- 1Graduate Program in Biological Sciences: Pharmacology and Therapeutics, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
- 2Graduate Program in Biological Sciences: Physiology, Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil
- 3Center for Genetic Medicine Research, Children’s National Research Institute, Washington, DC, United States
- 4Division of Emergency Medicine, Children’s National Hospital, Washington, DC, United States
- 5Department of Pediatrics, The George Washington University School of Medicine and Health Sciences, Washington, DC, United States
Physical activity and exercise have been widely related to prevention, treatment, and control for several non-communicable diseases. In this context, there are innumerous pre-clinical and clinical evidence indicating the potential role of exercise, beyond cancer prevention and survival, improved quality of life, including on psychological components, bone health and cachexia, from cancer survivors is described as well. This mini-review raises the potential role of circulating extracellular and particles vesicles (EVPs) cargo, as exerkines, conducting several positive effects on adjacent and/or distant tissues such as tumor, immune, bone and muscle cells. We highlighted new perspectives about microRNAs into EVPs changes induced by exercise and its benefits on malignancies, since microRNAs can be implicated with intricated physiopathological processes. Potential microRNAs into EVPs were pointed out here as players spreading beneficial effects of exercise, such as miR-150-5p, miR-124, miR-486, and miRNA-320a, which have previous findings on involvement with clinical outcomes and as well as tumor microenvironment, regulating intercellular communication and tumor growth. For example, high-intensity interval aerobic exercise program seems to increase miR‐150 contents in circulating EVPs obtained from women with normal weight or overweight. In accordance circulating EVPs miR-150-5p content is correlated with prognosis colorectal cancer, and ectopic expression of miR-150 may reduce cell proliferation, invasion and metastasis. Beyond the involvement of bioactive miRNAs into circulating EVPs and their pathways related to clinical and preclinical findings, this mini review intends to support further studies on EVPs cargo and exercise effects in oncology.
Introduction
Exercise and/or physical activity are associated with a reduced risk of developing cancer and the improvement of well-being and survival in cancer patients (1). According to the Physical Activity Guidelines for Americans Advisory Committee, physical activity is linked to a reduced risk of several types of cancer, including breast, colon, kidney, endometrial, bladder, esophageal, and stomach cancers (2). Moreover, (3) reported that exercise reduced the risk (approximately 20%) of colorectal cancer, endometrial cancer, esophageal cancer, and kidney cancer. Preclinical studies corroborate the potential role of exercise in cancer prevention. For example, Nilsson et al. (4) reported that aged mice submitted to lifelong, voluntary wheel-running (aerobic exercise) did not exhibit malignant tumors (e.g., brain, liver, spleen, and intestinal). Cormie et al. (5) suggested a consistent trend for reduced risk of cancer-specific mortality (28%–44%), cancer recurrence (21%–35%), and all-cause mortality (25%–48%) in patients who had higher exercise levels. In addition to reducing cancer risk exercise can counteract cancer-related comorbid conditions such as cachexia, sarcopenia, osteoporosis, metabolic imbalance, and cardiovascular diseases, including those caused by cancer-related management, such as chemotherapy and radiation (5, 6).
It is noteworthy that exercise is widely accepted for the prevention and treatment of several diseases, such as cardiovascular, psychiatric, neurodegenerative, and bone, joint, and muscle disorders (7). In this context, we have focused on the role of circulating extracellular vesicles and particles (EVPs) as a potential mechanism for modulating physiological and biochemical functions in adjacent tissues and/or distant sites via spreading exercise-induced molecules, such as microRNAs (miRNAs). These miRNA-induced changes may promote a healthier global status (8).
Several subclasses of EVPs, such as exosomes, microvesicles, and apoptotic bodies have been described (9–11) and have the ability to transfer bioactive molecules, such as proteins, lipids, messenger RNAs (mRNAs), and miRNA (12). Recently, Sadovska et al., (13), suggested EVPs as the central mechanism of exercise induced changes in prostate cancer, specifically on the progression of cancer in a metastatic prostate cancer model. They showed that administration (i.v.) of EVPs obtained from rats submitted to regular exercise (5 weeks) reduced the tumor size (primary tumor volume by 35%) and lung metastasis using a syngeneic orthotopic prostate cancer model (13).
This mini-review will highlight the potential relationship between EVPs cargo and exercise-induced benefits on cancer conditions, given that cancer is the second-leading cause of death worldwide. We will focus on the exercise effects on EVPs cargo, bringing a new perspective on the physiological role of circulating EVPs as exerkines that spread bioactive compounds in cancer diseases.
