
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 03 May 2023
Sec. Endocrinology of Aging
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1086924
This article is part of the Research Topic Ovarian Aging: Pathophysiology and Recent Development of Maintaining Ovarian Reserve, volume III View all 10 articles
Objectives: This study aimed to investigate whether the FSH (follicle-stimulating hormone)/LH (Luteinizing hormone) ratio correlates with ovarian response in a cross-sectional retrospective study of a population with normal levels of anti-Müllerian hormone (AMH).
Methods: This was a retrospective cross‐sectional study with data obtained from medical records from March 2019 to December 2019 at the reproductive center in the Affiliated Hospital of Southwest Medical University. The Spearmans correlation test evaluated correlations between Ovarian sensitivity index (OSI) and other parameters. The relationship between basal FSH/LH and ovarian response was analyzed using smoothed curve fitting to find the threshold or saturation point for the population with mean AMH level (1.1<AMH<6μg/L). The enrolled cases were divided into two groups according to AMH threshold. Cycle characteristics, cycle information and cycle outcomes were compared. The Mann-Whitney U test was used to compare different parameters between two groups separated by basal FSH/LH in the AMH normal group. Univariate logistic regression analysis and multivariate logistic regression analysis were performed to find the risk factor for OSI.
Results: A total of 428 patients were included in the study. A significant negative correlation was observed between OSI and age, FSH, basal FSH/LH, Gn total dose, and Gn total days, while a positive correlation was found with AMH, AFC, retrieved oocytes, and MII egg. In patients with AMH <1.1 ug/L, OSI values decreased as basal FSH/LH levels increased, while in patients with 1.1<AMH<6 ug/L, OSI values remained stable with increasing basal FSH/LH levels. Logistic regression analysis identified age, AMH, AFC, and basal FSH/LH as significant independent risk factors for OSI.
Conclusions: We conclude that increased basal FSH/LH in the AMH normal group reduces the ovarian response to exogenous Gn. Meanwhile, basal FSH/LH of 3.5 was found to be a useful diagnostic threshold for assessing ovarian response in people with normal AMH levels. OSI can be used as an indicator of ovarian response in ART treatment.
In recent years, infertility, which affects human development and health, has become a global medical and sociological problem (1). Assisted reproductive technologies (ART) have been developed for more than 40 years. In recent decades, assisted reproduction techniques have evolved. However, even when good quality embryos are selected for transfer to the uterus, the implantation rate remains low. Sunderam et al. showed that despite a gradual increase in clinical pregnancy rates among infertile women treated with ART over the past decades, the live birth rate per in vitro fertilization-embryo transfer (IVF-ET) was only 38.1% (2). Fertility practitioners should be fully aware of the failure of IVF cycles to improve the success rate of ART. Controlled ovarian hyperstimulation (COH) is critical to the success of IVF-ET (3). However, COH can lead to two adverse outcomes (high ovarian response or low ovarian response) due to the different ovarian responses to COH (4). Accurate prediction of ovarian response is critical to improve in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) (5). Currently, there are no relevant informative markers that directly predict ovarian response. The ovarian response is predicted based on the assessment of ovarian reserve indicators (6).
Anti-Müllerian hormone (AMH) levels are positively correlated with follicle number and decrease with increasing age and decreasing follicle number. AMH levels are constant throughout the menstrual cycle and its serum levels are not affected by FSH, LH, and E2 levels. These unique characteristics make AMH a good predictor of ovarian reserve (7). In addition, many studies have shown that age, AMH levels and antral follicle count (AFC) may be predictors of ovarian response (8). However, in clinical practice, the above parameters may not always be evaluated satisfactorily and accurately, and there is a need for more reliable factors to evaluate ovarian reserve. Several potential indicators of ovarian function are influenced by both cyclic variability and aging, and both factors must be taken into account in assessing ovarian function, which makes interpretation a challenge. Du et al. have demonstrated no factors can unconditionally assess ovarian reserve (9). Although AMH and AFC are widely considered as ovarian markers, they do not correctly detect hyporesponsive patients with normal ovarian reserve markers (10–12). A study related to the basal FSH/LH ratio predicting in vitro fertilization outcome showed that the basal FSH/LH was associated with poor outcome of in vitro fertilization treatment and may be a predictor of decreased ovarian reserve (13).
It has been observed that both the absolute number of oocytes retrieved and total gonadotrophin dose are essential measures of ovarian responsiveness, and the ratio of the two is a better representation of ovarian responsiveness than either parameter alone.
Ovarian sensitivity index (OSI), was first proposed by Biasoni et al. (14). OSI has been found correlated to AMH and AFC, which have been suggested as predictors of ovarian responsiveness (15, 16). Using OSI as a measure of ovarian responsiveness would be better than the number of retrieved oocytes for different gonadotrophin dosages applied to different subjects daily. Pan et al. showed that when OSI values were low, ovarian sensitivity was lower and pregnancy rates were lower; when OSI values were high, the incidence of OHSS was higher and pregnancy rates were lower (17). Huber et al. showed that an OSI below 1.7 was considered a low ovarian response (18). We defined OSI as the number of retrieved oocytes/the total dose of administered gonadotrophins. The use of gonadotropins for ovulation induction is related to a variety of factors, including the patient’s age, body mass index (BMI), ovarian function, hormone levels, personal and family history, and the patient’s personal preferences and treatment goals. The use of gonadotropins may also be influenced by the patient’s lifestyle and environmental factors. Therefore, when using gonadotropins for ovulation induction, these factors should be considered to ensure the treatment’s effectiveness and safety. Therefore, searching for new accurate, safe and effective markers is very important.
In the present study, we focused our research mainly on normal AMH population. The study aims were: (1) to detect the association between ovarian sensitivity index (OSI) and varieties of ovarian reserve, (2) to examine whether serum basal FSH/LH is corelated to OSI, (3) to assess whether OSI affects ovarian response, and (4) to find the threshold/saturation point in the study population.
