
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 April 2023
Sec. Pediatric Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1137940
This article is part of the Research TopicFertility Preservation in the Pediatric and Adolescent Populations, volume IIView all 10 articles
 Xiangyan Ruan1,2*†
Xiangyan Ruan1,2*† Jiaojiao Cheng1†
Jiaojiao Cheng1† Juan Du1
Juan Du1 Fengyu Jin1
Fengyu Jin1 Muqing Gu1
Muqing Gu1 Rui Ju1
Rui Ju1 Yurui Wu3
Yurui Wu3 Long Li4
Long Li4 Yuejiao Wang1
Yuejiao Wang1 Lingling Jiang1
Lingling Jiang1 Yu Yang1
Yu Yang1 Yanqiu Li1
Yanqiu Li1 Zecheng Wang1
Zecheng Wang1 Jun Ma1
Jun Ma1 Mingzhen Zhang1
Mingzhen Zhang1 Alfred O. Mueck1,2
Alfred O. Mueck1,2Background: There is limited information about the efficacy of ovarian tissue cryopreservation (OTC) in children. In the present study, we report eight patients with rare diseases who underwent OTC in China’s first and largest ovarian tissue cryobank.
Procedure: Data from girls with rare diseases who underwent OTC between September 2020 and November 2022 were retrospectively analyzed. We also compared the number of cryopreserved cortex pieces, follicle number, and AMH in those with rare diseases and age-matched children with non-rare diseases who also underwent OTC in our cryobank.
Results: The median age of the children was 5.88 ± 3.52 (range 2-13) years old. Unilateral oophorectomy was undertaken via laparoscopy in all of the children. The diseases in the 8 patients were: 4 mucopolysaccharidoses (MPS I two cases, IVA two cases), 1 Diamond-Blackfan anemia (DBA), 1 Fanconi anemia (FA), 1 hyperimmunoglobulin E syndrome (HIES), 1 Niemann-Pick disease. The number of cryopreserved cortex pieces was 17.13 ± 6.36, and the follicle count per 2 mm biopsy was 447.38 ± 524.35. No significant difference in age, the count of cryopreserved cortex pieces, follicle number per 2 mm biopsy, and AMH level was seen between the 20 children with non-rare diseases and those with rare diseases.
Conclusions: The reports help practitioners counsel girls with rare diseases about fertility preservation. The demand for OTC in pediatrics will likely grow as a standard of care.
Diseases with an incidence of fewer than 1/10,000 newborns, a prevalence of less than 1/10,000, and a number of patients less than 140,000 are classified as rare diseases (RD). As of February 2022, there are more than 7,000 known rare diseases worldwide, accounting for about 10% of all human diseases. About 72%-80% of rare diseases are caused by structural changes or abnormal regulation of genetic material (1). China has over 20 million rare disease patients, with over 200,000 new patients yearly (2). Around the world, the treatment of rare diseases is probably the most significant medical challenge facing humanity today. Treatment of some rare diseases requires hematopoietic stem cell therapy (HSCT) (3). Advances in HSCT technology and supportive care have increased the number of long-term survivors (4), so fertility preservation (FP) for these patients is also essential.
Ovarian tissue cryopreservation (OTC) is the only FP method for prepubertal girls (5). OTC includes laparoscopic surgery to remove part or the whole ovary, followed by transport to the ovarian tissue cryobank for cryopreservation and storage (6, 7). Professional institutions must have the necessary equipment, trained personnel, and sufficient frozen stock to conduct OTC safely and with consistent quality. More than 200 babies worldwide have been born through this technique, including reports of frozen ovarian tissue collected before puberty and frozen-thawed ovarian tissue transplantation (OTT) after puberty (8–10). In 2019, the American Society for Reproductive Medicine (ASRM) recommended that OTC technology no longer be considered experimental (11). Minimal complications have been reported after laparoscopic surgery (12).
The Oncofertility Consortium’s National Physicians’ Cooperative (ON-NPC) published its experience with OTC in 114 girls<15 years old in 2018 (13). Germany’s UniCareD cryobank stored frozen ovarian tissue from 104 girls with a mean age of 14 years between 2018 and May 2022 (14). Our center has cryopreserved ovarian tissue from more than 50 children (12).
Studies on FP in RD are limited. Because of the high incidence of POI for patients with genetic abnormalities, such as galactosemia and Turner syndrome (TS), experts recommend starting FP as soon as possible (15–19). FP counseling is also needed for children with RD who plan to undergo HSCT. This paper reports on the cryopreservation of ovarian tissue in 8 patients with RD to give medical workers more information and confidence and introduce patients to the FP center for consultation. A multidisciplinary team should always be involved in treating and managing RD patients.
The Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University, approved the provision of centralized OTC (2017-KY-020-01; March 15, 2017). Ovarian tissue was received from different hospitals and transported to the centralized cryobank. The parents of each patient signed an agreement and informed consent for their child.
Eight girls with RD underwent OTC in our cryobank between September 2020 and November 2022 (mean ± SD, range, 5.88 ± 3.52 years, 2-13 years). Unilateral oophorectomy was undertaken via laparoscopy in the eight children. No complications were reported after laparoscopic surgery in the eight children.
After retrieval, the ovarian tissue was immediately put into the cooled Custodiol (HTK). The temperature was maintained at 4-8 °C during ovarian tissue transportation. The mean temperature on reaching the cryobank was 5.44 °C, and the transport time was no more than 12 hours. In a sterile laminar flow cabinet, ovarian tissue was prepared at HTK solution, maintained at 4 °C. The ovarian cortex was handled to be 1 mm thick, cut to 6 mm x 3 mm slices, and cryopreserved. After slow programmed freezing, the tubes were stored in a liquid nitrogen tank with a gas phase. In ovarian tissue preparation, standardized cortical biopsies with a diameter of 2 mm from different areas were evaluated for follicle density and viability assay. The procedures were according to the previous publications (12, 20).
Twenty age-matched children who underwent OTC because of non-rare diseases were selected to compare the number of cryopreserved ovarian cortex pieces, follicle number per 2 mm biopsy, and the level of AMH between patients with RD and those with non-rare diseases. They did not undergo gonadotoxic treatment before OTC.
The levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and AMH in serum before OTC were evaluated. The details can be seen in our previous publication (15).
The number of surviving resting follicles was evaluated in biopsies by Calcein-AM assay, which method has been described in the previous publication (21).
SPSS 22.0 was applied for analysis. The data following normal distribution were expressed by “mean ± Standard deviation (SD)”; otherwise, “mean ± Standard Error of Mean (SEM)”. The differences between groups were compared by independent sample t-test. P < 0.05 indicates that the difference was statistically significant.
Characteristics of the 8 girls with OTC are shown in Table 1. The age of the 8 children was 5.88 ± 3.52 years old, range 2 to 13 years. The diseases in the 8 patients were: 4 mucopolysaccharidoses (MPS I two cases, IVA two cases), 1 Diamond-Blackfan Aanemia (DBA), 1 Fanconi anemia (FA), 1 hyperimmunoglobulin E syndrome (HIES), 1 Niemann-Pick disease. Ovarian tissue was cryopreserved because of planned HSCT.
Unilateral oophorectomy was undertaken via laparoscopy in the children. The temperature during transport to a centralized cryobank was 5.44 ± 0.96°C. The number of cryopreserved cortex pieces was 17.13 ± 6.36 (mean ± SD), the follicle number per 2 mm biopsy was 447.38 ± 524.35 (mean ± SD) (Figure 1). The FSH was 3.46 ± 2.14 IU/L (mean ± SD), LH was 0.55 ± 0.53 IU/L (mean ± SEM), and AMH was 1.60 ± 1.21 ng/ml (mean ± SD).
