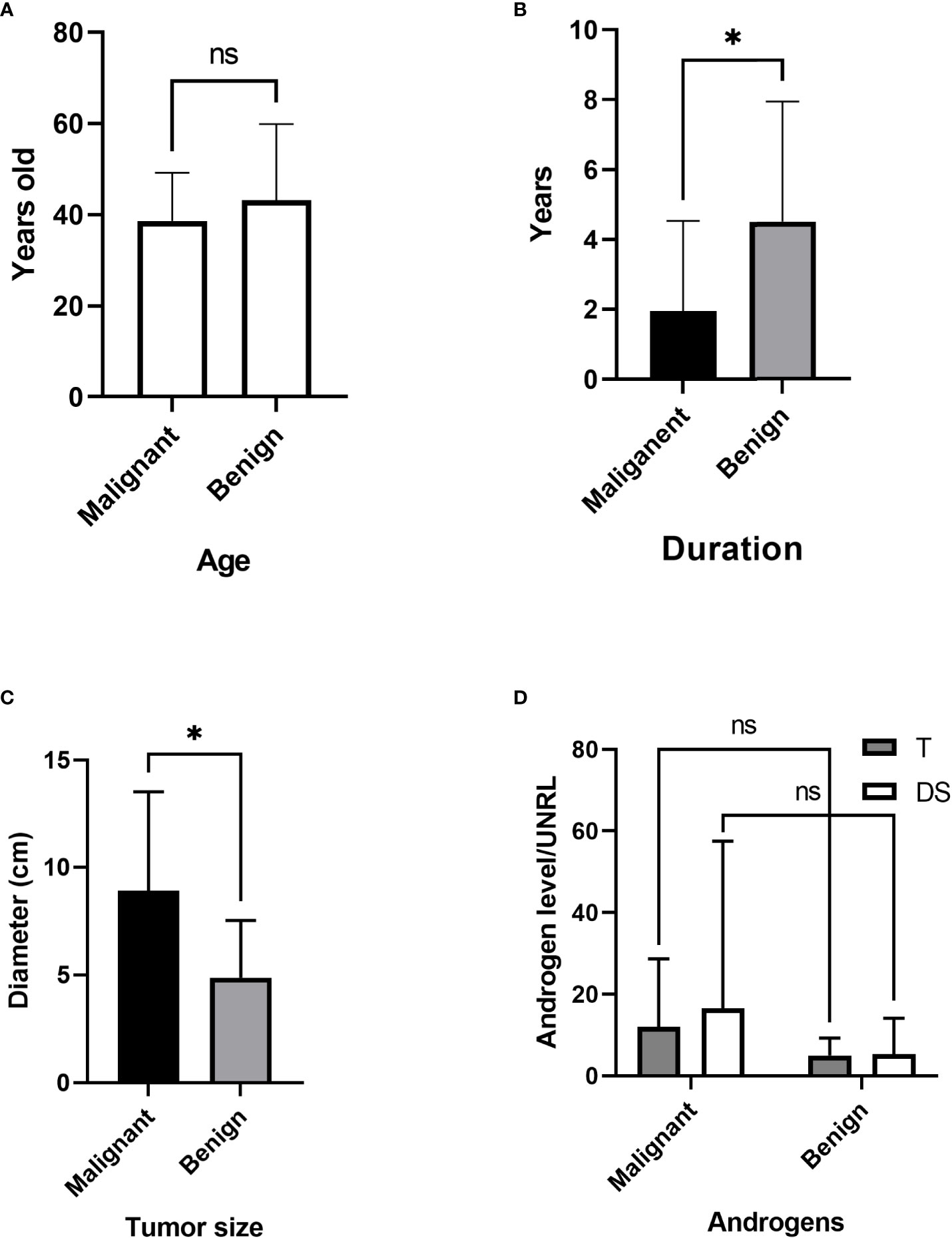
Figure 6 Comparison of malignant and benign tumors. (A) Age differences. (B) Duration before diagnosis. (C) Tumor size. (D) Androgen level. T, testosterone; DS, dehydroepiandrosterone sulfate; UNRL, upper normal range limit. ns, not significant; * P<0.05.
The average ages of all patients, the malignant group and the benign group were 40.48 ± 15.80, 38.64 ± 10.62 and 43.17 ± 16.73 years, respectively. The incidence of adult PASAT peaked at 21-30 years old, followed by 41-50 years old, while the incidence of malignant PASAT peaked at 41-50 years old (Figure 5A). In addition, PASATs were predominantly found in women (40/42, 95.23%; Figure 5B).