In the cross-sectional retrospective study, infertile women underwent IVF/ICSI treatment at the reproductive center in the Affiliated Hospital of Southwest Medical University between March 2019 and December 2019.
(1) Aged<40 years;
(2) FSH < 25 U/L;
(3) Patients received IVF/ICSI treatment;
(4) Complete case information.
(1) Patients with polycystic ovarian syndrome (PCOS), endometriosis, premature ovarian insufficiency (POI);
(2) Patients with a high incidence of ovarian stimulation hyperresponsiveness;
(3) Patients with a history of ovarian tumors and other malignancies;
(4) Patients with a history of endocrine abnormalities such as diabetes, hyperthyroidism, and hypothyroidism.
Collected data included age, duration of infertility, BMI, AFC, AMH, basal FSH, basal LH, basal estradiol (E2) and basal P (progesterone), total Gn dose, total Gn days, oocytes recovered, number of embryos transferred, number of MII eggs, HCG day E2 level, HCG day LH level and HCG day P level.
Ovarian sensitivity index (OSI) was calculated by the following formula: OSI= Retrieved oocytes×1000/total Gn doses
Venous blood was collected into plain serum tubes and all samples were centrifuged (2–8°C, 2,000 g, 10 min) within 1 h of blood collection to separate the serum. In order to separate serum from venous blood, all samples were centrifuged (2–8°C, 2,000 g, 10 minutes) within 1 h of blood collection. Each aliquot from each patient was evaluated in random order in the same run, and all hormones were analyzed simultaneously. Each hormone was measured with an Elecsys® assay in conjunction with a cobas e 601 module of a cobas® 6000 analyzer (Roche Diagnostics, Mannheim, Germany) according to the producer’s instructions.
All patients received the same ovulation promotion protocols, using the same hormones and the same dose adjustment criteria. Ovulation was induced using antagonists or long-term protocols. Recombinant follicle-stimulating hormone (rFSH, Gonal-F, Merck-Serono, Brazil) was given daily on day 2 of the menstrual cycle as the start of the antagonist protocol. The dose of rFSH was adjusted according to the ovarian response measured by estradiol serum concentrations, and follicular growth was monitored by vaginal ultrasound. When follicles reached 14 mm, patients started receiving gonadotropin-releasing hormone (GnRH) antagonists (Cetrotide, MerckSerono, Brazil) associated with rFSH. For the long-term regimen, treatment began with subcutaneous administration of 3.75 mg of GnRH agonist (Gonapeptyl, Ferring, Brazil) on day 21 of their menstrual cycle to suppress pituitary function. To confirm the downregulation of estradiol, serum estradiol concentrations and vaginal ultrasonography were performed approximately 10 days later. If the estradiol concentration was <30 pg/ml and ultrasound showed an endometrial thickness of <3 mm, patients were considered ready to start ovulation induction. After confirmation of suppression, patients received daily doses of rFSH for ovulation induction. In both regimens, oocyte maturation was induced with recombinant human chorionic gonadotrophin (hCG, Ovidrel, Merck-Serono, Brazil) when at least two follicles reached a mean size of 17 mm with concordant estradiol levels (approximately 200 pg/ml).
All embryo transfers were performed under ultrasound control. Therefore, patients were asked to fill their bladders to provide an acoustic window for uterine visualization. The catheter tip (Wallace, Smits-Medical, Dublin, Ireland) was placed 1.0–2.0 cm below the apex of the uterine cavity. Avoiding uterine contractions, a pipette was inserted slowly from the cervical os into the uterine cavity until it reached the fundus uteri.
The pregnancy diagnosis was made by a positive hCG test on Day 14 after embryo transfer. The patient underwent transrectal ultrasonography to monitor the gestational sac and the clinical pregnancy diagnosis was confirmed on day 28 post-transfer. Luteal phase support was continued until 12 weeks of gestation. The ratio of basal FSH/LH was computed to detect the turning point of OSI.
SPSS-22.0 software (SPSS Inc. Chicago, IL, USA) was used for statistical analysis. Continuous variables were expressed as median scores and compared using the Mann-Whitney U test. Categorical variables were applied as percentages and compared using Fisher’s exact test. Median [P25%, P75%] and Mann-Whitney U tests were used to represent and compare continuous variables. The t-test (2-tailed) was used for comparison between groups of measures, and the Kruskal-Wallis test was used when normality was not satisfied for comparison between groups. Enumeration data were expressed as percentages using the χ2 test. The Spearman correlation coefficient was applied to explore the correlation between variables. Differences were considered statistically significant at a P-value < 0.05. An additional logistic regression analysis was performed, and the outcome was a binary OSI variable obtained using the detected turning point as cutoff, which differs from the continuous OSI mentioned elsewhere.
Four hundred twenty-eight patients who met the selection criteria were included in this study. Figure 1 shows the study procedure flowchart. Patient information included in this study is shown in Table 1. The median age of the patients was 31 years and the median duration of infertility was 3 years. The median AMH and AFC are 4.06 ug/L and 8, respectively. The clinical pregnancy rate in this study was 28%. Additional patient information is shown in Table 1.
The results of the correlation analysis between OSI and other parameters in this study are shown in Table 2. There was a significant negative correlation as follows, for OSI with Age (rs=-0.115, p=0.017) (Figure 2A), FSH (rs=-0.267, P<0.001) (Figure 2D), basal FSH/LH (rs=-0.203, P<0.001) (Figure 2E), Gn total dose (rs=-0.551, P<0.001) (Figure 2F), and Gn total days (rs=-0.319, P=0.004) (Figure 2G). There is also a significant positive correlation as follows, for OSI with AMH (rs= 0.340, P<0.001) (Figure 2B), AFC(rs=0.223, P<0.001) (Figure 2C), Retrieved oocytes (rs=0.789, P<0.001) (Figure 2H) and MII egg (rs=0.099, P=0.040) (Figure 2I). More detailed results were shown in Table 2.