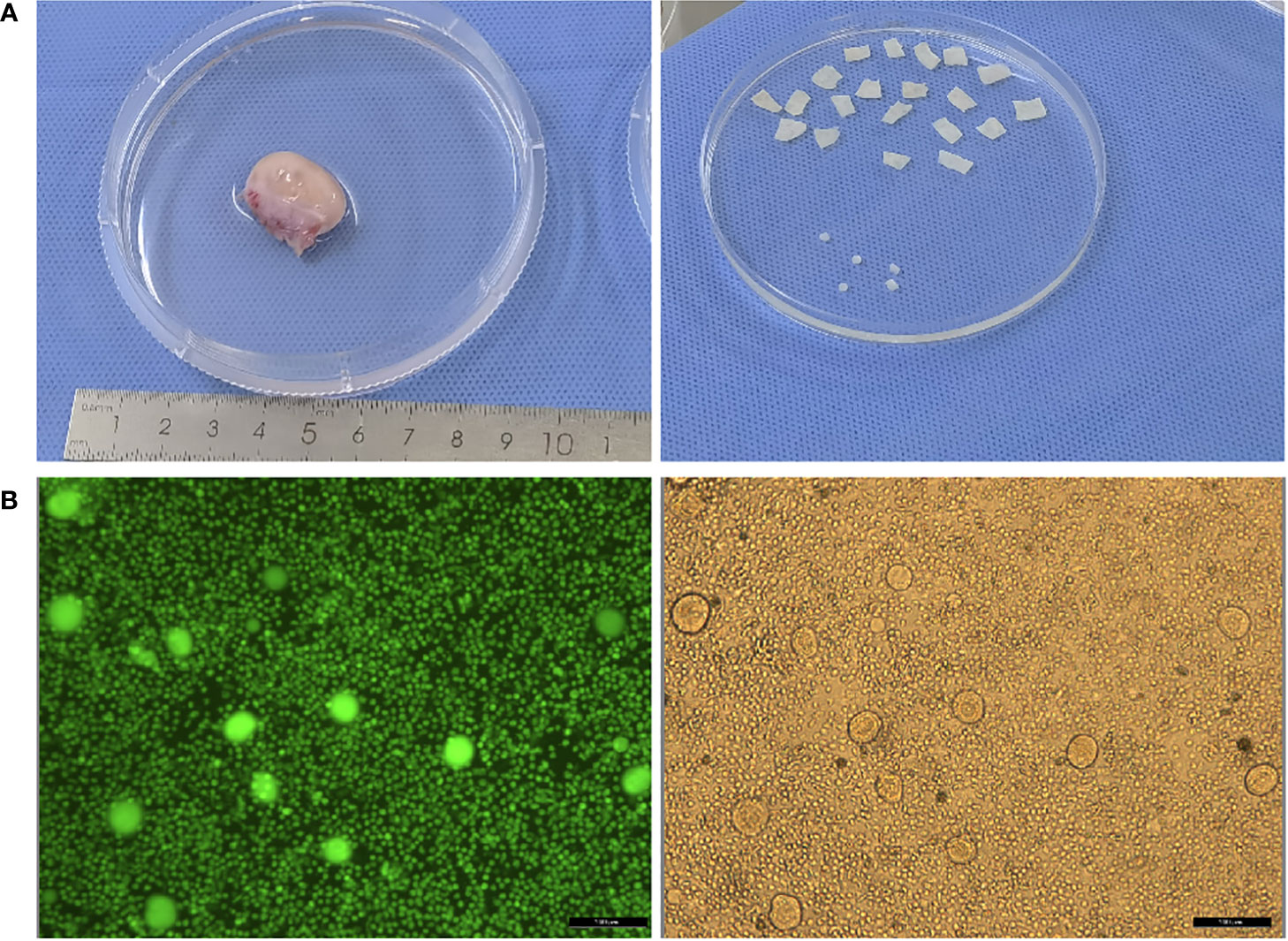
Figure 1 (A) Photos of ovary before and after ovarian tissue cortex preparation; (B) Detection of follicular activity in ovarian cortex.
In Figure 2, no significant difference in age, the number of cryopreserved cortex pieces, follicle number per 2 mm biopsy, and AMH level between 20 children with non-rare diseases and those with rare diseases (mean ± SD, 7.05 ± 3.16 vs. 5.88 ± 3.52, P=0.396; mean ± SD, 20.70 ± 7.39 vs. 17.13 ± 6.36, P=0.241; mean ± SEM, 947.35 ± 200.30 vs. 447.38 ± 185.39, P=0.153; mean ± SEM, 2.15 ± 0.36 vs. 1.60 ± 0.43, P=0.616, respectively).