Role of EVPs cargo as potential mechanisms for the beneficial effects of exercise on tumor growth and metastasis
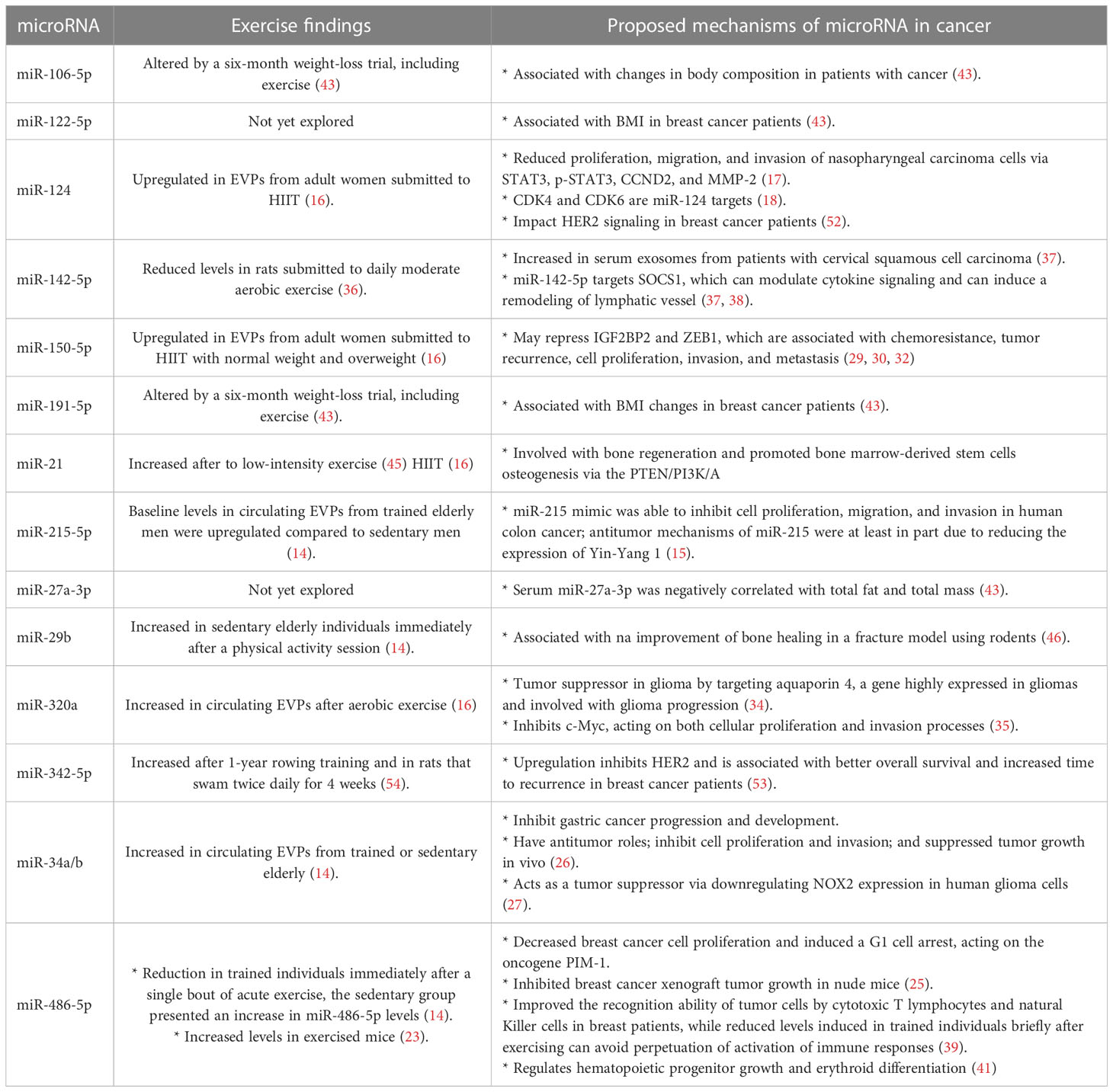
Several miRNAs in circulating EVPs impacted by exercise may be candidates for the beneficial effects of exercise in tumor growth and metastasis. Baseline levels of miR-215-5p in circulating EVPs from trained elderly men were upregulated compared to sedentary men (14). An in vitro study conducted by Chen et al., (15) showed that the miR-215 mimic was able to inhibit cell proliferation, migration, and invasion in human colon cancer, and these effects were reversed by its inhibitor (Figure 1). These authors suggested the antitumor mechanisms of miR-215 were at least in part due to reducing the expression of Yin-Yang 1 (YY1), a transcription factor belonging to the family of zinc finger proteins (15).
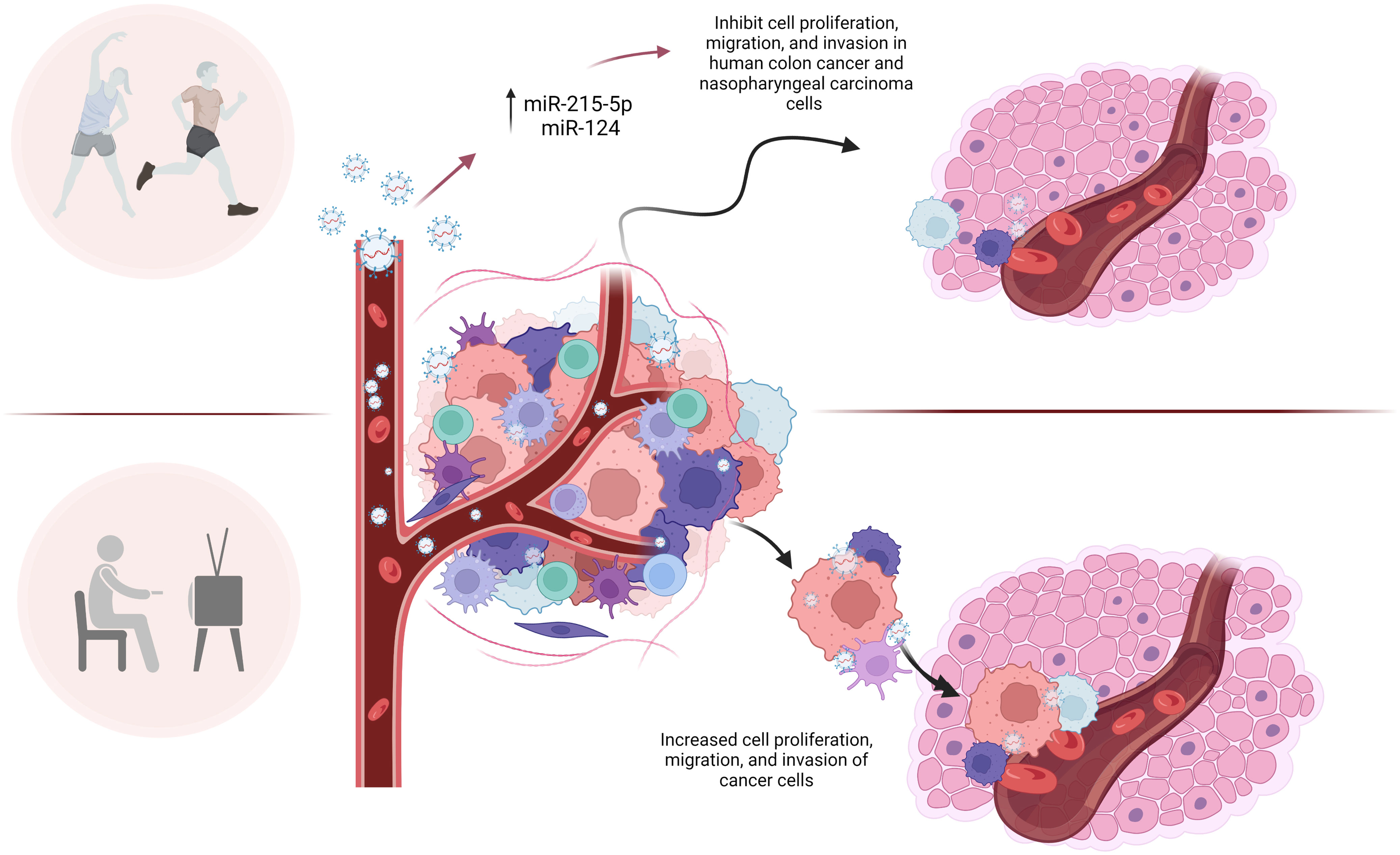
Figure 1 microRNAs in circulating EVPs are altered by exercise and may be candidates for the beneficial effects of exercise in tumor growth, metastasis and proliferation. microRNAs in circulating EVPs, such as miR-215-5p and miR-124, are upregulated in response to exercise in men and women, respectively. Many authors reported that those microRNAs target important molecules involved in cell growth, division, migration, and invasion of tumor, such as Yin-Yang 1 (miR-215-5p), cyclin-dependent kinase (miR-124), MMP-2 (miR-124), STAT3, p-STAT3, G1/S-specific cyclin-D2 (miR-124). Although given current data, it is impossible to affirm exactly the role of microRNAs from circulating EVPs in exercised cancer patients, it can be inferred that these molecules are involved in the effects of exercise on the tumor invasion process.
