
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 06 March 2023
Sec. Neuroendocrine Science
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1085950
This article is part of the Research TopicGlucocorticoids and Cognition: Recent Advances in Understanding Their Interaction, With a Particular Focus on Clinical Applicability for the Treating EndocrinologistView all 5 articles
Stress is viewed as a state of real or perceived threat to homeostasis, the management of which involves the endocrine, nervous, and immune systems. These systems work independently and interactively as part of the stress response. The scientific stress literature, which spans both animal and human studies, contains heterogeneous findings about the effects of stress on the brain and the body. This review seeks to summarise and integrate literature on the relationships between these systems, examining particularly the roles of physiological and psychosocial stress, the stress hormone cortisol, as controlled by the hypothalamic-pituitary-adrenal (HPA) axis, and the effects of stress on cognitive functioning. Health conditions related to impaired HPA axis functioning and their associated neuropsychiatric symptoms will also be considered. Lastly, this review will provide suggestions of clinical applicability for endocrinologists who are uniquely placed to measure outcomes related to endocrine, nervous and immune system functioning and identify areas of intervention.
Intuitively, we know what stress is, and we know that stress is bad for us. However, the current scientific literature contains varying explanations of this construct, which has led to ongoing debates about the effects of stress on the mind and the body (1–5). A useful starting point, therefore, would be to go back to the early definitions of stress.
Hans Selye, a pioneering physician and endocrinologist known in many circles as the founder of stress theory, defined stress as “the state manifested by a specific syndrome which consists of all the nonspecifically-induced changes within a biologic system” (6, p. 64). Hence, he suggested that stress has its own constitution, but without one particular cause, that produces visible changes in response to stress. Selye also wrote about a stress condition known as the General Adaptation Syndrome (GAS). Selye’s view of this syndrome, which he regarded as a form of defence similar to immunity, was that it represented the attempts of an organism to adapt itself to new conditions; he also clarified the role of the adrenal glands in adaptive reactions (7) (the role of the adrenal glands in the stress response will be discussed more in the later section on the hypothalamic-pituitary-adrenal axis). Selye described the stereotypical response pattern that occurs in situations of stress. Stated simply, this pattern included an initial shock phase and an alarm reaction, followed by adaptation to the stressor, and then an exhaustion phase, where persistent aversive stimulation can overwhelm an organism’s ability to resist the stressor (6). Additionally, Selye identified two categories of stress; namely distress and eustress (8). The former refers to stress that leads to one feeling overwhelmed and has a negative impact, and the latter meaning good stress, or stress that has a positive effect. Eustress, which can be brought on by facing an enjoyable challenge, involves a moderate amount of stress with beneficial outcomes. It has the ability to energize and motivate us to overcome illness and obstacles and produce positive feelings of excitement, fulfilment and satisfaction. These categories inform the concept that not all stress is experienced in the same way, which adds to the complexity of understanding varying definitions of stress and its impact on physical and mental health.
Amid these complexities, however, there is general consensus in the neuroscientific literature, that stress can be considered as a stimulus, a reaction to a stimulus, or the physiological effects of that reaction (9, 10). Within this explanatory framework, the neurobiological stress response occurs when the organism is exposed to stressors (i.e., life experiences that threaten a primary goal). By this definition, stressors are categorised as being either physiological (i.e., presenting a threat to one’s physical integrity) or psychological (i.e., presenting a threat to one’s mental well-being) in nature (11, 12). Stressors can be acute or chronic in presentation. An acute stressor (e.g., having a near-miss motor vehicle accident) triggers an immediate stress response that usually subsides shortly after the stressor itself has ceased to exist or is no longer present in the individual’s life. On the other hand, a chronic stressor (e.g., ongoing financial problems or a lengthy illness) occurs over a prolonged period of time, where a simple or quick solution is not available (13). Both acute and chronic stressors may lead to a range of physiological and psychological impairments (14–16).
In recent decades, research has sought to elucidate the effects of physiological and psychological stress on physical and mental health. This research has confirmed that the experience of stress can be helpful in circumstances in which immediate reaction to a stimulus is necessary for survival and continued wellbeing (17–19). However, when stress is excessive and prolonged, it can affect physical and mental health negatively.
Regarding the physical effects, exposure to chronically stressful conditions can decrease resistance to the effects of the stressor and take a toll on the body in general (and even, under some circumstances, lead to irreversible physiological damage). Hence, people experiencing prolonged periods of stress are at increased risk of, for instance, digestive and gastrointestinal problems, hypertension, diabetes, cardiovascular disease, loss of bone minerals, immunosuppression, and asthma (20–22). In one example of a typical cross-sectional study in this literature, Almuneef et al. (23) used questionnaire-based methods to measure associations between adverse childhood events (ACEs; for example, physical neglect, sexual abuse, exposure to household dysfunction) and chronic disease in a Saudi Arabian sample of 931 adults aged 18 years and older. They found significant associations between self-reports of ACES and those of chronic diseases such as diabetes, hypertension, and respiratory illness. Participants who reported exposure to ≥ 4 ACES had a 2–11 times greater risk of developing a chronic disease during adulthood. Longitudinal studies have confirmed these associations. For instance, Harris et al. (24) explored data from the Australian Longitudinal Study on Women’s Health (N = 12,844) and found that moderate-to-high levels of perceived stress in middle-aged adults were associated with a two-fold increase in risk of diabetes diagnosis 3 years after initial reporting of perceived stress (see also, 25). It is recognised that the effects of stress can have significantly detrimental effects on several biological systems (the cardiovascular system being a major one). However, it is beyond the scope of this review to provide more details on the links between stress and all of the biological systems affected.
The experience of chronic stress is also associated with negative mental health outcomes. There is a well-established link between prolonged exposure to psychosocial stress and risk for major depressive disorder (MDD; 26, 27). Animal models indicate that different sources of stress (e.g., learned helplessness, social defeat and social isolation) can alter brain structure and function, thus causing behaviours typically associated with depression (for a review see 28–30). For instance, Peric et al. (31) found that chronic social isolation stress affected the down-regulation of proteins involved in mitochondrial transport and energy processes in the rat hippocampus, causing decreased sucrose preference (a classic marker of anhedonia). Treatment with the antidepressant fluoxetine modified this behaviour, with rats’ subsequent intake of sucrose approaching that of non-stressed controls. Human studies have reported similar patterns of data, particularly with respect to early childhood adversity (32, 33). For instance, Mall et al. (34) found that childhood experiences of bullying, emotional neglect and emotional abuse, as well as recent relationship and academic stressors, were significant predictors of past-year depression in a sample of first-year university students (N= 686).