Hirsutism was the most common symptom, and almost all female PASAT patients (37/39, 94.87%) presented with hirsutism (one patient’s virilism was not described in detail), followed by clitoral enlargement, alopecia, voice deepening and acne (Figure 5C). Menstrual dysfunction, including oligomenorrhea, irregularity and even amenorrhea, was reported in 42.5% of female patients, while 15% of female patients showed normal menstruation (Figure 5D). There was no difference in tumor location (left or right side) (Figure 5E), but there were only two bilateral PASATs, including the present case. Tumors showing benign or malignant biological behavior accounted for 71.43% and 26.19%, respectively, with 2 uncertain tumors (Figure 5F).
The age differences between the malignant and benign groups were not significant (Figure 6A). The duration in the malignant group was significantly shorter (Figure 6B) than that in the benign group (1.96 vs. 4.51 years, P=0.025), while the tumor diameter in the malignant group was significantly increased (8.9 vs. 4.9 cm, p=0.011) (Figure 6C). For comparison, the androgen level was demonstrated as androgen concentration/upper normal range limit (UNRL). T was elevated in 36 adult PASAT patients (85.71%). The androgen levels, shown as T/UNRL (11.94 vs. 4.943, P=0.770) and DS/UNRL (16.5 vs. 5.28, P=0.625), in the malignant group were much higher than those in the benign group, but the differences were not significant (Figure 6D).
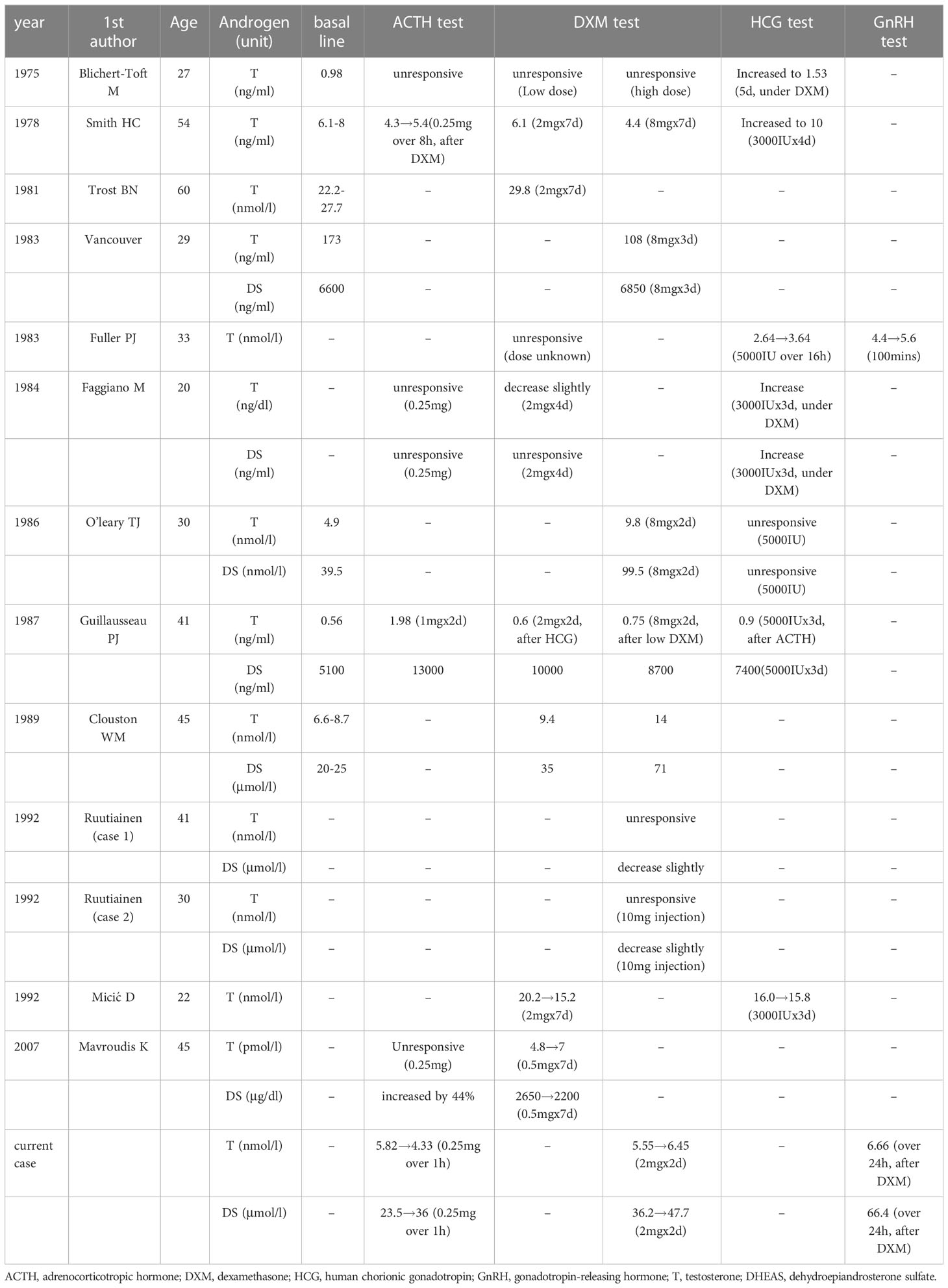
The responses of T and DS to the endocrine test are shown in Table 4. In the ACTH/synacthen test, the androgen levels increased in two patients but did not change in two patients. In another patient, the level of DS increased, but T was unresponsive. In the current patient, T decreased, but DS increased. The androgen response to the low-dose or high-dose dexamethasone test was not coordinated in different patients, and there were also paradoxical T and DS responses in the same patient. Regarding HCG (human chorionic gonadotropin) stimulation, most patients (5/7) showed an increase in DS or T. Furthermore, T and DS were slightly increased in the gonadotropin-releasing hormone (GnRH) test in two patients.
