- 1Department of Ultrasound, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China
- 2Key Laboratory of Endocrinology, Department of Endocrinology, National Commission of Health, Peking Union Medical College Hospital, Chinese Academy of Medical Science, Beijing, China
- 3Department of General Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Science & Peking Union Medical College, Beijing, China
Background: Identification of multigland disease (MGD) in primary hyperparathyroidism (PHPT) patients is essential for minimally invasive surgical decision-making.
Objective: To develop a nomogram based on US findings and clinical factors to predict MGD in PHPT patients.
Materials and Methods: Patients with PHPT who underwent surgery between March 2021 and January 2022 were consecutively enrolled. Biochemical and clinicopathologic data were recorded. US images were analyzed to extract US features. Logistic regression analyses were used to identify the risk factors for MGD. The nomogram was constructed based on the factors. Nomogram performance was evaluated by area under the receiver operating characteristic curve (AUC), calibration curve, the Hosmer–Lemeshow test, and decision curve analysis.
Results: A total of 102 PHPT patients were included. 82 (80.4%) had the single-gland disease (SGD) and 20 (19.6%) had MGD. Using multivariate analysis, the MGD was positively correlated with age (OR = 1.033, 96%CI = 0.985-1.092), PTH level (OR = 1.001, 95% CI = 1.000–1.002), MEN-1 (OR = 29.730, 95% CI = 3.089-836.785), US size (OR = 1.198, 95% CI = 0.647–2.088) and US texture (cystic-solid) (OR = 5.357, 95% CI = 0.499–62.912). And negatively correlated with gender (OR = 0.985, 95% CI = 0.190–4.047), calcium level (OR = 0.453, 95% CI = 0.070–2.448), and symptoms(yes) (OR = 0.935, 95%CI = 0.257–3.365). The nomogram showed good discrimination with an AUC of 0.77 (0.68-0.85) and good agreement for predicting MGD in PHPT patients. And 65 points was recommended as a cut-off value with a specificity of 0.94 and a sensitivity of 0.50.
Conclusion: US provided useful features for evaluating MGD. Combining the US and clinical features in a nomogram showed good diagnostic performance for predicting MGD.
1 Introduction
Primary Hyperparathyroidism (PHPT) is a common endocrine disorder, presenting as abnormal bone metabolism and hypercalcemia. About 80% of patients with PHPT have a single parathyroid adenoma, and 15-20% of patients encountered a multigland disease (MGD) (1). Parathyroidectomy is the only curative and definitive treatment for PHPT (2, 3). The surgical procedure for PHPT has evolved considerably in the past few years, with the development of preoperative localizing tools and intraoperative io-PTH. Minimally invasive and unilateral surgical approaches had replaced the traditional bilateral neck exploration (BNE) for being with a smaller scar, reduced incidence of postoperative hypocalcemia, lower rates of recurrent laryngeal injury, and shorter length of hospital stay, but with equally high cure rates (4–7). Thus, accurate preoperative localization of MGD is of great value for minimally invasive surgical decisions (5, 8).
For the preoperative location, ultrasound (US) and technetium-99m sestamibi are the first lines of imaging (9). However, the sensitivity for multigland disease is limited with 15%–35% in US, and 30%–44% in 99mTc-sestamibi imaging (10). The underestimated MGD may lead to incomplete surgical excision, and result in persistent and recurrent hyperparathyroidism (11). Previous studies have explored the risk factors of MGD and developed prediction models for improving SGD and MGD identification. CaPTHUS, a scoring system for single gland disease combining preoperative biochemical and imaging study results achieved a positive predictive value of 100% for a total score of 3 or higher (12). Wisconsin Index (WIN) was established for predicting the probability of the presence of additional hyperfunctioning glands during surgery, using preoperative parathyroid hormone (PTH) level and weight of surgically removed lesions (13). Subsequently, the efficacy of the two classical models have been validated (14, 15), both of which provide good diagnostic efficacy for single gland disease with PPV of 84.6-96.6%, but neither could be adopted to improve the diagnostic efficacy of MGD, The WIN could not be incorporated into the preoperative evaluation of MGD.Ultrasound is preferred for preoperative localization of primary hyperparathyroidism with high sensitivity, noninvasive, and free of radiation. To date, no comprehensive studies have evaluated the performance of specific US features in combination with clinical data to predict an MGD. This study aimed to identify ultrasound features associated with MGD and to assess the predictive value of a combination of US and clinical data for determining an MGD in PHPT patients.
2 Material & method
2.1 Patients
This retrospective study was approved by the Institutional Review Board of Perking Union Medical College Hospital.
All patients with PHPT who underwent parathyroidectomy at Peking Union Medical College Hospital between March 2021 and January 2022 were enrolled. The included criteria were: 1) diagnosed as primary hyperparathyroidism (PHPT). PHPT is diagnosed after comprehensive evaluation of biochemical testing. Classic PHPT presents as elevated serum calcium levels and serum parathyroid hormone (PTH) levels (calcium>2.5mmol/L; PTH>65pg/mL) (2).; 2) successful surgery (normal calcium level at least six months after surgery). The excluded criteria were incomplete data, including calcium and PTH determination, ultrasound examination, and pathological results.
