
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 05 April 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1126637
 Elahe Meftah1*
Elahe Meftah1* Rahem Rahmati2
Rahem Rahmati2 Fatemeh Zari Meidani2
Fatemeh Zari Meidani2 Sanaz Khodadadi3
Sanaz Khodadadi3 Kosar Chitzan-Zadeh4*
Kosar Chitzan-Zadeh4* Fatemeh Esfahanian5
Fatemeh Esfahanian5 Shiva Afshar6
Shiva Afshar6Background: Subacute thyroiditis (SAT) is a self-limiting thyroid inflammatory disease occurring specifically after upper respiratory tract infections. Since COVID-19 is a respiratory disease leading to multi-organ involvements, we aimed to systematically review the literature regarding SAT secondary to COVID-19.
Methods: We searched Scopus, PubMed/MEDLINE, Cochrane, Web of Science, ProQuest, and LitCovid databases using the terms “subacute thyroiditis” and “COVID-19” and their synonyms from inception to November 3, 2022. We included the original articles of the patients with SAT secondary to COVID-19. Studies reporting SAT secondary to COVID-19 vaccination or SAT symptoms’ manifestation before the COVID-19 infection were not included.
Results: Totally, 820 articles were retained. Having removed the duplicates, 250 articles remained, out of which 43 articles (40 case reports and three case series) with a total of 100 patients, were eventually selected. The patients aged 18–85 years (Mean: 42.70, SD: 11.85) and 68 (68%) were women. The time from the onset of COVID-19 to the onset of SAT symptoms varied from zero to 168 days (Mean: 28.31, SD: 36.92). The most common symptoms of SAT were neck pain in 69 patients (69%), fever in 54 (54%), fatigue and weakness in 34 (34%), and persistent palpitations in 31 (31%). The most common ultrasonographic findings were hypoechoic regions in 73 (79%), enlarged thyroid in 46 (50%), and changes in thyroid vascularity in 14 (15%). Thirty-one patients (31%) were hospitalized, and 68 (68%) were treated as outpatients. Corticosteroids were the preferred treatment in both the inpatient and outpatient settings (25 inpatients (81%) and 44 outpatients (65%)). Other preferred treatments were nonsteroidal anti-inflammatory drugs (nine inpatients (29%) and 17 outpatients (25%)) and beta-blockers (four inpatients (13%) and seven outpatients (10%)). After a mean duration of 61.59 days (SD: 67.07), 21 patients (23%) developed hypothyroidism and thus, levothyroxine-based treatment was used in six of these patients and the rest of these patients did not receive levothyroxine.
Conclusion: SAT secondary to COVID-19 seems to manifest almost similarly to the conventional SAT. However, except for the case reports and case series, lack of studies has limited the quality of the data at hand.
Although COVID-19 was initially considered a respiratory tract disease, it is currently identified as a systemic infection affecting multiple organs. Some organs that may be affected by COVID-19 are the respiratory, cardiovascular, hematopoietic, gastrointestinal, urinary, nervous, musculoskeletal, and endocrine systems (1). Despite this fact that several preventive measures have brought the COVID-19 pandemic almost under control, there are some uncertainties regarding the potentially prolonged sequela of COVID-19.
A significant proportion of COVID-19 patients report symptoms known as long-term COVID-19 symptoms that persist long after recovery. The term “long-term COVID-19” or “long COVID” refers to lack of complete recovery within two or three weeks after the initial manifestations of COVID-19 (2). Multi-organ involvement is responsible for long-term COVID-19 symptoms, including fatigue, dyspnea, alopecia, hyperhidrosis, insomnia, anxiety, arthralgia, weight loss, fever, body pains, carditis, and persistent reduction of lung function (3), some of which are common among other conditions, such as subacute thyroiditis. This overlap might result in misdiagnosing those other conditions as long-term COVID-19 symptoms and the patients receiving ill-suited managements (4).
Subacute thyroiditis (SAT) is a self-limiting thyroid inflammatory disease characterized by symptoms like anterior neck pain that may radiate to the ears and jaw (4). The potential systemic and laboratory findings of SAT are low-grade fever, fatigue, elevated C-reactive protein (CRP), increased erythrocyte sedimentation rate (ESR), and the suppression of thyroid-stimulating hormone (TSH) (5). Thyroid gland tenderness and small diffuse goiter may be detected in the physical examination. The clinical course of SAT is divided into three phases: thyrotoxicosis, hypothyroidism, and normal thyroid function. At the time of diagnosis, most patients display mild to moderate thyrotoxicosis manifestations, such as weight loss, palpitations, and tremors (6).
SAT is most commonly associated with a viral infection. The evidence of this association includes multiple antibodies for certain viruses, a prior upper respiratory tract infection, seasonal patterns, and case clusters (7). SAT has been linked with several respiratory viruses, including Epstein-Barr virus (8), coxsackievirus (9), and influenza virus (10). Nevertheless, there is currently no clear evidence as to decide if the follicular damage in SAT is caused by a direct viral infection of the gland or by an immune response to the virus (4). Angiotensin-converting enzyme 2 (ACE2), the receptor through which SARS-CoV-2 invades the cells, is expressed in many extrapulmonary tissues like thyroid follicular cells. Accordingly, the thyroid gland may be a potential target for viral damage because of its high ACE2 expression. COVID-19 can also affect thyroid function through other mechanisms, i. e, the autoimmune effects on the thyroid mediated by cytokine storm (11–13).
Because of the high susceptibility of thyroid involvement and inflammation during COVID-19, it is required to find out the relationship between these two disorders. As a result, the current systematic review targets to investigate and summarize the published articles exclusively focusing on COVID-19 infection-associated SAT.
