
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 June 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1126592
This article is part of the Research Topic Novel Treatment Strategies for Thyroid Autoimmunity and Thyroid Cancer View all 23 articles
 Ruo-Yun Zhou1
Ruo-Yun Zhou1 Ning Li1
Ning Li1 Hai-Long Tan1
Hai-Long Tan1 Neng Tang1
Neng Tang1 Pei Chen1
Pei Chen1 Mian Liu1
Mian Liu1 Deng-Jie Ou-Yang1
Deng-Jie Ou-Yang1 Zi-En Qin1
Zi-En Qin1 Lei Ai1
Lei Ai1 Bo Wei1
Bo Wei1 Ya-Xin Zhao1
Ya-Xin Zhao1 Shi Chang1,2,3,4
Shi Chang1,2,3,4 Peng Huang1*
Peng Huang1*Background: Guidelines widely recommend thyrotropin suppression to reduce the risk of recurrence in intermediate- and high-risk papillary thyroid cancer (PTC) after total thyroidectomy. However, an insufficient or excessive dosage may result in a number of symptoms/complications especially in older patients.
Patients and methods: We constructed a retrospective cohort including 551 PTC patient encounters. Using propensity score matching and logistic regression models, we determined the independent risk factors affecting levothyroxine therapy at different ages. Our outcomes included: expected TSH level and an unexpected TSH level, which was based on the initial thyroid-stimulating hormone (TSH) goal< 0.1 mIU/L with usual dosage of L-T4 (1.6 μg/kg/day).
Results: From our analysis, more than 70% of patients undergoing total thyroidectomy did not achieve the expected TSH level using an empirical medication regimen, and the effect of the drug was affected by age (odds ratio [OR], 1.063; 95% CI, 1.032-1.094), preoperative TSH level (OR, 0.554; 95% CI, 0.436-0.704) and preoperative fT3 level (OR, 0.820; 95% CI, 0.727-0.925). In patients with age < 55 years old, preoperative TSH level (OR, 0.588; 95% CI, 0.459-0.753), and preoperative fT3 level (OR, 0.859; 95% CI, 0.746-0.990) were two independent protective factors, while, in patients with age ≥ 55 years old, only preoperative TSH level (OR, 0.490; 95% CI, 0.278-0.861) was the independent protective factors to achieve expected TSH level.
Conclusion: Our retrospective analysis suggested the following significant risk factors of getting TSH suppression in PTC patients: age (≥55 years), lower preoperative TSH and fT3 levels.
Over the past several decades, there has been an increase in the incidence of thyroid cancer, with the vast majority being papillary thyroid cancer (PTC) (1). Although the 5-year survival rate of PTC is 90% or more, the risk of cancer recurrence is 5-20% or even higher in patients judged to be at intermediate to high risk. Total thyroidectomy is recommended by the American Thyroid Association (ATA, 2015 edition) to reduce the risk of recurrence (2). After total thyroidectomy, levothyroxine (LT4) is prescribed to replace the function of the removed thyroid gland. Meanwhile, a supraphysiological dose of LT4 is applied to suppress the secretion of thyroid-stimulating hormone (TSH), which further reduces the risk of recurrence (3, 4).
For patients with different levels of predicted risks, different postoperative TSH targets are recommended. In addition, the benefits of TSH suppression treatment could be outweighed by the side effects as age advances. It is widely acknowledged that iatrogenic thyrotoxicosis resulting from aggressive suppression therapy is associated with an increased risk of fracture and cardiovascular events (5, 6), 2- to 4-fold higher in elderly patients (7, 8). Organ declines and adaptive changes in the hypothalamic-pituitary-thyroid axis of aged individuals, who are at high risk of thyroid cancer (2, 9) and consequently the main group receiving thyroidectomy (1), complicate the selection of the individual dosage of LT4. Even though optimal TSH goals were set to balance the adverse effects of TSH suppression and recurrence risk, individual variations in responses to LT4 remain unresolved, and there is no appropriate LT4 dosage prediction strategy for patients, especially for the aged.
In clinical routine, patients are usually prescribed an initial dosage LT4 of 1.6 μg/kg/day at the beginning of suppression therapy (10). However, the required LT4 dosage is affected by more than just body weight (BW). It has been suggested that body mass index (BMI) and body surface area (BSA) may predict the initial dosage more effectively (11, 12). Sex, age, and autoimmune diseases have also been reported to affect the LT4 requirement (13–16). Drugs and comorbidities can interfere with the treatment effect by disturbing the absorption or metabolism of LT4 (17–19). In addition, LT4 requirements are apparently affected by environmental temperature since thyroid hormones play an important role in regulating metabolism. The metabolism rate and function of the endocrine system vary with advancing age (20, 21), which accounts for different responses to LT4 in people of different ages. It is necessary to develop a specific strategy for LT4 dosage selection for elderly patients.