The androgen level decreased in 36 patients in a short time after surgery, and virilism symptoms were also partly or completely relieved. However, details regarding androgens after surgery were not reported in six patients. All ten patient with malignancy underwent surgery, except for one patient with nonresectable disease with multiple metastases at diagnosis (Table 2). Mitotane was applied to seven malignant patients (5 post-surgically, 1 with nonresectable disease and 1 after recurrence). Five patients with malignancy (including the one with nonresectable disease) were reported to have experienced recurrence, metastasis, or death.
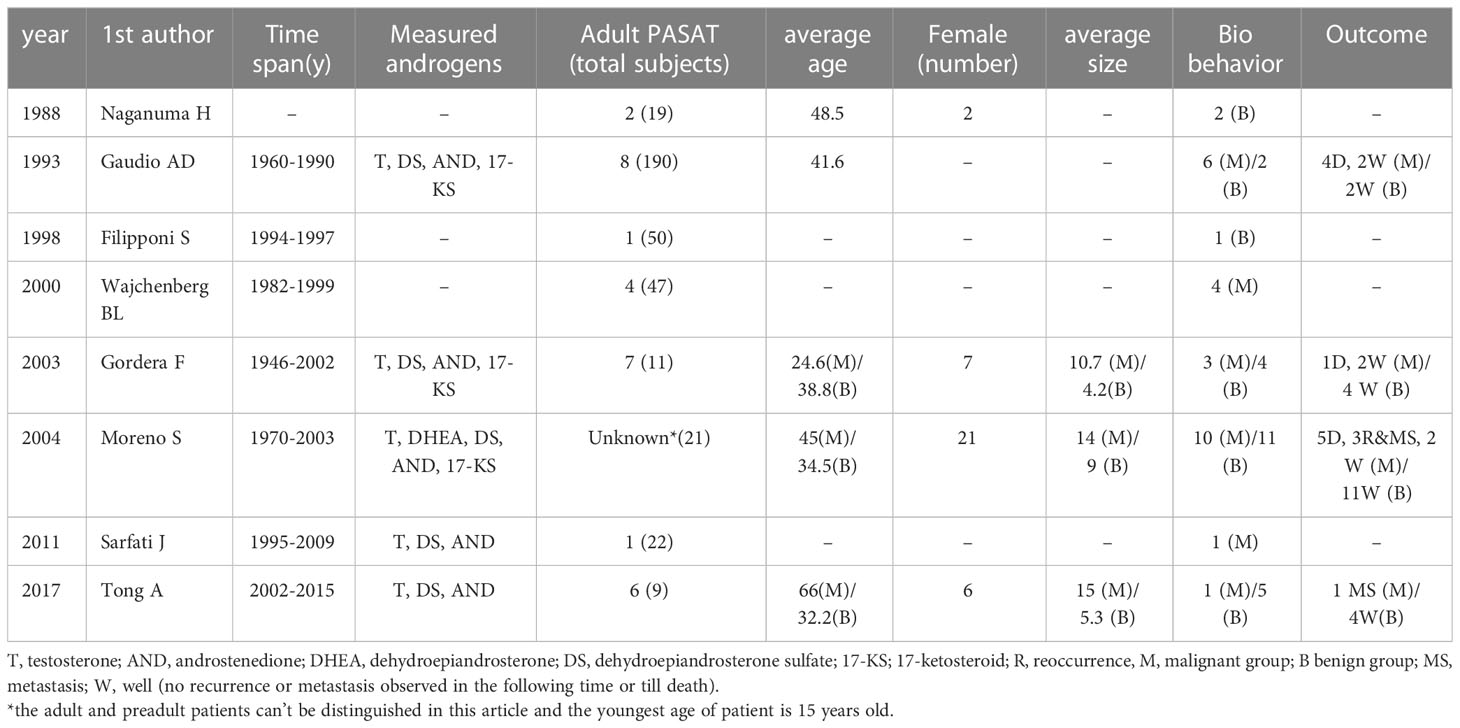
The articles containing adult PASAT were summarized Table 3. T and DS were the most frequent androgens used for hormone evaluation, and urinary 17-ketosteroid was a significant indicator of hyperandrogenism in the earlier studies. The age distribution of the patients with malignant or benign tumors varied among the different studies. The age of the malignant group was younger than that of the benign group in the study by Gordera F but older in the study by Moreno S. The average size of the malignant tumors was larger than that of the benign tumors. Most malignancy patients (14/20) had a negative outcome (recurrence, metastasis or death), while no patient with benign conditions were reported to have a negative outcome during the follow-up period.