Another potential modulator of cell growth and division, migration, and invasion altered by exercise in circulating EVPs is miR-124 (Figure 1). This miRNA was upregulated in EVPs from adult women submitted to a high-intensity interval aerobic exercise program (16). The upregulation of miR-124 has also been associated with reduced proliferation, migration, and invasion of nasopharyngeal carcinoma cells (C666-1 cells) via downregulation of STAT3, p-STAT3, G1/S-specific cyclin-D2 (CCND2) and Matrix Metalloproteinase-2 (MMP-2) expression in C666-1 cells (17). Several molecular targets of miR-124 are key players in cell cycle progression and DNA replication, including cyclin-dependent kinase (CDK) 4 and CDK6 (18).
In addition, MMP-2, a proteinase that degrades the components of the extracellular matrix (ECM), is a target of miR-124. MMP-2 is related to the invasion of tumor cells by facilitating the movement of tumor cells across the ECM and the basal membrane of the blood vessel wall, so we can infer that MMP-2 suppression in recipient cells by higher levels of miR-124 delivered by EVPs could be involved with prevention of distant metastasis induced by exercise. MMP-2 levels in circulating blood have been considered as a breast cancer metastasis marker and are related to mortality rates (19, 20). 90% of patients that had worse prognoses (metastases and mortality), in a Brazilian cohort, had positive staining for MMP-2 in their breast cancer tumors (21), indicating they were in a “high-risk group”. Clinical and preclinical studies have demonstrated MMP-2 involvement in several human cancers, including prostate, gastric, and pancreatic cancers. In accordance, even in patients with hormone receptor-negative tumors, which are usually considered high-risk, reduced MMP-2 levels have been linked to a better prognosis (for review 22). Although given current data, it is impossible to affirm exactly the relationship between circulating miR-124 in EVPs and MMP-2 in the tumor microenvironment, it can be inferred that these molecules are involved in the effects of exercise on the tumor invasion process.
Exercise-induced miRNA changes in circulating EVPs, and repression of downstream targets, such as cyclins and CDKs mRNA, are associated with negative regulation of cell cycle progression in recipient tumor cells. Both pre-clinical and clinical evidence has demonstrated an increase in miR-486 levels in circulating EVPs from exercised mice (14, 23). Similarly, a higher level of total circulating miR-486 has been shown in resting athletes (24). Increased expression of miR-486-5p significantly decreased breast cancer cell proliferation and induced a G1 arrest, acting on its direct target the oncogene PIM-1. In addition, miR-486-5p inhibited breast cancer xenograft tumor growth in nude mice (25).
Immediately after exercise, miR-34b levels increased in circulating EVPs from trained or sedentary elderly (14). There is in vitro and in vivo evidence that miR-34-containing exosomes derived from cancer-associated fibroblasts can inhibit gastric cancer progression and development. These exosomes were taken up by gastric cancer cells, showing antitumor roles; inhibition of cell proliferation and invasion; and suppressed tumor growth in vivo (26). Interestingly, miR-34a acts as a tumor suppressor via downregulating NOX2 expression in human glioma cells showing that the miR-34a/NOX2 pathway has a relevant role in tumor proliferation (27).
Dimassi et al., (16) also described that adult Caucasian women with normal weight or overweight submitted to a high-intensity interval aerobic exercise program had increased miR‐150 levels in circulating EVPs. Decreased serum exosomal miR-150-5p expression was correlated with poor prognosis in colorectal cancer (28). In accordance, the miR-150 expression in osteosarcoma tissues was significantly decreased compared with adjacent noncancerous tissues while the expression levels of a direct target of miR-150, insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) were markedly increased in osteosarcoma tissues (29) and oral cancer (30). IGF2BP2, which may be controlled by miR-150, seems to be involved with maintenance of cancer stem cells, and consequently with chemoresistance and tumor recurrence (31). Jin et al., (32) described that epithelial ovarian cancer tissues and serum from patients had dramatically downregulated levels of this miRNA. The authors also found that ectopic expression of miR-150 may reduce cell proliferation, invasion, and metastasis by suppressing the expression of Zinc Finger E-Box Binding Homeobox 1 (ZEB1), a crucial regulator of epithelial-to-mesenchymal transition. Exercise-induced higher levels of miR-150 in circulating EVPs may repress IGF2BP2 and ZEB1 expression in target tissues, which may mediate the effects of exercise on some cancer types.
Moreover, miR-150 is able to improve endothelial barrier function by suppressing angiopoietin-2 (Ang2), an endothelial growth factor; miR-150 and/or Ang2 modulation have been considered promising tools for promoting improvements of endothelial barrier dysfunction in different conditions, such as sepsis and lung injury (33). Interestingly, Ang2 may be relevant to breast cancer metastases formation, and because miRNA can downregulate Ang2, this potential mechanism might be responsible for the lower breast cancer risk and the improved outcomes (i.e. decreased metastasis formation) associated with increased physical activity.
Another miRNA that may be related to decreased cell invasion and migration is miR-320a. Interestingly, this miRNA is increased in circulating EVPs after aerobic exercise (16). Xiong et al., (34) described miR-320a as a tumor suppressor in glioma by targeting aquaporin 4, a gene that is highly expressed in gliomas and is involved with glioma progression. The ability of this miRNA to suppress tumor invasion has been described in hepatocellular carcinoma cells as well, since upregulation of miR-320a inhibits c-Myc, acting on both cellular proliferation and invasion processes (35).