There are also well established associations between stress and neurodegenerative disease. It appears that the experience of chronic stress increases the risk of developing dementia and that individuals who experience cognitive decline in later life are more likely to have been exposed to chronically stressful events (35–37). Rodent models of Alzheimer’s disease (AD) have been particularly useful in delineating putative mechanisms underlying the relationship between stress and dementia (38–40). For instance, Han et al. (41) reported that chronic stress applied over 4 weeks led to impaired cognitive function (as measured by Morris Water Maze performance) and increased beta-amyloid (Aβ) deposition in APP/PS1 mice, and that these impairments and increases were related to particular metabolic changes (e.g., among amino acids and ketone bodies). In older adult humans, there are significant associations between experiences of stress and impaired cognitive functioning. For instance, Koyanagi et al. (42) investigated associations between stress (as measured by questions about inability to cope with or control everyday events or activities) and clinically diagnosed mild cognitive impairment (MCI) in six low-and-middle-income countries (China, Ghana, India, Mexico, Russia, and South Africa; N= 32715; age= ≥50 years). Those with higher levels of perceived stress had an increased risk of MCI. Similarly, James et al. (43) demonstrated that older adults with mild-to-moderate AD reported significantly higher levels of current psychosocial stress than healthy age-matched controls, and that perceived stress levels were significantly correlated with performance on a standardised episodic memory test.
Finally, large-scale epidemiological studies also suggest that exposure to various kinds of stress (e.g., everyday life stress, work stress, traumatic events and major life stressors) may increase the risk for developing dementia. For instance, Johansson et al. (44) investigated relations between self-reported stress at three time points during mid-life and dementia diagnosis at 35-year follow-up (N= 1462 women; age range at initial assessment = 38- 60 years). Relative to those who reported no significant period of everyday stress during mid-life, those who reported work, health and/or family stressors were 1.10-2.51 times more likely to be diagnosed with dementia at follow-up (with a higher risk of dementia being associated with more consistent reporting of lifetime stressful experiences).
Individual factors can moderate the effects of stress on an organism’s functioning. One such factor is resilience. Resilience is viewed as the maintenance of physical and psychological health in the face of threat and/or adversity (45, 46). Not every person will experience a particular stressor in the same way. A key factor in this is the causal role of stimulus appraisal; that is to say, how one evaluates and interprets a situation or event with respect to one’s wellbeing (47). Research has indicated that differences in psychological resilience account for meaningful variation in daily emotional responses to stress. Further, it has been shown that the experience of positive emotions aids high-resilient individuals in their ability to recover effectively from daily stress (48).
Resilience has also been associated with other important factors that moderate the stress effect, namely age and sex. For instance, older adults seem to possess higher levels of resilience than younger adults, and females tend to have higher levels of resilience and access more social support than males do; however, females have increased vulnerability to stress-induced ailments (49, 50). Aside from their associations with resilience, both age and sex have been identified as independent and joint moderators of the stress response.
With regard to age, the animal literature indicates that advancing age is associated with a decreased stress response (see e.g., 51, 52). Using a longitudinal design, Lendvai et al. (53) measured the stress response of a sample of house sparrows (Passer domesticus; age-range = 1-8 years) twice in consecutive years. They found that the birds’ response to a standardised stressor decreased in magnitude as they increased in age. Human literature has delivered less consistent findings, with some studies finding no age effects on stress responses, others finding that stress responses decline with age, and still others reporting that the stress response increased with age (see review on stress and resilience in older adults; 54). One indication of this complexity in the literature is provided by Scott et al. (55), who found that, in contrast to older adults, the immediate responses to everyday stressors of younger adults contained stronger elements of negative affect. However, there were no age differences in responses to those stressors 3–6 hours later.
With regard to sex, animal studies have investigated biological factors that underlie and explain a greater vulnerability of females to stress-related disorders (e.g., MDD and post-traumatic stress disorder, PTSD; 56). For instance, Weisbrod et al. (57) reported that two forms of stress (chronic unpredictable mild stress administered at least once a day for two weeks and shock stress administered for 2 hours on 3 consecutive days) altered certain behavioural (open field activity) and biological (body weight) indicators of depression in female, but not male, Sprague-Dawley rats. In humans, analyses of physiological and emotional responses to stress indicate that women may have a greater stress response than men (58, 59). Data combined from three national surveys analysing psychological stress at three time points (1983, 2006, and 2009) indicated higher levels of stress in women than in men at all time points (60). Likewise, Matud (61) found that women scored significantly higher than men on measures of chronic stress and minor daily stressors, even after adjusting for sociodemographic variables. Further, the lifetime risk for depression and for PTSD in women is twice that of men (62, 63). Although some of this discrepancy in diagnostic rates might be attributed to socio-cultural factors and gender- informed perceptions of illness, it appears that there are credible biological reasons underlying greater female vulnerability to the negative effects of stress. For instance, Herbison et al. (64) found that, for women, medium to high chronic stress exposure or exposure during puberty/adolescence predicted depression and anxiety symptoms, while low or reduced stress exposure during the life course did not. In contrast, for men, prenatal stress (as reported by their parents/caregivers) was a strong and independent contributor to later-life depression, notwithstanding any experiences of postnatal stress.
Implicit in much of the reviewed literature is that the magnitude of stress effects is, to a great degree, dependent on when stress occurs (i.e., at what point in the organism’s lifespan the stress is experienced). Exposure to stress during developmentally critical or sensitive periods of early life increases the risk for developing poor physical and mental health outcomes (see, e.g., 65–68). For instance, Schalinski et al. (69) reported that, among a number of different forms of childhood trauma and ACEs experienced by a sample of young adults (N = 180, mean age = 28.6 years), (a) the experience of emotional and physical neglect at age 10 years bore a strong relationship to severity of positive psychotic symptoms, and (b) the experience of physical and/or sexual abuse at age 12 years bore a strong relationship to severity of negative psychotic symptoms.
A large proportion of the stress-related literature has focused on understanding the physiological nature of stress and how the brain and body interact to respond to and cope with exposure to a stressor.
There are two neuroendocrine systems that play a significant role in responding to and coping with stressful conditions: the sympathetic adrenal medullary system (SAM) and the hypothalamic-pituitary-adrenal (HPA) axis (the main stress response system of interest in this review).