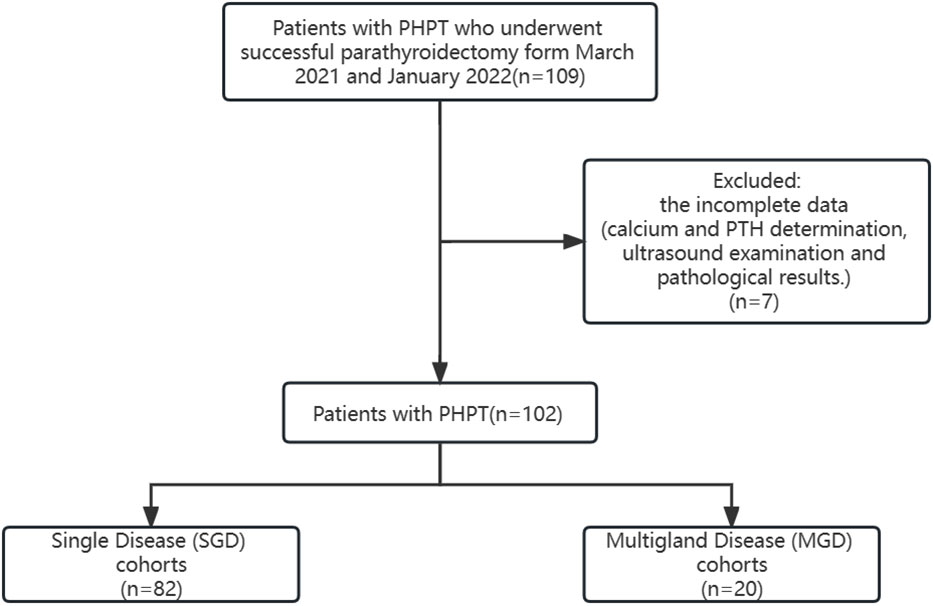
According to operative records and pathological results, the cohort was divided into two groups: single-gland disease (SGD) and multi-gland disease (MGD). The flowchart of study participants can be seen in Figure 1.
Demographic and biochemical data were reviewed, including age, gender, symptom, preoperative PTH and calcium, pathology reports, and operative notes. To note, the patient was defined as “symptomatic” if the patient presents with at least the one of the following symptoms: skeletal symptoms (bone pain, fractures), urinary symptom (nephrolithiasis), gastrointestinal symptoms (anorexia, nausea, constipation).
2.2 Ultrasound technique and image analysis
All patients underwent neck ultrasound scanning by an experienced radiologist before surgery. The ultrasound examination was performed using IU22 (Philips, Netherlands) and Aixplorer (Supersonic Imagine, France) with Linear probes (3-12 MHz, centered at 10 MHz). For each suspicious lesion, the grey scale ultrasound images of both the longitudinal section and cross-section were acquired. The largest US size of the dominant candidate lesion in the longitudinal section was measured. the location, including the side and quadrant, were labeled. The recorded imaging data of the patients were independently reviewed and selected for further analysis by two experienced radiologists, blinded to the pathological results. The location and US size were recorded. The US texture of suspicious lesions evaluated accordingly. If there were multiple lesions in US, the dominant lesion was evaluated. The US texture is mainly divided into two categories according to the lesion echogenicity, 1) solid (hypoechoic, isoechoic, hyperechoic); 2) cystic-solid (lesion containing fluid content (anechoic)).
2.3 Operative regimens
The surgical approach depends on preoperative localization study findings, typically two concordant preoperative imaging studies are required. When first-line modalities, US and parathyroid scintigraphy, are negative or discordant, multiphase computed tomography (CT), positron emission tomography (PET) by using 11C-methionine, or magnetic resonance imaging (MRI) are considered. Histopathological examinations were performed for all lesions removed during the surgery. The histopathological diagnosis was performed according to the WHO classification criteria (16). All histopathological results were recorded as the golden standard.
2.4 Statistical analysis
All data were classified as categorical or quantitative variables. Categorical variables were reported as frequencies and percentages and analyzed using Pearson Chi-square. Quantitative variables were characterized by median (minimum-maximum) or by the mean and standard deviation (SD) and compared using Student’s t-test for parametric data and Mann-Whitney U test or Kruskal–Wallis test for non-parametric data where appropriate. Univariable and multivariate logistic regression was performed on the clinical and US characteristics. Based on the multivariate analysis, all risk factors were included in the nomogram construction. The performance of the nomogram was evaluated by calculating the area under the ROC curve (AUC). The Hosmer–Lemeshow test and calibration plots were used to assess the model fit. Decision curve analysis was used to evaluate the clinical benefits of the nomogram.
p < 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS (SPSS 20.0, USA) software package and the packages in R 4.1.0 (https://www.r-project.org/), such as “rms,” “pROC,” “regplot” and “ggDCA”. The inter-operator agreement of the two readers was measured using the κ value, which was interpreted as follows: κ < 0, poor agreement; 0 < κ < 0.20, slight agreement; 0.20 < κ < 0.40, fair agreement; 0.40 < κ < 0.60, moderate agreement; 0.60 < κ < 0.80, substantial agreement; and 0.80 < κ < 1, perfect agreement.
3 Results
3.1 Clinical characteristics and ultrasonic features of the cohorts
A total of 102 patients with PHPT who underwent parathyroidectomy have been retrospectively collected. The mean age was 53 ± 12 years, and 77.5% of patients were female. 52 patients had obvious clinical symptoms, including 30 with urinary symptoms, 26 with skeletal symptoms, and 7 with gastrointestinal symptoms. The remaining 50 patients were asymptomatic. All patients underwent neck US, and 99 (97.1%) underwent 99mTc-MIBI in addition to US. 7 (6.9%) underwent 4D-CT, 7(6.9%) underwent PET-CT, and 1 (1.0%) underwent MRI. Of which 82 (80.4%) patients had SGD and 20 (19.6%) patients had MGD according to pathological findings.
