- 1Department of Breast and Thyroid Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 2Department of Ultrasonography, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 3Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Introduction: The parathyroid glands are important endocrine glands for maintaining calcium and phosphorus metabolism, and they are vulnerable to accidental injuries during thyroid cancer surgery. The aim of this retrospective study was to investigate the application of high-frequency ultrasound imaging for preoperative anatomical localization of the parathyroid glands in patients with thyroid cancer and to analyze the protective effect of this technique on the parathyroid glands and its effect on reducing postoperative complications.
Materials and methods: A total of 165 patients who were operated for thyroid cancer in our hospital were included. The patients were assigned into two groups according to the time period of surgery: Control group, May 2018 to February 2021 (before the application of ultrasound localization of parathyroid in our hospital); PUS group, March 2021 to May 2022. In PUS group, preoperative ultrasound was used to determine the size and location of bilateral inferior parathyroid glands to help surgeons identify and protect the parathyroid glands during operation. We compared the preoperative ultrasound results with the intraoperative observations. Preoperative and first day postoperative serum calcium and PTH were measured in both groups.
Results: Our preoperative parathyroid ultrasound identification technique has more than 90% accuracy (true positive rate) to confirm the location of parathyroid gland compared to intraoperative observations. Postoperative biochemical results showed a better Ca2+ [2.12(0.17) vs. 2.05(0.31), P=0.03] and PTH [27.48(14.88) vs. 23.27(16.58), P=0.005] levels at first day post-operation in PUS group compared to control group. We also found a reduced risk of at least one type of hypoparathyroidism after surgery in control group:26 cases (31.0%) vs. 41 cases (50.6%), p=0.016.
Conclusion: Ultrasound localization of the parathyroid glands can help in the localization, identification and in situ preservation of the parathyroid glands during thyroidectomy. It can effectively reduce the risk of hypoparathyroidism after thyroid surgery.
1 Introduction
Thyroid cancer is one of the most rapidly increasing malignancies in the world in recent years, and more than 90% of its pathological type is papillary carcinoma, for which surgical resection represents the most effective treatment (1). Hypoparathyroidism caused by surgical damage is one of the most common comorbidities of thyroid surgery (2, 3).
The parathyroid gland (PG) is an important endocrine gland. The chief cells of PG secrete parathyroid hormone (PTH), an important hormone that maintaining calcium and phosphorus homeostasis in the body (4, 5). Due to different migration patterns during embryonic development, Inferior parathyroid glands (IPG) ectopia is observed frequently with a fragile blood supply and anatomical variability (6, 7). These locations include paraoesophageal, mediastinal, intrathoracic, intrathyroidal and/or around the carotid sheath (8). Additionally, the number of PGs is also varying (9). Therefore, it is crucial to identify and protect the PGs in situ during thyroidectomy (10, 11), because incidental resection of one or more PGs or disruption of parathyroid blood supply may lead to hypocalcemia and hypoparathyroidism (12, 13), causing symptoms such as numbness sensation in the hands and feet and even paralysis of the laryngeal and respiratory muscles, which seriously affects the quality of life of patients. It is also a major cause of doctor-patient tension after thyroid surgery.
Ultrasound diagnostic techniques is a common examination of thyroid and cervical lymph node lesions (14, 15). Compared to other diagnostic techniques, ultrasound diagnosis has several advantages including dynamic, radiation-free, reproducible, high-resolution, low-cost, and is accepted by a larger number of patients (16, 17). However, studies on the characteristics of preoperative normal parathyroid ultrasound images are not very prominent (18, 19), and it is desirable to improve the clinical knowledge and application of preoperative normal parathyroid ultrasound images.
The purpose of this work is to evaluate the protective effect of the application of preoperative high-frequency ultrasound imaging of parathyroid in patients with thyroid cancer and its effect on reducing postoperative complications.
2 Materials and methods
2.1 Patience
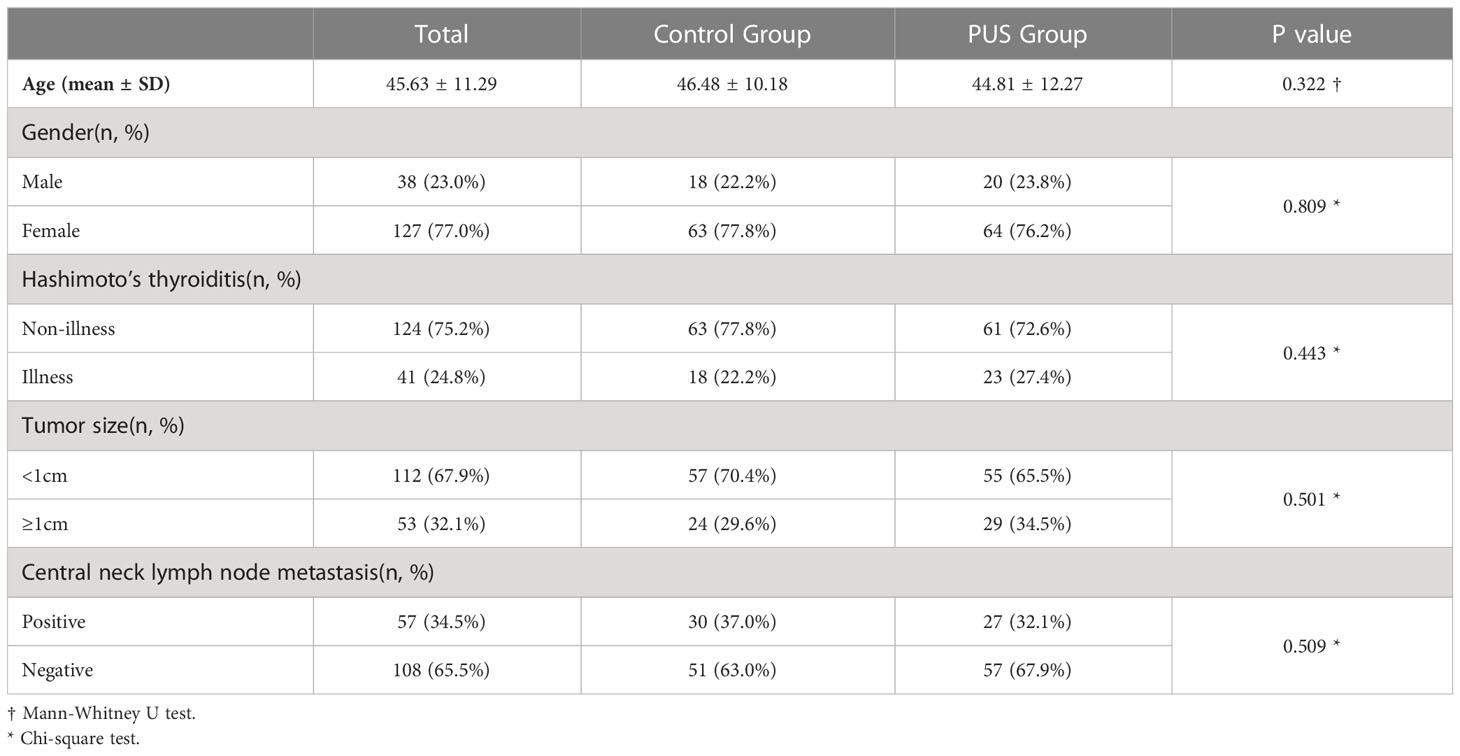
A total of 165 patients with thyroid cancer surgery in our hospital from May 2018 to May 2022 were included, with a male to female ratio of 38:127 (1:3.34), aged from 21 to 71 years, with a mean of 45.63 ± 11.29 years.