Discussion
Androgen-secreting adrenal tumors are rare. A recent population-based study of 1287 patients diagnosed with adrenal tumors reported only one androgen-excess adrenal tumor (0.1%) (56). In another study of 1205 patients with androgen excess, 20 (1.7%) individuals were identified as having androgen-secreting adrenal tumors (57). Androgen-secreting adrenocortical tumors, especially adult PASATs, remain a challenge in clinical diagnosis and treatment due to their rarity. Here, we report a rare case of adult bilateral PASATs, and to our knowledge, only one other case of bilateral PASATs has been reported since 1970. Furthermore, we conducted a systematic review of adult PASATs to increase the understanding of adult PASATs and provide guidelines for the diagnosis and treatment of adult PASATs.
The incidence peak for adult PASAT occurred at 21-30 years of age, while the incidence peak for malignant PASAT occurred at 41-50 years of age. Hirsutism, as one of the initial complaints, was the most common symptom found in the present review and other PASAT studies (53, 55, 58). Young females might focus more on their appearance, and they may notice hirsutism once it occurs, prompting them to visit doctors and undergo medical screening for virilism-causing adrenal tumors. This may contribute to the peak incidence of adult PASAT at 21-30 years of age. The incidence of malignant PASAT peaked at 41-50 years of age, which is close to the age of incidence peak of adult adrenocortical carcinoma in a previous study (51). Several factors may be responsible for this. Above all, the incidence peak of malignant PASAT may be associated with age because the risk of malignant tumors is higher in older people (59). It may also be associated with menstrual dysfunction. It was indicated that the occurrence of menstrual dysfunction is associated with higher androgen levels in women with hirsutism (60), and our data analysis demonstrated that patients with malignant PASAT presented with higher androgen levels, which indicates that malignant PASAT might lead to more menstrual disorders. It is well known that females aged 41-50 years usually begin to suffer menstrual problems due to perimenopause, prompting them to seek medical advice and hormone evaluation. Therefore, androgen abnormalities that are related to potential virilism-causing malignant adrenal tumors are more likely to be diagnosed in patients aged 41-50 years. However, the potential factors responsible for the age distribution of PASAT need to be identified in further research.
In the present review, PASAT was predominant (40/42) in women. The low incidence of PASAT in men may be associated with clinical ignorance. It is difficult to ascertain the onset of androgen-excess symptoms in males, which are usually clinically ignored until the presence of a consistent peripheral androgen-to-estrogen conversion is determined (61). In addition to hirsutism and menstrual dysfunction, other virilism manifestations, such as clitoral enlargement, alopecia, voice deepening and acne, were found to be important signs for screening and diagnosis.
Malignant adrenal neoplasms were identified in 26% of adult PASAT patients, which was significantly higher than the incidence (8.6%) of malignant adrenal tumors in an adrenal tumor population (56). The average duration before diagnosis in the malignant group was significantly shorter than that in the benign group, which may be related to the rapid progression of the malignant adrenocortical tumor. The malignant adrenal tumor size was significantly larger than that of benign tumors, consistent with various studies on adrenal tumors. Testosterone is the most sensitive marker in the hormone evaluation of PASAT, as it is elevated in almost all PASAT patients and is the only increased androgen in certain patients (41, 62, 63). Some studies (64, 65) have demonstrated that increased DS (produced uniquely by the adrenal zona reticularis) and DHEA (produced mainly by the adrenal zona reticularis) are indicators of adrenal etiology, especially DS. In the current review, however, the DS level was normal in 6/32 patients, and the DHEA levels was normal in 3/6 patients. The levels of T and DS, which are presented as androgen levels/upper normal range limit in Table 2, were much higher in malignant cases, but these differences were not significant and the potential differences in androgens between malignant and benign PASAT need to be identified in future studies due to the limited number of cases in the present review.
The contribution of the ACTH/synacthen test and dexamethasone test (low dose or high dose) to the diagnosis of PASAT may have been limited because the T and DS responses varied among different patients. However, most female patients showed an increased T and DS level in the HCG test, which may potentially contribute to the diagnosis of PASAT. Leydig cells or features related to Leydig cells, which could increase testosterone secretion under the stimulation of HCG, have been reported in the pathological findings of PASAT neoplasm (11, 13, 16, 18), and this may be the possible reason why PASAT responds actively respond to HCG.