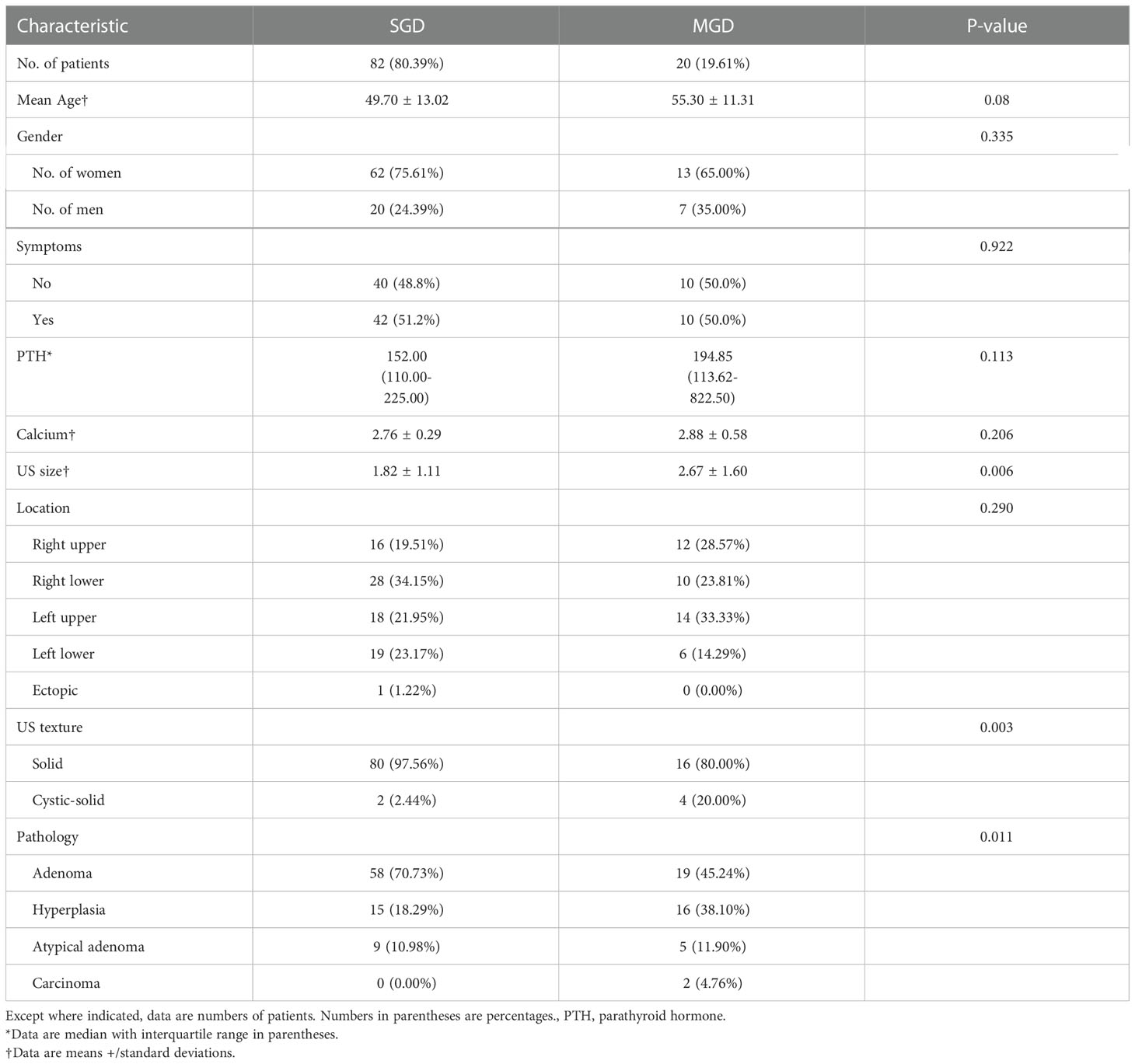
The baseline clinical and ultrasound features of PHPT patients with SGD and MGD were shown in Table 1. There was a statistically significant difference between SGD and MGD on size (p=0.006), US texture (p=0.003) and pathology (p=0.011). And there was no significant difference between SGD and MGD in terms of age (p=0.08) and gender (p=0.335), symptoms (p=0.992), PTH (p=0.113). MGD has a higher PTH level, a larger size than that SGD. The cystic-solid lesion was prone to be seen in MGD. In terms of pathological type, hyperplasia is more common in MGD. Of the MGD cohorts, 17 were double lesions and 3 were triple lesions. Of the 17 patients with double lesions, the pathologic findings included 7 adenoma complicated with a hyperplastic gland (41.2%), four double adenomas (23.5%), three two-gland hyperplasia (17.6%), one double atypical adenomas (5.9%), and one double carcinomas (5.9%), one an atypical adenoma complicated with an adenoma (5.9%). Three patients were confirmed with triple lesions including three adenomas(n=1), two glands hyperplasia with one adenoma(n=1), and two atypical adenomas with one hyperplasia (n=1).
The inter-operator agreement for the ultrasonic feature (US texture) was 0.98.