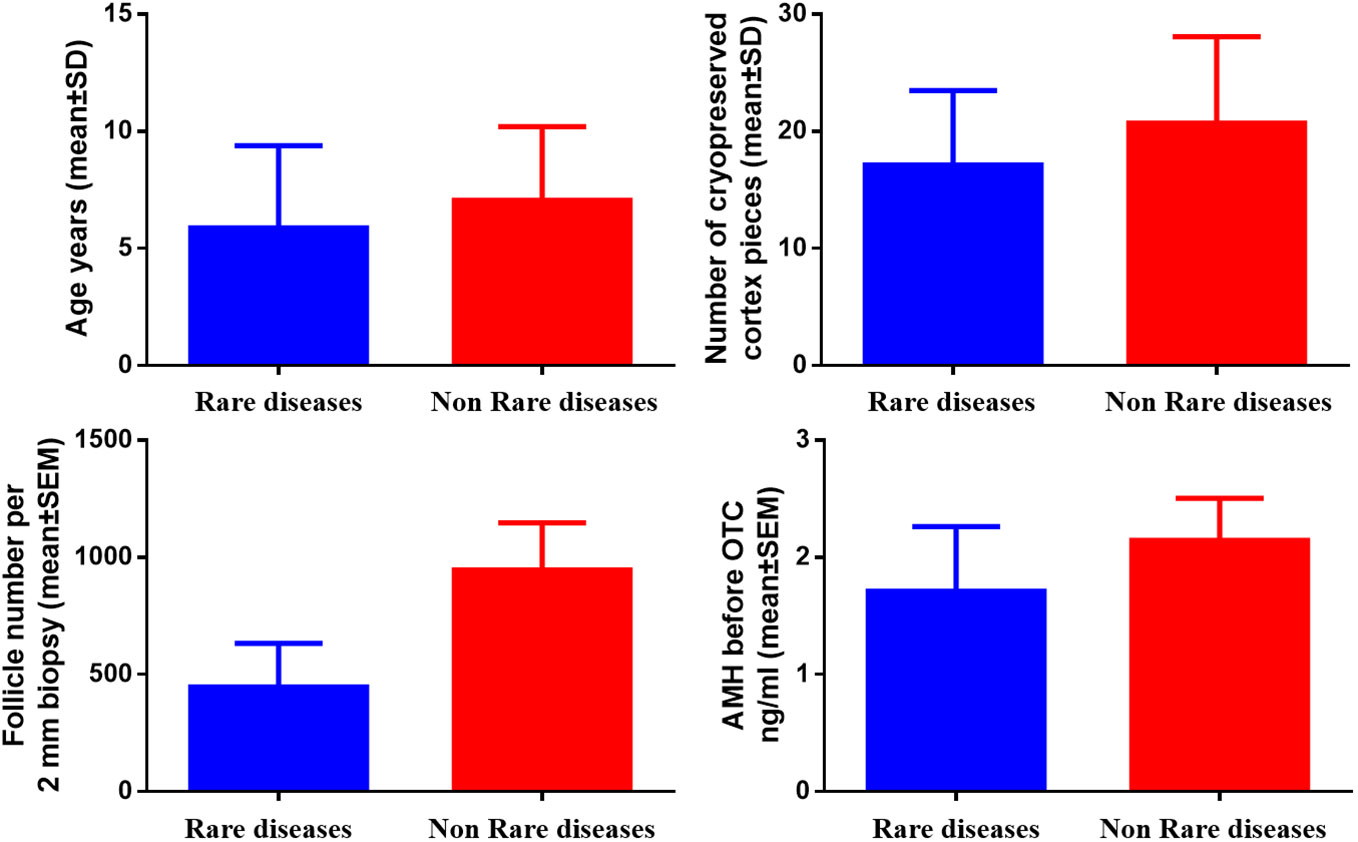
Figure 2 Comparison of age, number of cryopreserved cortex pieces, follicle number per 2 mm biopsy, and AMH in children with and without rare diseases.
No studies have described OTC for DBA, FA, MPS-I, MPS-IVA, HIES, and Niemann-Pick disease. Patients with these diseases required high-dose chemotherapy with alkylating agents and/or total body irradiation as pre-treatment for HSCT. Therefore, the present study includes important information for OTC in children with RD.
With the development of Assisted Reproductive Technology (ART), embryos that do not carry explicit disease-causing genes can be transferred back to the maternal uterine cavity after embryo preimplantation genetic testing (PGT) for families with evident genes for RD/genetic disorders. This is an essential technical tool for the primary prevention and control of congenital disabilities and can stop the transmission of RD/genetic disorders in the family from the source and produce healthy offspring (22). Secondary prevention is prenatal screening, which requires amniocentesis during pregnancy to ensure again that the fetus does not carry the disease-causing genes. Tertiary prevention is post-birth screening.
DBA is a rare congenital intrinsic erythroid hypoplasia, with 7 cases/million live births, acknowledged in 2005 as the first human ribosomopathy (23, 24). The median age of diagnosis is two to three months, with 95% of the DBA cases diagnosed before two years and 99% before five years of age (25, 26). HSCT is safe and efficient in DBA and should be considered if a matched sibling or unrelated donor is available (26, 27). FA is a challenging disease, and HSCT is the only curative therapy for the hematologic complications associated with this disease (28). The favorable mean overall survival was 80.9%, and event-free survival was 79.3% (29).
