
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 09 May 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1136120
This article is part of the Research Topic Gender Pressure in Cancer: Susceptibility, Progression and Treatment of the Neoplastic Disease View all 5 articles
Background: We attempted to examine the clinical characteristics in patients with breast cancer (BC) and thyroid cancer (TC); explore the potential mechanisms of tumorigenesis and progression.
Methods: Using the Surveillance, Epidemiology, and End Result Program-9 (SEER-9) database, a retrospective study (1975-2017) was conducted on patients with BC and TC. We identified the common differentially expressed genes involved in BC and TC using the Gene Expression Omnibus database (GEO). Immunohistochemical staining (IHC) was performed to verify the expression of the hit gene in patients with co-occurrence of BC and TC. Using The Cancer Genome Atlas (TCGA) database, the relationship between gene expression and clinicopathological characters was determined. Gene set enrichment analysis (GSEA) was used to identify the pathways enriched in BC and TC.
Results: BC patients had a higher predisposition to develop TC (standardized incidence ratio, SIR: 1.29) and vice-versa (SIR: 1.12). Most of these patients were differentiated thyroid carcinoma (DTC) and hormone receptor (HR) - positive BC. The mRNA expression of COMP (Cartilage oligomeric matrix protein) was significantly overexpressed in BC and TC by analyzing the GEO database. The protein expression of COMP was increased in both BC and TC tissues obtained from the same patients validated by IHC. COMP was correlated with worse OS in BC (stage II-IV) and TC; it was the independent factor for prognosis of BC. GSEA indicated that the estrogen response and epithelial-mesenchymal transition (EMT) pathways were significantly enriched in both TC- and BC- COMP overexpressed groups.
Conclusion: The co-occurrence risk of BC and TC in the same individual is higher than in the general population. Overexpression of COMP could promote oncogenesis and progression in patients with BC and TC through estrogen signaling and EMT pathways.
Globally, breast cancer (BC) is the most commonly diagnosed malignancy in females, accounting for about 25.1% of all cancer diagnoses (1). It is reported that BC survivors have an increased risk of developing a second primary tumor, in particular, thyroid cancer (TC) (2, 3). Although the global incidence of TC is on the rise in recent decades, the patients have relatively higher survival rates. However, survivors of TC have a higher risk of developing BC than the general population. The risk of BC in TC survivors increased from 21% to 89%, while that of TC in BC survivors increased from 31% to 73% (4).
Previous studies have reported co-incidences of BC and TC occurring either synchronously or as metachronous malignancies. A retrospective case-controlled study by An et al. (4) reports that in the five-year follow up of 4243 TC patients, 55 developed BC; the standardized incidence ratio (SIR) was 2.45. Conversely, in 6.2-year follow up of 6833 BC patients, 88 had subsequently developed TC; SIR of 2.18. The expression of estrogen and progesterone receptors (ER and PR) in the tissues of BC patients with TC was significantly higher than those of patients with BC only (5). In the case of TC co-occurring with BC, smaller tumor and higher follicular thyroid cancer (FTC) is reported in comparison with TC-only patients (6).
Taken together, these studies suggest that BC and TC may have some common etiologies, such as genetic susceptibility, hormonal and environmental factors. Previous studies have reported the association between thyroid hormones and breast cancer risk; abnormally high free-thyroxine is positively correlated while higher thyroid-stimulating hormone level in the euthyroid range is negatively correlated with breast cancer (7–9). Higher estrogen level and obesity are also implicated in the development of TC and BC (10). Studies also reported that there is neither any significant increase in BC incidence after radioactive iodine treatment nor any significant increase in the risk of TC after radiotherapy for BC (11, 12). As for the genetic correlation, previous studies report that the mutations in the tumor suppressor PTEN are correlated with Cowden syndrome, and significantly increases the risk of breast and thyroid cancers (13). In addition, PARP4 germline mutations are implicated as potential susceptibility factors in synchronous thyroid and breast cancers (14). Common pathogenic factors for BC and TC are not clearly understood. In the present study, we investigate the association between the two cancers and the underlying genetic factors for tumorigenesis and progression of the malignancies. We found that the risk of subsequently developing BC or TC in TC or BC patients, respectively, was higher than in general population. COMP (Cartilage oligomeric matrix protein) was significantly overexpressed in BC and TC, which was an independent factor for the prognosis of BC. COMP overexpression had significantly enriched the estrogen response and EMT pathways. These results of this study may have implications in clinical diagnosis, treatment, and prognosis of the associated tumors.
Data collection was done from the SEER-9 database (https://seer.cancer.gov/) using the SEER*Stat software. The database includes approximately 10% population of the United States. To analyze the association between TC and BC, we identified patients according to primary tumor site codes; C73.9 for TC and C50.0-C50.9 for BC. From 1975 to 2017, a total of 1982 TC patients had subsequently developed BC and 2098 BC patients had developed TC. Tumor histology, incidence rate, and survival information of the double primary malignancies were collected and analyzed.
SIR was calculated using the multiple primary-SIR program of SEER*Stat and used to compare the incidence risk of BC or TC in previously diagnosed BC or TC patients with that in normal population. SIR = standardized incidence rate of BC (TC) patients to develop TC (BC)/standardized incidence rate of the general population to develop TC (BC). SIR > 1 and p-value < 0.05 suggested that patients with BC or TC have a significantly higher risk of developing secondary tumors than the normal population.
Patients with coexisting breast and thyroid cancers were grouped by primary cancer, the histological subtype of TC, and the hormonal status of BC for survival analysis. Survival curves were plotted using the Kaplan-Meier method. Data analysis was performed using SPSS v25 (SPSS Inc. Chicago, Illinois, USA), and p < 0.05 was considered statistically significant.
Two gene expression datasets (GSE3467 (15) and GSE61304 (16)) were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Paired, 9-papillary thyroid carcinoma (PTC) and 9-normal tissue samples were obtained from GSE3467. Sixty-two-breast tumor samples and 4-normal breast epithelium samples were obtained from GSE61304. The platform used for both datasets was GPL570 [HG-U133_Plus_2] (17) Affymetrix Human Genome U133 Plus 2.0 Array.
NetworkAnalyst (https://www.networkanalyst.ca/) was used to identify the differentially expressed genes (DEGs) in the breast-, thyroid-cancer, and normal tissue samples (P-value <0.05; |logFC| ≥1). Volcano plots were generated in R v4.0.3 using the ggplot2 package. Common DEGs from two datasets were prioritized using Draw Venn Diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/).
COMP mRNA-seq data for THCA and BRCA were downloaded from the TCGA database (https://portal.gdc.cancer.gov/). The package edgeR in R v4.0.3 was used to normalize the raw RNA counts and identify the difference in COMP expression between the normal and tumor tissues. Further, diagnostic capability of COMP in TC and BC was calculated using ROC curves.
Clinical data for TC and BC patients were obtained from the TCGA database. A total of 501 TC patients and 1090 BC patients had both the mRNA data and complete clinic information. The clinicopathological characteristics included TNM stage, tumor size, lymph node metastasis, distant metastasis, ER status, PR status, human epidermal growth factor receptor 2 (HER2) status, age, and gender. According to the optimal threshold of COMP in BC and TC (51.85 and 17.81 FPKM, respectively), patients were classified into two groups (high/low). The correlation between COMP expression and clinical characteristics was calculated by Chi-square test.
Kaplan-Meier (K-M) analysis was used to compare the overall survival (OS) between high and low expression COMP groups. Univariate and multivariate COX analyses were also performed to identify the independent factors related to OS.
GSEA was performed using TCGA (THCA and BRCA) datasets to identify the potential enriched pathways which were correlated with COMP overexpression. Pathways with p-value < 0.05 and the false discovery rate (FDR) < 0.25 and | Normalized Enrichment Score (NES)| ≥1 were considered significantly enriched.
Based on the TCGA database, The correlation of genes in the enriched pathways with COMP in TC and BC was evaluated by Spearman correlation analysis. Further, the expression and the correlation of these genes with OS in TC and BC were examined.
