- 1Department of Radiotherapy, Cancer Center, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 2Department of Radiotherapy, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 3Key Laboratory of Radiation Biology of Fujian Higher Education Institutions, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China
Background: The optimal approach to assess the postoperative status of lymph nodes in differentiated thyroid cancer (DTC) remains controversial. Our aim was to determine if the log odds of negative lymph nodes/T stage ratio (LONT) could serve as a new prognostic and predictive tool for DTC without metastases in patients aged ≥ 55 years.
Methods: The Surveillance, Epidemiology, and End Results (SEER) database was used to study the role of LONT in patients aged ≥55 years diagnosed with DTC without metastases. The primary outcome was overall survival (OS). The Kaplan-Meier method and the Cox proportional hazard regression model were used to calculate the outcome. Moreover, the robustness of research findings was evaluated using sensitivity analyses.
Results: A total of 21,172 DTC patients aged ≥55 years without distant metastasis were enrolled. Multivariate Cox regression analyses and a “floating absolute risk” analysis showed that a LONT ≥0.920 (vs. -0.56 to 0.92) was a protective factor for OS in DTC patients. Sensitivity analyses revealed an E-value of 1.98 for the obtained LONT value. In subgroup analyses, LONT was correlated significantly with OS in different subgroups of negative lymph nodes, stage-I–II subgroups and the N0 subgroup. The conditional probability of survival of DTC improved with prolonged survival time in the LONT ≥0.920 group.
Conclusion: A high LONT was associated with longer OS compared with low LONT in patients aged ≥55 years with non-metastatic DTC. LONT could provide valuable information for undertaking postoperative evaluations.
1 Introduction
Thyroid cancer (TC) is the most common malignant tumor of the endocrine system. According to the origin of the tumor and the variability in differentiation, the pathologic type of >90% of TCs is differentiated thyroid cancer (DTC), including papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) (1). Thyroidectomy and standardized dissection of lymph nodes in the neck are the first-line treatments for DTC (2, 3).
Tumor-node-metastasis (TNM) staging for TC is important because it is used to guide the treatment and prognosis of patients. However, this system focuses only on the depth of tumor invasion, number of lymph-node metastases, and presence of distant metastases, while prognoses vary even among patients with TC at identical stages. Therefore, developing a more intricate and detailed method of prognostic evaluation may help to accurately predict the outcome, and choose a more targeted and rational individualized treatment protocol.
The main factors influencing DTC outcomes are age and TNM stage. Age is an independent risk factor for disease-specific survival (DSS) in TC patients (4, 5). The eighth version of the Thyroid Cancer Staging System set by the American Joint Committee on Cancer (AJCC) continues to adopt anatomic staging based on T, N and M. The diagnostic cutoff point for age required for the prognosis increases from 45 to 55 years (6). Metastasis in the lymph nodes in the neck is a risk factor for recurrence and shortened survival in patients with TC. However, the strategy for dissection of these lymph nodes is controversial (7–11). Often, dissection of regional lymph nodes for DTC is based on individualized treatment with varying degrees of dissection, which challenges the objective assessment of the postoperative status of lymph nodes. With respect to lymph-node management in DTC, postoperative evaluation of lymph-node status is influenced by multiple factors such as the surgical approach. The number of positive lymph nodes, examination of lymph nodes (ELN) and the number of negative lymph nodes (NLN) are classical evaluation indicators of lymph-node status, which are associated with survival in gastric (12), colorectal (13, 14), and breast cancer (15).
Log odds of negative lymph nodes/T stage ratio (LONT) refers to information on the disease stage and is used to quantify the degree of lymph-node dissection. NLNs represent the total level of lateral neck dissection (LND) and the T stage represents disease severity. NLNs adjusted according to the T stage denote the relative number of NLNs removed from each patient. A high value of LONT indicates more NLNs obtained, while a low LONT value means that fewer NLNs were obtained. Therefore, LONT can be used to compare the relative levels of LND among patients. Different TNM stages, ELNs, or NLNs, and the same LONT denote the same risk level (16). Studies have shown that risk stratification using LONT in patients after surgery for gastric cancer reflects disease severity and integrates the prognostic information of the degree of lymph-node dissection, while being closely related to the treatment outcome (16). However, studies using LONT to investigate TC are lacking, and the prognostic value of LONT in TC patients remains unknown.
To address this, we retrospectively analyzed clinical-pathology data in 21,172 patients with DTC who underwent resection based on the Surveillance, Epidemiology, and End Results (SEER) database of the US National Cancer Institute (Bethesda, MD, USA). We aimed to investigate the effect of LONT on the prognosis of DTC patients aged ≥55 years without metastasis at the first visit using the Kaplan–Meier method, and a Cox regression analysis. Moreover, the robustness of research findings was evaluated by sensitivity analysis and calculation of E-values. This is the minimum strength of association (risk ratio scale) that an unmeasured confounder would need to have with both exposure and the outcome to fully explain the specific exposure-outcome association.
2 Materials and methods
2.1 Data source and cohorts
A population-based study was undertaken retrospectively using data from the SEER database using SEER*Stat 8.3.9. From 2010 to 2015, patients aged 55 and older diagnosed with non-metastatic DTC were evaluated. The exploration was based on the SEER database, which discloses the demographic features, clinicopathological characteristics, and survival information of patients. As the data were derived from anonymous public databases, ethical approval was not sought or needed.
The inclusion criteria were: (1) diagnosis of TC without metastasis; (2) age ≥55 years; (3) histology type included International Classification of Diseases for Oncology, third edition (ICD-O-3) code 8050/3 (papillary carcinoma, not otherwise specified (NOS)), 8260/3 (papillary adenocarcinoma, NOS), 8290/3 (oxyphilic adenocarcinoma), 8330/3 (follicular adenocarcinoma, NOS), 8331/3 (follicular adenocarcinoma, well differentiated), 8335/3 (follicular carcinoma, minimally invasive), 8340/3 (papillary carcinoma, follicular variant), 8341/3 (papillary microcarcinoma), 8342/3 (papillary carcinoma, oxyphilic cell), 8343/3 (papillary carcinoma, encapsulated), and 8344/3 (papillary carcinoma, columnar cell); (4) history of total thyroidectomy.