In this study, we sought to identify the effect that age has on the treatment efficiency of LT4. Patients undergoing initial total thyroidectomy with intermediate to high risk were recruited and treated with LT4 following the guidance of the American Thyroid Association (ATA, 2015 edition) (2). We conducted propensity score matching (PSM) to diminish the effect of BW and ambient temperature. Other characteristics of the LT4-sensitive groups were explored, including age, sex, BMI, BSA, thyroid thickness, preoperative thyroid function, Hashimoto’s thyroiditis (HT), hypertension and diabetes mellitus to offer a theoretical basis for individual medicine care during suppression therapy.
This study is a single-center retrospective analysis that received approval from the medical ethics committee at Xiangya Hospital (20211245). Patients who met the following requirements were included in the study: (1) initially undergoing total thyroidectomy for thyroid cancer with an intermediate or high risk for recurrence (ATA, 2015 edition) (2); (2) taking LT4 per day based on the 1.6 µg/kg dosing regimen; (3) clinicopathologic data complete; and (4) no thyroid hormones or iodine given before surgery. The cohort was followed from January 2016 to October 2019 at the Department of General Surgery, Xiangya Hospital of Central South University.
A total of 551 patients met the criteria and were enrolled in this study. All the patients completed a physical examination, thyroid function test, ultrasonography of the thyroid, and measurements of serum thyroid peroxidase antibodies (TPOAb) and anti-thyroglobulin antibodies (TgAb) before surgery and were followed up at 1 month for postoperative TSH measurement. All tests used immunochemiluminometric assays conducted at the same laboratory. The reference ranges are 2.8-7.1pmol/l for serum free T3 (fT3), 12~22pmol/l for serum free T4 (fT4), and 0.27-4.2mIU/L for TSH.
According to the American Thyroid Association, initial TSH suppression to below 0.1 mIU/L is recommended among thyroid cancer patients at high risk of recurrence (ATA, 2015 edition) (2). At the first follow-up after surgery, the TSH suppression treatment effect was classified into the expected level or an unexpected level, corresponding to a serum TSH level <0.1 mIU/L and ≥0.1 mIU/L. We further divided the unexpected level into two groups, the replacement group and the hypothyroidism group, and the corresponding TSH levels were 0.1~4.2 mIU/L and ≥4.2 mIU/L, respectively.
To investigate the clinicopathological characteristics of patients who obtained different treatment effects, the variables collected included age, sex, BMI, BSA, average temperature during operation month, preoperative serum TSH, preoperative serum fT3, preoperative serum fT4, thyroid thickness, HT, and comorbidities including hypertension and diabetes mellitus. The mean temperature during the operative month is available on the website of the China Meteorological Administration. The thyroid thickness was calculated by the average of the left and right glands obtained from the preoperative ultrasound report.
SPSS 26.0 software was used to conduct the statistical analyses. Continuous data are presented as the mean and standard deviation (SD), and the Mann−Whitney U test was used for comparisons between groups. The binary data are expressed as the patient number with their percentages, and the chi square test was used for comparisons between two groups. PSM was performed by using the nearest-neighbor matching method with a caliper distance of 0.02 without replacement between chosen groups. Weight and ambient temperature were used to calculate the propensity score to perform the matching. Significant variables with P <0.05 after PSM were entered into the multivariate binary logistic regression analysis to identify the factors affecting the treatment outcome. The effect of the factors was presented with the OR and 95% confidence interval (CI). A level of P<0.05 was used to indicate significance, and all statistical tests were two-tailed. A receiver operating characteristic curve (ROC) analysis was carried out to quantify the prediction performance of the identified factors, and the value of the area under the ROC curve (AUC) was calculated.
We selected 551 patients from the database who took LT4 (Euthyrox, Merck KGaA) at 1.6 μg/kg per day after total thyroidectomy, including 425 women (76.2%) and 126 men (23.8%) at a ratio of 3.4:1. Their mean age was 43.93 ± 11.18 years, ranging from 18 to 73 years. The average weight, height, BMI, and BSA of the 551 patients were 61.61 ± 10.48 kg, 161.05 ± 6.75 m, 23.70 ± 3.26 kg/m2, and 1.66 ± 0.16 m2, respectively. Preoperative thyroid function included TSH (3.01 ± 2.57 mIU/L), fT3 (7.12 ± 5.02 pmol/L), and fT4 (13.67 ± 6.44 pmol/L). The average thyroid thickness was 16.38 ± 4.04 mm before surgery. Hashimoto’s thyroiditis, hypertension and diabetes mellitus were found in 127 patients (23%), 75 patients (13.6%), and 21 patients (3.8%), respectively (Table 1).