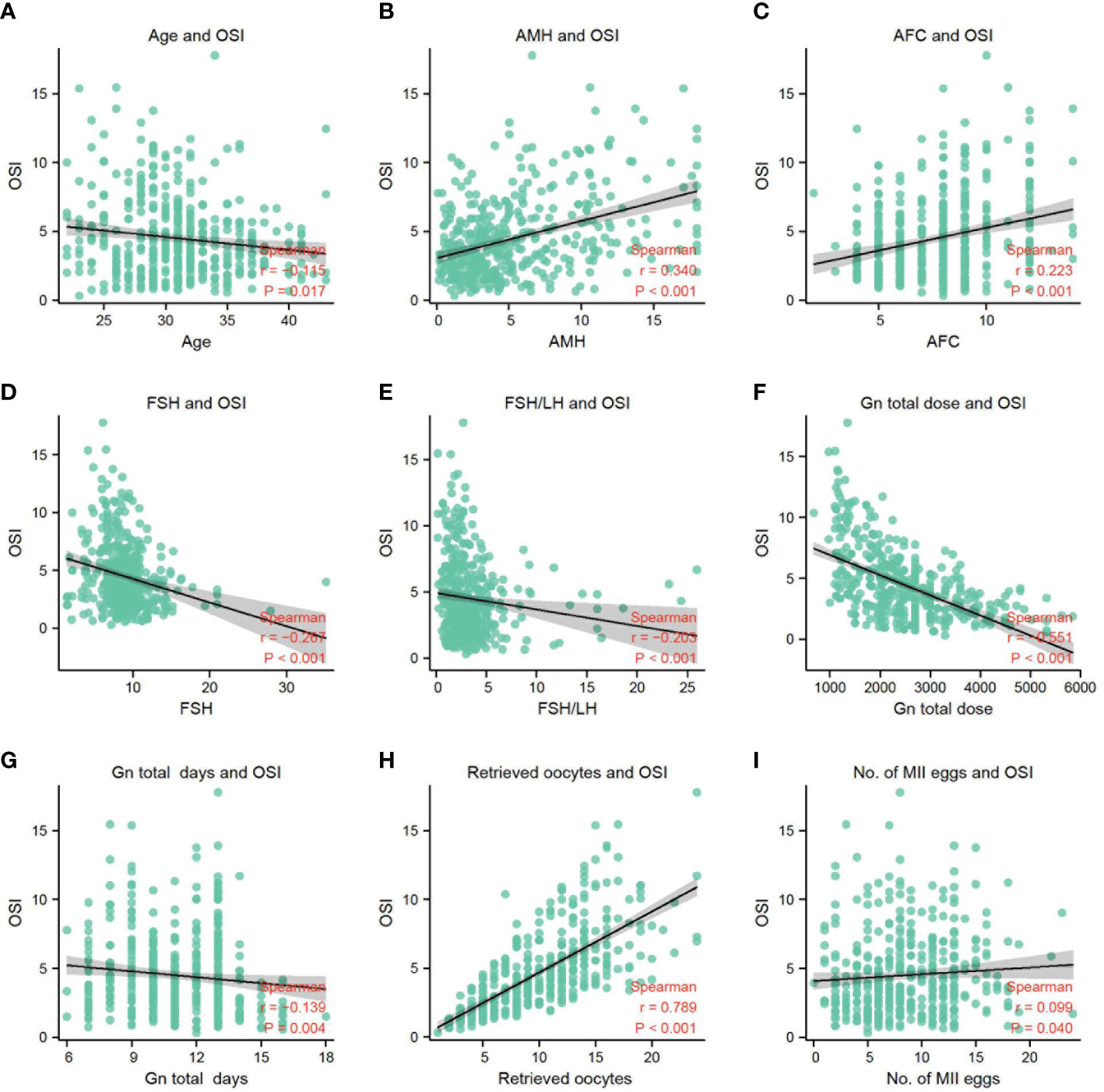
Figure 2 (A) The correlation between OSI and age. (B) The correlation between OSI and AMH. (C) The correlation between OSI and AFC. (D) The correlation between OSI and FSH. (E) The correlation between OSI and FSH/LH. (F) The correlation between OSI and Gn total dose. (G) The correlation between OSI and Gn total days. (H) The correlation between OSI and retrieved oocytes. (I) The correlation between OSI and No. of MII eggs.
A total of 50 patients with AMH < 1.1ug/L and 243 patients with 1.1<AMH<6ug/L were analyzed to examine the relationship between basal FSH/LH and OSI while excluding ovarian response-related factors such as age, BMI, AMH, E2, and AFC. In the AMH<1.1ug/L group, OSI values decreased as basal FSH/LH levels increased (Figure 3). In contrast, for the 1.1<AMH<6ug/L group, OSI values remained stable and the curve was smooth with increasing basal FSH/LH levels (Figure 4). Table 3 (revised) presents the threshold effect analysis for the association between FSH/LH and OSI in two groups with different AMH levels: AMH <1.1 ug/L and 1.1<AMH<6 ug/L. The table is divided into two sections, with one section for each group. Each section contains two models (Models I and II) and their respective adjusted beta coefficients (95% CI) and P-values. In Model I (linear analysis) for the group with AMH <1.1 ug/L, the one-line slope has an adjusted beta coefficient of -0.3 with a 95% CI of (-0.9, 0.3) and a P-value of 0.413. For the group with 1.1<AMH<6 ug/L, the one-line slope has an adjusted beta coefficient of -0.1 with a 95% CI of (-0.2, 0.1) and a P-value of 0.358. In Model II (non-linear analysis), a turning point is identified for each group. For the group with AMH <1.1 ug/L, the turning point is 2.3, with a slope1 of 2.1 (95% CI: -4.4, 8.7) and P-value of 0.513 for values below 2.3, and a slope2 of -0.1 (95% CI: -0.3, 0.3) and P-value of 0.316 for values above 2.3. For the group with 1.1<AMH<6 ug/L, the turning point is 3.5, with a slope1 of -0.2 (95% CI: -0.6, -0.1) and P-value of 0.049 for values below 3.5, and a slope2 of 0.1 (95% CI: -0.1, 0.3) and P-value of 0.052 for values above 3.5. The LRT test results indicate that there is a significant difference between Models I and II for both groups, with P-values of 0.03 for the AMH <1.1 ug/L group and 0.042 for the 1.1<AMH<6 ug/L group, suggesting a non-linear relationship between FSH/LH and OSI in both groups.