Exercise may induce changes in EVP cargo that modulate the immune system in cancer
In addition to altering tumor growth and metastasis, miRNA cargo in EVPs may also be responsible for changes in the immune system in cancer. Specifically, exercise may induce changes in miRNA levels that improve the immune response to tumors. Oliveira et al., (36) showed that rats submitted to daily moderate aerobic exercise showed reduced miR-142-5p levels in circulating EVPs. Interestingly, miR-142-5p is increased in serum exosomes from patients with cervical squamous cell carcinoma compared to healthy controls (37). As discussed by Siqueira et al., (8) reduced miR-142-5p levels in EVPs can be related to counteracting the immunosuppressive tumor microenvironment condition, since this miRNA targets SOCS1 that can modulate cytokine signaling (38) and can induce a remodeling lymphatic vessel (37).
Findings obtained by Nair et al. (14) revealed an interesting exercise-induced temporal pattern on circulating EVP miR-486-5p levels, depending on inactive or trained conditions. The heat map reveals that the circulating EVP miR-486-5p baseline level was increased in trained elderly men compared to the sedentary control group. Although there was a reduction in trained individuals immediately after a single bout of acute exercise, the sedentary group presented a modest increase in miR-486-5p levels (14). In addition, exercise also increases miR-486 levels in circulating EVPs from exercised mice (23). This dual effect can be related to adaptations since this miRNA induces a relevant modulation of the immune system (39). In agreement, miR-486-5p has been associated with immune regulation state, for example, in the breast cancer microenvironment. miR-486-5p might be involved in improving the recognition ability of tumor cells by cytotoxic T lymphocytes and natural Killer cells in breast patients (39), while reduced levels induced in trained individuals briefly after exercising can avoid perpetuation of activation of immune responses (Figure 2).
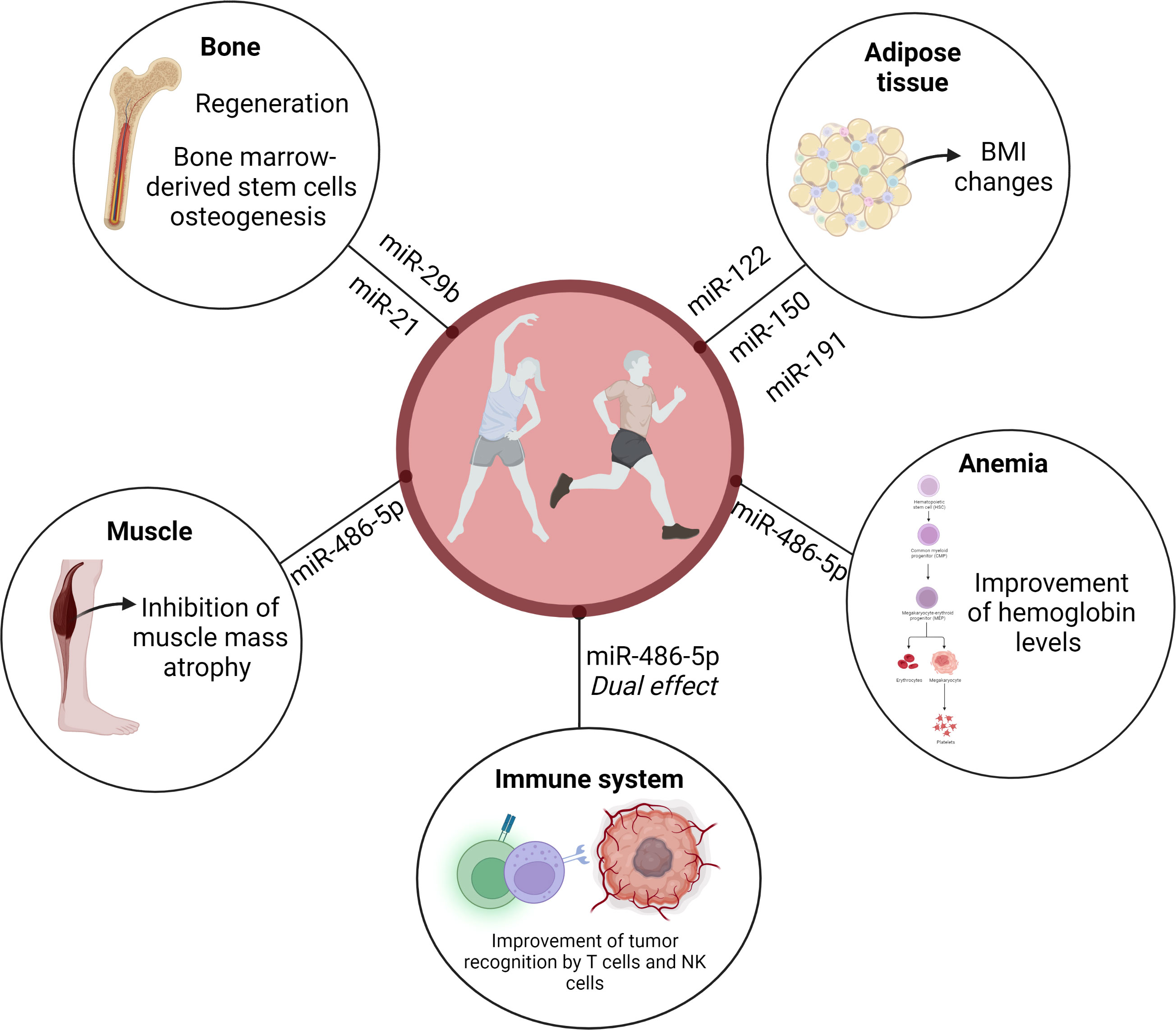
Figure 2 microRNA from EVP can be suggested as a possible mechanism for the beneficial effects of exercise on cancer. EVPs content may have a central role in spreading exercise-induced active molecules, such as microRNAs. miR-486 presented an exercise-induced temporal pattern, depending on inactive or trained conditions. This dual effect can be related to adaptations since this miRNA induces a relevant modulation of the immune system. miR-486-5p might be involved in improving the recognition ability of tumor cells by cytotoxic T lymphocytes and natural Killer cells in breast patients, while reduced levels induced in trained individuals briefly after exercising can avoid perpetuation of activation of immune responses. miR-486 may also regulate normal hematopoiesis. Anemia is one of the most common hematological conditions in cancer. Given the changes in this microRNA levels after exercise and the known role that miR-486-5p plays in hematopoietic progenitor growth and erythroid differentiation, we can infer that this miRNA may improve the hemoglobin levels seen in patients with cancer who exercise. As we described in this mini-review miR-29b, miR-21, miR-122, miR-150, miR-191, and miR-486-5p may be associated with changes in body composition as well as bone health in patients with cancer. Those exercise-responsive microRNAs may induce beneficial effects by improving bone healing, regeneration and promoting bone marrow-derived stem cells osteogenesis. In addition, circulating EVP miR-486 might be involved with exercise-induced muscle mass growth in cancer patients.