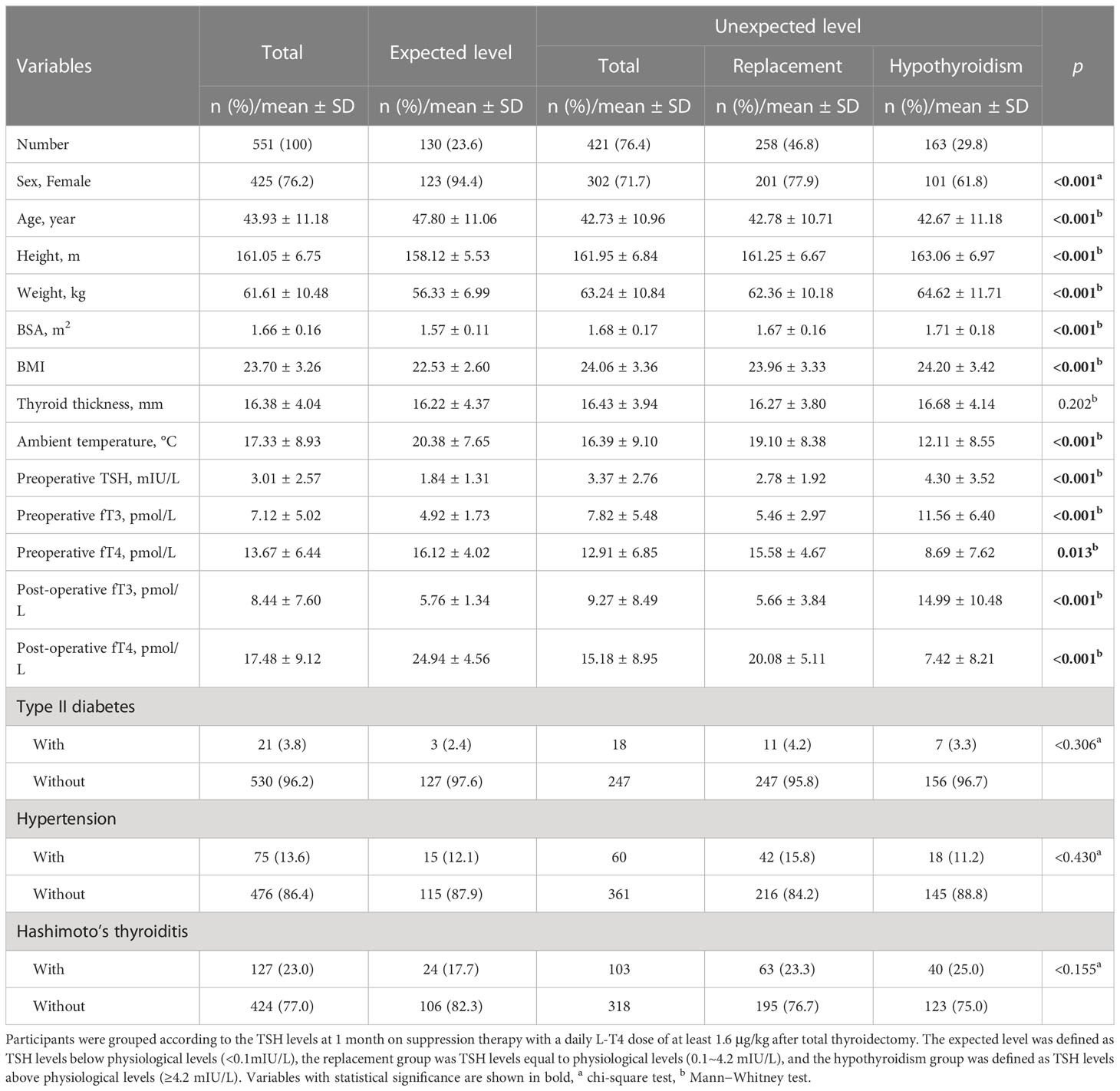
Table 1 Demographics and clinical characteristics of 551 PTC patients who underwent total thyroidectomy.