3.2 Factors associated with MGD patients
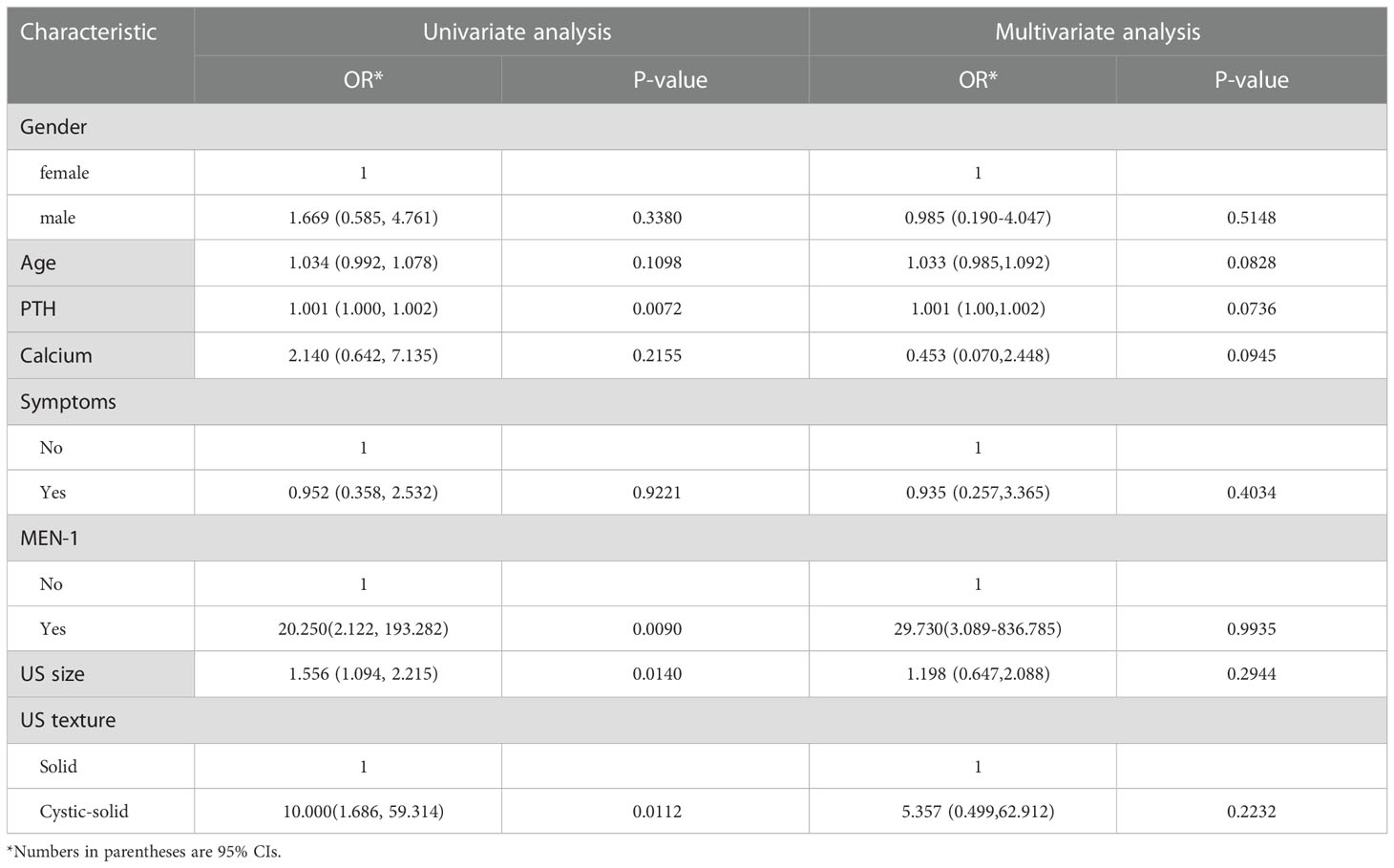
The univariate and multivariate logistic regression analyses were performed to identify the risk factors for MGD (Table 2). Univariate logistic regression analysis revealed that PTH level (odds ratio [OR] = 1.001, 95% CI: 1.000–1.002, P = 0.007), MEN-1 (OR = 20.250, 95% CI: 2.122–193.282, P =0.009), lesion US size (OR = 1.556, 95% CI: 1.094–2.215, P = 0.014) and US texture (OR = 10.000, 95% CI: 1.686–59.314, P = 0.011) were risk factors for MGD patients. Gender, age, preoperative calcium level, and symptoms were not significantly different between the two groups (p > 0.05).
All risk factors clinically relevant to MGD have been adopted in our model. The combined model (including clinical and US variables) showed that age (OR = 1.033, 95%CI = 0.985–1.092), MEN-1 (OR = 29.730, 95% CI = 3.089-836.785), US size (OR = 1.198, 95% CI = 0.647–2.088) and cystic-solid lesion (OR = 5.357, 95% CI = 0.499–62.912) were risk factors for MGD to some degree, and was used to develop a nomogram (Table 2).
3.3 Nomogram construction for MGD
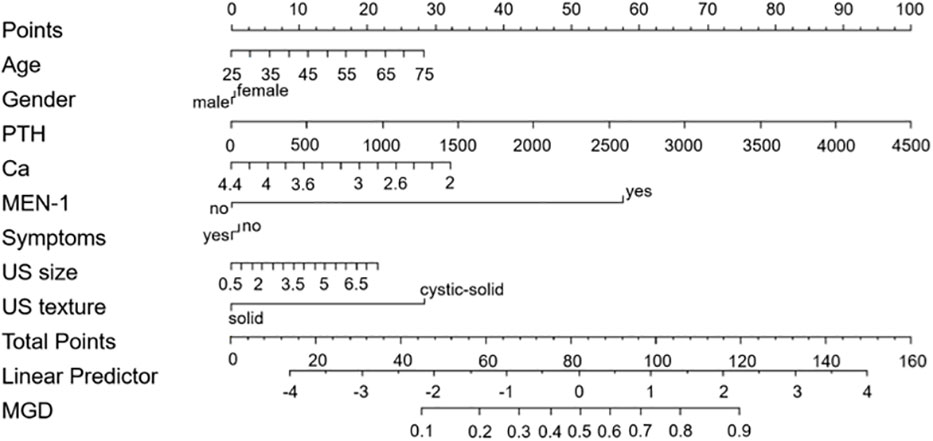
Based on the results of multivariate analysis, a nomogram was built for visual prediction of MGD (Figure 2). The cut-off value was 65 points through Youden’s index for MGD. In the prediction of MGD, the AUC was 0.773 (0.68-0.85) (Figure 3A). The calibration plots showed a good consistency between observed and predicted probability (Figure 3B). The Hosmer–Lemeshow test showed the goodness of fit and yielded chi-squares of 6.68 (P = 0.67) for the derivation cohorts. Further, the Decision curve analysis (Figure 3C) showed a positive net benefit for patients with PHPT.