The exclusion criteria were: (1) unknown number of examined or positive regional lymph nodes; (2) unknown T, N, and M stages; (3) incomplete information on age, sex, ethnicity, tumor diameter, survival, and vital signs; (4) T0 and T4 stages.
2.2 Definition of LONT
“LONT” is defined as the log odds of (negative lymph nodes +1)/T stage ratio. The description of LONT calculation is as follows:
T stages T1a, T1b, T2, T3, T4a, and T4b are assigned as 1, 2, 3, 4, 5, and 6, respectively. NLNs denote the ELNs minus the count of positive lymph nodes. The value of one is added for NLNs to avoid the occurrence of zero. A restricted cubic spline was used to assess the association between LONT and survival to avoid assuming it was a simple linear association. LONT was categorized if non-linearity was detected. The optimal thresholds for LONT were determined on the result of restricted cubic spline.
2.3 Selection of variables
The main variables extracted from the SEER database were “age at the diagnosis”, “sex”, “ethnicity”, “marital status at the diagnosis”, “year of the diagnosis”, “stage group, derived from AJCC seventh edition (2010–2015)”, “T stage derived from AJCC seventh edition (2010–2015)”, “N stage derived from AJCC seventh edition (2010–2015)”, “M stage derived from AJCC seventh edition (2010–2015)”, “tumor grade”, “radiotherapy recode”, “chemotherapy recode”, “CS tumor size”, “first indicator of primary malignancy”, “SEER cause-specific death classification”, “survival in months”, “vital signs”, and “histology type (based on ICD-O-3)”. The histology type includes papillary carcinoma (PC), follicular adenocarcinoma (FC), papillary carcinoma, follicular variant (PCFV), Papillary microcarcinoma (PMC).
2.4 Statistical analyses
Categorical demographic (ethnicity, sex) and clinical data was analyzed by the chi-square test. The designated outcome was overall survival (OS). Survival curves were constructed using the Kaplan-Meier method. The log-rank test was undertaken to compare various subgroups. Univariate and multivariate Cox proportional hazards tests were conducted for OS using the “survival” package in R 4.0.1 (R Institute for Statistical Computing, Vienna, Austria). For comparisons involving multiple groups, 95% confidence intervals (CIs) were calculated using the floating absolute risks (FAR) method to enable valid comparisons between any two groups and not only with the reference group (17). Stratified analyses were conducted with Cox models; hazard ratios (HR), and 95% CIs were calculated for subgroups. The “conditional probability of survival” was defined as the probability of surviving to “Y” years after the diagnosis given survival to “X” (X< Y) years. Additionally, it can describe in detail the survival of patients at different time stages (18). Further analyses of E-value sensitivity were conducted to evaluate the robustness of the association to unmeasured confounding using the “E-Value” package within R. Statistical analyses were also conducted employing R. P < 0.05 (two-tailed) was considered significant.
3 Results
3.1 The relationship between LONT and survival
The restricted cubic spline curve showed that LONT had a nonlinear association with survival. HR for death was lowest near the LONT of -0.586, with a gradual rise after 0.920 (Figure S1). Then, optimal LONT thresholds were identified and the cohort was separated into three groups: LONT< -0.586, -0.586≤ LONT<0.920, and LONT ≥0.920.
3.2 Baseline population demographics
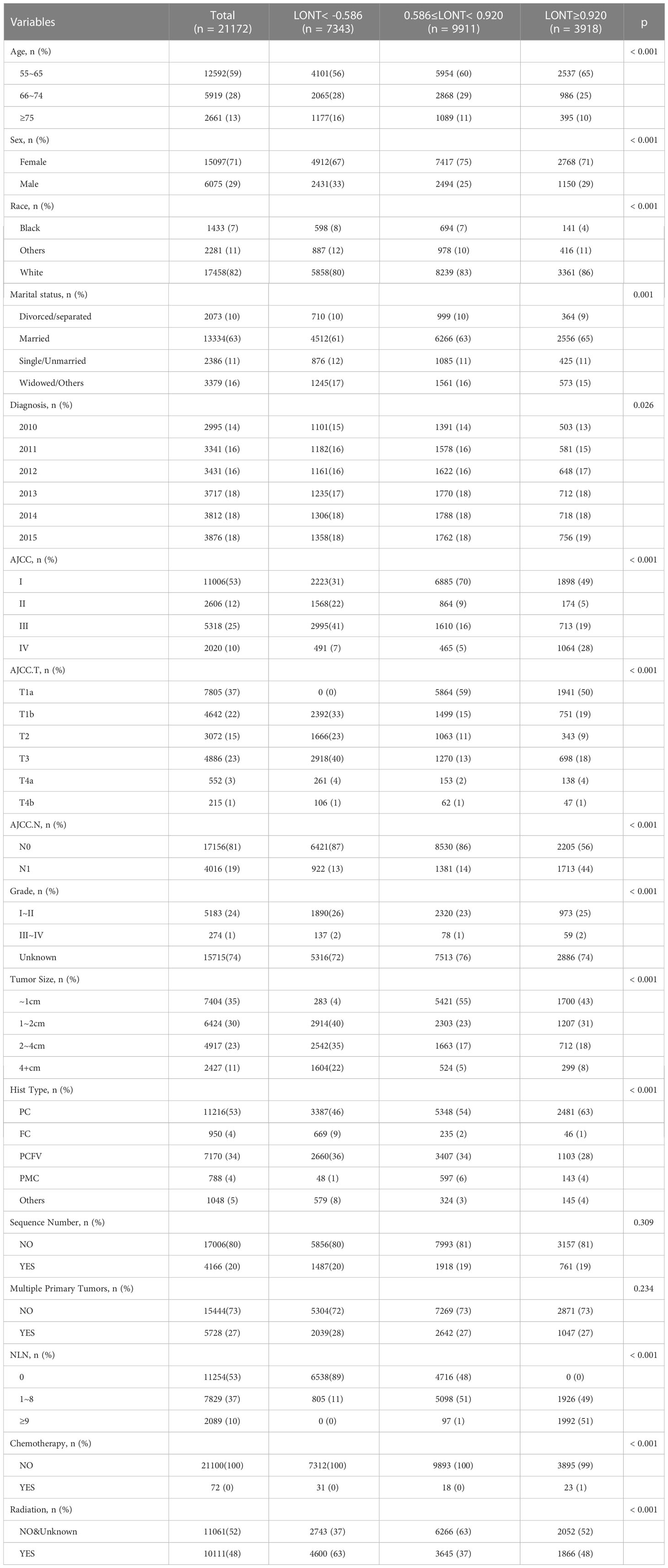
A total of 21,172 patients aged ≥55 years diagnosed with TC without metastasis between 2010 and 2015 were enrolled. There was no significant difference between the three groups in the distribution of sequence number and multiple primary tumors. Detailed information about the demographic and clinicopathological characteristics of patients is shown in Table 1.