The detailed characteristics of the different treatment effects are summarized in Table 1. A total of 23.6% of patients had their TSH suppressed under 0.1 mIU/L, 46.8% of the patients were euthyroid, and 29.8% were hypothyroid at the first follow-up. There were significant differences between the two groups with different TSH suppression levels, including age, sex, height, weight, BMI, BSA, ambient temperature, preoperative serum fT3 and preoperative serum TSH (all p<0.001), and preoperative serum fT4(p=0.013). Among patients who obtained the expected level, women distinctly made up the majority compared with patients who had an unexpected level (expected level 94.4% women vs. unexpected level 71.7% women). Patients in the unexpected level group had higher fT3 (expected level 4.92 ± 1.73 pmol vs. unexpected level 7.82 ± 5.48 pmol) and higher TSH (expected level 1.84 ± 1.31 mIU/L vs. unexpected level 3.37 ± 2.76 mIU/L) and lower fT4 (expected level 16.12± 4.02 pmol/l vs. unexpected level 12.91 ± 6.85 pmol/l). Their weight showed an increasing trend, while ambient temperature showed a decreasing trend as the treatment effect weakened. There were no significant differences in the categorical data except for sex between the two levels (diabetes mellitus, p = 0.306; hypertension, p = 0.430; Hashimoto’s thyroiditis, p = 0.155) (Table 1).
Ambient temperature (p <0.001) and weight (p <0.001) had significant differences between the expected level and unexpected level before PSM. All the other variables also showed significant differences between the two levels. After 1:1 PSM, weight and mean temperature reached an equilibrium (weight, p =0.338; ambient temperature, p = 0.236). Sex and BSA were not significantly different between the matched groups, while age, height, BMI and preoperative fT3, fT4, and TSH remained significantly different between the two groups. Age seemed to have a higher possibility of being different between the two groups (p<0.001).
We further explored the potential prognostic factors for obtaining the expected level using univariate binary logistic regression analysis. Age (p<0.001, OR=1.063, 95% CI, 1.032-1.094), preoperative TSH (p<0.001, OR=0.554, 95% CI, 0.436-0.704) and preoperative fT3 (p=0.001, OR=0.820, 95% CI, 0.727-0.925) were all significantly associated with the treatment effect (Table 2). ROC analyses were performed to quantify the prediction performance of the three identified factors. Preoperative fT3 was identified as the most effective prediction factor, with an AUC of 0.776, followed by age, with an AUC of 0.703. Using age and preoperative fT3 as co-prediction factors had the highest AUC of 0.825 (Figure 1).