The present systematic review was performed pursuant to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (14). The search strategy was developed using the Medical Subject Headings (MeSH) related terms/subheadings, including “Subacute Thyroiditis” and “COVID-19”. The following combination was searched for the subacute thyroiditis concept: “Subacute Thyroiditides” OR “Subacute Thyroiditis” OR “Subacute Painful Thyroiditis” OR “Painful Thyroiditis,” Subacute OR “Granulomatous Thyroiditides” OR “Subacute Nonsuppurative Thyroiditis” OR Thyroiditis, “Subacute Nonsuppurative” OR “De Quervain Thyroiditis” OR Thyroiditis, “De Quervain” OR “Giant Cell Thyroiditis” OR Thyroiditis, “Giant Cell.” Considering the concept of COVID-19, we searched the following combination: “COVID-19” OR COVID19 OR “SARS-CoV-2” OR “Coronavirus Disease-19” OR “SARS Coronavirus 2” OR “Coronavirus Disease 2019 Virus” OR “2019 Novel Coronavirus” OR “2019-nCoV” OR “Severe Acute Respiratory Syndrome Coronavirus 2”. The relevant studies published up to December 22, 2021, were searched through Scopus, PubMed/MEDLINE, Cochrane, Web of Science, ProQuest, and LitCovid databases. The search strategy was modified to fit each database. No restriction was imposed on the language or the time of publication. We updated the search on November 3, 2022, and extracted all the articles added to the above mentioned databases up to the stated date. Manual backward and forward citation searches were also conducted in order not to miss any of the publications.
Figure 1 depicts the completed search strategy results in the PRISMA flowchart. A total of 250 articles were retained after duplicate reports were removed by the Endnote bibliographic management application and then manually. The inclusion criteria of this systematic review are as follows:
1. The original articles describing the patients with confirmed SAT secondary to COVID-19.
2. Polymerase-Chain Reaction (PCR) and/or serologic confirmation of COVID-19.
3. SAT diagnosis by thyroid function tests and/or at least one imaging modality (thyroid ultrasound or radionuclide study) or cytology confirmation (fine needle aspiration).
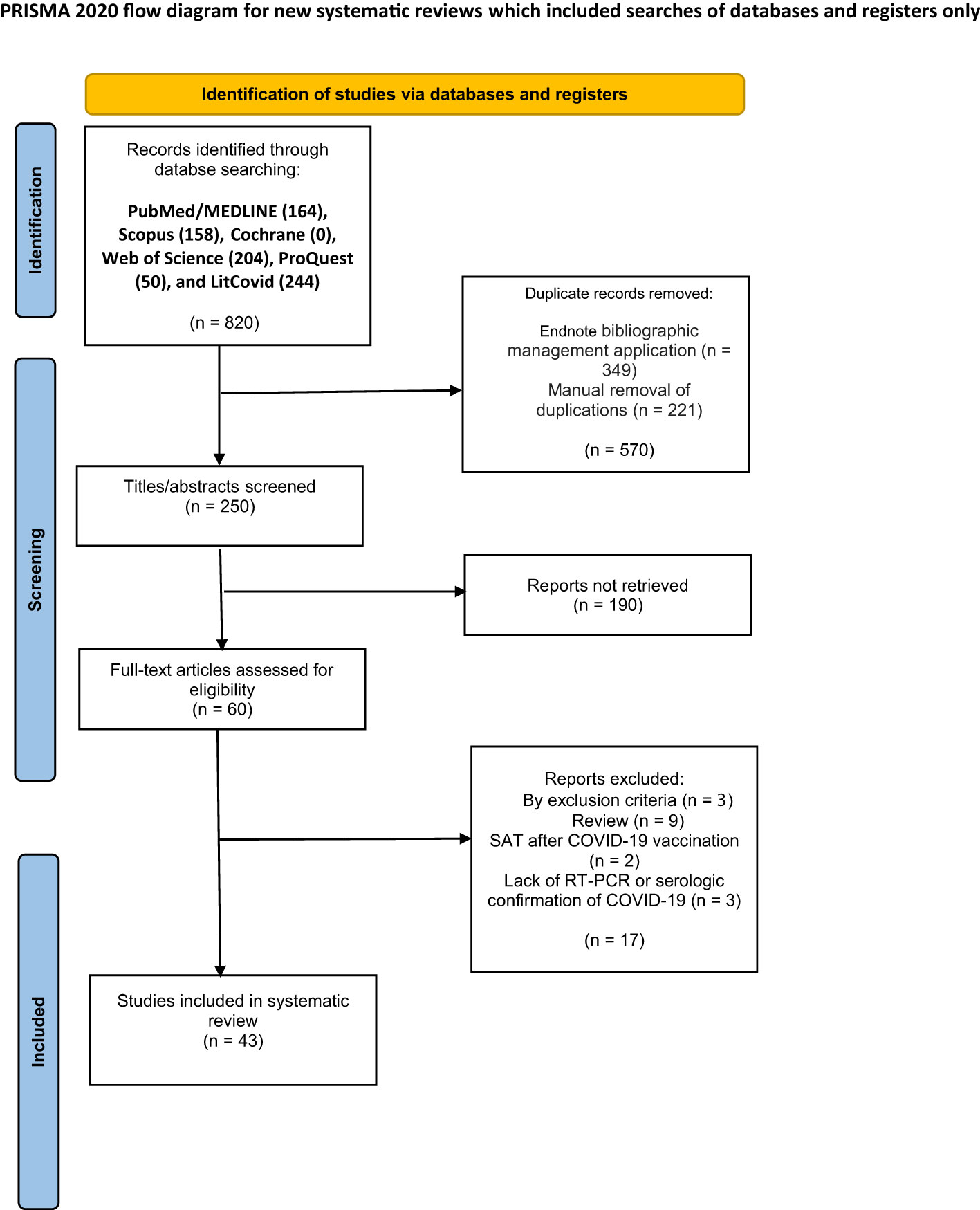
Figure 1 Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram of the study selection for the systematic review.
Reviews, comments, expert opinions, studies reporting SARS-CoV-2 vaccine-associated SAT, and studies with SAT symptoms manifesting before COVID-19 were not included.
The titles and abstracts of the retained publications were independently screened by three authors (EM, KC, and SA) in two different phases to determine whether they met the requirements to be included. Following that, the authors (EM, KC, SK, RR, FZM, and SA) screened the full texts of the retained publications to assess their eligibility for inclusion.
The relevant information was extracted from the included publications and sorted into three categories:
1. The general information of the study: document type, the year of publication, the number of the study patients, authors, and the country of origin.