Potential role of EVPs cargo as a player in exercise-induced body composition changes in cancer
miR-486-5p shuttled by EVPs may also regulate normal hematopoiesis. It is widely accepted that anemia is one of the most common hematological conditions in cancer, induced by both cancer per se and myelotoxic chemotherapy, and leads to a poorer prognosis. Mohamady et al. (40) described that moderate aerobic exercise for 25–40 min, 3 times/week for 12 weeks improved hemoglobin levels and red blood cell count in elderly women with breast cancer. miR-486-5p regulates normal erythropoiesis since an important role for miR-486-5p has been described in normal hematopoietic progenitor growth and erythroid differentiation (41). Given the increase in miR-486-5p in sedentary individuals after exercise, and the baseline increase in this miRNA in active people, as well as the known role that miR-486-5p plays in hematopoietic progenitor growth and erythroid differentiation, we can infer that this miRNA may improve the hemoglobin levels seen in patients with cancer who exercise (Figure 2).
Another remarkable point is that overweight and obesity are associated with tumor recurrence and cancer-related mortality (42). Siqueira et al. (8) have reviewed the role of EVPs miRNAs in exercise effects on adipose tissue and consequently, obesity and overweight. Adams et al. (43) described miRNAs associated with BMI in breast cancer patients, for example, miR-191-5p, miR-150-5p, and miR-122-5p. Interestingly miR-106-5p and miR-191-5p levels were altered by a six-month weight-loss trial, including exercise. Moreover, serum miR-27a-3p was negatively correlated with total fat and total mass. However, the impact of exercise on miRNA cargo in EVPs and its association with changes in body composition in patients with cancer has not been properly explored (Figure 2).
Exercise-induced EVP miRNA changes may also be involved in bone health. Regular exercise has been widely highlighted and recognized as a relevant intervention to maintain or increase bone mass and density. A potential miRNA affected by exercise and involved in bone health is miR-21, since in vitro findings showed that miR-21 is involved with bone regeneration and promoted bone marrow-derived stem cells osteogenesis via the PTEN/PI3K/Akt signaling pathway (44). Circulating EVPs miR-21 is increased in individuals submitted to low-intensity exercise (45) and to a high-intensity interval aerobic exercise program (16). In addition to miR-21, higher levels of circulating EVP miR-29b were reported in sedentary elderly individuals immediately after a physical activity session (14). This miRNA has also been associated with improved bone healing in a fracture model using rodents (46) (Figure 2).
In addition to bone health, muscle mass atrophy (as cachexia and sarcopenia) has also been related to cancer. Morley et al. (47) suggested that approximately 20% of deaths among cancer patients are related to cachexia and its associated morbidity. Cachexia is a hypercatabolic state, defined as a multifactorial syndrome characterized by critical body loss of body weight, fat, and muscle mass related to illness (48). Sarcopenia is defined as physical performance and muscle mass loss. These terms are commonly interchanged since there are several overlaps. Interestingly, EVPs derived from bone marrow mesenchymal stem cells inhibited in vitro and in vivo dexamethasone-induced muscle atrophy through miR-486-5p/FoxO1 (49). Reduced visceral fat and increased skeletal muscle rate were described in patients with head and neck cancer after 8 weeks of multimodal exercise (50). Resistance and aerobic training have been considered relevant treatment approaches for the management of cachexia and sarcopenia (51). As described above, circulating EVPs from exercised mice had increased miR-486 levels (23), and circulating EVP miR-486-5p was increased in both trained elderly men and sedentary ones submitted to a single training session (14). It is possible to infer the involvement of circulating EVP miR-486 on exercise-induced muscle mass growth even in diseases, such as cancer (Figure 2).
Factors that may alter the impact of exercised-induced EVPs mediated effects on cancer
Cancer outcomes are highly dependent on the genetic and molecular features of cancer as well as tumor cell subpopulations and staging. The literature indicates that the effects of exercise may be dependent on these factors. Jones et al. (52) described that smaller tumor sizes and lower-grade tumors can be more responsive to physical activity/exercise effects. Consequently, it is possible to infer that the impact of exercise on early tumor phases, such as inhibiting proliferation and invasion and/or improving immune response can be considered more effective in the management of cancer.
In addition, miRNAs and their targets may be involved in cancer subtype-related susceptibility to physical activity/exercise benefits. Jones et al. (52) showed a lack of benefit on mortality and risk of recurrence of post-diagnosis exercise in women diagnosed with early-stage hormone or human epidermal growth factor receptor (HER2) receptor–negative breast cancer. The hazard ratio for risk of death was 0.72 and 0.50 for exercising patients with, respectively, ER and HER2-positive tumors (52). Lindholm et al. (53) demonstrated the inhibitory effects of miR-342-5p on HER2 signaling and the association between high of miR-342-5p expression and better overall survival and increased time to recurrence in breast cancer patients. To the best of our knowledge, the impact of exercise on the miRNA profile in circulating EVPs from breast cancer patients has not been tested, however, Hou et al. (54) reported that exercise increased plasma exosomal miR-342-5p in human subjects submitted to 1-year rowing training and rats that swam twice daily for 4 weeks (Figure 3). Given that patients with HER2-positive tumors showed increased plasma MMP2 activity, additive molecular effects of exercise such as miRNA-124 and miR-342-5p in circulating EVPs on MMP-2 levels and HER2 signaling might be explored.