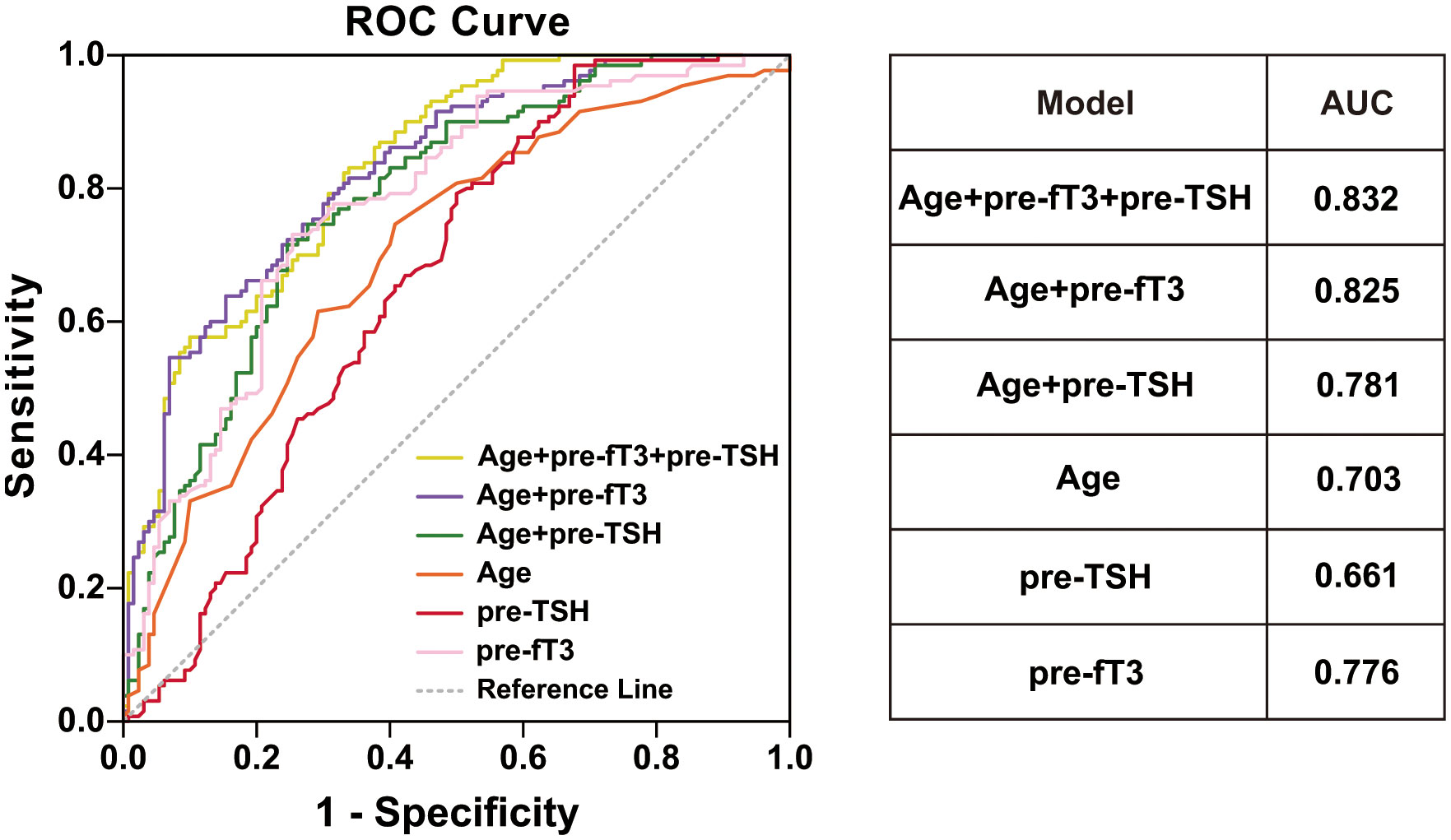
Figure 1 ROC curve and AUC for different model. age (orange line), preoperative TSH (red line), preoperative fT3 (pink line), age+preoperative fT3 (purple line), age+preoperative TSH (green line), age+preoperative TSH +preoperative fT3 (yellow line).
Between the replacement group and hypothyroid group, ambient temperature showed a significant difference (p<0.001), while weight was already equilibrated (p=0.065). After 1:1 PSM, the ambient temperature reached equilibrium. Other variables, including sex, height, BSA, BMI, preoperative serum TSH, preoperative serum fT3 and preoperative serum fT4 (all p<0.001), were significantly different before PSM. After PSM, only sex, preoperative serum TSH, preoperative serum fT3 and preoperative serum fT4 (all p<0.001) were distributed differently between the replacement and hypothyroidism groups. The multivariate binary logistic regression analysis showed that sex and preoperative fT3 were the two factors that affected the treatment effect. Male sex (p<0.001, OR=3.248, 95% CI, 1.708-6.177) and patients with higher preoperative fT3 (p=0.001, OR=1.248, 95% CI, 1.101-1.158) were more likely to be in above-target TSH state (Table 3).
It has now been established that age is a factor that influences whether the expected TSH level is met, and we identified additional factors that influence the treatment effect of LT4 in different age groups. We divided the patients into two age groups based on age, using 55 years as the cut-off value according to the 8th American Joint Committee on Cancer (AJCC) staging system (18). We also conducted PSM before multivariate binary logistic regression. In the group under 55 years old, sex, height, BMI, preoperative serum TSH and preoperative fT3 were included in the multivariate regression analysis. Only preoperative serum TSH (p<0.001, OR=0.588, 95% CI, 0.459-0.753) and preoperative fT3 (p=0.035, OR=859, 95% CI, 0.746-0.990) were significantly different (Table 4).
In the group 55 years of age and older, all of the variables were significantly different between the unexpected level and expected level groups before PSM. After PSM, weight (p=0.775) and ambient temperature (p=0.870) were equilibrated. Preoperative TSH was slightly higher after PSM 3.57 ± 2.10 mIU/L vs. before PSM 3.27 ± 1.74 mIU/L in the unexpected level group after PSM and was the only variable that remained significantly different after PSM (p=0.010). In binary logistic analysis, preoperative TSH was an independent protective factor (p=0.013, OR=0.490, 95% CI, 0.278-0.861) (Table 5).