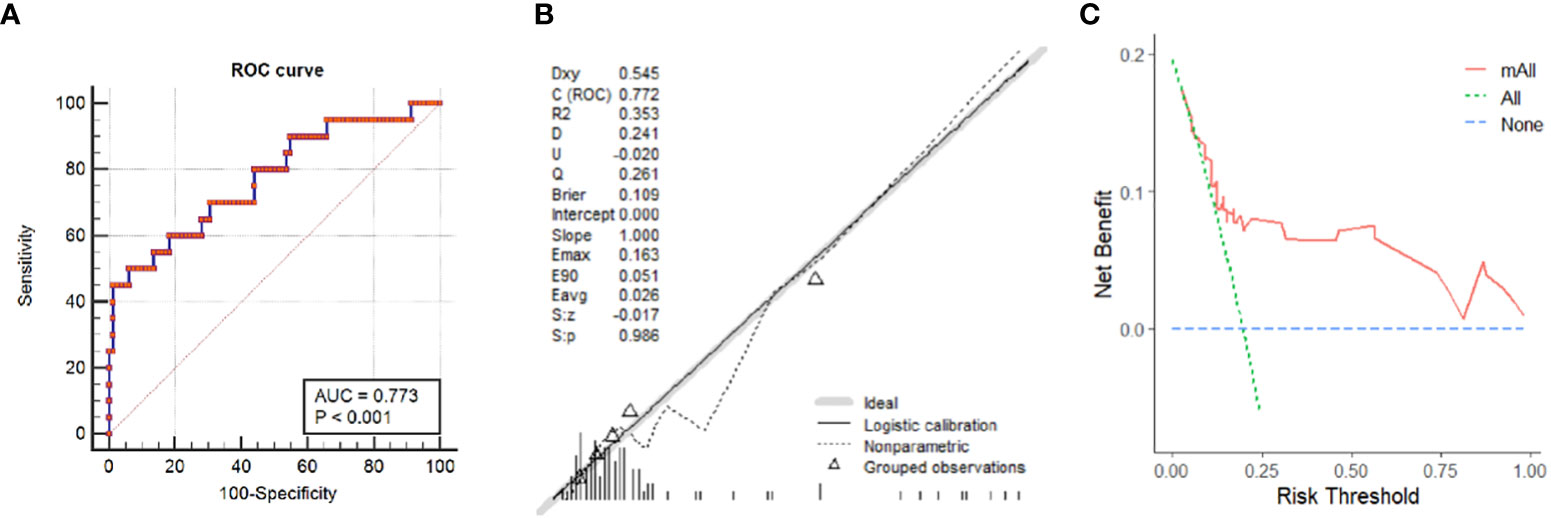
Figure 3 Prediction performance of the nomogram. (A) Receiver operating characteristic (ROC) curve of the nomogram. (B) Calibration Curve of the nomogram obtained by comparing the observed and predicted probability in PHPT patients. (C) Decision curve analysis for having MGD in PHPT patients.
The 65 points was defined as the threshold with a specificity of 93.9% and a sensitivity of 50%. Representative examples of clinical use of the nomogram for MGD and SGD are given in Figures 4, 5, respectively.
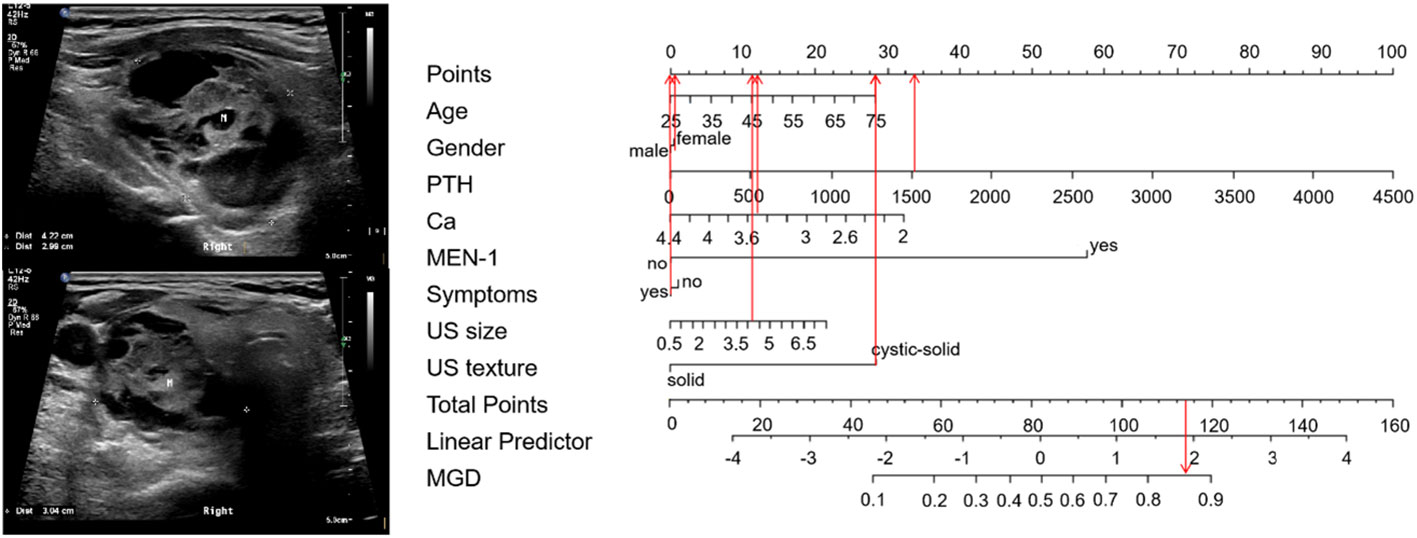
Figure 4 Examples of clinical use the nomogram for MGD. A75-year-old woman with PHPT complained bone pain in both lower extremities. The PTH and serum calcium level were 1526 pg/ml and 3.45 mmol/L respectively. MEN-1 was not diagnosed. The neck ultrasound showed cystic-solid nodule with 4.2cm in size in the posterior aspect of her right thyroid. The total score is 117, which corresponds to an MGD risk of greater than 0.85. Pathology confirmed that there were two glandular involved (an adenoma with cystic degeneration on the right side, and a hyperplasia on the left side), with an adenoma and a hyperplasia. M, mass.
