- 1National Centre for Healthcare Research and Pharmacoepidemiology, Laboratory of the University of Milano-Bicocca, Milan, Italy
- 2Department of Statistics and Quantitative Methods, Unit of Biostatistics, Epidemiology, and Public Health, University of Milano-Bicocca, Milan, Italy
- 3Rheumatology Unit, University of Verona, Verona, Italy
- 4Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy
- 5Local Health Unit (USL) Umbria, Perugia, Italy
- 6Department of Pharmacology, School of Medicine, University of Messina, Messina, Italy
- 7Italian Bone Disease Research Foundation, Fondazione Italiana Ricerca sulle Malattie dell’Osso (FIRMO), Florence, Italy
- 8Department of Medicine, Surgery and Neurosciences, Rheumatology Unit, University of Siena, Azienda Ospedaliero-Universitaria Senese, Siena, Italy
- 9Department of Medicine, Surgery and Neuroscience, Policlinico Le Scotte, University of Siena, Siena, Italy
- 10Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 11Department of Experimental Medicine, Sapienza University of Rome, Viale del Policlinico, Rome, Italy
- 12AMICI Onlus, Associazione Nazionale per le Malattie Infiammatorie Croniche dell’Intestino, Milan, Italy
- 13Italian Society of General Medicine and Primary Care Società Italiana di Medicina Generale e delle cure primarie (SIMG), Florence, Italy
- 14Department of Movement, Human and Health Sciences, Foro Italico University, Rome, Italy
- 15CnAMC, Coordinamento nazionale delle Associazioni dei Malati Cronici e rari di Cittadinanzattiva, Rome, Italy
- 16Department of Clinical Sciences and Translational Medicine, University of Rome “Tor Vergata”, Rome, Italy
- 17Department of Orthopedics and Traumatology, “Policlinico Tor Vergata” Foundation, Rome, Italy
Background: Fragility fractures are a major public health concern owing to their worrying and growing burden and their onerous burden upon health systems. There is now a substantial body of evidence that individuals who have already suffered a fragility fracture are at a greater risk for further fractures, thus suggesting the potential for secondary prevention in this field.
Purpose: This guideline aims to provide evidence-based recommendations for recognizing, stratifying the risk, treating, and managing patients with fragility fracture. This is a summary version of the full Italian guideline.
Methods: The Italian Fragility Fracture Team appointed by the Italian National Health Institute was employed from January 2020 to February 2021 to (i) identify previously published systematic reviews and guidelines on the field, (ii) formulate relevant clinical questions, (iii) systematically review literature and summarize evidence, (iv) draft the Evidence to Decision Framework, and (v) formulate recommendations.
Results: Overall, 351 original papers were included in our systematic review to answer six clinical questions. Recommendations were categorized into issues concerning (i) frailty recognition as the cause of bone fracture, (ii) (re)fracture risk assessment, for prioritizing interventions, and (iii) treatment and management of patients experiencing fragility fractures. Six recommendations were overall developed, of which one, four, and one were of high, moderate, and low quality, respectively.
Conclusions: The current guidelines provide guidance to support individualized management of patients experiencing non-traumatic bone fracture to benefit from secondary prevention of (re)fracture. Although our recommendations are based on the best available evidence, questionable quality evidence is still available for some relevant clinical questions, so future research has the potential to reduce uncertainty about the effects of intervention and the reasons for doing so at a reasonable cost.
Background
Fragility fractures result from mechanical forces that would not ordinarily result in fracture, known as low-level (or “low energy”) trauma (1). The World Health Organization (WHO) has quantified this as forces equivalent to a fall from a standing height or less (2).
Fragility fractures have garnered great attention as a public health concern. Worldwide, approximately 200 million women have osteoporosis and an increased risk of fragility fracture (3). It was estimated that 2.7 million new fragility fractures occurred in 2017 in the five largest EU countries (France, Germany, Italy, Spain, and UK) plus Sweden (overall referred to as EU6) (4).
The worrying burden of fragility fractures on individuals attributable to the high number of fracture-related annual losses of quality-adjusted (QALYs) and disability-adjusted life years (5–7), of sick days (8, 9), and of healthcare costs totalled an estimated €37.5 billion across the EU6 countries (10, 11). It should be emphasized that with aging populations, EU6 countries should expect increases in the number of fragility fractures (+23%), QALY loss (+26%), and fracture-related costs (+27%) from 2017 to 2030 (6).
There is now a substantial body of evidence that individuals who have already suffered a fragility fracture are at greater risk for further fractures (12–21), particularly in the 2 years following an initial fracture (22). This suggests that there is a potential for optimizing the benefits of secondary fracture prevention by recognition that it is due to fragility, rather than other causes, and treating patients as soon as possible after occurrence of a fracture (6). Nevertheless, the treatment gap (i.e., the proportion of patients who did not receive appropriate drug therapy), in EU6 in 2017 is estimated to be 73% for women and 63% for men (6). Compared with analysis from the year 2010, this indicates a marked increase from 56% in women and 47% in men (23, 24).
Given these premises, that is by considering that secondary prevention of fragility fracture is a huge concern for public health which needs to be addressed as a priority, the Italian National Health Institute, in accordance with the recently founded (2020) Italian Fragility Fracture Observatory (monitoring centre of the epidemiology of fragility fractures in Italy), encouraged the establishment of a working group to draft guidelines in this field (i.e., the Italian Guidelines for “Diagnosis, risk stratification and continuity of care of fragility fractures” (25)). The primary objective was to provide support so that healthcare professionals from several disciplines, including non-specialist physicians, nurses, and patients’ organizations, could make appropriate decisions to improve the outcomes of secondary fragility fractures in adherence with standards for trustworthy guidelines and the GRADE (Grading of Recommendations Assessment, Development and Evaluation) system (26, 27). The current guidelines cover a wide range of areas, including recognition of fragility as the cause of bone fracture, assessment of the risk (including the imminent risk) of secondary fractures, the choice, sequence and timing of drug therapy, and the management of clinical pathway.
The current manuscript is a translated summary of the full version of the Italian Guidelines for “Diagnosis, risk stratification and continuity of care of fragility fractures” (25). We hope that the worldwide audience of healthcare professionals and policymakers takes advantage of the Italian experience.
Guideline development process
Who contributed to guideline accomplishment: the Fragility Fracture Team
The Fragility Fracture Team (FFT) was made up of professionals appointed by speciality and primary care scientific societies and the National College of Nursing Professions, representatives of patients’ associations, in addition to a team of clinical epidemiologists and biostatisticians directly appointed by the Italian National Health Institute (please see Supplementary material, Table S1, for the complete list of experts involved).
FFT took office in January 2020 for establishing the team arrangement by assigning each member to one or more panels including (i) the executive committee (MLB, GC, SL, MR, UT) for leading the FFT, and for convocation, and coordination of plenary meetings; (ii) the evidence review team (AB, LC, DG, SM, EP, GP, MP, RR) for defining the clinical questions, developing the literature search strategies, querying the bibliographic databases, and assessing the quality of the evidence; (iii) the skilled/stakeholder panel whose members (GA, RA, RB, MLB, LC, DG, SG, GI, AL, SL, RM, SM, TN, MP, AP, EP, MR, UT) consulted the preliminary versions of the guidelines and expressed opinions, comments and viewpoints according to their own experience, and made recommendations for subsequent versions; and (iv) the quality assurance team (MLB, GC, SL, MR, UT) responsible for ensuring that the Guideline Development Process complied with methodological standards. FFT members met via webinar and corresponded through e-mail. Once the Guidelines were definitively drafted, a peer review was requested from two external experts (APC, BF). The final document was signed by all FFT members, submitted for its endorsement to the National Centre for Clinical Excellence, Healthcare Quality and Safety, and approved by the Italian National Health Institute in October 2021.
Identifying previously published systematic reviews and guidelines
The GRADE-ADOLOPMENT approach (based on the GRADE EtD frameworks) was used to determine whether to develop a new guideline or adopt existing recommendations (28). Through databases developed by international health agencies (29–32), guidelines published in the last 10 years were preliminarily searched. Experts in the sector were also asked to report any other documents of interest. Only evidence-based guidelines ensuring editorial independence and reporting the adopted methods were included. Guidelines developed by regional, peripheral, or local agencies or bodies or by a single author and guidelines containing recommendations limited to a single intervention were excluded. In addition, systematic reviews on the issues of interest were also identified from those cited in guidelines, through the more widespread biomedical research databases (33, 34), and those reporting systematic reviews (35–37), as well as by means of hand-checking to identify additional relevant publications. Only systematic reviews published in the last 10 years were included. When data were published more than once, we considered the most recent and complete publication. Supplementary material, Figure S1, describes the results of the guideline/systematic review selection process. Overall, eight documents were selected (four guidelines and four systematic reviews) (38–45). Their critical analysis in terms of quality, topicality, and content was presented to the entire FFT. Because no document addressed the full spectrum of recommendations for secondary prevention of fragility fractures, the FFT opted to develop new recommendations, i.e., of developing the current guidelines.
Formulating clinical questions
Topics to be considered in the current guidelines were established in a plenary session by the entire FFT. They covered three clinical issues, namely, (i) recognition of frailty as the cause of bone fracture, (ii) the (re)fracture risk assessment for prioritizing interventions, and (iii) the treatment and management of patients experiencing a fragility fracture. Clinical Questions (CQ) covering the abovementioned clinical issues were organized according to the PICO model against which we issued the recommendations (46). PICO stands for patient/population, intervention, comparison, outcome. The PICO questions were formulated by the skilled/stakeholder panel and the evidence review team.
Systematically reviewing literature and building evidence synthesis
For each CQ, a literature search was conducted using PubMed/Medline, Embase, and the Cochrane Library (33–35), as well as original articles reported through guidelines and systematic reviews. All databases were queried, and specific search strategies were adopted for each CQ. A two-step procedure (i.e., article screening by title and abstract followed by review of entire main text) was performed in a double-blind fashion by the evidence review team. Discrepancies between readers were resolved in conference. The quality of each individual study included was evaluated using validated tools, such as the revised Cochrane ROB (risk of bias) for RCTs (randomized controlled trials) (47), the NOS (Newcastle–Ottawa Scale) for observational studies (48), and the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) for accuracy diagnostic studies (49).
After making a final decision regarding the quality of evidence and conducting the corresponding meta-analytic syntheses, the SoF (summary of finding) table was developed for each combination of CQ and outcome. The GRADE evidence profile table was consistently built (50). The GRADE quality assessment labels (i.e., high, moderate, low, and very low) were assigned to each outcome through five dimensions (risk of bias, consistency of effect, imprecision, indirectness, and publication bias).
Evidence-to-Decision (EtD) Framework achievement
The process of moving from evidence to recommendations represents a cornerstone of guideline development (51, 52). Among the broad variety of criteria for consideration suggested by international organizations for reaching a decision (53, 54), those included in the most popular framework known as GRADE-EtD (55, 56) were adopted for building the current recommendations. Details about the development process of the GRADE-EtD framework are available elsewhere (57). Briefly, the GRADE-EtD framework aims to help panel members use evidence in a structured and transparent way to inform healthcare decisions and help guideline development teams consider the most relevant criteria influencing decisions by shaping discussions (58).
Formulating recommendations
When a new systematic review was conducted, or when existing systematic reviews were evaluated and their results adapted, the FFT collaborated ahead of the recommendation decision according with the GRADE-EtD framework, developing drafts of the evidence for a decision table and recommendations’ text. Ratings for recommendation type and strength (i.e., 1 recommended/recommended against, 2 suggested/suggested against) together with GRADE quality assessment labels (i.e., A = high, B = moderate, C = low, and D = very low) were assigned. The balance of effects, values and acceptability, and feasibility were also considered. The manual from the Italian National System for Guidelines (National Health Institute 2019) (58) was referenced in developing the recommendations.
Results
Overall, 351 original papers were included in our systematic review (10, 11, 13, 16, 18, 42–44, 59–74–389), selected to answer six clinical questions. One of the six (CQ1) refers to the issue concerning frailty recognition as the cause of bone fracture (Might the recognition of frailty as the cause or contributing cause of fracture improve the patient’s prognosis)?. Two of the six questions (CQ2 and CQ3) refer to the issue concerning (re)fracture risk assessment for prioritizing interventions (What operational characteristics and applicability do the available risk assessment tools and algorithms show? and How can we identify patients at imminent risk of (re)fracture? Three of the six questions (CQ4, CQ5, and CQ6) refer to the issue concerning the treatment and management of patients experiencing fragility fracture (Which therapeutic strategy should be recommended in the short- and long-term treatment of patients at high or imminent risk of (re)fracture? Might it be advisable to discontinue a drug aimed at reducing the risk of adverse events in a patient at high risk of (re)fracture? Is the use of clinical governance models, such as the so-called Fracture Liaison Services, suitable for the post-fracture patient’s management)?. For each CQ, we have formulated one to three recommendations, which are synthesized in the corresponding visual summaries (Figures 1–6) whose footnotes report a broad and detailed description of rationale, clinical benefits, values and preferences, and understanding recommendations. Moreover, specific sections related to (i) search strategies, (ii) study selection flowchart, (iii) complete meta-analytic results, (iv) quality of evidence, and (v) SoF are reported for each CQ in the Supplemental Material.
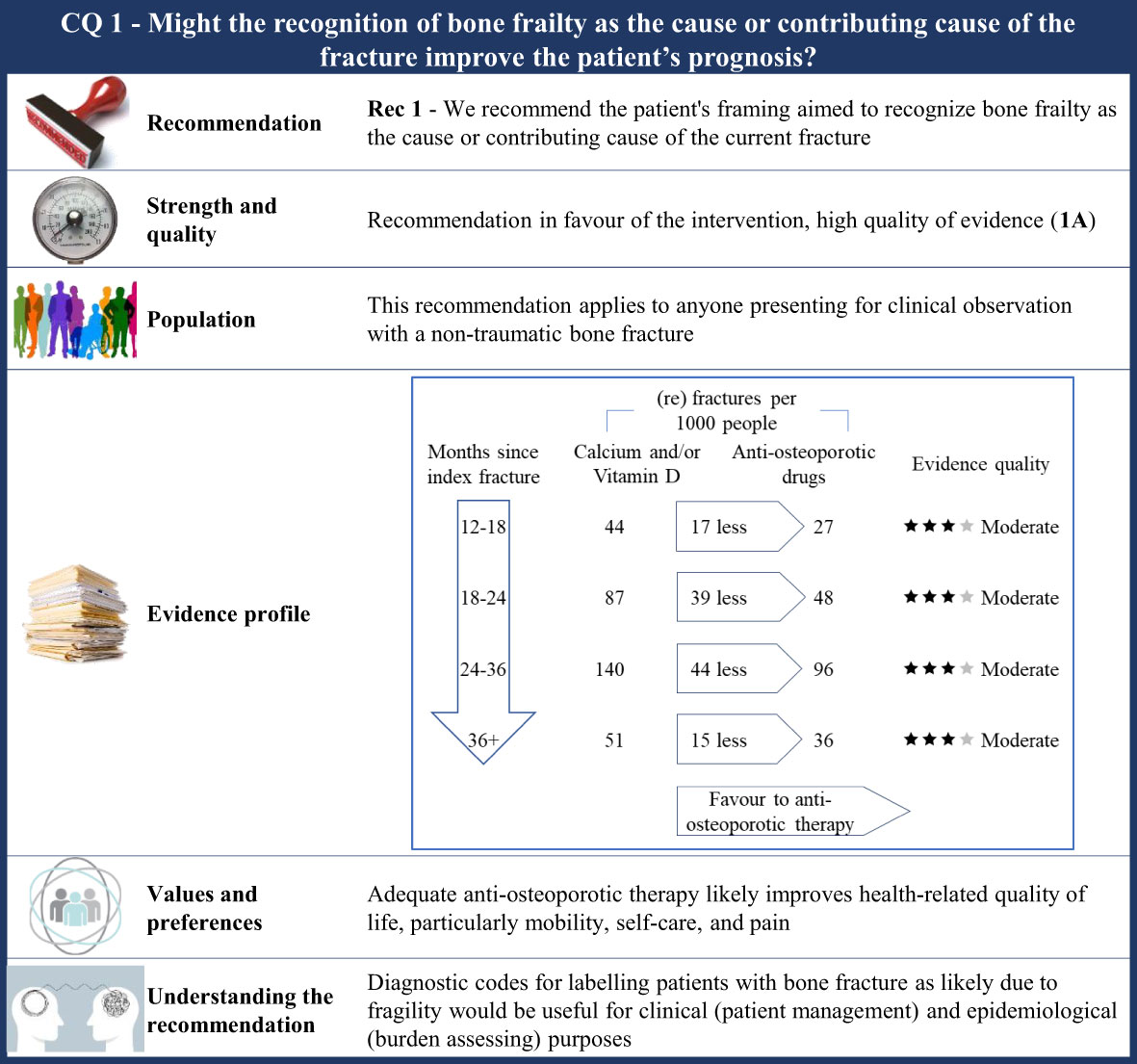
Figure 1 Visual summary for CQ1 (Might the recognition of frailty as the cause or contributing cause of the fracture improve the patient’s prognosis)?. Rationale. As ethical concerns hinder carrying out clinical studies by randomizing patients to obtain an adequate comparator (i.e., patients who have no tools to recognize bone frailty were included), the CQ was indirectly investigated. RCTs comparing outcome occurrence (refracture) among patients who received any anti-osteoporotic drug therapy and those who received calcium and/or vitamin D were included. The underlying assumption is that all the included patients were indicated for anti-osteoporotic drug therapy (i.e., the bone frailty was the cause or contributing cause of the current fracture), but some of them did not receive effective drug therapy (so surrogating those patients for whom no tools recognizing bone frailty are used, i.e., the comparator of interest). Through the updating of the most recently published systematic review on this issue (42), our systematic review included 46 RCTs (59–74, 89–104). Critical outcomes of interest pertained the rate of refracture at 12–18 months, 18–24 months, 24–36 months, and 3 years or more from the index fracture. Clinical benefits. Although the quality of evidence was moderate within each time category, a clear advantage favouring anti-osteoporotic drug therapy was observed. Between-rate absolute difference (RD) ranged from 15 to 44 (re)fractures avoided with therapy every 1,000 fractured patients, respectively, 36 months or more and 24–36 months after the index fracture. Values and preferences. Osteoporotic fractures have a negative impact on Health-related Quality of Life (HRQoL), particularly for mobility, self-care, and pain. Patients over 50 years of age treated with anti-osteoporotic therapy showed a significant improvement in HRQoL at 24 months (105). Increased quality of life as detected by the QUALIOST questionnaire was obtained through treatment of postmenopausal women (90), although no significant differences were found for the Short-Form or SF-36. At last, a higher Osteoporosis Quality of Life Scale score was reached after 12 months of drug therapy (99). Understanding the recommendation. The FFT noted that there were strong clinical benefits associated with anti-osteoporotic therapy and, consequently, agreed to upgrade up to high evidence quality despite the moderate certainty evidence. A combination of the evidence, values, and preferences also contributed to the strong recommendation in favour of the anti-osteoporotic treatment in patients with fragility fractures. Diagnostic codes for label patients who have bone fracture is likely due to fragility would be useful for clinic (patients’ managing) and epidemiologic (burden assessing) purposes.