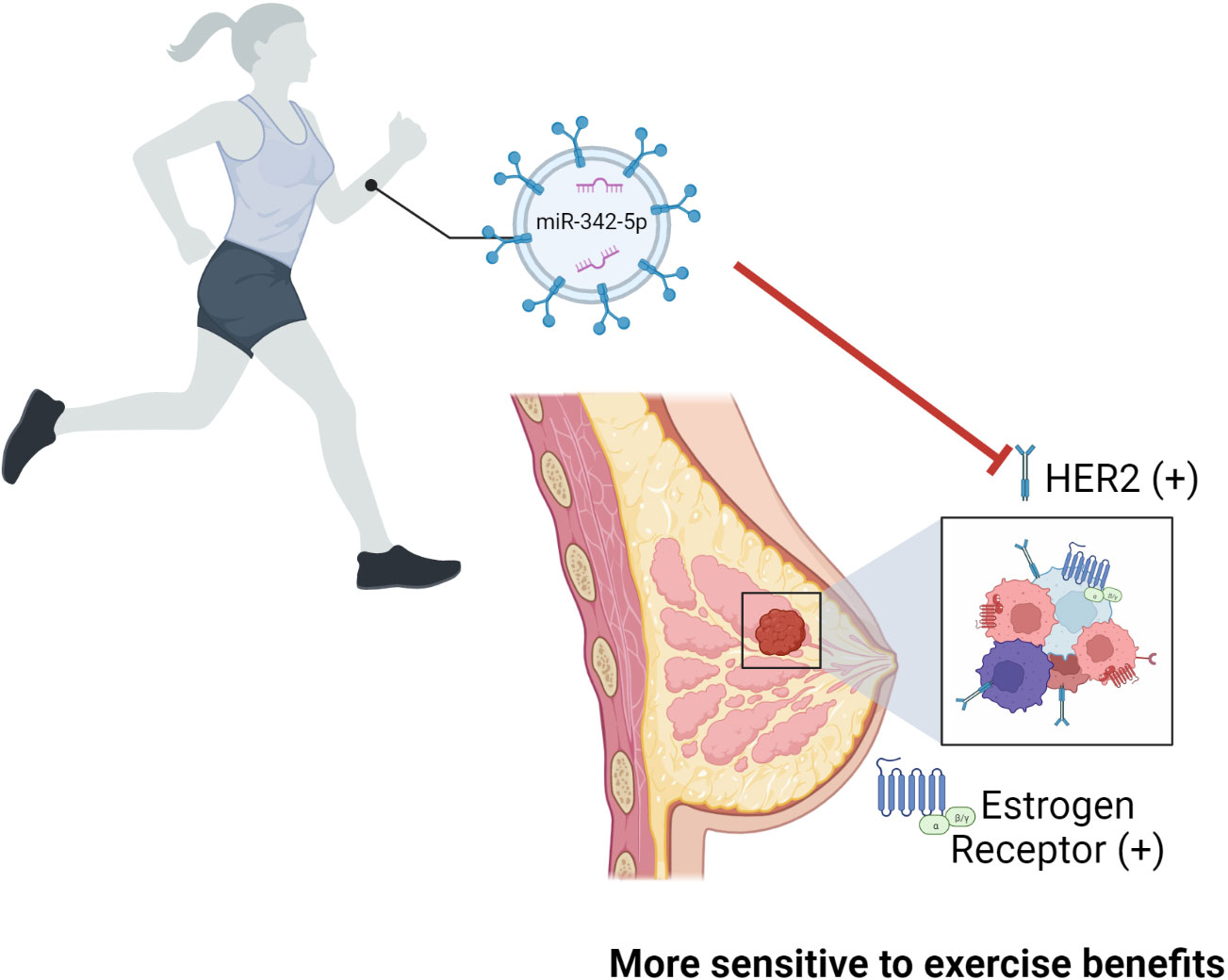
Figure 3 The role of miR-342-5p in breast cancer patients and its effect after exercise. Exercise was shown to increase the levels of exosomal miR-342-5p in humans submitted to 1-year rowing and rats that swam twice daily for 4 weeks. Authors reported an inhibitory effect of miR-342-5p on HER2 signaling which was associated with better overall survival and increased time to recurrence in breast cancer patients. It is relevant to point out that women diagnosed with early-stage breast cancer with estrogen receptor, progesterone receptor, or HER2 negative tumors showed reduced benefit on mortality and risk of recurrence of post-diagnosis exercise. Given that, it is possible to infer that patients with HER2-positive tumors will have higher susceptibility to physical activity/exercise benefits, although this was not tested.
Discussion
EVP cargo can be considered as a possible mechanism for the beneficial effects of exercise on cancer. We suggest that EVPs have a central role in spreading exercise-induced active molecules, such as miRNAs, modulating healthier global condition in cancer patients. In this mini-review, we show that exercise-responsive EVP miRNAs can act synergistically to promote changes in the tumor microenvironment. The synergistic effect of these miRNAs – miR-215, miR-124, miR-486, and miR-142 – can amplify the known benefits of exercise (effects were listed on Table 1). In this context, miR-486 seems to have multitarget actions, such as inhibition of cell proliferation, induction of cell cycle arrest, modulation of the immune system, increase in muscle mass growth, and improvement in erythropoiesis. This mini-review intends to support further studies on EVP cargo and the effects of exercise interventions on oncologic outcomes, which may open promising avenues for further studies and perspectives on exercise in oncology.
Author contributions
Conceptualization, IS, LC, RB, and RF; Writing and literature review, IS and LC; Critical revision of the manuscript, IS, LC, RB, and RF. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship (Dr. I.R. Siqueira 307980/2018-9).