The paraffin-embedded BC, TC, and paired normal tissues of 7-double malignancies from patients coexisting with BC and TC who underwent surgeries were collected. Tissue sections were deparaffinized and antigen retrieval was done using citrate buffer. The sections were incubated overnight at 4°C with a rabbit polyclonal antibody against COMP (1:400; Abcam, Cambridge, UK). Diaminobenzidine was added to the sections and incubated at room temperature. Counterstaining was done with hematoxylin. Image-Pro Plus 6.0 was used to quantitatively analyze IHC images. The COMP level was represented by the average optical density (AOD). AOD = integrated optical density (IOD)/area.
R v4.0.3 and SPSS v25 (SPSS Inc. Chicago, Illinois, USA) were used for statistical analysis. Count data were expressed as rate (%), and measurement data were expressed as mean ± standard deviation. Chi-square analysis and Fisher’s exact test were used to compare categorical variables. The Wilcoxon and Kruskal-Wallis tests are used to compare continuous variables. The ROC curve was used to calculate the diagnostic capability of COMP for TC and BC. The survival curve was plotted using the Kaplan-Meier method. Univariate and multivariate COX regression were used to analyze the clinical features affecting the survival of BC and TC patients. Spearman correlation analysis was used to evaluate the correlation between genes in the COMP enrichment pathway and COMP in TC and BC. Image-Pro Plus 6.0 was used for the quantitative analysis of IHC images. COMP levels are expressed as average optical density (AOD). IHC results are plotted by GraphPad Prism 9. P <0.05 was the threshold of significance.
From 1975 to 2017, 2098 out of 87,1359 BC patients subsequently developed TC (BC-1st); median time between diagnosis of BC and subsequent diagnosis of TC was 69 months. During the same period, 1982 out of 97,530 TC patients subsequently developed BC (TC-1st). There were 72,896 female TC patients, of which 1975 were subsequently diagnosed with BC. Seven of 2463 male TC patients were diagnosed with BC. The median between the diagnoses was 111 months. Among BC-1st patients (Table 1), 55.1% BC patients were HR-positive and 91.3% of them subsequently developed DTC. In 73.5% TC-1st patients (Table 2), the subsequent BC subtype was HR-positive.
SIR was used to examine the theoretical linkages in the etiology of the two cancers, by comparison of subsequent cancer experiences with the number of cancers that would be expected based on incidence rates in the general population. SIR>1 and P<0.05 indicate that BC or TC survivors have a higher risk of developing another primary cancer than the general population. For BC-1st group (Table 3), the SIR was 1.29 (95% CI: 1.23-1.35) for both females and males. Male BC patients had higher SIR of 3.0 (95% CI: 1.50-5.37), while that of the females was 1.28 (95% CI: 1.22-1.35). On comparing the hormone receptor (HR) status, HR-positive BC had a higher risk of subsequent TC development as compared to HR-negative BC (1.37 and 1.18, respectively). For the TC-1st group (Table 4), the SIR was 1.12 (95% CI: 1.07-1.17) for both females and males. However, only the female TC patients had a significant SIR of 1.12 (95% CI: 1.07-1.17). Additionally, DTC patients had a higher risk of developing BC. Among them, the SIR of FTC patients was 1.23; the excess risk was 4.51, which was higher than that in PTC patients (1.11). Our results also indicated that in the advanced BC stages, SIR was higher for subsequent TC; in TC, stage III patients had significantly higher risk of developing BC.
Kaplan-Meier analyses were used to analyze the survival of patients in the BC-1st and TC-1st groups. Either the overall survival curves (Figure 1A) or cancer-specific (Death due to TC or BC) (Figure 1B) survival curves for BC-1st patients declined faster than that for TC-1st patients. The three-year survival rates of BC-1st and TC-1st patients were 97.6% and 98.9%, the five-year survival rates were 95.7% and 97.8%, and the ten-year survival rates were 88.2% and 92.5%, respectively.
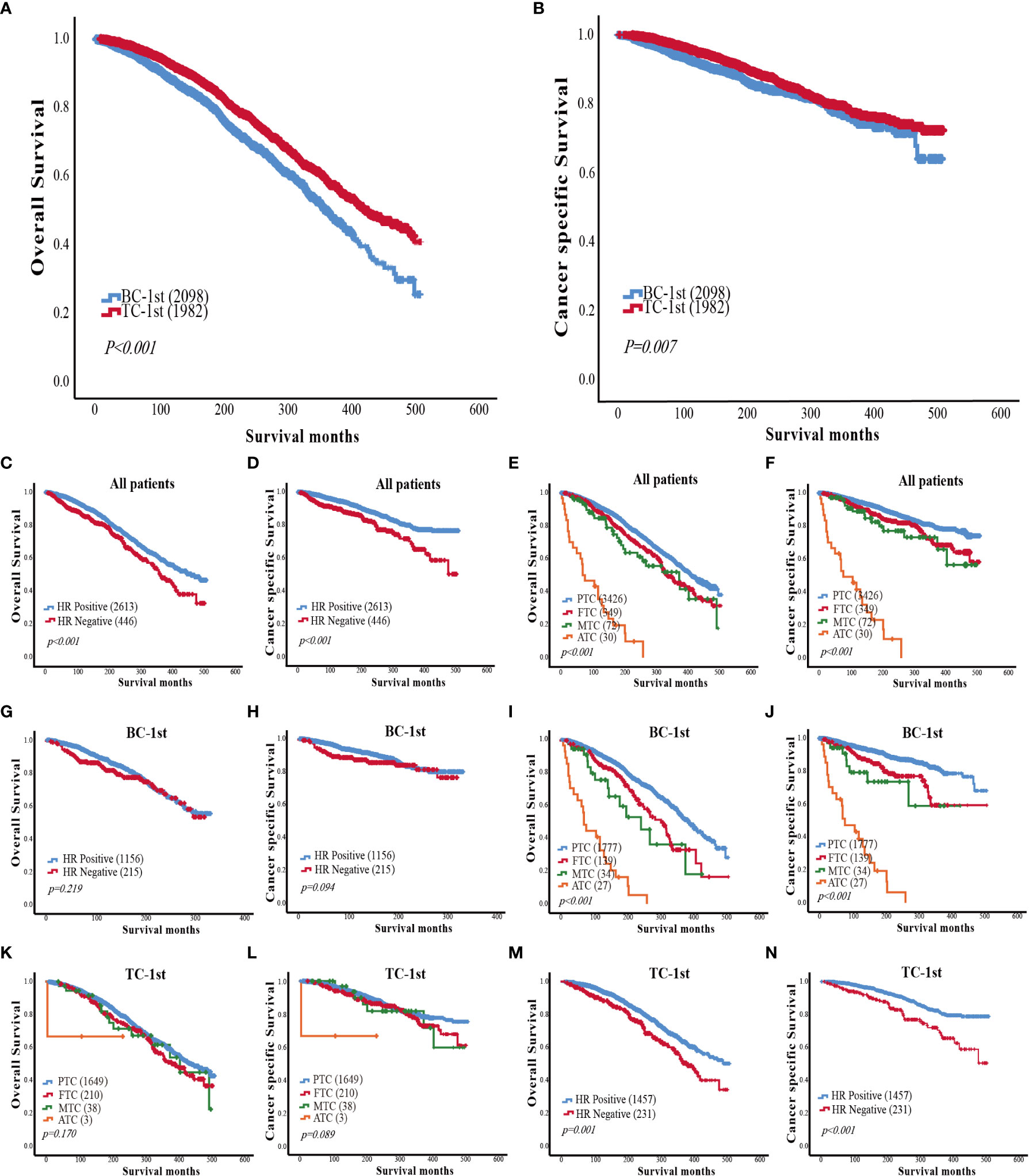
Figure 1 (A) Comparison of overall survival curves between BC-1st and TC-1st patients. (B) Comparison of cancer-specific survival curves between BC-1st and TC-1st patients. (C) Overall survival curves based on the HR status of patients with BC and TC. (D) Cancer-specific survival curves based on the HR status of patients with BC and TC. (E) Overall survival curves based on TC histological subtype of patients with BC and TC. (F) Cancer-specific survival curves based on TC histological subtype of patients with BC and TC. (G) Overall survival curves based on the HR status of BC-1st patients. (H) Cancer-specific survival curves based on the HR status of BC-1st patients. (I) Overall survival curves based on TC histological subtype of BC-1st patients. (J) Cancer-specific survival curves based on TC histological subtype of BC-1st patients. (K) Overall survival curves based on TC histological subtype of TC-1st patients. (L) Cancer-specific survival curves based on TC histological subtype of TC-1st patients. (M) Overall survival curves based on the HR status of TC-1st patients. (N) Cancer-specific survival curves based on the HR status of TC-1st patients.
