- 1Phase 1 Clinical Trial Laboratory, Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China
- 2Department of Pharmacy, Guangxi Academy of Medical Sciences and the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, China
Background: The optimal first-line immune checkpoint inhibitor (ICI) treatment strategy for metastatic or early triple-negative breast cancer (TNBC) has not yet been determined as a result of various randomized controlled trials (RCTs). The purpose of this study was to compare the efficacy and safety of ICIs in patients with metastatic or early TNBC.
Methods: RCTs comparing the efficacy and safety of ICIs in patients with TNBC were included in the studies. Based on PRISMA guidelines, we estimated pooled hazard ratios (HRs) and odds ratios (ORs) using random-effects models of Bayesian network meta-analysis. Primary outcomes were progression-free survival (PFS) and overall survival (OS). Secondary outcomes included pathologic complete response rate (pCR), grade ≥ 3 treatment-related adverse events (trAEs), immune-related adverse events (irAEs), and grade ≥ 3 irAEs.
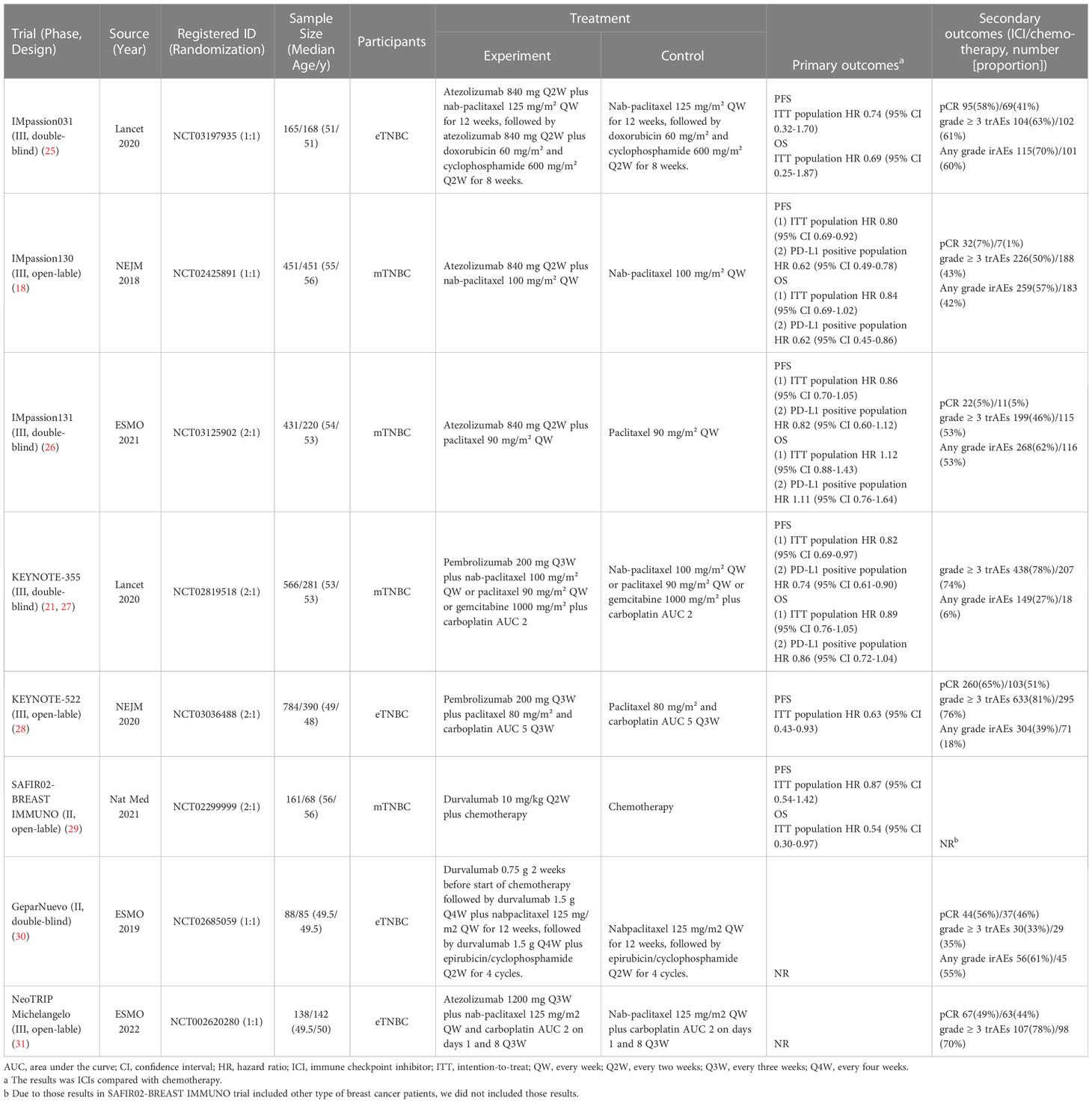
Results: The criteria for eligibility were met by a total of eight RCTs involving 4,589 patients with TNBC. When ICIs were used in patients without programmed death-ligand 1 (PD-L1) selection, there was a trend toward improved PFS, OS, and pCR, without significant differences. Pembrolizumab plus chemotherapy is superior to other treatment regimens in terms of survival for TNBC patients based on Bayesian ranking profiles. Subgroup analysis by PD-L1 positive population indicated similar results, and atezolizumab plus chemotherapy provided better survival outcomes. Among grade ≥ 3 trAEs and any grade irAEs, there was no statistically significant difference among different ICI agents. The combination of ICIs with chemotherapy was associated with a higher incidence of grade ≥ 3 irAEs. Based on rank probability, the ICI plus chemotherapy group was more likely to be associated with grade ≥ 3 trAEs, any grade irAEs, and grade ≥ 3 irAEs. Hypothyroidism and hyperthyroidism were the most frequent irAEs in patients receiving ICI.
Conclusions: ICI regimens had relatively greater efficacy and safety profile. Pembrolizumab plus chemotherapy and atezolizumab plus chemotherapy seem to be superior first-line treatments for intention-to-treat and PD-L1-positive TNBC patients, respectively. It may be useful for making clinical decisions to evaluate the efficacy and safety of different ICIs based on our study.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022354643.
Introduction
The triple-negative breast cancer (TNBC) is associated with aggressive histology, a poor prognosis, and nonresponsiveness to hormonal therapy (1). High-risk early breast cancer (BC) with a neoadjuvant treatment is used widely to shrink tumors and reduce their size. Currently, the most common neoadjuvant therapies include chemotherapy, anti-human epidermal growth receptor 2 (HER2) therapy, endocrine therapy, and the co-administration of chemotherapy and HER2. Despite the lack of an anti-HER2 therapy and the potential antagonism between endocrine therapy and chemotherapy, anthracycline plus cyclophosphamide plus taxane neoadjuvant chemotherapy remains the preferred treatment option for patients with TNBC (2, 3). Despite the lack of clear overall survival (OS) benefits, bevacizumab is commonly used in combination with chemotherapy as a maintenance therapy in several countries (4, 5).