3.3 OS
Survival analyses using the Kaplan-Meier method revealed a significantly poor OS (P < 0.001) for patients with low LONT (Figure 1). Univariate and multivariate analyses were undertaken using data from all patients to assess the potential prognostic factors. In the univariate Cox regression analysis, the following factors were associated with shortened OS: age groups of 66–74 years and >75 years (vs. 55–65 years), male sex, black ethnicity (vs. white ethnicity), marital status of widowed/other (vs. married), grade of II, III, or IV (vs. I), stage of T2, T3, T4a, or T4b (vs. Ia), N1 stage (vs. N0), grade of III–IV (vs. I–II), tumor diameter of 2–4 cm or >4 cm (vs.<1 cm), multiple primary tumors, and high NLNs. LONT >0.920 was an independent protective factor for OS (HR, 0.756; 95%CI, 0.589–0.97, P =0.028) based on multivariate Cox regression analyses after adjustment for baseline demographic and clinical features (Figure 2). The floating absolute risk method provides the variance in the logarithm of the hazard ratio for each category (including the reference category) to facilitate comparisons among the different LONT categories (Table 2). Compared with the OS values of the -0.586 to 0.920 group, the adjusted HR for LONT >0.920 was 0.756 (95%CI, 0.589–0.97). Stratified analyses showed the HRs for OS across two LONT groups stratified by demographic and clinicopathologic features (Figure S2). The association between LONT and OS remained significant for male sex (P =0.026), black ethnicity (P =0.033), marital status divorced/separated (P =0.015), T4b stage (P =0.005), N status (P<0.001), grade of III–IV (P<0.001), and tumor diameter >4 cm (P<0.001).
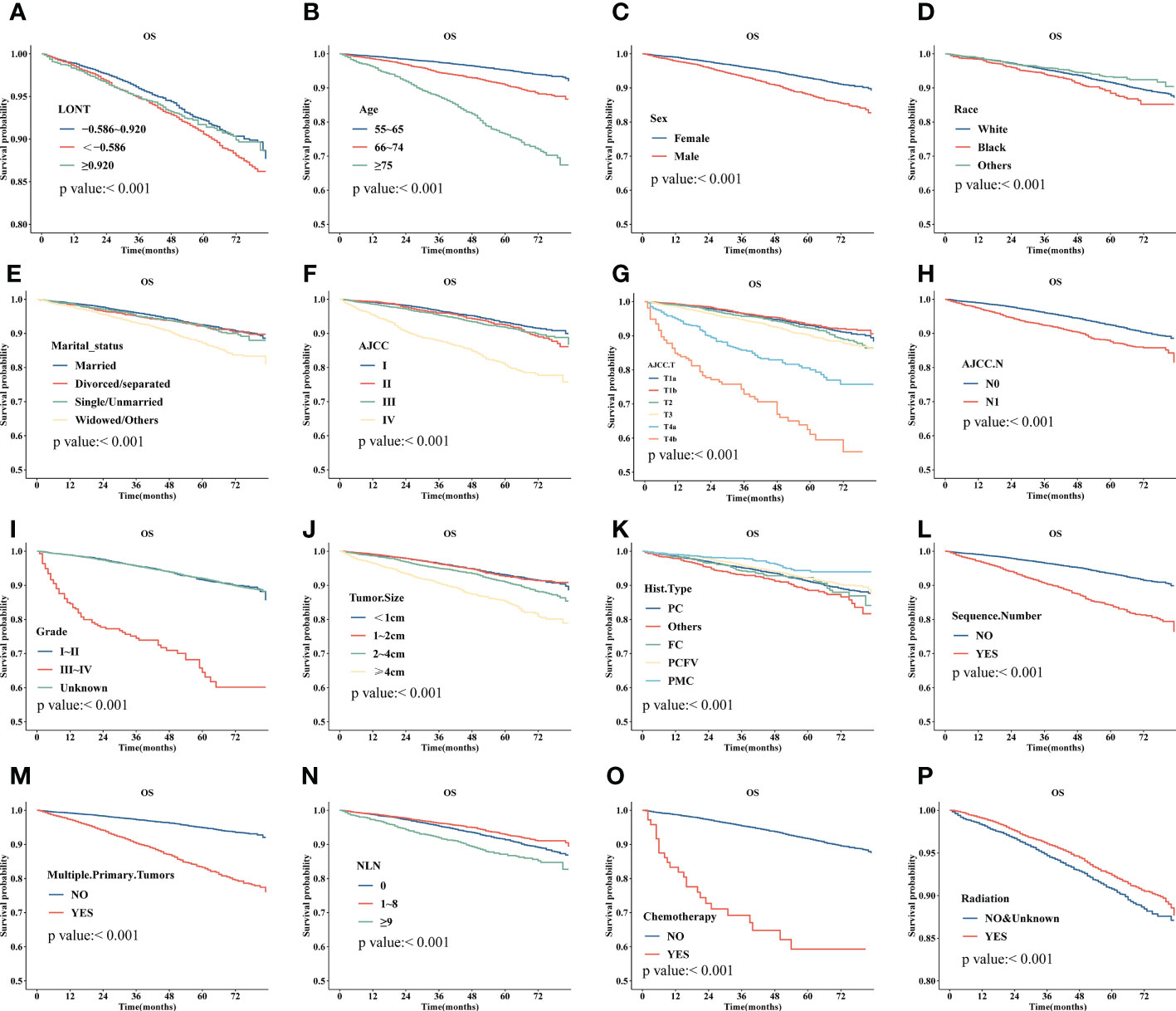
Figure 1 Kaplan–Meier plot for overall survival (OS) for LONT (A), age (B), sex (C), ethnicity (D), marital status (E), stage (based on AJCC system) (F), T stage (based on AJCC system) (G), N (based on AJCC system) (H), tumor grade (I), tumor diameter (J), histology type (K), sequence number (L), multiple primary tumors (M), NLNs (N), chemotherapy (O), and radiotherapy (P) of patients with differentiated thyroid cancer without metastases aged 55 years and older.