Acknowledgments
Figures were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exercise (2019) 51(11):2391. doi: 10.1249/MSS.0000000000002117
2. Campbell KL, Winters-Stone K, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exercise (2019) 51(11):2375. doi: 10.1249/MSS.0000000000002116
3. Wang Q, Zhou W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J Sport Health Sci (2021) 10(2):201–10. doi: 10.1016/j.jshs.2020.07.008
4. Nilsson MI, Bourgeois JM, Nederveen JP, Leite MR, Hettinga BP, Bujak AL, et al. Lifelong aerobic exercise protects against inflammaging and cancer. PloS One (2019) 14(1):e0210863. doi: 10.1371/journal.pone.0210863
5. Cormie P, Zopf EM, Zhang X, Schmitz KH. The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol Rev (2017) 39(1):71–92. doi: 10.1093/epirev/mxx007
6. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res (2018) 20(1):1–10. doi: 10.1186/s13058-018-1051-6
7. Warburton DE, Bredin SS. Health benefits of physical activity: A systematic review of current systematic reviews. Curr Opin Cardiol (2017) 32(5):541–56. doi: 10.1097/HCO.0000000000000437
8. Siqueira IR, Palazzo RP, Cechinel LR. Circulating extracellular vesicles delivering beneficial cargo as key players in exercise effects. Free Radical Biol Med (2021) 172:273–85. doi: 10.1016/j.freeradbiomed.2021.06.007
9. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracellular Vesicles (2018) 7(1):1535750. doi: 10.1080/20013078.2018.1535750
10. Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc (2019) 14(4):1027–53. doi: 10.1038/s41596-019-0126-x
11. Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell (2020) 182(4):1044–61. doi: 10.1016/j.cell.2020.07.009
12. Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, et al. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol (2020) 17(4):323–34. doi: 10.1038/s41423-020-0391-1
13. Sadovska L, Auders J, Keiša L, Romanchikova N, Silamiķele L, Kreišmane M, et al. Exercise-induced extracellular vesicles delay the progression of prostate cancer. Front Mol Biosci (2021) 8. doi: 10.3389/fmolb.2021.784080
14. Nair VD, Ge Y, Li S, Pincas H, Jain N, Seenarine N, et al. Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol (2020) 11:605. doi: 10.3389/fphys.2020.00605
15. Chen Z, Han S, Huang W, Wu J, Liu Y, Cai S, et al. MicroRNA-215 suppresses cell proliferation, migration and invasion of colon cancer by repressing yin-yang 1. Biochem Biophys Res Commun (2016) 479(3):482–8. doi: 10.1016/j.bbrc.2016.09.089
16. Dimassi S, Karkeni E, Laurant P, Tabka Z, Landrier JF, Riva C. Microparticle miRNAs as biomarkers of vascular function and inflammation response to aerobic exercise in obesity? Obesity (2018) 26(10):1584–93. doi: 10.1002/oby.22298
17. Xu S, Zhao N, Hui L, Song M, Miao ZW, Jiang XJ. MicroRNA-124-3p inhibits the growth and metastasis of nasopharyngeal carcinoma cells by targeting STAT3. Oncol Rep (2016) 35(3):1385–94. doi: 10.3892/or.2015.4524
18. Agirre X, Vilas-Zornoza A, Jiménez-Velasco A, Martin-Subero JI, Cordeu L, Gárate L, et al. Epigenetic silencing of the tumor suppressor microRNA hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res (2009) 69(10):4443–53. doi: 10.1158/0008-5472.CAN-08-4025
19. Liu SC, Yang SF, Yeh KT, Yeh CM, Chiou HL, Lee CY, et al. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clinica Chimica Acta (2006) 371(1-2):92–6. doi: 10.1016/j.cca.2006.02.026
20. Feng Y, Sun B, Li X, Zhang L, Niu Y, Xiao C, et al. Differentially expressed genes between primary cancer and paired lymph node metastases predict clinical outcome of node-positive breast cancer patients. Breast Cancer Res Treat (2007) 103(3):319–29. doi: 10.1007/s10549-006-9385-7
21. Ramos EA, Silva CTD, Manica G, Pereira IT, Klassen L, Ribeiro EM, et al. Worse prognosis in breast cancer patients can be predicted by immunohistochemical analysis of positive MMP-2 and negative estrogen and progesterone receptors. Rev da Associação Médica Bras (2016) 62:774–81. doi: 10.1590/1806-9282.62.08.774
22. Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med Sci Monitor (2009) 15(2):RA32–40.
23. Di W, Amdanee N, Zhang W, Zhou Y. Long-term exercise-secreted extracellular vesicles promote browning of white adipocytes by suppressing miR-191a-5p. Life Sci (2020) 263:118464. doi: 10.1016/j.lfs.2020.118464
24. Denham J, Prestes PR. Muscle-enriched microRNAs isolated from whole blood are regulated by exercise and are potential biomarkers of cardiorespiratory fitness. Front Genet (2016) 7:196. doi: 10.3389/fgene.2016.00196
25. Zhang G, Liu Z, Cui G, Wang X, Yang Z. MicroRNA-486-5p targeting PIM-1 suppresses cell proliferation in breast cancer cells. Tumor Biol (2014) 35(11):11137–45. doi: 10.1007/s13277-014-2412-0
26. Shi L, Wang Z, Geng X, Zhang Y, Xue Z. Exosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells in vitro and in vivo. Aging (Albany NY) (2020) 12(9):8549. doi: 10.18632/aging.103157
27. Li SZ, Hu YY, Zhao J, Zhao YB, Sun JD, Yang YF, et al. MicroRNA-34a induces apoptosis in the human glioma cell line, A172, through enhanced ROS production and NOX2 expression. Biochem Biophys Res Commun (2014) 444(1):6–12. doi: 10.1016/j.bbrc.2013.12.136
28. Zou SL, Chen YL, Ge ZZ, Qu YY, Cao Y, Kang ZX. Downregulation of serum exosomal miR-150-5p is associated with poor prognosis in patients with colorectal cancer. Cancer Biomarkers (2019) 26(1):69–77. doi: 10.3233/CBM-190156
29. Wang L, Aireti A, Aihaiti A, Li K. Expression of microRNA-150 and its target gene IGF2BP1 in human osteosarcoma and their clinical implications. Pathol Oncol Res (2019) 25(2):527–33. doi: 10.1007/s12253-018-0454-0
30. Chou CH, Chang CY, Lu HJ, Hsin MC, Chen MK, Huang HC, et al. IGF2BP2 polymorphisms are associated with clinical characteristics and development of oral cancer. Int J Mol Sci (2020) 21(16):5662. doi: 10.3390/ijms21165662
31. Dai FQ, Li CR, Fan XQ, Tan L, Wang RT, Jin H. miR-150-5p inhibits non-small-cell lung cancer metastasis and recurrence by targeting HMGA2 and β-catenin signaling. Mol Therapy-Nucleic Acids (2019) 16:675–85. doi: 10.1016/j.omtn.2019.04.017
32. Jin M, Yang Z, Ye W, Xu H, Hua X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PloS One (2014) 9(8):e103965. doi: 10.1371/journal.pone.0103965
33. Rajput C, Tauseef M, Farazuddin M, Yazbeck P, Amin MR, Avin BR V, et al. MicroRNA-150 suppression of angiopoetin-2 generation and signaling is crucial for resolving vascular injury. Arteriosclerosis thrombosis Vasc Biol (2016) 36(2):380–8. doi: 10.1161/ATVBAHA.115.306997
34. Xiong W, Ran J, Jiang R, Guo P, Shi X, Li H, et al. miRNA-320a inhibits glioma cell invasion and migration by directly targeting aquaporin 4. Oncol Rep (2018) 39(4):1939–47. doi: 10.3892/or.2018.6274
35. Xie F, Yuan Y, Xie L, Ran P, Xiang X, Huang Q, et al. miRNA-320a inhibits tumor proliferation and invasion by targeting c-myc in human hepatocellular carcinoma. OncoTargets Ther (2017) 10:885. doi: 10.2147/OTT.S122992
36. Oliveira GP Jr., Porto WF, Palu CC, Pereira LM, Petriz B, Almeida JA, et al. Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Front Physiol (2018) 9:532. doi: 10.3389/fphys.2018.00532
37. Zhou C, Zhang Y, Yan R, Huang L, Mellor AL, Yang Y, et al. Exosome-derived miR-142-5p remodels lymphatic vessels and induces IDO to promote immune privilege in the tumour microenvironment. Cell Death Diff (2021) 28(2):715–29. doi: 10.1038/s41418-020-00618-6
38. Talebi F, Ghorbani s, Chan WF, Boghozian R, Masoumi F, Ghasemi S, et al. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J. Neuroinflammation (2017) 14:1–14.