MPS is a group of rare genetic diseases with abnormal glycosaminoglycan (GAG) catabolism (30). The overall incidence of MPS is estimated to be 1/100,000 live births, which varies according to region and race (31). HSCT has been used in patients with MPS I, II, IVA, VI, and VII, showing increased acceptance and therapeutic benefits (32). In 2011, a retrospective study evaluated the outcome of HSCT in 45 patients with MPS VI, with a 1-and 3-year survival rate of 66%. Patients who received HSCT had a longer life expectancy than those who did not receive treatment or enzyme replacement therapy (ERT) (33). The overall survival rate after transplantation was 90% (30). Standardized follow-up and a multidisciplinary team help accurately assess long-term post-transplantation outcomes and improve the quality of life (34).
HIES is primary immunodeficiency that results from heterozygous mutations in the signal transducer and activator of the transcription 3 genes. Some patients with HIES have been reported to be treated with HSCT. However, the efficacy of HSCT for autosomal dominant HIES is inconsistent (35). HSCT potentially benefits the severe phenotype of Niemann-Pick disease patients (36). However, pre-treatment with radiotherapy and high-dose chemotherapy before HSCT can seriously harm the ovaries, and 70~100% of young females develop premature ovarian insufficiency (POI) (37, 38).
Our study found no significant difference in ovarian size, follicle number, and AMH level before OTC between children with rare diseases and age-matched children with non-rare diseases. However, it did not include children with Turner syndrome who have accelerated follicle depletion prior to puberty (39–41). Also, further evaluation is necessary after increasing the sample size. The measurement of AMH levels is controversial because the assessment of ovarian reserve in children and adolescents using AMH levels has limitations (9, 42).
Ovarian size varies with age and pubertal development. The prepubertal ovary is smaller than the reproductive ovary; therefore, the entire unilateral ovary is usually removed (43). Many pediatric surgeons and gynecologists perform unilateral laparoscopic oophorectomy, using an ultrasonic advanced energy device to segment the ovarian artery and mesovarium. No major surgical complications have been observed with this technique (44).
With increasing evidence of live births and recovery of endocrine function, a British Fertility Society guideline concluded that prepubertal girls should be considered for OTC (45, 46). OTC and transplantation are now the only FP option for prepubertal girls (47, 48).
Our center is the first and largest ovarian tissue cryobank in China. Nearly 500 cases of ovarian tissue have been successfully preserved, 10 cases have been successfully transplanted, and the ovarian function has recovered after OTC and OTT (49). One patient with MDS successfully conceived naturally and delivered a healthy baby girl after OTC and OTT (50). With the cooperation of pediatrics, OTC in children in our center has increased in the last two years (12).
The limitation of this study is the lack of data regarding outcomes such as later fertility following the use of cryopreserved ovarian tissues. This should be mentioned when counseling by practitioners. The outcome in these patients will hopefully be reported on long-term follow-up. It should stimulate further research within this challenging scientific field.
To conclude, reporting information helps practitioners counsel girls with RD about FP and the preservation of ovarian endocrine function supported by OTC. The demand for OTC in pediatrics will likely grow as a standard of care.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
All authors qualify for authorship by contributing substantially to this article. XR: project leader, and project supervisor, evaluated the ovarian reserve function of each child, supervised and guided ovarian tissue biopsy, transport, preparation, cryopreservation, follow-up, interpretation of results, and provided critical comments and revised the first draft. JC: article preparation, ovarian tissue transportation, preparation, and cryopreservation. JD, FJ, and MG: ovarian tissue preparation and cryopreservation. RJ, YRW, and LL: biopsied ovarian tissue. YJW, LJ, YY, YL, ZW, JM, and MZ: ovarian tissue transportation, AM: experimental supervision, interpretation of results, and article revision. All authors reviewed the article’s final version and approved it for publication.
This study was supported by Capital’s Funds for Health Improvement and Research (2020-2-2112), Beijing Natural Science Foundation (7202047), and Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181401).