2. Patient data: the evaluation setting, gender, age, comorbidities, COVID-19 symptoms, and PCR or serology results for COVID-19. If the details of the COVID-19 symptoms were not reported, we only extracted the severity of COVID-19 (mild, moderate, or severe) as the classification by the World Health Organization required (15).
3. SAT Characteristics: the symptoms, physical examination findings, relevant laboratory tests, thyroid ultrasound, thyroid scan findings, fine needle aspiration (FNA) findings, the timing between respiratory and thyroid manifestations, management, outcome, and follow-up duration.
Having extracted the above-mentioned data, the publications were screened regarding their quality of conduction and reporting. We used two relevant checklists, i. e., the Joanna Briggs Institute Critical Appraisal tools for use in Systematic Reviews—the Checklist for Case Reports and the Checklist for Case Series (16), based on which, the case reports and the case series were scored from zero to eight and ten, respectively. The quality of each publication was assessed by two authors individually, and a third author would decide on the score in case of disagreement.
Forty-three articles [40 case reports (17–56) and three case series (57–59)] with 100 cases of SAT following COVID-19 were included in the present systematic review (Figure 1). The patients’ age ranged from 18 to 85 years (Mean: 42.70, SD: 11.85), and 68 (68%) of them were women. Comorbidities were reported in 10 patients, as stated in Supplementary Table 1. Eleven patients were reported as free from any comorbidities, and the presence or absence of comorbidities was not reported in the remaining 79 patients (79%). The cases were reported from Europe (36 cases, 36%), the Middle East (35 cases, 35%), Asia (21 cases, 21%), North America (seven cases, 7%), and South America (three cases, 3%). The details regarding the distribution of the countries are listed in Supplementary Table 2.
The time from the onset of COVID-19 to the onset of SAT symptoms varied from zero to 168 days (Mean: 28.31, SD: 36.92). In 9 patients (10%), COVID-19 and SAT were simultaneously diagnosed. Among the remaining 83 patients (89%), the mean duration between COVID-19 and SAT symptoms was reported as 33.51 days (SD: 33.69). The diagnosis was confirmed by SARS-CoV-2 PCR and the presence of IgG/IgM against SAR-COV-2 in 83 and 17 cases, respectively. In 65 patients, the following COVID-19 symptoms were detected: fever (27 cases, 36%), cough (20 cases, 27%), fatigue (14 cases, 19%), myalgia (12 cases, 16%), dyspnea (12 cases, 16%), anosmia (eight cases, 11%), sore throat (seven cases, 9%), headache (seven cases, 9%), and other less frequent symptoms as listed in Supplementary Table 1. The symptoms of 22 other patients were divided into three groups of mild (10 cases), moderate (seven cases), and severe COVID-19 (five cases). Ten patients (13%) had no symptoms of COVID-19, and the symptoms of COVID-19 were not reported in three patients.
Based on the data provided in Supplementary Table 3, the most prevalent symptoms in COVID-19-associated SAT were neck pain (69 cases, 69%), fever (54 cases, 54%), fatigue and weakness (34 cases, 34%), persistent palpitations (31 cases, 31%), tachycardia (15 cases, 15%), sweating (14 cases, 14%), and thyroid enlargement (14 cases, 14%). Mild to severe unilateral or bilateral neck pain radiating to the ear, jaw, or even the submandibular area was the main feature of the neck pain. Psychiatric symptoms such as agitation, irritability, anxiety, and mood changes were reported by three (3%), three (3%), one (1%), and one (1%) of the patients, respectively. Other less frequent symptoms were weight loss, tremor, myalgia, sore throat, cough, odynophagia, dysphagia, diarrhea, insomnia, chills, heat intolerance, and alopecia. The details of the symptoms are displayed in Supplementary Table 1. The symptoms of SAT were not mentioned in two patients (numbers 57 and 82). One of the patients out of 100 ones (number 40) had no symptoms associated with SAT. In this patient, the diagnosis was confirmed by the thyroid ultrasound and fine needle aspiration (FNA).
Heterogeneous findings were obtained from the thyroid ultrasonography on 92 patients. The results were as it follows: bilateral hypoechoic regions (61 cases, 66%), enlarged thyroid (46 cases, 50%), reduced vascularity (12 cases, 13%), isolated unilateral hypoechoic regions (12 cases, 13%), increased vascularity (two cases, 2%), and normal appearance (one case, 1%). In two (2%) of the 92 cases with reported ultrasonography results, it was only reported that the findings were “compatible with SAT” without any other details of the ultrasonography findings. Thyroid scintigraphy was performed in 34 cases (34%), demonstrating significantly-reduced or absent uptake in the thyroid gland in all these cases. In 15 cases (15%), the FNA of the thyroid gland was utilized in order to confirm the diagnosis.
As mentioned in Supplementary Table 4, the CRP level was measured in 87 patients and ranged from 1.05 mg/L to 347.22 mg/L (Mean: 64.00, SD: 71.49). The CRP was reported qualitatively “high” in five more patients. The CRP increased in 74 patients (85%), while ESR got elevated in 82 patients out of 83 ones (99%) with a reported ESR level and ranged from 18 to 140 mm/h (Mean: 65.87, SD: 28.30). In one patient with a normal ESR, the CRP was significantly increased. Thirteen individuals had normal white blood cell (WBC) count, 15 had raised WBC levels, and two had leukopenia. All patients had abnormal thyroid function tests at the onset of the disease, with TSH levels ranging from less than 0.001 to 1.53 mIU/L (Mean: 0.08, SD: 0.23), the FT4 levels from 0.96 to 7.7 ng/dl, and the FT3 levels from 2.07 to 21.6 ng/dL. Twenty-five patients had the reported serum thyroglobulin levels ranging from 2.4 to 187 ng/ml. Thyroglobulin was low in two patients who had auto-antibodies against thyroglobulin. Thyroid antibodies were reported as thyroid peroxidase antibody (anti-TPO) in 48, thyroglobulin antibody (anti-Tg) in 32, thyroid-stimulating immunoglobulin (TSI) in 16, and thyrotropin receptor antibody (TRAb) in 24 patients. Anti-TPO and anti-Tg were positive in eight (17%) and six patients (18%), respectively, with a maximum of 96.71 IU/ml and 512 IU/ml, respectively. TRAb and TSI results were all negative.