Then we analyzed the impact of HR status and TC histological subtype on the survival of patients with BC and TC. The overall and cancer-specific survival curves of HR-negative patients declined significantly faster as compared to HR-positive patients (p<0.001) (Figures 1C, D). In the TC histological subtype, patients with PTC had the best survival, while patients with anaplastic thyroid carcinoma (ATC) had the worst survival. The survival of FTC patients was worse than that of PTC patients but better than the medullary thyroid carcinoma (MTC) patients (p<0.001) (Figures 1E, F).
In the BC-1st patients, the effects of HR status on overall survival and cancer-specific survival were not statistically significant (Figures 1G, H). However, PTC was correlated with a better OS in BC-1st patients (Figures 1I, J). In the TC-1st patients, there was no significant difference in survival among different histological subtypes of TC (Figures 1K, L). However, in the TC-1st patients, HR-positive BC patients had a better OS (p<0.001) (Figures 1M, N).
To explore the common genetic mechanism of BC and TC, differential expression analysis was performed. Using GSE61304, a total of 123 DEGs were identified; 29 genes were up-regulated while 94 were down-regulated in BC (Figure 2A). In the GSE3467 dataset, a total of 287 DEGs were identified; 161 genes were up-regulated while 126 were down-regulated in TC (Figure 2B). Common DEGs in BC and TC are depicted using a Venn diagram as shown in Figure 2C. A total of 16 common DEGs were obtained, including 5 genes which were up-regulated (COMP, CKS2, CLEC5A, LAMP5, INHBA) and 9 genes which were down-regulated (LIFR, LRRC2, CAVIN2, OCA2, CA4, SCN4B, EDN3, MAMDC2, CFD). Two DEGs were up-regulated in TC but down-regulated in BC (PHYHIP, LAMB3). A heat map was generated to represent the expression of these DEGs in the two datasets as shown in Figure 2D. Noticeable, COMP is significantly highly expressed in both BC and TC.
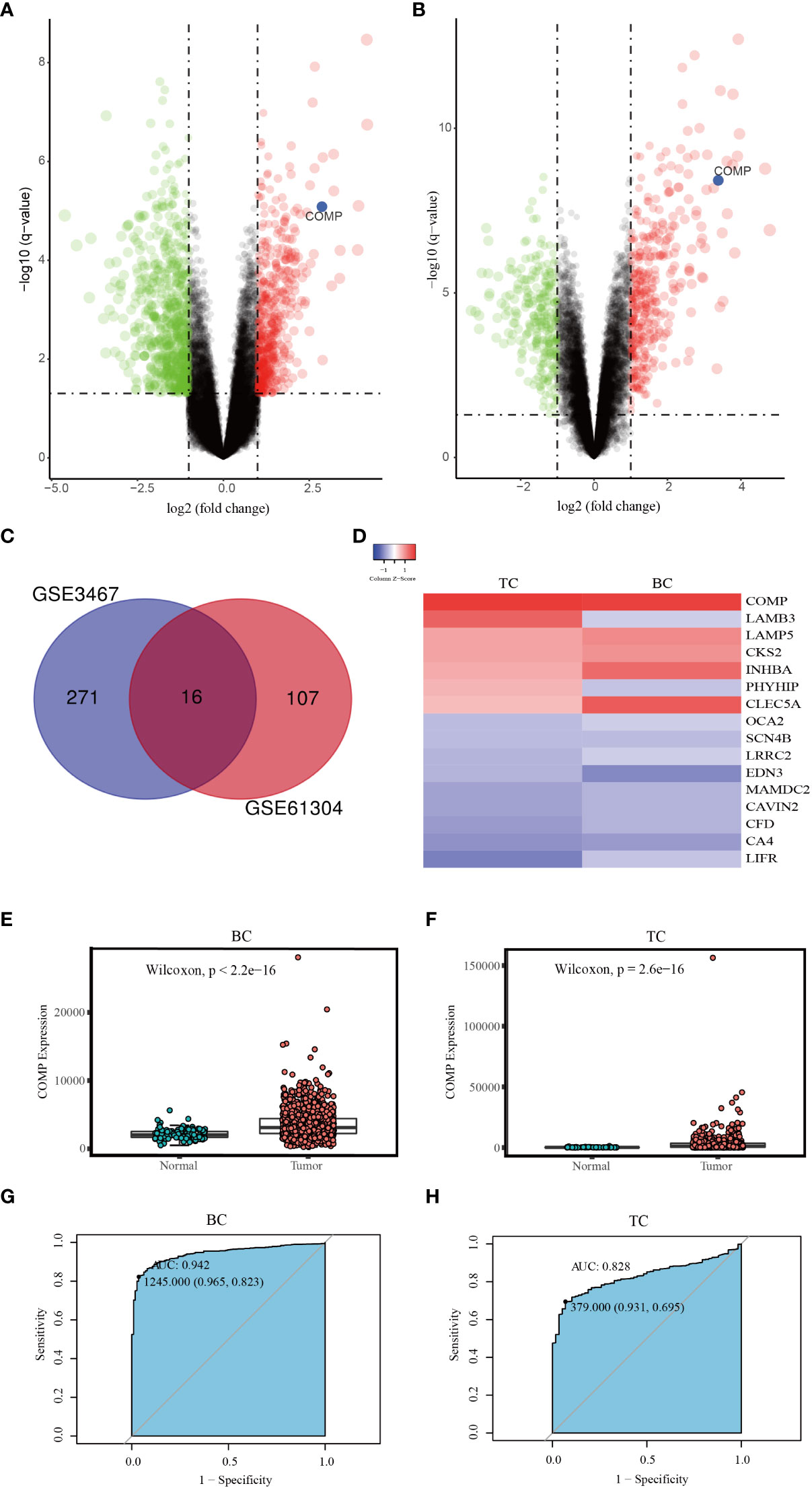
Figure 2 (A) Volcano map of DEGs between BC and normal tissues (GSE61304). (B) Volcano map of DEGs between TC and normal tissues (GSE3467). (C) The Venn diagram of all DEGs in the two datasets; (D) The expression level of DEGs in two datasets. (E) The differential expression of COMP between BC and normal tissues. (F) The differential expression of COMP between TC and normal tissues. (G) ROC curve of COMP upregulation for BC. (H) ROC curve of COMP upregulation for TC.
Then using data from the TCGA database, we further confirmed that the expression of COMP was significantly upregulated in BC as well as in the TC patients as compared to the normal tissues (Figures 2E, F). Thus, the diagnostic value of COMP for BC and TC patients was evaluated using ROC curves. The AUC of COMP for BC and TC was 0.942 and 0.828, respectively (Figures 2G, H).
In BC patients, there was a significant correlation between the expression of COMP and ER or PR status (Table 5). As shown in Table 6 COMP was significantly correlated with the TNM stage, tumor size, lymph nodes metastasis, and age of TC patients. Furthermore, the association between COMP expression and clinic characteristics of BC or TC patients was verified using the COMP mRNA expression data as a continuous variable (Figure 3).