There is a poor survival outcome among patients with TNBC following standard neoadjuvant chemotherapy (6). To further increase survival outcomes for patients with TNBC, new strategies and agents are urgently needed. There has been a significant amount of research on the role of immune checkpoint molecules in preventing immune system suppression in tumor microenvironments (7). These molecules include cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1). The effect of these molecules on tumor cells and/or tumor-infiltrating lymphocytes (TILs) in metastatic TNBC has been evaluated (7). Research on immune checkpoint inhibitors (ICIs) has been a major focus in the last few years for patients with TNBC. Based on the updated guidelines, ICI plus chemotherapy is recommended as a treatment option for TNBC (8, 9). An important characteristic of cancer is its ability to evade immune destruction, which has led to the identification of escape mechanisms for cancer cells. Immunotherapeutic approaches to TNBC are rationalized by several factors. There is a strong correlation between a high level of TILs in TNBC and a positive response to ICI in other types of cancer (10). Additionally, immune evasion molecules such as PD-L1 are highly expressed in both tumors and immune cells (11), and the presence of many non-synonymous mutations that produce tumor-specific neoantigens may enhance the anti-tumor immune response and enhance the rationale for ICI treatment (12, 13). The treatment of early and metastatic TNBC using immunotherapeutic agents such as atezolizumab, durvalumab, and pembrolizumab showed that ICI had a superior effect in earlier treatment lines as well as in tumors positive for PD-L1 (14–21). TNBC poses a challenge for treatment because there are no specific targets for therapeutic intervention (22). Although previous published RCTs showed the superior efficiency of these novel ICIs for TNBC and the efficacy of recommended ICIs for TNBC has mainly been evaluated in clinical trials, there remains a lack of evidence to evaluate the relative effectiveness and safety according to classes of immunotherapies and targeted therapies.
Traditional pairwise meta-analysis can only compare two drug classes that have already been evaluated in previously published studies. However, the optimal types of ICIs for the treatment of TNBC are various, and the efficacy and safety of different types of ICIs remain inconclusive. In a complex choice with several optional strategies for treatment, and some therapeutic strategies have not been directly compared, a Bayesian network meta-analysis (NMA) can compare direct and indirect comparisons of different treatment strategies simultaneously within a single network and rank optional treatments according to comparative efficacy and safety. The 95% intervals generated by the Bayesian NMA are slightly wider than those under the traditional pairwise meta-analysis, and the primary reasons including the size of the discrepancies was heavily dependent on the number of included studies and the heterogeneity of the results, another key driver for the difference was the “prior information” used in the Bayesian analyses for random-effects model. However, from a practical standpoint, most health technology assessment bodies see little harm in being conservative by risking an overestimation of the width of 95% intervals as opposed to risking underestimation. Considering this background, we performed the present systematic review and Bayesian NMA of randomized controlled trials (RCTs) that assessed the efficacy and safety of ICI in patients with TNBC. In this study, we aim to determine the magnitude of benefit and safety that can be derived from different types of first-line ICIs in order to guide decision-making in clinical practice and future research on novel immunotherapeutic agents.
Methods
In this study, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines with an extension for NMAs (23). The study protocol was registered with PROSPERO (CRD42022354643).
Data sources and search strategy
MEDLINE (via OVID SP), Embase (via OVID SP), and PubMed (via OVID SP) databases were searched systematically for articles published up to October 8, 2022. The following search terms were used: breast cancer, TNBC, immune checkpoint inhibitor, programmed cell death protein-1, PD-1, programmed death ligand 1, PD-L1, atezolizumab, durvalumab, nivolumab, pembrolizumab, cytotoxic T-lymphocyte-associated antigen 4, CTLA-4, ipilimumab, and the name of other ICIs. We identified studies that were eligible and did not impose any language restrictions. We reviewed the reference lists of the identified trials, reviews, and meta-analyses in order to identify additional resources. Trials enrolled in the study were approved by all authors.
Selection criteria
Potentially eligible studies had to satisfy the following criteria in order to be included in the systematic review: (i) RCTs that study evaluated ICI, (ii) in TNBC patients previously untreated with ICI and (iii) with available results on primary outcomes or secondary outcomes. We looked at the two primary outcomes included OS and progression-free survival (PFS), as well as the secondary outcomes, included pathological complete response (pCR), grade ≥ 3 treatment-related adverse events (trAEs), immune-related adverse events (irAEs), and grade ≥ 3 irAEs. Exclusion criteria were: (i) non-RCTs studies such as single-arm trials or retrospective studies, (ii) no ICI treatments were used in the treatment arm of these trials, and (iii) the literature search was conducted in the context of ongoing studies with unpublished results. The analysis included all studies that met the inclusion criteria.
Data extraction
The following variables were extracted if available: name of the clinical trial, name of the first author, year of publication, study sample size, ICI used in combination with chemotherapy, chemotherapy regimen used as a control arm, and clinical outcomes of intention-to-treat (ITT) and PD-L1 status subgroups, including median and hazard ratios (HRs) and 95% confidence intervals (CIs) for PFS and OS, and the incidence of pCR and AEs. A message was sent by e-mail to the author when a primary or secondary outcome had not been reported.
Assessment of the risks of bias
In order to assess the methodological quality of the included RCTs, the Risk of Bias 2 tool was used to grade each trial as having low bias, high bias, or some concerns regarding bias (24).
Statistical analysis
In this study, we compared multiple trials involving different ICIs for the treatment of TNBC using NMA. Random-effects models were used to pool outcome measures. In the random-effects model approach, it is assumed that different studies estimate effects that are related to intervention but differ from each other. By using this approach, we are able to explain heterogeneity that cannot be simply explained by other factors. OS and PFS outcomes were expressed as HRs with 95% CIs. pCR, grade ≥ 3 trAEs any grade irAEs, and grade ≥ 3 irAEs were expressed as odds ratios (OR) and 95% CIs.
Bayesian NMA was performed using WinBUGS 1.4.3 software (MRC Biostatistics Unit, Cambridge, UK). It was conducted for each treatment comparison 100,000 times, with the first 10,000 iterations being discarded. To estimate the rank probabilities and assess the likelihood of each treatment regimen from best to worst, we used the surface under the cumulative ranking probabilities. As a result of the connections between the included studies and the number of participants, network plots were constructed based on the number of studies and participants.