In the event of experiencing acute stress, the initial response to this is facilitated via the SAM, which regulates the release of catecholamines (including noradrenaline, adrenaline, and small amounts of dopamine) and ultimately triggers the “fight or flight” response (70, 71). These processes lead to activation of the HPA axis. With activation of the HPA axis, the hypothalamus and anterior pituitary are triggered to secrete corticotropin-releasing hormone (CRH) and produce adrenocorticotropic hormone (ACTH), respectively. These processes stimulate the zona fasciculata of the adrenal cortex to release glucocorticoids, of which cortisol is the principal human glucocorticoid (in rodents, this is corticosterone), into the bloodstream (11, 72). The release of this hormone is the best characterised marker of the HPA axis response to psychosocial stress. Cortisol is regulated by a negative feedback system, involving the hippocampus, in which circulating glucocorticoids down-regulate CRH (73) and ACTH (74) secretions from the anterior pituitary and hypothalamus, respectively (see Figure 1.).
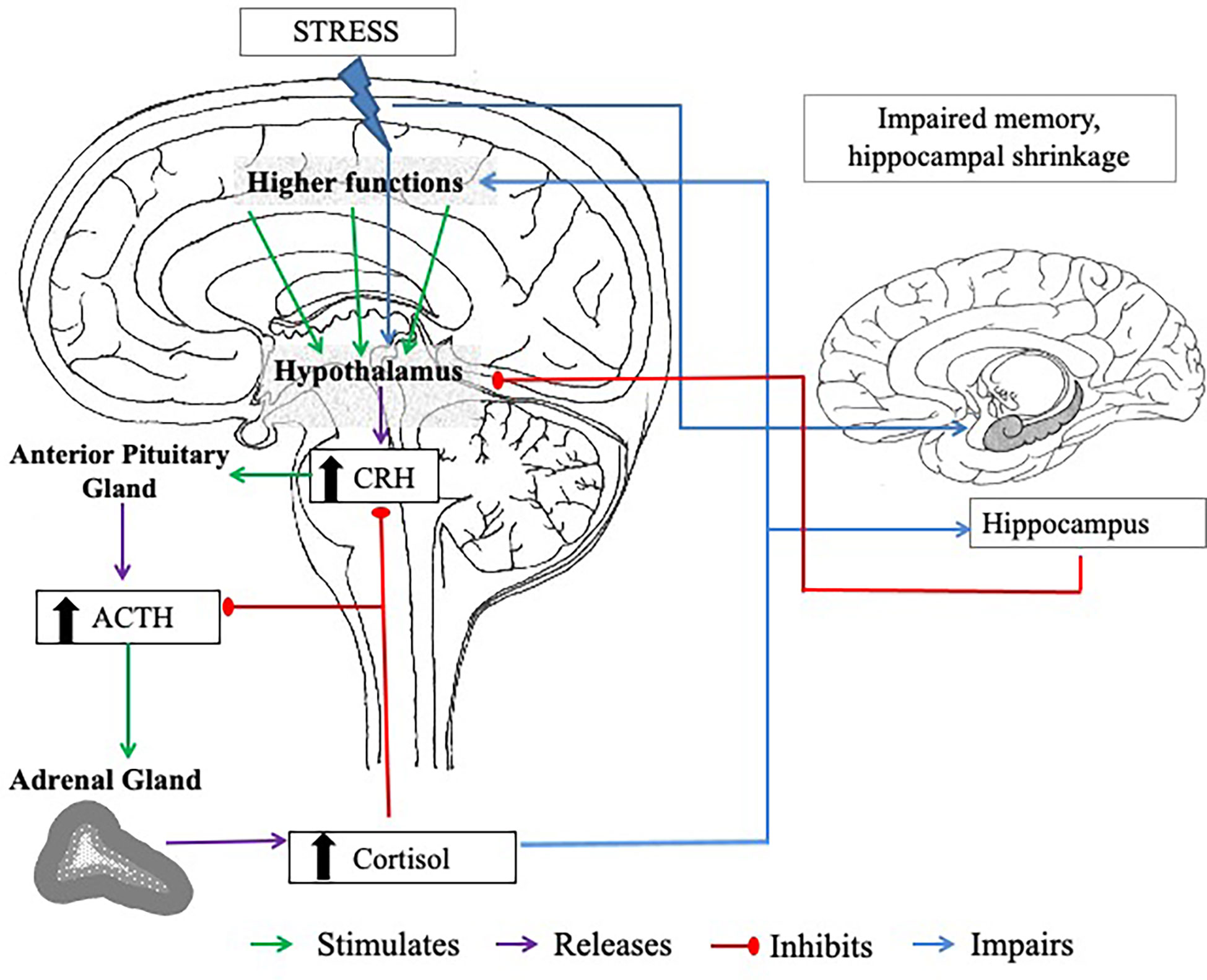
Figure 1 The negative feedback loop of the HPA axis. CRH = corticotropin-releasing hormone. ACTH = adrenocorticotropic hormone. The body experiences a stressor; this triggers the hypothalamus to release CRH, which stimulates the anterior lobe of the pituitary gland to release ACTH. The adrenal cortex of the adrenal glands responds to stimulation by the ACTH and produces cortisol. High plasma cortisol stimulates the hippocampus (a brain region involved in memory processes, that has an abundance of corticosteroid receptor sites), which also has inhibitory control over the HPA axis to prevent excess cortisol release. When stress is chronic, high levels of circulating cortisol can cause long-term damage to the hippocampus, including hippocampal shrinkage, which can impair the actions of the negative feedback loop. Cortisol also has a negative feedback on the hypothalamus and the pituitary gland, inhibiting CRH and ACTH secretion.
It should also be noted that cortisol is not the only steroid released from the adrenal cortex; sex hormones (androgens and oestrogens) and mineralocorticoids (the most important of which is aldosterone) are also secreted from the adrenal glands. More specifically related to the stress response, in addition to the release of cortisol, is the release of dehydroepiandrosterone (DHEA) and its sulphated form, DHEA-S. DHEA is understood as a precursor to the production of sex hormones (testosterone, oestrogen, progesterone) but it is also produced in greater quantities as a moderator of the stress response (75, 76). Interestingly, the DHEA-to-cortisol ratio has been associated with increased stress tolerance; in other words, individuals who have a higher ratio (DHEA levels are higher than cortisol levels), seem to tolerate stress better and experience less negative effects from the same stressors as those with a lower ratio.