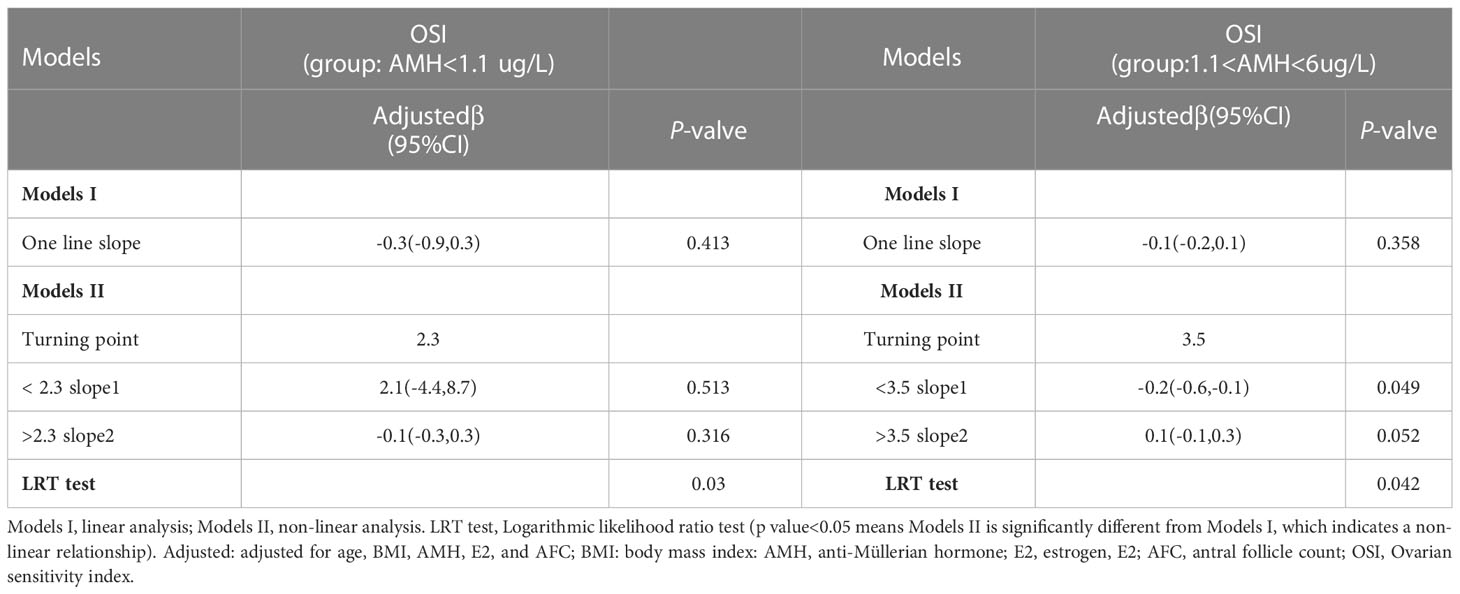
Table 3 Threshold effect analysis for the association between FSH/LH and OSI in AMH <1.1 ug/L and 1.1<AMH<6 ug/L group.
The results comparing age, infertility duration, BMI, AFC, AMH, and FSH (basal FSH/LH<3.5 and basal FSH/LH≥3.5) in the two groups are shown in Table 4. The following variables were statistically significant: Age, BMI, AFC, AMH, FSH, LH, E2, total Gn dose, and retrieved oocytes. Age, BMI, FSH, and total Gn dose were significantly higher in the basal FSH/LH≥3.5 group than in the basal FSH/LH<3.5 group (P<0.05). AFC, AMH, LH, and E2 were significantly lower in the basal FSH/LH≥3.5 group than in the basal FSH/LH<3.5 group (P<0.05). Although the pregnancy rate was also significantly higher than in the basal FSH/LH<3.5 group, the difference in pregnancy rate was not statistically significant(P=0.66). The retrieved oocytes, MII eggs, E2, and P on HCG day were lower than those in the basal FSH/LH<3.5 group (P<0.05).
In the univariate and multivariate logistic regression analyses, there were finally four parameters significantly correlated with OSI (Table 5), namely age (odds ratio (OR) 0.72, 95% CI 0.66–0.94, P = 0.026), AMH (odds ratio (OR): 1.32, 95% CI 1.26–1.74, P<0.001), AFC (odds ratio (OR): 1.55, 95% CI 1.46–1.88, P<0.001), and basal FSH/LH (odds ratio (OR): 0.84, 95% CI 0.72–0.94, P=0.042). The logistics regression model showed Age, AMH, AFC, FSH, and basal FSH/LH were independent risk factors of OSI (P<0.05 for all, shown in Table 5; Figures 5A, B).