Results
Systematic review and characteristics of selected trials
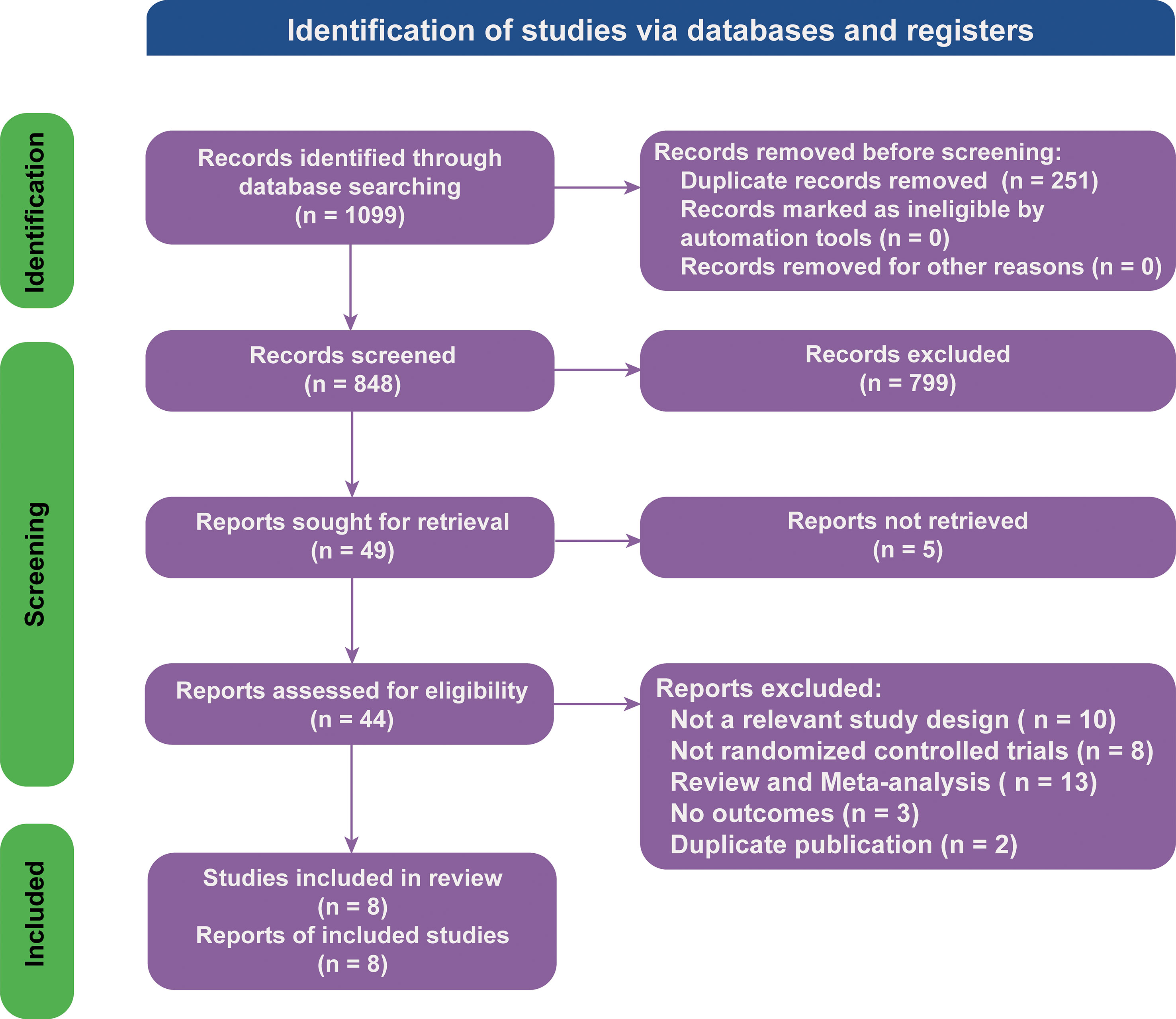
There were 1,099 articles found in the literature search. The NMA included 8 RCTs (18, 21, 25–31) involving 4,589 participants based on an assessment of 44 full-text articles (Figure 1; details of included studies are shown in Table 1). There were four treatment regimens recorded, including the following: atezolizumab plus chemotherapy (Atezo-Chemo), durvalumab plus chemotherapy (Durva-Chemo), pembrolizumab plus chemotherapy (Pemb-Chemo), and chemotherapy (Chemo). The Risk of Bias 2 tool was used to evaluate the quality of the trial (Supplementary Figure 1).
Comparisons of progression-free survival
NMA included 4 treatments for PFS in patients with TNBC (Figure 2). No significant differences were found among the treatment regimens in the ITT population for improvement of PFS (Figure 3). Based on Bayesian ranking profiles, Pemb-Chemo was ranked first for PFS, followed by Atezo-Chemo and Durva-Chemo (Figure 4).
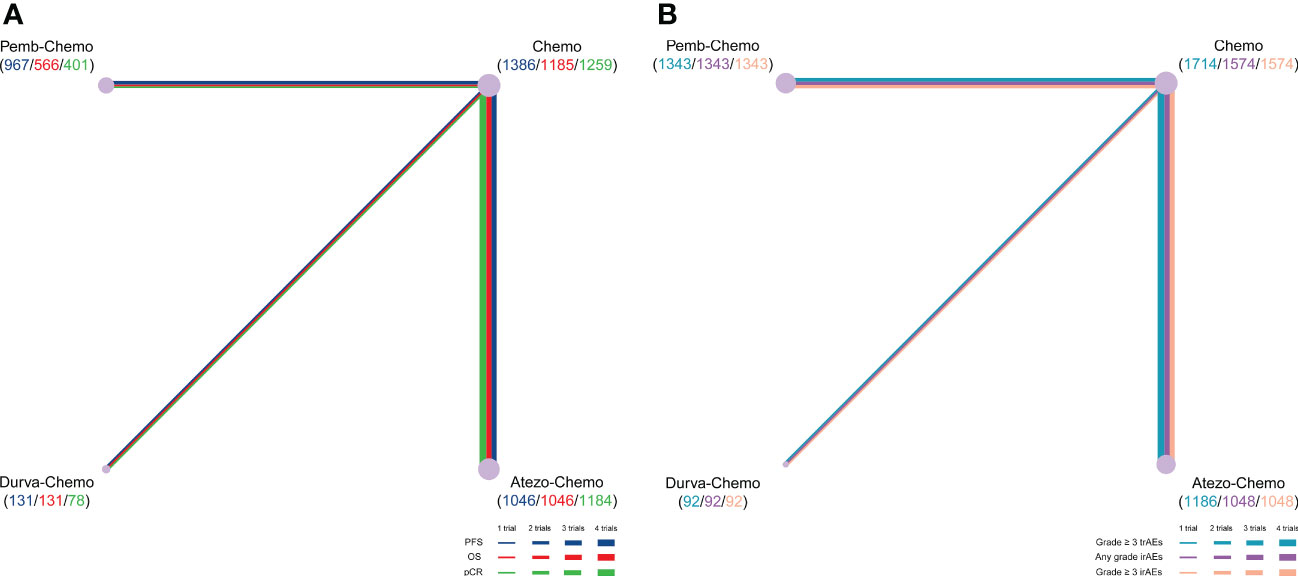
Figure 2 Comparative network plots for efficacy and toxicity of immunotherapy in patients with TNBC. Lines represent trials comparing 2 drugs or drugs for different outcomes. The nodes indicate the drug treatments assessed in existing trials. The size of the node and the width of the line are proportional to the number of randomized controlled trials and comparisons, respectively. Comparisons were generated by using the Bayesian framework on (A) PFS, OS, pCR, (B) any-grade trAEs, any-grade irAEs and grade ≥ 3 irAEs. Atezo, atezolizumab; Chemo, chemotherapy; Durva, durvalumab; irAEs, immune-related adverse events; OS, overall survival; pCR, pathologic complete response; Pemb, pembrolizumab; PFS, progression-free survival; TNBC, triple-negative breast cancer; trAEs, treatment-related adverse events.