A previous study indicated that as many as 45% of female patients with androgen-producing adrenal neoplasms underwent needless ovarian surgical treatment before obtaining the correct diagnosis (66). Polycystic ovarian morphology, a potential factor in hyperandrogenemia, has been reported in several PASAT patients (11, 24, 28, 38, 39) and was present in the current case, but it was identified as an irrelevant factor. Unfortunately, two PASAT patients included in the current review also underwent an unnecessary oophorectomy (11, 28). It is unclear whether polycystic ovarian morphology is caused by hyperandrogenemia itself, but it does lead to difficulties in identifying the source of hyperandrogenism in terms of location. Notably, adrenal venous sampling (AVS) or ovarian venous sampling (OVS) played a significant role in accurately identifying the aetiological location in some PASAT patients (9, 13, 21, 24, 34) and in the current case. Thus, AVS and OVS are recommended for the identification of the location of hyperandrogenemia aetiology, especially when clinical findings are inconclusive.
Conclusion
Adult PASATs are extremely rare, and their characteristics are largely unknown. The diagnosis and treatment of adult PASATs remain a clinical challenge. Adult PASATs are predominant in women and most frequently characterized by hirsutism symptoms and other virilism signs. Serum testosterone is the most sensitive indicator, and elevated DS could contribute to the diagnosis of PASAT specifically. HCG stimulation test might also be of help in diagnosis. Individuals with malignant PASATs have a shorter disease duration before diagnosis, larger tumor sizes and relatively higher androgen levels. AVS and OVS may be important approaches for the location diagnosis. Surgery is recommended for all patients with local PASATs, whether benign or malignant. Tumor malignancy should be fully considered in patients PASATs due to the high risk of malignancy, poor prognosis and limited effective treatment approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization, ZL, YG, and YSZ. Methodology, ZL and YZ. Validation, ZW and XW. Data analysis, ZL, YG, and JZ. Writing—original draft preparation, ZL. Writing—review and editing, YSZ. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by the National Natural Science Foundation of China (81670611).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lyraki R, Schedl A. Adrenal cortex renewal in health and disease. Nat Rev Endocrinol (2021) 17(7):421–34. doi: 10.1038/s41574-021-00491-4
2. Bonfig W, Bittmann I, Bechtold S, Kammer B, Noelle V, Arleth S, et al. Virilising adrenocortical tumours in children. Eur J Pediatr (2003) 162(9):623–8. doi: 10.1007/s00431-003-1230-y
3. Ribeiro R, Figueiredo B. Childhood adrenocortical tumours. Eur J Cancer (2004) 40(8):1117–26. doi: 10.1016/j.ejca.2004.01.031
4. Honour J, Price D, Taylor N, Marsden H, Grant D. Steroid biochemistry of virilising adrenal tumours in childhood. Eur J Pediatr (1984) 142(3):165–9. doi: 10.1007/BF00442442
5. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2010) 95(9):4133–60. doi: 10.1210/jc.2009-2631
6. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2018) 103(11):4043–88. doi: 10.1210/jc.2018-01865
7. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2016) 101(5):1889–916. doi: 10.1210/jc.2015-4061
8. Blichert-Toft M, Vejlsted H, Hehlet H, Albrechtsen R. Virilizing adrenocortical adenoma responsive to gonadotrophin. Acta Endocrinol (Copenh) (1975) 78(1):77–85. doi: 10.1530/acta.0.0780077
9. Larson BA, Vanderlaan WP, Judd HL, McCullough DL. A testosterone-producing adrenal cortical adenoma in an elderly woman. J Clin Endocrinol Metab (1976) 42(5):882–7. doi: 10.1210/jcem-42-5-882
10. Nogeire C, Fukushima DK, Hellman L, Boyar RM. Virilizing adrenal cortical carcinoma. Cancer (1977) 40(1):307–13. doi: 10.1002/1097-0142(197707)40:1<307::aid-cncr2820400143>3.0.co;2-2
11. Smith HC, Posen S, Clifton-Bligh P, Casey J. A testosterone-secreting adrenal cortical adenoma. Aust N Z J Med (1978) 8(2):171–5. doi: 10.1111/j.1445-5994.1978.tb04506.x