The authors acknowledge Markus Montag and Jana Liebenthron for their technical assistance. The authors thank Liu Rong, Li Junhui, and Shi Xiaodong (Department of Hematology, Children’s Hospital, Capital Institute of Pediatrics), Qin Maoquan and Yang Jun (Hematology Oncology Center, Beijing Children’s Hospital, Capital Medical University), and Sun Yuan, Sun Qingzhen, and Chen Jiao (Beijing JingDu Children’s Hospital) for referring patients for fertility preservation counseling and performing ovarian tissue biopsy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chung C, Chu A, Chung B. Rare disease emerging as a global public health priority. Front Public Health (2022) 10:1028545. doi: 10.3389/fpubh.2022.1028545
2. Ying Z, Gong L, Li C. An update on china's national policies regarding rare diseases. Intractable Rare Dis Res (2021) 10:148–53. doi: 10.5582/irdr.2021.01027
3. Stoller JK. The challenge of rare diseases. Chest (2018) 153:1309–14. doi: 10.1016/j.chest.2017.12.018
4. Majhail NS, Rizzo JD. Surviving the cure: Long term followup of hematopoietic cell transplant recipients. Bone Marrow Transplant (2013) 48:1145–51. doi: 10.1038/bmt.2012.258
5. Jadoul P, Dolmans MM, Donnez J. Fertility preservation in girls during childhood: Is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update (2010) 16:617–30. doi: 10.1093/humupd/dmq010
6. Ruan X. Chinese Society of gynecological endocrinology affiliated to the international society of gynecological endocrinology guideline for ovarian tissue cryopreservation and transplantation. Gynecol Endocrinol (2018) 34:1005–10. doi: 10.1080/09513590.2018.1488957
7. Hinkle K, Orwig KE, Valli-Pulaski H, Taylor S, van Leeuwen K, Carpentieri D, et al. Cryopreservation of ovarian tissue for pediatric fertility. Biopreserv Biobank (2021) 19:130–5. doi: 10.1089/bio.2020.0124
8. Dolmans MM, von Wolff M, Poirot C, Diaz-Garcia C, Cacciottola L, Boissel N, et al. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil Steril (2021) 115:1102–15. doi: 10.1016/j.fertnstert.2021.03.008
9. Takae S, Furuta S, Iwahataa H, Iwahata Y, Keino D, Kanamori R, et al. Cryopreservation of paediatric ovarian tissue with an updated version of the Edinburgh criteria for appropriate patient selection. Reprod BioMed Online (2021) 44:667–76. doi: 10.1016/j.rbmo.2021.10.009
10. Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet (2012) 379:588. doi: 10.1016/S0140-6736(11)61781-9
11. Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril (2019) 112:1022–33. doi: 10.1016/j.fertnstert.2019.09.013
12. Ruan X, Cheng J, Du J, Jin F, Gu M, Li Y, et al. Analysis of fertility preservation by ovarian tissue cryopreservation in pediatric children in China. Front Endocrinol (2022) 13:930786. doi: 10.3389/fendo.2022.930786
13. Armstrong AG, Kimler BF, Smith BM, Woodruff TK, Pavone ME, Duncan FE. Ovarian tissue cryopreservation in young females through the oncofertility consortium's national physicians cooperative. Future Oncol (2018) 14:363–78. doi: 10.2217/fon-2017-0410
14. Baston-Bust DM, Bielfeld AP. Fertility preservation in the pediatric population-experience from a German cryobank for ovarian tissue. Front Endocrinol (Lausanne) (2022) 13:995172. doi: 10.3389/fendo.2022.995172
15. Bedoschi G, Navarro PA. Oncofertility programs still suffer from insufficient resources in limited settings. J Assist Reprod Genet (2022) 39:953–5. doi: 10.1007/s10815-022-02452-w
16. Gornet ME, Chen LX, Christianson MS. Ovarian tissue cryopreservation for emerging primary ovarian insufficiency: Expanding indications outside of cancer to preserve fertility and increase access to care. Fertil Steril (2022) 118:985–6. doi: 10.1016/j.fertnstert.2022.09.002
17. Gravholt CH, Viuff M, Just J, Sandahl K, Brun S, van der Velden J, et al. The changing face of turner syndrome. Endocr Rev (2023) 44:33–69. doi: 10.1210/endrev/bnac016
18. Cheng J, Ruan X, Du J, Jin F, Gu M, Wu Y, et al. Ovarian tissue cryopreservation for a 3-year-old girl with mosaic turner syndrome in China: First case report and literature review. Front Endocrinol (Lausanne) (2022) 13:959912. doi: 10.3389/fendo.2022.959912