The patients were assigned into two groups according to the time period of surgery: Control group, May 2018 to February 2021 (before the application of ultrasound localization of parathyroid in our hospital); PUS group, March 2021 to May 2022 (After the application of ultrasound localization of parathyroid). Among them, 81 cases were in the control group and 84 cases in the preoperative ultrasound group (PUS group).
Inclusion criteria: (1) Subject is received total thyroidectomy and bilateral central node dissection, and is diagnosed as papillary thyroid cancer by postoperative pathology. (2) Subject is treated for the first time with thyroid surgery. (3) Preoperative parathyroid hormone levels, serum calcium levels, liver function and kidney function were within normal range. No history of suspected parathyroid pathology such as chronic kidney disease, urinary tract stones, increased or decreased bone density, pathological fracture, etc.
Exclusion criteria: (1) Subjects is received lymph node dissection in the lateral neck area. (2) Subjects is received intraoperative parathyroid auto-transplantation.
All subjects were informed and the informed consent form were signed. The study was approved by the ethics committee of our hospital (NO.2022-301).
2.2 Identification of normal Inferior parathyroid glands using ultrasonography
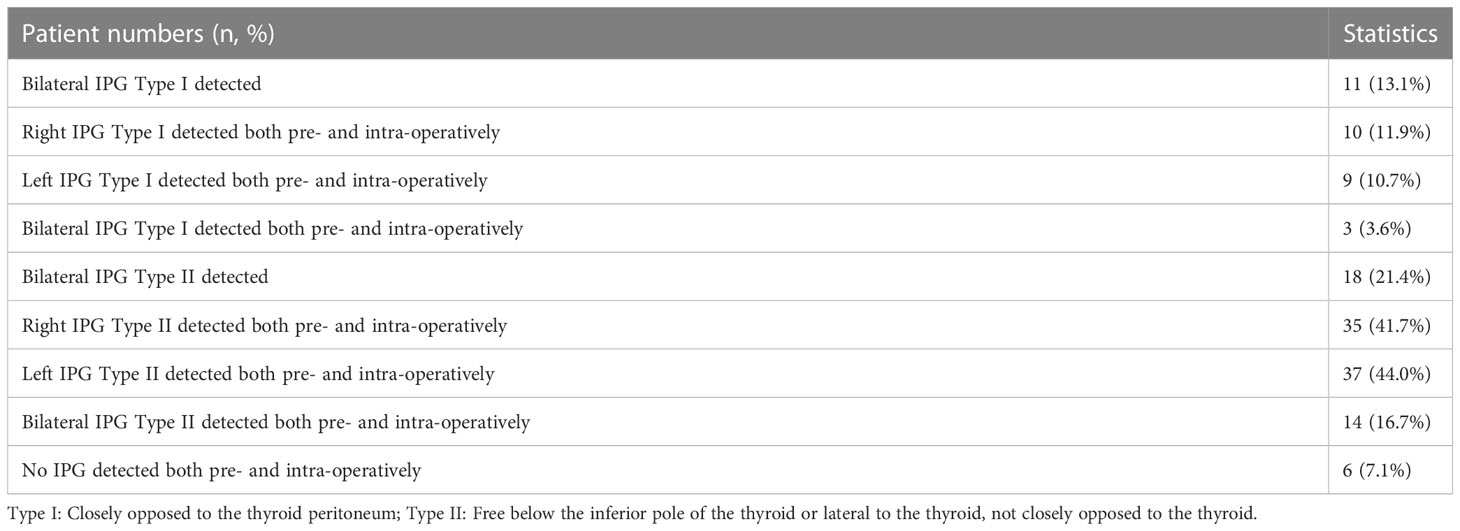
We developed a ultrasonic identification method of the IPGs. The IPGs was scaned using Aplio 500 Doppler ultrasonography (Toshiba) with a 5 to 14 MHz frequency probe. During the examination, the patient was placed in a supine position, and the head was tilted back to fully expose the anterior neck, with the head tilted to the side if necessary (Figure 1). The number, location, size, and internal echogenicity of the IPGs are observed (Figure 2).
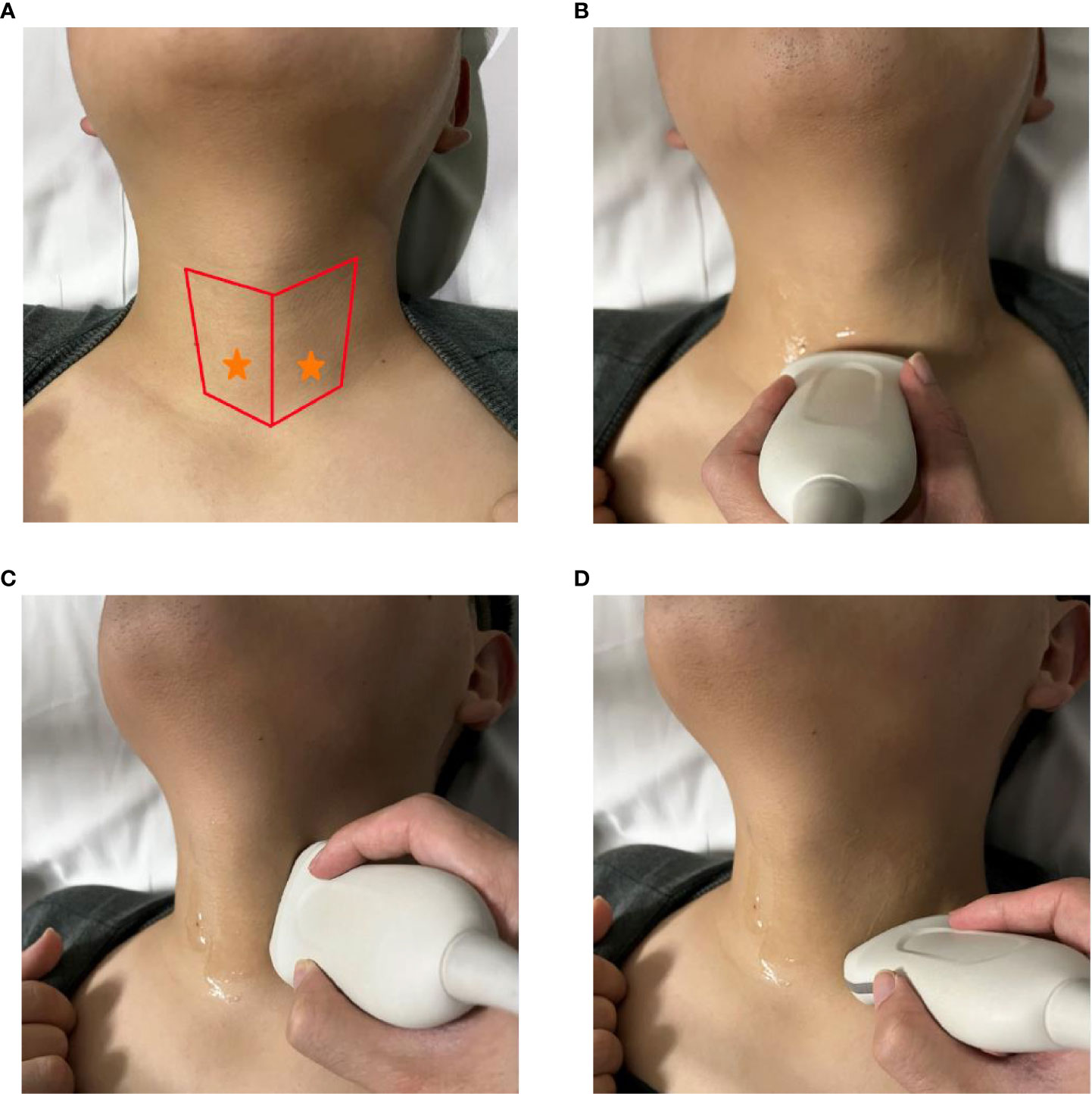
Figure 1 Identification of normal Inferior parathyroid glands using ultrasonography. (A) red area: main scanning range, yellow star: expected location of IPGs; (B–D) operation method of scanning IPGs.