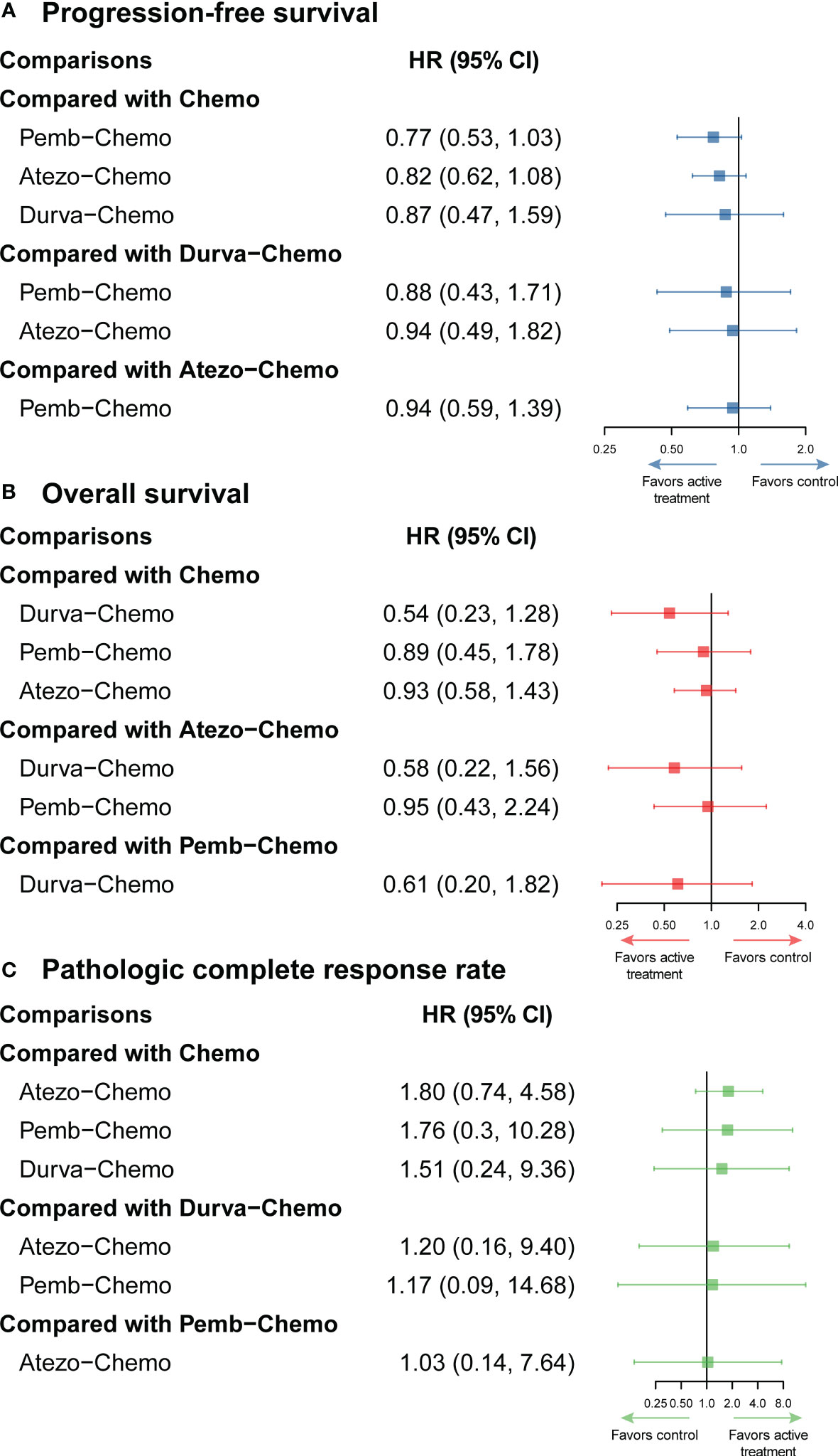
Figure 3 Efficacy profiles of the Bayesian network meta-analysis in patients with TNBC. (A) forest plot of progression-free survival; (B) forest plot of overall survival; (C) forest plot of pathologic complete response. Atezo, atezolizumab; Chemo, chemotherapy; CI confidence interval; Durva, durvalumab; HR, hazard ratio; Pemb, pembrolizumab; TNBC, triple-negative breast cancer.