Thirty-one patients (31%) were admitted to the hospital, with 24 ones hospitalized because of SAT symptoms and six hospitalized due to COVID-19. Most of these cases were treated with prednisolone 20 to 50 mg daily (19 patients, 61%), aspirin combined with other drugs (three cases, 9%), aspirin alone (three cases, 9%), atenolol combined with corticosteroids (three cases, 9%), and dexamethasone 8 to 24 mg daily (three cases, 9%). Other prescribed drugs were methimazole (two cases, 6%), propranolol (one case, 3%), ibuprofen (one case, 3%), naproxen (one case, 3%), celecoxib (one case, 3%), and paracetamol (one case, 3%).
Sixty-eight patients (68%) were treated as outpatients, most of whom were treated with corticosteroids like prednisone and methylprednisolone (43 patients, 63%) or nonsteroidal anti-inflammatory drugs (NSAIDs) such as naproxen, ibuprofen, and aspirin (17 patients, 25%). The other medications utilized in the treatment of these patients were beta-blockers (seven cases, 10%), paracetamol (one case, 2%), colchicine (one case, 2%), and dexamethasone (one case, 2%). Two patients (3%) did not receive any treatment, and the treatment plan was not described for three other patients. The duration of the follow-up was reported in 86 patients and ranged from 4 days to 12 months (Mean: 61.59 days, SD: 67.07). The follow-ups included evaluating the clinical symptoms, imaging, and laboratory studies. The findings of the follow-ups were reported in 90 patients. Of twenty-one patients (23%) who developed hypothyroidism in the follow-up, six ones (6% of the total population) were treated with levothyroxine and the rest of the patients received no medication for hypothyroidism.
Thyroid dysfunction has been reported in numerous patients during the COVID-19 pandemic, indicating that SARS-CoV-2 may have played a role in thyroid damage either during or after the infection. Compared to other subtypes of thyroiditis, SAT has been more commonly linked to viral infections, particularly those of the upper respiratory tract, such as coronaviruses (11). Thyrotoxicosis, hypothyroidism, and sick euthyroid syndrome are three common thyroid complications among the effects of SARS-CoV-2 on the thyroid gland (12). Consequently, predicting thyroid function in COVID-19 patients is challenging.
SARS-CoV-2 has the potential to destroy the thyroid tissue directly or indirectly. The most accepted mechanism involves direct viral damage through transmembrane serine protease 2 and angiotensin-converting enzyme 2 receptors on the surface of the thyroid follicular cells (60, 61). In addition to the mentioned mechanism, other mechanisms, such as indirect immune-mediated responses and medicines prescribed for COVID-19, are also thought to be responsible for thyroid damage (11–13).
Prior viral infection is considered a risk factor in genetically susceptible individuals. As argued by Stasiak et al., the presence of either HLA-B*35 or HLA-C*04:01 alleles or both are genetic markers of susceptibility to SAT. As this theory states, HLA-mediated tissue destruction occurs through either cross-reaction with host antigens or auto-antibody formation against the large amount of self-antigens released from the damaged thyroid tissue. Although no HLA testing was performed on any of the cases we reviewed, Stasiak et al. suggest that the presence of novel high-risk alleles, including HLA-B*18:01 and HLA-DRB1*01, may deteriorate the clinical course of SAT (62, 63). Another hypothesis involves molecular mimicry; a recent in-vitro investigation revealed that antibodies against SARS-CoV-2 antigens may cross-react with self-tissue antigens, such as TPO. This mechanism is a putative pathogenic trigger for immune-mediated adverse effects following SARS-CoV-2 infection and immunization (63).
Similar to classic SAT and prior reviews on COVID-19-associated SAT (12), the current systematic review study was performed on the patients aged 18 to 85 years, with a majority of women (68%). The age range in the present study was longer than that of several previously-published reviews (4, 11, 64–66). In line with the most prevalent COVID-19 symptoms (66, 67), fever and cough were the two most frequent complaints of the patients during COVID-19 infection and before SAT manifestations. Moreover, the asymptomatic patients and those with severe COVID-19 symptoms, as defined by the WHO (15), had similar SAT presentations.
In the present study, the time from the beginning of COVID-19 infection to the onset of SAT ranged from zero to 168 days, which is longer than that of previous studies (4, 11, 59, 65, 66). There were two patients with a lag of 168 days between COVID-19 diagnosis and SAT manifestation. Although the relatively prolonged lag may question the correlation between these two entities, we included these two cases based on their positive serology (IgM, IgG) when SAT manifested (58). Similar to classic SAT and prior reviews (4, 11, 64–66), the most prevalent symptom was neck pain (69%) in the present study. Fever (54%) was the second most common symptom in the present study, congruent with merely two of the previously conducted studies (11, 66). The early cases of COVID-19-associated SAT generally reported suffering from severe neck discomfort. In contrast, the authors of observational studies of COVID-19 patients in intensive care units described uncommon cases of painless SAT with no local symptoms (30, 59). Although we discovered heterogeneity among the inpatient cases, it is proposed that insufficient lymphocytic infiltration within the thyroid gland due to COVID-19-associated lymphopenia results in the absence of tension of the thyroid capsule and, thus, painless presentations of thyroiditis (59). Furthermore, the growing evidence supports the complicated connection between thyroid hormones and CNS neurotransmitters, which has the potential to bring about neuropsychiatric symptoms and raise patient risks (68, 69). Nevertheless, the outcomes in our cases with the stated disorders were similar, and the treatment was successful.
Consistent with early reviews (4, 11, 64–66), we observed at least one of the ESR or CRP variables elevating in all patients. The ESR increased in the majority of the cases (99%), while the CRP increased in 85%. The mean ESR observed in our study was 65.87 mm/hr; however, ESR in SAT might increase up to three-digit values (70). Additionally, nearly 60% had low TSH levels during the early phase of the illness, with high free T3 and free T4 levels. Following the classic SAT (11, 25, 71), 13 individuals had normal WBC levels, 15 cases had elevated WBC levels, as being also indicated in a few patients in another study (65), while two revealed leukopenia in contrast.