Figure 2 Preoperative ultrasound identification of the IPG. (A) yellow arrow shows the IPG; (B) yellow arrow and circle show the IPG, blue arrow and circle show the thyroid, red arrow and circle show the muscle.
Operation of ultrasonography: The upper and lower range is from the middle and lower 1/2 of the dorsal thyroid to the level of the sternoclavicular joint, and the right and left range is between the trachea and the lateral border of the sternocleidomastoid (Figure 1).
Ultrasound features of the IPGs we developed are: (1) The morphology is mostly oval, a few narrow. (2) The size is about (6-8 mm) x (4-5 mm) x (3-4 mm). (3) The overall echogenicity is higher than that of the surrounding muscle tissue and is slightly higher than that of the thyroid tissue. The internal echogenicity is homogenous and a border with the surrounding tissue is visible (Figure 2). We have grouped the location of parathyroid into two categories, type I: closely opposed to the thyroid peritoneum; type II: free below the inferior pole of the thyroid gland or lateral to the thyroid gland, not closely opposed to the thyroid gland.
We have focused on measuring the following three distances of the IPGs in cross-section and longitudinal section: (A) The distance of the IPGs from the cricoid cartilage; (B) The distance of the IPGs from the midline of the trachea; (C)The distance of the IPGs from the anterior tracheal section. (It should be noted that this study did not involve bilateral superior parathyroid glands(SPG); please see the Conclusions and Discussion section for details). The diameters of three axes of the IPG were also measured (Figure 3). In addition, our IPG ultrasound images were independently reviewed and determined by three senior ultrasound experts individually. We included the PUS group for their agreement to confirm the IPG images.

Figure 3 Measurement of the relative location of the IPG to the trachea. (A) Diagram of IPG location; (B) The distance of the IPG from the cricoid cartilage (A, PCC); (C) The distance of the IPG from the midline of the trachea (B, P-MT) ; (D) The distance of the IPG from the anterior tracheal section (C, P-ATS).
2.3 Operation and measurement
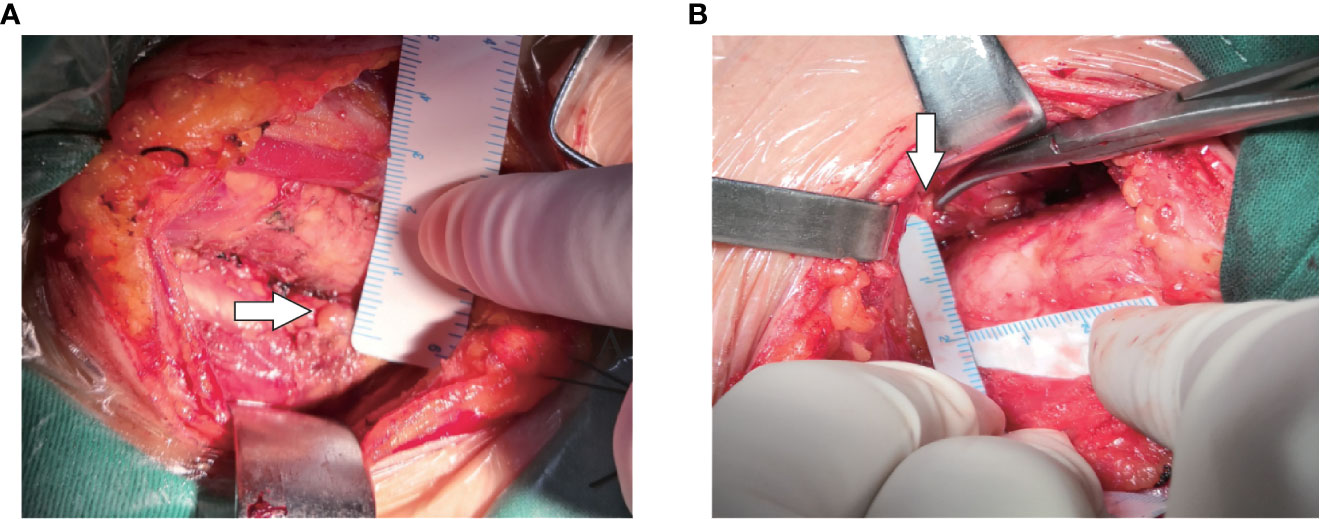
Intraoperatively, after carefully searching of the IPGs according the preoperative ultrasound localization, a sterile ruler was used to measure the distance A and B without touching the PGs (Figure 4). We didn’t measure the distance C (i.e., the depth), because significant variation due to the pulling of muscles during the anatomical exposure of the thyroid gland. We do not measure the size of the PGs for the sake of unnecessary manipulation.