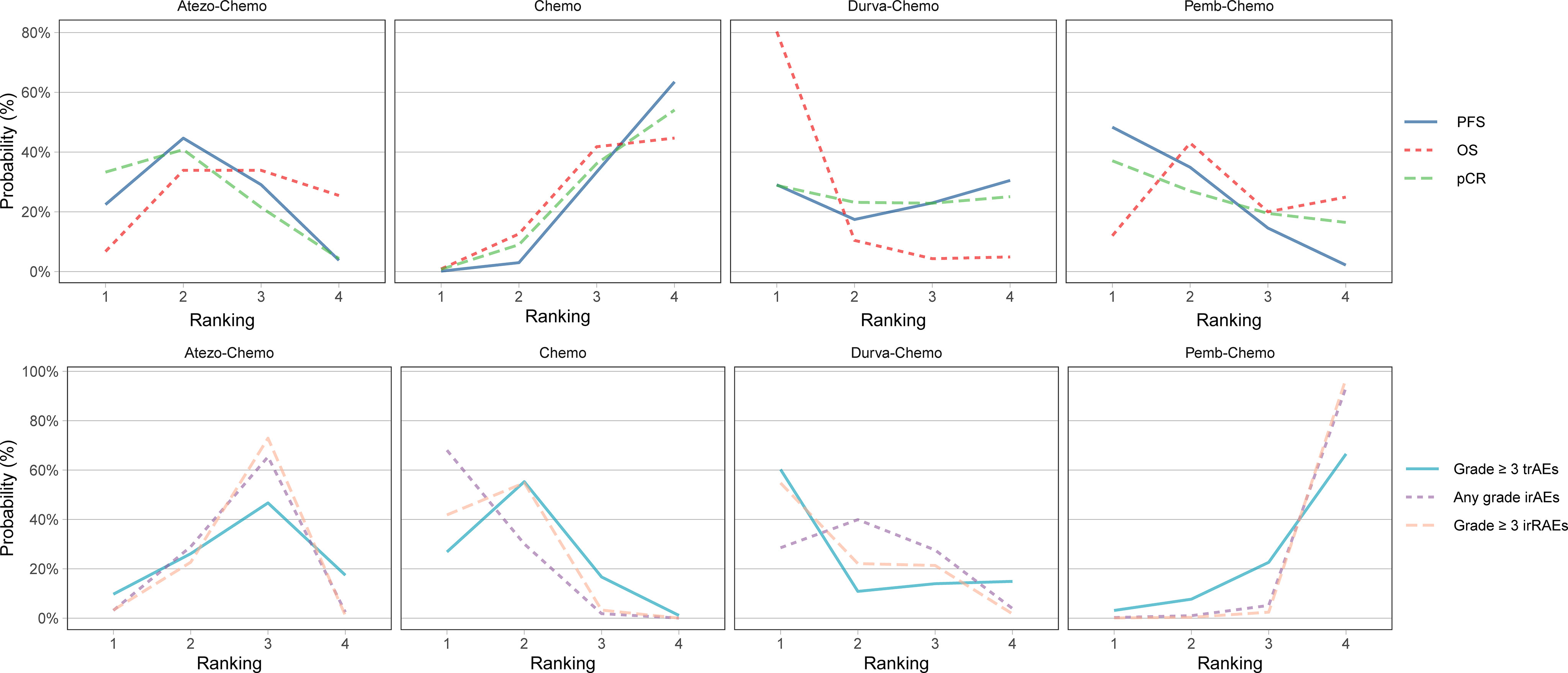
Figure 4 Bayesian ranking profiles for ICIs on efficacy and safety for patients with TNBC. Ranking plots indicate the probability of each comparable immunotherapy combination being ranked from first to last on PFS, OS, pCR, any-grade trAEs, any-grade irAEs and grade ≥ 3 irAEs. The graphs display the distribution of probabilities of treatment ranking from best through worst for each outcome. Ranking indicates the probability that drug class is first “best,” second “best,” etc. Atezo, atezolizumab; Chemo, chemotherapy; Durva, durvalumab; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; OS, overall survival; pCR, pathologic complete response; Pemb, pembrolizumab; PFS, progression-free survival; TNBC, triple-negative breast cancer; trAEs, treatment-related adverse events.
A specific subgroup analysis for the PFS endpoint was conducted in the PD-L1 positive population (Supplementary Figure 2). Similar results revealed that no significant improvement was found among the treatment agents in the PD-L1-positive population (Supplementary Figure 3). Based on Bayesian ranking profiles, Atezo-Chemo was ranked first for PFS, followed by Pemb-Chemo (Supplementary Figure 4). Further subgroup analysis was performed in patients with metastatic TNBC, and the results showed no significant improvement among the comparisons (Supplementary Figure 5).
Comparisons of overall survival
NMA included 4 treatments for OS in patients with TNBC (Figure 2). All of the evaluated comparisons for OS were statistically non-significant in the ITT population (Figure 3). Based on Bayesian ranking profiles, Durva-Chemo was ranked first for OS, followed by Pemb-Chemo and Atezo-Chemo (Figure 4).
A specific subgroup analysis was carried out in the PD-L1 positive population, the HRs of OS were not statistically significant among the comparison (Supplementary Figure 6). Based on Bayesian ranking profiles, Atezo-Chemo was ranked first for OS, followed by Pemb-Chemo (Supplementary Figure 4). Further subgroup analysis was performed in patients with metastatic TNBC, and the results showed no significant improvement among the comparisons (Supplementary Figure 7).
Comparisons of pathologic complete response rate
The pCR rate was analyzed in 6 studies with 4 treatments in patients with TNBC (Figure 2). However, the results presented no significance in the comparison of the ITT population (Figure 3). Based on Bayesian ranking profiles, Atezo-Chemo was ranked first for pCR, followed by Pemb-Chemo and Durva-Chemo (Figure 4).
The subgroup analysis was performed in the PD-L1 positive population and the favorable tendency of ICI for the treatment of pCR; however, the results showed no significantly different (Supplementary Figure 8). Based on Bayesian ranking profiles, Atezo-Chemo was ranked first for pCR, followed by Pemb-Chemo and Durva-Chemo (Supplementary Figure 4).
Safety analysis
Overall, 7 studies including 4335 patients were included in the safety analysis (Figure 2).
In accordance with the safety analysis, an analysis of grade ≥ 3 trAEs has been conducted. No significant difference was found among the treatment agents in grade ≥ 3 trAEs (Figure 5). Based on Bayesian ranking profiles, Pemb-Chemo was ranked worst for grade ≥ 3 trAEs, followed by Atezo-Chemo (Figure 4).