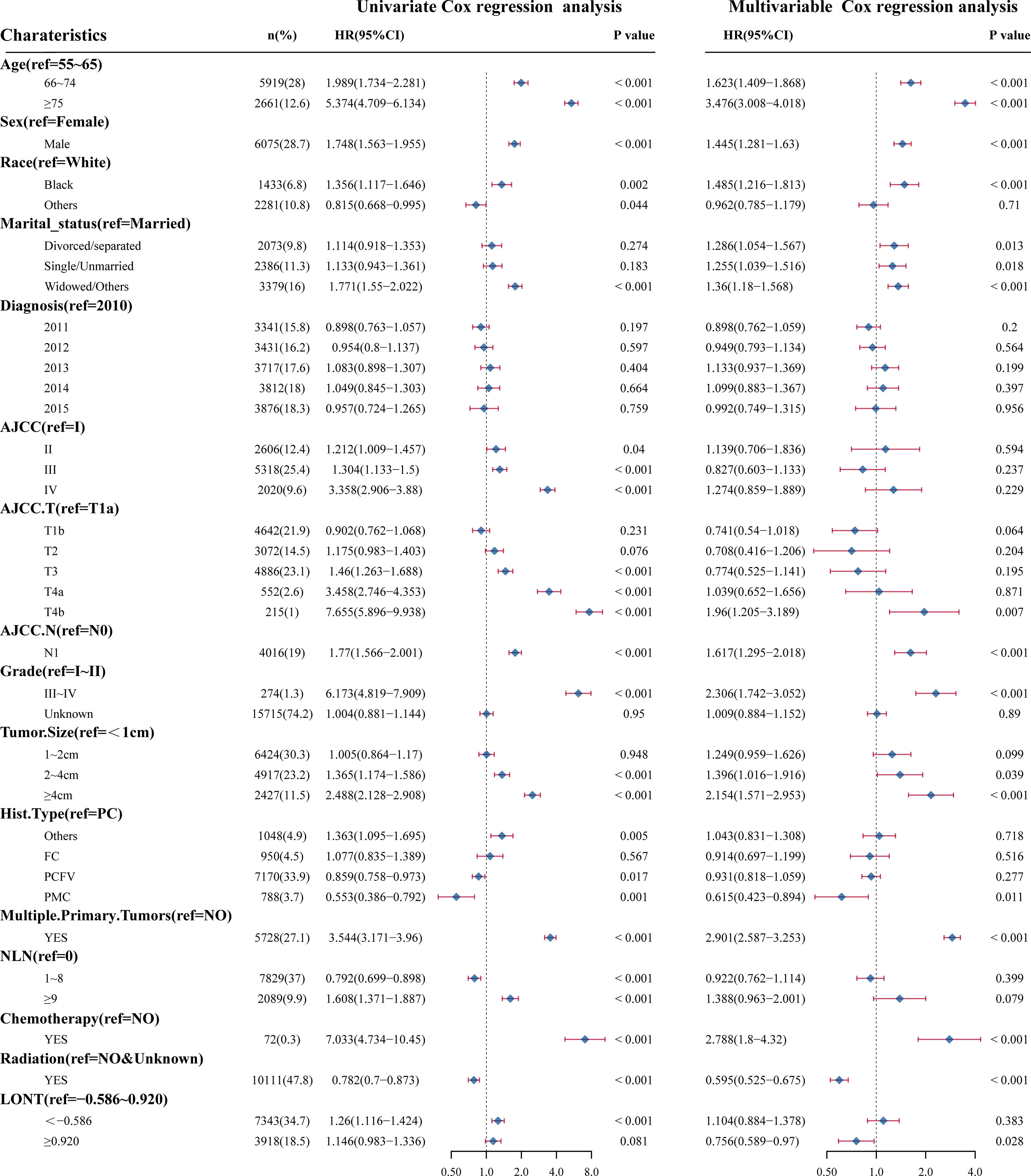
Figure 2 Univariate Cox regression analyses and multivariate Cox regression analyses for LONT and clinical features. Forest plot of hazard ratios demonstrating the results of Cox regression analyses. The dashed line denotes where HR = 1.