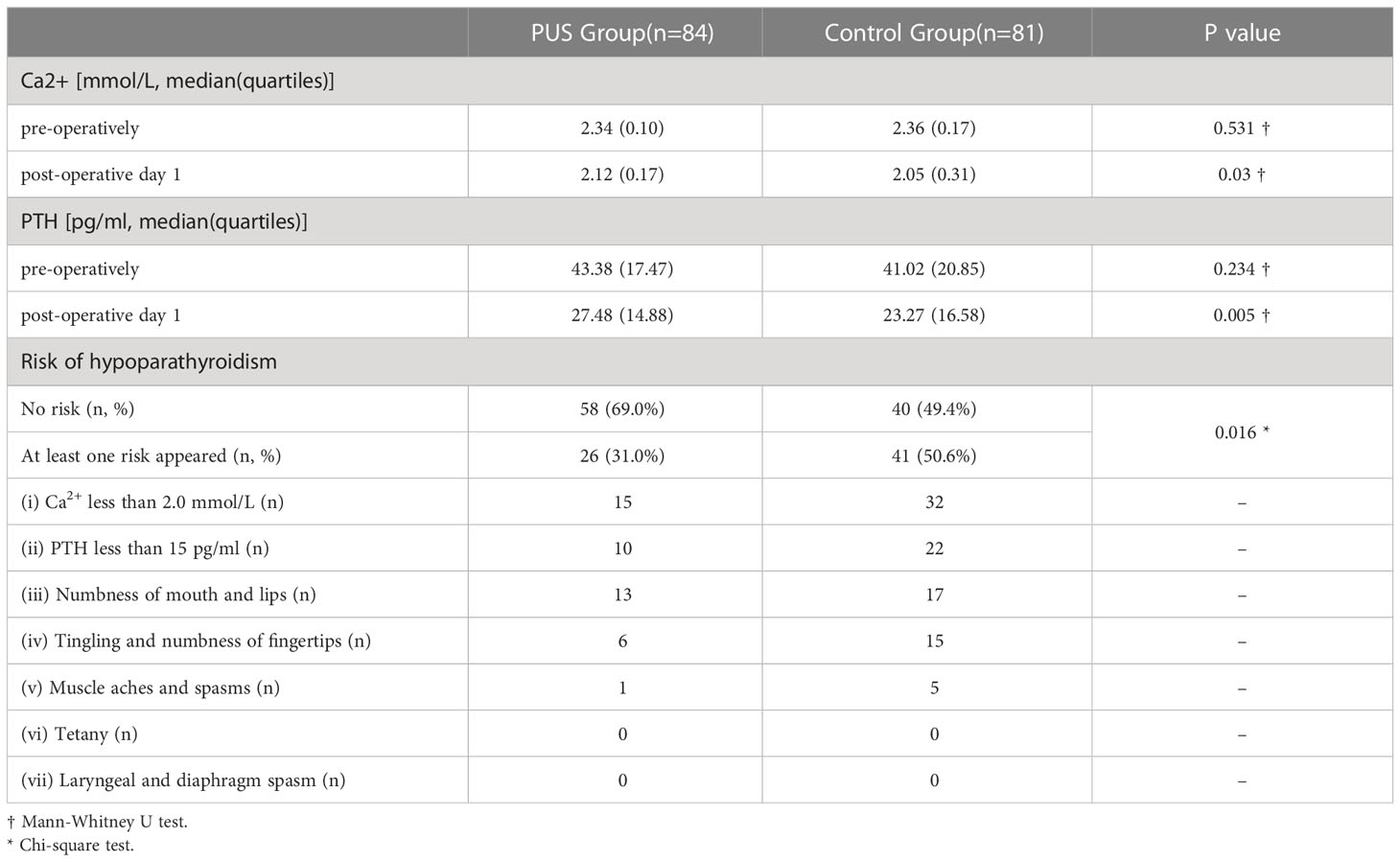
The Ca2+ (SIEMENS, ADVIA2400) and PTH (ROCHE, Cobas e 601) levels were measured preoperatively and on postoperative day 1. Patients with the following conditions are considered to be at risk for hypoparathyroidism: (i) Ca2+ less than 2.0 mmol/L; (ii) PTH less than 15 pg/ml; (iii) numbness of mouth and lips; (iv) Tingling and numbness of fingertips; (v) Muscle aches and spasms; (vi) tetany; (vii) laryngeal spasm, diaphragm spasm, and other severe spasms in both skeletal and smooth muscles throughout the body.
2.4 Statistical analysis
SPSS 25.0 and Graphpad prism 8.0.2 software were used to process the data. The Mann-Whitney U test was used. Data were expressed as mean ± standard deviation (X ± S) or median (quartiles); Spearman correlation was used for bivariate correlation analysis; Pearson X2 test was used for categorical variables. p<0.05 was considered statistically significant.
3 Result
3.1 Patient characteristics
A total of 165 eligible patients were included in this study. There were 84 in the preoperative ultrasound group and 81 in the control group. There were no significant differences between the preoperative ultrasound groups and control groups in parameters such as age, sex, prevalence of Hashimoto’s thyroiditis, tumor size, and lymph node metastasis in the central region (Table 1).
3.2 Preoperative ultrasound localization of IPGs
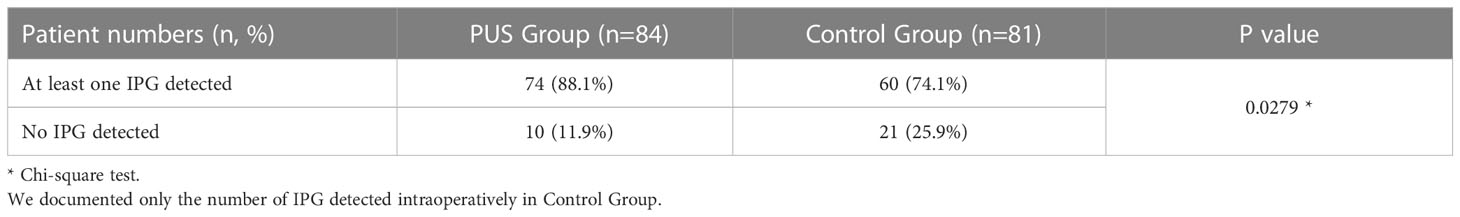
Our data showed that the left side IPGs were found in 51 (60.7%) patients and 48 (57.1%) for the right side by ultrasonography. A total of 31 (36.9%) IPGs were found bilaterally, and 68 (81.0%) IPGs were found on at least one side. This indicates that we had a high detection rate of the IPGs on at least one side (Table 2). The average size observed in ultrasonography of the IPGs was: (7.25 ± 1.38) x (3.88 ± 0.92) x (4.65 ± 1.04) mm, while the three-dimensional spatial coordinate A, B, and C were (36.70 ± 5.75) mm, (15.85 ± 3.82) mm, and (5.29 ± 2.93) mm, respectively. This is an important reference for finding the IPGs during surgery (Table 2; Figure 5).