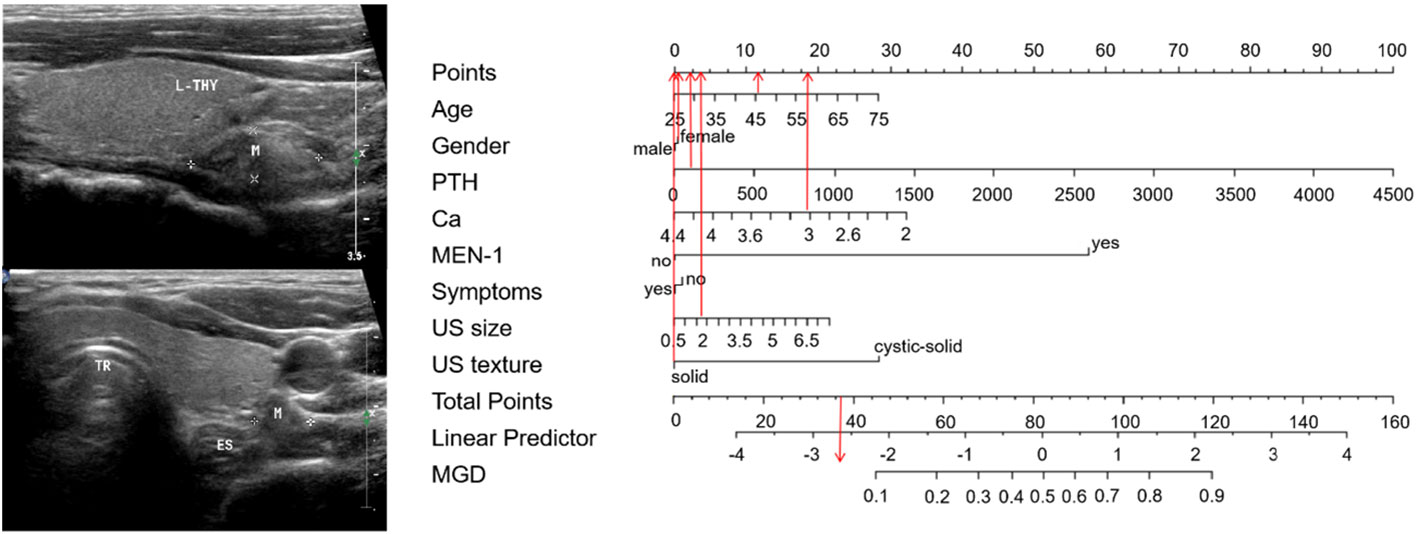
Figure 5 Examples of clinical use the nomogram for SGD. A 46-year-old man with PHPT complained recurrent urinary stones. The PTH and serum calcium level were 201 pg/ml and 3.08mmol/L respectively. MEN-1 was not diagnosed. The neck ultrasound showed 1.7cm-sized solid nodule in his left lower parathyroid area. The total score is 37, which corresponds to an MGD risk of less than 0.10. Pathology confirmed that the PHPT patients with SGD (a hyperplasia). Abbreviations:. L-THY=left thyroid, M=mass, TR=trachea, ES=esophagus.
4 Discussion
Multiglandular disease represents a recognized diagnostic challenge in primary hyperparathyroidism. The preoperative localization of MGD in PHPT patients is a key issue in the minimally invasive surgical decisions. Our study developed a nomogram based on the clinical and US variables, which demonstrated good efficacy in discrimination of MGD with an AUC of 0.773 (0.68-0.85). When taking 65 points as a diagnostic standard, it achieved a good distinguish capability with a specificity of 0.939 and sensitivity of 0.500. Good consistency was observed in the calibration curve, and the DCA curve also showed an efficient clinical applicability. Thus, the nomogram can effectively predict MGD and aid in treatment strategies.