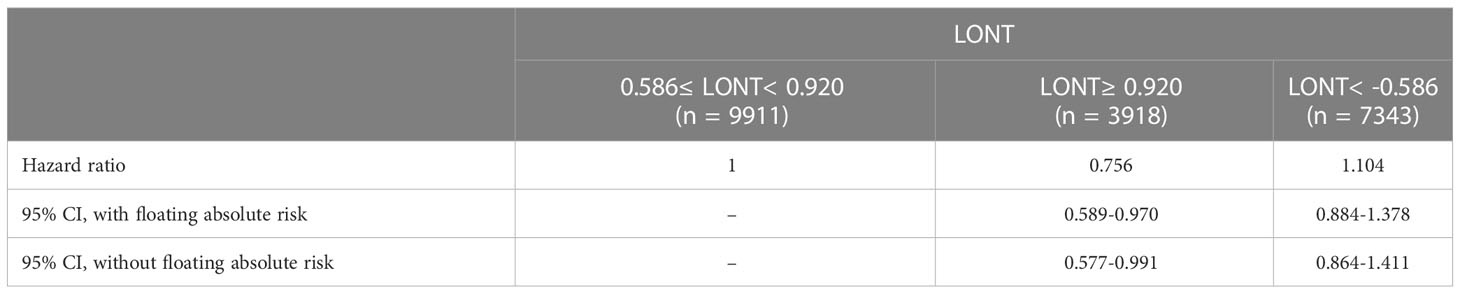
Table 2 Adjusted Hazard Ratios for OS to patients with different LONT by floating absolute risk methods.
3.4 Sensitivity analyses
Analyses undertaken using the FAR method yielded results similar to multivariate Cox regression analyses for OS. Sensitivity analyses for E-values were also conducted to evaluate the robustness of the association to unmeasured confounding (Figure S3). E-values (95% CI) were calculated for LONT ≥0.920 (vs. -0.586 to 0.920), 75 years of age and 66–74 years (vs. 55–65 years), sex, black ethnicity (vs. white ethnicity), married marital status (vs. other), multiple primary tumors, tumor diameter of >4 cm and 2–4 cm (vs.<1 cm), histology type of papillary microcarcinoma (vs. papillary carcinoma, NOS, and papillary adenocarcinoma, NOS), grade of III–IV (vs. I–II), T4b stage (vs. T1a stage), N status, chemotherapy, and radiotherapy. The respective values were as follows:1.976(1.209-2.786), 6.410(5.466-7.5), 2.629(2.168-3.141), 2.247(1.881-2.643), 2.334(1.728-3.027), 2.06(1.641-2.512), 1.892(1.293-2.51), 1.821(1.24-2.4), 5.249(4.613-5.96), 3.731(2.518-5.355), 2.140(1.143-3.241), 2.635(1.483-4.16), 4.041(2.879-5.555), 5.021(3-8.107), 3.332(1.702-5.831), 2.616(1.913-3.451), 5.021(3-8.107), and 2.750(2.326-3.218).
3.5 Conditional probability of survival
Figure 3 shows the conditional probability of survival at various time points for the study cohort combining LONT< -0.586 (Figure 3A), -0.586≤ LONT<0.920 (Figure 3B), and LONT ≥0.920 (Figure 3C) separately. In the LONT ≥0.920 group, the annual conditional probability of survival increased with OS. From a 92% chance of survival immediately after the diagnosis, the probability of OS at 1, 2, 3, and 4 years after the diagnosis increased by 93%, 95%, 97%, and 98%, respectively. The probability of OS the following year decreased from 98% to 96% at 3 years and 5 years, respectively.

Figure 3 The conditional probability of survival of LONT ≥0.920 (A), -0.586≤ LONT<0.920 (B), and LONT<-0.586 (C).
4 Discussion
The TNM staging system contains only certain characteristic indicators of tumors. For example, lymph-node status is one of the most important predictors for patients with DTC. We comprehensively analyzed the information on the number of NLNs and tumor stage after surgery. LONT was proposed as an indicator to quantify the degree of lymph-node dissection and disease severity intraoperatively and to predict the survival of patients with DTC. We undertook retrospective data analyses of 21,172 patients with DTC aged ≥55 years without distant metastasis. Multivariate COX regression analyses and FAR analyses showed that LONT ≥0.920 (vs. -0.56 to 0.92) was a protective factor for OS in patients with DTC. Sensitivity analyses demonstrated that with an E-value of 1.98 for LONT ≥0.920 (vs. -0.56 to 0.92), the results were stable even in the presence of unmeasured confounding factors. Subgroup analyses revealed that LONT correlated significantly with OS in different NLN subgroups, stage-I–II subgroups, and the N0 subgroup. The conditional probability of survival of patients with DTC improved with a prolonged survival time in the LONT ≥0.920 group.
Furthermore, an accurate prognosis for patients with TC s crucial for the postoperative treatment and follow-up plan. The TNM staging system set by the AJCC is the most widely used system for prognostic assessment. Age at TC diagnosis is an independent predictor of DSS (19, 20). The eighth version of the AJCC staging system for TC continues to adopt anatomic staging based on T, N and M. However, a cutoff for the age at the DTC diagnosis from 45 to 55 years is the best single time point for the prognostic model (4–6, 21). Patients with DTC aged ≥55 years with stage-I–IVa disease were included in our study; their prognosis is quite heterogeneous, with 10-year DSS fluctuating between 50% and 100% according to data from the AJCC staging system. These patients may achieve optimal surgical results and longer OS after additional treatment, but older patients with DTC tend to seek medical treatment late and often have comorbidities. Therefore, the risk of metastasis and disease recurrence is higher in older patients. Certain studies have found that survival in DTC patients aged<55 years is similar to that in patients with stage-III TC, and significantly lower than that in patients aged ≥55 years with stage-II TC (22, 23).
In the present study, age remained an independent predictor in the multivariate Cox regression mode. Older patients with DTC had to be stratified further according to the diameter of the primary tumor and lymph-node status. The location of metastatic lymph nodes does not affect the prognosis of older patients, while other indicators such as the number and maximum diameter of the primary tumor, maximum diameter of metastases, and external invasion of lymph nodes have been postulated as potential prognostic factors (24, 25).
Studies have shown (26–28) that the prognosis of patients with N1b-stage disease is significantly worse than that of patients with N1a-stage disease. Metastasis to lateral-neck lymph nodes is an independent risk factor for death. Moreover, indicators related to lymph-node metastasis are associated with outcomes in patients with DTC (29–31). The number of lymph nodes and the number of NLNs may reflect the degree of lymph-node dissection required. Certain studies have suggested that with greater ELNs (32) or NLNs (33), fewer micro-metastases will be missed in the lymph nodes. ELNs and NLNs are independent prognostic factors for lung (34), breast (35), and esophageal cancer (32). However, whether there are differences in DTC outcomes for different combinations of lymph-node metastasis-related indicators remains unknown.