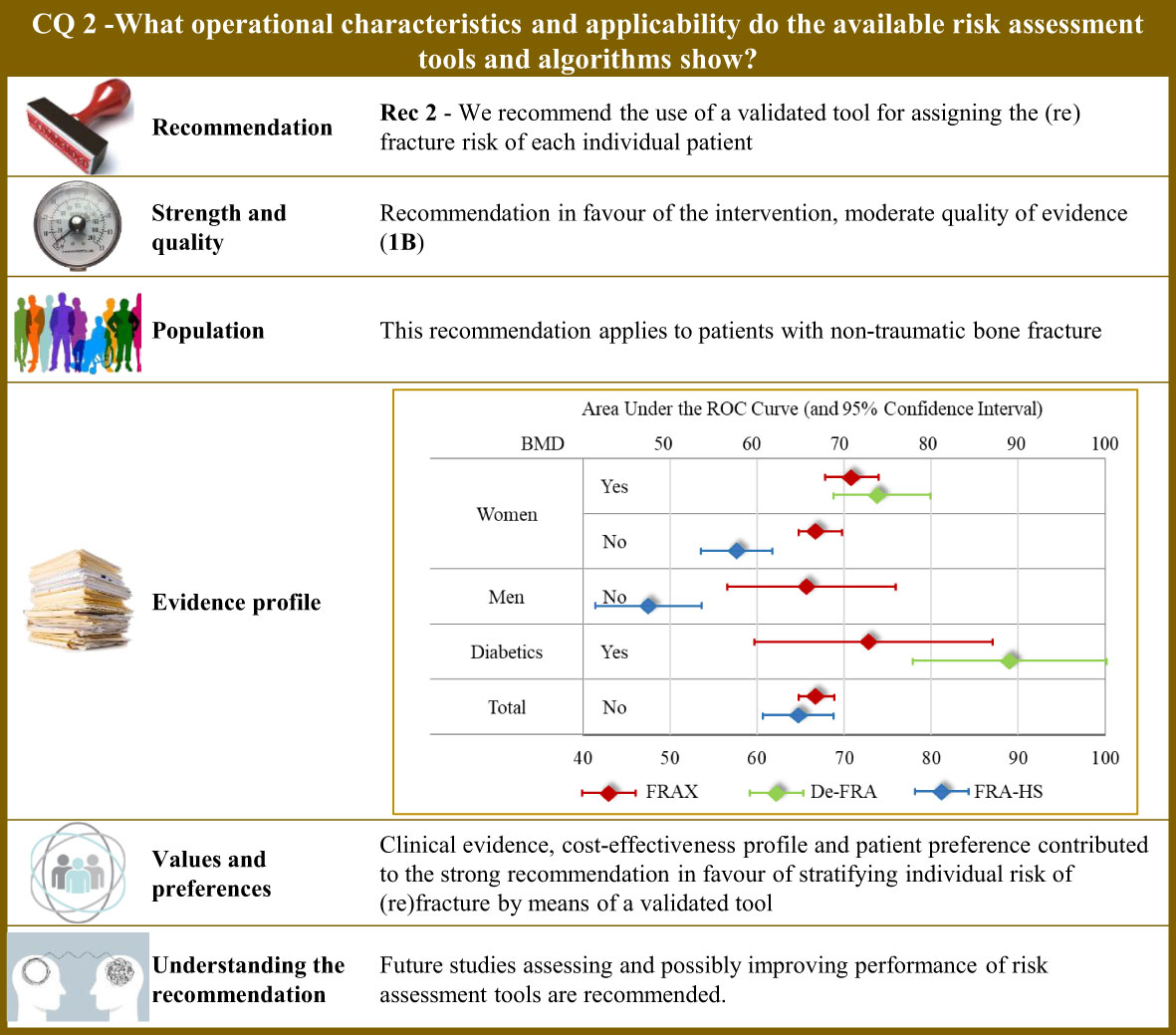
Figure 2 Visual summary for CQ2 (What operational characteristics and applicability do the available risk assessment tools and algorithms show)?. Rationale. The most common tool used worldwide for assessing the fracture risk is the so-called FRAX®, which was developed at the University of Sheffield, United Kingdom, and is based on individual patient models that integrate the risk associated with several individual features (i.e., gender, age, body mass index, personal history of fragility fracture; parental history of proximal femur fracture; current smoking status; prolonged use of glucocorticoids; rheumatoid arthritis; secondary causes of osteoporosis; and alcohol consumption ≥ 3 units per day), with or without including bone mineral density at the femoral neck (106). The model was externally validated (107) and calibrated from country-specific fracture data covering more than 80% of the world population (108). The FRAX® algorithm give the 10-year probability of fracture (106). Although the FRAX® tool is the most popular predictive tool, it nevertheless presents some application concerns, and above all access problems for regulatory use. For this reason, national versions have been developed such as the QFracture algorithm to predict risk of osteoporotic fracture in primary care in the UK (109). In Italy, three algorithms have been developed, nominally: (i) the DeFRA developed by the Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases in collaboration with the Italian Society of Rheumatology (110), and made available online (111); (ii) its updated version (DeFRAcalc79) defined according to drug reimbursement rules from the Italian Drug Agency (112); (iii) and the FRActure Health Search (FRA-HS) developed by the Italian Society of General Medicine and Primary Care (113). As studies directly comparing reliability and applicability of available tools are lacking, a systematic revision of literature was carried out for obtaining and comparing meta-analytic estimates of discriminatory powers through the AUC (area under the receiver operating curve) (114). Through the updating of the NICE guidelines (115), our systematic review included 47 original papers investigating operative characteristics of FRAX® (116–162) and added three papers pertaining Italian instruments (two for DeFRA (163, 164) and one for FRA-HS (165)). Operative characteristics pertain the 10-year predicted and observed fracture risk (major osteoporotic or proximal femur) in all the included papers. Tools performance. Meta-analytic AUC estimates (and 95% confidence intervals) for FRAX were 0.66 (0.57 to 0.76) and 0.67 (0.65 to 0.70) in women and men, respectively. By including body mineral density among the considered items, the AUC of FRAX® improved to 0.71 (0.68 to 0.74) and 0.73 (0.60 to 0.87) in women and diabetics, respectively. DeFRA had better performance than FRAX® for both women (0.74, 0.69 to 0.80) and diabetics (0.89, 0.78 to 1.00). Conversely, FRA-HS discriminated worse than other tools with the AUC estimates 0.58 (0.54 to 0.62) and 0.48 (0.42 to 0.54) in women and men, respectively. Clinical and value issues. Ten-year fracture risk perceived by patients and that estimated by the predictive tool (specifically by FRAX®) was found to be highly disagreeing among patients at high fracture risk, women, elderly, and patients treated with anti-osteoporotic medications or calcium/vitamin D (166), thus making implementation of fracture prediction tools in clinical practice highly to be hoped for. Efficient screening strategies may support fragility fracture prevention as shown by a Sweden study that administered the FRAX® to postmenopausal women via e-mail, online or screening mammography (167). Patients at high risk of fracture should be earlier identified to reduce mortality, comorbidities, and costs (177). Suitable cost-effectiveness profiles for fracture risk screening (169), and for consequent therapy of high-risk patients with any anti-osteoporotic treatment (170–176), and other drugs (176) were consistently reported from several European countries. Understanding the recommendation. Although clinical evidence, cost-effectiveness profile, and patient’s preference contributed to the strong recommendation in favours of stratifying the individual risk of (re)fracture, concerns persist about quality of evidence of studies investigating predictivity of available tools, including FRAX®. Although FRAX® might be used in any healthcare settings (168, 177), cautions should be taken in its use in specific countries by adopting tools built and validated in the target population. Future studies assessing and possibly improving performance of risk assessment tools are recommended.
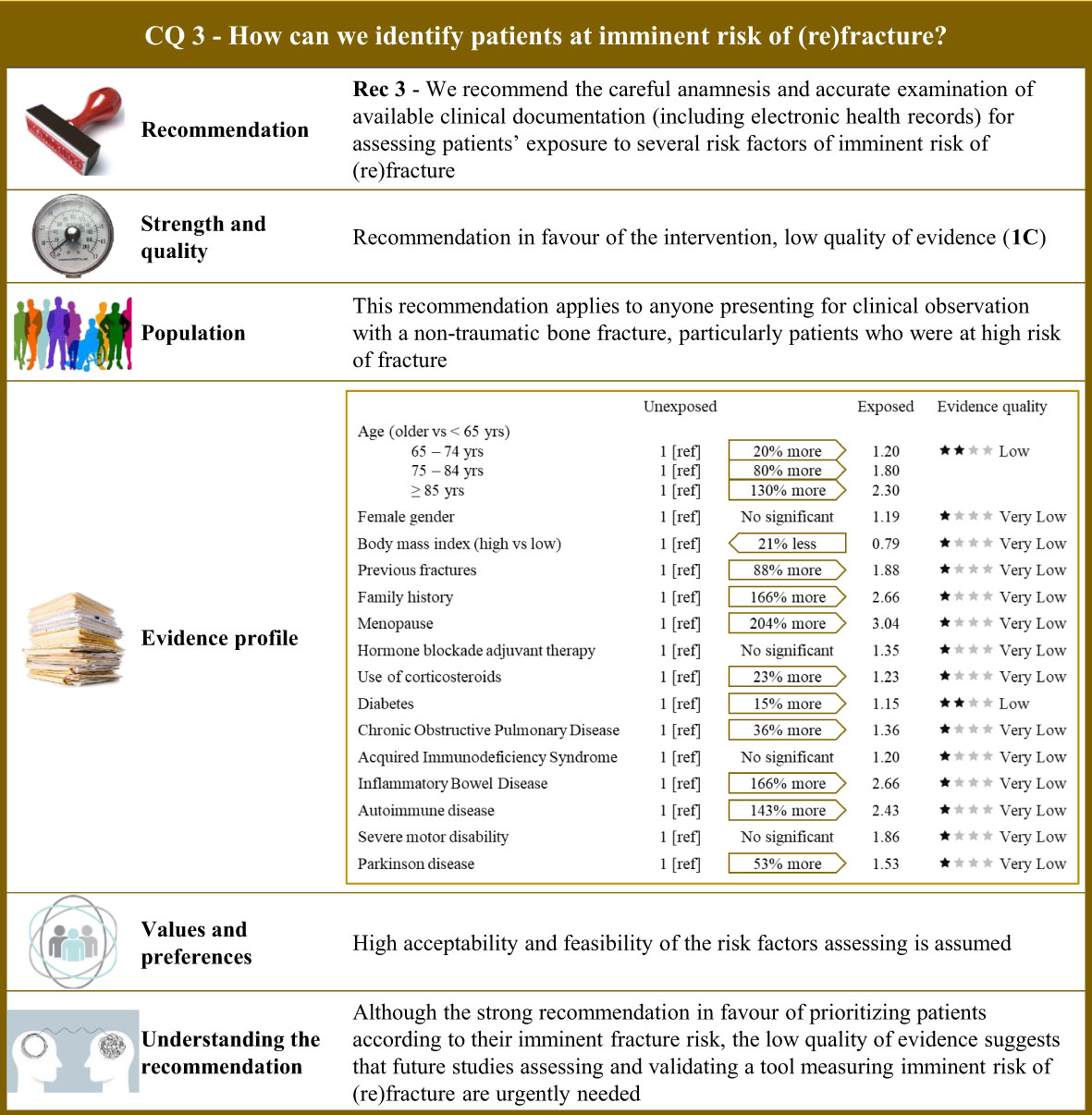
Figure 3 Visual summary for CQ3 (How can we identify patients at imminent risk of (re)fracture)?. Rationale. As the risk of bone fracture after experiencing a fracture is on average doubled in the 2 years that follow (11, 13, 16, 178–185), a period which has been defined “imminent,” particular attention should be placed on identifying patients at higher risk of imminent (re)fractures (10, 186–188). Our systematic review included 46 observational studies comparing the risk of imminent (re)fracture of patients who were exposed and not exposed to a series of potential risk factors (18, 181, 186, 187, 189–230). Risk factor profile. Among the 15 factors included, 11 showed evidence of increasing the imminent risk of fracture, with relative risk excess ranging from 20% (acquired immunodeficiency syndrome) to 204% (menopausal status). Values and preferences. High acceptability and feasibility of the assessment risk factors is assumed. Understanding the recommendation. Despite the strong recommendation in favour of prioritizing patients according to their imminent fracture risk, uncertainty due to serious/very serious risk of bias of observational designs and imprecision and inconsistency of estimates strongly affected the quality of evidence. Future studies assessing and validating a tool measuring imminent risk of (re)fracture are therefore urgently needed.
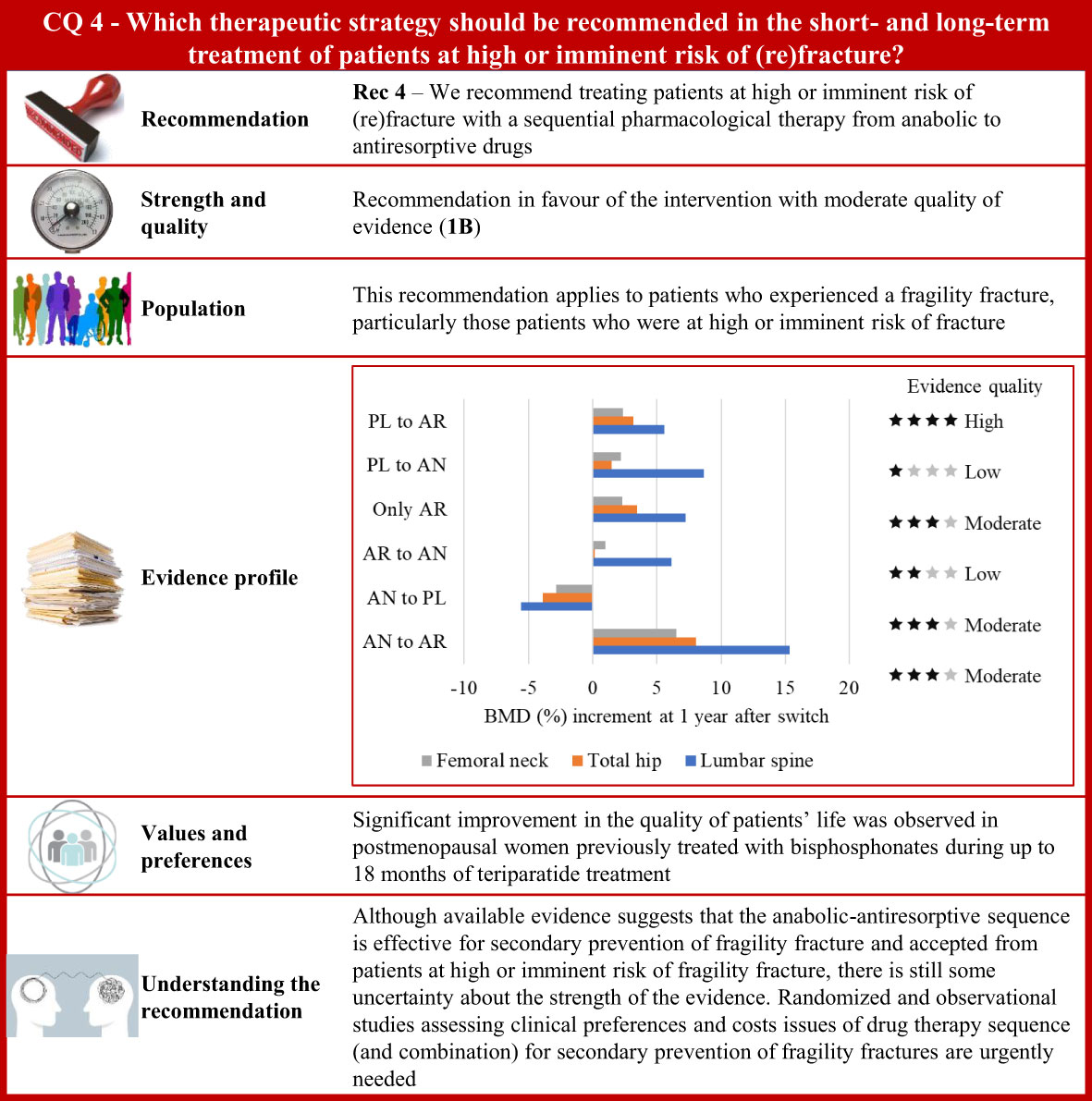
Figure 4 Visual summary for CQ4 (Which therapeutic strategy should be recommended in the short- and long-term treatment of patients at high or imminent risk of (re)fracture)?. Rationale. Nowadays, pharmacological options have been developed for the treatment of osteoporosis and fragility fractures. Bisphosphonates and denosumab are potent antiresorptive drugs (AR). In particular, denosumab is a fully monoclonal antibody, directed against the receptor activator of nuclear factor-κB ligand that inhibits the differentiation, activation, and survival of osteoclasts (231). Anabolic drugs (AN) are teriparatide and abaloparatide (the latter not yet available in Italy). These treatments, typically taken intermittently, act through the parathyroid hormone receptor and stimulate osteoblast activity for bone formation. Romosozumab is the newer anabolic drug made available (also in Italy) (232), acting with a dual mechanism of action since it stimulates bone formation and inhibits bone resorption (231). Anabolic treatment is time-limited (12 to 24 months) and consequently its beneficial effects against bone loss, which could increase the fracture risk. For this reason, treatment that includes antiresorptive agents should be considered (231). However, the optimal treatment strategy for fracture needs to be identified based on sequential or combined therapies whose clinical efficacy should be assessed according with their antifracture potential and harm profile (231–233). Our systematic review included 17 RCTs (234–250). Between-arm comparison of bone mineral density changes and the fracture risk during follow-up were the critical outcomes of interest. Clinical benefits. Only one study compared bone mineral density changes among patients randomized to the anabolic–antiresorptive sequence or vice versa (241). Increasing values of bone mineral density were obtained for the entire observation time-window (that is, from baseline to 24 months after the drug switch was scheduled, and 24 months after the start of the second sequential drug) for all the considered bone sites. Conversely, among patients who started with an antiresorptive medication, decreasing values of bone mineral density were observed once the switch was made to an anabolic medication, suggesting that anabolic drugs in the second phase can compromise the effect of antiresorptive taken initially. Several other comparisons are available, but the corresponding findings (available in the Supplementary material) do not offer clear evidence at favour of a specific sequence. The risk of fracture was found to be lower 12 and 24 months after switching from anabolic to antiresorptive compared with both placebo (PL)–antiresorptive sequence (235, 242), or treatment with alendronate only (245, 250). Twelve months after switching from placebo to anabolic (teriparatide) the risk of fracture was found to be lower than switching from antiresorptive (bisphosphonate) to anabolic teriparatide (240). The certainty for fracture risk was moderate because of risk of bias and imprecision. Values and preferences. A prospective, multicentre observational study conducted in eight European countries (Austria, Denmark, France, Germany, Greece, Ireland, The Netherlands, and Sweden) after teriparatide was approved by regulatory agencies reported that postmenopausal women previously treated with bisphosphonates had significant improvement in HRQoL during up to 18 months of teriparatide treatment (251). Understanding the recommendation. Although available evidence suggests that the anabolic–antiresorptive sequence is effective for secondary prevention of fragility fracture and accepted from patients at high or imminent risk of fragility fracture, there is still some uncertainty about the strength of the evidence. Randomized and observational studies assessing clinical preferences and cost issues of drug therapy sequence (and combination) for secondary prevention of fragility fractures are however urgently need.