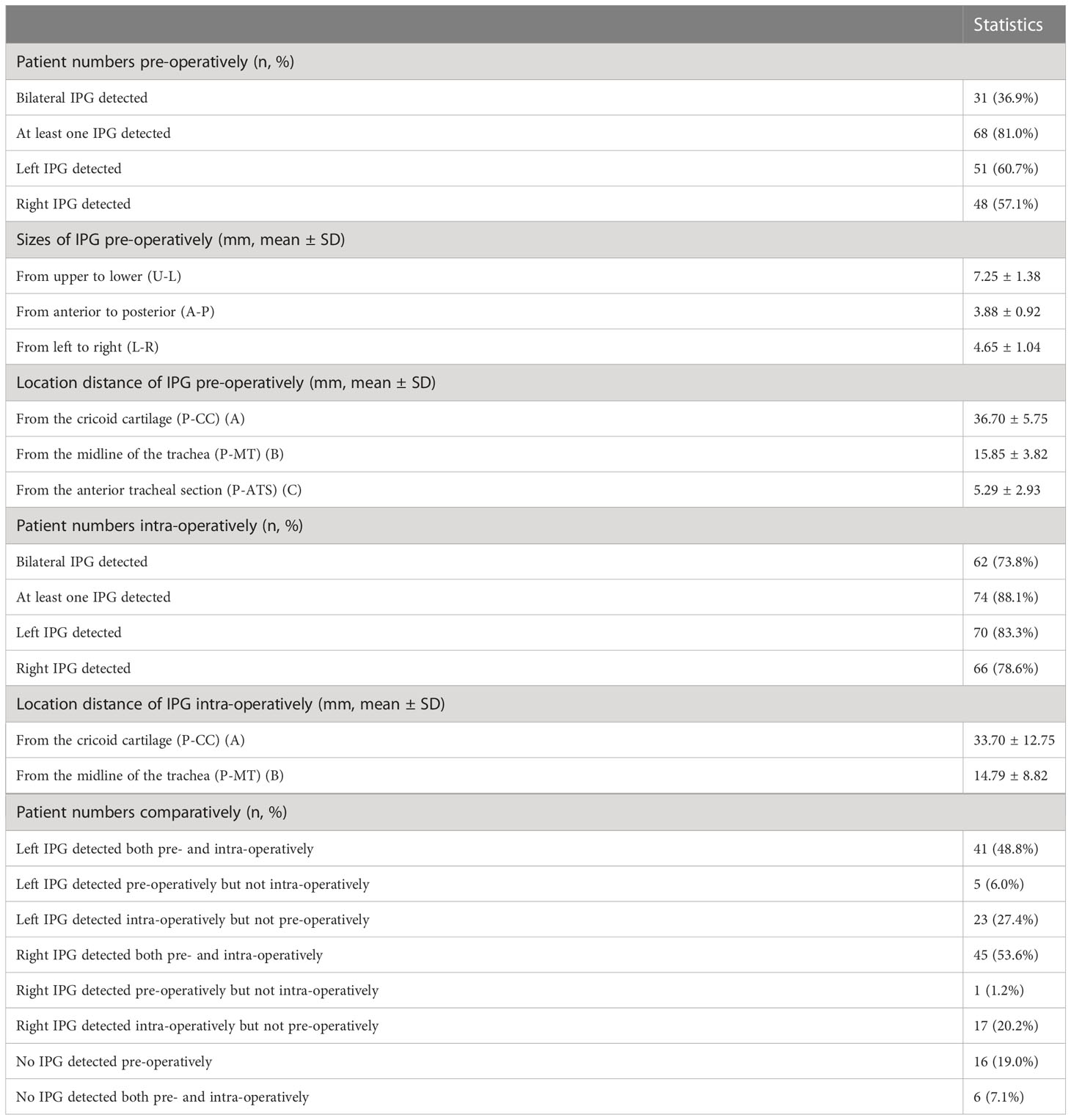
Table 2 Details of pre-operative ultrasound evaluation and intra-operative detection of IPG in PUS Group.
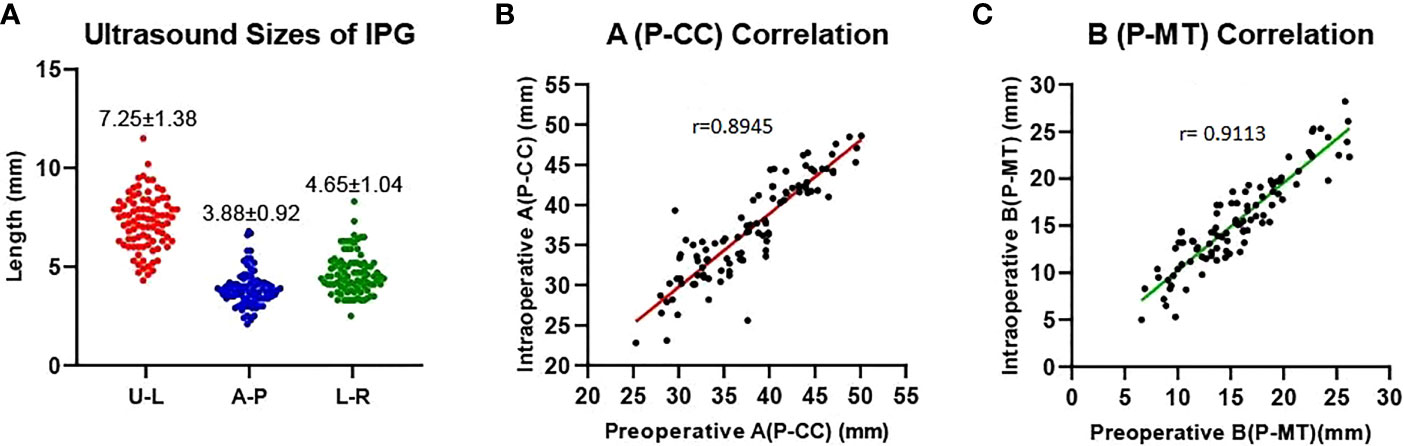
Figure 5 Preoperative ultrasound measurements of the IPG. (A) Ultrasound sizes of IPG (U-L: upper-lower diameters, A-P: anterior-posterior diameters, L-R: left-right diameters); (B, C) Preoperative and intraoperative distance correlation. Distance of the IPG from the cricoid cartilage (A, P-CC), distance of the IPG from the midline of the trachea (B, P-MT).
We have classified the position of IPGs into two categories (Figure 6): type I, where the IPGs were in close proximity to the thyroid gland and adhered to the thyroid peritoneum, on the inferior thyroid position or to the dorsal lateral thyroid peritoneum; type II, where the IPGs were not adhered to the thyroid peritoneum and separated from the thyroid gland for a certain distance.