Decades of research has contributed to the understanding that disruption of the HPA axis can result in dysregulated stress response phenotypes that demand a physiological cost that is referred to as allostatic load. Organisms that experience a high allostatic load may be at increased risk of further challenges. This was demonstrated in a model of non-invasive chronic corticosterone treatment in mice. Results from that study indicated that dysregulation of the HPA axis led to incongruity between the hormonal and neural response to acute stress; this resulted in abnormal behavioural coping strategies that would have negatively impacted on their chance of survival (77). Further, cortisol (corticosterone in animals) augments gluconeogenesis, suppresses immune response, and increases the metabolism of fat and protein. Circadian variation in the HPA axis is the driving force in the overall regulation of adrenal glucocorticoid secretion.
Circadian rhythms are endogenous processes with a periodicity of approximately 24 hours. Circadian rhythmicity stimulates anticipation of repetitive daily events that occur at approximately the same time of day. This process enables the upregulation of most of the major physiological systems in mammals. Cortisol is a foundational element of human physiology and it is present even during foetal life in healthy pregnancies (78, 79). It has been suggested that the foetus is a peripheral oscillator within the maternal system where the peripheral circadian clocks are largely controlled by the maternal circadian rhythm. After birth, a crucial transition takes place involving postnatal integration of the dispersed foetal circadian clocks, that will then imitate the adult-like circadian rhythmicity that is controlled by the suprachiasmatic nucleus of the hypothalamus (80). It is understood that this circadian rhythm develops within the first six months of life; recent evidence suggests that this rhythm is sometimes established as early as one month of age in healthy, full-term infants (81, 82).
Along with melatonin, cortisol is the most widely used biological phase marker to study circadian rhythm; these hormones are both highly rhythmic (83). Cortisol levels peak in the latter half of the night and are highest in the early morning; this is known as the cortisol awakening response (CAR). The CAR usually lasts for about 30-45 minutes immediately after waking. Thereafter, cortisol levels decrease throughout the day, to half of its peak value in the afternoon, with the lowest production at midnight (84).
Cortisol has been studied extensively and is an important factor in psychosocial, physiological, developmental, clinical, experimental, and behavioural studies. What is clear from reviewing the literature is that there are a variety of ways in which cortisol is obtained and measured, and this, in part, contributes to some of the challenges of direct comparisons between studies.
Cortisol has been examined via several different methods using a range of substrates including blood (serum or plasma), cerebrospinal fluid (CSF; 85, 86), urine (87), sweat (88, 89), interstitial fluid (90), hair (91), fingernails (92), and most prominently, saliva (25).
In the blood, cortisol exists in two forms: the majority of cortisol is bound to carrier proteins, and a smaller portion exists in a soluble free form. When choosing to study cortisol, it is imperative to consider the varying cortisol fractions, the biological functions of their binding proteins, and the relationship to the HPA axis. Measuring cortisol in serum is useful in the diagnosis of conditions such as hypercortisolism and adrenal insufficiency. However, many factors can affect serum cortisol concentration and particularly episodic cortisol secretion. This, therefore, makes the interpretation of a single cortisol value questionable, at best, and hazardous, at worst. Salivary measurements of cortisol may provide some advantages over serum measurements, including providing a more appropriate measure of adrenal cortical function (93).
Salivary measurements of cortisol are favoured due to their simple, quick, and non-invasive technique. These factors also make the measurement of salivary cortisol in infants and children an easier process (94). Long-term storage of saliva samples of cortisol at room temperature has been found to be detrimental to the integrity of the samples, thus freezing is recommended (95). Salivary cortisol samples have been shown to withstand repeated freezing and thawing of samples up to four times before analysis, with no effect on the cortisol concentrations. Further, centrifuged saliva samples of cortisol can be stored at 5°C for up to 3 months or at −20°C or −80°C for at least one year. However, measuring cortisol concentrations in saliva is not without its complications. Salivary cortisol concentrations can be affected by several factors including body weight and body mass (96), daily rhythm (97), caffeine ingestion (98, 99), alcohol consumption (100), antibiotic intake (101), and recent infection (97). Ideally, these factors should be controlled to improve reliability and accuracy of cortisol readings.
Salivary cortisol levels have been measured across the age range, including in infants, children, adolescents, and adults. Kiess et al. (96) measured salivary cortisol in samples from all these groups (138 healthy infants, children and adolescents and 14 adults). They found that cortisol levels were age-dependent. Other interesting findings to emerge from their study demonstrated that the highest cortisol levels were measured in saliva of children under the age of 1 year, with no circadian variation before the age of 9 months. They also reported that circadian rhythmicity of salivary cortisol levels only emerged after 1 year of age, and they observed a correlation of cortisol levels after the age of 6 years with pubertal stages. More recent evidence from a prospective longitudinal study of 24-hour urinary free cortisol excretion, in people aged 20 to 90 years old, highlights important questions about the impact of age-related changes in cortisol in predicting medical, physiological and behavioural changes. Findings from that study showed that 24-hour urinary free cortisol to creatinine ratio (UFC/Cr) followed a U-shaped pattern across the life span. This pattern indicated a decrease in the second and third decades of life, relative stability in people in their 40s and 50s, and increases thereafter (102). The impact of age on cortisol levels is clearly an important consideration.
In normal ageing, several endocrine changes occur, including those linked with changes in the structure and function of the adrenal gland. Such changes are associated with alterations in hormonal output, such as gradual sustained elevation in glucocorticoid secretion (although a similar alteration in normal circadian rhythm has not been observed; 103). Veldhuis et al. (104) described the relationship between ACTH and rising cortisol levels as humans age; this was confirmed by Roelfsema et al. (105), who demonstrated how increasing age was linked with elevated cortisol concentrations in the late evening and early night and advanced the timings of the peak diurnal rhythm. These findings correspond with earlier studies that reported significant associations between older age and higher baseline cortisol (106, 107). Furthermore, the capacity to secrete DHEA and DHEA-S in response to acute stress declines with age; therefore reducing the potential beneficial effect of the DHEA-cortisol ratio discussed earlier in this article (76, 103).