The predictive value of tumor diameter-related parameters is controversial, but most scholars agree that a large primary tumor diameter is a clinicopathologic risk factor associated with poor DTC outcomes (36). The tumor diameter was<2 cm in 65% of patients in the present study. One meta-analysis (36) showed that patients with a tumor diameter between 1 cm and 2 cm had a greater risk of postoperative recurrence and death compared to that in patients with a tumor diameter<1 cm. Bilimoria et al. (37) undertook a retrospective study involving 52,173 patients with PTC. Compared with those who underwent total thyroidectomy, patients with a tumor diameter 1–2 cm had insufficient initial treatment, suffered recurrence, and had a poor prognosis. In the AJCC staging system, tumor diameter is not completely equivalent to the T stage, which integrates tumor diameter and gross extrathyroidal extension. Song and colleagues performed a study involving 3,104 patients undergoing thyroid surgery. The DSS of patients with gross extrathyroidal extension was significantly lower than that of patients with stage-T3 disease (38). The authors suggested adjusting the classification of patients with tumor diameter ≤4 cm from stage T3b toT2 to obtain more accurate survival predictions.
In our study, LONT comprised the number of lymph-nodes examined, the number of positive lymph nodes, and the T stage, which integrated the details of tumor diameter and gross extrathyroidal extension. LONT reflected the disease status after surgery and provided information on the degree of lymph-node dissection. In TC, the information required for complete staging may not be available for perioperative thyroidectomy. The eighth version of TNM staging by the AJCC states that information obtained within four months after the surgical procedure can be used to update T, N, and M stages. It also promotes clinical application of LONT and provides additional data for the individualized diagnosis and treatment of TC.
The current study is the first to apply LONT to quantify the relative degree of lymph-node dissection. LONT provides information on postoperative lymph-node dissection and T stage. The prognostic evaluation of patients with M0 disease and identical ELNs or NLNs is a powerful tool. The pattern of lymph-node metastasis varies among different pathologic types. More than 90% of DTC is PTC. Compared with FTC, metastasis to lymph nodes in the neck in PTC is more common and may often occur early. In one large-cohort study, the prevalence of lymph-node metastasis in PTC cases was ≤22% (39). Treatment of metastases in lymph nodes in the lateral neck in patients with DTC remains controversial. With respect to analyses of efficacy and economic perspectives, prospective studies to demonstrate the impact of different surgical scopes on the prognosis are difficult to conduct. Glover et al. (24) stated that safe implementation of prophylactic central neck dissection by an experienced surgeon can compensate for the unreliability of preoperative and intraoperative assessments of lymph-node metastasis in the central compartment. This approach can avoid the regional recurrence caused by insufficient dissection due to a narrow surgical scope and obtain more accurate PTC stages (40), reduce postoperative thyroglobulin levels, reduce the requirement for reoperation (41), reduce the risk of recurrence, and improve the OS (42). However, some studies have shown occult lymph-node metastases have a limited impact upon survival and recurrence (9). Prophylactic LND in patients with negative clinical lymph-node metastasis (cN0) expands the surgical scope and increases the risk of injuring blood vessels, nerves, and lymphatic vessels in the lateral neck (43). Guidelines for the Diagnosis and Treatment of Adult Thyroid Nodules and Differentiated Thyroid Cancer (44) published by the American Thyroid Association in 2015 recommend that conventional therapeutic LND of lymph nodes should not be carried out for patients with cN0 disease. Moreover, therapeutic LND should be undertaken for patients with DTC exhibiting lateral-neck-lymph node metastases (cN1b) by clinical and/or preoperative and intraoperative assessments. In contrast, this study proposes LONT as a new prognostic index, which does not consider the surgical approach/procedure, thus avoiding the impact of different surgical methods, while focusing on postoperative outcomes. Subgroup analyses in the present study showed a survival benefit with high LONT in patients with stage-I–II disease and N0 status. Hence, disease severity should not be underestimated in low-risk patients, and follow-up monitoring must be strengthened.
In addition, we revealed that high LONT prolonged OS significantly through adjustment of demographic and clinicopathologic factors. A high LONT value suggested a significant survival benefit. Specifically, higher the LONT values reflected, lower T stages, and fewer lymph nodes involved. Stroup and colleagues (45) retrospectively evaluated 20,513 women with DTC aged ≥40 years. They found OS and DSS to be shorter in the “regional” group (tumor confined to the thyroid gland and soft tissue) than in the “localized” group (confined to the thyroid gland). The difference in life expectancy of patients with DTC (pT1–3, pN0–1, M0) is not significant compared with that in the treated general population (46). The reason for this observation may be that the long-term survival of TC patients has been improved along with the screening and early treatment of TC. Furthermore, most studies have not distinguished subgroups by age.
Nevertheless, our study had several limitations. First, despite being based on a large cohort, our study was limited by its retrospective nature. Second, there was a selection bias for patients with TC. Third, LONT accounts for the degree of lymph-node dissection but cannot fully evaluate the success of surgical treatment or provide information on complications. Fourth, information on subsequent treatment, such as specific regimen and dose of radiotherapy and chemotherapy was not available. Fifth, the population consisted of middle-aged and older patients (≥55 years). Therefore, the results cannot be generalized to younger patient groups. Sixth, whether patients received targeted therapy or immunotherapy based on the molecular properties of TC was unknown, as we did not possess the information on their biomolecular markers. Finally, the postoperative pathology data on vascular invasion, nerve invasion, and condition of the incisal edge were not available.