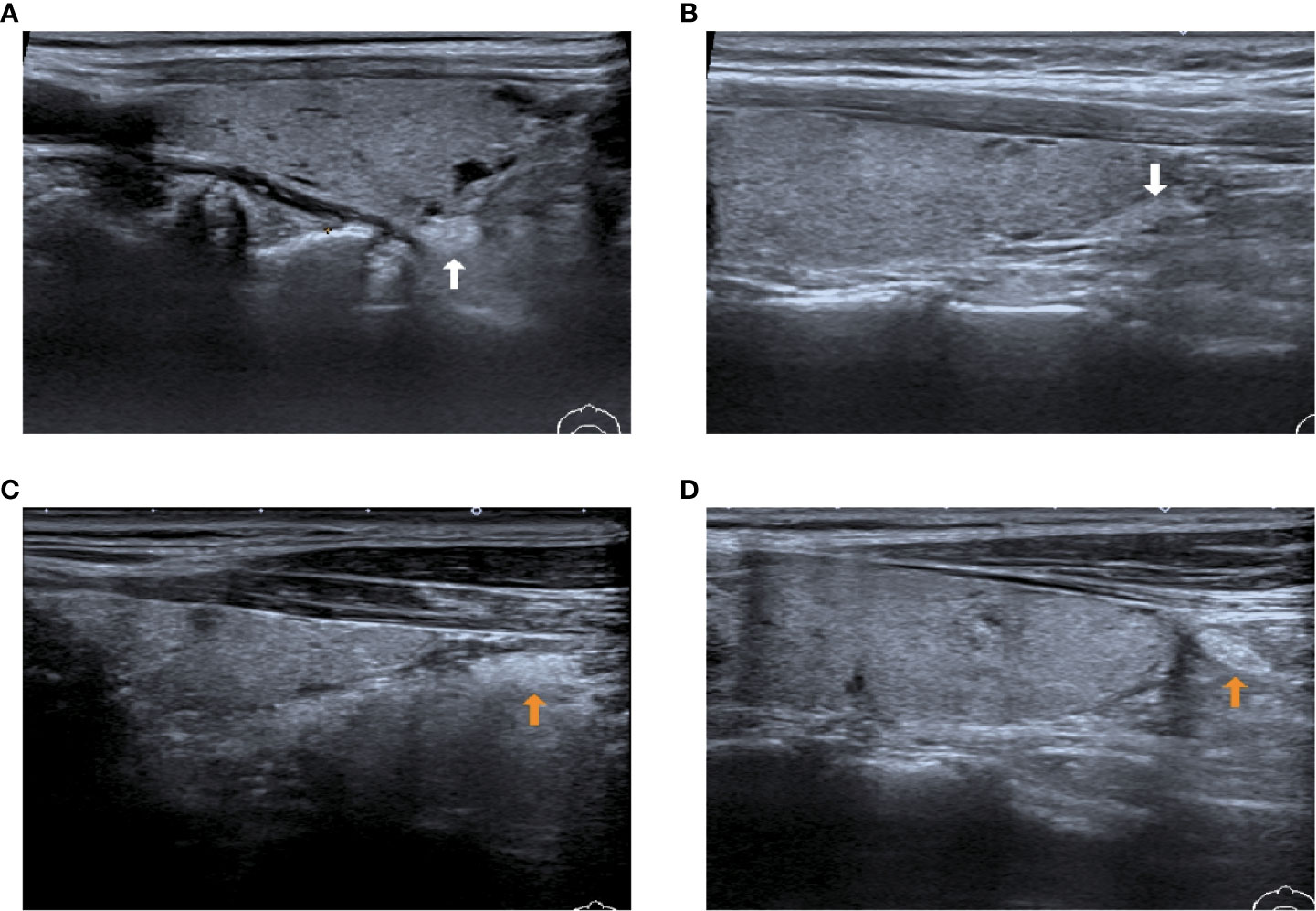
Figure 6 Two types of IPG observed by preoperative ultrasound images. (A, B): White arrow shows type I PG, where the PGs are in close contact with the thyroid gland; (C, D): Yellow arrow shows type II PG, where the PGs are free of the thyroid gland and not in close contact with the thyroid gland.
3.3 Intraoperative comparison for IPG
After carefully searching the IPGs according to the preoperative ultrasound position, the coordinate parameters of parathyroid (A and B) were measured with a sterile scale, which were (33.70 ± 12.75) mm and (14.79 ± 8.82) mm, respectively, we found a good correlation (r= 0.8945; r= 0.9113) with the preoperative ultrasound measured spatial position (Figure 5). This indicates that our preoperative ultrasound-measured three-dimensional spatial coordinate was very helpful in finding identification during surgery.
Bilateral IPGs could be explored intraoperatively in 62 cases (73.8%) and at least one IPG was found in 74 cases (88.1%) in the preoperative ultrasound (Table 2). The percentage of those who could be found on preoperative ultrasonography but cannot be found intraoperatively, whether left or right sides, was less than 10% (6.0%, 1.2%). This indicates t that our preoperative ultrasonic identification technique has more than 90% (94%, 98.8%) accuracy (true positive rate) in identifying the tissue as a PG. Those who were not identified by preoperative ultrasound evaluation but had found intraoperatively were: 17 cases (20.2%) in the right side and 23 cases (27.4%) in the left side (false negative rate).
In addition, in the preoperative ultrasound group versus the control group, 74 (88.1%) vs. 60 (74.1%) IPGs could be detected intraoperatively on at least one side; 10 (11.9%) vs. 21 (25.9%) IPGs were not detected intraoperatively on both sides, P=0.0279 (Table 3).
We can conclude that ultrasonic localization of the PGs can help in the search, identification and in situ protection of the PGs during thyroidectomy.
3.4 Evaluation of IPG protection after surgery
At first day postoperative, patients in the preoperative ultrasound group had better postoperative biochemical parameters including Ca2+: 2.12 (0.17) vs. 2.05 (0.31), P=0.03; PTH: 27.48 (14.88) vs. 23.27 (16.58), P=0.005, than the control group (Table 4). The risk of at least one type of reduced parathyroid function postoperatively: 26 (31.0%) vs. 41 (50.6%), P= 0.016.
In addition, only 6 patients in the preoperative ultrasound group complained of slight numbness in both hands-on postoperative day 1, and only 1 complained of numbness and tingling with spasm in the fingertips; none of them complained of the above-mentioned numbness on postoperative day 3 when they were left the hospital. In contrast, 15 patients in the control group complained of slight numbness in both hands first day after surgery, and 5 patients complained of numbness and tingling in the with spasm; 2 patients complained of numbness in both hands and around the mouth again on the third day after surgery. There were no critical symptoms such as hand-foot convulsions and laryngeal diaphragm spasms in the study and control groups after surgery.
4 Discussion
In present study, we evaluated the effect of the application of preoperative ultrasound localization techniques on reducing the risk of postoperative hypoparathyroidism. We found that our preoperative ultrasonic identification technique has more than 90% (94%, 98.8%) accuracy (true positive rate) in identifying the tissue as a PG. Postoperative biochemical result showed a better Ca2+ and PTH levels at first day post-operation in PUS group compared to control group.