39. ElKhouly AM, Youness RA, Gad MZ. MicroRNA-486-5p and microRNA-486-3p: Multifaceted pleiotropic mediators in oncological and non-oncological conditions. Non-coding RNA Res (2020) 5(1):11–21. doi: 10.1016/j.ncrna.2020.01.001
40. Mohamady HM, Elsisi HF, Aneis YM. Impact of moderate intensity aerobic exercise on chemotherapy-induced anemia in elderly women with breast cancer: A randomized controlled clinical trial. J Advanced Res (2017) 8(1):7–12. doi: 10.1016/j.jare.2016.10.005
41. Wang LS, Li L, Li L, Chu S, Shiang KD, Li M, et al. MicroRNA-486 regulates normal erythropoiesis and enhances growth and modulates drug response in CML progenitors. Blood J Am Soc Hematol (2015) 125(8):1302–13. doi: 10.1182/blood-2014-06-581926
42. Greenlee H, Shi Z, Molmenti CLS, Rundle A, Tsai WY. Trends in obesity prevalence in adults with a history of cancer: Results from the US national health interview survey 1997 to 2014. J Clin Oncol (2016) 34(26):3133. doi: 10.1200/JCO.2016.66.4391
43. Adams BD, Arem H, Hubal MJ, Cartmel B, Li F, Harrigan M, et al. Exercise and weight loss interventions and miRNA expression in women with breast cancer. Breast Cancer Res Treat (2018) 170(1):55–67. doi: 10.1007/s10549-018-4738-6
44. Yang C, Liu X, Zhao K, Zhu Y, Hu B, Zhou Y, et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone regeneration in critical size defects. Stem Cell Res Ther (2019) 10(1):1–11. doi: 10.1186/s13287-019-1168-2
45. Karvinen S, Sievänen T, Karppinen JE, Hautasaari P, Bart G, Samoylenko A, et al. MicroRNAs in extracellular vesicles in sweat change in response to endurance exercise. Front Physiol (2020) 11:676. doi: 10.3389/fphys.2020.00676
46. Lee WY, Li N, Lin S, Wang B, Lan HY, Li G. miRNA-29b improves bone healing in mouse fracture model. Mol Cell Endocrinol (2016) 430:97–107. doi: 10.1016/j.mce.2016.04.014
47. Morley JE, Thomas DR, Wilson MMG. Cachexia: Pathophysiology and clinical relevance. Am J Clin Nutr (2006) 83(4):735–43. doi: 10.1093/ajcn/83.4.735
48. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr (2008) 27(6):793–9. doi: 10.1016/j.clnu.2008.06.013
49. Li Z, Liu C, Li S, Li T, Li Y, Wang N, et al. BMSC-derived exosomes inhibit dexamethasone-induced muscle atrophy via the miR-486-5p/FoxO1 axis. Front Endocrinol (2021) 12. doi: 10.3389/fendo.2021.681267
50. Yen CJ, Hung CH, Kao CL, Tsai WM, Chan SH, Cheng HC, et al. Multimodal exercise ameliorates exercise responses and body composition in head and neck cancer patients receiving chemotherapy. Supportive Care Cancer (2019) 27(12):4687–95. doi: 10.1007/s00520-019-04786-1
51. Dunne RF, Mustian KM, Garcia JM, Dale W, Hayward R, Roussel B, et al. Research priorities in cancer cachexia: The university of rochester cancer center NCI community oncology research program (NCORP) research base symposium on cancer cachexia and sarcopenia. Curr Opin Supportive Palliative Care (2017) 11(4):278. doi: 10.1097/SPC.0000000000000301
52. Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, et al. Exercise and prognosis on the basis of clinicopathologic and molecular features in early-stage breast cancer: The LACE and pathways Studies Exercise, tumor features, and outcomes in breast cancer. Cancer Res (2016) 76(18):5415–22. doi: 10.1158/0008-5472.CAN-15-3307
53. Lindholm EM, Leivonen SK, Undlien E, Nebdal D, Git A, Caldas C, et al. miR-342-5p as a potential regulator of HER2 breast cancer cell growth. Microrna (2019) 8(2):155–65. doi: 10.2174/2211536608666181206124922
Keywords: physical activity, exosomes, microvesicles, microparticles (MP), tumor, cachexia, obesity
Citation: Siqueira IR, Batabyal RA, Freishtat R and Cechinel LR (2023) Potential involvement of circulating extracellular vesicles and particles on exercise effects in malignancies. Front. Endocrinol. 14:1121390. doi: 10.3389/fendo.2023.1121390
Received: 11 December 2022; Accepted: 07 February 2023;
Published: 03 March 2023.
Edited by:
Joanna Jaworska, Medical University of Gdansk, PolandReviewed by:
Mauricio Rodriguez-Dorantes, National Institute of Genomic Medicine (INMEGEN), MexicoCopyright © 2023 Siqueira, Batabyal, Freishtat and Cechinel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ionara Rodrigues Siqueira, aW9uYXJhQHVmcmdzLmJy
 Ionara Rodrigues Siqueira
Ionara Rodrigues Siqueira Rachael A. Batabyal
Rachael A. Batabyal Robert Freishtat
Robert Freishtat Laura Reck Cechinel
Laura Reck Cechinel