The majority of studies on cortisol have included smaller, selected samples which have limited generalisability of the findings. Larsson et al. (107) aimed to address this in a cross-sectional study of 1811 men and women (30-75 years of age) that specifically investigated age differences in diurnal cortisol patterns. They found that in males and females, there was a significant association between age and increased cortisol on all measures (this association was most consistent with evening cortisol). The elevation of cortisol levels in older adults is of particular relevance due to the effect of cortisol on several systems including cognition, and the known relationships between chronic stress, raised cortisol levels and ageing. Furthermore, excessive cortisol levels can lead to changes in the structural and functional integrity of several important brain regions such as the hippocampus, amygdala, and prefrontal cortex (108). The subsequent impact on cognitive functioning as a result of these changes will be discussed in more detail in later sections of this article. It is also pertinent to note the impact of chronically high cortisol levels on the stress response in older adults, which can result in impaired ability to recover from stressful stimuli (103).
In addition to age differences, several studies report sex differences in cortisol levels (105, 107, 109). These largely exist due to biological sex differences in the HPA axis itself, which leads to sex-dependent responses to stress. Further, sex steroids are considered to play an important modulatory role in the sex-differentiated stress response (110, 111). For instance, testosterone has been negatively correlated with salivary cortisol in men, while progesterone was negatively correlated with cortisol responses in women, suggesting inhibitory functions of these steroids in men and women, respectively (112). Furthermore, cortisol levels in older women in response to stress have been found to be higher than in males (106). Interestingly, and supported by findings from Seeman et al. (106) and Hidalgo et al. (113), younger women appear to produce lower levels of cortisol in response to stress than young men. This finding is contradicted by Batabyal et al. (114) who reported that in a sample of university-age students, men showed significantly lower levels of salivary cortisol than women over the period of one year. Despite these varying results, there is evidence to suggest significant age-by-sex interactions regarding patterns of stress response and cortisol secretion (106, 115).
As inferred from the above discussion, the measurement of cortisol is complex and affected by many factors. Single-point measurements are not that helpful and cortisol assessments are only performed in laboratories which also make them less accessible. Given that cortisol levels can provide indicators of HPA dysfunction and the presence of underlying, potentially treatable conditions, it would be helpful to be able to measure cortisol more efficiently. Improved accuracy and faster detection of cortisol may have significant implications for preventing, diagnosing and treating stress-related disorders, as well as for those experiencing adrenal insufficiencies. Recent investigations point to technological advances and the development of promising proto-types, including wearable devices with electrochemical sensors (89, 116). Future developments in this vein may lead to the provision of rapid, accurate, and repeated cortisol assessment in everyday life. It would be premature to over-interpret this information at this stage but it is something to bear in mind for the future.
Cortisol is known to permeate the blood brain barrier (3). Research shows that this is particularly true when the stressor persists longer than 15-60 minutes. In the context of acute stress, glucocorticoids interact with corticosteroid receptors (mineralocorticoid and glucocorticoid receptors) located throughout the brain. There is an abundance of both mineralocorticoid and glucocorticoid receptor in the hippocampus, amygdala, and in the prefrontal cortex (117–119). These receptors play a key role in regulating the neural circuits and neuroendocrine systems that instigate behavioural responses to stress (120).
Glucocorticoid receptors play a role in both short- and long-term neuro-biological changes found in response to stressors (121). For example, environmental circumstances that occur early in life can lead to permanent changes in (a) the development of glucocorticoid expression in the hippocampus, and (b) HPA axis responses to chronic stress (122). Further, glucocorticoids can alter the hippocampus in several ways: they can reduce excitability of some hippocampal neurons, they can lead to atrophy of dendritic branches in pyramidal cells within the CA3 area, and they can stunt the growth of new neurons in the dentate gyrus (123). Of note, these areas have been implicated in many cognitive processes including executive functioning, memory, and learning (124). High levels of cortisol are associated with impaired memory; however, ongoing evidence suggests that low elevations of cortisol may also lead to deleterious effects on memory (125, 126).
While the physiological response to acute psychological stress is well documented, the response to prolonged psychological stress is less well known. Žarković et al. (127) designed a prospective assessment of cortisol secretion during prolonged psychological stress that was induced via continuous air raids and after cessation of the stressor. Psychological, endocrine and psychiatric assessments were conducted at two months and at 18 months after cessation of the stressor. In their small sample of healthy participants (aged 34-39 years old), they found that prolonged psychological stress was associated with a transient suppression of the HPA axis. This was manifested by low morning cortisol and reduced cortisol response to ACTH. They concluded that the reduced cortisol response was sufficient to result in a false diagnosis of HPA insufficiency. Such findings support the “adrenal fatigue” theory that prolonged exposure to stress can drain the adrenals (due to an inability to keep pace with the demands of an ongoing perceptual fight-or-flight arousal state) leading to a low cortisol state. However, validity of this theory has been disputed and remains unresolved due to a lack of hard evidence to support it (128).
Cortisol depletion subsequent to prolonged or excessive secretion can contribute to its dysfunction, but alternate explanations should also be considered. In addition to depletion of cortisol, cortisol dysfunction may arise from several other neuroendocrine alterations such as impaired cortisol secretion, a deficiency in free (unbound) cortisol, glucocorticoid receptor resistance, or dysregulation of the negative feedback system (129–132). Irrespective of which neuroendocrine mechanism is responsible for ensuing changes, the long-term effect of chronic stress is the same; namely, that cortisol fails to function.
Another factor that appears to influence the HPA axis response to stress and circulating cortisol levels is the є4 variant of apolipoprotein E (APOE-є) (133, 134). APOE-є is a protein that is involved in lipid transport and metabolism. It is produced and secreted in the brain and is involved in neuronal regeneration (135). In humans, the apolipoprotein E phenotype is coded by a gene that has three common allelic variants: є2, є3, є4 (136, 137). Rodents express only one form of APOE-є.
Several animal studies have explored how stress and APOE genotype interact to influence glucocorticoid levels. For example, one study found that elevations in glucocorticoid levels after constraint stress were markedly lower in APOE-deficient mice than in control wild-type mice (138). Findings from another study involving predator stress suggest that the effect of stress on cognition is mediated by corticosterone, and that this effect is modified by APOE presence (139). Further, increased levels of cortisol in APOE-є deficient mice suggest that APOE genotype may influence cortisol concentrations in neurological disorders such as AD (140). Rodent studies have also suggested an important role for APOE-є in regulating adrenal steroidogenesis, and have alluded to the possibility that APOE-є activity may be involved in disorders of glucocorticoid hypersecretion (141).