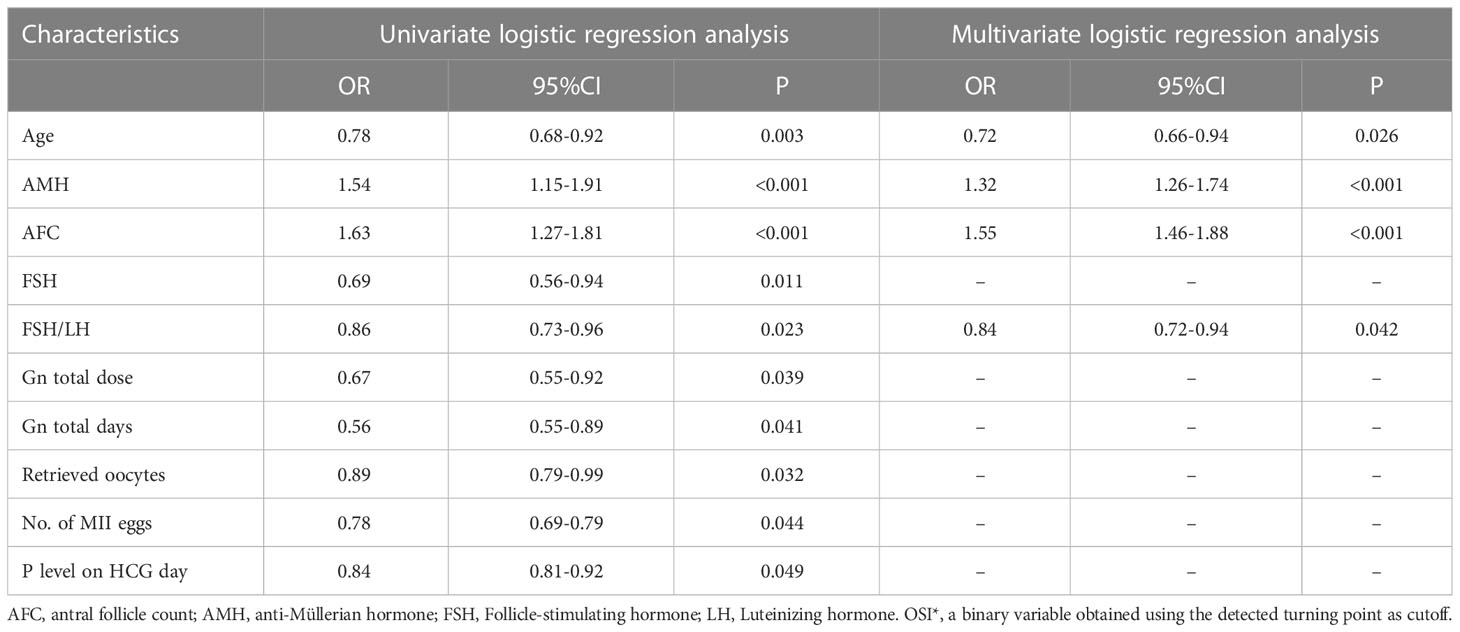
Table 5 Risk factors for OSI* identified by univariate logistic regression analysis and multivariate logistic regression analysis.
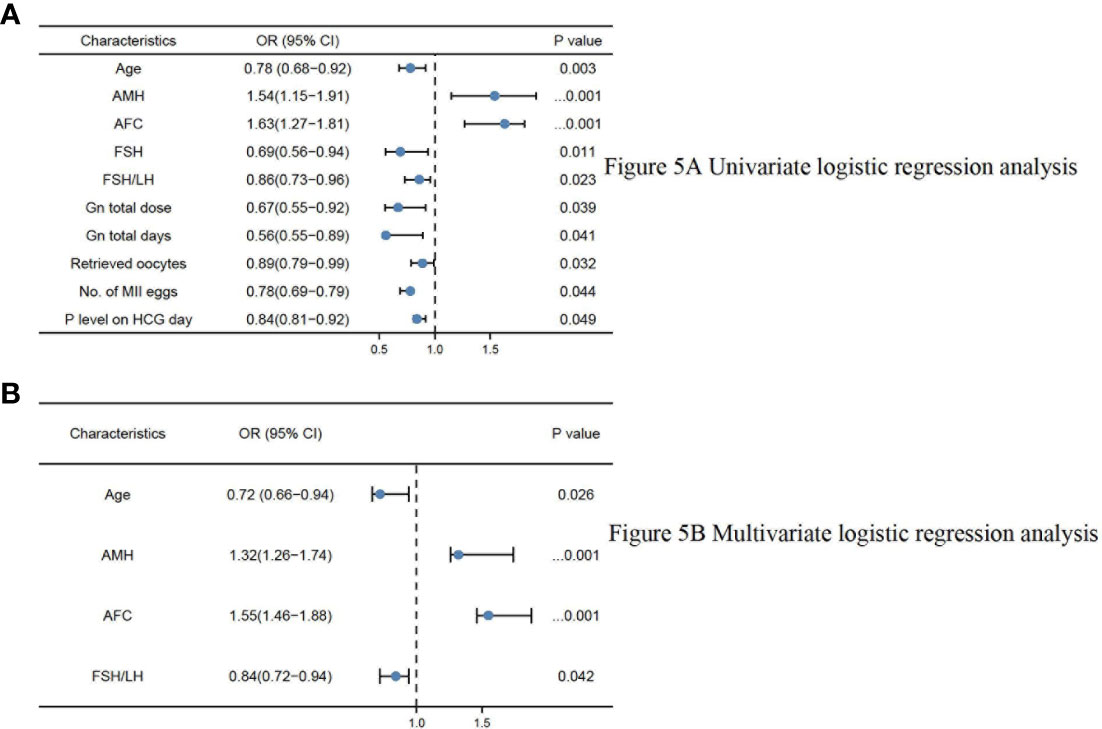
Figure 5 (A) Univariate logistic regression analysis. (B) Multivariate logistic regression analysis.
In vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in Assisted reproductive technology (ART) are effective methods for the treatment of infertile women (19). However, the response to exogenous gonadotropins (Gn) may vary between women undergoing controlled ovarian hyperstimulation (COH), which is associated with patient prognosis, including cycle cancellation rate, exogenous gonadotropin dose, and pregnancy outcome (20). Poor ovarian response (POR) is an important issue in clinic infertility treatment. Even when appropriate ovarian stimulation is given, the prognosis for poor ovarian response (POR) remains unfavorable pregnancy outcome (21). Fertility declines gradually with age in women, starting to decline significantly around the age of 32 years and accelerating significantly after the age of 37 years (22). Screening for individuals and groups at risk of declining fertility is critical. In clinical practice, it is crucial to identify patients at risk of low ovarian response, and individualized ovulatory treatment for different ovarian responses may improve clinical pregnancy rates in infertile patients.