Previous studies established prediction models for predicting SGD and MGD. CaPTHUS is a classic scoring system proposed by Kebebew et al. in 2006 (12). By setting 5 standards to calculate a risk score for SGD. (1) preoperative total serum calcium level (≥3 mmol/L [≥12 mg/dL]); (2) intact PTH level (≥2 times the upper limit of normal PTH levels); (3) sestamibi scan results positive for 1 enlarged parathyroid gland; (4) neck ultrasound results positive for 1 enlarged parathyroid gland; and (5) concordant sestamibi and neck ultrasound study results (identifying 1 enlarged gland on the same side of the neck). The results showed that with a score of ≥3 the PPV increased to 91% for predicting SGD. However, the preoperative diagnostic efficacy of MGD has not been addressed. Mazeh et al. created the Wiscons Index (WIN) (13), to predict the likelihood of the presence of additional hyperfunctioning glands during surgery. According to the results, the WIN score was related with the weight of parathyroid lesion, based on these, it created an intraoperative nomogram for stratifying patients into categories of high, medium, or low risk of MGD to assist surgical decision. Another two studies have also concluded that the pathologic weight of resected parathyroid lesions could inform the likelihood of the presence of additional hyperfunctioning parathyroid glands (17, 18).Whereas, the weight of the lesion only can be obtained intraoperatively. Recently, other imaging methods have been used to identify hyperfunctioning glands, Sepahdari et al (19). built a scoring model based on the features of four-dimensional computed tomography (4D-CT) images, including largest lesion size and the number of suspicious lesions, combining with biochemical information, such as preoperative calcium and PTH, and WIN. The 4D-CT MGD score achieved a good specificity of 81-96% and variable sensitivity of 39-64% for predicting MGD. Sho et al (20) prospectively validated the efficacy of the 4D-CT scoring system and showed that the system had lower specificity (74%-88%) and similar sensitivity (32-75%) compared to retrospective data. Based on the 4D-CT MGD score, Bunch et al (21). considered the size of the second-largest high-confidence candidate lesion, either through estimated volume or maximum diameter, for identifying MGD by setting varying threshold. It still has a relatively limited sensitivity in MGD (53-67%), More important, it’s only available for the patients who show a second lesion on 4D-CT. In our combined model, not only the accompanied MEN-1 and cystic-solid lesion, but also the lesion size and patient age were associated with MGD. This indicates that associated MEN represent MGD status, consistent with prior studies (22–24).With regard to the relationship between the patient age and MGD, multiple studies have reported that PHPT patient with older ages is more likely to MGD (25). It was also associated with MGD in our study. In addition, we found that US size was also associated with the occurrence of MGD. The results suggests that MGD is tend to have a larger lesion, and previous studies (16–18) have found a correlation between size of the lesions and MGD, but the conclusion is controversial and needs to be confirmed by further studies. Interestingly, in the present study we found that US texture, was also an important factor. Cystic-solid lesions are commonly found in MGD. This observation has not been reported before and needs to be further demonstrated in more studies. The underlying pathological basis is worth exploring. Our nomogram incorporating refined US and clinical variables more accurately predicted multiglandular parathyroid disease in primary hyperparathyroidism patients than consideration of only the clinical variables. And it is relatively simple and easy to apply preoperatively.
There are some limitations in our study. Firstly, this is a retrospective and single-center study. The sample size is limited, and the efficacy of our nomogram needs to be validated in a larger sample size data or multicenter data. In addition, only gray-scale ultrasound image features were included in this study, and semi-or quantitative ultrasound parameters, such as color Doppler, elastography, and contrast-enhanced ultrasound, could be considered for future prospective trials.
5 Conclusion
In summary, our study indicated that US findings, including US size and texture of the dominant lesion, can serve as risk factors for MGD. And constructed a novel nomogram combing clinical and US characteristics to predict MGD in PHPT patients preoperatively. It could be helpful to assist treatment regimens for PHPT patients with multigland disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Perking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HL, QZ, and YJ put up the research concept and design. SF, YH and YL took part in the collection of the data. SJ, OW, and YL completed data analysis. QL, JL performed data interpretation. YL wrote the article. HL and QZ made revision of the article and provided the final approval of it. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National High level Hospital Clinical Research Funding (2022-PUMCH-B-065).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Insogna KL. Primary hyperparathyroidism. N Engl J Med (2018) 379:1050–9. doi: 10.1056/NEJMcp1714213
2. Zhu CY, Sturgeon C, Yeh MW. Diagnosis and management of primary hyperparathyroidism. JAMA (2020) 323:1186–7. doi: 10.1001/jama.2020.0538
3. Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, et al. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg (2016) 151:959–68. doi: 10.1001/jamasurg.2016.2310