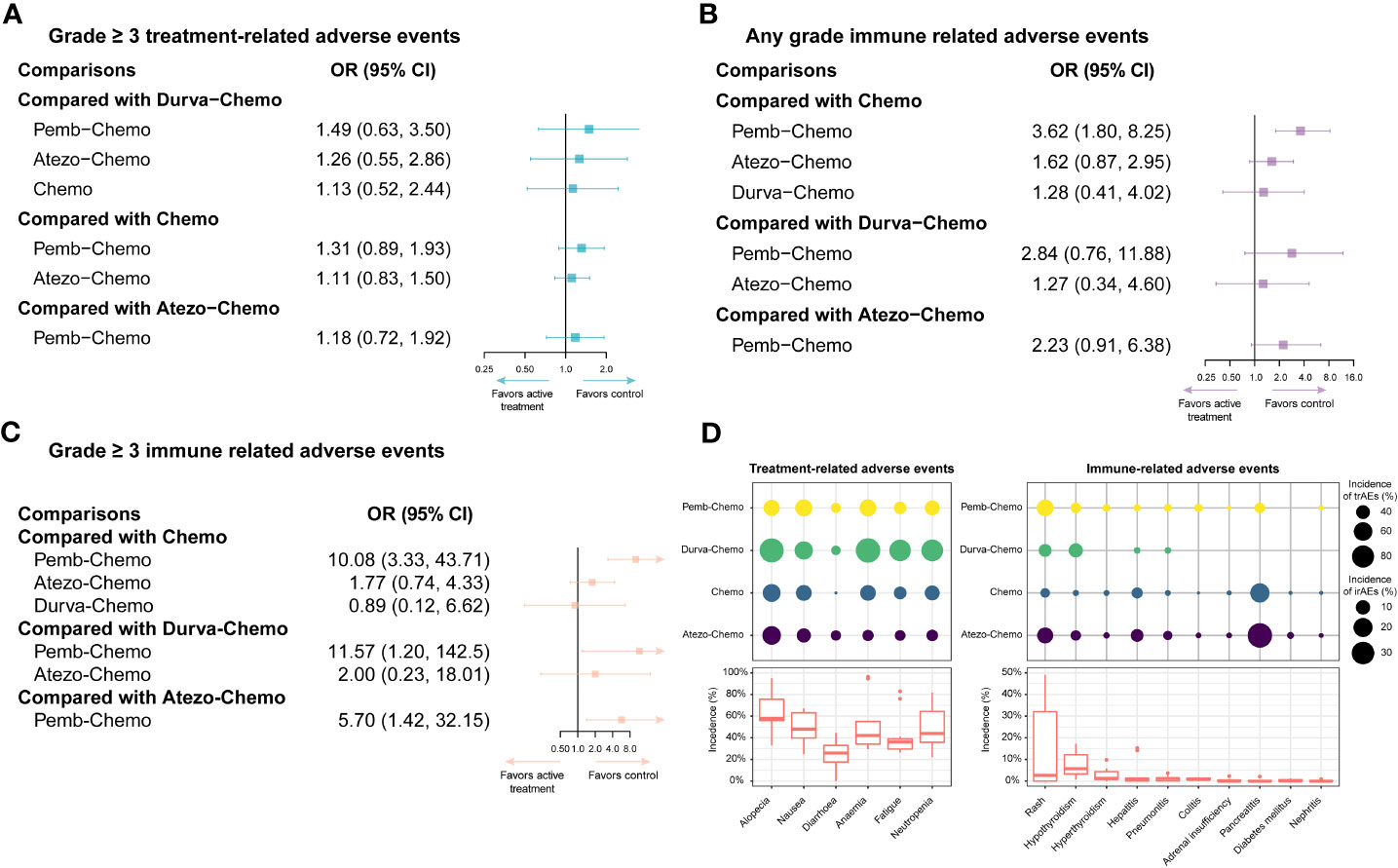
Figure 5 Safety profiles of the Bayesian network meta-analysis in patients with TNBC. (A) forest plot of any-grade trAEs; (B) forest plot of any-grade irAEs; (C) forest plot of grade ≥ 3 irAEs; (D) incidence of treatment-related and immune-related AEs. Atezo, atezolizumab; Chemo, chemotherapy; CI confidence interval; Durva, durvalumab; irAEs, immune-related adverse events; OS, overall survival; HR, hazard ratio; pCR, pathologic complete response; Pemb, pembrolizumab; PFS, progression-free survival; TNBC, triple-negative breast cancer; trAEs, treatment-related adverse events.
In terms of any grade irAEs, compared with Chemo, Pemb-Chemo was related higher incidence of irAEs and the result was statistically significant (Figure 5). Based on Bayesian ranking profiles, Pemb-Chemo was ranked worst for any grade irAEs, followed by Atezo-Chemo and Durva-Chemo (Figure 4).
In terms of grade ≥ 3 irAEs, compared with Chemo, Durva-Chemo, and Atezo-Chemo, Pemb-Chemo was significantly related to a higher incidence of grade ≥ 3 irAEs (Figure 5). Based on Bayesian ranking profiles, Pemb-Chemo was ranked worst for grade ≥ 3 irAEs, followed by Atezo-Chemo and Durva-Chemo (Figure 4).
We conducted a comprehensive analysis of the included studies in order to estimate the frequency of certain trAEs and irAEs. The most frequent trAEs were alopecia (60.20% with Atezo-Chemo, 48.92% with Pemb-Chemo and 92.93% with Durva-Chemo) followed by nausea and anemia (Figure 5). There was a relatively uniform frequency of the most common trAEs of any grade between the treatment arms (Figure 5). In addition, the most common irAEs associated with ICI were hypothyroidism (13.24% with Atezo-Chemo, 14.59% with Pemb-Chemo, and 7.61% with Durva-Chemo) followed by hyperthyroidism (Figure 5).
Discussion
To the best of our knowledge, this is the first NMA to evaluate the efficacy and safety profiles of currently available ICI treatments for patients with TNBC. As part of our study, we aimed to discuss the potential clinical implications of ICIs in this setting as well as some controversial aspects, such as PD-L1 positive patients. A controversial issue is whether immunotherapy can enhance the therapeutic effect of original standard chemotherapy (8, 9). The present study evaluated ICIs in combination with chemotherapy as the first-line treatment for TNBC in all included studies. Concomitant chemotherapy has been shown to enhance the anticancer effect of ICI by increasing the release of cancer antigens (32). In spite of this, the question of which immunomodulatory therapy will be more effective when combined with chemotherapy remains an open question. Several research studies are underway in an effort to find treatments that mobilize and activate antitumor T cells and/or move immune suppression in the direction of immune activation.
Considering this background, to determine the efficacy and safety of ICIs in patients with TNBC, a systematic review and NMA of RCTs were performed. This NMA yielded two key findings regarding the efficacy and safety of ICIs for the treatment of TNBC. First, ICIs plus chemotherapy showed a trend toward better outcomes in terms of PFS, OS, and pCR, especially for the PD-L1 positive population. However, the results of this study were shown no significant difference among the comparison. It is clear that ICI plus chemotherapy had a trend of better survival advantage than chemotherapy alone as survival increased with the extension of OS and PFS. There was a higher incidence of grade ≥ 3 trAEs, any grade irAEs, and grade ≥ 3 irAEs in the ICI plus chemotherapy group compared to the control group. Second, Bayesian ranking is a useful tool to rank the probability of each treatment. Based on the Bayesian ranking profile, Atezo-Chemo was related to higher rank probability in terms of PFS, OS, and pCR in the PD-L1 positive population. Pemb-Chemo was associated with a relatively higher probability in terms of PFS, OS, and pCR in the ITT population. As for the safety profile, Pemb-Chemo was related relatively to higher risk in terms of grade ≥ 3 trAEs any grade irAEs, and grade ≥ 3 irAEs, followed by Atezo-Chemo. Therefore, we suggested that Pemb-Chemo and Atezo-Chemo are better with longer survival for ITT TNBC population or PD-L1 positive TNBC population; however, those treatments had less favorable safety profiles. Hence, ICIs plus chemotherapy should be used cautiously. Based on the results, it appears that the combination of ICIs and chemotherapy trend to the better OS, PFS, and pCR rates. Immunotherapeutic approaches to TNBC are justified by a number of factors. There are two possible explanations for the benefit of ICIs in combination with chemotherapy. First, it is important to note that ICIs kill tumor cells by activating tumor immunity, which is different from chemotherapy and plays a synergetic role in the treatment of TNBC, especially in PD-L1 positive patients (28, 33). Second, in early breast cancer, the antitumor effect may be more significant than in metastatic disease due to the robustness of the tumor’s immune microenvironment (34).