Reduced ovarian reserve is the dominant factor for poor ovarian response, and clinical indicators reflecting ovarian reserve include age, AMH AFC basic FSH level, and other relevant indicators (23). However, the evaluation of ovarian response is unsatisfactory and even the results can be inaccurate (24). Some new indicators such as AFC/TOC and FSH/LH may be a recent approaches in treating ovarian stimulation on COH therapy (10, 25). In early clinical practice, basic FSH was often used as an index to assess ovarian reserve, but ovarian response has been found to be lower in patients with normal FSH (26). The AFC is susceptible to human factors, resulting in a lack of accuracy and objectivity. In recent years, the combined use of AMH and AFC has allowed the assessment ovarian reserve. Patients with the low response, normal or high to exogenous Gn, can be identified by AFC and AMH (27). Mutlu et al. has shown that AMH is less sensitive in predicting low ovarian response (28). Overall, there are no specific markers to evaluate ovarian reserve and response independently, and a combined application for evaluation is still needed. Basal FSH/LH reflects ovarian response to exogenous Gn and is also associated with the length of the menstrual cycle prior to IVF/ICSI-ET (29). Kofinas et al. showed that elevated basal FSH/LH ratio >3 was more likely to result in individual menstrual cycle cancellation (15 vs 5.24%; p = 0.0001) in a total of 676 patients in the USA involved (30). Seckin et al. demonstrated that older women with a high basal FSH/LH (n = 23) had a significantly lower number of good grade embryos transferred (p = 0.04) and a significantly lower pregnancy rate (p = 0.03) compared to older women with a low basal FSH/LH ratio. However, in younger women, treatment outcomes were similar in both subgroups (31). Thus, they concluded that basal FSH/LH ratio is useful in predicting IVF outcomes in older women but does not appear to be an accurate predictor in younger women.
Patients with normal serum AMH levels but low ovarian response still exist and are easily overlooked by clinicians in clinical practice. In this study, we found that AMH and AFC decreased with increasing basal FSH/LH with increasing age by analyzing the normal AMH group. Therefore, we believe that the basal FSH/LH levels can reflect the reserve function of ovaries to some extent. Also, patients with elevated basal FSH/LH levels had higher total Gn doses but significantly fewer MII eggs than those with low basal FSH/LH levels in this study. Thus, patients with elevated basal FSH/LH levels had reduced sensitivity to exogenous Gn and reduced ovarian response. Previous studies found that the number of mature oocytes was reduced in those with elevated basal FSH/LH (23) levels and suggested that elevated basal FSH/LH levels were associated with a decreased final pregnancy rate (13). However, Arat et al. confirmed basal FSH/LH levels were not associated with the final cycle outcome (23) and that age and number of embryos transferred were independent factors affecting the final live birth rate (30). In the present study, we found no significant reduction in the number of mature eggs, number of embryos transferred, and final pregnancy rate in the population with basal FSH/LH ≥3.5. Therefore, we concluded that the number of mature eggs and the number of embryos transferred were not related to the level of basal FSH/LH. There was no significant difference in the number of mature eggs and final cycle outcomes. However, due to the small sample size, further follow-up is needed to calculate the cumulative pregnancy rate to determine whether the pregnancy outcome is affected by basal FSH/LH. In the present study, we found a decreasing trend in LH levels from the basal FSH/LH<3.5 group to the basal FSH/LH>3.5 group. We can further speculate that the decrease in ovarian response to exogenous Gn may be related to the increase in FSH and the decrease in LH level. A study found that a decrease in the basal LH level on the third day of the menstrual cycle reduced the number of retrieved oocytes and decreased the risk of hyperstimulation syndrome (OHSS) (32). Also, a decrease in LH may lead to a decrease in the number of antral follicles (33), as studied at the genetic level in rats. Noel et al. also demonstrated a reduced requirement for exogenous Gn during COH (34) in individuals with elevated endogenous LH levels to a certain extent. A complex interaction of molecular pathways occurs between female and male gametes during clinical pregnancies and live births. Olszewska et al. have demonstrated the relationship between methylation (5mC) and hydroxymethylation (5hmC) in sperm DNA concerning sperm chromatin protamination in three subpopulations of fertile normozoospermic controls and infertile patients with oligo-/oligoasthenozoospermia (35). Furthermore, Giebler et al. showed that PIWI-LIKE 1 and 2 transcript levels in the spermatozoa of the swim-up fraction were positively correlated with each other by analyzing how PIWI-LIKE 1-4 mRNA expression in ejaculated spermatozoa predicts outcomes of assisted reproductive techniques (ART), evaluating swim-up spermatozoa used for fertilization from 160 in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycles (36). In conclusion, our study sheds light on the potential impact of basal FSH/LH levels on ovarian response and ART outcomes, but it is essential to recognize the multifactorial nature of infertility and the diverse molecular pathways that come into play during the process of fertilization and embryo development. By expanding our knowledge in this area and exploring additional factors such as sperm DNA methylation and PIWI-LIKE transcript levels, we may be able to develop a more comprehensive understanding of infertility and improve the prognosis and treatment options for infertile couples seeking assistance through ART.
This study has the advantage of focusing on a specific population in Southwest China, an area that may not be economically developed but has an increasing trend of infertility patients. Additionally, these measurements were made in the same laboratory using the same equipment. As a result, laboratory testing is much less likely to be variable. There are three limitations to our study. First, we generated our findings from a relatively small number of individuals, which should be validated in larger cohorts of Chinese Han patients. Second, this study is limited by its retrospective nature and its confinement to a single center. In the future, the sample size will be expanded, or multicenter studies will be performed for further validation. Third, in this study, only associations between ART pregnancy outcomes and basal FSH/LH and OSI were investigated without addressing other confounders’ impacts.