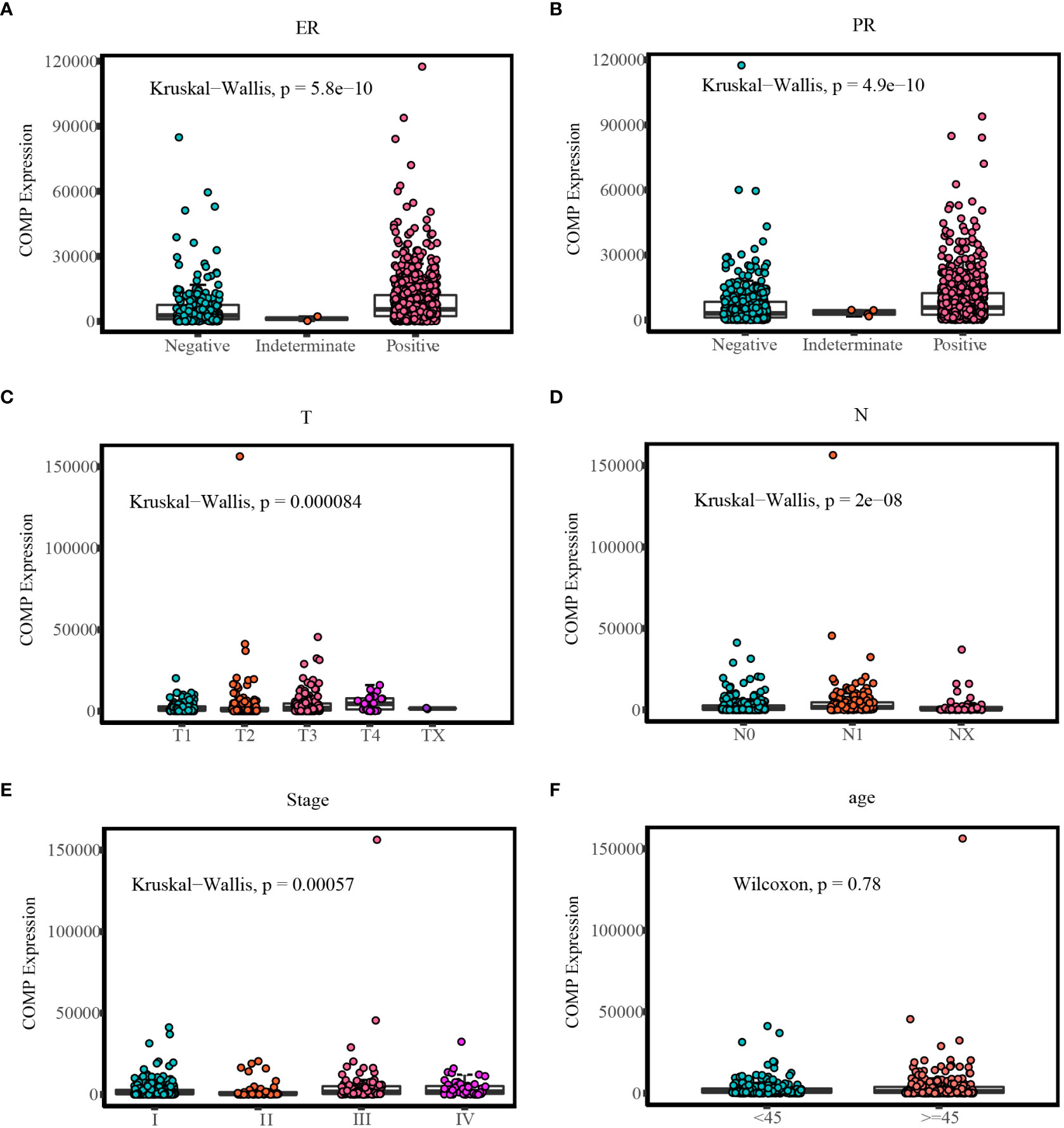
Figure 3 Comparison of COMP expression in different clinic groups: (A) Different COMP expression levels in ER status of BC; (B) Different COMP expression levels in PR status of BC; (C) Different COMP expression levels in T stages of TC; (D) Different COMP expression levels in N stages of TC; (E) Different COMP expression levels in TNM stages of TC; (F) Different COMP expression levels in age groups of TC.
Survival analysis based on TCGA data indicated that the expression of COMP was correlated with OS of patients in stage II-IV BC (p=0.048) (Figure 4A). Overexpression of COMP was significantly associated with poor OS of TC patients (p=0.025) (Figure 4B). In addition, Cox regression analysis showed that COMP levels, age, PR status, and lymph nodes metastasis were independent factors of poor OS in BC patients (Table 7). In the TC patients, univariate analysis showed that COMP, age, tumor size, distance metastasis, and TNM stage were correlated with OS. However, in multivariate analysis, COMP was not an independent factor of OS (Table 8).
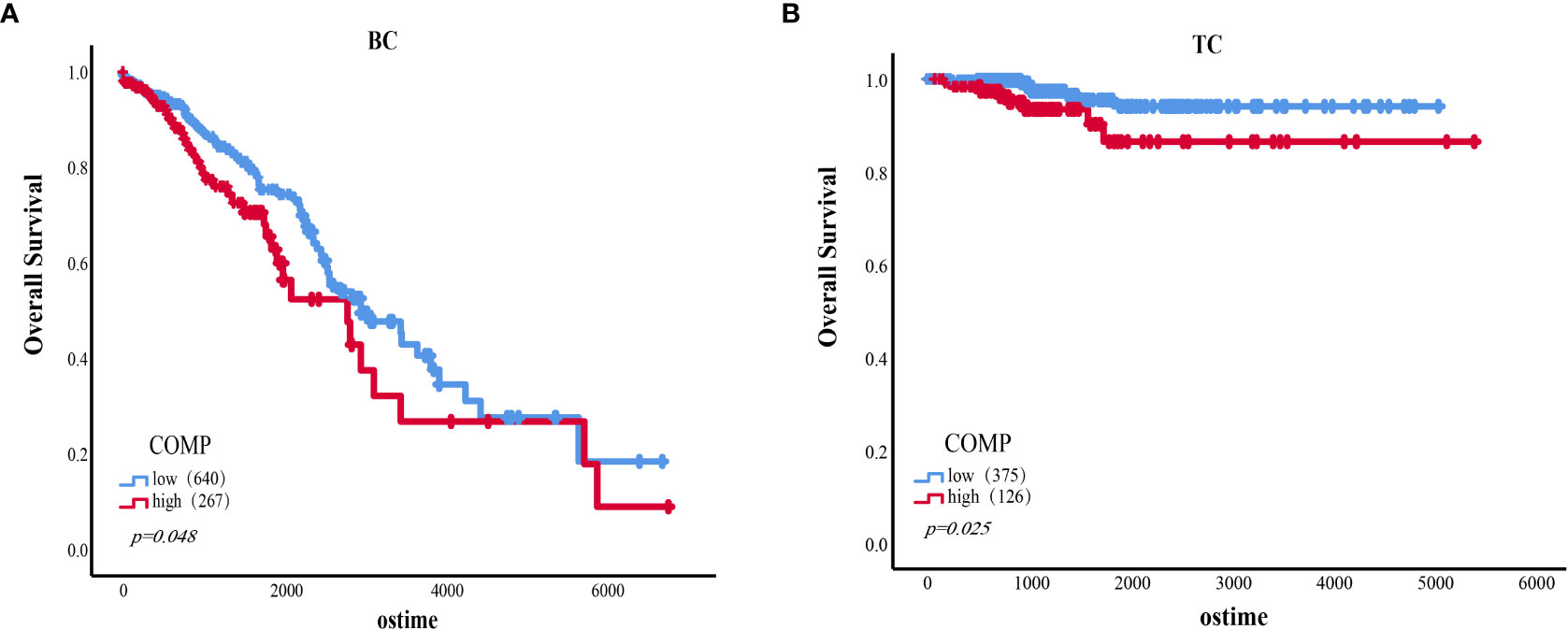
Figure 4 (A) The association of the expression level of COMP with OS of BC patients (Stage II-IV). (B) The association of the expression level of COMP with OS of TC patients.
GSEA was performed to identify the enriched pathways associated with upregulated COMP in TC and BC (Figure 5). Results indicated that the estrogen response early and estrogen response late pathways were enriched in BC and TC, respectively; the epithelial-mesenchymal transition (EMT) pathways were significantly enriched in both BC and TC.