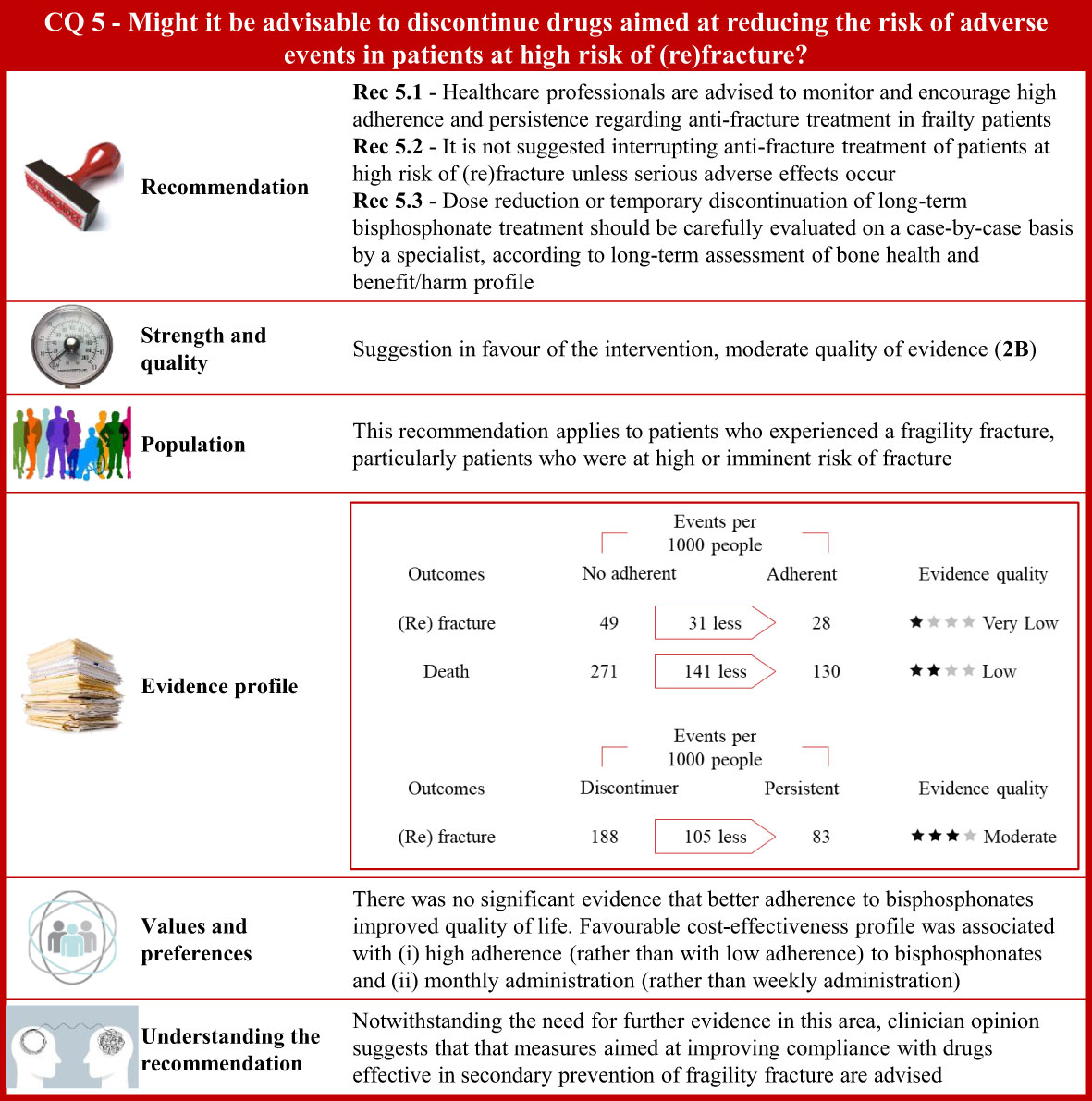
Figure 5 Visual summary for CQ5 (Might it be advisable to discontinue drugs aimed at reducing the risk of adverse events in patients at high risk of (re)fracture)?. Rationale. Secondary fragility fractures may be prevented by improving long-term adherence to anti-osteoporotic drugs (252–254). However, low adherence could be induced by adverse events (255–257), inadequate drug dosage regimens (255, 257), or asymptomatic disease (255, 256). Less frequent or intermittent dosing schedules may promote medications adherence to long-term therapies and improve health outcomes in postmenopausal women (74, 258). Through updates of the most recently published systematic reviews on this issue (259–261), and specific manual research, our systematic review included 15 publications investigating the association between continuity of treatment (persistence and adherence) and several outcomes (changes in bone mineral density and fracture risk) in patients with fragility fracture (62, 74, 252, 258, 262–272). Clinical benefits. Three comparisons evaluated the medication vacation in patients with osteoporosis, nominal adherence vs. no adherence, persistence vs. discontinuity, and continuous vs. cyclical treatment. Adherence was defined by the number of doses dispensed with respect to the observation time and calculated by the medication possession ratio (MPR). A reduced risk of vertebral (risk ratio 0.74; 95% confidence interval 0.60 to 0.91), non-vertebral (0.42; 0.20 to 0.87), or any fracture (0.82; 0.72 to 0.93) was detected among patients with MPR greater than 80% compared to non-adherent subjects. Adherence was associated with lower mortality (0.47; 0.35 to 0.64). Reduced vertebral fracture risk (0.81; 0.66 to 0.99) was found among patients who adhered to therapy for more than 12 months with respect to those whose adherence was less than 12 months. Persistence was defined by at least 30 days of drug therapy interruption. Reduced vertebral fracture risk was found in persistent patients compared to discontinuers (0.85; 0.75 to 0.96). No significant decreased risk of vertebral, non-vertebral, or any fracture was detected among patients who persisted with therapy for more than 12 months compared to patients who persisted for less than 12 months. Consistently, there was no evidence of a reduced mortality risk. Extension trials were included in this comparison. Patients randomized to receive placebo or anti-osteoporotic drugs after 5 years were re-randomized to respectively receive anti-osteoporotic drugs or placebo. A reduced risk of non-vertebral fracture was associated with the continuous treatment (0.37; 0.26 to 0.54), whereas there was no evidence of decreased risk of vertebral or any fracture. There was no reduced risk of adverse events associated with the continuous anti-osteoporotic therapy. Finally, studies that randomly assigned patients to daily anti-osteoporotic therapy or cyclical treatment found no statistical evidence of difference in fracture risk and adverse events (upper gastrointestinal or oesophageal disorders). Quality of evidence was low for studies investigating MPR and persistence, and moderate for studies balancing fracture risk and adverse events. Values and preferences. Only one RCT compared patients who had long-term adherence to oral bisphosphonate with those who did not adhere to therapy, without finding any significant difference in health-related quality of life at baseline and 12–24 months afterward (270). Postmenopausal women discontinued anti-osteoporotic treatment due to drug-related/fear of side effects or insufficient motivation (273). Poor compliance was related to benzodiazepine and gastroprotective use, whereas persistence to treatment was higher in patients with previous vertebral fractures, early menopause, or low bone mass values or treated with corticosteroid or anti-inflammatory medications. Higher education level and disease awareness were associated with better adherence to long-term treatment with alendronate, whereas onset of new diseases had induced treatment interruption (274). Medication routes of administration may directly influence adherence. Specifically, self-administered teriparatide injection was well tolerated (275). Subcutaneous injection of parathyroid hormone was shown to be correctly administered to elderly patients with trochanteric hip fracture (276). Treatment adherence to denosumab administered subcutaneously every 6 months was greater than adherence to oral alendronate taken once a week in postmenopausal women (277). Conversely, a Chinese study showed that patients with a history of fracture had a stronger preference for weekly oral tablets compared to other modes of administration such as annual intravenous infusion or 6-month subcutaneous injection (278). Finally, there was no statistical evidence of differences of administration route preference in patients undergoing a standardized educational session regarding the pathophysiology of osteoporosis and its complications (279). Although poor compliance might be associated with reduced clinical benefits and increased mortality (280), it would not necessarily affect the actual cost per fracture avoided (281). Based on model estimates, more fractures were avoided with monthly bisphosphonate (58.1 per 1,000 treated women) than with weekly bisphosphonates (33.8 per 1,000 treated women), resulting in lower incremental cost per QALY gained (282). Costs per QALY gained were estimated to increase with higher adherence to oral bisphosphonates whereas poor compliance would result in a decreased cost-effectiveness of drug therapy (283). Included studies might have limited generalizability across different countries because of distinctive resources and prioritized treatment options available in various healthcare settings. Understanding the recommendation. Notwithstanding questionable quality of available evidence, clinician opinion suggests that measures aimed at improving compliance with drugs effective in secondary prevention of fragility fracture are advised.
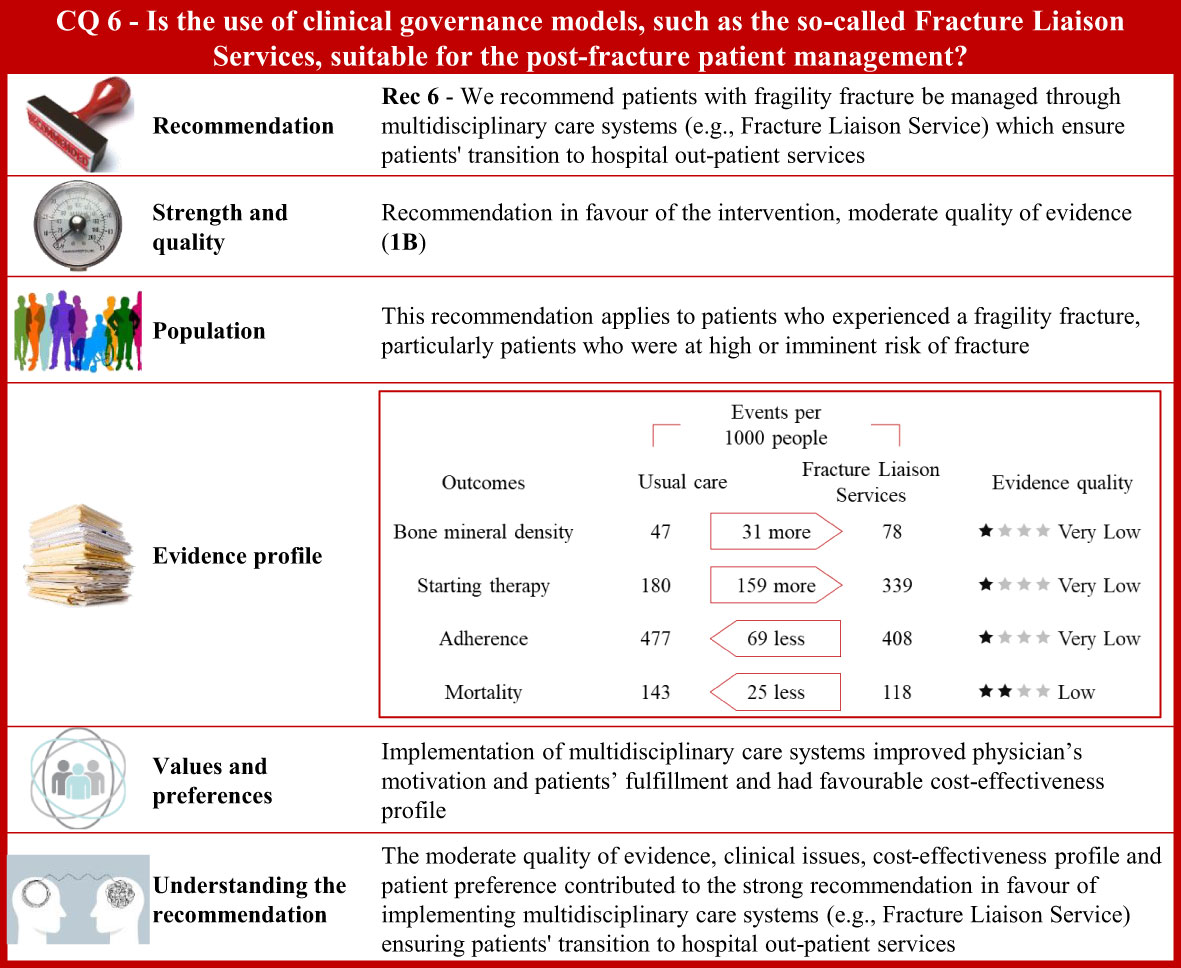
Figure 6 Visual summary for CQ6 (Is the use of clinical governance models, such as the so-called Fracture Liaison Services, suitable for patients’ post-fracture management)?. Rationale. Patients who experienced a fragility fracture should receive correct care planning after hospital discharge to ensure continuity of care through shared diagnostic-therapeutic pathways (284). According to the Fragility Fracture Network (285), global multidisciplinary collaborations should be carried out to improve the care of patients with fragility fractures (286). The Fracture Liaison Service (FLS) is a model of care designed to prevent recurrent fractures (44, 287–289) with coordinated strategies (290) and achieve optimal adherence to anti-osteoporotic medications (291, 292). The multidisciplinary team should be formed by the bone specialist (FLS coordinator), the orthopaedic surgeon, and the specialized bone nurse (43, 293, 294). Through updates of the most recently published systematic reviews on this issue (290, 295–298), and specific manual research, our systematic review included 77 publications on multidisciplinary care systems, such as nurse-led clinics, structured service delivery models, and FLS (299–375). Bone mineral density values, anti-osteoporotic therapy initiation, adherence to anti-osteoporotic therapy, and (re)fracture and mortality risk were the outcomes of interest. Clinical benefits. Compared with usual care, the multidisciplinary programs significantly increased body mineral density values, initiation to anti-osteoporotic treatment, and adherence to anti-osteoporotic therapy. Moreover, these coordinated models showed a reduction of fractures and a significant decrease in mortality risk. Quality of evidence was very low for body mineral density values, initiation to anti-osteoporotic treatment, and adherence to anti-osteoporotic therapy, and low for mortality rate. Values and preferences. Physicians’ motivation in implementing FLS is justified given barriers in treating fractures, gaps in osteoporosis knowledge, and difficulty in managing patients presenting with a fragility fracture observed by healthcare professionals (376). Chinese orthopaedic surgeons reported low sensitivity to the concept of fracture prevention as well as in the effectiveness of preventive measures for fragility fractures (377). Only 25% of patients who contacted their physician received anti-osteoporotic treatment, according to an RCT (378). Conversely, 61% of subjects with a low-trauma fracture were treated with an anti-osteoporotic medication according to a study investigating performance of FLS (Yates 2015) (379). However, the included studies considered various healthcare settings; thus, resource requirements might have limited generalizability across different countries. A decreased refracture risk and favourable cost-effectiveness profile of FLS models compared to usual care was reported from a systematic review evaluating FLS programs in the Asia-Pacific region (298). Compared to usual care or no treatment, FLS resulted in a favourable cost-effectiveness profile from another systematic review (Wu 2018) including osteoporotic patients aged 50 and above from Canada, Australia, the US, UK, Japan, Taiwan, and Sweden (380). FLS-based management of fragility fractures had been reported to be cost-effective in Canada with the reduction of subsequent hip fractures and a net hospital cost savings (381). The implementation of hip FLS co-managed by a nurse and physician showed a $54 incremental cost/patient with a modest gain of eight QALYs/1,000 patients (382). For every 10,000 patients that participated in FLS, an additional 400 patients would be treated with bisphosphonates, resulting in the avoidance of around four hip fractures. Furthermore, the proportion of patients who appropriately received bisphosphonate treatment increased in the year following fracture, from 4.3% to 17.5% (383). The FLS implementation in the USA resulted in 153 fewer fractures, 37.4 QALY gained, and $66,879 in cost savings for every 10,000 patients (384). An FLS implemented in UK was estimated to prevent at least 18 fractures and save £21,000 for every 1,000 patients (385). FLS organizations ensure that patients, affected by osteoporosis or fractures, receive appropriate evaluation and treatment (386, 387) although failures in entry registration, male gender, frailty, education level, living alone, or lack of motivation could be independent factors for FLS non-attendance (388). Additional potential barriers include lack of communication between patients and physicians or the need for patient education intervention (389). Some patients might refuse treatment because of concerns with costs or side effects. Thus, person-centred care should support the interaction between patients and healthcare professionals (389). Understanding the recommendation. The moderate quality of evidence, clinical issues, cost-effectiveness profile, and patient’s preference contributed to the strong recommendation in favour of implementing multidisciplinary care systems (e.g., Fracture Liaison Service) ensuring patients’ transition to hospital outpatient services.
Briefly, we recommend (i) recognizing bone fragility as the cause or contributing cause of the current fracture, (ii) measuring the individual (re)fracture risk using a validated tool, (iii) assessing the patient’s exposure to several factors associated with imminent (re)fracture risk, (iv) using a sequential pharmacologic scheme from anabolic to antiresorptive drugs, mainly in patients at higher/imminent risk of fracture, (v) avoiding treatment interruption, except for serious adverse events that occur, and (vi) implementing multidisciplinary care systems (e.g., Fracture Liaison Service), for ensuring patients’ transition to hospital outpatient services. Of these six recommendations, one was of high quality, another one of low quality, the remaining four being of moderate quality.
Perspectives
From now on, as current delivery of secondary fracture prevention globally is lamentably suboptimal and taking into account the availability of guideline-based recommendations, the key challenge facing us all is how to ensure that guidelines-based care becomes usual care. The promotion of widespread awareness of the new guidelines, must necessarily be accompanied by a robust evaluation plan aimed of (i) monitoring the quality of services for secondary fracture prevention (are we providing healthcare according to recognized quality standards? what critical issues arise)? and (ii) assessing their impact (how and how much the guidelines adoption prevents the occurrence of secondary fractures and improve quality of life of patients? at what cost)?. In this regard, the combination of national clinical care standards and registries to enable benchmarking against such standards provides an opportunity to undertake so-called “real-world data” analyses for monitoring the changes and assessing the impact of usual care. There are currently 20 national hip fracture registries established worldwide, and the China National Hip Fracture Registry is at an advanced stage of development (390). Furthermore, there are currently national FLS registries at various stages of development in Australia and New Zealand (391, 392), Ireland (393), the UK (394), and USA (395). Several “real-world” evidence from the UK National Hip Fracture Database and Best Practice Tariff for hip fracture care have provided valuable insights (396–398). In Italy, the “real-world” monitoring changes and assessing impact of the new guidelines will be made possible by the “Italian Fragility Fracture Observatory,” a structure recently founded for bridging the gap between health institution and academy in generating knowledge (399) in the field of fragility fractures.