Several traditional pairwise meta-analyses that evaluated the efficacy and safety profiles of ICI for patients with TNBC have been published. It has been reported in two previous publications that ICIs added to chemotherapy are associated with improved PFS and OS in TNBC patients with PD-L1 positivity, and those findings were similar to our findings. ICIs plus chemotherapy appears to be better than chemotherapy alone for TNBC treatment, with better OS and PFS, especially for PD-L1 positive population, and its high rates of serious AEs need to be taken seriously (35, 36). In another study, PD-1 and PD-L1 inhibitors were combined with chemotherapy as a treatment for TNBC (37). The results of this study demonstrated a significant pCR benefit and confirmed that PD-1/PD-L1 ICIs plus chemotherapy may improve the PFS of PD-1/PD-L1 patients in both neoadjuvant and adjuvant settings, with tolerable safety events. In the last study, platinum-based chemotherapy and immunotherapy were evaluated in early TNBC, and the results showed that ICIs plus chemotherapy increased the pCR rate and reduced adverse effects when compared to platinum-based chemotherapy (38).
There are several differences between the previously published and the current publication mainly in the following aspects. First, our study is an NMA, standard pairwise meta-analysis is limited to comparing two drug categories that have been evaluated in head-to-head trials. Several treatment options exist for a complex condition, many of which have not been directly compared in clinical trials. The theoretical advantage of NMA is based on the integration of direct evidence from studies directly comparing a particular treatment comparison with indirect evidence from pathways with at least one intermediate comparator within a single framework that ranks treatments by efficacy and safety (39). Second, compared with chemotherapy alone, single ICIs plus chemotherapy showed a trend of benefit without significant differences. Third, subgroup analyses of PD-L1-positive TNBC patients treated with ICIs plus chemotherapy also showed a trend of benefit with statistically non-significant. Fourth, our study included and analyzed both early and metastatic TNBC. Lastly, the discrepancies between trAEs and irAEs in these treatments were examined in detail in order to identify those AEs that require additional attention when they are combined with ICIs.
Implications
The purpose of this study is to summarize the findings of RCTs in order to provide clinicians with a reference source to evaluate the strengths and weaknesses of several promising options that are available for practice. When taking both efficacy and safety into consideration, Pemb-Chemo and Atezo-Chemo seem to be superior first-line treatments for patients with TNBC with ITT and PD-L1 positive, respectively. Nevertheless, there is a need for further trials in which head-to-head comparisons are conducted, such as Pemb-Chemo versus Atezo-Chemo versus Durva-Chemo or ICIs monotherapy. In addition, these findings may help answer the question of whether ICIs should be included in the standard care of patients with TNBC and which treatment is most appropriate for those with no actionable mutations.
Limitations
This study has some limitations that need to be addressed. First, we analyzed published results rather than individual patient data. Second, In the included trials, the method of assessing PD-L1 was different. For example, whereas PD-L1 was evaluated in the KEYNOTE 355 trial by IHC 22C3 pharmDx assay and characterized by CPS, PD-L1 positivity in IMpassion trials was determined by immune cell staining of 1% in accordance with VENTANA PD-L1 SP142 immunohistochemical testing. As a result, it is necessary to harmonize PD-L1 testing across clinical trials in order to address this issue in clinical studies of immunotherapy for both early and metastatic breast cancer. This suggests that it is prudent to interpret the aggregate findings of PD-L1 positive populations cautiously. Third, PFS and OS were not primary outcomes in GeparNuevo and NeoTRIP Michelangelo trials and subgroup analysis data for PFS and OS has not been reported for half of the included trials. Therefore, in order to strengthen the results of this study, future studies should include RCTs with a greater number of participants. Fourth, patients with TNBC who lack genetic alterations can normally be treated with taxane or platinum-based chemotherapy as a first-line standard systemic treatment. The chemotherapeutic strategies in this study were varied and included both taxane and platinum chemotherapy. The combination of different chemotherapy regimens with immunotherapy may therefore have different synergistic effects. Finally, the 95% intervals generated by the Bayesian approach are slightly wider than those under the traditional pairwise meta-analysis a function of the prior used. While the size of the discrepancies was heavily dependent on the number of studies available and the amount of heterogeneity in the data, another key driver for the difference was the “prior information” used in the Bayesian analyses for random-effects variance. In this study, due to the limitation of the number of included studies, no statistically significant differences in most of outcomes. So generally, little is lost in basing conclusions about the treatment comparisons on the potentially more conservative 95% intervals generated by the Bayesian approach.
Conclusion
In summary, our study demonstrated the efficacy and safety of different ICIs in the treatment of TNBC. When taking both efficacy and safety into consideration, Pemb-Chemo and Atezo-Chemo seem to be superior first-line treatments for patients with TNBC with ITT and PD-L1-positive populations, respectively. Nevertheless, both ICIs plus chemotherapy schedules were associated with a higher risk of trAEs or irAEs. It may be useful for making clinical decisions based on this information.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Study concept and design: XL and YL. Data acquisition and management: XL and XC. Statistical analysis: XL and XC. Interpretation of data: HL. Drafting of the manuscript: XL. Critical revision of the manuscript for important intellectual content: XC and YL. Study supervision: YL and HL.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82160763).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1137464/full#supplementary-material
Supplementary Figure 1 | Assessment of Risk of Bias using Cochrane Risk of Bias Tool 2.0.
Supplementary Figure 2 | Comparative network plots for efficacy of ICIs in patients with PD-L1-positive TNBC.
Supplementary Figure 3 | Forest plot of progression-free survival of ICIs in patients with PD-L1-positive TNBC.
Supplementary Figure 4 | Bayesian ranking profiles for ICIs on efficacy and safety for patients with PD-L1-positive TNBC.
Supplementary Figure 5 | Forest plot of progression-free survival of ICIs in patients with metastatic TNBC.
Supplementary Figure 6 | Forest plot of overall survival of ICIs in patients with PD-L1-positive TNBC.
Supplementary Figure 7 | Forest plot of overall survival of ICIs in patients with metastatic TNBC.
Supplementary Figure 8 | Forest plot of pathologic complete response rate of ICIs in patients with PD-L1-positive TNBC.