Firstly, in individuals with normal AMH levels, we observed that an increase in basal FSH/LH leads to a reduced ovarian response to exogenous Gn. Secondly, the OSI exhibited a strong correlation with female parameters associated with ovarian reserve. Thirdly, we identified threshold effects for basal FSH/LH and OSI in both normal and low anti-Müllerian hormone populations, with turning points at 3.5 and 2.3, respectively. Additionally, the ovarian sensitivity index (OSI) independently impacted the ovarian response. These findings could assist clinicians in evaluating ovarian response in patients with normal AMH levels undergoing assisted reproductive technology (ART) treatments for infertility. By employing factor analysis, we may be able to better understand the underlying relationships among variables like AMH, AFC, FSH/LH, and others, and potentially reveal novel patterns or factors that contribute to ovarian reserve and response. Consequently, further research is required to elucidate the relevant underlying mechanisms.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (ethics code number: KY2022300). Informed consent from study participants was not required.
XS and CY: Conceptualization. XS, LL, FY, and CS: Data curation, Writing-Original draft preparation. YH, MM, and YL: Revising the manuscript critically for important intellectual content. All authors agree with the contents of the manuscript.
We thank the survey participants and all members involved in this study for their painstaking efforts in conducting the data collection. All authors gave informed consent prior to the start of the study and agreed with the contents of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PloS Med (2012) 9(12):e1001356. doi: 10.1371/journal.pmed.1001356
2. Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance — united states, 2018. MMWR Surveill Summ (2022) 71(4):1–19. doi: 10.15585/mmwr.ss7104a1
3. Penzias AS. Improving results with assisted reproductive technologies: individualized patient-tailored strategies for ovulation induction. Reprod BioMed Online (2011) 22 Suppl 1:S83–6. doi: 10.1016/S1472-6483(11)60013-8
4. Chalumeau C, Moreau J, Gatimel N, Cohade C, Lesourd F, Parinaud J, et al. Establishment and validation of a score to predict ovarian response to stimulation in IVF. Reprod BioMed Online (2018) 36(1):26–31. doi: 10.1016/j.rbmo.2017.09.011
5. Kim SW, Kim YJ, Shin JH, Kim H, Ku SY, Suh CS, et al. Correlation between ovarian reserve and incidence of ectopic pregnancy after in vitro fertilization and embryo transfer. Yonsei Med J (2019) 60(3):285–90. doi: 10.3349/ymj.2019.60.3.285
6. Saleh BO, Ibraheem WF, Ameen NS. The role of anti-mullerian hormone and inhibin b in the assessment of metformin therapy in women with polycystic ovarian syndrome. Saudi Med J (2015) 36(5):562–7. doi: 10.15537/smj.2015.5.11112
7. Josso N. WOMEN IN REPRODUCTIVE SCIENCE: anti-müllerian hormone: a look back and ahead. Reproduction (2019) 158(6):F81–9. doi: 10.1530/REP-18-0602
8. Brodin T, Hadziosmanovic N, Berglund L, Olovsson M, Holte J. Comparing four ovarian reserve markers–associations with ovarian response and live births after assisted reproduction. Acta Obstet Gynecol Scand (2015) 94(10):1056–63. doi: 10.1111/aogs.12710
9. Du YY, Guo N, Wang YX, Hua X, Deng TR, Teng XM, et al. Urinary phthalate metabolites in relation to serum anti-müllerian hormone and inhibin b levels among women from a fertility center: a retrospective analysis. Reprod Health (2018) 15(1):33. doi: 10.1186/s12978-018-0469-8
10. Alvin C, Conforti A, Esteves SC, Vallone R, Venturella R, Staiano S, et al. Understanding ovarian hypo-response to exogenous gonadotropin in ovarian stimulation and its new proposed marker-the follicle-To-Oocyte (FOI) index. Front Endocrinol (Lausanne) (2018) 9:589. doi: 10.3389/fendo.2018.00589
11. Conforti A, Esteves SC, Di Rella F, Strina I, De Rosa P, Fiorenza A, et al. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol (2019) 17(1):18. doi: 10.1186/s12958-019-0460-4
12. Alvin C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, et al. Clinical relevance of genetic variants of gonadotropins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod Update (2018) 24(5):599–614. doi: 10.1093/humupd/dmy019
13. Prasad S, Gupta T, Divya A. Correlation of the day 3 FSH/LH ratio and LH concentration in predicting IVF outcome. J Reprod Infertil (2013) 14(1):23–8.