In humans, a growing field of research has explored the impact of APOE genotype on stress response-related processes, identifying strong links between mitochondrial function, endoplasmic reticulum stress, and the immune response (see mini review by 142). Additionally, carrying the APOE-є4 allele may pose a vulnerability factor in the negative effects of HPA axis dysregulation on cognition during ageing (143). An increased frequency of the APOE-є4 allele has been associated with higher CSF cortisol concentrations in individuals with AD relative to controls (140). It has been suggested that the neurodegenerative process of AD may be initiated by gene-environment interactions that involve APOE-є4 (144–146). For example, Peavy et al. (147) examined the interaction between an environmental factors (i.e., “real-life” stress) and a genetic risk factor for AD (i.e., the є4 allele) in explaining cognitive performance in a sample of 91 non-demented older adults (mean age: 78.8 years). Low-stress participants demonstrated better cognitive performance than high-stress participants on tests of delayed recall, list learning, and visual memory. Participants without the є4 allele obtained better scores than participants with є4 on tests of immediate and delayed recall of visual designs. The authors also reported significant stress by APOE-є4 interaction effects on memory and cortisol in the high-stress, є4-positive group. Those participants displayed consistently poorer memory performance compared to the high stress, є4-negative group, the low-stress, є4-positive group, and the low-stress, є4-negative group. Participants in this group also had higher cortisol levels than those in the low-stress, є4-positive group; cortisol levels did not differ in the high- and low-stress groups who were є4-negative. Similarly, Lee et al. (137) investigated APOE-є4 carrier status, cortisol levels, and cognitive function in community-dwelling older adults. Their results showed that, even though a higher cortisol level was associated with lower cognitive scores, the slopes of the adverse relations were steeper in individuals with at least one APOE-є4 allele. The authors suggested that cortisol’s relationship with cognitive functioning is modified by APOE-є4, such that the є4 allele increases the vulnerability of the ageing brain to the negative effects of stress.
It is well understood that the health of the body and mind are inextricably linked; the most well established example of this is the association between psychological stress and psychological ill-health. There is a significant body of existing and ongoing evidence that suggests a link between HPA axis dysregulation and the risk of developing psychiatric disorders, including depression, schizophrenia, and anxiety disorders (148–150). While it is beyond the scope of this review to report comprehensively on the associations between HPA axis functioning and mental health disorders, it is salient to provide a brief overview of the more general psychological implications of HPA axis alterations.
Chronic stress plays a significant role in the development of depressive disorders and it is associated with elevated cortisol levels. Individuals with MDD have significantly higher stress and cortisol levels when compared with controls (151). Associations have been demonstrated between cortisol hypersecretion and both acute and more severe subtypes of MDD (29). The association between increased cortisol levels and the development of depression may relate to excessive adrenal activity acting destructively on the hippocampus and increasing one’s vulnerability to depression (152, 153). Despite known associations between cortisol and MDD, the current general consensus appears to remain uncertain and cautious about the value of assessing cortisol as a biological guideline for the pathophysiology or treatment of MDD. Furthermore, the absolute level of cortisol is viewed as unreliable in predicting the efficacy of antidepressant treatment.
To further complicate matters, questions have been raised about the linearity of the relationship between stress and mental health disorders, such as depression. The early stress literature examining the links between stress and depression was largely based on the tacit assumption of a unidirectional relationship (the stress model of depression: where the experience of stress was assumed to increase susceptibility to depression). However, a comprehensive review in this area (154) concluded that later stress research supported the role of depression in predicting generated stress (the stress generation model; 155); further research is still required in this domain. Recent evidence has pointed to genetic variations in the HPA axis functioning as moderating the effects of psychosocial susceptibility markers on the development of proximal life events (156).
It is salient to note that the psychological determinants of an individual’s response to stress are important predictors of stress-related outcomes. Not all people will experience a stressor in the same way subjectively or experience an endocrine response to a stressor; what may be a stressful experience for one person may be a non-event for another. Exploring endocrine stress reactivity, Fischer et al. (157) measured subjective perceptions of stress and salivary cortisol levels in nurses and physicians working in neonatal and paediatric critical care environments. They found that even though there were frequent stress-related cortisol surges in these participants, the cortisol increases were independent of subjective stress perception and professional experience did not reduce stress reactivity. Additionally, from a biological perspective, the release of cortisol in response to a stressor may attenuate the unpleasant feelings associated with the stressor and ultimately provide beneficial outcomes. For example, small doses of cortisol release in healthy individuals can improve memory and motivation, enhance one’s immune system and increased pain threshold (158–160). Some researchers have reported associations between stress-induced salivary cortisol level elevations and reduced negative affect (161). Such findings suggest a potential mood-buffering cortisol effect and this contributes to the divergence in the literature seeking to associate stress-induced psychological reactions and cortisol levels. Another challenge in interpreting the impact of stress exposure, is that the majority of daily life stressors that are studied, are relatively mild in biological terms. This raises questions about their detectable influence on cortisol secretions and leads to its own interpretation difficulties (162). An additional factor for consideration is the potential for habituation; whereby individuals repeatedly exposed to mild or moderate stressors often adapt to the stress. Such adaptation consequently leads to a reduced cortisol response (163). Such factors result in interpretation difficulties regarding the relationships between psychological stress and cortisol.
Neuropsychiatric effects have also been reported in conditions of adrenal insufficiency, such as in the case of Addison’s disease. Addison’s disease is a medical condition marked by chronic adrenal insufficiency resulting in a failure of glucocorticoid secretion. It presents with a constellation of primary signs and symptoms including but not limited to: extreme fatigue, weight loss, hyperpigmentation, hypoglycaemia, nausea and muscle or joint pain. Other symptoms can include psychosis, irritability, depression and behavioural symptoms; however, the neuropsychiatric component of the condition is less well understood (164). It is relevant for physicians to be aware of the neuropsychiatric symptoms that can manifest in patients with Addison’s disease, because on occasion, these symptoms may be the only manifestations of a potentially life-threatening Addisonian crisis. Additionally, motivation and behaviour during an Addisonian crisis are two of the more unusual presentations, which can make the diagnostic process more challenging (164, 165).