Conclusion
The current guidelines provide guidance to support individualized management of patients experiencing non-traumatic bone fracture aimed of secondary prevention of (re)fracture. Although our recommendations are based on the best available evidence, questionable quality evidence is still available for some relevant clinical questions, so future research has the potential to reduce uncertainty about the effects of intervention and the reasons for doing so at a reasonable cost.
Author contributions
All authors contributed to the preparation of the guidelines, all participated in the data collection, drafting, writing and editing the manuscript. Concept and design: MLB, GC, SL, MR, UT. Acquisition, analysis, or interpretation of data: AB, LC, DG, SM, EP, GP, MP, RR. Statistical analysis: AB, GP, RR. MLB, GC, SL, MR, UT take responsibility for the integrity of the data and the data analysis. The external experts APC, BF peer reviewed the guidelines. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from ALTIS Omnia Pharma Service. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
We thank the Charlesworth Author Services for the English Academic Editing.
Conflict of interest
GC received research support from the European Community EC, the Italian Agency of Drug AIFA, and the Italian Ministry for University and Research MIUR. He took part to a variety of projects that were funded by pharmaceutical companies i.e., Novartis, GSK, Roche, AMGEN, and BMS. He also received honoraria as member of Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MLB has received i honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB; ii grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma; and iii honoraria as consultant for Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB Pharma. LC has received honoraria as member of the Advisory Board from UCB Pharma and speaking fee of Dynamicom Education and took part to the Italian project for the introduction of Fracture Liaison Service. GA has received honoraria as consultant for Theramex. He took part to a project funded by the Italian Society of Rheumatology. DG has received honoraria as consultant for Eli-Lilly, Organon, and MSD Italia. SG has received honoraria as consultant for UCB Pharma. SM has received honoraria as consultant for UCB, Eli-Lilly, and Amgen. MR has received honoraria as consultant for UCB, Eli-Lilly, Theramex, and Amgen. He took part to a project funded by Savio Pharma Italia and UCB Pharma. RM took part to a project funded by Abiogen Pharma. GI received honoraria as speaker by Eli-Lilly, Menarini, and UCB Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1137671/full#supplementary-material
References
1. Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, et al. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess (2016) 20:1–406. doi: 10.3310/hta20780
2. WHO. Report of a World Health Organization Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series, no. 843 (1994). Geneva. Available at: https://apps.who.int/iris/bitstream/handle/10665/39142/WHOTRS843eng.pdf?sequence=1&isAllowed=y (Accessed August 2022).
3. Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int (1992) 2:285–9. doi: 10.1007/BF01623184
4. International Osteoporosis Foundation. Broken bones, broken lives – the fragility fracture crisis in six European countries (2018). Available at: https://www.iofbonehealth.org/broken-bones-broken-lives (Accessed August 2022).
5. National Institute for Health and Care Excellence. Glossary . Available at: https://www.nice.org.uk/glossary (Accessed August 2022).
6. Borgström F, Karlsson L, Ortsäter G, Norton N, Halbout P, Cooper C, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos (2020) 15:59. doi: 10.1007/s11657-020-0706-y
7. Institute for Health Metrics and Evaluation (IHME). GBD compare data visualization (2016). Available at: https://vizhub.healthdata.org/gbd-compare/ (Accessed August 2022).
8. National Osteoporosis Society. Employment and osteoporosis . Available at: https://nos.org.uk/help-and-support/living-with-osteoporosis/employment-and-osteoporosis/ (Accessed August 2022).
9. Cooper C, Ferrari S, on behalf of the International Osteoporosis Foundation (IOF) Board and Executive Committee. IOF Compendium of Osteoporosis. Available at: https://share.osteoporosis.foundation/WOD/Compendium/IOF-Compendium-of-Osteoporosis-WEB.pdf. (last accessed August 2022).
10. Roux C, Briot K. Imminent fracture risk. Osteoporos Int (2017) 28:1765–9. doi: 10.1007/s00198-017-3976-5
11. Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos (2016) 11:26. doi: 10.1007/s11657-016-0280-5
12. Johnell O, Oden A, Caulin F, Kanis JA. Acute and long-term increase in fracture risk after hospitalization for vertebral fracture. Osteoporos Int (2001) 12:207–14. doi: 10.1007/s001980170131
13. Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C. Fracture risk following an osteoporotic fracture. Osteoporos Int (2004) 15(3):175–9. doi: 10.1007/s00198-003-1514-0
14. Nymark T, Lauritsen JM, Ovesen O, Röck ND, Jeune B. Short time-frame from first to second hip fracture in the funen county hip fracture study. Osteoporos Int (2006) 17:1353–7. doi: 10.1007/s00198-006-0125-y
15. Giangregorio LM, Leslie WD. Manitoba Bone density program. time since prior fracture is a risk modifier for 10-year osteoporotic fractures. J Bone Miner Res (2010) 25:1400–5. doi: 10.1002/jbmr.35
16. van Geel TACM, van Helden S, Geusens PP, Winkens B, Dinant G-J. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis (2009) 68:99–102. doi: 10.1136/ard.2008.092775
17. Dretakis KE, Dretakis EK, Papakitsou EF, Psarakis S, Steriopoulos K. Possible predisposing factors for the second hip fracture. Calcif Tissue Int (1998) 62:366–9. doi: 10.1007/s002239900446
18. Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA (2001) 285:320–3. doi: 10.1001/jama.285.3.320
19. Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P. Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J Bone Miner Res (2009) 24:1299–307. doi: 10.1359/jbmr.090207
20. Banefelt J, Åkesson KE, Spångéus A, Ljunggren O, Karlsson L, Ström O, et al. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int (2019) 30:601–9. doi: 10.1007/s00198-019-04852-8
21. Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle SG, Grauer A, et al. Risk of subsequent fracture after prior fracture among older women. Osteoporos Int (2019) 30:79–92. doi: 10.1007/s00198-018-4732-1
22. Kanis JA, Johansson H, Odén A, Harvey NC, Gudnason V, Sanders KM, et al. Characteristics of recurrent fractures. Osteoporos Int (2018) 29:1747–57. doi: 10.1007/s00198-018-4502-0
23. Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European union: medical management, epidemiology and economic burden. Arch Osteoporos (2013) 8:136. doi: 10.1007/s11657-013-0136-1
24. Strom O, Borgstrom F, Kanis JA, Compston J, Cooper C, McCloskey EV, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the international osteoporosis foundation (IOF) and the European federation of pharmaceutical industry associations (EFPIA). Arch Osteoporos (2011) 6:59–155. doi: 10.1007/s11657-011-0060-1
25. Sistema Nazionale per le Linee Guida. Diagnosi, stratificazione del rischio e continuità assistenziale delle fratture da fragilità . Available at: https://snlg.iss.it/wp-content/uploads/2022/01/LG-392_Fratture-da-Fragilit%C3%A0_v2.pdf (Accessed August 2022).
26. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
27. Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P, et al. Guidelines international network: toward international standards for clinical practice guidelines. Ann Intern Med (2012) 156:525–31. doi: 10.7326/0003-4819-156-7-201204030-00009
28. Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol (2017) 81:101–10. doi: 10.1016/j.jclinepi.2016.09.009
29. The National Institute for Health and Care Excellence. NICE guidance . Available at: www.nice.org.uk/guidance/ (Accessed August 2022).
30. Agency for Healthcare Research and Quality. Guidelines and measures . Available at: https://www.ahrq.gov/gam/index.html (Accessed August 2022).
31. Scottish Intercollegiate Guidelines Network (SIGN). Our guidelines . Available at: https://www.sign.ac.uk/our-guidelines/ (Accessed August 2022).
32. Guidelines International Network (GIN). International guidelines library . Available at: https://g-i-n.net/international-guidelines-library/ (Accessed August 2022).
34. Embase . Elsevier. Available at: https://www.embase.com/landing?status=grey (Accessed August 2022).
35. Cochrane library . Available at: https://www.cochranelibrary.com/ (Accessed August 2022).
36. The Campbell Collaboration. Database of abstracts of reviews of effects (DARE): Quality-assessed reviews . Available at: https://www.ncbi.nlm.nih.gov/books/NBK285222/ (Accessed August 2022).
37. Public Health Agency of Canada. Health evidence . Available at: https://www.healthevidence.org/ (Accessed August 2022).
38. Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, et al. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int (2011) 22:2051–65. doi: 10.1007/s00198-011-1642-x
39. Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int (2011) 22:2067–82. doi: 10.1007/s00198-011-1544-y
40. National Clinical Guideline Centre (UK). Osteoporosis: Fragility fracture risk: Osteoporosis: Assessing the risk of fragility fracture. London: Royal College of Physicians (UK (2012).
41. Scottish Intercollegiate Guidelines Network (SIGN). Management of osteoporosis and the prevention of fragility fractures: A national clinical guideline. Scottish Intercollegiate Guidelines Network (2015).
42. Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern MP, Chung KC. Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporos Int (2017) 28:3289–300. doi: 10.1007/s00198-017-4175-0
43. Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian society for orthopaedics and traumatology. J Orthop Traumatol (2017) 18(Suppl 1):3–36. doi: 10.1007/s10195-017-0474-7
44. Lems WF, Dreinhöfer KE, Bischoff-Ferrari H, Blauth M, Czerwinski E, da Silva J, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis (2017) 76:802–10. doi: 10.1136/annrheumdis-2016-210289
45. Lee SY, Jung SH, Lee SU, Ha YC, Lim JY. Can bisphosphonates prevent recurrent fragility fractures? a systematic review and meta-analysis of randomized controlled trials. J Am Med Dir Assoc (2018) 19:384–90. doi: 10.1016/j.jamda.2018.02.005
46. Luijendijk HJ. How to create PICO questions about diagnostic tests. BMJ Evid Based Med (2021) 26:155–7. doi: 10.1136/bmjebm-2021-111676
47. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
48. Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, in: Secondary the Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (2011). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed August 2022).
49. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
50. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
51. Schunemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ (2014) 186:E123–42. doi: 10.1503/cmaj.131237
52. Meneses-Echavez JF, Bidonde J, Yepes-Nuñez JJ, Poklepović Peričić T, Puljak L, Bala MM, et al. Evidence to decision frameworks enabled structured and explicit development of healthcare recommendations. J Clin Epidemiol (2022) 150:51–62. doi: 10.1016/j.jclinepi.2022.06.004
53. IOM (Institute of Medicine). Clinical practice guidelines we can trust. Washington, DC: The National Academies Press (2011).
54. World Health Organization. WHO handbook for guideline development. 2nd ed. Geneva: World Health Organization (2014).
55. Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, et al. GRADE: assessing the quality of evidence in environmental and occupational health. Environ Int (2016) 92-93:611–6. doi: 10.1016/j.envint.2016.01.004
56. Rehfuess EA, Stratil JM, Scheel IB, Portela A, Norris SL, Baltussen R. The WHO-INTEGRATE evidence to decision framework version 1.0: integrating WHO norms and values and a complexity perspective. BMJ Glob Health (2019) 4:e000844. doi: 10.1136/bmjgh-2018-000844
57. Rosenbaum SE, Moberg J, Glenton C, Schünemann HJ, Lewin S, Akl E, et al. Developing evidence to decision frameworks and an interactive evidence to decision tool for making and using decisions and recommendations in health care. Glob Challenges (2018) 2:1–9. doi: 10.1002/gch2.201700081
58. SNLG ISS. Sistema nazionale per le linee guida-Istituto superiore di sanità. Come produrre, diffondere e aggiornare raccomandazioni per la pratica clinica. manuale metodologico (2019). Roma: PNLG. Available at: http://www.snlg-iss.it/manuale_metodologico_SNLG (Accessed August 2022).
59. Reid IR, Wattie DJ, Evans MC, Gamble GD, Stapleton JP, Cornish J. Continuous therapy with pamidronate, a potent bisphosphonate, in postmenopausal osteoporosis. J Clin Endocrinol Metab (1994) 79:1595–9.
60. Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. the alendronate phase III osteoporosis treatment study group. N Engl J Med (1995) 333:1437–43. doi: 10.1056/NEJM199511303332201
61. Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. fracture intervention trial research group. Lancet (1996) 348:1535–41. doi: 10.1016/S0140-6736(96)07088-2
62. Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY, et al. A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int (1997) 7:488–95. doi: 10.1007/PL00004152
63. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA (1999) 282:637–45. doi: 10.1001/jama.282.7.637
64. Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA (1999) 282:1344–52. doi: 10.1001/jama.282.14.1344
65. Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN study group. J Clin Endocrinol Metab (2000) 85:1895–900.
66. Chesnut CH 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF study group. Am J Med (2000) 109:267–76. doi: 10.1016/S0002-9343(00)00490-3
67. Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. vertebral efficacy with risedronate therapy (VERT) study group. Osteoporos Int (2000) 11:83–91. doi: 10.1007/s001980050010
68. McClung MR, Geusens P, Miller PD, Hooper M, Roux C, Brandi ML, et al. Effect of risedronate on the risk of hip fracture in elderly women. hip intervention program study group. N Engl J Med (2001) 344:333–40. doi: 10.1056/NEJM200102013440503
69. Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med (2001) 344:1434–41. doi: 10.1056/NEJM200105103441904
70. Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med (2002) 162:1140–3. doi: 10.1001/archinte.162.10.1140
71. Brumsen C, Papapoulos SE, Lips P, Geelhoed-Duijvestijn PH, Hamdy NA, Landman JO, et al. Daily oral pamidronate in women and men with osteoporosis: a 3-year randomized placebo-controlled clinical trial with a 2-year open extension. J Bone Miner Res (2002) 17:1057–64. doi: 10.1359/jbmr.2002.17.6.1057
72. Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone (2003) 33:522–32. doi: 10.1016/S8756-3282(03)00241-2
73. Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, et al. Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab (2004) 22:462–8. doi: 10.1007/s00774-004-0508-0
74. Chesnut CH 3rd, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Oral ibandronate osteoporosis vertebral fracture trial in north America and Europe (BONE). effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res (2004) 19:1241–9. doi: 10.1359/JBMR.040325
75. Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med (2004) 350:459–68. doi: 10.1056/NEJMoa022436
76. McCloskey E, Selby P, Davies M, Robinson J, Francis RM, Adams J, et al. Clodronate reduces vertebral fracture risk in women with postmenopausal or secondary osteoporosis: results of a double-blind, placebo-controlled 3-year study. J Bone Miner Res (2004) 19:728–36. doi: 10.1359/jbmr.040116
77. Chesnut CH 3rd, Majumdar S, Shields A, Van Pelt J, Laschansky E, et al. Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Miner Res (2005) 20:1548–61. doi: 10.1359/JBMR.050411
78. Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int (2005) 16:510–6. doi: 10.1007/s00198-004-1713-3
79. Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, Phenekos C, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of peripheral osteoporosis (TROPOS) study. J Clin Endocrinol Metab (2005) 90:2816–22. doi: 10.1210/jc.2004-1774
80. Siris ES, Harris ST, Eastell R, Zanchetta JR, Goemaere S, Diez-Perez A, et al. Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to evista (CORE) study. J Bone Miner Res (2005) 20:1514–24. doi: 10.1359/JBMR.050509
81. Kanis JA, Barton IP, Johnell O. Risedronate decreases fracture risk in patients selected solely on the basis of prior vertebral fracture. Osteoporos Int (2005) 16:475–82. doi: 10.1007/s00198-004-1698-y
82. Quandt SA, Thompson DE, Schneider DL, Nevitt MC, Black DM, Fracture Intervention Trial Research Group. Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of-1.6 to –2.5 at the femoral neck: the fracture intervention trial. Mayo Clin Proc (2005) 80:343–9. doi: 10.4065/80.3.343
83. Nakamura T, Liu JL, Morii H, Huang QR, Zhu HM, Qu Y, et al. Effect of raloxifene on clinical fractures in Asian women with postmenopausal osteoporosis. J Bone Miner Metab (2006) 24:414–8. doi: 10.1007/s00774-006-0702-3
84. Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med (2007) 357:1799–809. doi: 10.1056/NEJMoa074941
85. Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1– 84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med (2007) 146:326–39. doi: 10.7326/0003-4819-146-5-200703060-00005
86. Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw KT, et al. Effects of raloxifene on fracture risk in postmenopausal women: the raloxifene use for the heart trial. J Bone Miner Res (2008) 23:112–20. doi: 10.1359/jbmr.070904
87. Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res (2008) 23:1923–34. doi: 10.1359/jbmr.080710
88. Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, et al. Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: A randomized placebo-controlled double-blind study. Osteoporos Int (2009) 20:1429–37. doi: 10.1007/s00198-008-0816-7
89. Cecilia D, Jodar E, Fernandez C, Resines C, Hawkins F. Effect of alendronate in elderly patients after low trauma hip fracture repair. Osteoporos Int (2009) 20:903–10. doi: 10.1007/s00198-008-0767-z
90. Meunier PJ, Roux C, Ortolani S, Diaz-Curiel M, Compston J, Marquis P, et al. Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos Int (2009) 20:1663–73. doi: 10.1007/s00198-008-0825-6
91. Sontag A, Wan X, Krege JH. Benefits and risks of raloxifene by vertebral fracture status. Curr Med Res Opin (2010) 26:71–6. doi: 10.1185/03007990903427082
92. Beaupre LA, Morrish DW, Hanley DA, Maksymowych WP, Bell NR, Juby AG, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int (2011) 22:983–91. doi: 10.1007/s00198-010-1411-2
93. Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab (2011) 96:1727–36. doi: 10.1210/jc.2010-2784
94. Krege JH, Wan X. Teriparatide and the risk of nonvertebral fractures in women with postmenopausal osteoporosis. Bone (2012) 50:161–4. doi: 10.1016/j.bone.2011.10.018
95. Frankel B, Krishna V, Vandergrift A, Bauer DC, Nicholas J. Natural history and risk factors for adjacent vertebral fractures in the fracture intervention trial. Spine (Phila Pa 1976) (2013) 38:2201–7. doi: 10.1097/BRS.0000000000000025
96. Nakano T, Shiraki M, Sugimoto T, Kishimoto H, Ito M, Fukunaga M, et al. Once-weekly teriparatide reduces the risk of vertebral fracture in patients with various fracture risks: subgroup analysis of the teriparatide once- weekly efficacy research (TOWER) trial. J Bone Miner Metab (2014) 32:441–6. doi: 10.1007/s00774-013-0505-2
97. Palacios S, Silverman SL, de Villiers TJ, Levine AB, Goemaere S, Brown JP, et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: effects on bone density and fracture. Menopause (2015) 22:806–13. doi: 10.1097/GME.0000000000000419
98. Palacios S, Kalouche-Khalil L, Rizzoli R, Zapalowski C, Resch H, Adachi JD, et al. Treatment with denosumab reduces secondary fracture risk in women with postmenopausal osteoporosis. Climacteric (2015) 18:805–12. doi: 10.3109/13697137.2015.1045484
99. Li Y, Zhao WB, Wang DL, He Q, Li Q, Pei FX, et al. Treatment of osteoporotic intertrochanteric fractures by zoledronic acid injection combined with proximal femoral nail anti-rotation. Chin J Traumatol (2016) 19:259–63. doi: 10.1016/j.cjtee.2016.07.001
100. Cosman F, Hattersley G, Hu MY, Williams GC, Fitzpatrick LA, Black DM, et al. Effects of abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res (2017) 32:17–23. doi: 10.1002/jbmr.2991
101. Kendler DL, Chines A, Brandi ML, Papapoulos S, Lewiecki EM, Reginster JY, et al. The risk of subsequent osteoporotic fractures is decreased in subjects experiencing fracture while on denosumab: results from the FREEDOM and FREEDOM extension studies. Osteoporos Int (2019) 30:71–8. doi: 10.1007/s00198-018-4687-2
102. Watts NB, Hattersley G, Fitzpatrick LA, Wang Y, Williams GC, Miller PD, et al. Abaloparatide effect on forearm bone mineral density and wrist fracture risk in postmenopausal women with osteoporosis. Osteoporos Int (2019) 30:1187–94. doi: 10.1007/s00198-019-04890-2
103. Sugimoto T, Shiraki M, Nakano T, Kishimoto H, Ito M, Fukunaga M, et al. A randomized, double-blind, placebo-controlled study of once weekly elcatonin in primary postmenopausal osteoporosis. Curr Med Res Opin (2019) 35:447–54. doi: 10.1080/03007995.2018.1498780
104. Schemitsch EH, Miclau T, Karachalios T, Nowak LL, Sancheti P, Poolman RW, et al. A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J Bone Joint Surg Am (2020) 102:693–702. doi: 10.2106/JBJS.19.00790
105. Adachi JD, Lyles KW, Colón-Emeric CS, Boonen S, Pieper CF, Mautalen C, et al. Zoledronic acid results in better health-related quality of life following hip fracture: the HORIZON-recurrent fracture trial. Osteoporos Int (2011) 22:2539–49. doi: 10.1007/s00198-010-1514-9
106. FRAX. fracture risk assessment tool . Available at: https://www.sheffield.ac.uk/FRAX/ (Accessed August 2022).
107. Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int (2007) 18:1033–46. doi: 10.1007/s00198-007-0343-y
108. Kanis JA, Harvey NC, Cooper C, Johansson H, Odén A, McCloskey EV, et al. A systematic review of intervention thresholds based on FRAX: A report prepared for the national osteoporosis guideline group and the international osteoporosis foundation. Arch Osteoporos (2016) 11:25. doi: 10.1007/s11657-016-0278-z
109. Johansen A. QFracture is better than FRAX tool in assessing risk of hip fracture. BMJ (2012) 345:e4988. doi: 10.1136/bmj.e4988
110. Adami S, Bianchi G, Brandi ML, Di Munno O, Frediani B, Gatti D, et al. Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: project rationale and description. Clin Exp Rheumatol (2010) 28:561–70.