19. Vash-Margita A, Szymanska-Vandendriessche K, Gunther K, Rodriguez-Buritica DF, Christison-Lagay E, Saluja S, et al. Laparoscopic ovarian tissue harvesting for cryopreservation from a child with galactosemia. Fertil Steril (2022) 118:982–4. doi: 10.1016/j.fertnstert.2022.08.005
20. Liebenthron J, Montag M. Chapter 15 development of a nationwide network for ovarian tissue cryopreservation. Methods Mol Biol (2017) 1568:205–20. doi: 10.1007/978-1-4939-6828-2_15
21. Li Y, Ruan X, Liebenthron J, Montag M, Zhou Q, Kong W, et al. Ovarian tissue cryopreservation for patients with premature ovary insufficiency caused by cancer treatment: Optimal protocol. Climacteric (2019) 22:383–9. doi: 10.1080/13697137.2018.1554644
22. van der Schoot V, Dondorp W, Dreesen J, Coonen E, Paulussen ADC, de Wert G, et al. Preimplantation genetic testing for more than one genetic condition: Clinical and ethical considerations and dilemmas. Hum Reprod (2019) 34:1146–54. doi: 10.1093/humrep/dez059
23. Leger-Silvestre I, Caffrey JM, Dawaliby R, Alvarez-Arias DA, Gas N, Bertolone SJ, et al. Specific role for yeast homologs of the diamond blackfan anemia-associated Rps19 protein in ribosome synthesis. J Biol Chem (2005) 280:38177–85. doi: 10.1074/jbc.M506916200
24. Liu JM, Ellis SR. Ribosomes and marrow failure: Coincidental association or molecular paradigm? Blood (2006) 107:4583–8. doi: 10.1182/blood-2005-12-4831
25. Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, et al. Diagnosing and treating diamond blackfan anaemia: Results of an international clinical consensus conference. Br J Haematol (2008) 142:859–76. doi: 10.1111/j.1365-2141.2008.07269.x
26. Da CL, Leblanc T, Mohandas N. Diamond-blackfan anemia. Blood (2020) 136:1262–73. doi: 10.1182/blood.2019000947
27. Strahm B, Loewecke F, Niemeyer CM, Albert M, Ansari M, Bader P, et al. Favorable outcomes of hematopoietic stem cell transplantation in children and adolescents with diamond-blackfan anemia. Blood Adv (2020) 4:1760–9. doi: 10.1182/bloodadvances.2019001210
28. Bonfim C, Nichele S, Loth G, Funke VAM, Nabhan SK, Pillonetto DV, et al. Transplantation for fanconi anaemia: Lessons learned from Brazil. Lancet Haematol (2022) 9:e228–36. doi: 10.1016/S2352-3026(22)00032-1
29. Xu L, Lu Y, Hu S, Li C, Tang Y, Wang H, et al. Unmanipulated haploidentical haematopoietic cell transplantation with radiation-free conditioning in fanconi anaemia: A retrospective analysis from the Chinese blood and marrow transplantation registry group. Br J Haematol (2022) 199:401–10. doi: 10.1111/bjh.18408
30. Sawamoto K, Stapleton M, Almeciga-Diaz CJ, Espejo-Mojica AJ, Losada JC, Suarez D, et al. Therapeutic options for mucopolysaccharidoses: Current and emerging treatments. Drugs (2019) 79:1103–34. doi: 10.1007/s40265-019-01147-4
31. Burlina AB, Gragnaniello V. Newborn screening of mucopolysaccharidosis type I. Crit Rev Clin Lab Sci (2022) 59:257–77. doi: 10.1080/10408363.2021.2021846
32. Kharbanda S, Dvorak CC. The beginning of the end of allogeneic transplantation for hurler syndrome? N Engl J Med (2021) 385:2003–4. doi: 10.1056/NEJMe2116020
33. Turbeville S, Nicely H, Rizzo JD, Pedersen TL, Orchard PJ, Horwitz ME, et al. Clinical outcomes following hematopoietic stem cell transplantation for the treatment of mucopolysaccharidosis VI. Mol Genet Metab (2011) 102:111–5. doi: 10.1016/j.ymgme.2010.09.010
34. Wang J, Luan Z, Jiang H, Fang J, Qin M, Lee V, et al. Allogeneic hematopoietic stem cell transplantation in thirty-four pediatric cases of mucopolysaccharidosis-a ten-year report from the China children transplant group. Biol Blood Marrow Transplant (2016) 22:2104–8. doi: 10.1016/j.bbmt.2016.08.015
35. Yanagimachi M, Ohya T, Yokosuka T, Kajiwara R, Tanaka F, Goto H, et al. The potential and limits of hematopoietic stem cell transplantation for the treatment of autosomal dominant hyper-IgE syndrome. J Clin Immunol (2016) 36:511–6. doi: 10.1007/s10875-016-0278-1