As a side note, low levels of cortisol have been associated with fibromyalgia, chronic fatigue, chronic pain syndromes, and other functional somatic disorders. However, the relatively moderate cortisol reductions are generally not considered to represent Addisonian insufficiency (166, 167). Lower levels of cortisol may be due to underactivity of the HPA axis, partial primary adrenal insufficiency, increased negative feedback sensitivity and/or altered glucocorticoid metabolism. The majority of research examining lower cortisol levels has been conducted in the context of PTSD, where lower levels of cortisol have been associated with early-life physical or sexual abuse (168, 169). This stands in paradox to a wealth of evidence demonstrating associations between severe stress-related psychiatric disorders and elevated levels of cortisol. PTSD arises from exposure to extremely traumatic experiences but it only occurs in a proportion of people exposed to such events. Suggested explanations for this speak to individual variation in vulnerability and resilience.
Another factor to consider in relation to HPA axis alterations and brain and mental health outcomes, is removal of the adrenal glands. Adrenalectomy surgery involves removing one or both adrenal glands. This is often due to the presence of a tumour (generally benign) that is either large in size or is producing excess hormones, which creates problematic health conditions. The majority of adrenalectomy-related research has been conducted in rodents, exploring a number of outcomes such as gene transcription and expression (170), alterations in hippocampal neuronal activity (171), and psychostimulant sensitivity (172).
In humans, there has been an increase in the prevalence of incidental adrenal masses found on routine imaging, as well as increased rates of adrenal operations (173). The standard treatment procedure for such masses involves hospitalisation and endoscopic adrenalectomy. Evidence suggests that adrenalectomy in the context of lateralised primary aldosteronism has beneficial effects not only in terms treating the biological condition, but also in terms of improving mental health outcomes. For example, Citton et al. (174) reported improved postoperative mental health outcomes as evidenced by scores on the Mental Component Summary and Depression Scale questionnaire; these improvements held at early (one month postoperatively) and late follow-up (at least 6 months postoperatively).
Adrenalectomy surgery might also be performed in the context of conditions such as Cushing’s syndrome (hypercortisolism). This syndrome can occur when the adrenal glands produce too much cortisol and it is a potentially life-threatening condition if untreated. In the case of adrenalectomy, glucocorticoid secretion decreases and this leads to increased neuropeptide expression and secretion by the paraventricular nucleus. These processes can occur in both basal and stress-induced states and can make stressors less well-tolerated (175).
In addition to the presence of several physical symptoms, patients with Cushing’s syndrome experience a range of neuropsychiatric and cognitive symptoms, as well as brain atrophy, indicating involvement of the central nervous system. Cushing (176) provided the first description of psychiatric disturbances in Cushing’s syndrome where he highlighted the presence of “emotional disturbances” as a pathologic feature of this syndrome (177). Further studies have been conducted to better characterise the spectrum and frequency of psychiatric and neurocognitive symptoms linked with Cushing’s syndrome (178, 179). The most important of these clinical symptoms include MDD (prevalence 50-81%), bipolar disorders (prevalence 30%), anxiety (prevalence 66%) (180), and neurocognitive impairment (most frequent reported alterations are memory impairment, approximately 83% of cases, and reduced concentration, 66% of cases) (179). Of these, atypical depression is the most common.
Dorn et al. (181) examined the longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism in patients with active CS. Before cure, 66.7% of patients had significant psychopathology (mainly atypical depressive disorder). Post-cure, overall psychopathology decreased significantly to 53.6% at 3 months, 36% at 6 months and 24.1% at 12 months. Atypical depressive disorder remained as the prevailing diagnosis and an increased frequency of suicidal ideation and panic was reported.
Brain structural abnormalities associated with Cushing’s syndrome have been found to include smaller hippocampal volumes, enlarged ventricles, and cerebral atrophy (182). The impact on brain volume is partially reversible after correction of hypercortisolism, but still persists to some extent (183). Furthermore, there is evidence to suggest that after long-term remission, patients with Cushing’s disease still experience chronic fatigue (184), reduced quality of life, and impaired cognitive functioning (particularly in the domains of attention, visuospatial orientation, reasoning, working and speed memory, verbal fluency and executive functions (179, 185). Thus, the picture of whether CS remission completely resolves psychiatric and neurocognitive disorders remains uncertain. Such findings speak to the importance of considering the psychological, psychiatric, cognitive and behavioural implications of HPA axis dysfunction and adrenalectomy. While psychopathology generally seems to improve with time post-surgery, healthcare practitioners should remain alert to any changes in psychiatric and cognitive symptomology including changes in mood, suicidal ideation, panic and cognitive functioning. The findings also highlight the importance of follow-up checks and monitoring of symptoms; the management of which should be considered as one of the essential outcomes of CS patients.
Stress affects cognition in several ways depending on its intensity, timing, duration, and origin (186, 187). The literature, which spans both animal and human studies, differentiates between mild-to-severe stressors, time of stress exposure, acute and chronic exposure to stress, and different origins of stress (physiological or psychological).
Animal studies play an important role in advancing the understanding of numerous medical conditions. There exists a vast literature documenting the responses to and effects of stress in laboratory animals. Rodent studies (including both rats and mice) have been invaluable in the study of stress and stress-related conditions as they provide insight into the effects of stress on mental and physical functioning (188). Humans share approximately 90% of their genes with rodents and there is significant overlap in terms of organ systems and functions performed; this makes mice an effective model for human biology and pathology (189, 190). A common method involves exposing animals to one or more stressors that are uncontrollable and often unpredictable. Such exposures might include isolation, confinement or restraint, noise, and separation from companions. Both acute and chronic stress conditions can be studied in rodents; these specific stressors include the forced swim test, inescapable tail-shock, immobilisation, and cold and ether stress. As with humans, the HPA axis in rodents controls their adaptation to stress via the actions of the corticosteroid receptor systems in the CNS. The stress manipulations used in rodent studies tend to increase behaviours that are similar to processes observed in human psychopathology, such as anxiety disorders, depressive illnesses, and PTSD (191).