The clinical SAT symptoms might not be typical; thus, thyroid ultrasonography may help to get to the correct diagnosis. In this regard and in agreement with almost all the previous cases (4, 11, 64–66), 92 patients in this review had the classic sonographic findings of SAT, which include bilateral or unilateral, localized or multifocal, and poorly defined hypoechoic regions (72). The previously conducted studies also found that in order to assess thyroid function, radionuclide scanning combined with ultrasound examination could significantly increase the diagnostic rate of SAT. In the mentioned instances, radionuclide uptake in the thyroid gland ranges from very low or absent in the hyperthyroid phase to a normal appearance in the late recovery phase (73, 74). Confirming the findings of the previous studies (4, 64, 65), there was a significant decrease in radionuclide uptake in all 34 cases undergoing thyroid scintigraphy. A previously firm thyroid nodule can rapidly grow in SAT setting, initially suggesting thyroid malignancy (70). Thus, the clinicians are obliged to be aware of this matter that SAT might present as suspicious nodular lesions on ultrasound and may require thyroid FNA, which is rarely used in diagnostic workups. However, one study suggested that margin, vascularity, and echogenicity may help differentiate the characteristics of SAT and malignancy and thus, the requirement for FNA gets reduced (75). Fifteen of the patients we reviewed underwent an FNA to confirm the diagnosis.
The serologic evaluations from previous studies frequently reveal normal thyroid antibody levels in cases with classic SAT. In fact, some trials reported positive transient results of anti-TPO, TSI, anti-Tg, and TRAb in some non-autoimmune thyroid diseases, including SAT, suggesting that immunological hyperactivity in COVID-19 might have led to the formation of these antibodies (4, 12). In our study, TRAb and TSI were negative in all 24 and 16 cases being screened in this respect. However, anti-TPO was assessed in 48 cases and was found positive in eight patients. Moreover, out of 32 individuals who had anti-Tg results, six were positive. Two of these patients with positive anti-Tg had normal thyroglobulin levels, possibly due to the presence of this autoantibody and its effect on thyroglobulin (76). Similarly, some relevant reviews reported the new detection of autoantibodies in SAT patients (4, 11, 64, 65). Based on our findings and consistent with a previous study (4), the patients with positive and negative auto-antibodies received various treatments, and all recovered comparably.
Thirty-one patients had to be hospitalized due to their risk factors or the severity of their symptoms. However, their medical procedures and results were comparable to those of the patients with less severe conditions. Therefore, NSAIDs, beta-blockers, and glucocorticoids may occasionally be advised due to the self-limiting nature of SAT (11). In this respect, 87 patients of 100 cases (87%) in our review received either of these three categories of drugs. Sixty-six received glucocorticoids, 26 NSAIDs, 11 beta-blockers, two methimazole, and one colchicine. For three patients, the treatment was not reported, and two patients did not receive any treatment. Based on the literature, glucocorticoids (4, 11, 64–66) are the preferred treatment for SAT secondary to COVID-19, and beta-blockers (4, 11, 64, 65) and NSAIDs (4, 64) are other prescribed medications, respectively.
The three clinical manifestations of SAT are hyperthyroidism, transient hypothyroidism, and normal thyroid function (13). SAT patients often require three months to return to a normal euthyroid condition (25, 59). Nevertheless, with the available medications, the duration from the symptom improvement to complete recovery ranges from two days to three months. Moreover, only about one-fourth of our patients showed temporary hypothyroidism until the end of their follow-up. Previous reviews with smaller sample sizes (4, 11, 64–66) revealed 10% to 36.5% hypothyroidism incidence during follow-up. Thus, about 10% of the individuals could develop persistent hypothyroidism (12), which was not reported in the studied cases of the present study. Besides, a follow-up period of up to twelve months was taken into account to prevent the potential adverse outcomes. However, no serious adverse event was reported.
The present study reviewed the current literature systematically and comprehensively regarding the characteristics and manifestations of subacute thyroiditis in COVID-19. However, this study had its own limitations. The case reports and the case series constituted the majority of the included articles, affecting the quality of the results in our study. Additionally, some variables of interest were not reported in the included studies, which limited an otherwise more comprehensive review.