36. Quarello P, Spada M, Porta F, Vassallo E, Timeus F, Fagioli F, et al. Hematopoietic stem cell transplantation in niemann-pick disease type b monitored by chitotriosidase activity. Pediatr Blood Cancer (2018) 65:e26811. doi: 10.1002/pbc.26811
37. Ruan X. Expert consensus on fertility preservation in hematopoietic stem cell transplantation in girls in China. Gynecol Endocrinol (2022). doi: 10.1080/09513590.2022.2146671
38. Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE consortium and the international late effects of childhood cancer guideline harmonization group. Lancet Oncol (2021) 22:e45–56. doi: 10.1016/S1470-2045(20)30594-5
39. Mastellari E, Marca A LA. Genetic conditions impairing female fertility. Panminerva Med (2020) 62:260–7. doi: 10.23736/S0031-0808.20.04208-1
40. Nourollahpour SM, Cassinerio E, Modarres M, Zareiyan A, Hamzehgardeshi Z, Moghadam ZB. Reproductive health issues in female patients with beta-thalassaemia major: A narrative literature review. J Obstet Gynaecol (2020) 40:902–11. doi: 10.1080/01443615.2019.1692802
41. La Marca A, Mastellari E. Fertility preservation for genetic diseases leading to premature ovarian insufficiency (POI). J Assist Reprod Genet (2021) 38:759–77. doi: 10.1007/s10815-021-02067-7
42. Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM, et al. Anti-mullerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: A systematic review. Hum Reprod Update (2022) 28:417–34. doi: 10.1093/humupd/dmac004
43. Takae S, Furuta S, Keino D, Shiraishi E, Iwahata Y, Oyama K, et al. Surgical management of unilateral oophorectomy for ovarian tissue cryopreservation in high-risk children and adolescents with varied backgrounds. Pediatr Surg Int (2021) 37:1021–9. doi: 10.1007/s00383-021-04900-7s
44. Rowell EE, Corkum KS, Even KA, Laronda MM. Ovarian tissue health after laparoscopic unilateral oophorectomy: A porcine model for establishing optimized fertility preservation techniques in children. J Pediatr Surg (2020) 55:1631–8. doi: 10.1016/j.jpedsurg.2019.12.014
45. Khattak H, Malhas R, Craciunas L, Afifi Y, Amorim CA, Fishel S, et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: A systematic review and individual patient data meta-analysis. Hum Reprod Update (2022) 28(3):400–16. doi: 10.1093/humupd/dmac003
46. Yasmin E, Balachandren N, Davies MC, Jones GL, Lane S, Mathur R, et al. Fertility preservation for medical reasons in girls and women: British fertility society policy and practice guideline. Hum Fertil (Camb) (2018) 21:3–26. doi: 10.1080/14647273.2017.1422297
47. Masciangelo R, Chiti MC, Camboni A, Amorim CA, Donnez J, Dolmans MM. Mitochondrial content, activity, and morphology in prepubertal and adult human ovaries. J Assist Reprod Genet (2021) 38:2581–90. doi: 10.1007/s10815-021-02282-2
48. Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med (2017) 377:1657–65. doi: 10.1056/NEJMra1614676
49. Ruan X, Cheng J, Korell M, Du J, Kong W, Lu D, et al. Ovarian tissue cryopreservation and transplantation prevents iatrogenic premature ovarian insufficiency: First 10 cases in China. Climacteric (2020) 23:574–80. doi: 10.1080/13697137.2020.1767569
Keywords: ovarian tissue cryopreservation, children, fertility preservation, rare diseases, hematopoietic stem cell therapy
Citation: Ruan X, Cheng J, Du J, Jin F, Gu M, Ju R, Wu Y, Li L, Wang Y, Jiang L, Yang Y, Li Y, Wang Z, Ma J, Zhang M and Mueck AO (2023) Ovarian tissue cryopreservation in the pediatric with rare diseases- experience from China’s first and the largest ovarian tissue cryobank. Front. Endocrinol. 14:1137940. doi: 10.3389/fendo.2023.1137940
Received: 05 January 2023; Accepted: 22 March 2023;
Published: 03 April 2023.
Edited by:
Yasmin Jayasinghe, The University of Melbourne, AustraliaReviewed by:
Thomas Rabe, Heidelberg University Hospital, GermanyCopyright © 2023 Ruan, Cheng, Du, Jin, Gu, Ju, Wu, Li, Wang, Jiang, Yang, Li, Wang, Ma, Zhang and Mueck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangyan Ruan, cnVhbnhpYW5neWFuQGNjbXUuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.