For patients with an intermediate to high risk of recurrence, suppression of TSH is required to reduce the risk of recurrence (22–24). In elderly patients, it is essential to balance the benefits of suppression treatment with the side effects of iatrogenic hyperthyroidism. At present, the factors influencing LT4 medication based on age stratification remain unclear. In this study, we analyzed the factors influencing postoperative LT4 medication based on age in PTC patients with intermediate to high risks of recurrence, which has important value for the formulation of personalized LT4 medication regimens.
Consistent with a previous study, we observed that several factors affected the treatment effect, such as ambient temperature and BMI. Since thyroid hormone is required for normal development as well as regulating metabolism in the adult (25, 26), previous studies usually focused on the effects of body weight (27, 28). In our study, the patients all took LT4 at an empirical regimen of 1.6 µg/kg, and more than 70% did not achieve the expected TSH suppression level. This suggests that there are many factors other than body weight that affect the effect of LT4. Ambient temperature is an important factor affecting the body’s metabolism. Therefore, our study considered the influence of ambient temperature on the effect of LT4 medication. We used BW and ambient temperature in the calculation of the propensity score to minimize their impact on the results. Surprisingly, although the effects of weight and ambient temperature were diminished, age was still an independent factor that affected the treatment effect of suppressing TSH levels among patients with an intermediate to high risk of recurrence.
It has been proven that the homeostasis of the endocrine system changes with the advancement of age, as does thyroid hormone regulation. The suppression of TSH by thyroid hormone is greater in elderly individuals (7, 29), which means that under a state of hypothyroidism, elderly individuals have a smaller rise in TSH concentration. In other words, a given dose suppresses the secretion of TSH more with advancing age. Age-related differences in treatment outcomes may be associated with changes in the hypothalamic-pituitary-thyroid axis in different organ hierarchies (20). For such changes, there is no specific age at which the effect begins. Several previous studies indicated that 97.5th percentile of serum TSH was noted to increase by 0.1-0.3 mU/L every 10 years after 30~39 years of age (21, 30, 31). The functional decline of the thyroid gland, causing it to secrete less thyroid hormone, could be the cause of upper reference limit of TSH in elderly individuals. The longer period of elimination of thyroid hormone in elderly individuals seems to result in a better suppression effect of LT4 (32).
Preoperative TSH was an independent factor in both elderly and younger patients after we stratified patients based on age. Our results showed that patients with lower preoperative TSH levels are more likely to achieve a state of TSH suppression after surgery. We suspect that variable changes in the sensitivity or numbers of thyroid hormone receptors in the pituitary and hypothalamus explain this correlation.
The preoperative fT3 level was also shown to be an independent factor among patients under 55 years old. In our study, patients with higher preoperative fT3 were less likely to suppress the secretion of TSH, which may be the result of the adaptation of the pituitary to higher fT3. An interesting correlation between presurgical fT3 and the treatment effect was also seen in Vincenzo Di Donna’s study (11). This result indicates that iodothyronine deiodinases may also function in the suppression of TSH secretion. Since the secretion of TSH is suppressed by fT4 and fT3, the meaning of fT3 or TSH as a predictor may be the same. It is also interesting that fT3 did not show significance among patients aged 55 and over, which is expected due to their decreased metabolism of thyroid hormone (33, 34) or the changes in the sensitivity of the hypothalamic-pituitary-thyroid axis during aging.
Previous studies have shown that age-related thyroid changes differ by sex (35) and sex was indicated to be relative with lymph node metastasis in PTC (36). So, is the age-related level of TSH suppression after LT4 medication also related to sex? In our analysis, sex was not an independent influencing factor for either people aged 55 years older or those younger than 55 years old. We considered that this was because sex hormones have little effect on the response to thyroid hormones in the hypothalamus and pituitary gland.
Even though sex was not identified as an independent factor affecting the TSH suppression level, it was shown to be a factor that led to a worse treatment effect. Among patients with unexpected levels, men were more likely to have TSH levels beyond the physiological range. This means that men require a larger LT4 dose if they cannot suppress TSH in the first month after surgery on the usual dose.
There are some limitations in our study. First, although we tried to determine the relationship between treatment outcome and some comorbidities, the number of patients who had comorbidities was too small and even smaller after PSM to reveal any relationship. In addition, patient adherence to LT4 is uncertain, which may bias our results. In our study, we only tried to determine the predictors of TSH suppression degree. It remains to be studied further how to adjust the initial levothyroxine dose according to known factors to obtain a satisfactory targeting suppression degree.