111. DEFRA. l’algoritmo per la stima del rischio di frattura . Available at: https://defra-osteoporosi.it/ (Accessed August 2022).
112. Adami G, Gatti D, Rossini M, Giollo A, Bertoldo E, Viapiana O, et al. Factors associated with referral for osteoporosis care in men: a real-life study of a nationwide dataset. Arch Osteoporos (2021) 16:56. doi: 10.1007/s11657-021-00915-8
113. SIMG sicilia (2016). Available at: https://www.simg.it/sicilia/frahs-il-nuovo-score-per-la-valutazione-del-rischio-di-frattura-osteoporotica/.
114. Kester AD, Buntinx F. Meta-analysis of ROC curves. Med Decis Making (2000) 20:430–9. doi: 10.1177/0272989X0002000407
115. UK, National Clinical Guideline Centre. Osteoporosis: Fragility fracture risk: Osteoporosis: Assessing the risk of fragility fracture. (2012).
116. Ensrud KE, Lui LY, Taylor BC, Schousboe JT, Donaldson MG, Fink HA, et al. A comparison of prediction models for fractures in older women: is more better? Arch Intern Med (2009) 169:2087–94. doi: 10.1001/archinternmed.2009.404
117. Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ (2009) 339:b4229. doi: 10.1136/bmj.b4229
118. Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Manitoba Bone density program. independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res (2010) 25:2350–8. doi: 10.1002/jbmr.123
119. Pluskiewicz W, Adamczyk P, Franek E, Leszczynski P, Sewerynek E, Wichrowska H, et al. Ten-year probability of osteoporotic fracture in 2012 polish women assessed by FRAX and nomogram by Nguyen et al.-conformity between methods and their clinical utility. Bone (2010) 46:1661–7. doi: 10.1016/j.bone.2010.02.012
120. Sandhu SK, Nguyen ND, Center JR, Pocock NA, Eisman JA, Nguyen TV. Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and garvan nomogram. Osteoporos Int (2010) 21:863–71. doi: 10.1007/s00198-009-1026-7
121. Sornay-Rendu E, Munoz F, Delmas PD, Chapurlat RD. The FRAX tool in French women: How well does it describe the real incidence of fracture in the OFELY cohort? J Bone Miner Res (2010) 25:2101–7. doi: 10.1002/jbmr.106
122. Tanaka S, Yoshimura N, Kuroda T, Hosoi T, Saito M, Shiraki M. The fracture and immobilization score (FRISC) for risk assessment of osteoporotic fracture and immobilization in postmenopausal women–a joint analysis of the nagano, miyama, and taiji cohorts. Bone (2010) 47:1064–70. doi: 10.1016/j.bone.2010.08.019
123. Trémollieres FA, Pouillès JM, Drewniak N, Laparra J, Ribot CA, Dargent-Molina P. Fracture risk prediction using BMD and clinical risk factors in early postmenopausal women: sensitivity of the WHO FRAX tool. J Bone Miner Res (2010) 25:1002–9. doi: 10.1002/jbmr.12
124. Bolland MJ, Siu AT, Mason BH, Horne AM, Ames RW, Grey AB, et al. Evaluation of the FRAX and garvan fracture risk calculators in older women. J Bone Miner Res (2011) 26:420–7. doi: 10.1002/jbmr.215
125. Cummins NM, Poku EK, Towler MR, O'Driscoll OM, Ralston SH. Clinical risk factors for osteoporosis in Ireland and the UK: a comparison of FRAX and QFractureScores. Calcif Tissue Int (2011) 89:172–7. doi: 10.1007/s00223-011-9504-2
126. Fraser LA, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, et al. Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int (2011) 22:829–37. doi: 10.1007/s00198-010-1465-1
127. Henry MJ, Pasco JA, Merriman EN, Zhang Y, Sanders KM, Kotowicz MA, et al. Fracture risk score and absolute risk of fracture. Radiology (2011) 259:495–501. doi: 10.1148/radiol.10101406
128. Sambrook PN, Flahive J, Hooven FH, Boonen S, Chapurlat R, Lindsay R, et al. Predicting fractures in an international cohort using risk factor algorithms without BMD. J Bone Miner Res (2011) 26:2770–7. doi: 10.1002/jbmr.503
129. Tamaki J, Iki M, Kadowaki E, Sato Y, Kajita E, Kagamimori S, et al. Fracture risk prediction using FRAX®: a 10-year follow-up survey of the Japanese population-based osteoporosis (JPOS) cohort study. Osteoporos Int (2011) 22:3037–45. doi: 10.1007/s00198-011-1537-x
130. Cheung EY, Bow CH, Cheung CL, Soong C, Yeung S, Loong C, et al. Discriminative value of FRAX for fracture prediction in a cohort of Chinese postmenopausal women. Osteoporos Int (2012) 23:871–8. doi: 10.1007/s00198-011-1647-5
131. González-Macías J, Marin F, Vila J, Díez-Pérez A. Probability of fractures predicted by FRAX® and observed incidence in the Spanish ECOSAP study cohort. Bone (2012) 50:373–7. doi: 10.1016/j.bone.2011.11.006
132. Briot K, Paternotte S, Kolta S, Eastell R, Felsenberg D, Reid DM, et al. FRAX®: prediction of major osteoporotic fractures in women from the general population: the OPUS study. PloS One (2013) 8:e83436.
133. Czerwiński E, Borowy P, Kumorek A, Amarowicz J, Górkiewicz M, Milert A, et al. Fracture risk prediction in outpatients from Krakow region using FRAX tool versus fracture risk in 11-year follow-up. Ortop Traumatol Rehabil (2013) 15:617–28. doi: 10.5604/15093492.1091517
134. Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Manitoba Bone density program. selection of women aged 50-64 yr for bone density measurement. J Clin Densitom (2013) 16:570–8. doi: 10.1016/j.jocd.2013.01.004
135. Rubin KH, Abrahamsen B, Friis-Holmberg T, Hjelmborg JV, Bech M, Hermann AP, et al. Comparison of different screening tools (FRAX®, OST, ORAI, OSIRIS, SCORE and age alone) to identify women with increased risk of fracture. a population-based prospective study. Bone (2013) 56:16–22. doi: 10.1016/j.bone.2013.05.002
136. Tebé Cordomí C, Del Río LM, Di Gregorio S, Casas L, Estrada MD, Kotzeva A, et al. Validation of the FRAX predictive model for major osteoporotic fracture in a historical cohort of Spanish women. J Clin Densitom (2013) 16:231–7. doi: 10.1016/j.jocd.2012.05.007
137. Cheung E, Cheung CL, Kung AW, Tan KC. Possible FRAX-based intervention thresholds for a cohort of Chinese postmenopausal women. Osteoporos Int (2014) 25:1017–23. doi: 10.1007/s00198-013-2553-9
138. Crandall CJ, Larson J, Gourlay ML, Donaldson MG, LaCroix A, Cauley JA, et al. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US preventive services task force strategy and two traditional strategies in the women's health initiative. J Bone Miner Res (2014) 29:1661–6. doi: 10.1002/jbmr.2174
139. Friis-Holmberg T, Rubin KH, Brixen K, Tolstrup JS, Bech M. Fracture risk prediction using phalangeal bone mineral density or FRAX(®)?-a Danish cohort study on men and women. J Clin Densitom (2014) 17:7–15. doi: 10.1016/j.jocd.2013.03.014
140. El Maghraoui A, Sadni S, Jbili N, Rezqi A, Mounach A, Ghozlani I. The discriminative ability of FRAX, the WHO algorithm, to identify women with prevalent asymptomatic vertebral fractures: a cross-sectional study. BMC Musculoskelet Disord (2014) 15:365. doi: 10.1186/1471-2474-15-365
141. Pluskiewicz W, Adamczyk P, Franek E, Sewerynek E, Leszczynski P, Wichrowska H, et al. FRAX calculator and garvan nomogram in male osteoporotic population. Aging Male (2014) 17:174–82. doi: 10.3109/13685538.2013.875991
142. van Geel TA, Eisman JA, Geusens PP, van den Bergh JP, Center JR, Dinant GJ. The utility of absolute risk prediction using FRAX® and garvan fracture risk calculator in daily practice. Maturitas (2014) 77:174–9. doi: 10.1016/j.maturitas.2013.10.021
143. Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc (2014) 15:918–23. doi: 10.1016/j.jamda.2014.07.011
144. Azagra R, Zwart M, Encabo G, Aguyé A, Martin-Sánchez JC, Puchol-Ruiz N, et al. Rationale of the Spanish FRAX model in decision-making for predicting osteoporotic fractures: an update of FRIDEX cohort of Spanish women. BMC Musculoskelet Disord (2016) 17:262. doi: 10.1186/s12891-016-1096-6
145. Esmaeilzadeh S, Cesme F, Oral A, Yaliman A, Sindel D. The utility of dual-energy X-ray absorptiometry, calcaneal quantitative ultrasound, and fracture risk indices (FRAX® and osteoporosis risk assessment instrument) for the identification of women with distal forearm or hip fractures: A pilot study. Endocr Res (2016) 41:248–60. doi: 10.3109/07435800.2015.1120744
146. Indhavivadhana S, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Tanmahasamut P, Leerasiri P. Validation of osteoporosis risk assessment tools in middle-aged Thai women. Climacteric (2016) 19:588–93. doi: 10.1080/13697137.2016.1231176
147. Lin J, Yang Y, Fei Q, Zhang X, Ma Z, Wang Q, et al. Validation of three tools for identifying painful new osteoporotic vertebral fractures in older Chinese men: bone mineral density, osteoporosis self-assessment tool for asians, and fracture risk assessment tool. Clin Interv Aging (2016) 11:461–9.
148. Villa P, Lassandro AP, Moruzzi MC, Amar ID, Vacca L, Di Nardo F, et al. A non-invasive prevention program model for the assessment of osteoporosis in the early postmenopausal period: a pilot study on FRAX(®) and QUS tools advantages. J Endocrinol Invest (2016) 39:191–8. doi: 10.1007/s40618-015-0341-4
149. Dagan N, Cohen-Stavi C, Leventer-Roberts M, Balicer RD. External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort study. BMJ (2017) 356:i6755. doi: 10.1136/bmj.i6755
150. Hoff M, Meyer HE, Skurtveit S, Langhammer A, Søgaard AJ, Syversen U, et al. Validation of FRAX and the impact of self-reported falls among elderly in a general population: the HUNT study, Norway. Osteoporos Int (2017) 28:2935–44. doi: 10.1007/s00198-017-4134-9
151. Kharroubi A, Saba E, Ghannam I, Darwish H. Evaluation of the validity of osteoporosis and fracture risk assessment tools (IOF one minute test, SCORE, and FRAX) in postmenopausal Palestinian women. Arch Osteoporos (2017) 12:6. doi: 10.1007/s11657-016-0298-8
152. Kral R, Osima M, Borgen TT, Vestgaard R, Richardsen E, Bjørnerem Å. Increased cortical porosity and reduced cortical thickness of the proximal femur are associated with nonvertebral fracture independent of fracture risk assessment tool and garvan estimates in postmenopausal women. PloS One (2017) 12:e0185363. doi: 10.1371/journal.pone.0185363
153. Marques A, Lucas R, Simões E, Verstappen SMM, Jacobs JWG, da Silva JAP. Do we need bone mineral density to estimate osteoporotic fracture risk? a 10-year prospective multicentre validation study. RMD Open (2017) 3:e000509. doi: 10.1136/rmdopen-2017-000509
154. Su Y, Leung J, Hans D, Lamy O, Kwok T. The added value of trabecular bone score to FRAX® to predict major osteoporotic fractures for clinical use in Chinese older people: the mr. OS and ms. OS cohort study in Hong Kong. Osteoporos Int (2017) 28:111–7. doi: 10.1007/s00198-016-3741-1
155. Bansal B, Mithal A, Chopra SR, Bhanot S, Kuchay MS, Farooqui KJ. Judicious use of DXA-BMD in assessing fracture risk by using clinical risk factors in the Indian population. Arch Osteoporos (2018) 13:115. doi: 10.1007/s11657-018-0536-3
156. Chandran M, McCloskey EV, Thu WPP, Logan S, Hao Y, Tay D, et al. FRAX® based intervention thresholds for management of osteoporosis in Singaporean women. Arch Osteoporos (2018) 13:130. doi: 10.1007/s11657-018-0542-5
157. Cherian KE, Kapoor N, Shetty S, Naik D, Thomas N, Paul TV. Evaluation of different screening tools for predicting femoral neck osteoporosis in rural south Indian postmenopausal women. J Clin Densitom (2018) 21:119–24. doi: 10.1016/j.jocd.2017.08.002
158. Goldshtein I, Gerber Y, Ish-Shalom S, Leshno M. Fracture risk assessment with FRAX using real-world data in a population-based cohort from Israel. Am J Epidemiol (2018) 187:94–102. doi: 10.1093/aje/kwx128
159. Zhang X, Lin J, Yang Y, Wu H, Li Y, Yang X, et al. Comparison of three tools for predicting primary osteoporosis in an elderly male population in Beijing: a cross-sectional study. Clin Interv Aging (2018) 13:201–9. doi: 10.2147/CIA.S145741
160. Crandall CJ, Schousboe JT, Morin SN, Lix LM, Leslie W. Performance of FRAX and FRAX-based treatment thresholds in women aged 40 years and older: The Manitoba BMD registry. J Bone Miner Res (2019) 34:1419–27. doi: 10.1002/jbmr.3717
161. Singh V, Pal AK, Biswas D, Ghosh A, Singh BP. Identification of patients at high risk of fragility fractures in an Indian clinical setting using FRAX. Arch Osteoporos (2020) 15:131. doi: 10.1007/s11657-020-00807-3
162. Liu S, Chen R, Ding N, Wang Q, Huang M, Liu H, et al. Setting the new FRAX reference threshold without bone mineral density in Chinese postmenopausal women. J Endocrinol Invest (2021) 44:347–52. doi: 10.1007/s40618-020-01315-4
163. Bonaccorsi G, Fila E, Cervellati C, Romani A, Giganti M, Rossini M, et al. Assessment of fracture risk in a population of postmenopausal Italian women: A comparison of two different tools. Calcif Tissue Int (2015) 97:50–7. doi: 10.1007/s00223-015-0009-2
164. Bonaccorsi G, Messina C, Cervellati C, Maietti E, Medini M, Rossini M, et al. Fracture risk assessment in postmenopausal women with diabetes: comparison between DeFRA and FRAX tools. Gynecol Endocrinol (2018) 34:404–8. doi: 10.1080/09513590.2017.1407308
165. Francesco L, Elisa B, Raffaella M, Alessandro P, Iacopo C, Giampiero M, et al. Assessing risk of osteoporotic fractures in primary care: Development and validation of the FRA-HS algorithm. Calcif Tissue Int (2017) 100:537–49. doi: 10.1007/s00223-016-0230-7
166. Grover ML, Edwards FD, Chang YH, Cook CB, Behrens MC, Dueck AC. Fracture risk perception study: patient self-perceptions of bone health often disagree with calculated fracture risk. Womens Health Issues (2014) 24:e69–75. doi: 10.1016/j.whi.2013.11.007
167. Moberg LME, Nilsson PM, Holmberg AH, Samsioe G, Borgfeldt C. Primary screening for increased fracture risk by the FRAX® questionnaire-uptake rates in relation to invitation method. Arch Osteoporos (2019) 14:51. doi: 10.1007/s11657-019-0603-4
168. Edmonds SW, Cram P, Lu X, Roblin DW, Wright NC, Saag KG, et al. Improving bone mineral density reporting to patients with an illustration of personal fracture risk. BMC Med Inform Decis Mak (2014) 14:101. doi: 10.1186/s12911-014-0101-y
169. Turner DA, Khioe RFS, Shepstone L, Lenaghan E, Cooper C, Gittoes N, et al. The cost-effectiveness of screening in the community to reduce osteoporotic fractures in older women in the UK: Economic evaluation of the SCOOP study. J Bone Miner Res (2018) 33(5):845–51. doi: 10.1002/jbmr.3381
170. Makras P, Athanasakis K, Boubouchairopoulou N, Rizou S, Anastasilakis AD, Kyriopoulos J, et al. Cost-effective osteoporosis treatment thresholds in Greece. Osteoporos Int (2015) 26:1949–57. doi: 10.1007/s00198-015-3055-8
171. Martin-Sanchez M, Comas M, Posso M, Louro J, Domingo L, Tebé C, et al. Cost-effectiveness of the screening for the primary prevention of fragility hip fracture in Spain using FRAX®. Calcif Tissue Int (2019) 105:263–70. doi: 10.1007/s00223-019-00570-9
172. Lippuner K, Johansson H, Borgström F, Kanis JA, Rizzoli R. Cost-effective intervention thresholds against osteoporotic fractures based on FRAX® in Switzerland. Osteoporos Int (2012) 23:2579–89. doi: 10.1007/s00198-011-1869-6
173. Alzahouri K, Bahrami S, Durand-Zaleski I, Guillemin F, Roux C. Cost-effectiveness of osteoporosis treatments in postmenopausal women using FRAX™ thresholds for decision. Joint Bone Spine (2013) 80:64–9. doi: 10.1016/j.jbspin.2012.01.001
174. Ström O, Jönsson B, Kanis JA. Intervention thresholds for denosumab in the UK using a FRAX®-based cost-effectiveness analysis. Osteoporos Int (2013) 24:1491–502. doi: 10.1007/s00198-012-2115-6
175. Marques A, Lourenço Ó, Ortsäter G, Borgström F, Kanis JA, da Silva JA. Cost-effectiveness of intervention thresholds for the treatment of osteoporosis based on FRAX(®) in Portugal. Calcif Tissue Int (2016) 99:131–41. doi: 10.1007/s00223-016-0132-8
176. Kim K, Svedbom A, Luo X, Sutradhar S, Kanis JA. Comparative cost-effectiveness of bazedoxifene and raloxifene in the treatment of postmenopausal osteoporosis in Europe, using the FRAX algorithm. Osteoporos Int (2014) 25:325–37. doi: 10.1007/s00198-013-2521-4
177. MacLean FR, Thomson SA, Gallacher SJ. Using WHO-FRAX to describe fracture risk: experience in primary care. Scott Med J (2012) 57:8–13. doi: 10.1258/smj.2011.011185
178. Haentjens P, Johnell O, Kanis JA, Bouillon R, Cooper C, Lamraski G, et al. Evidence from data searches and life-table analyses for gender-related differences in absolute risk of hip fracture after colles' or spine fracture: Colles' fracture as an early and sensitive marker of skeletal fragility in white men. J Bone Miner Res (2004) 19:1933–44. doi: 10.1359/jbmr.040917
179. Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone (2004) 35:375–82. doi: 10.1016/j.bone.2004.03.024
180. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res (2000) 15:721–39. doi: 10.1359/jbmr.2000.15.4.721
181. Sheer RL, Barron RL, Sudharshan L, et al. Validated prediction of imminent risk of fracture for older adults. Am J Manag Care (2020) 26(3):e91–7.
182. Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E, et al. Imminent risk of fracture after fracture. Osteoporos Int (2017) 28:775–80. doi: 10.1007/s00198-016-3868-0
183. Schnell AD, Curtis JR, Saag KG. Importance of recent fracture as predictor of imminent fracture risk. Curr Osteoporos Rep (2018) 16:738–45. doi: 10.1007/s11914-018-0487-z
184. Söreskog E, Ström O, Spångéus A, Åkesson KE, Borgström F, Banefelt J, et al. Risk of major osteoporotic fracture after first, second and third fracture in Swedish women aged 50 years and older. Bone (2020) 134:115286. doi: 10.1016/j.bone.2020.115286
185. Toth E, Banefelt J, Åkesson K, Spångeus A, Ortsäter G, Libanati C. History of previous fracture and imminent fracture risk in Swedish women aged 55 to 90 Years presenting with a fragility fracture. J Bone Miner Res (2020) 35:861–8. doi: 10.1002/jbmr.3953
186. Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos (2016) 11:26. doi: 10.1007/s11657-016-0280-5
187. Hannan MT, Weycker D, McLean RR, Sahni S, Bornheimer R, Barron R, et al. Predictors of imminent risk of nonvertebral fracture in older, high-risk women: The framingham osteoporosis study. JBMR Plus (2019) 3:e10129. doi: 10.1002/jbm4.10129
188. Kanis JA, Cooper C, Rizzoli R, Abrahamsen B, Al-Daghri NM, Brandi ML, et al. Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int (2017) 28:2023–34. doi: 10.1007/s00198-017-4009-0
189. Cooper C, Coupland C, Mitchell M. Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann Rheum Dis (1995) 54:49–52. doi: 10.1136/ard.54.1.49
190. Meyer HE, Henriksen C, Falch JA, Pedersen JI, Tverdal A. Risk factors for hip fracture in a high incidence area: a case-control study from Oslo, Norway. Osteoporos Int (1995) 5:239–46. doi: 10.1007/BF01774013
191. Torgerson DJ, Campbell MK, Thomas RE, Reid DM. Prediction of perimenopausal fractures by bone mineral density and other risk factors. J Bone Miner Res (1996) 11:293–7. doi: 10.1002/jbmr.5650110219
192. Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord (1998) 36:790–6. doi: 10.1038/sj.sc.3100648
193. Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Blue mountains eye study. diabetes and risk of fracture: The blue mountains eye study. Diabetes Care (2001) 24:1198–203. doi: 10.2337/diacare.24.7.1198
194. Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the national osteoporosis risk assessment. JAMA (2001) 286:2815–22. doi: 10.1001/jama.286.22.2815
195. Simonelli C, Chen YT, Morancey J, Lewis AF, Abbott TA. Evaluation and management of osteoporosis following hospitalization for low-impact fracture. J Gen Intern Med (2003) 18:17–22. doi: 10.1046/j.1525-1497.2003.20387.x
196. Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest (2005) 128:2099–107. doi: 10.1378/chest.128.4.2099
197. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia (2005) 48:1292–9. doi: 10.1007/s00125-005-1786-3
198. Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int (2006) 70:1358–66. doi: 10.1038/sj.ki.5001754
199. Krege JH, Siminoski K, Adachi JD, Misurski DA, Chen P. A simple method for determining the probability a new vertebral fracture is present in postmenopausal women with osteoporosis. Osteoporos Int (2006) 17:379–86. doi: 10.1007/s00198-005-2005-2
200. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the united states. J Am Soc Nephrol (2006) 17:3223–32. doi: 10.1681/ASN.2005111194
201. Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest (2007) 132:1599–607. doi: 10.1378/chest.07-1092
202. Loh KY, Shong KH, Lan SN, Lo WY, Woon SY. Risk factors for fragility fracture in seremban district, Malaysia: a comparison of patients with fragility fracture in the orthopedic ward versus those in the outpatient department. Asia Pac J Public Health (2008) 20:251–7. doi: 10.1177/1010539508317130
203. Rhew EY, Lee C, Eksarko P, Dyer AR, Tily H, Spies S, et al. Homocysteine, bone mineral density, and fracture risk over 2 years of follow-up in women with and without systemic lupus erythematosus. J Rheumatol (2008) 35:230–6.
204. Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int (2009) 20:385–92. doi: 10.1007/s00198-008-0671-6
205. Weiss RJ, Wick MC, Ackermann PW, Montgomery SM. Increased fracture risk in patients with rheumatic disorders and other inflammatory diseases – a case-control study with 53,108 patients with fracture. J Rheumatol (2010) 37:2247–50. doi: 10.3899/jrheum.100363
206. Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, Greenspan S, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med (2011) 124:1043–50. doi: 10.1016/j.amjmed.2011.06.013
207. Ruan WD, Wang P, Ma XL, Ge RP, Zhou XH. Analysis on the risk factors of second fracture in osteoporosis-related fractures. Chin J Traumatol (2011) 14:74–8.
208. Dennison EM, Compston JE, Flahive J, Siris ES, Gehlbach SH, Adachi JD, et al. Effect of co-morbidities on fracture risk: findings from the global longitudinal study of osteoporosis in women (GLOW). Bone (2012) 50:1288–93. doi: 10.1016/j.bone.2012.02.639
209. Gehlbach S, Saag KG, Adachi JD, Hooven FH, Flahive J, Boonen S, et al. Previous fractures at multiple sites increase the risk for subsequent fractures: the global longitudinal study of osteoporosis in women. J Bone Miner Res (2012) 27:645–53. doi: 10.1002/jbmr.1476
210. Sosa-Henriquez M, Gomez de Tejada-Romero MJ, Saavedra-Santana P, Blazquez-Cabrera JA, Garcia-Vadillo JA, Valdes-Llorca C, et al. Prevalence and risk factors for non-vertebral fractures in patients receiving oral glucocorticoids. Int J Endocrinol Metab (2012) 10:480–5. doi: 10.5812/ijem.3442
211. Vochteloo AJ, Borger van der Burg BL, Röling MA, van Leeuwen DH, van den Berg P, Niggebrugge AH, et al. Contralateral hip fractures and other osteoporosis-related fractures in hip fracture patients: incidence and risk factors. an observational cohort study of 1,229 patients. Arch Orthop Trauma Surg (2012) 132:1191–7. doi: 10.1007/s00402-012-1520-9
212. Waterloo S, Ahmed LA, Center JR, Eisman JA, Morseth B, Nguyen ND, et al. Prevalence of vertebral fractures in women and men in the population-based tromsø study. BMC Musculoskelet Disord (2012) 13:3. doi: 10.1186/1471-2474-13-3
213. Atteritano M, Sorbara S, Bagnato G, Miceli G, Sangari D, Morgante S, et al. Bone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: a case control study. PloS One (2013) 8:e66991. doi: 10.1371/journal.pone.0066991
214. Pouwels S, Bazelier MT, de Boer A, Weber WE, Neef C, Cooper C, et al. Risk of fracture in patients with parkinson's disease. Osteoporos Int (2013) 24:2283–90. doi: 10.1007/s00198-013-2300-2
215. Anpalahan M, Morrison SG, Gibson SJ. Hip fracture risk factors and the discriminability of hip fracture risk vary by age: a case-control study. Geriatr Gerontol Int (2014) 14:413–9. doi: 10.1111/ggi.12117
216. Gibson-Smith D, Klop C, Elders PJ, Welsing PM, van Schoor N, Leufkens HG, et al. The risk of major and any (non-hip) fragility fracture after hip fracture in the united kingdom: 2000-2010. Osteoporos Int (2014) 25:2555–63. doi: 10.1007/s00198-014-2799-x
217. Prieto-Alhambra D, Güerri-Fernández R, De Vries F, Lalmohamed A, Bazelier M, Starup-Linde J, et al. HIV Infection and its association with an excess risk of clinical fractures: a nationwide case-control study. J Acquir Immune Defic Syndr (2014) 66:90–5. doi: 10.1097/QAI.0000000000000112
218. Prieto-Alhambra D, Muñoz-Ortego J, De Vries F, Vosse D, Arden NK, Bowness P, et al. Ankylosing spondylitis confers substantially increased risk of clinical spine fractures: a nationwide case-control study. Osteoporos Int (2015) 26:85–91. doi: 10.1007/s00198-014-2939-3
219. Weycker D, Edelsberg J, Barron R, Li X, Crittenden DB, Chandler D. Predictors of near-term fracture in osteoporotic women aged ≥65 years, based on data from the study of osteoporotic fractures. Osteoporos Int (2017) 28:2565–71. doi: 10.1007/s00198-017-4103-3
220. Lee CK, Choi SK, Shin DA, Yi S, Kim KN, Kim I, et al. Parkinson's disease and the risk of osteoporotic vertebral compression fracture: a nationwide population-based study. Osteoporos Int (2018) 29:1117–24. doi: 10.1007/s00198-018-4409-9
221. Reber KC, König HH, Becker C, Rapp K, Büchele G, Mächler S, et al. Development of a risk assessment tool for osteoporotic fracture prevention: A claims data approach. Bone (2018) 110:170–6. doi: 10.1016/j.bone.2018.02.002
222. Adachi JD, Berger C, Barron R, Weycker D, Anastassiades TP, Davison KS, et al. Predictors of imminent non-vertebral fracture in elderly women with osteoporosis, low bone mass, or a history of fracture, based on data from the population-based Canadian multicentre osteoporosis study (CaMos). Arch Osteoporos (2019) 14:53. doi: 10.1007/s11657-019-0598-x
223. Adas-Okuma MG, Maeda SS, Gazzotti MR, Roco CM, Pradella CO, Nascimento OA, et al. COPD as an independent risk factor for osteoporosis and fractures. Osteoporos Int (2020) 31:687–97. doi: 10.1007/s00198-019-05235-9
224. Malmgren L, McGuigan FE, Christensson A, Akesson KE. Kidney function and its association to imminent, short- and long-term fracture risk-a longitudinal study in older women. Osteoporos Int (2020) 31:97–107. doi: 10.1007/s00198-019-05152-x
225. Shim YB, Park JA, Nam JH, Hong SH, Kim JW, Jeong J, et al. Incidence and risk factors of subsequent osteoporotic fracture: a nationwide cohort study in south Korea. Arch Osteoporos (2020) 15:180. doi: 10.1007/s11657-020-00852-y
226. Tedeschi SK, Kim SC, Guan H, Grossman JM, Costenbader KH. Comparative fracture risks among united states Medicaid enrollees with and those without systemic lupus erythematosus. Arthritis Rheumatol (2019) 71:1141–6. doi: 10.1002/art.40818
227. Yusuf AA, Hu Y, Chandler D, Crittenden DB, Barron RL. Predictors of imminent risk of fracture in Medicare-enrolled men and women. Arch Osteoporos (2020) 15:120. doi: 10.1007/s11657-020-00784-7
228. Inose H, Kato T, Ichimura S, Nakamura H, Hoshino M, Togawa D, et al. Risk factors for subsequent vertebral fracture after acute osteoporotic vertebral fractures. Eur Spine J (2021) 30:2698–707. doi: 10.1007/s00586-021-06741-3
229. Yu WY, Hwang HF, Chen CY, Lin MR. Situational risk factors for fall-related vertebral fractures in older men and women. Osteoporos Int (2021) 32:1061–70. doi: 10.1007/s00198-020-05799-x
230. Zhou J, Liu B, Qin MZ, Liu JP. A prospective cohort study of the risk factors for new falls and fragility fractures in self-caring elderly patients aged 80 years and over. BMC Geriatr (2021) 21:116. doi: 10.1186/s12877-021-02043-x
231. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: Anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res (2017) 32:198–202. doi: 10.1002/jbmr.3051
232. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: An endocrine society guideline update. J Clin Endocrinol Metab (2020) 105:587–94. doi: 10.1210/clinem/dgaa048
233. Anastasilakis AD, Polyzos SA, Yavropoulou MP, Makras P. Combination and sequential treatment in women with postmenopausal osteoporosis. Expert Opin Pharmacother (2020) 21:477–90. doi: 10.1080/14656566.2020.1717468
234. Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. PaTH study investigators. one year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med (2005) 353:555–65. doi: 10.1056/NEJMoa050336
235. Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res (2005) 20:1507–13. doi: 10.1359/JBMR.050501
236. Gonnelli S, Martini G, Caffarelli C, Salvadori S, Cadirni A, Montagnani A, et al. Teriparatide's effects on quantitative ultrasound parameters and bone density in women with established osteoporosis. Osteoporos Int (2006) 17:1524–31. doi: 10.1007/s00198-006-0157-3
237. Middleton ET, Steel SA, Doherty SM. The effect of prior bisphosphonate exposure on the treatment response to teriparatide in clinical practice. Calcif Tissue Int (2007) 81:335–40. doi: 10.1007/s00223-007-9066-5
238. Boonen S, Marin F, Obermayer-Pietsch B, Simões ME, Barker C, Glass EV, et al. EUROFORS investigators. effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab (2008) 93:852–60. doi: 10.1210/jc.2007-0711
239. Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, et al. Open-label study to determine how prior therapy with alendronate or risedronate in postmenopausal women with osteoporosis influences the clinical effectiveness of teriparatide investigators. early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab (2008) 93:3785–93. doi: 10.1210/jc.2008-0353
240. Obermayer-Pietsch BM, Marin F, Hadji P, Farrerons J, Boonen S, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res (2008) 23:1591–600. doi: 10.1359/jbmr.080506
241. Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-switch study): extension of a randomised controlled trial. Lancet (2015) 386:1147–55. doi: 10.1016/S0140-6736(15)61120-5
242. Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med (2016) 375:1532–43. doi: 10.1056/NEJMoa1607948
243. Fahrleitner-Pammer A, Burr D, Dobnig H, Stepan JJ, Petto H, Li J, et al. Improvement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatment. Bone (2016) 89:16–24. doi: 10.1016/j.bone.2016.05.004
244. Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet (2017) 390:1585–94. doi: 10.1016/S0140-6736(17)31613-6
245. Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med (2017) 377:1417–27. doi: 10.1056/NEJMoa1708322
246. Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: Results of the FRAME extension study. J Bone Miner Res (2019) 34:419–28. doi: 10.1002/jbmr.3622
247. Niimi R, Kono T, Nishihara A, et al. Efficacy of switching from teriparatide to bisphosphonate or denosumab: A prospective, randomized, open-label trial. JBMR Plus (2018) 2:289–94. doi: 10.1002/jbm4.10054
248. Kendler DL, Bone HG, Massari F, Gielen E, Palacios S, Maddox J, et al. Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos Int (2019) 30:2437–48. doi: 10.1007/s00198-019-05146-9
249. Miyauchi A, Dinavahi RV, Crittenden DB, Yang W, Maddox JC, Hamaya E, et al. Increased bone mineral density for 1 year of romosozumab, vs placebo, followed by 2 years of denosumab in the Japanese subgroup of the pivotal FRAME trial and extension. Arch Osteoporos (2019) 14:59. doi: 10.1007/s11657-019-0608-z
250. Cosman F, Lewiecki EM, Ebeling PR, Hesse E, Napoli N, Matsumoto T, et al. T-Score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res (2020) 35:1333–42. doi: 10.1002/jbmr.3996
251. Jakob F, Oertel H, Langdahl B, Ljunggren O, Barrett A, Karras D, et al. Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European forsteo observational study. Eur J Endocrinol (2012) 166:87–97. doi: 10.1530/EJE-11-0740
252. Sheehy O, Kindundu C, Barbeau M, LeLorier J. Adherence to weekly oral bisphosphonate therapy: cost of wasted drugs and fractures. Osteoporos Int (2009) 20:1583–94. doi: 10.1007/s00198-008-0829-2
253. McClung MR. Bisphosphonates in osteoporosis: recent clinical experience. Expert Opin Pharmacother (2000) 1:225–38. doi: 10.1517/14656566.1.2.225
254. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med (2004) 350:1189–99. doi: 10.1056/NEJMoa030897
255. Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int (2005) 16:78–85. doi: 10.1007/s00198-004-1646-x
256. Blouin J, Dragomir A, Ste-Marie LG, Fernandes JC, Perreault S. Discontinuation of antiresorptive therapies: a comparison between 1998–2001 and 2002–2004 among osteoporotic women. J Clin Endocrinol Metab (2007) 92:887–94. doi: 10.1210/jc.2006-1856
257. Cramer JA, Gold DT, Silverman SL, Lewiecki EM. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int (2007) 18:1023–31. doi: 10.1007/s00198-006-0322-8
258. Miller PD, Watts NB, Licata AA, Harris ST, Genant HK, Wasnich RD, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: efficacy and safety after seven years of treatment. Am J Med (1997) 103:468–76. doi: 10.1016/S0002-9343(97)00278-7
259. Diab DL, Watts NB. Bisphosphonate drug holiday: who, when and how long. Ther Adv musculoskeletal Dis (2013) 5:3:107–11. doi: 10.1177/1759720X13477714
260. Adler RA, El-Hajj Fuleihan G, Camacho PM, Clarke BL, Clines GA, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American society for bone and mineral research. J Bone Miner Res (2016) 31:16–35. doi: 10.1002/jbmr.2708
261. Nayak S, Greenspan SL. A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk. Osteoporos Int (2019) 30:705–20. doi: 10.1007/s00198-018-4791-3
262. Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. FLEX research group. effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA (2006) 296:2927–38. doi: 10.1001/jama.296.24.2927
263. Lin TC, Yang CY, Yang YH, Lin SJ. Alendronate adherence and its impact on hip-fracture risk in patients with established osteoporosis in Taiwan. Clin Pharmacol Ther (2011) 90:109–16. doi: 10.1038/clpt.2011.62
264. Soong YK, Tsai KS, Huang HY, Yang RS, Chen JF, Wu PC, et al. Risk of refracture associated with compliance and persistence with bisphosphonate therapy in Taiwan. Osteoporos Int (2013) 24:511–21. doi: 10.1007/s00198-012-1984-z
265. Cosman F, Cauley JA, Eastell R, Boonen S, Palermo L, Reid IR, et al. Reassessment of fracture risk in women after 3 years of treatment with zoledronic acid: when is it reasonable to discontinue treatment? J Clin Endocrinol Metab (2014) 99:4546–54. doi: 10.1210/jc.2014-1971
266. Chan DC, Chang CH, Lim LC, Brnabic AJM, Tsauo JY, Burge R, et al. Association between teriparatide treatment persistence and adherence, and fracture incidence in Taiwan: analysis using the national health insurance research database. Osteoporos Int (2016) 27:2855–65. doi: 10.1007/s00198-016-3611-x
267. Chen YC, Lin WC. Poor 1st-year adherence to anti-osteoporotic therapy increases the risk of mortality in patients with magnetic resonance imaging-proven acute osteoporotic vertebral fractures. Patient Prefer Adherence (2017) 11:839–43. doi: 10.2147/PPA.S131564
268. Keshishian A, Boytsov N, Burge R, Krohn K, Lombard L, Zhang X, et al. Examining the effect of medication adherence on risk of subsequent fracture among women with a fragility fracture in the U.S. Medicare population. J Manag Care Spec Pharm (2017) 23:1178–90. doi: 10.18553/jmcp.2017.17054
269. Adams AL, Adams JL, Raebel MA, Tang BT, Kuntz JL, Vijayadeva V, et al. Bisphosphonate drug holiday and fracture risk: A population-based cohort study. J Bone Miner Res (2018) 33:1252–9. doi: 10.1002/jbmr.3420
270. McAlister FA, Ye C, Beaupre LA, Rowe BH, Johnson JA, Bellerose D, Hassan I, Majumdar SR. Adherence to osteoporosis therapy after an upper extremity fracture: a pre-specified substudy of the c-STOP randomized controlled trial. Osteoporos Int (2019) 30:127–34. doi: 10.1007/s00198-018-4702-7
271. Yu SF, Cheng JS, Chen YC, Chen JF, Hsu CY, Lai HM, et al. Adherence to anti-osteoporosis medication associated with lower mortality following hip fracture in older adults: a nationwide propensity score-matched cohort study. BMC Geriatr (2019) 19:290. doi: 10.1186/s12877-019-1278-9
272. Hsu CL, Chen HM, Chen HJ, Chou MY, Wang YC, Hsu YH, et al. A national study on long-term osteoporosis therapy and risk of recurrent fractures in patients with hip fracture. Arch Gerontol Geriatr (2020) 88:104021. doi: 10.1016/j.archger.2020.104021
273. Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, et al. Treatment of osteoporosis in clinical practice (TOP) study group. determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int (2006) 17(6):914–21.