14. Biasoni V, Patriarca A, Dalmasso P, Bertagna A, Manieri C, Benedetto C, et al. Ovarian sensitivity index is strongly related to circulating AMH and may be used to predict ovarian response to exogenous gonadotropins in IVF. Reprod Biol Endocrinol (2011) 9:112. doi: 10.1186/1477-7827-9-112
15. Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril (2009) 91(3):705–14. doi: 10.1016/j.fertnstert.2007.12.013
16. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update (2011) 17(1):46–54. doi: 10.1093/humupd/dmq034
17. Pan W, Tu H, Jin L, Hu C, Xiong J, Pan W, et al. Comparison of recombinant and urinary follicle-stimulating hormones over 2000 gonadotropin-releasing hormone antagonist cycles: a retrospective study. Sci Rep (2019) 9(1):5329. doi: 10.1038/s41598-019-41846-2
18. Huber M, Hadziosmanovic N, Berglund L, Holte J. Using the ovarian sensitivity index to define poor, normal, and high response after controlled ovarian hyperstimulation in the long gonadotropin-releasing hormone agonist protocol: suggestions for a new principle to solve an old problem. Fertil Steril (2013) 100(5):1270–6. doi: 10.1016/j.fertnstert.2013.06.049
19. van Hoogenhuijze NE, Torrance HL, Mol F, Laven JSE, Scheenjes E, Laven MAF, et al. Endometrial scratching in women with implantation failure after a first IVF/ICSI cycle; does it lead to a higher live birth rate? the SCRaTCH study: a randomized controlled trial (NTR 5342). BMC Women's Health (2017) 17(1):47. doi: 10.1186/s12905-017-0378-y
20. Gingold JA, Lee JA, Whitehouse MC, Rodriguez-Purata J, Sandler B, Grunfeld L, et al. Maximum basal FSH predicts reproductive outcome better than cycle-specific basal FSH levels: waiting for a "better" month conveys limited retrieval benefits. Reprod Biol Endocrinol (2015) 13:91. doi: 10.1186/s12958-015-0078-0
21. Özkan ZS. Ovarian stimulation modalities in poor responders. Turk J Med Sci (2019) 49(4):959–62. doi: 10.3906/sag-1905-179
22. Sun YF, Zhang J, Xu YM, Luo ZY, Sun Y, Hao GM, et al. Effects of age on pregnancy outcomes in patients with simple tubal factor infertility receiving frozen-thawed embryo transfer. Sci Rep (2020) 10(1):18121. doi: 10.1038/s41598-020-75124-3
23. Arat Ö, Deveci D, Özkan ZS, Tuncer Can S. What is the effect of the early follicular phase FSH/LH ratio on the number of mature oocytes and embryo development? Turk J Med Sci (2020) 50(2):420–5. doi: 10.3906/sag-1910-234
24. Zhou SJ, Zhao MJ, Li C, Su X. The comparison of evaluative effectiveness between antral follicle count/age ratio and ovarian response prediction index for the ovarian reserve and response functions in infertile women. Med (Baltimore) (2020) 99(36):e21979. doi: 10.1097/MD.0000000000021979
25. Scheinhardt MO, Lerman T, König IR, Griesinger G. Performance of prognostic modelling of high and low ovarian response to ovarian stimulation for IVF. Hum Reprod (2018) 33(8):1499–505. doi: 10.1093/humrep/dey236
26. Kumba B, Oral E, Kahraman S, Karlikaya G, Karagozoglu H. Young patients with diminished ovarian reserve undergoing assisted reproductive treatments: a preliminary report. Reprod BioMed Online (2005) 11(3):294–9. doi: 10.1016/s1472-6483(10)60836-x
27. Polyzos NP, Tournaye H, Guzman L, Camus M, Nelson SM. Predictors of ovarian response in women treated with corifollitropin alfa for in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril (2013) 100(2):430–7. doi: 10.1016/j.fertnstert.2013.04.029
28. Mutlu MF, Erdem M, Erdem A, Yildiz S, Mutlu I, Arisoy O, et al. Antral follicle count determines poor ovarian response better than anti-müllerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet (2013) 30(5):657–65. doi: 10.1007/s10815-013-9975-3
29. Brodin T, Bergh T, Berglund L, Hadziosmanovic N, Holte J. Menstrual cycle length is an age-independent marker of female fertility: results from 6271 treatment cycles of in vitro fertilization. Fertil Steril (2008) 90(5):1656–61. doi: 10.1016/j.fertnstert.2007.09.036
30. Kofinas JD, Elias RT. Follicle-stimulating hormone/luteinizing hormone ratio as an independent predictor of response to controlled ovarian stimulation. Women's Health (Lond) (2014) 10(5):505–9. doi: 10.2217/whe.14.31
31. Seckin B, Turkcapar F, Ozaksit G. Elevated day 3 FSH/LH ratio: a marker to predict IVF outcome in young and older women. J Assist Reprod Genet (2012) 29(3):231–6. doi: 10.1007/s10815-011-9695-5
32. Vivian K, Isik AZ. Low day 3 luteinizing hormone values are predictive of reduced response to ovarian stimulation. Hum Reprod (1999) 14(3):863–4. doi: 10.1093/humrep/14.3.863
33. Mature A, Tenino I, Varik I, Kruse S, Tiido T, Kristjuhan A, et al. FSH/LH-dependent upregulation of ahr in murine granulosa cells is controlled by PKA signaling and involves epigenetic regulation. Int J Mol Sci (2019) 20(12):3068. doi: 10.3390/ijms20123068
34. Noel SD, Kaiser UB. G Protein-coupled receptors involved in GnRH regulation: molecular insights from human disease. Mol Cell Endocrinol (2011) 346(1-2):91–101. doi: 10.1016/j.mce.2011.06.022
35. Olszewska M, Kordyl O, Kamieniczna M, Fraczek M, Jędrzejczak P, Kurpisz M. Global 5mC and 5hmC DNA levels in human sperm subpopulations with differentially protaminated chromatin in normo- and oligoasthenozoospermic males. Int J Mol Sci (2022) 23(9):4516. doi: 10.3390/ijms23094516
Keywords: FSH/LH, ovarian sensitivity index (OSI), anti-Mullerian hormone, pregnancy, assisted reproductive technology
Citation: He Y, Liu L, Yao F, Sun C, Meng M, Lan Y, Yin C and Sun X (2023) Assisted reproductive technology and interactions between serum basal FSH/LH and ovarian sensitivity index. Front. Endocrinol. 14:1086924. doi: 10.3389/fendo.2023.1086924
Received: 01 November 2022; Accepted: 10 April 2023;
Published: 03 May 2023.
Edited by:
Osamu Hiraike, The University of Tokyo, JapanReviewed by:
Amelia Caruana, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyCopyright © 2023 He, Liu, Yao, Sun, Meng, Lan, Yin and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengliang Yin, Y2hlbmdsaWFuZ3lpbkAxNjMuY29t; Xingyu Sun, c3h5NjYzNkB5ZWFoLm5ldA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.