For PTC patients with intermediate to high risk of recurrence, age is an independent risk factor for achieving the target TSH levels through usual dosage of LT4. Under the condition of the same body weight and ambient temperature, it is easier to achieve the target TSH suppression level in patients aged less than 55 years old with lower preoperative TSH levels and fT3 and in patients aged 55 and older with lower preoperative TSH levels (Table 6).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study received approval from the medical ethical committee at Xiangya Hospital (20211245).Written informed consent to participate in this study was waived because of the retrospective and anonymous nature of the study.
Study concepts, R-YZ and PH. Study design, PH and SC. Data acquisition, LA, NT, and NL. Quality control of data and algorithms, PC, ML, and D-JO-Y. Data analysis and interpretation, LA, BW, and Y-XZ. Statistical analysis, Z-EQ and H-LT. Manuscript preparation, R-YZ and PH. Manuscript editing, R-YZ and PH. Manuscript review, PH and SC. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant number 81974423), the Special Funding for the Construction of Innovative Provinces in Hunan (Grant number 2020SK4003), the China Postdoctoral Science Foundation (Grant numbers 2020M672517, 2021T140749), the Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Grant number 2021KFJJ03), the Fundamental Research Funds for the Central Universities of Central South University (Grant number 2022ZZTS0945, CX20210354).
We wish to thank all the patients who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PTC, papillary thyroid cancer; ATA, the American Thyroid Association; LT4, levothyroxine; TSH, thyroid-stimulating hormone; BW, body weight; BMI, body mass index; BSA, body surface area; PSM, propensity score matching; HT, Hashimoto’s thyroiditis; TPOAb, thyroid peroxidase antibodies; TgAb, anti-thyroglobulin antibodies; fT3, free T3; fT4, free T4; SD, standard deviation; CI, confidence interval; ROC, receiver operating characteristic curve; AUC, the area under the ROC curve; AJCC, American Joint Committee on Cancer.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
3. McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med (2002) 34(7-8):554–64. doi: 10.1080/078538902321117760
4. Grani G, Ramundo V, Verrienti A, Sponziello M, Durante C. Thyroid hormone therapy in differentiated thyroid cancer. Endocrine (2019) 66(1):43–50. doi: 10.1007/s12020-019-02051-3
5. Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev (2014) 35(3):433–512. doi: 10.1210/er.2013-1083
6. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur J Endocrinol (2019) 180(6):407–16. doi: 10.1530/eje-19-0006
7. Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med (1994) 331(19):1249–52. doi: 10.1056/NEJM199411103311901
8. Bauer DC, Ettinger B, Nevitt MC, Stone KL, Study of Osteoporotic Fractures Research G. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med (2001) 134(7):561–8. doi: 10.7326/0003-4819-134-7-200104030-00009
9. Wei X, Wang X, Xiong J, Li C, Liao Y, Zhu Y, et al. Risk and prognostic factors for Braf(V600e) mutations in papillary thyroid carcinoma. BioMed Res Int (2022) 2022:9959649. doi: 10.1155/2022/9959649
10. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. role of triiodothyronine in pituitary feedback in humans. N Engl J Med (1987) 316(13):764–70. doi: 10.1056/NEJM198703263161302
11. Di Donna V, Santoro MG, de Waure C, Ricciato MP, Paragliola RM, Pontecorvi A, et al. A new strategy to estimate levothyroxine requirement after total thyroidectomy for benign thyroid disease. Thyroid (2014) 24(12):1759–64. doi: 10.1089/thy.2014.0111
12. Ojomo KA, Schneider DF, Reiher AE, Lai N, Schaefer S, Chen H, et al. Using body mass index to predict optimal thyroid dosing after thyroidectomy. J Am Coll Surg (2013) 216(3):454–60. doi: 10.1016/j.jamcollsurg.2012.12.002
13. Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid (2011) 21(8):821–7. doi: 10.1089/thy.2011.0029
14. Jonklaas J. Sex and age differences in levothyroxine dosage requirement. Endocr Pract (2010) 16(1):71–9. doi: 10.4158/Ep09257.Or
15. Olubowale O, Chadwick DR. Optimization of thyroxine replacement therapy after total or near-total thyroidectomy for benign thyroid disease. Br J Surg (2006) 93(1):57–60. doi: 10.1002/bjs.5157
16. Checchi S, Montanaro A, Pasqui L, Ciuoli C, De Palo V, Chiappetta MC, et al. L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab (2008) 93(2):465–9. doi: 10.1210/jc.2007-1544
17. Babic Leko M, Gunjaca I, Pleic N, Zemunik T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int J Mol Sci (2021) 22(12):6521. doi: 10.3390/ijms22126521
18. Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Delivery (2017) 14(4):467–72. doi: 10.1080/17425247.2017.1290604
19. Ott J, Promberger R, Kober F, Neuhold N, Tea M, Huber JC, et al. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid (2011) 21(2):161–7. doi: 10.1089/thy.2010.0191
20. Mooradian AD. Age-related resistance to thyroid hormone action. Drugs Aging (2019) 36(11):1007–14. doi: 10.1007/s40266-019-00711-7
21. Raverot V, Bonjour M, Abeillon du Payrat J, Perrin P, Roucher-Boulez F, Lasolle H, et al. Age- and sex-specific tsh upper-limit reference intervals in the general French population: there is a need to adjust our actual practices. J Clin Med (2020) 9(3):792. doi: 10.3390/jcm9030792
22. Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the national thyroid cancer treatment cooperative registry. Thyroid (1998) 8(9):737–44. doi: 10.1089/thy.1998.8.737
23. Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J, Jaffiol C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab (1996) 81(12):4318–23. doi: 10.1210/jcem.81.12.8954034
24. Xiang Y, Xu Y, Bhandari A, Sindan N, Hirachan S, Yang Q, et al. Serum tsh levels are associated with postoperative recurrence and lymph node metastasis of papillary thyroid carcinoma. Am J Transl Res (2021) 13(6):6108–16.