Interestingly, As shown in Table 5, we found a higher detection rate of type II PG than type I pg. We suppose the reasons are as follows: Firstly, type II IPG are not closely adhered to thyroid, which makes them easier to identify; Secondly, most type II IPG were enveloped by a “fat capsule” (Figure 7).
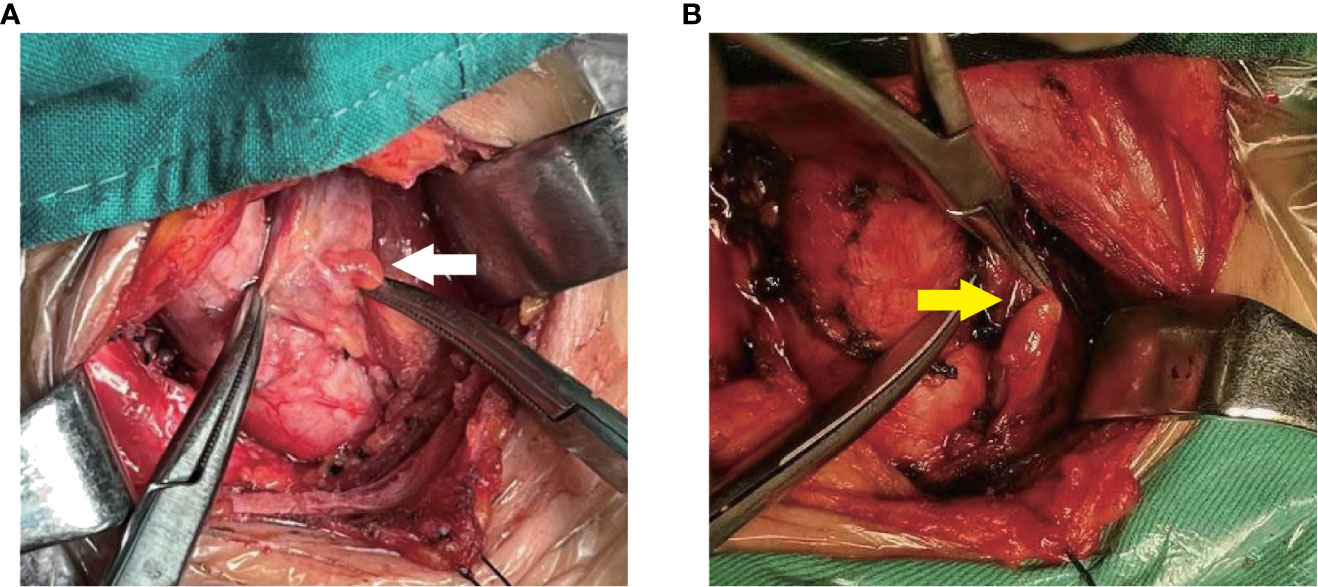
Figure 7 Two types of IPG observed during surgery. (A): White arrow shows Type I PG; (B): yellow arrow shows Type II PG.
The reasons for the high false-negative rate are as follows: (1) A small percentage of suspicious IPGs on ultrasound do not have clear borders and echogenicity and are similar to surrounding fat, lymph, and connective tissue. The three senior ultrasound specialists in our team did not include ambiguous tissues in the positive criteria. (2) A small percentage of IPGs do not visible clearly on ultrasound and may be covered by other tissues. For example, they are extremely close to the true peritoneum of the thyroid gland, far from the thyroid gland, or encapsulated in lymph nodes in the central region and not clearly detected.
At present, the SPGs are very challenging to visualize on ultrasonography, the result as follows: (1) We have tried to obtain satisfactory ultrasound images for the SPGs from experienced ultrasonographers. Very few of the SPGs are clearly visualized on ultrasound. This may be due to the complex anatomy surrounding the SPGs, such as the thyroid cartilage, which affects the visualization on ultrasound. (2) The location of the SPGs is relatively fixed and without surrounding fat or lymph nodes. As a result, the SPGs are easy to find during surgery. (3) The location of the IPGs is extremely variable, with a complex blood supply and surrounding fat or lymph nodes. During the operation, it is difficult to find, which increased the risk of accidental removal or blood supply damage. (4) In our prior experience, the probability of accidental removal of the IPGs is much higher than the SPGs. Therefore, our study focused on bilateral inferior parathyroid glands and excluded bilateral superior parathyroid glands.
How it helps to reduce the damage to the parathyroid glands and their blood supply by anticipation the location of the inferior parathyroid glands? Since we anticipate their location, we can protect the parathyroid glands and their blood supply before they are fully exposed. Our technique reduces the window period to discover the parathyroid glands. More importantly, our technique avoids unnecessary manipulations to search and expose parathyroid (this is also the main reason of accidently damage by experienced surgeon), that effectively reduces the risk of the direct damage of the parathyroid glands. Because while we were searching for it during surgery, we had already damaged it or its blood supply.
For the parathyroid glands that closely attached to thyroid (type I IPG), we can try to anticipately preserve their blood supply before thyroidectomy. For the parathyroid glands that positioned at a certain distance from the thyroid gland (type II IPG), due to their high similarity to surrounding lymph nodes and fatty granules, surgeon should well expose the suspicious structures one by one to identify the parathyroid. Our preoperative localization technique allows us to anticipate its location and avoid exposing every suspicious structure, which reduces the risk of accidental removal or blood supply damage.
Additionally, for the parathyroid glands that closely attached to thyroid glands and without independent blood supply. This type of parathyroid glands could not be preserved in situ, and the only solution is auto-transplantation. We have excluded these cases in the present works. This is because survival rates are different after auto-transplantation. Serum Ca2+ and PTH may be affected.
In our study, in 10 cases (11.9%) we did not find IPG either by preoperative ultrasound localization or intraoperatively. The IPG and SPG have distinct embryonal origin: The IPG originates from the third pharyngeal bursa together with the thymus, whereas the SPG originates from the fourth pharyngeal bursa (20, 21), which explains the variation in the location of the IPGs. The 10 cases in which we did not find the IPGs could localized around the vascular sheaths of the neck, in the thymus, mediastinum or thyroid parenchyma. This category may be called “type III” PG.
Currently, 99mTc-MIBI is only used to localize parathyroid adenomas but is not recommended for the identification of normal PGs during surgery (22, 23). Although exogenous dyes such as indocyanine green have been used intraoperatively to localize PGs, it may cause toxicity, pain, allergy (24). In addition, there is the parathyroid near-infrared autofluorescence technique. However, it is difficult to determine type II PG, which is covered by fat, connective tissue or the thyroid gland. In other word, the type II PGs cannot be identified with autofluorescence unless the glands are exposed (25, 26). More importantly, these techniques require additional manipulations and increase overall time cost of surgery.