12. Spaulding SW, Masuda T, Osawa Y. Increased 17 beta-hydroxysteroid dehydrogenase activity in a masculinizing adrenal adenoma in a patient with isolated testosterone overproduction. J Clin Endocrinol Metab (1980) 50(3):537–40. doi: 10.1210/jcem-50-3-537
13. Trost BN, Koenig MP, Zimmermann A, Zachmann M, Müller J. Virilization of a post-menopausal woman by a testosterone-secreting leydig cell type adrenal adenoma. Acta Endocrinol (Copenh) (1981) 98(2):274–82. doi: 10.1530/acta.0.0980274
14. Ho Yuen B, Moon YS, Mincey EK, Li D. Adrenal and sex steroid hormone production by a virilizing adrenal adenoma and its diagnosis with computerized tomography. Am J Obstet Gynecol (1983) 145(2):164–9. doi: 10.1016/0002-9378(83)90484-2
15. Fuller PJ, Pettigrew IG, Pike JW, Stockigt JR. An adrenal adenoma causing virilization of mother and infant. Clin Endocrinol (Oxf) (1983) 18(2):143–53. doi: 10.1111/j.1365-2265.1983.tb03197.x
16. Aguirre P, Scully RE. Testosterone-secreting adrenal ganglioneuroma containing leydig cells. Am J Surg Pathol (1983) 7(7):699–705. doi: 10.1097/00000478-198310000-00010
17. Faggiano M, Criscuolo T, Sinisi AA, Scialdone A, Bellastella A, Cuccurullo L. Virilization syndrome in a young woman due to an androgen-secreting adenoma. J endocrinological Invest (1984) 7(1):41–5. doi: 10.1007/bf03348374
18. Vasiloff J, Chideckel EW, Boyd CB, Foshag LJ. Testosterone-secreting adrenal adenoma containing crystalloids characteristic of leydig cells. Am J Med (1985) 79(6):772–6. doi: 10.1016/0002-9343(85)90531-5
19. O'Leary TJ, Ooi TC, Miller JD, Branchaud CL, Kalra J. Virilization of two siblings by maternal androgen-secreting adrenal adenoma. J Pediatr (1986) 109(5):840–2. doi: 10.1016/s0022-3476(86)80707-7
20. Pollock WJ, McConnell CF, Hilton C, Lavine RL. Virilizing leydig cell adenoma of adrenal gland. Am J Surg Pathol (1986) 10(11):816–22. doi: 10.1097/00000478-198611000-00009
21. Guillausseau PJ, Boitard C, Le Charpentier Y, Cedard L, Nahoul K, Blacker C, et al. Androgen producing adrenal adenoma. Rep Case associated hyperparathyroidism. J Endocrinol Invest (1987) 10(6):593–9. doi: 10.1007/bf03347005
22. Clouston WM, Cannell GC, Fryar BG, Searle JW, Martin NI, Mortimer RH. Virilizing adrenal adenoma in an adult with the beckwith-wiedemann syndrome: paradoxical response to dexamethasone. Clin Endocrinol (Oxf) (1989) 31(4):467–73. doi: 10.1111/j.1365-2265.1989.tb01270.x
23. Jackson JA, Mellinger RC. Hyperandrogenemia and virilization with simultaneous pituitary and adrenal adenomas. Henry Ford Hosp Med J (1991) 39(1):22–4.
24. Micić D, Zorić S, Popović V, Janković R, Jancić M, Han R, et al. Androgen-producing bilateral large cortical adrenal adenomas associated with polycystic ovaries in a young female. Postgrad Med J (1992) 68(797):219–22. doi: 10.1136/pgmj.68.797.219
25. Ruutiainen K, Satokari K, Anttila L, Erkkola R. Adrenal- and ovarian-vein steroids and LH response to GnRH in two patients with virilizing adrenocortical adenoma studied by selective catheterizations. Horm Res (1992) 37(1-2):49–53. doi: 10.1159/000182281
26. Coonrod DV, Rizkallah TH. Virilizing adrenal carcinoma in a woman of reproductive age: a case presentation and literature review. Am J Obstet Gynecol (1995) 172(6):1912–1914; discussion 1914-1915. doi: 10.1016/0002-9378(95)91431-5
27. Bozbora A, Erbil Y, Ozbey N, Kapran Y, Ozarmagan S, Berber E, et al. A young female patient with an androgen-secreting tumor: A rare malignant disease. Tumori (2000) 86(6):487–8. doi: 10.1177/030089160008600612
28. Mavroudis K, Aloumanis K, Papapetrou PD, Voros D, Spanos I. Virilization caused by an ectopic adrenal tumor located behind the iliopsoas muscle. Fertil Steril (2007) 87(6):1468.e1413–1466. doi: 10.1016/j.fertnstert.2006.08.106
29. Amano T, Imao T, Seki M, Takemae K, Yamauchi K, Hata S. Normal delivery following resection of an androgen-secreting adrenal carcinoma. Reprod Med Biol (2011) 10(1):55–8. doi: 10.1007/s12522-010-0071-4
30. Surrey LF, Thaker AA, Zhang PJ, Karakousis G, Feldman MD. Ectopic functioning adrenocortical oncocytic adenoma (oncocytoma) with myelolipoma causing virilization. Case Rep Pathol (2012) 2012(326418). doi: 10.1155/2012/326418