25. Brent GA. Mechanisms of thyroid hormone action. J Clin Invest (2012) 122(9):3035–43. doi: 10.1172/jci60047
26. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev (2010) 31(2):139–70. doi: 10.1210/er.2009-0007
27. Jin J, Allemang MT, McHenry CR. Levothyroxine replacement dosage determination after thyroidectomy. Am J Surg (2013) 205(3):360–3. doi: 10.1016/j.amjsurg.2012.10.015
28. Younis IR, Ahmed MA, Burman KD, Soldin OP, Jonklaas J. Stable isotope pharmacokinetic studies provide insight into effects of age, sex, and weight on levothyroxine metabolism. Thyroid (2018) 28(1):41–9. doi: 10.1089/thy.2017.0380
29. Over R, Mannan S, Nsouli-Maktabi H, Burman KD, Jonklaas J. Age and the thyrotropin response to hypothyroxinemia. J Clin Endocrinol Metab (2010) 95(8):3675–83. doi: 10.1210/jc.2010-0281
30. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the us population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab (2007) 92(12):4575–82. doi: 10.1210/jc.2007-1499
31. Surks MI, Boucai L. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab (2010) 95(2):496–502. doi: 10.1210/jc.2009-1845
32. Davis JD, Stern RA, Flashman LA. Cognitive and neuropsychiatric aspects of subclinical hypothyroidism: significance in the elderly. Curr Psychiatry Rep (2003) 5(5):384–90. doi: 10.1007/s11920-003-0073-6
33. Chaker L, Korevaar TI, Medici M, Uitterlinden AG, Hofman A, Dehghan A, et al. Thyroid function characteristics and determinants: the Rotterdam study. Thyroid (2016) 26(9):1195–204. doi: 10.1089/thy.2016.0133
34. Waring AC, Arnold AM, Newman AB, Buzkova P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab (2012) 97(11):3944–50. doi: 10.1210/jc.2012-2481
35. Cho BA, Yoo SK, Song YS, Kim SJ, Lee KE, Shong M, et al. Transcriptome network analysis reveals aging-related mitochondrial and proteasomal dysfunction and immune activation in human thyroid. Thyroid (2018) 28(5):656–66. doi: 10.1089/thy.2017.0359
Keywords: thyrotropin suppression, intermediate-and high-risk differentiated thyroid cancer, individualized medication regimens, age stratification, thyroid cancer
Citation: Zhou R-Y, Li N, Tan H-L, Tang N, Chen P, Liu M, Ou-Yang D-J, Qin Z-E, Ai L, Wei B, Zhao Y-X, Chang S and Huang P (2023) Age-based factors modulating the required thyroxine dose to achieve thyrotropin suppression in intermediate-and high-risk papillary thyroid cancer. Front. Endocrinol. 14:1126592. doi: 10.3389/fendo.2023.1126592
Received: 18 December 2022; Accepted: 23 May 2023;
Published: 14 June 2023.
Edited by:
Malgorzata Gabriela Wasniewska, University of Messina, ItalyCopyright © 2023 Zhou, Li, Tan, Tang, Chen, Liu, Ou-Yang, Qin, Ai, Wei, Zhao, Chang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Huang, eGlhbmd5YWhwQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.