We reviewed all the relevant cases with the required details—including history, physical examination, lab tests, and imaging—to be able to ascertain the earlier relevant findings. Although we found no significant clinical differences between the typical and post-COVID-19 SAT, the immunological, viral, or pharmaceutical reactions may cause minor discrepancies between the classic and COVID-19-associated SAT. The literature is controversial on how post-viral SAT symptoms, thyroid function problems, and medications may affect the findings.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
EM and KC-Z designed the study. EM performed the bibliographic search, prepared the tables, and critically revised the manuscript. EM, SK, RR, FZM, KC-Z, and SA screened the articles, extracted the data, and assessed the quality of the included articles. KC-Z, SK, RR, and FZM wrote the initial version of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1126637/full#supplementary-material
1. Temgoua MN, Endomba FT, Nkeck JR, Kenfack GU, Tochie JN, Essouma M. Coronavirus disease 2019 (COVID-19) as a multi-systemic disease and its impact in low- and middle-income countries (LMICs). SN Compr Clin Med (2020) 2(9):1377–87. doi: 10.1007/s42399-020-00417-7
2. Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: The persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne) (2021) 8:653516. doi: 10.3389/fmed.2021.653516
3. Chakraborty T, Jamal RF, Battineni G, Teja KV, Marto CM, Spagnuolo G. A review of prolonged post-COVID-19 symptoms and their implications on dental management. Int J Environ Res Public Health (2021) 18(10). doi: 10.3390/ijerph18105131
4. Trimboli P, Cappelli C, Croce L, Scappaticcio L, Chiovato L, Rotondi M. COVID-19-Associated subacute thyroiditis: Evidence-based data from a systematic review. Front Endocrinol (Lausanne) (2021) 12:707726. doi: 10.3389/fendo.2021.707726
5. Alfadda AA, Sallam RM, Elawad GE, Aldhukair H, Alyahya MM. Subacute thyroiditis: Clinical presentation and long term outcome. Int J Endocrinol (2014) 2014:794943. doi: 10.1155/2014/794943
6. Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord (2021) 22(4):1027–39. doi: 10.1007/s11154-021-09648-y
7. Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med (2008) 47(8):725–9. doi: 10.2169/internalmedicine.47.0740
8. Nakamura T, Kakimoto H, Morita S, Mizota M, Iwamoto J. Subacute thyroiditis presenting with creeping in a 6-year-old boy. Clin Pediatr Endocrinol (2021) 30(1):75–8. doi: 10.1297/cpe.30.75
9. Volpé R, Row VV, Ezrin C. Circulating viral and thyroid antibodies in subacute thyroiditis. J Clin Endocrinol Metab (1967) 27(9):1275–84. doi: 10.1210/jcem-27-9-1275
10. Dimos G, Pappas G, Akritidis N. Subacute thyroiditis in the course of novel H1N1 influenza infection. Endocrine (2010) 37(3):440–1. doi: 10.1007/s12020-010-9327-3
11. Aemaz Ur Rehman M, Farooq H, Ali MM, Ebaad Ur Rehman M, Dar QA, Hussain A. The association of subacute thyroiditis with COVID-19: A systematic review. SN Compr Clin Med (2021) 3(7):1515–27. doi: 10.1007/s42399-021-00912-5
12. Popescu M, Ghemigian A, Vasile CM, Costache A, Carsote M, Ghenea AE. The new entity of subacute thyroiditis amid the COVID-19 pandemic: From infection to vaccine. Diagnostics (2022) 12(4):960–. doi: 10.3390/diagnostics12040960
13. Sariyildiz GT, ÇİÇEk C, Arslan Aİ, DemİR ME. COVID-19 and COVID-19 vaccines-related subacute thyroiditis: analysis of a case series. Anatolian Curr Med J (2022) 4(2):138–45. doi: 10.38053/acmj.1061185
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. System Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
15. Who. Coronavirus disease (COVID-19) [Internet]. (2022) [cited 2022 Sep 20]. Available from: https://www.who.int/health-topics/coronavirus#tab=tab_3.
16. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute. (2017). Available from https://reviewersmanual.joannabriggs.org/.
17. Abreu R, Miguel R, Saieg M. Subacute (De quervain) thyroiditis during the COVID-19 pandemic. Cancer Cytopathol (2021) 129(11):844–6. doi: 10.1002/cncy.22449
18. Al-Shammaa MS, Abdlkadir AS. A case of post COVID-19 subacute thyroiditis. Clin Case Rep (2022) 10(7):e6092. doi: 10.1002/ccr3.6092
19. Álvarez Martín MC, Del Peso Gilsanz C, Hernández López A. Subacute de quervain thyroiditis after SARS-CoV-2 infection. Endocrinol Diabetes Nutr (Engl Ed) (2021) 68(10):754–755. doi: 10.1016/j.endien.2020.10.010
20. Ashraf S, Imran MA, Ashraf S, Hafsa H-T, Khalid S, Akram MK, et al. COVID-19: A potential trigger for thyroid dysfunction. Am J Med Sci (2021) 362(3):303–7. doi: 10.1016/j.amjms.2021.05.013
21. Ayhan M, Guner R. A subacute thyroiditis case after SARS-CoV-2 infection: A case report and current literature review. Haseki Tip Bulteni (2021) 59:54–6. doi: 10.4274/haseki.galenos.2021.7126
22. Baykan EK, Carlioglu A. Subacute thyroiditis secondary to SARS-CoV-2 infection; case studies from Turkey. Turkish J Endocrinol Metab (2021) 25(2):232–7. doi: 10.25179/tjem.2020-80585
23. Brancatella A, Ricci D, Cappellani D, Viola N, Sgrò D, Santini F, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? insights from a case series. J Clin Endocrinol Metab (2020) 105(10). doi: 10.1210/clinem/dgaa537
24. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after sars-COV-2 infection. J Clin Endocrinol Metab (2020) 105(7). doi: 10.1210/clinem/dgaa276
25. Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M. Subacute thyroiditis associated with COVID-19. Case Rep Endocrinol (2020) 2020:8891539. doi: 10.1155/2020/8891539
26. Chakraborty U, Ghosh S, Chandra A, Ray AK. Subacute thyroiditis as a presenting manifestation of COVID-19: A report of an exceedingly rare clinical entity. BMJ Case Rep (2020) 13(12):e239953. doi: 10.1136/bcr-2020-239953
27. Chong WH, Shkolnik B, Saha B, Beegle S. Subacute thyroiditis in the setting of coronavirus disease 2019. Am J Med Sci (2020) 361(3):400–402. doi: 10.1016/j.amjms.2020.09.011
28. Davoodi L, Oladi Z, Jafarpour H, Zakariaei Z, Soleymani E, Razavi A. A 33-year-old man with COVID-19 presented with subacute thyroiditis: A rare case report and literature review. New Microbes New Infect (2021) 41:100871. doi: 10.1016/j.nmni.2021.100871
29. de la Higuera López-Frías M, Perdomo CM, Galofré JC. Subacute thyroiditis following COVID-19 infection. Rev Clin Esp (2021) 221(6):370–2. doi: 10.1016/j.rceng.2021.01.002
30. Dolkar T, Jitidhar F, Patel MJ, Hamad AM, Salauddin F, Shiferaw-Deribe Z, et al. Painless subacute thyroiditis in a patient with acute COVID-19 infection: A transient event. Cureus (2022) 14(7):e26924. doi: 10.7759/cureus.26924
31. Elawady SS, Phuyal D, Shah RK, Mirza L. A case of subacute thyroiditis following COVID-19 infection. Case Rep Endocrinol (2022) 2022:2211061. doi: 10.1155/2022/2211061
32. Feghali K, Atallah J, Norman C. Manifestations of thyroid disease post COVID-19 illness: Report of hashimoto thyroiditis, graves' disease, and subacute thyroiditis. J Clin Transl Endocrinol Case Rep (2021) 22:100094. doi: 10.1016/j.jecr.2021.100094
33. Guven M. Subacute thyroiditis in the course of coronavirus disease 2019: A case report. J Endocrinol Metab (2020) 10(3-4):110–2. doi: 10.14740/jem678
34. Hajósi-Kalcakosz S, Dénes J, Góth M. Subacute thyroiditis associated with COVID-19 infection: A report of an increasing entity. Arch Endocrinol Metab (2022) 66(1):118–28. doi: 10.20945/2359-3997000000446
35. Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? insights from a case report. J Endocrinol Invest (2020) 43(8):1171–2. doi: 10.1007/s40618-020-01312-7
36. Jakovac H, Ferencic A, Stemberger C, Mohar Vitezic B, Cuculic D. Detection of sars-Cov-2 antigens in thyroid gland showing histopathological features of subacute thyroiditis. Eur Thyroid J (2022) 11(2):e220005. doi: 10.1530/ETJ-22-0005
37. Khatri A, Charlap E, Kim A. Subacute thyroiditis from COVID-19 infection: A case report and review of literature. Eur Thyroid J (2021) 9(6):324–8. doi: 10.1159/000511872
38. Kliem T, Strobel D, Heinke F, Pavel M, Neurath MF, Neufert C. Fallbericht: Persistierendes Fieber nach SARS-CoV-2-Infektion [Case report: persistent fever after SARS-CoV-2 infection]. Inn Med (Heidelb). (2022) 63(6):662–5. German. doi: 10.1007/s00108-022-01315-x.