31. Rodríguez-Gutiérrez R, Bautista-Medina MA, Teniente-Sanchez AE, Zapata-Rivera MA, Montes-Villarreal J. Pure androgen-secreting adrenal adenoma associated with resistant hypertension. Case Rep Endocrinol (2013) 2013(356086). doi: 10.1155/2013/356086
32. Varma T, Panchani R, Goyal A, Maskey R. A case of androgen-secreting adrenal carcinoma with non-classical congenital adrenal hyperplasia. Indian J Endocrinol Metab (2013) 17(Suppl 1):S243–245. doi: 10.4103/2230-8210.119585
33. Galketiya KP, Ranjikumar S, Majeed U. Androgen-secreting adrenocortical carcinoma. Lancet Diabetes Endocrinol (2013) 1(1):e10. doi: 10.1016/s2213-8587(13)70005-6
34. Tetsi Nomigni M, Ouzounian S, Benoit A, Vadrot J, Tissier F, Renouf S, et al. Steroidogenic enzyme profile in an androgen-secreting adrenocortical oncocytoma associated with hirsustism. Endocr Connect (2015) 4(2):117–27. doi: 10.1530/ec-15-0014
35. Uruc F, Urkmez A, Yuksel OH, Sahin A, Verit A. Androgen secreting giant adrenocortical carcinoma with no metastases: A case report and review of the literature. Can Urol Assoc J (2015) 9(9-10):E644–647. doi: 10.5489/cuaj.2867
36. El Ghorayeb N, Grunenwald S, Nolet S, Primeau V, Côté S, Maugard CM, et al. First case report of an adrenocortical carcinoma caused by a BRCA2 mutation. Med (Baltimore) (2016) 95(36):e4756. doi: 10.1097/md.0000000000004756
37. Carré J, Grunenwald S, Vezzosi D, Mazerolles C, Bennet A, Meduri G, et al. Virilizing oncocytic adrenocortical carcinoma: Clinical and immunohistochemical studies. Gynecol Endocrinol (2016) 32(8):662–6. doi: 10.3109/09513590.2016.1149811
38. LaVoie M, Constantinides V, Robin N, Kyriacou A. Florid hyperandrogenism due to a benign adrenocortical adenoma. BMJ Case Rep (2018) 2018. doi: 10.1136/bcr-2018-224804
39. Karimi F, Dehghanian A, Fallahi M, Dalfardi B. Pure androgen-secreting adrenocortical carcinoma presenting with hypoglycemia. Arch Iran Med (2019) 22(9):527–30.
40. Dotto RS, Marx G, Bastos M, Machado JL, Glufke V, de Oliveira Freitas DM. A rare case of virilizing adult ectopic adrenal tumor. Urol Case Rep (2019) 27(100907). doi: 10.1016/j.eucr.2019.100907
41. Zhou WB, Chen N, Li CJ. A rare case of pure testosterone-secreting adrenal adenoma in a postmenopausal elderly woman. BMC endocrine Disord (2019) 19(1):14. doi: 10.1186/s12902-019-0342-y
42. Sailo SL, Sailo L. Primary amenorrhoea & virilization induced by pure testosterone-secreting adrenocortical adenoma. Indian J Med Res (2020) 152(Suppl 1):S53–s54. doi: 10.4103/ijmr.IJMR_1852_19
43. Pingle SR, Jalil F, Millar D, Malchoff CD, Ristau BT. Isolated DHEAS production by an adrenal neoplasm: Clinical, biochemical and pathologic characteristics. Urol Case Rep (2020) 31(101148). doi: 10.1016/j.eucr.2020.101148
44. Prachi, Aiyer HM, Jain V. Adrenocortical oncocytoma associated with androgen excess: A rare cause of hirsutism. Indian J Urol (2021) 37(3):277–80. doi: 10.4103/iju.IJU_636_20
45. Correia DN, Carvalho IR, Mangi JA. Virilising adrenocortical carcinoma. BMJ Case Rep (2021) 14(6). doi: 10.1136/bcr-2021-242895
46. Bao Z, He W, Di W, Gao H. Hyperandrogenism caused by a rare adrenocortical oncocytic neoplasm with uncertain malignant potential: A case report and review of the literature. Endocr J (2022). doi: 10.1507/endocrj.EJ22-0277
47. Gopinath C, Shekar S, Acharya M, Pattan V, Sundaresh V. Pure androgen-secreting radiologically suspicious adrenal mass: Benign or malignant? Cureus (2022) 14(6):e26234. doi: 10.7759/cureus.26234
48. Naganuma H, Ojima M, Sasano N. 11 beta-hydroxylase in mitochondrial fractions of functioning and non-functioning adrenocortical tumors. Tohoku J Exp Med (1988) 155(1):81–96. doi: 10.1620/tjem.155.81
49. Del Gaudio AD, Del Gaudio GA. Virilizing adrenocortical tumors in adult women. report of 10 patients, 2 of whom each had a tumor secreting only testosterone. Cancer (1993) 72(6):1997–2003. doi: 10.1002/1097-0142(19930915)72:6<1997::aid-cncr2820720634>3.0.co;2-1
50. Filipponi S, Guerrieri M, Arnaldi G, Giovagnetti M, Masini AM, Lezoche E, et al. Laparoscopic adrenalectomy: A report on 50 operations. Eur J Endocrinol (1998) 138(5):548–53. doi: 10.1530/eje.0.1380548
51. Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer (2000) 88(4):711–36.