274. Pepe J, Cipriani C, Cecchetti V, Ferrara C, Della Grotta G, Danese V, et al. Patients' reasons for adhering to long-term alendronate therapy. Osteoporos Int (2019) 30:1627–34. doi: 10.1007/s00198-019-05010-w
275. Adachi JD, Hanley DA, Lorraine JK, Yu M. Assessing compliance, acceptance, and tolerability of teriparatide in patients with osteoporosis who fractured while on antiresorptive treatment or were intolerant to previous antiresorptive treatment: an 18-month, multicenter, open-label, prospective study. Clin Ther (2007) 29:2055–67. doi: 10.1016/j.clinthera.2007.09.024
276. Chesser TJ, Fox R, Harding K, Halliday R, Barnfield S, Willett K, et al. The administration of intermittent parathyroid hormone affects functional recovery from trochanteric fractured neck of femur: a randomised prospective mixed method pilot study. Bone Joint J (2016) 98-B:840–5. doi: 10.1302/0301-620X.98B6.36794
277. Kendler DL, Macarios D, Lillestol MJ, Moffett A, Satram-Hoang S, Huang J, et al. Influence of patient perceptions and preferences for osteoporosis medication on adherence behavior in the denosumab adherence preference satisfaction study. Menopause (2014) 21:25–32. doi: 10.1097/GME.0b013e31828f5e5d
278. Si L, Tu L, Xie Y, Palmer AJ, Gu Y, Zheng X, et al. Chinese Patients' preference for pharmaceutical treatments of osteoporosis: a discrete choice experiment. Arch Osteoporos (2019) 14:85. doi: 10.1007/s11657-019-0624-z
279. Fraenkel L, Gulanski B, Wittink D. Patient treatment preferences for osteoporosis. Arthritis Rheum (2006) 55:729–35. doi: 10.1002/art.22229
280. Cotté FE, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res (2011) 11:151. doi: 10.1186/1472-6963-11-151
281. Francis RM, Anderson FH, Torgerson DJ. A comparison of the effectiveness and cost of treatment for vertebral fractures in women. Br J Rheumatol (1995) 34:1167–71. doi: 10.1093/rheumatology/34.12.1167
282. Earnshaw SR, Graham CN, Ettinger B, Amonkar MM, Lynch NO, Middelhoven H. Cost-effectiveness of bisphosphonate therapies for women with postmenopausal osteoporosis: implications of improved persistence with less frequently administered oral bisphosphonates. Curr Med Res Opin (2007) 23:2517–29. doi: 10.1185/030079907X226339
283. Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY. Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int (2010) 86:202–10. doi: 10.1007/s00223-009-9329-4
284. Ministero della salute. quaderni del ministero della salute (2010). Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1701_allegato.pdf (Accessed August 2022).
285. Fragility Fracture Network. Guide to the formation of national fragility fracture networks . Available at: https://www.fragilityfracturenetwork.org/wp-content/uploads/2019/07/National_FFN_Guide.pdf (Accessed August 2022).
286. Dreinhöfer KE, Mitchell PJ, Bégué T, Cooper C, Costa ML, Falaschi P, et al. A global call to action to improve the care of people with fragility fractures. Injury (2018) 49:1393–7. doi: 10.1016/j.injury.2018.06.032
287. McLellan AR, Gallacher SJ, Fraser M, McQuillian C. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int (2003) 14:1028–340. doi: 10.1007/s00198-003-1507-z
288. Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE Jr, McLellan A, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res (2012) 27:2039–60. doi: 10.1002/jbmr.1698
289. Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, et al. Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int (2013) 24:2135–52. doi: 10.1007/s00198-013-2348-z
290. Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int (2013) 24:393–406. doi: 10.1007/s00198-012-2090-y
291. Luc M, Corriveau H, Boire G, Filiatrault J, Beaulieu MC, Gaboury I. Patient-related factors associated with adherence to recommendations made by a fracture liaison service: A mixed-method prospective study. Int J Environ Res Public Health (2018) 15:944. doi: 10.3390/ijerph15050944
292. Swart KMA, van Vilsteren M, van Hout W, Draak E, van der Zwaard BC, van der Horst HE, et al. Factors related to intentional non-initiation of bisphosphonate treatment in patients with a high fracture risk in primary care: a qualitative study. BMC Fam Pract (2018) 19:141. doi: 10.1186/s12875-018-0828-0
293. Miller AN, Lake AF, Emory CL. Establishing a fracture liaison service: an orthopaedic approach. J Bone Joint Surg Am (2015) 97:675–81. doi: 10.2106/JBJS.N.00957
294. Noordin S, Allana S, Masri BA. Establishing a hospital based fracture liaison service to prevent secondary insufficiency fractures. Int J Surg (2018) 54(Pt B):328–32. doi: 10.1016/j.ijsu.2017.09.010
295. Wu CH, Tu ST, Chang YF, Chan DC, Chien JT, Lin CH, et al. Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: A systematic literature review and meta-analysis. Bone (2018) 111:92–100. doi: 10.1016/j.bone.2018.03.018
296. Wu CH, Chen CH, Chen PH, Yang JJ, Chang PC, Huang TC, et al. Identifying characteristics of an effective fracture liaison service: systematic literature review. Osteoporos Int (2018) 29:1023–47. doi: 10.1007/s00198-017-4370-z
297. Bell K, Strand H, Inder WJ. Effect of a dedicated osteoporosis health professional on screening and treatment in outpatients presenting with acute low trauma non-hip fracture: a systematic review. Arch Osteoporos (2014) 9:167. doi: 10.1007/s11657-013-0167-7
298. Chang Y-, Huang C-, Hwang J-, Kuo J-, Lin K-, Huang H-, et al. Fracture liaison services for osteoporosis in the Asia-pacific region: current unmet needs and systematic literature review. Osteoporos Int (2018) 29:779–92. doi: 10.1007/s00198-017-4347-y
299. Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med (2000) 109:326–8. doi: 10.1016/S0002-9343(00)00457-5
300. Rolnick SJ, Kopher R, Jackson J, Fischer LR, Compo R. What is the impact of osteoporosis education and bone mineral density testing for postmenopausal women in a managed care setting? Menopause (2001) 8:141–8. doi: 10.1097/00042192-200103000-00010
301. Hawker G, Ridout R, Ricupero M, Jaglal S, Bogoch E. The impact of a simple fracture clinic intervention in improving the diagnosis and treatment of osteoporosis in fragility fracture patients. Osteoporos Int (2003) 14:171–8. doi: 10.1007/s00198-003-1377-4
302. Jachna CM, Whittle J, Lukert B, Graves L, Bhargava T. Effect of hospitalist consultation on treatment of osteoporosis in hip fracture patients. Osteoporos Int (2003) 14:665–71. doi: 10.1007/s00198-003-1413-4
303. Majumdar SR, Rowe BH, Folk D, Johnson JA, Holroyd BH, Morrish DW, et al. A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Ann Intern Med (2004) 141:366–73. doi: 10.7326/0003-4819-141-5-200409070-00011
304. Sidwell A, Wilkinson T, Hanger H. Secondary prevention of fractures in older people: evaluation of a protocol for the investigation and treatment of osteoporosis. Intern Med J (2004) 34:129–32. doi: 10.1111/j.1444-0903.2004.00554.x
305. Brankin E, Mitchell C, Munro R, Lanarkshire Osteoporosis Service. Closing the osteoporosis management gap in primary care: a secondary prevention of fracture programme. Curr Med Res Opin (2005) 21:475–82. doi: 10.1185/030079905X38150
306. Harrington JT, Barash HL, Day S, Lease J. Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum (2005) 53:198–204. doi: 10.1002/art.21072
307. Johnson SL, Petkov VI, Williams MI, Via PS, Adler RA. Improving osteoporosis management in patients with fractures. Osteoporos Int (2005) 16:1079–85. doi: 10.1007/s00198-004-1814-z
308. Jones G, Warr S, Francis E, Greenaway T. The effect of a fracture protocol on hospital prescriptions after minimal trauma fractured neck of the femur: a retrospective audit. Osteoporos Int (2005) 16:1277–80. doi: 10.1007/s00198-005-1960-y
309. Murray AW, McQuillan C, Kennon B, Gallacher SJ. Osteoporosis risk assessment and treatment intervention after hip or shoulder fracture. a comparison of two centres in the united kingdom. Injury (2005) 36:1080–4. doi: 10.1016/j.injury.2005.03.012
310. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc (2005) 53:1476–82. doi: 10.1111/j.1532-5415.2005.53466.x
311. Fisher AA, Davis MW, Rubenach SE, Sivakumaran S, Smith PN, Budge MM. Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare. J Orthop Trauma (2006) 20:172–8. doi: 10.1097/01.bot.0000202220.88855.16
312. Streeten EA, Mohamed A, Gandhi A, Orwig D, Sack P, Sterling R, et al. The inpatient consultation approach to osteoporosis treatment in patients with a fracture. is automatic consultation needed? J Bone Joint Surg Am (2006) 88:1968–74. doi: 10.2106/00004623-200609000-00010
313. Davis JC, Guy P, Ashe MC, Liu-Ambrose T, Khan K. HipWatch: osteoporosis investigation and treatment after a hip fracture: a 6-month randomized controlled trial. J Gerontol A Biol Sci Med Sci (2007) 62:888–91. doi: 10.1093/gerona/62.8.888
314. Laslett LL, Whitham JN, Gibb C, Gill TK, Pink JA, McNeil JD, et al. Improving diagnosis and treatment of osteoporosis: evaluation of a clinical pathway for low trauma fractures. Arch Osteoporos (2007) 2:1–6. doi: 10.1007/s11657-007-0010-0
315. van Helden S, Cauberg E, Geusens P, Winkes B, van der Weijden T, Brink P. The fracture and osteoporosis outpatient clinic: an effective strategy for improving implementation of an osteoporosis guideline. J Eval Clin Pract (2007) 13:801–05. doi: 10.1111/j.1365-2753.2007.00784.x
316. Cranney A, Lam M, Ruhland L, Brison R, Brison R, Godwin M, Harrison MM, et al. A multifaceted intervention to improve treatment of osteoporosis in postmenopausal women with wrist fractures: a cluster randomized trial. Osteoporos Int (2008) 19:1733–40. doi: 10.1007/s00198-008-0669-0
317. Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ (2008) 178:569–75. doi: 10.1503/cmaj.070981
318. Miki RA, Oetgen ME, Kirk J, Insogna KL, Lindskog DM. Orthopaedic management improves the rate of early osteoporosis treatment after hip fracture. a randomized clinical trial. J Bone Joint Surg Am (2008) 90:2346–53. doi: 10.2106/JBJS.G.01246
319. Tosi LL, Gliklich R, Kannan K, Koval KJ. The American orthopaedic association's "own the bone" initiative to prevent secondary fractures. J Bone Joint Surg Am (2008) 90:163–73. doi: 10.2106/JBJS.G.00682
320. Jaglal SB, Hawker G, Bansod V, Salbach NM, Zwarenstein M, Carroll J, et al. A demonstration project of a multi-component educational intervention to improve integrated post-fracture osteoporosis care in five rural communities in Ontario, Canada. Osteoporos Int (2009) 20:265–74. doi: 10.1007/s00198-008-0654-7
321. Morrish DW, Beaupre LA, Bell NR, Cinats JG, Hanley DA, Harley CH, et al. Facilitated bone mineral density testing versus hospital-basedcase management to improve osteoporosis treatment for hip fracture patients: additional results from a randomized trial. Arthritis Rheum (2009) 61:209–15. doi: 10.1002/art.24097
322. Yuksel N, Majumdar SR, Biggs C, Tsuyuki RT. Community pharmacist-initiated screening program for osteoporosis: randomized controlled trial. Osteoporos Int (2010) 21:391–8. doi: 10.1007/s00198-009-0977-z
323. Huntjens KM, van Geel TC, Geusens PP, Winkens B, Willems P, van den Bergh J, et al. Impact of guideline implementation by a fracture nurse on subsequent fractures and mortality in patients presenting with non-vertebral fractures. Injury (2011) 42(Suppl 4):S39–43. doi: 10.1016/S0020-1383(11)70011-0
324. Lih A, Nandapalan H, Kim M, Yap C, Lee P, Ganda K, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int (2011) 22:849–58. doi: 10.1007/s00198-010-1477-x
325. Majumdar SR, Johnson JA, Bellerose D, McAlister FA, Russell AS, Hanley DA, et al. Nurse case-manager vs multifaceted intervention to improve quality of osteoporosis care after wrist fracture: randomized controlled pilot study. Osteoporos Int (2011) 22:223–30. doi: 10.1007/s00198-010-1212-7
326. Roy A, Heckman MG, O'Connor MI. Optimizing screening for osteoporosis in patients with fragility hip fracture. Clin Orthop Relat Res (2011) 469:1925–30. doi: 10.1007/s11999-011-1839-5
327. Wallace I, Callachand F, Elliott J, Gardiner P. An evaluation of an enhanced fracture liaison service as the optimal model for secondary prevention of osteoporosis. JRSM Short Rep (2011) 2:8. doi: 10.1258/shorts.2010.010063
328. Astrand J, Nilsson J, Thorngren KG. Screening for osteoporosis reduced new fracture incidence by almost half: a 6-year follow-up of 592 fracture patients from an osteoporosis screening program. Acta Orthop (2012) 83:661–5. doi: 10.3109/17453674.2012.747922
329. Heilmann RM, Friesleben CR, Billups SJ. Impact of a pharmacist-directed intervention in postmenopausal women after fracture. Am J Health Syst Pharm (2012) 69:504–9. doi: 10.2146/ajhp110309
330. Leslie WD, LaBine L, Klassen P, Dreilich D, Caetano PA. Closing the gap in postfracture care at the population level: a randomized controlled trial. CMAJ (2012) 184:290–6. doi: 10.1503/cmaj.111158
331. Goltz L, Degenhardt G, Maywald U, Kirch W, Schindler C. Evaluation of a program of integrated care to reduce recurrent osteoporotic fractures. Pharmacoepidemiol Drug Saf (2013) 22:263–70. doi: 10.1002/pds.3399
332. Queally JM, Kiernan C, Shaikh M, Rowan F, Bennett D. Initiation of osteoporosis assessment in the fracture clinic results in improved osteoporosis management: a randomised controlled trial. Osteoporos Int (2013) 24:1089–94. doi: 10.1007/s00198-012-2238-9
333. Roux S, Beaulieu M, Beaulieu MC, Cabana F, Boire G. Priming primary care physicians to treat osteoporosis after a fragility fracture: an integrated multidisciplinary approach. J Rheumatol (2013) 40:703–11. doi: 10.3899/jrheum.120908
334. Ganda K, Schaffer A, Pearson S, Seibel MJ. Compliance and persistence to oral bisphosphonate therapy following initiation within a secondary fracture prevention program: a randomised controlled trial of specialist vs. non-specialist management. Osteoporos Int (2014) 25:1345–55. doi: 10.1007/s00198-013-2610-4
335. Hofflich HL, Oh DK, Choe CH, Clay B, Tibble C, Kulasa KM, et al. Using a triggered endocrinology service consultation to improve the evaluation, management, and follow-up of osteoporosis in hip-fracture patients. Jt Comm J Qual Patient Saf (2014) 40:228–34. doi: 10.1016/S1553-7250(14)40030-8
336. Huntjens KM, van Geel TA, van den Bergh JP. Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am (2014) 96:e29. doi: 10.2106/JBJS.L.00223
337. Van der Kallen J, Giles M, Cooper K, Gill K, Parker V, Tembo A, et al. A fracture prevention service reduces further fractures two years after incident minimal trauma fracture. Int J Rheum Dis (2014) 17:195–203. doi: 10.1111/1756-185X.12101
338. Chan T, de Lusignan S, Cooper A, Elliott M. Improving osteoporosis management in primary care: An audit of the impact of a community based fracture liaison nurse. PloS One (2015) 10:e0132146. doi: 10.1371/journal.pone.0132146
339. Ruggiero C, Zampi E, Rinonapoli G, Baroni M, Serra R, Zengarini E, et al. Fracture prevention service to bridge the osteoporosis care gap. Clin Interv Aging (2015) 10:1035–42.