References
1. Scott LC, Mobley LR, Kuo TM, Il'yasova D. Update on triple-negative breast cancer disparities for the united states: a population-based study from the united states cancer statistics database, 2010 through 2014. Cancer (2019) 125(19):3412–17. doi: 10.1002/cncr.32207
2. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med (2010) 363(20):1938–48. doi: 10.1056/NEJMra1001389
3. Wild SA, Cannell IG, Nicholls A, Kania K, Bressan D, Hannon GJ, et al. Clonal transcriptomics identifies mechanisms of chemoresistance and empowers rational design of combination therapies. Elife (2022) 11:e80981. doi: 10.7554/eLife.80981
4. Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol (2014) 15(12):1351–60. doi: 10.1016/s1470-2045(14)70444-9
5. Brodowicz T, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, et al. Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses. Br J Cancer (2014) 111(11):2051–7. doi: 10.1038/bjc.2014.504
6. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (2014) 384(9938):164–72. doi: 10.1016/s0140-6736(13)62422-8
7. Sadeghalvad M, Mohammadi-Motlagh HR, Rezaei N. Immune microenvironment in different molecular subtypes of ductal breast carcinoma. Breast Cancer Res Treat (2021) 185(2):261–79. doi: 10.1007/s10549-020-05954-2
8. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(6):691–722. doi: 10.6004/jnccn.2022.0030
9. Denduluri N, Somerfield MR, Chavez-MacGregor M, Comander AH, Dayao Z, Eisen A, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO guideline update. J Clin Oncol (2021) 39(6):685–93. doi: 10.1200/jco.20.02510
10. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol (2014) 25(8):1544–50. doi: 10.1093/annonc/mdu112
11. Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev (2014) 23(12):2965–70. doi: 10.1158/1055-9965
12. Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov (2020) 10(12):1808–25. doi: 10.1158/2159-8290
13. Wein L, Luen SJ, Savas P, Salgado R, Loi S. Checkpoint blockade in the treatment of breast cancer: current status and future directions. Br J Cancer (2018) 119(1):4–11. doi: 10.1038/s41416-018-0126-6
14. Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): where to go from here. Cancer (2018) 124(10):2086–103. doi: 10.1002/cncr.31272
15. Michel LL, von Au A, Mavratzas A, Smetanay K, Schütz F, Schneeweiss A. Immune checkpoint blockade in patients with triple-negative breast cancer. Target Oncol (2020) 15(4):415–28. doi: 10.1007/s11523-020-00730-0
16. Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol (2019) 5(1):74–82. doi: 10.1001/jamaoncol.2018.4224
17. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort a of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30(3):397–404. doi: 10.1093/annonc/mdy517
18. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
19. Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol (2021) 32(8):983–93. doi: 10.1016/j.annonc.2021.05.355
20. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2020) 21(1):44–59. doi: 10.1016/s1470-2045(19)30689-8
21. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomized, placebo-controlled, double-blind, phase 3 clinical trial. Lancet (2020) 396(10265):1817–28. doi: 10.1016/s0140-6736(20)32531-9
22. Franzoi MA, Romano E, Piccart M. Immunotherapy for early breast cancer: too soon, too superficial, or just right? Ann Oncol (2021) 32(3):323–36. doi: 10.1016/j.annonc
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
24. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (2019) 366:l4898. doi: 10.1136/bmj.l4898
25. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (2020) 396(10257):1090–100. doi: 10.1016/s0140-6736(20)31953-x
26. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol (2021) 32(8):994–1004. doi: 10.1016/j.annonc.2021.05.801
27. Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med (2022) 387(3):217–26. doi: 10.1056/NEJMoa2202809
28. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
29. Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, et al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med (2021) 27(2):250–5. doi: 10.1038/s41591-020-01189-2
30. Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol (2019) 30(8):1279–88. doi: 10.1093/annonc/mdz158
31. Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol (2022) 33(5):534–43. doi: 10.1016/j.annonc.2022.02.004
32. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
33. Li S, Liu M, Do MH, Chou C, Stamatiades EG, Nixon BG, et al. Cancer immunotherapy via targeted TGF-β signaling blockade in T(H) cells. Nature (2020) 587(7832):121–5. doi: 10.1038/s41586-020-2850-3
34. Hutchinson KE, Yost SE, Chang CW, Johnson RM, Carr AR, McAdam PR, et al. Comprehensive profiling of poor-risk paired primary and recurrent triple-negative breast cancers reveals immune phenotype shifts. Clin Cancer Res (2020) 26(3):657–68. doi: 10.1158/1078-0432.Ccr-19-1773
35. Villacampa G, Tolosa P, Salvador F, Sánchez-Bayona R, Villanueva L, Dienstmann R, et al. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: a systematic review and meta-analysis. Cancer Treat Rev (2022) 104:102352. doi: 10.1016/j.ctrv.2022.102352
36. Ji Q, Ding J, Hao M, Luo N, Huang J, Zhang W. Immune checkpoint inhibitors combined with chemotherapy compared with chemotherapy alone for triple-negative breast cancer: a systematic review and meta-analysis. Front Oncol (2021) 11:795650. doi: 10.3389/fonc.2021.795650
37. Qi Y, Zhang W, Jiang R, Xu O, Kong X, Zhang L, et al. Efficacy and safety of PD-1 and PD-L1 inhibitors combined with chemotherapy in randomized clinical trials among triple-negative breast cancer. Front Pharmacol (2022) 13:960323. doi: 10.3389/fphar.2022.960323
38. He Q, Peng Y, Sun J, Liu J. Platinum-based chemotherapy and immunotherapy in early triple-negative breast cancer: a meta-analysis and indirect treatment comparison. Front Oncol (2021) 11:693542. doi: 10.3389/fonc.2021.693542
Keywords: immune checkpoint inhibitors, atezolizumab, pembrolizumab, durvalumab, triple-negative breast cancer, network meta-analysis, anti-PD1/PD-L1
Citation: Liang X, Chen X, Li H and Li Y (2023) Immune checkpoint inhibitors in first-line therapies of metastatic or early triple-negative breast cancer: a systematic review and network meta-analysis. Front. Endocrinol. 14:1137464. doi: 10.3389/fendo.2023.1137464
Received: 04 January 2023; Accepted: 28 April 2023;
Published: 09 May 2023.
Edited by:
Zoe Quandt, University of California, San Francisco, United StatesReviewed by:
Ronald Cohen, The University of Chicago, United StatesAgostina Stradella, Catalan Institute of Oncology, Spain
Shuvadeep Ganguly, All India Institute of Medical Sciences, India
Copyright © 2023 Liang, Chen, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, bGl5YW4yMDEwMjAxMEBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Xueyan Liang, orcid.org/0000-0002-4043-6066
Xiaoyu Chen, orcid.org/0000-0002-2924-3618
Huijuan Li, orcid.org/0000-0002-1070-3776
Yan Li, orcid.org/0000-0003-3901-0727
 Xueyan Liang
Xueyan Liang Xiaoyu Chen
Xiaoyu Chen Huijuan Li1‡
Huijuan Li1‡ Yan Li
Yan Li