39. Kong M, Sarah La P. Case report: De quervain’s thyroiditis as a long-term sequelae complication to SARS-CoV-2 infection. Case Rep Acute Med (2021) 4(2):64–70. doi: 10.1159/000517705
40. Mathews SE, Castellanos-Diaz J, Srihari A, Kadiyala S, Leey-Casella J, Ghayee HK, et al. Subacute thyroiditis and heart failure in a patient presenting with COVID-19. J Investig Med High Impact Case Rep (2021) 9:23247096211009412. doi: 10.1177/23247096211009412
41. Mattar SAM, Koh SJQ, Chandran SR, Cherng BPZ. Subacute thyroiditis associated with COVID-19. BMJ Case Rep (2020) 13(8):e237336. doi: 10.1136/bcr-2020-237336
42. Mehmood MA, Bapna M, Arshad M. A case of post-COVID-19 subacute thyroiditis. Cureus (2020) 12(12):e12301. doi: 10.7759/cureus.12301
43. Osorio Martínez A, González-Razo VT, Navarro-Sánchez V, Souto Meiriño CA, Ahumada-Ayala M. SARS-CoV-2-Related subacute thyroiditis, myocarditis, and hepatitis after full resolution of COVID-19 serum markers. Am J Case Rep (2021) 22:e932321. doi: 10.12659/AJCR.932321
44. Poudel Jaishi P, Neupane SK, Neupane PK, Koirala D, Kandel A, Joshi K. Case report: Paradoxical manifestation of covid 19 induced thyroiditis in the unvaccinated patient. Ann Med Surg (Lond) (2022) 80:104204. doi: 10.1016/j.amsu.2022.104204
45. Ruano R, Zorzano-Martinez M, Campos A, Rius F, Hernández M. Subacute thyroiditis might be a complication triggered by SARS-CoV-2. Endocrinol Diabetes Nutr (Engl Ed) (2020) 68(10):755–6. doi: 10.1016/j.endinu.2020.09.002
46. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens) (2021) 20(1):219–21. doi: 10.1007/s42000-020-00230-w
47. Salehi AM, Salehi H, Mohammadi HA, Afsar J. SARS-CoV-2 and subacute thyroiditis: A case report and literature review. Case Rep Med (2022) 2022:6013523. doi: 10.1155/2022/6013523
48. San Juan MDJ, Florencio MQV, Joven MH. SUBACUTE THYROIDITIS IN a PATIENT WITH CORONAVIRUS DISEASE 2019. AACE Clin Case Rep (2020) 6(6):e361-e364. doi: 10.4158/ACCR-2020-0524
49. Sato D, Nishiguchi S, Tanaka E. Successful management of subacute thyroiditis following SARS-CoV-2 infection. Intern Med (2021) 60(22):3573–6. doi: 10.2169/internalmedicine.7913-21
50. Semikov VI, Aghayan DL, Shulutko AM, Khorobrykh TV, Aleksandrov YK, Mansurova GT, et al. Subacute thyroiditis after SARS-CoV-2 infection. Clin Case Rep (2021) 9(11):e05109. doi: 10.1002/ccr3.5109
51. Seyed Resuli A, Bezgal M. Subacute thyroiditis in COVID-19 patients. Ear Nose Throat J (2021) 101(8):501–5. doi: 10.1177/01455613211012114
52. Sohrabpour S, Heidari F, Karimi E, Ansari R, Tajdini A, Heidari F. Subacute thyroiditis in COVID-19 patients. Eur Thyroid J (2021) 9(6):321–3. doi: 10.1159/000511707
53. Stasiak M, Zawadzka-Starczewska K, Lewiński A. Clinical manifestation of subacute thyroiditis triggered by SARS-CoV-2 infection can be HLA-dependent. Viruses (2021) 13(12):2447. doi: 10.3390/v13122447
54. Timofeeva LA, Aleksandrov YK, Aleshina TN, Yusova MA. Subacute thyroiditis associated with covid-19. Russ Electron J Radiol (2021) 11(3):15–24. doi: 10.21569/2222-7415-2021-11-3-15-24
55. Tjønnfjord E, Moe RB, Ghanima W, Aballi S. Subacute thyroiditis after COVID-19. Tidsskr Nor Laegeforen (2021) 141(10). doi: 10.4045/tidsskr.21.0169
56. Ünübol M, Erdemir Z, Çolak G, Ünsal MG, Güney E. Subacute thyroiditis associated with COVID-19 infection: Two case reports. Turkish J Endocrinol Metab (2021) 25(3):344–8. doi: 10.25179/tjem.2021-84279
57. Brancatella A, Viola N, Rutigliano G, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis during the SARS-CoV-2 pandemic. J Endocr Soc (2021) 5(10):bvab130. doi: 10.1210/jendso/bvab130
58. Bahçecioğlu AB, Karahan ZC, Aydoğan BI, Kalkan IA, Azap A, Erdoğan MF. Subacute thyroiditis during the COVID-19 pandemic: a prospective study. J Endocrinol Invest (2022) 45(4):865–74. doi: 10.1007/s40618-021-01718-x
59. Mondal S, DasGupta R, Lodh M, Ganguly A. Subacute thyroiditis following recovery from COVID-19 infection: novel clinical findings from an Eastern Indian cohort. Postgraduate Med J (2022) 15:postgradmedj-2021-141429. doi: 10.1136/postgradmedj-2021-141429
60. Poma A, Bonuccelli D, Giannini R, Macerola E, Vignali P, Ugolini C, et al. COVID-19 autopsy cases: Detection of virus in endocrine tissues. J Endocrinol Invest (2022) 45(1):209–14. doi: 10.1007/s40618-021-01628-y
61. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu S, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: A clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest (2021) 44:1085–90. doi: 10.1007/s40618-020-01436-w
62. Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewiński A. Subacute thyroiditis is associated with HLA-b* 18: 01,-DRB1* 01 and-c* 04: 01–the significance of the new molecular background. J Clin Med (2020) 9(2):534. doi: 10.3390/jcm9020534
63. Lanzo N, Patera B, Fazzino GFM, Gallo D, Lai A, Piantanida E, et al. The old and the new in subacute thyroiditis: An integrative review. Endocrines (2022) 3(3):391–410. doi: 10.3390/endocrines3030031
64. Viola N, Brancatella A, Sgrò D, Santini F, Latrofa F. Clinical, biochemical features and functional outcome of patients with SARS-CoV-2-related subacute thyroiditis: a review. Endocrine (2022) 79(3):448–54. doi: 10.1007/s12020-022-03247-w
65. Christensen J, O'Callaghan K, Sinclair H, Hawke K, Love A, Hajkowicz K, et al. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID-19: a systematic review. Internal Med J (2022) 52(4):522–9. doi: 10.1111/imj.15432
66. Ando Y, Ono Y, Sano A, Fujita N, Ono S. Subacute thyroiditis after COVID-19: A literature review. Am J Trop Med Hygi (2022) 107(5):1074. doi: 10.4269/ajtmh.21-1223
67. Willis C, Chalder T. Concern for covid-19 cough, fever and impact on mental health. what about risk of somatic symptom disorder? J Ment Health (2021) 30(5):551–5. doi: 10.1080/09638237.2021.1875418
68. Swee DS, Chng CL, Lim A. Clinical characteristics and outcome of thyroid storm: A case series and review of neuropsychiatric derangements in thyrotoxicosis. Endocr Practi (2015) 21(2):182–9. doi: 10.4158/EP14023.OR
69. Gonzalez R, Nemeroff CB. Inflammatory diseases and their psychiatric manifestations. In: Textbook of medical psychiatry. Washington, DC: American Psychiatric Association Publishing (2020). p. 229–63.
70. Stasiak M, Tymoniuk B, Stasiak B, Lewiński A. The risk of recurrence of subacute thyroiditis is HLA-dependent. Int J Mol Sci (2019) 20(5):1089. doi: 10.3390/ijms20051089
71. Ray I, D’Souza B, Sarker P, Agarwal P. Management of subacute thyroiditis–a systematic review of current treatment protocols. Int J Gen Med (2022) 15:6425. doi: 10.2147/IJGM.S366784
72. Stasiak M, Tymoniuk B, Adamczewski Z, Stasiak B, Lewiński A. Sonographic pattern of subacute thyroiditis is HLA-dependent. Front Endocrinol (2019) 10:3–. doi: 10.3389/fendo.2019.00003
73. Wang L, Xiong X, Hao T, Zhao Y. Evaluation on the diagnostic value of nuclear medicine and ultrasound examination in subacute thyroiditis. Indian J Pharm Sci (2021) 83(1):215–9. doi: 10.36468/pharmaceutical-sciences.spl.200
74. Mariani G, Tonacchera M, Grosso M, Fiore E, Falcetta P, Montanelli L, et al. The role of nuclear medicine in the clinical management of benign thyroid disorders, part 2: Nodular goiter, hypothyroidism, and subacute thyroiditis. J Nucl Med (2021) 62(7):886 LP–95. doi: 10.2967/jnumed.120.251504
75. Zeng W, Tan S, King TFJ. Subacute thyroiditis presenting as a painful suspicious thyroid nodule. Endocrinol Diabetes Metab Case Rep (2022) 2022:21–0135. doi: 10.1530/EDM-21-0135. Epub ahead of print.
Keywords: subacute thyroiditis (SAT), De Quervain thyroiditis, COVID-19, SARS-CoV-2, thyroid, long-term COVID-19 symptoms, long COVID, systematic review
Citation: Meftah E, Rahmati R, Zari Meidani F, Khodadadi S, Chitzan-Zadeh K, Esfahanian F and Afshar S (2023) Subacute thyroiditis following COVID-19: A systematic review. Front. Endocrinol. 14:1126637. doi: 10.3389/fendo.2023.1126637
Received: 18 December 2022; Accepted: 24 March 2023;
Published: 05 April 2023.
Edited by:
Carlo Cappelli, University of Brescia, ItalyReviewed by:
Nicola Viola, University of Pisa, ItalyCopyright © 2023 Meftah, Rahmati, Zari Meidani, Khodadadi, Chitzan-Zadeh, Esfahanian and Afshar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elahe Meftah, ZS1NZWZ0YWhAc3R1ZGVudC50dW1zLmFjLmly; Kosar Chitzan-Zadeh, Q2hpdHphbnphZGVoLmtAYWp1bXMuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.