5 Conclusions
LONT is a new prognostic indicator reflecting the relative degree of LND in different patients. It can predict the OS in patients aged ≥55 years without distant metastases undergoing surgical treatment regardless of preoperative and intraoperative outcomes. It could provide more valuable information for clinicians to conduct postoperative evaluations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XW: Writing – original draft (lead); writing – review and editing (equal input); formal analysis (equal input). YW: Visualization (equal input); formal analysis (equal input). XL: Visualization (equal input); formal analysis (equal input). JH: Writing – review and editing (equal input). MZ: Conceptualization (lead); writing – review and editing (equal input). All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Professor Hong’s team members (Key Laboratory of Radiation Biology of Fujian Higher Education Institutions, The First Affiliated Hospital, Fujian Medical University, Fuzhou, China) for helpful discussion and their critical reading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1132687/full#supplementary-material
Supplementary Figure 1 | Association of LONT with OS.
Supplementary Figure 2 | Subgroup analyses for LONT.
Supplementary Figure 3 | E-value of LONT and clinical features in sensitivity analyses.
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388(10061):2783–95. doi: 10.1016/s0140-6736(16)30172-6
2. Kostov GG, Dimov RS, Doykov MI. Prophylactic central lymph node dissection in differentiated thyroid cancer - benefits and risk. Folia Med (2022) 64(3):430–6. doi: 10.3897/folmed.64.e64030
3. Bulfamante AM, Lori E, Bellini MI, Bolis E, Lozza P, Castellani L, et al. Advanced differentiated thyroid cancer: A complex condition needing a tailored approach. Front Oncol (2022) 12:954759. doi: 10.3389/fonc.2022.954759
4. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, et al. Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid (2015) 25(10):1106–14. doi: 10.1089/thy.2015.0104
5. Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, et al. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol (2016) 23(2):410–5. doi: 10.1245/s10434-015-4762-2
6. Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the Ajcc/Uicc staging system for well-differentiated thyroid cancer. Thyroid (2016) 26(3):373–80. doi: 10.1089/thy.2015.0315
7. Lim YC, Choi EC, Yoon YH, Koo BS. Occult lymph node metastases in neck level V in papillary thyroid carcinoma. Surgery (2010) 147(2):241–5. doi: 10.1016/j.surg.2009.09.002
8. Palestini N, Borasi A, Cestino L, Freddi M, Odasso C, Robecchi A. Is central neck dissection a safe procedure in the treatment of papillary thyroid cancer? our experience. Langenbeck's Arch Surg (2008) 393(5):693–8. doi: 10.1007/s00423-008-0360-0
9. Hughes DT, Rosen JE, Evans DB, Grubbs E, Wang TS, Solórzano CC. Prophylactic central compartment neck dissection in papillary thyroid cancer and effect on locoregional recurrence. Ann Surg Oncol (2018) 25(9):2526–34. doi: 10.1245/s10434-018-6528-0
10. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol Off J Eur Soc Med Oncol (2019) 30(12):1856–83. doi: 10.1093/annonc/mdz400
11. Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: Clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab (2015) 100(4):1316–24. doi: 10.1210/jc.2014-3825
12. Gulmez S, Senger AS, Uzun O, Omeroglu S, Ofluoglu C, Sert ZO, et al. Prognostic significance of the metastatic lymph node ratio compared to the tnm classification in stage iii gastric cancer. Niger J Clin Pract (2021) 24(11):1602–8. doi: 10.4103/njcp.njcp_345_20
13. Li Destri G, Barchitta M, Pesce A, Latteri S, Bosco D, Di Cataldo A, et al. Predictive value of the number of harvested lymph nodes and cut-off for lymph node ratio in the prognosis of stage ii and iii colorectal cancer patients. J Invest Surg (2019) 32(1):1–7. doi: 10.1080/08941939.2017.1369605
14. İmamoğlu G, Oğuz A, Cimen S, Eren T, Karacin C, Colak D, et al. The impact of lymph node ratio on overall survival in patients with colorectal cancer. J Cancer Res Ther (2021) 17(4):1069–74. doi: 10.4103/jcrt.JCRT_11_19
15. Tausch C, Taucher S, Dubsky P, Seifert M, Reitsamer R, Kwasny W, et al. Prognostic value of number of removed lymph nodes, number of involved lymph nodes, and lymph node ratio in 7502 breast cancer patients enrolled onto trials of the Austrian breast and colorectal cancer study group (Abcsg). Ann Surg Oncol (2012) 19(6):1808–17. doi: 10.1245/s10434-011-2189-y
16. Xie J, Pang Y, Li X, Wu X. The log odds of negative lymph Nodes/T stage: A new prognostic and predictive tool for resected gastric cancer patients. J Cancer Res Clin Oncol (2021) 147(8):2259–69. doi: 10.1007/s00432-021-03654-y
17. Easton DF, Peto J, Babiker AG. Floating absolute risk: An alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med (1991) 10(7):1025–35. doi: 10.1002/sim.4780100703
18. Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Conditional probability of survival in patients with newly diagnosed glioblastoma. J Clin Oncol (2011) 29(31):4175–80. doi: 10.1200/jco.2010.32.4343
19. Kim M, Kim YN, Kim WG, Park S, Kwon H, Jeon MJ, et al. Optimal cut-off age in the tnm staging system of differentiated thyroid cancer: Is 55 years better than 45 years? Clin Endocrinol (2017) 86(3):438–43. doi: 10.1111/cen.13254
20. Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on Cancer/Tumor-Node-Metastasis staging system for differentiated and anaplastic thyroid cancer (Eighth edition): What changed and why? Thyroid (2017) 27(6):751–6. doi: 10.1089/thy.2017.0102
21. Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: Rethinking current staging systems. J Clin Oncol (2016) 34(36):4415–20. doi: 10.1200/jco.2016.68.9372
22. Berenson RA, Horvath J. Confronting the barriers to chronic care management in Medicare. Health Aff (2003), W3–37-53. doi: 10.1377/hlthaff.w3.37
23. Liu Z, Shen X, Liu R, Zhu G, Huang T, Xing M. Stage ii differentiated thyroid cancer is a high-risk disease in patients <45/55 years old. J Clin Endocrinol Metab (2019) 104(11):4941–8. doi: 10.1210/jc.2018-02809
24. Glover AR, Gundara JS, Norlén O, Lee JC, Sidhu SB. The pros and cons of prophylactic central neck dissection in papillary thyroid carcinoma. Gland Surg (2013) 2(4):196–205. doi: 10.3978/j.issn.2227-684X.2013.10.05
25. Jeon MJ, Yoon JH, Han JM, Yim JH, Hong SJ, Song DE, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol (2013) 168(2):219–25. doi: 10.1530/eje-12-0744
26. Wu MH, Shen WT, Gosnell J, Duh QY. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer. Head Neck (2015) 37(9):1336–43. doi: 10.1002/hed.23747
27. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol (2015) 33(21):2370–5. doi: 10.1200/jco.2014.59.8391
28. Kim HI, Kim TH, Choe JH, Kim JH, Kim JS, Oh YL, et al. Restratification of survival prognosis of N1b papillary thyroid cancer by lateral lymph node ratio and largest lymph node size. Cancer Med (2017) 6(10):2244–51. doi: 10.1002/cam4.1160
29. Jeon MJ, Kim WG, Kim TH, Kim HK, Kim BH, Yi HS, et al. Disease-specific mortality of differentiated thyroid cancer patients in Korea: A multicenter cohort study. Endocrinol Metab (2017) 32(4):434–41. doi: 10.3803/EnM.2017.32.4.434
30. Kim HI, Kim K, Park SY, Choe JH, Kim JH, Kim JS, et al. Refining the eighth edition ajcc tnm classification and prognostic groups for papillary thyroid cancer with lateral nodal metastasis. Oral Oncol (2018) 78:80–6. doi: 10.1016/j.oraloncology.2018.01.021
31. Kim M, Jeon MJ, Oh HS, Park S, Song DE, Sung TY, et al. Prognostic implication of N1b classification in the eighth edition of the tumor-Node-Metastasis staging system of differentiated thyroid cancer. Thyroid (2018) 28(4):496–503. doi: 10.1089/thy.2017.0473
32. Wu LL, Zhong JD, Zhu JL, Kang L, Huang YY, Lin P, et al. Postoperative survival effect of the number of examined lymph nodes on esophageal squamous cell carcinoma with pathological stage T1-3n0m0. BMC Cancer (2022) 22(1):118. doi: 10.1186/s12885-022-09207-x
33. Ogino S, Nosho K, Irahara N, Shima K, Baba Y, Kirkner GJ, et al. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol (2010) 105(2):420–33. doi: 10.1038/ajg.2009.578
34. Huang X, Hu P, Yan F, Zhang J. Establishment and validation of a nomogram based on negative lymph nodes to predict survival in postoperative patients with non-small cell lung cancer. Technol Cancer Res Treat (2022) 21:15330338221074506. doi: 10.1177/15330338221074506
35. Singh D, Mandal A. The prognostic value of lymph node ratio in survival of non-metastatic breast carcinoma patients. Breast Cancer Res Treat (2020) 184(3):839–48. doi: 10.1007/s10549-020-05885-y
36. Zhang TT, Li CF, Wen SS, Huang DZ, Sun GH, Zhu YX, et al. Effects of tumor size on prognosis in differentiated thyroid carcinoma smaller than 2 Cm. Oncol Lett (2019) 17(5):4229–36. doi: 10.3892/ol.2019.10088
37. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg (2007) 246(3):375–81. doi: 10.1097/SLA.0b013e31814697d9
38. Song E, Lee YM, Oh HS, Jeon MJ, Song DE, Kim TY, et al. A relook at the T stage of differentiated thyroid carcinoma with a focus on gross extrathyroidal extension. Thyroid (2019) 29(2):202–8. doi: 10.1089/thy.2018.0300
39. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery (2008) 144(6):1070–7. doi: 10.1016/j.surg.2008.08.034
40. Shindo M, Wu JC, Park EE, Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg (2006) 132(6):650–4. doi: 10.1001/archotol.132.6.650
41. Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for Cn0 papillary thyroid cancer. Surgery (2011) 150(6):1048–57. doi: 10.1016/j.surg.2011.09.003
42. Barczyński M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg (2013) 100(3):410–8. doi: 10.1002/bjs.8985
43. Bozec A, Dassonville O, Chamorey E, Poissonnet G, Sudaka A, Peyrottes I, et al. Clinical impact of cervical lymph node involvement and central neck dissection in patients with papillary thyroid carcinoma: A retrospective analysis of 368 cases. Eur Arch Otorhinolaryngol (2011) 268(8):1205–12. doi: 10.1007/s00405-011-1639-2
44. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
45. Stroup AM, Harrell CJ, Herget KA. Long-term survival in young women: Hazards and competing risks after thyroid cancer. J Cancer Epidemiol (2012) 2012:641372. doi: 10.1155/2012/641372
Keywords: DTC, LONT, negative lymph nodes, negative lymph nodes/T stage, prognosis
Citation: Wang X, Wu Y, Li X, Hong J and Zhang M (2023) Log odds of negative lymph nodes/T stage ratio (LONT): A new prognostic tool for differentiated thyroid cancer without metastases in patients aged 55 and older. Front. Endocrinol. 14:1132687. doi: 10.3389/fendo.2023.1132687
Received: 27 December 2022; Accepted: 01 March 2023;
Published: 22 March 2023.
Edited by:
Carla Colombo, University of Milan, ItalyReviewed by:
Loredana De Pasquale, San Paolo Hospital, ItalyZbigniew Adamczewski, Medical University of Lodz, Poland
Copyright © 2023 Wang, Wu, Li, Hong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinsheng Hong, MTM3OTkzNzU3MzJAMTYzLmNvbQ==; Mingwei Zhang, emhhbmdtaW5nd2VpMjhAc2luYS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xuezhen Wang
Xuezhen Wang Yufan Wu1,2†
Yufan Wu1,2† Jinsheng Hong
Jinsheng Hong Mingwei Zhang
Mingwei Zhang