4. Barczynski M, Konturek A, Cichon S, Hubalewska-Dydejczyk A, Golkowski F, Huszno B. Intraoperative parathyroid hormone assay improves outcomes of minimally invasive parathyroidectomy mainly in patients with a presumed solitary parathyroid adenoma and missing concordance of preoperative imaging. Clin Endocrinol (Oxf) (2007) 66:878–85. doi: 10.1111/j.1365-2265.2007.02827.x
5. Ishii H, Mihai R, Watkinson JC, Kim DS. Systematic review of cure and recurrence rates following minimally invasive parathyroidectomy. BJS Open (2018) 2:364–70. doi: 10.1002/bjs5.77
6. Jinih M, O'Connell E, O'Leary DP, Liew A, Redmond HP. Focused versus bilateral parathyroid exploration for primary hyperparathyroidism: A systematic review and meta-analysis. Ann Surg Oncol (2017) 24:1924–34. doi: 10.1245/s10434-016-5694-1
7. Palazzo FF, Sadler GP. Minimally invasive parathyroidectomy. Bmj (2004) 328:849–50. doi: 10.1136/bmj.328.7444.849
8. Chen H, Pruhs Z, Starling JR, Mack E. Intraoperative parathyroid hormone testing improves cure rates in patients undergoing minimally invasive parathyroidectomy. Surgery (2005) 138:583–7. doi: 10.1016/j.surg.2005.06.046
9. Egan RJ, Scott-Coombes DM. The surgical management of sporadic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab (2018) 32:847–59. doi: 10.1016/j.beem.2018.12.001
10. Kuzminski SJ, Sosa JA, Hoang JK. Update in parathyroid imaging. Magn Reson Imaging Clin N Am (2018) 26:151–66. doi: 10.1016/j.mric.2017.08.009
11. Bergenfelz AO, Wallin G, Jansson S, Eriksson H, Mårtensson H, Christiansen P, et al. Results of surgery for sporadic primary hyperparathyroidism in patients with preoperatively negative sestamibi scintigraphy and ultrasound. Langenbecks Arch Surg (2011) 396:83–90. doi: 10.1007/s00423-010-0724-0
12. Kebebew E, Hwang J, Reiff E, Duh QY, Clark OH. Predictors of single-gland vs multigland parathyroid disease in primary hyperparathyroidism: A simple and accurate scoring model. Arch Surg (2006) 141:777–82. doi: 10.1001/archsurg.141.8.777
13. Mazeh H, Chen H, Leverson G, Sippel RS. Creation of a "Wisconsin index" nomogram to predict the likelihood of additional hyperfunctioning parathyroid glands during parathyroidectomy. Ann Surg (2013) 257:138–41. doi: 10.1097/SLA.0b013e31825ffbe1
14. Edafe O, Collins EE, Ubhi CS, Balasubramanian SP. Current predictive models do not accurately differentiate between single and multi gland disease in primary hyperparathyroidism: A retrospective cohort study of two endocrine surgery units. Ann R Coll Surg Engl (2018) 100:140–5. doi: 10.1308/rcsann.2017.0112
15. Serradilla-Martín M, Palomares-Cano A, Cantalejo-Díaz M, Mogollón-González M, Brea-Gómez E, Muñoz-Pérez NV, et al. Usefulness of the Wisconsin and CaPTHUS indices for predicting multiglandular disease in patients with primary hyperparathyroidism in a southern European population. Gland Surg (2021) 10:861–9. doi: 10.21037/gs-20-857
17. McCoy KL, Chen NH, Armstrong MJ, Howell GM, Stang MT, Yip L, et al. The small abnormal parathyroid gland is increasingly common and heralds operative complexity. World J Surg (2014) 38:1274–81. doi: 10.1007/s00268-014-2450-1
18. Norlén O, Glover A, Zaidi N, Aniss A, Sywak M, Sidhu S, et al. The weight of the resected gland predicts rate of success after image-guided focused parathyroidectomy. World J Surg (2015) 39:1922–7. doi: 10.1007/s00268-015-3017-5
19. Sepahdari AR, Bahl M, Harari A, Kim HJ, Yeh MW, Hoang JK. Predictors of multigland disease in primary hyperparathyroidism: A scoring system with 4D-CT imaging and biochemical markers. AJNR Am J Neuroradiol (2015) 36:987–92. doi: 10.3174/ajnr.A4213
20. Sho S, Yilma M, Yeh MW, Livhits M, Wu JX, Hoang JK, et al. Prospective validation of two 4D-CT-Based scoring systems for prediction of multigland disease in primary hyperparathyroidism. AJNR Am J Neuroradiol (2016) 37:2323–7. doi: 10.3174/ajnr.A4948
21. Bunch PM, Goyal A, Valenzuela CD, Randle RW. Parathyroid 4D CT in primary hyperparathyroidism: Exploration of size measurements for identifying multigland disease and guiding biochemically successful parathyroidectomy. AJR Am J Roentgenol (2022) 218:888–97. doi: 10.2214/AJR.21.26935
22. Hellman P, Skogseid B, Oberg K, Juhlin C, Akerström G, Rastad J. Primary and reoperative parathyroid operations in hyperparathyroidism of multiple endocrine neoplasia type 1. Surgery (1998) 124:993–9. doi: 10.1016/S0039-6060(98)70040-6
23. Lambert LA, Shapiro SE, Lee JE, Perrier ND, Truong M, Wallace MJ, et al. Surgical treatment of hyperparathyroidism in patients with multiple endocrine neoplasia type 1. Arch Surg (2005) 140:374–82. doi: 10.1001/archsurg.140.4.374
24. Arnalsteen LC, Alesina PF, Quiereux JL, Farrel SG, Patton FN, Carnaille BM, et al. Long-term results of less than total parathyroidectomy for hyperparathyroidism in multiple endocrine neoplasia type 1. Surgery (2002) 132:1119–24. doi: 10.1067/msy.2002.128607
Keywords: multiglandular parathyroid disease, nomogram, ultrasound, primary hyperparathyroidism, prediction
Citation: Luo Y, Jin S, He Y, Fang S, Wang O, Liao Q, Li J, Jiang Y, Zhu Q and Liu H (2023) Prediction of multiglandular parathyroid disease in primary hyperparathyroidism using ultrasound and clinical features. Front. Endocrinol. 14:1088045. doi: 10.3389/fendo.2023.1088045
Received: 02 November 2022; Accepted: 26 January 2023;
Published: 08 February 2023.
Edited by:
Takahisa Hiramitsu, Japanese Red Cross Nagoya Daini Hospital, JapanCopyright © 2023 Luo, Jin, He, Fang, Wang, Liao, Li, Jiang, Zhu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He Liu, bGl1aGViakAxMjYuY29t; Qingli Zhu, enFscHVtY2hAMTI2LmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Qingli Zhu, orcid.org/0000-0002-0618-2381
He Liu, orcid.org/0000-0002-7038-3946
 Yanwen Luo
Yanwen Luo Siqi Jin1
Siqi Jin1 Quan Liao
Quan Liao Qingli Zhu
Qingli Zhu He Liu
He Liu