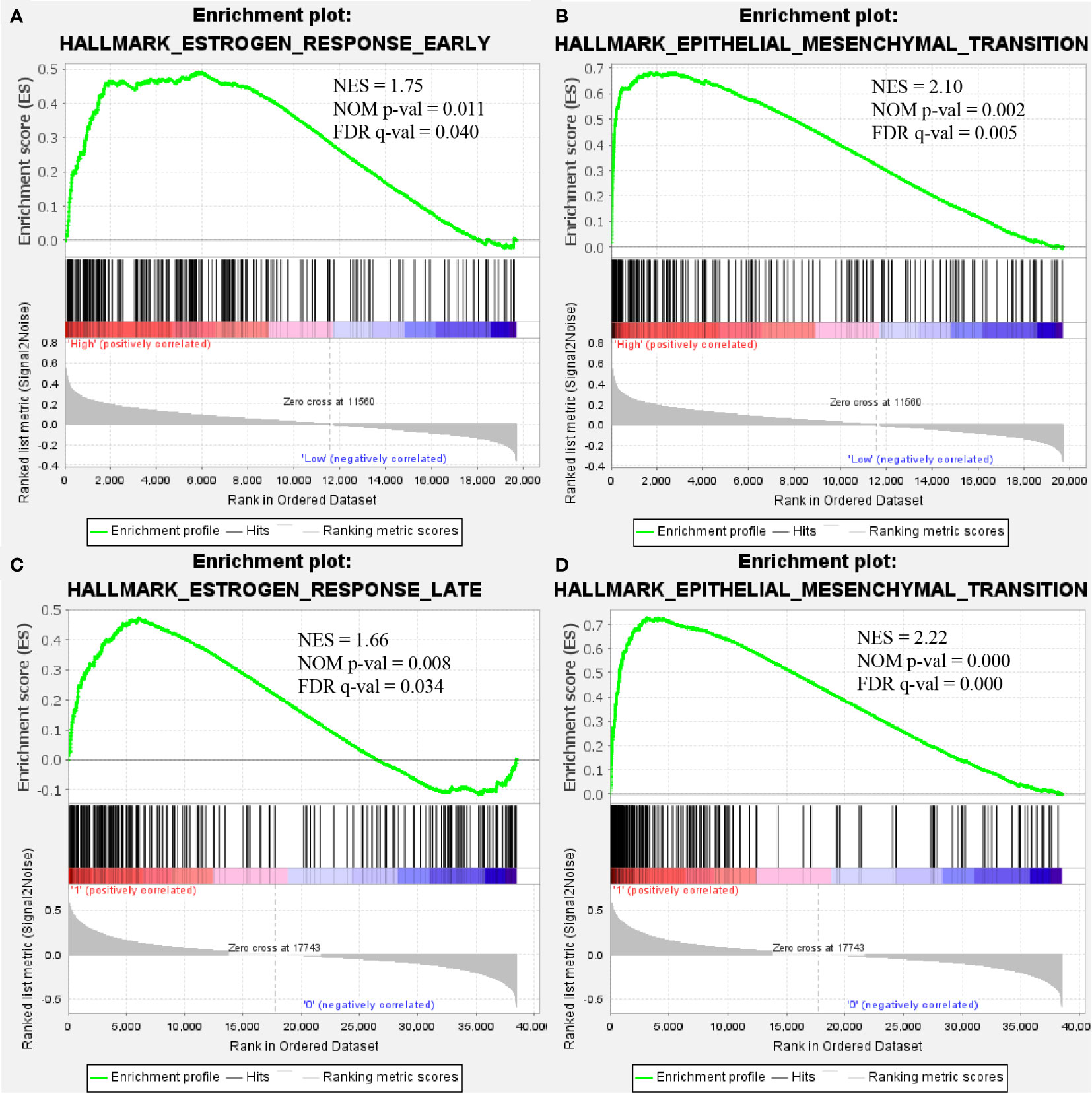
Figure 5 COMP overexpression was significantly associated with “estrogen response early” (A) and “epithelial-mesenchymal transition” (B) in BC. COMP overexpression was also associated with “estrogen response late” (C) and “epithelial-mesenchymal transition” (D) in TC.
INHBA and MMP14 in EMT pathways were correlated with COMP expression (Figures 6A–D) for both BC and TC. INHBA and MMP14 were significantly overexpressed in BC and TC tissues as compared to normal tissues (Figures 6E–H). K-M analysis showed that MMP14 overexpression was significantly correlated with unfavorable OS in TC (p = 0.009; Figure 6L). INHBA expression and OS were significantly correlated in both BC and TC (p < 0.05, Figures 6I, J). However, the high expression of MMP14 was not significantly correlated with survival in BC (Figure 6K).
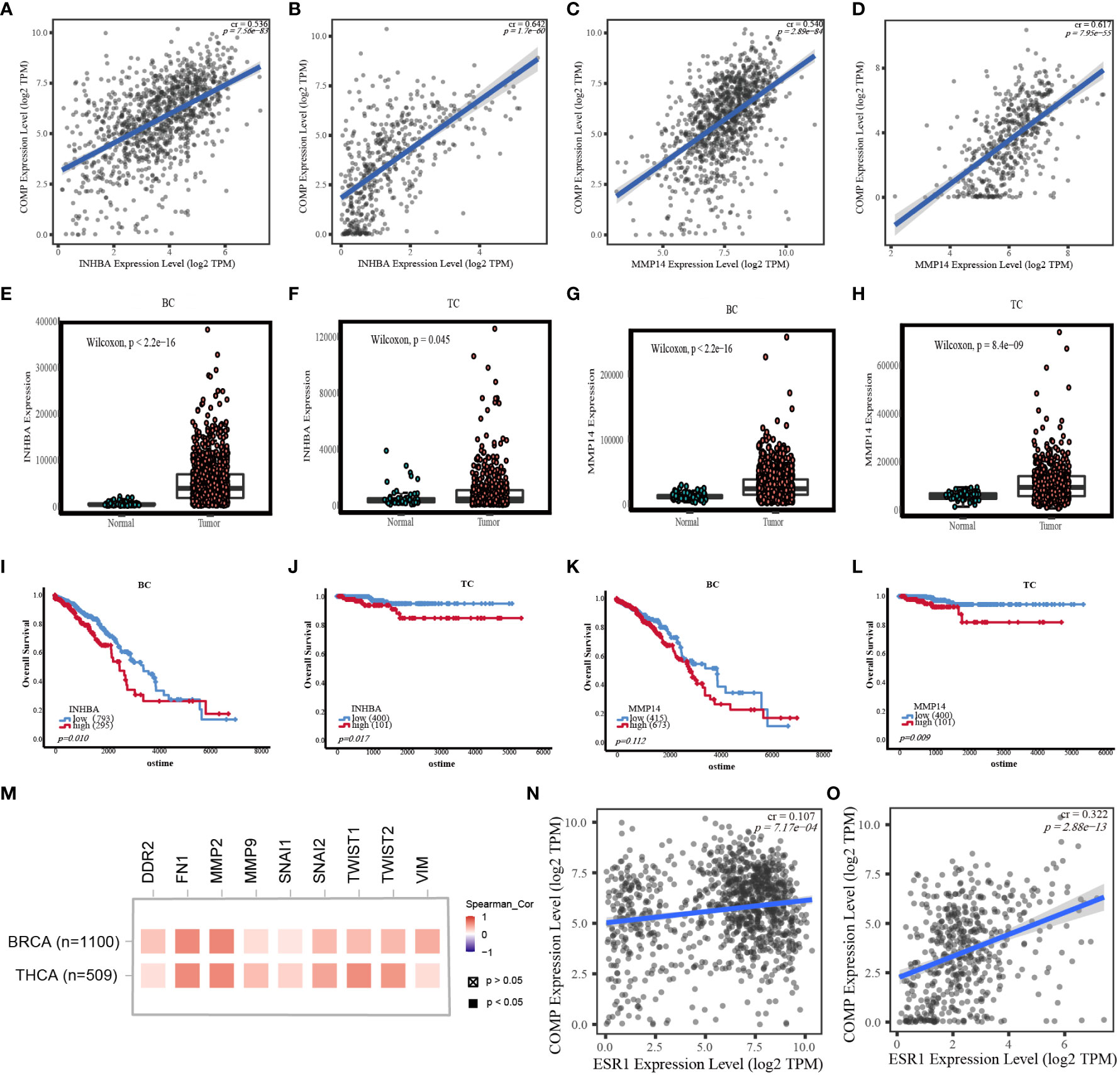
Figure 6 (A) Correlation between INHBA expression and COMP mRNA expression in BC. (B) Correlation between INHBA expression and COMP mRNA expression in TC. (C) Correlation between MMP14 expression and COMP mRNA expression in BC. (D) Correlation between MMP14 expression and COMP mRNA expression in TC. (E) Differential expression of INHBA between BC and normal tissues. (F) Differential expression of INHBA between TC and normal tissues. (G) Differential expression of MMP14 between BC and normal tissues. (H) Differential expression of MMP14 between TC and normal tissues. (I) Correlation between the expression level of INHBA and OS in BC patients. (J) Correlation between the expression level of INHBA and OS in TC patients. (K) Correlation between the expression level of MMP14 and OS in BC patients. (L) Correlation between the expression level of MMP14 and OS in TC patients. (M) Correlation between COMP expression and EMT marks expression. (N) Correlation between ESR1 expression and COMP mRNA expression in BC. (O) Correlation between ESR1 expression and COMP mRNA expression in TC.