5 Conclusion
In summary, our ultrasonography identification and localization help to anticipate the location of parathyroid gland before surgery. Our technique reduces intraoperative damage to the parathyroid glands by reducing unnecessary manipulations during the search of the parathyroid glands, which reduces the incidence of postoperative hypoparathyroidism. We have confirmed its effect by postoperative biochemical analysis. In addition, this technique also reduces the psychological stress of the surgeon during surgery about the unknown location of the parathyroid glands.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CG designed and developed whole project and modified original article. RC, KZ reviewed and editing preparation, creation and presentation of the work of the research group. RC written the original article and completed the statistical work. JL, LG, and KL assisted measurement of IPGs in surgery. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Giuffrida D, Giuffrida R, Puliafito I, Vella V, Memeo L, Puglisi C, et al. Thyroidectomy as treatment of choice for differentiated thyroid cancer. Int J Surg Oncol (2019) 2019:2715260. doi: 10.1155/2019/2715260
2. Jin S, Sugitani I. Narrative review of management of thyroid surgery complications. Gland Surg (2021) 10(3):1135–46. doi: 10.21037/gs-20-859
3. Christou N, Mathonnet M. Complications after total thyroidectomy. J Visc Surg (2013) 150(4):249–56. doi: 10.1016/j.jviscsurg.2013.04.003
4. Goltzman D. Physiology of parathyroid hormone. Endocrinol Metab Clin North Am (2018) 47(4):743–58. doi: 10.1016/j.ecl.2018.07.003
5. Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol (2015) 22:41–50. doi: 10.1016/j.coph.2015.03.005
6. Fancy T, Gallagher D 3rd, Hornig JD. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am (2010) 43(2):221–vii. doi: 10.1016/j.otc.2010.01.001
7. Mohebati A, Shaha AR. Anatomy of thyroid and parathyroid glands and neurovascular relations. Clin Anat (2012) 25(1):19–31. doi: 10.1002/ca.21220
8. Tjahjono R, Nguyen K, Phung D, Riffat F, Palme CE. Methods of identification of parathyroid glands in thyroid surgery: A literature review. ANZ J Surg (2021) 91(9):1711–6. doi: 10.1111/ans.17117
9. Gschwandtner E, Seemann R, Bures C, Preldzic L, Szucsik E, Hermann M. How many parathyroid glands can be identified during thyroidectomy?: evidence-based data for medical experts. Eur Surg (2018) 50(1):14–21. doi: 10.1007/s10353-017-0502-0
10. Xing Z, Qiu Y, Fei Y, Xia B, Abuduwaili M, Zhu J, et al. Protective strategy of parathyroid glands during thyroid lobectomy: A retrospective cohort and case-control study. Med (Baltimore) (2021) 100(14):e21323. doi: 10.1097/MD.0000000000021323
11. Lorente-Poch L, Sancho JJ, Ruiz S, Sitges-Serra A. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg (2015) 102(4):359–67. doi: 10.1002/bjs.9676
12. Păduraru DN, Ion D, Carsote M, Andronic O, Bolocan A. Post-thyroidectomy hypocalcemia - risk factors and management. Chirurgia (Bucur) (2019) 114(5):564–70. doi: 10.21614/chirurgia.114.5.564
13. Dedivitis RA, Aires FT, Cernea CR. Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg (2017) 25(2):142–6. doi: 10.1097/MOO.0000000000000346
14. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
15. Mathonnet M. Exploration des nodules thyroïdiens: rôle de l'échographie préopératoire [Role of preoperative ultrasonography in the management of thyroid nodules]. Ann Chir (2006) 131(10):577–82. doi: 10.1016/j.anchir.2006.01.008
16. Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American Thyroid association statement on preoperative imaging for thyroid cancer surgery. Thyroid (2015) 25(1):3–14. doi: 10.1089/thy.2014.0096
17. Milas M, Stephen A, Berber E, Wagner K, Miskulin J, Siperstein A. Ultrasonography for the endocrine surgeon: a valuable clinical tool that enhances diagnostic and therapeutic outcomes. Surgery (2005) 138(6):1193–201. doi: 10.1016/j.surg.2005.08.032
18. Andrioli M, Valcavi R. Sonography of normal and abnormal thyroid and parathyroid glands. Front Horm Res (2016) 45:1–15. doi: 10.1159/000442273
19. Xia C, Zhu Q, Li Z, Hu M, Fang J, Zhong Q, et al. Study of the ultrasound appearance of the normal parathyroid using an intraoperative procedure. J Ultrasound Med (2019) 38(2):321–7. doi: 10.1002/jum.14691
20. Guilmette J, Sadow PM. Parathyroid pathology. Surg Pathol Clin (2019) 12(4):1007–19. doi: 10.1016/j.path.2019.08.006
21. Policeni BA, Smoker WR, Reede DL. Anatomy and embryology of the thyroid and parathyroid glands. Semin Ultrasound CT MR (2012) 33(2):104–14. doi: 10.1053/j.sult.2011.12.005
22. Kunstman JW, Kirsch JD, Mahajan A, Udelsman R. Clinical review: Parathyroid localization and implications for clinical management. J Clin Endocrinol Metab (2013) 98(3):902–12. doi: 10.1210/jc.2012-3168
23. Baj J, Sitarz R, Łokaj M, Forma A, Czeczelewski M, Maani A, et al. Preoperative and intraoperative methods of parathyroid gland localization and the diagnosis of parathyroid adenomas. Molecules (2020) 25(7):1724. doi: 10.3390/molecules25071724
24. Lavazza M, Liu X, Wu C, Anuwong A, Kim HY, Liu R, et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg (2016) 5(5):512–21. doi: 10.21037/gs.2016.10.06
25. Solórzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A. Detecting the near infrared autofluorescence of the human parathyroid: Hype or opportunity? Ann Surg (2020) 272(6):973–85. doi: 10.1097/SLA.0000000000003700
26. Benmiloud F, Godiris-Petit G, Gras R, Gillot JC, Turrin N, Penaranda G, et al. Association of autofluorescence-based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: Results of the PARAFLUO multicenter randomized clinical trial. JAMA Surg (2020) 155(2):106–12. doi: 10.1001/jamasurg.2019.4613
Keywords: thyroid cancer, parathyroid gland, hypoparathyroidism, ultrasound localization, clinical effect
Citation: Chen R, Zhang K, Liu J, Guo L, Liu K and Geng C (2023) Preoperative ultrasound identification and localization of the inferior parathyroid glands in thyroid surgery. Front. Endocrinol. 14:1094379. doi: 10.3389/fendo.2023.1094379
Received: 10 November 2022; Accepted: 08 February 2023;
Published: 27 February 2023.
Edited by:
Kyung Tae, Hanyang University, Republic of KoreaReviewed by:
Xuedong Wang, Tsinghua University, ChinaXianqiang Wang, PLA General Hospital, China
Jun Liu, Sichuan University, China
Bin Lv, Qilu Hospital, Shandong University, China
Copyright © 2023 Chen, Zhang, Liu, Guo, Liu and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong Geng, Y2hvbmdnZW5nXzE5ODJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Ruyue Chen1†
Ruyue Chen1† Chong Geng
Chong Geng