52. Cordera F, Grant C, van Heerden J, Thompson G, Young W. Androgen-secreting adrenal tumors. Surgery (2003) 134(6):874–880; discussion 880. doi: 10.1016/s0039-6060(03)00410-0
53. Moreno S, Montoya G, Armstrong J, Leteurtre E, Aubert S, Vantyghem MC, et al. Profile and outcome of pure androgen-secreting adrenal tumors in women: Experience of 21 cases. Surgery (2004) 136(6):1192–8. doi: 10.1016/j.surg.2004.06.046
54. Sarfati J, Bachelot A, Coussieu C, Meduri G, Touraine P. Impact of clinical, hormonal, radiological, and immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol (2011) 165(5):779–88. doi: 10.1530/eje-11-0542
55. Tong A, Jiang J, Wang F, Li C, Zhang Y, Wu X. Pure androgen-producing adrenal tumor: Clinical features and pathogenesis. Endocrine Pract (2017) 23(4):399–407. doi: 10.4158/ep161580.or
56. Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, et al. Epidemiology of adrenal tumours in Olmsted county, Minnesota, USA: A population-based cohort study. Lancet Diabetes Endocrinol (2020) 8(11):894–902. doi: 10.1016/s2213-8587(20)30314-4
57. Elhassan YS, Idkowiak J, Smith K, Asia M, Gleeson H, Webster R, et al. Causes, patterns, and severity of androgen excess in 1205 consecutively recruited women. J Clin Endocrinol Metab (2018) 103(3):1214–23. doi: 10.1210/jc.2017-02426
58. Sciarra F, Tosti-Croce C, Toscano V. Androgen-secreting adrenal tumors. Minerva endocrinologica (1995) 20(1):63–8.
59. Doll R. The age distribution of cancer: Implications for models of carcinogenesis. J R Stat Society: Series A (General) (1971) 134(2):133–55. doi: 10.2307/2343871
60. Redmond GP, Bergfeld W, Gupta M, Bedocs NM, Skibinski C, Gidwani G. Menstrual dysfunction in hirsute women. J Am Acad Dermatol (1990) 22(1):76–8. doi: 10.1016/0190-9622(90)70011-6
61. Di Dalmazi G. Hyperandrogenism and adrenocortical tumors. Front Horm Res (2019) 53(92-99). doi: 10.1159/000494905
62. Kamilaris TC, DeBold CR, Manolas KJ, Hoursanidis A, Panageas S, Yiannatos J. Testosterone-secreting adrenal adenoma in a peripubertal girl. Jama (1987) 258(18):2558–61. doi: 10.1001/jama.1987.03400180092034
63. Sorgo W, Meyer D, Rodens K, Homoki J, Heinze E, Heymer B, et al. Testosterone-secreting adrenocortical tumor in a pubertal girl. case report and review of the literature. Horm Res (1988) 30(6):217–23. doi: 10.1159/000181067
64. Witchel SF, Pinto B, Burghard AC, Oberfield SE. Update on adrenarche. Curr Opin Pediatr (2020) 32(4):574–81. doi: 10.1097/mop.0000000000000928
65. Burger HG. Androgen production in women. Fertility sterility (2002) 77(3-5). doi: 10.1016/s0015-0282(02)02985-0
Keywords: pure androgen-secreting adrenal tumor (PASAT), clinical characteristics, diagnosis and treatment, adrenal venous sampling (AVS), ovarian venous sampling (OVS)
Citation: Liao Z, Gao Y, Zhao Y, Wang Z, Wang X, Zhou J and Zhang Y (2023) Pure androgen-secreting adrenal tumor (PASAT): A rare case report of bilateral PASATs and a systematic review. Front. Endocrinol. 14:1138114. doi: 10.3389/fendo.2023.1138114
Received: 05 January 2023; Accepted: 01 March 2023;
Published: 22 March 2023.
Edited by:
Yuxuan Song, Peking University People’s Hospital, ChinaReviewed by:
Ruizhi Xue, First Hospital of Shanxi Medical University, ChinaAndreas G. Moraitis, Corcept, United States
Ana Valea, Iuliu Hatieganu University of Medicine and Pharmacy Cluj-Napoca, Romania
Xiang Chen, Xiangya Hospital, Central South University, China
Copyright © 2023 Liao, Gao, Zhao, Wang, Wang, Zhou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yushi Zhang, cHVtY2h6aGFuZ3l1c2hpQDEyNi5jb20=
 Zhangcheng Liao
Zhangcheng Liao Yuting Gao
Yuting Gao Yang Zhao
Yang Zhao Zhan Wang1
Zhan Wang1 Yushi Zhang
Yushi Zhang