In addition, in breast and thyroid cancers, we also found that COMP was positively associated with other EMT markers (TWIST1, TWIST2, SNAI1, SNAI2, DDR2, MMP2, MMP9, FN1, VIM) and estrogen receptor-α (ESR1) (Figures 6M–O).
To further validate the expression profile of COMP, immunohistochemical staining for tissues of patients with co-occurrence of BC and TC was performed. COMP was significantly overexpressed in BC and TC as compared to normal tissues, consistent with the gene expression pattern (p<0.0001) (Figure 7).
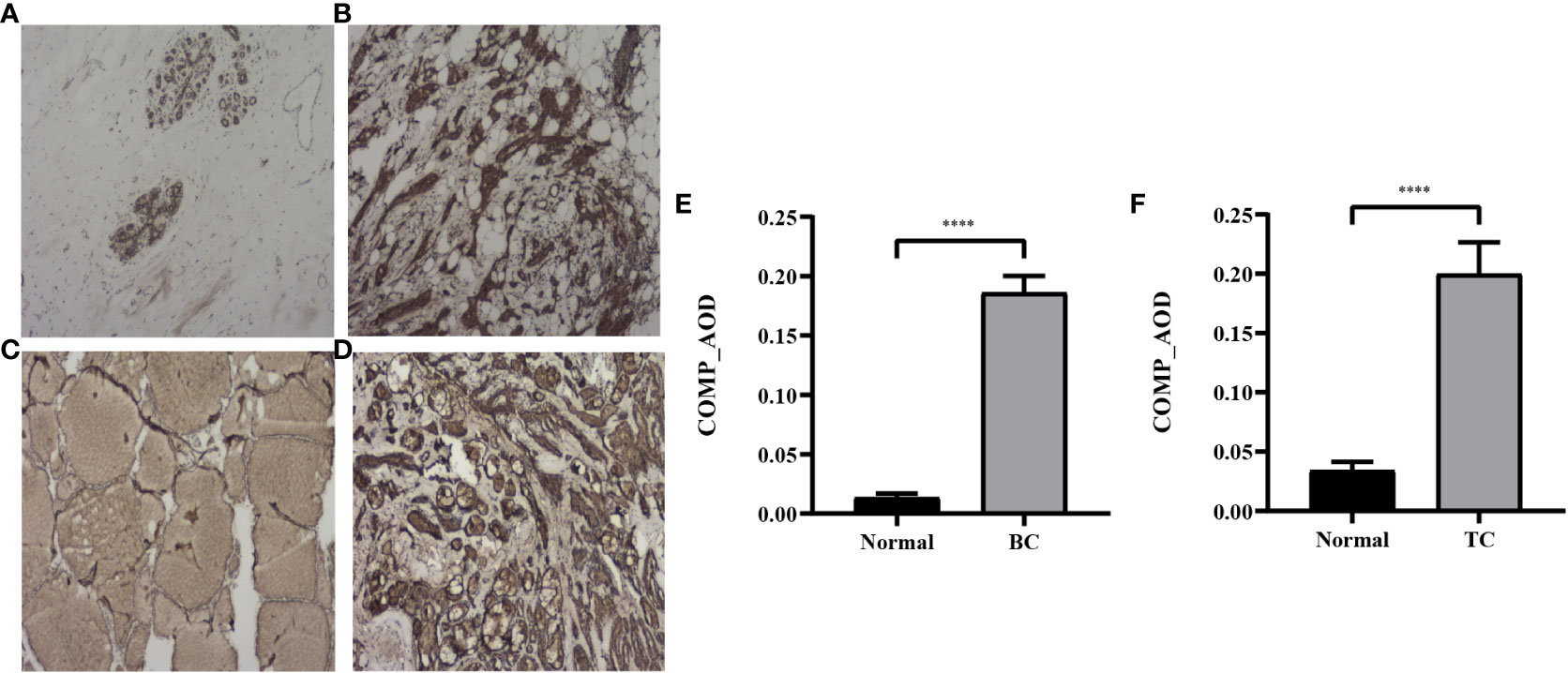
Figure 7 Representative IHC images of COMP in normal breast tissues (A), BC tissues (B), normal thyroid tissues (C) and TC tissues (D). Magnification: ×40. (E) AOD of COMP in breast cancer vs normal breast tissues; (F) AOD of COMP in thyroid cancer vs normal thyroid tissues. ****p<0.0001.
In this study, we showed that the risk of subsequently developing BC or TC in TC or BC patients, respectively, was higher than in general population by analyzing double primary cancer (BC and TC) data using the SEER-9 database. And the pathological or molecular subtype of patients with BC and TC was mostly DTC and HR-positive BC, respectively. Therefore, DTC and HR-positive BC may be closely related. In BC-1st patients, the SIR of HR-positive BC patients was higher than that of HR-negative BC patients. DTC patients also had a significantly higher risk of developing BC. In addition, patients with higher TNM stages had a higher risk of developing another primary cancer.
We also analyzed the survival of BC-1st and TC-1st patients. K-M analysis indicated that the overall survival and cancer-specific survival of BC-1st was poorer than that of TC-1st patients. This may be attributed to the better prognosis of TC patients. Additionally, the median time for TC-1st (111 months) was longer than the median time for BC-1st (60 months). Further, the impact of HR status and TC subtype on the survival of double primary cancer patients were also analyzed. For all patients with BC and TC, HR status and TC histological subtype were correlated with the survival of patients. However, for BC-1st patients, only the TC histological subtype was significantly associated with the survival of patients. Only the HR status was correlated with the OS of TC-1st patients. Therefore, the OS of patients with BC and TC were correlated with the molecular or pathological subtype of the second primary cancer rather than the first primary cancer.
The association of TC and BC is complex; previous studies have aimed at evaluating this relationship. What more, An et al. (4) showed that the expression of ER and PR in co-occurrence of BC and TC was higher than in BC-only patients, suggesting that there is underlying molecular pathogenesis for BC and TC double primary cancers. We aimed to analyze the genetic mechanisms underlying the association of BC and TC. We obtained 16 common differentially expressed genes in BC and TC. In the preliminary analysis, other genes except COMP were not associated with the survival prognosis of BC or TC. In addition, COMP is highly expressed in both BC and TC, which may be a potential pathogenic gene, so we conducted further analysis on COMP.