340. Amphansap T, Stitkitti N, Dumrongwanich P. Evaluation of police general hospital's fracture liaison service (PGH's FLS): The first study of a fracture liaison service in Thailand. Osteoporos Sarcopenia (2016) 2:238–43. doi: 10.1016/j.afos.2016.09.002
341. Axelsson KF, Jacobsson R, Lund D, Lorentzon M. Effectiveness of a minimal resource fracture liaison service. Osteoporos Int (2016) 27:3165–75. doi: 10.1007/s00198-016-3643-2
342. Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing (2016) 45:236–42. doi: 10.1093/ageing/afv204
343. Nakayama A, Major G, Holliday E, Attia J, Bogduk N. Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int (2016) 27:873–9. doi: 10.1007/s00198-015-3443-0
344. Soong C, Cram P, Chezar K, Tajammal F, Exconde K, Matelski J, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma (2016) 30:647–52. doi: 10.1097/BOT.0000000000000691
345. Anderson ME, Mcdevitt K, Cumbler E, Bennett H, Robison Z, Gomez B, et al. Geriatric hip fracture care: Fixing a fragmented system. Perm J (2017) 21:16–104. doi: 10.7812/TPP/16-104
346. Bachour F, Rizkallah M, Sebaaly A, Barakat A, Razzouk H, El Hage R, et al. Fracture liaison service: report on the first successful experience from the middle East. Arch Osteoporos (2017) 12:79. doi: 10.1007/s11657-017-0372-x
347. Beaton DE, Vidmar M, Pitzul KB, Sujic R, Rotondi NK, Bogoch ER, et al. Addition of a fracture risk assessment to a coordinator's role improved treatment rates within 6 months of screening in a fragility fracture screening program. Osteoporos Int (2017) 28:863–9. doi: 10.1007/s00198-016-3794-1
348. Beaton DE, Mamdani M, Zheng H, Jaglal S, Cadarette SM, Bogoch ER, et al. Improvements in osteoporosis testing and care are found following the wide scale implementation of the Ontario fracture clinic screening program: An interrupted time series analysis. Med (Baltimore) (2017) 96:e9012. doi: 10.1097/MD.0000000000009012
349. Coventry LS, Nguyen A, Karahalios A, Roshan-Zamir S, Tran P. Comparison of 3 different perioperative care models for patients with hip fractures within 1 health service. Geriatr Orthop Surg Rehabil (2017) 8:87–93. doi: 10.1177/2151458517692651
350. Cosman F, Nicpon K, Nieves JW. Results of a fracture liaison service on hip fracture patients in an open healthcare system. Aging Clin Exp Res (2017) 29:331–4. doi: 10.1007/s40520-016-0545-2
351. Davidson E, Seal A, Doyle Z, Fielding K, McGirr J. Prevention of osteoporotic refractures in regional Australia. Aust J Rural Health (2017) 25:362–8. doi: 10.1111/ajr.12355
352. Henderson CY, Shanahan E, Butler A, Lenehan B, O'Connor M, Lyons D, et al. Dedicated orthogeriatric service reduces hip fracture mortality. Ir J Med Sci (2017) 186:179–84. doi: 10.1007/s11845-016-1453-3
353. Lamb LC, Montgomery SC, Wong Won B, Harder S, Meter J, Feeney JM. A multidisciplinary approach to improve the quality of care for patients with fragility fractures. J Orthop (2017) 14:247–51. doi: 10.1016/j.jor.2017.03.004
354. Merle B, Chapurlat R, Vignot E, Thomas T, Haesebaert J, Schott AM, et al. Post-fracture care: do we need to educate patients rather than doctors? the PREVOST randomized controlled trial. Osteoporos Int (2017) 28:1549–58. doi: 10.1007/s00198-017-3953-z
355. Naranjo A, Fernández-Conde S, Ojeda S, Torres-Hernández L, Hernández-Carballo C, Bernardos I, et al. Preventing future fractures: effectiveness of an orthogeriatric fracture liaison service compared to an outpatient fracture liaison service and the standard management in patients with hip fracture. Arch Osteoporos (2017) 12:112. doi: 10.1007/s11657-017-0373-9
356. Vaculík J, Stepan JJ, Dungl P, Majerníček M, Čelko A, Džupa V. Secondary fracture prevention in hip fracture patients requires cooperation from general practitioners. Arch Osteoporos (2017) 12:49. doi: 10.1007/s11657-017-0346-z
357. Aubry-Rozier B, Stoll D, Gonzalez Rodriguez E, Hans D, Prudent V, Seuret A, et al. Impact of a fracture liaison service on patient management after an osteoporotic fracture: the CHUV FLS. Swiss Med Wkly (2018) 148:w14579.
358. Brañas F, Ruiz-Pinto A, Fernández E, Del Cerro A, de Dios R, Fuentetaja L, et al. Beyond orthogeriatric co-management model: benefits of implementing a process management system for hip fracture. Arch Osteoporos (2018) 13:81. doi: 10.1007/s11657-018-0497-6
359. Greenspan SL, Singer A, Vujevich K, Marchand B, Thompson DA, Hsu YJ, et al. Implementing a fracture liaison service open model of care utilizing a cloud-based tool. Osteoporos Int (2018) 29:953–60. doi: 10.1007/s00198-017-4371-y
360. Inderjeeth CA, Raymond WD, Briggs AM, Geelhoed E, Oldham D, Mountain D. Implementation of the Western Australian osteoporosis model of care: a fracture liaison service utilising emergency department information systems to identify patients with fragility fracture to improve current practice and reduce re-fracture rates: a 12-month analysis. Osteoporos Int (2018) 29:1759–70. doi: 10.1007/s00198-018-4526-5
361. Majumdar SR, McAlister FA, Johnson JA, Rowe BH, Bellerose D, Hassan I, et al. Comparing strategies targeting osteoporosis to prevent fractures after an upper extremity fracture (C-STOP trial): A randomized controlled trial. J Bone Miner Res (2018) 33:2114–21. doi: 10.1002/jbmr.3557
362. Rotman-Pikielny P, Frankel M, Lebanon OT, Yaacobi E, Tamar M, Netzer D, et al. Orthopedic-metabolic collaborative management for osteoporotic hip fracture. Endocr Pract (2018) 24:718–25. doi: 10.4158/EP-2018-0082
363. Sietsema DL, Araujo AB, Wang L, Boytsov NN, Pandya SA, Haynes VS, et al. The effectiveness of a private orthopaedic practice-based osteoporosis management service to reduce the risk of subsequent fractures. J Bone Joint Surg Am (2018) 100:1819–28. doi: 10.2106/JBJS.17.01388
364. Sofie S, Yves P, Barbara V, Margareta L, Raf VH, Bruno V, et al. Building for better bones: evaluation of a clinical pathway in the secondary prevention of osteoporotic fractures. Eur J Hosp Pharm (2018) 25:210–3. doi: 10.1136/ejhpharm-2016-000906
365. Abrahamsen C, Nørgaard B, Draborg E, Nielsen MF. The impact of an orthogeriatric intervention in patients with fragility fractures: a cohort study. BMC Geriatr (2019) 19:268. doi: 10.1186/s12877-019-1299-4
366. Baroni M, Serra R, Boccardi V, Ercolani S, Zengarini E, Casucci P, et al. The orthogeriatric comanagement improves clinical outcomes of hip fracture in older adults. Osteoporos Int (2019) 30:907–16. doi: 10.1007/s00198-019-04858-2
367. Sanli I, van Helden SH, Ten Broeke RHM, Geusens P, Van den Bergh JPW, Brink PRG, et al. The role of the fracture liaison service (FLS) in subsequent fracture prevention in the extreme elderly. Aging Clin Exp Res (2019) 31:1105–11. doi: 10.1007/s40520-018-1054-2
368. Shigemoto K, Sawaguchi T, Goshima K, Iwai S, Nakanishi A, Ueoka K. The effect of a multidisciplinary approach on geriatric hip fractures in Japan. J Orthop Sci (2019) 24:280–5. doi: 10.1016/j.jos.2018.09.012
369. Singh S, Whitehurst DG, Funnell L, Scott V, MacDonald V, Leung PM, et al. Breaking the cycle of recurrent fracture: implementing the first fracture liaison service (FLS) in British Columbia, Canada. Arch Osteoporos (2019) 14:116. doi: 10.1007/s11657-019-0662-6
370. Wasfie T, Jackson A, Brock C, Galovska S, McCullough JR, Burgess JA. Does a fracture liaison service program minimize recurrent fragility fractures in the elderly with osteoporotic vertebral compression fractures? Am J Surg (2019) 217:557–60. doi: 10.1016/j.amjsurg.2018.09.027
371. Amphansap T, Stitkitti N, Arirachakaran A. The effectiveness of police general hospital's fracture liaison service (PGH's FLS) implementation after 5 years: A prospective cohort study. Osteoporos Sarcopenia (2020) 6:199–204. doi: 10.1016/j.afos.2020.11.004
372. Anighoro K, Bridges C, Graf A, Nielsen A, Court T, McKeon J, et al. From ER to OR: Results after implementation of multidisciplinary pathway for fragility hip fractures at a level I trauma center. Geriatr Orthop Surg Rehabil (2020) 11:2151459320927383. doi: 10.1177/2151459320927383
373. Beaupre LA, Moradi F, Khong H, Smith C, Evens L, Hanson HM, et al. Implementation of an in-patient hip fracture liaison services to improve initiation of osteoporosis medication use within 1-year of hip fracture: a population-based time series analysis using the RE-AIM framework. Arch Osteoporos (2020) 15:83. doi: 10.1007/s11657-020-00751-2
374. Schuijt HJ, Kusen J, van Hernen JJ, van der Vet P, Geraghty O, Smeeing DPJ, et al. Orthogeriatric trauma unit improves patient outcomes in geriatric hip fracture patients. Geriatr Orthop Surg Rehabil (2020) 11:2151459320949476. doi: 10.1177/2151459320949476
375. Svenøy S, Watne LO, Hestnes I, Westberg M, Madsen JE, Frihagen F. Results after introduction of a hip fracture care pathway: comparison with usual care. Acta Orthop (2020) 91:139–45. doi: 10.1080/17453674.2019.1710804
376. Giangregorio L, Fisher P, Papaioannou A, Adachi JD. Osteoporosis knowledge and information needs in healthcare professionals caring for patients with fragility fractures. Orthop Nurs (2007) 26:27–35. doi: 10.1097/00006416-200701000-00009
377. Mo J, Huang K, Wang X, Sheng X, Wang Q, Fang X, et al. The sensitivity of orthopaedic surgeons to the secondary prevention of fragility fractures. J Bone Joint Surg Am (2018) 100:e153. doi: 10.2106/JBJS.17.01297
378. Bliuc D, Eisman JA, Center JR. A randomized study of two different information-based interventions on the management of osteoporosis in minimal and moderate trauma fractures. Osteoporos Int (2006) 17:1309–17. doi: 10.1007/s00198-006-0078-1
379. Yates CJ, Chauchard MA, Liew D, Bucknill A, Wark JD. Bridging the osteoporosis treatment gap: performance and cost-effectiveness of a fracture liaison service. J Clin Densitom (2015) 18:150–6. doi: 10.1016/j.jocd.2015.01.003
380. Wu CH, Kao IJ, Hung WC, Lin SC, Liu HC, Hsieh MH, et al. Economic impact and cost-effectiveness of fracture liaison services: a systematic review of the literature. Osteoporos Int (2018) 29:1227–42. doi: 10.1007/s00198-018-4411-2
381. Sander B, Elliot-Gibson V, Beaton DE, Bogoch ER, Maetzel A. A coordinator program in post-fracture osteoporosis management improves outcomes and saves costs. J Bone Joint Surg Am (2008) 90:1197–205. doi: 10.2106/JBJS.G.00980
382. Beaupre LA, Lier D, Smith C, Evens L, Hanson HM, Juby AG, et al. A 3i hip fracture liaison service with nurse and physician co-management is cost-effective when implemented as a standard clinical program. Arch Osteoporos (2020) 15:113. doi: 10.1007/s11657-020-00781-w
383. Majumdar SR, Lier DA, Hanley DA, Juby AG, Beaupre LA, STOP-PRIHS Team. Economic evaluation of a population-based osteoporosis intervention for outpatients with non-traumatic non-hip fractures: the "Catch a break" 1i [type c] FLS. Osteoporos Int (2017) 28:1965–77. doi: 10.1007/s00198-017-3986-3
384. Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am (2008) 90(Suppl 4):188–94. doi: 10.2106/JBJS.H.00628
385. Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res (2014) 29:1667–74. doi: 10.1002/jbmr.2180
386. Bogoch ER, Elliot-Gibson V, Beaton DE, Jamal SA, Josse RG, Murray TM. Effective initiation of osteoporosis diagnosis and treatment for patients with a fragility fracture in an orthopaedic environment. J Bone Joint Surg Am (2006) 88:25–34.
387. Che M, Ettinger B, Liang J, Pressman AR, Johnston J. Outcomes of a disease-management program for patients with recent osteoporotic fracture. Osteoporos Int (2006) 17:847–54. doi: 10.1007/s00198-005-0057-y
388. van den Berg P, van Haard PMM, Geusens PP, van den Bergh JP, Schweitzer DH. Challenges and opportunities to improve fracture liaison service attendance: fracture registration and patient characteristics and motivations. Osteoporos Int (2019) 30:1597–606. doi: 10.1007/s00198-019-05016-4
389. Seuffert P, Sagebien CA, McDonnell M, O'Hara DA. Evaluation of osteoporosis risk and initiation of a nurse practitioner intervention program in an orthopedic practice. Arch Osteoporos (2016) 11:10. doi: 10.1007/s11657-016-0262-7
390. Asia Pacific Fragility Fracture Alliance-Fragility Fracture Network. The hip fracture registry (HFR) toolbox (2020). Available at: https://apfracturealliance.org/hfr-toolbox/ (Accessed 2 March 2023).
391. New Zealand and Australia. Australian And new Zealand fragility fracture registry (2020). Available at: https://fragilityfracture.co.nz/ (Accessed 2 March 2023).
392. Australian Fragility Fracture Foundation. Australian And new Zealand fragility fracture registry (2020). Available at: https://fragilityfracture.com.au/ (Accessed 2 March 2023).
393. The Irish Institute of Trauma and Orthopaedic Surgery. Fracture liaison service database (2018). Available at: https://iitos.com/fracture-liaison-service-database-2/ (Accessed 2 March 2023).
394. Royal College of Physicians. Fracture liaison service database (FLS-DB) (2016). Available at: https://www.rcplondon.ac.uk/projects/fracture-liaison-service-database-fls-db (Accessed 2 March 2023).
395. American Orthopaedic Association. Own the bone (2009). Available at: https://www.ownthebone.org/ (Accessed 2 March 2023).
396. Zogg CK, Metcalfe D, Judge A, Perry DC, Costa ML, Gabbe BJ, et al. Learning from england's best practice tariff: Process measure pay-for-Performance can improve hip fracture outcomes. Ann Surg (2022) 275:506–14. doi: 10.1097/SLA.0000000000004305
397. Metcalfe D, Zogg CK, Judge A, Perry DC, Gabbe B, Willett K, et al. Pay for performance and hip fracture outcomes: an interrupted time series and difference-in-differences analysis in England and Scotland. Bone Joint J (2019) 101-B:1015–23. doi: 10.1302/0301-620X.101B8.BJJ-2019-0173.R1
398. Patel R, Judge A, Johansen A, Marques EMR, Griffin J, Bradshaw M, et al. Multiple hospital organisational factors are associated with adverse patient outcomes post-hip fracture in England and Wales: the REDUCE record-linkage cohort study. Age Ageing (2022) 51:afac183. doi: 10.1093/ageing/afac183
Keywords: evidence-based guideline, fragility fracture, secondary prevention, systematic review, grade
Citation: Corrao G, Biffi A, Porcu G, Ronco R, Adami G, Alvaro R, Bogini R, Caputi AP, Cianferotti L, Frediani B, Gatti D, Gonnelli S, Iolascon G, Lenzi A, Leone S, Michieli R, Migliaccio S, Nicoletti T, Paoletta M, Pennini A, Piccirilli E, Rossini M, Tarantino U and Brandi ML (2023) Executive summary: Italian guidelines for diagnosis, risk stratification, and care continuity of fragility fractures 2021. Front. Endocrinol. 14:1137671. doi: 10.3389/fendo.2023.1137671
Received: 04 January 2023; Accepted: 27 March 2023;
Published: 18 April 2023.
Edited by:
Jonathan H Tobias, University of Bristol, United KingdomReviewed by:
Jan Josef Stepan, Charles University, CzechiaPaul Mitchell, University of Notre Dame Australia, Australia
Copyright © 2023 Corrao, Biffi, Porcu, Ronco, Adami, Alvaro, Bogini, Caputi, Cianferotti, Frediani, Gatti, Gonnelli, Iolascon, Lenzi, Leone, Michieli, Migliaccio, Nicoletti, Paoletta, Pennini, Piccirilli, Rossini, Tarantino and Brandi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Corrao, Z2lvdmFubmkuY29ycmFvQHVuaW1pYi5pdA==; Maria Luisa Brandi, bWFyaWFsdWlzYS5icmFuZGlAdW5pZmkuaXQ=
 Giovanni Corrao
Giovanni Corrao Annalisa Biffi1,2
Annalisa Biffi1,2 Gloria Porcu
Gloria Porcu Giovanni Adami
Giovanni Adami Davide Gatti
Davide Gatti Giovanni Iolascon
Giovanni Iolascon Andrea Lenzi
Andrea Lenzi Salvatore Leone
Salvatore Leone Raffaella Michieli
Raffaella Michieli Marco Paoletta
Marco Paoletta Eleonora Piccirilli
Eleonora Piccirilli Maurizio Rossini
Maurizio Rossini Umberto Tarantino
Umberto Tarantino Maria Luisa Brandi
Maria Luisa Brandi