We found that ROC curves suggested better diagnostic values of COMP in TC and BC. Studies (18, 19) have shown that COMP promotes the proliferation and migration of PTC and BC cells. Meanwhile, high COMP expression was found to be associated with poor prognosis in TC and BC patients in our study. The correlation between COMP and survival of stage II-IV BC patients may be due to the oncogenic role of COMP in advance stage BC. Papadakos et al. (20) show that serum COMP in metastatic BC patients was higher as compared to those in the early stages of BC and was significantly correlated with bone and liver metastasis. In this study, multivariate Cox analysis also confirmed that COMP overexpression was an independent factor of unfavorable OS in BC (stage II-IV). However, in TC, only univariate analysis showed that COMP overexpression correlated with poor OS; this probably contributes to the better prognosis of TC patients (16 of 501 deceased patients during the follow-up). Thus, COMP may be a potential biomarker for the diagnosis and prognosis of TC and BC patients. Besides we also found that stage III BC or TC survivors are more likely to develop another second primary cancer. Therefore, COMP may promote the progression of TC and BC, thereby increasing the risk of secondary primary cancer.
COMP also known as TSP5, is a member of the thrombospondin family (21). which is highly expressed in colon cancer, hepatocellular carcinoma, prostate cancer, lung cancer, and bladder cancer (22–24). COMP can enhance the ability of tumor cells to degrade ECM by upregulating the expression of MMP9, thereby, promoting EMT, which in turn promotes tumor invasion and metastasis (25). In addition, COMP promotes cell growth and proliferation by activating the MEK/ERK and PI3K/AKT signaling pathways (26). These observations are consistent with our results; COMP is overexpressed in BC patients and associated with ER and PR (19); GSEA indicated that COMP overexpression had significantly enriched the estrogen response and EMT pathways in both TC and BC and may promote TC and BC progression, thereby, increasing the risk of these double primary malignancies.
Our results also showed that INHBA and MMP14 in the EMT pathway were positively correlated with COMP overexpression. Both INHBA and MMP14 were upregulated in BC and TC as compared to the normal tissues. Additionally, INHBA overexpression was associated with unfavorable OS of BC and TC; high expression of MMP14 was associated with the poorer prognosis of TC patients. INHBA was one of 16 common DEGs which was upregulated in BC and TC. Activin A, a homodimer composed of two inhibin βA subunits is encoded by INHBA. Recent studies (27–29), indicate the importance of activin signaling in the progression of BC and TC. High expression of activin A induces EMT. MMP14 is a member of the membrane-anchored metalloproteinases (MMP) subfamily. This protein not only degrades extracellular matrix protein but also acts as an activator of other MMP zymogen including MMP2 and MMP9, playing a key role in tumor invasion (30).
EMT is important for the process of tumor metastasis. The activation of EMT causes the cancer cells to migrate and invade. Decrease in epithelial markers, including E-cadherin and occludin, and an increase in the mesenchymal markers (N-cadherin, vimentin, and fibronectin) are considered as characteristics of EMT (31). A previous study (25) shows co-expression of COMP with mesenchymal markers and its negative correlation with the expression of epithelial markers. In the present study, we found that COMP was enriched in the EMT pathway in both BC and TC and correlated with some EMT markers. Therefore, COMP plays a role in the occurrence and progression of BC and TC through the EMT pathway.
Estrogen plays an important role in BC and TC. Estrogen acts via ER. In TC and BC, ERα enhances the migration and invasion of tumor cells by upregulating MMP-9 and downregulating E-cadherin (32–35). In addition (36, 37), estrogen can activate the MAPK pathway, the phosphatidylinositol 3-kinase (PI3K) signaling pathway, and MMPs by binding to membrane-associated estrogen receptor. Our analysis based on the SEER database found that HR-positive BC patients have higher risk of developing TC. Meanwhile, we found that COMP was up-regulated in ER-positive breast cancer. COMP was enriched in the estrogen response pathway in both BC and TC, and positively correlated with ESR1 expression. COMP may promote the development and progression of double primary cancer through the estrogen response pathway.
Based on the above findings, COMP is highly expressed in both TC and BC and has the same pathogenic pathway. Therefore, we hypothesized that COMP expression may also be elevated when patients developed BC or TC, thereby increasing the risk of another primary cancer through the same pathogenic pathway.
However, there are some limitations to this study. Compared with the general population, patients with BC or TC may undergo more examinations, thus, it is easier to find that they subsequently suffer from another cancer, which has a certain selection bias. However, how determining the true incidence of double primary cancer needs further designing of a more reasonable research program that includes appropriate research subjects. Such as prospective follow-up of healthy physical examination population. The function of COMP and the correlation of COMP with ER and EMT also need further experimental investigation. Moreover, the sodium iodide symporter in most DTC and BC are expressed and mediated by the radioactive iodine treatment. Therefore, the expression of sodium iodide symporter in double primary cancer and its relationship with COMP need to be further studied.
In conclusion, we found that the co-occurrence risk of BC and TC in the same individual is higher than in the general population. And double primary cancers share a common pathogenic mechanism. Overexpression of COMP could promote oncogenesis and progression of BC and TC patients through estrogen signaling and EMT pathways and may increase the risk of TC in BC patients or BC in TC patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All experimental procedures involving humans in this study were reviewed and approved by the Ethics Committee of China-Japan Union Hospital of Jilin University. Written informed consent was obtained from all patients.
JF and MH performed statistical analysis and write the manuscript. QW performed bioinformatics analysis and did experiments. XZ collected clinical data and prepared some figures. XQ and KS collected part of the clinical data. XW participated in research conception, critical reading, and correction of the manuscript. GZ participated in research conception, supervised and coordinate research, and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Program of Norman Bethune of Jilin University (2020B45, MH), Program of Jilin Provincial Health Commission (2021LC022, MH) and Program of The Education Department of Jilin Province (JJKH20201055KJ, GZ).
We thank all the researchers and the funding of Program of Norman Bethune of Jilin University (2020B45, MH), Program of Jilin Provincial Health Commission (2021LC022, MH) and Program of JiLin Provincial Department of Education (JJKH20201055KJ, GZ)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1136120/full#supplementary-material
Supplementary Figure 1 | (A) Correlation between SLC5A5 expression and COMP mRNA expression in BC. (B) Correlation between SLC5A5 expression and COMP mRNA expression in TC. (C) Correlation between SLC5A5 expression and ESR1 mRNA expression in BC. (D) Correlation between SLC5A5 expression and ESR1 mRNA expression in TC. (E) Correlation between SLC5A5 expression and ESR2 mRNA expression in BC. (F) Correlation between SLC5A5 expression and ESR2 mRNA expression in TC.
1. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pacific J Cancer Prev (2016) 17(S3):43–6. doi: 10.7314/apjcp.2016.17.s3.43
2. Corso G, Veronesi P, Santomauro GI, Maisonneuve P, Morigi C, Peruzzotti G, et al. Multiple primary non-breast tumors in breast cancer survivors. J Cancer Res Clin Oncol (2018) 144(5):979–86. doi: 10.1007/s00432-018-2621-9
3. Lei K, He X, Yu L, Ni C, Chen H, Guan D, et al. Breast cancer prognosis is better in patients who develop subsequent metachronous thyroid cancer. PloS One (2019) 14(5):e0215948–e0215948. doi: 10.1371/journal.pone.0215948
4. An JH, Hwangbo Y, Ahn HY, Keam B, Lee KE, Han W, et al. A possible association between thyroid cancer and breast cancer. Thyroid (2015) 25(12):1330–8. doi: 10.1089/thy.2014.0561
5. Zhang L, Wu Y, Liu F, Fu L, Tong Z. Characteristics and survival of patients with metachronous or synchronous double primary malignancies: breast and thyroid cancer. Oncotarget (2016) 7(32):52450–9. doi: 10.18632/oncotarget.9547
6. Kuo JH, Chabot JA, Lee JA. Breast cancer in thyroid cancer survivors: an analysis of the surveillance, epidemiology, and end results-9 database. Surgery (2016) 159(1):23–30. doi: 10.1016/j.surg.2015.10.009
7. Kim EY, Chang Y, Lee KH, Yun J-S, Park YL, Park CH, et al. Serum concentration of thyroid hormones in abnormal and euthyroid ranges and breast cancer risk: a cohort study. Int J Cancer (2019) 145(12):3257–66. doi: 10.1002/ijc.32283
8. Khan SR, Chaker L, Ruiter R, Aerts JGJV, Hofman A, Dehghan A, et al. Thyroid function and cancer risk: the Rotterdam study. J Clin Endocrinol Metab (2016) 101(12):5030–6. doi: 10.1210/jc.2016-2104
9. Mette S, Dóra Körmendiné F, Vera E, Jens Otto Lunde J, Olaf MD, Henrik Toft S. Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. Eur J Endocrinol (2016) 174(4):409–14. doi: 10.1530/EJE-15-0989
10. Bolf EL, Sprague BL, Carr FE. A linkage between thyroid and breast cancer: a common etiology? Cancer Epidemiol Biomarkers & Prev (2019) 28(4):643. doi: 10.1158/1055-9965.EPI-18-0877
11. Reiners C, Schneider R, Platonova T, Fridman M, Malzahn U, Mäder U, et al. Breast cancer after treatment of differentiated thyroid cancer with radioiodine in young females: what we know and how to investigate open questions. review of the literature and results of a multi-registry survey. Front Endocrinol (Lausanne) (2020) 11:381. doi: 10.3389/fendo.2020.00381
12. Sun L-M, Lin C-L, Liang J-A, Huang W-S, Kao C-H. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: a nationwide population-based cohort study. Int J Cancer (2015) 137(12):2896–903. doi: 10.1002/ijc.29667
13. Ng EK, Shin VY, Leung CP, Chan VW, Law FB, Siu MT, et al. Elevation of methylated DNA in KILLIN/PTEN in the plasma of patients with thyroid and/or breast cancer. Onco Targets Ther (2014) 7:2085–92. doi: 10.2147/OTT.S53597
14. Ikeda Y, Kiyotani K, Yew PY, Kato T, Tamura K, Yap KL, et al. Germline PARP4 mutations in patients with primary thyroid and breast cancers. Endocr Relat Cancer (2016) 23(3):171–9. doi: 10.1530/ERC-15-0359
15. He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA (2005) 102(52):19075–80. doi: 10.1073/pnas.0509603102
16. Aswad L, Yenamandra SP, Ow GS, Grinchuk O, Ivshina AV, Kuznetsov VA. Genome and transcriptome delineation of two major oncogenic pathways governing invasive ductal breast cancer development. Oncotarget (2015) 6(34):36652–74. doi: 10.18632/oncotarget.5543
17. Li F, Teng H, Liu M, Liu B, Zhang D, Xu Z, et al. Prognostic value of immune-related genes in the tumor microenvironment of bladder cancer. Front Oncol (2020) 10:1302. doi: 10.3389/fonc.2020.01302
18. Zhang J, Wang H, Lv C, Han J, Hao M, Li J, et al. Cartilage oligomeric matrix protein affects the biological behavior of papillary thyroid carcinoma cells by activating the PI3K/AKT/Bcl-2 pathway. J Cancer (2021) 12(6):1623–33. doi: 10.7150/jca.49144
19. Englund E, Bartoschek M, Reitsma B, Jacobsson L, Escudero-Esparza A, Orimo A, et al. Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene (2016) 35(43):5585–96. doi: 10.1038/onc.2016.98
20. Papadakos KS, Darlix A, Jacot W, Blom AM. High levels of cartilage oligomeric matrix protein in the serum of breast cancer patients can serve as an independent prognostic marker. Front Oncol (2019) 9:1141. doi: 10.3389/fonc.2019.01141
21. Acharya C, Yik JHN, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol (2014) 37:102–11. doi: 10.1016/j.matbio.2014.06.001
22. T-t L, X-s L, Zhang M, X-n L, F-x Z, F-m Z, et al. Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the akt pathway. J Cancer Res Clin Oncol (2018) 144(6):1049–63. doi: 10.1007/s00432-018-2626-4
23. Li Q, Wang C, Wang Y, Sun L, Liu Z, Wang L, et al. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J Exp Clin Cancer Res (2018) 37(1):231–1. doi: 10.1186/s13046-018-0908-y
24. Zheng C, Li X, Ren Y, Yin Z, Zhou B. Coexisting EGFR and TP53 mutations in lung adenocarcinoma patients are associated with COMP and ITGB8 upregulation and poor prognosis. Front Mol Biosci (2020) 7:30. doi: 10.3389/fmolb.2020.00030
25. Zhong W, Hou H, Liu T, Su S, Xi X, Liao Y, et al. Cartilage oligomeric matrix protein promotes epithelial-mesenchymal transition by interacting with transgelin in colorectal cancer. Theranostics (2020) 10(19):8790–806. doi: 10.7150/thno.44456
26. Nfonsam VN, Jecius HC, Janda J, Omesiete PN, Elquza E, Scott AJ, et al. Cartilage oligomeric matrix protein (COMP) promotes cell proliferation in early-onset colon cancer tumorigenesis. Surg Endoscopy (2020) 34(9):3992–8. doi: 10.1007/s00464-019-07185-z
27. Bashir M, Damineni S, Mukherjee G, Kondaiah P. Activin-a signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ Breast Cancer (2015) 1:15007–7. doi: 10.1038/npjbcancer.2015.7
28. Kalli M, Mpekris F, Wong CK, Panagi M, Ozturk S, Thiagalingam S, et al. Activin a signaling regulates IL13Rα2 expression to promote breast cancer metastasis. Front Oncol (2019) 9:32. doi: 10.3389/fonc.2019.00032
29. Shibata M, Inaishi T, Ichikawa T, Shimizu D, Soeda I, Takano Y, et al. Identifying the tumor-progressive gene expression profile in high-risk papillary thyroid cancer. Surg Today (2021) 51(10):1703–12. doi: 10.1007/s00595-021-02262-0
30. Lu H, Hu L, Yu L, Wang X, Urvalek AM, Li T, et al. KLF8 and FAK cooperatively enrich the active MMP14 on the cell surface required for the metastatic progression of breast cancer. Oncogene (2014) 33(22):2909–17. doi: 10.1038/onc.2013.247
31. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol: Mech Disease (2018) 13(1):395–412. doi: 10.1146/annurev-pathol-020117-043854
32. Rajoria S, Suriano R, George AL, Shanmugam A, Jussim C, Shin EJ, et al. Estrogen activity as a preventive and therapeutic target in thyroid cancer. BioMed Pharmacother (2012) 66(2):151–8. doi: 10.1016/j.biopha.2011.11.010
33. Sharma D, Kumar S, Narasimhan B. Estrogen alpha receptor antagonists for the treatment of breast cancer: a review. Chem Cent J (2018) 12(1):107–7. doi: 10.1186/s13065-018-0472-8
34. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocrine-Related Cancer (2014) 21(5):T273–83. doi: 10.1530/ERC-14-0053
35. Zhang Y, Wei F, Zhang J, Hao L, Jiang J, Dang L, et al. Bisphenol a and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway. Arch Biochem Biophys (2017) 633:29–39. doi: 10.1016/j.abb.2017.09.002
36. Vannucchi G, De Leo S, Perrino M, Rossi S, Tosi D, Cirello V, et al. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur J Endocrinol (2015) 173(1):29–36. doi: 10.1530/EJE-15-0054
Keywords: breast cancer, thyroid cancer, COMP, prognosis, standardized incidence ratio (SIR)
Citation: Fu J, He M, Wu Q, Zhang X, Qi X, Shen K, Wang X and Zhang G (2023) The clinical and genetic features in patients coexisting primary breast and thyroid cancers. Front. Endocrinol. 14:1136120. doi: 10.3389/fendo.2023.1136120
Received: 02 January 2023; Accepted: 26 April 2023;
Published: 09 May 2023.
Edited by:
Marina Bacci, University of Florence, ItalyReviewed by:
Francesca Coperchini, University of Pavia, ItalyCopyright © 2023 Fu, He, Wu, Zhang, Qi, Shen, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochun Wang, OTQ3MTY3OTU2QHFxLmNvbQ==; Guang Zhang, emhhbmdndWFuZ0BqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.