Rodent studies have also demonstrated that stress exposure can alter important cognitive functions such as learning and memory processes (192). For example, rat pups deprived of early support from their dams later exhibited hippocampal-dependent cognitive impairment (193, 194), as well as marked anxious and depressive behavioural changes and deficits in working memory and attention set-shifting in their adult years (195, 196). Additionally, rats that were separated from their mothers early on in their lives were found to later develop AD-like pathology (197–199). In a similar rodent model of PTSD, rats exposed to single prolonged stress, beginning at 25 days old, equivalent to childhood in humans, showed anxious-like behaviours at day 32 and 60 which later expressed as depression by day 90 (200). Short term memory deficits were also observed at day 32 and 60 but not day 90. In terms of timing, age of stress onset is a mediating factor in animal models worthy of further investigation. Ricon et al. (193) found that onset of stress, in the form of food deprivation, elevated heights and forced swimming, affected learning dependent on whether the rat was between 30-90 or 60-90 days old; learning and memory were negatively affected by adult-onset stress but not by juvenile-onset stress. This suggests that juvenile exposure to stress may induce resilience rather than impairment. This is in contrast to literature indicating that ELS leads to cognitive impairment in later life. It also raises questions about the role of resilience and whether this is an intrinsic or acquired trait.
Sex also appears to be a mediating factor of stress in rat models, where sex hormones (testosterone and oestrogen) play an influential role in HPA axis activity (201). Both acute and chronic stress as measured by electrical foot shock and the paradigm for chronic unpredictable mild stress, respectively, produced differences in plasma sex hormone levels (202). Acute stress decreased these hormone levels in female rats while it produced no effect or increased effects in the male rats. The chronic stress paradigm produced greater changes via hypothalamic stress-related molecule expression in female rats than male ones, which was associated with greater depression and anxious-like behaviour. The effect of chronic stress on corticosterone concentrations and corticosteroid-binding globulin further strengthen these findings (203).
Similarly, HPA axis hormone plasma levels, as well as corticosterone concentrations, were significantly increased for female rats as compared to their male counterparts, following an acute period of physical spatial restraint (204). Differences in ACTH concentration were not observed at baseline, suggesting that there is an interaction effect between sex and ACTH concentrations in rats. While females expressed greater corticosterone concentrations before and after restraint, corticosterone differences were significantly emphasised following the stress condition. Similar findings by Figueiredo et al. (205) and Heck and Handa (201) suggest that inhibitory inputs to the HPA axis are reduced in females as compared to males following acute restraint. In contrast to this, rats exposed to 10 consecutive days of loud noise for 30 minutes resulted in no differences in ACTH levels and corticosterone concentrations between female and male rats (206). The variance in the effects of sex on stress responses in the above-stated findings is interesting. It may represent inconclusive findings about the overall effect of sex or it may have occurred due to differences in stress techniques used (e.g. physical restraint versus noise).
Animal studies have also examined the effects of stress on brain regions in rats. Stress has been found to cause atrophy in a number of hippocampal subregions (207, 208), as well as in the thalamus and the visual cortex in rats (209). The chronic stress effect is mediated by length of exposure to the stressor according to one study by Luine et al. (210) who found that exposure lasting from 3-6 weeks caused deleterious effects in rats while exposure lasting 3-10 consecutive days improved explicit memory and spatial learning. Furthermore, there are sex differences in the effects of chronic stress on brain regions in rodents; Lin et al. (211) reported abnormalities in brain morphology (atrophy in CA1, CA2, CA3 and amygdala, anterior cingulate area, dorsal part regions) following chronic stress exposure in male rodents but not in females. Such findings suggest a sexual dimorphism in the molecular response to stress in rodents.
As mentioned earlier, prolonged stress has been identified as a contributing factor to the development of neurodegenerative diseases such as AD. This was demonstrated empirically by Carroll et al. (212) in a restraint/isolation-induced chronic stress model that aggravated the accumulation of Aβ in transgenic mice with an APP mutation (Tg2576). The researchers then used this stress paradigm in a tau transgenic mouse model with the P301S mutation (PS19). The combined findings indicated that prolonged stress, arising from a dysregulated HPA axis, increased the production and deposition of Aβ, as well as tau phosphorylation. Such findings provide support for the hypothesis that prolonged stress may contribute to the neuropathogenesis of AD.
Chronic stress has also been found to downregulate glucocorticoid expression in the prefrontal cortex (PFC), which has been linked to cognitive dysfunction in animal models (213–215). In these same models, dendritic shrinking and the remodelling of cell structures was found within the PFC (187). While reversible, following cessation of a stressor, the formation of the affected dendrites shifted in position to the cell body, possibly affecting cell connectivity and subsequent gene expression (216, 217). Specifically, these findings and subsequent cognitive impairment are comparable with those following a medial PFC lesion (187). Conversely, chronic stress appears to lead to dendritic expansion in the orbitofrontal cortex as well as the basolateral amygdala (218–220).
Based on evidence from animal studies, it is clear that brain regions are affected differentially by exposure to stress. What remains unclear is how variables such as sex, type of stressor, age of organism at time of stressor and duration of stressor impact on the brain and body of the organism. Further, the applicability of findings from animal studies to humans remains a complex task. One of the challenges for future stress-related research will be to identify links between the cellular changes observed in animal models of chronic stress to behavioural effects, and to understand the risks they pose for humans for the precipitation of stress-related disorders.
Evidence provided by animal studies conducted over numerous decades has confirmed the significant effect of stress and stress hormones on the brain and body. In recent decades, researchers have explored similar effects in humans, largely through the use of laboratory-induced psychosocial stressors such as the Trier Social Stress Test (109). An important area of such research has focused specifically on the relationship between stress and cognition.
Long-term or chronic stress, particularly in childhood and adolescence, consistently affects cognitive mechanisms. It is widely accepted that experiencing early life stress can result in deleterious effects such as psychiatric disorders, depression, anxiety and PTSD, (213, 221–225). Research has also linked early life stress to dementia, cognitive impairment and chronic inflammation in later life (226). In one systematic review conducted by Seifan et al. (227), AD progression was particularly linked to stress associated with early life socioeconomic status. This finding is further supported by a study investigating the role of childhood trauma in Australian Aboriginals, a historically, socially and economically disadvantaged group. Those results demonstrated a significant correlation between childhood trauma and the development of dementia in later life (228). This same finding was reported in a longitudinal design following two different German cohorts (229).
Most studies exploring the effects of stress and cognition using laboratory-induced stressors and pharmacological approaches have focused on global cognition or basic memory functions such as memory consolidation. For example, Leng et al. (230) in drawing data from the EPIC-Norfolk study, recruited 5129 middle-to-older aged men and women (48-90 years) and found that self-perceived stress and stressful life experiences were negatively correlated with scores of global cognition as measured by the Mini Mental State Examination. However, more recent studies have expanded their research approach to include investigating the impact of stress on other cognitive domains. For example, acute episodes of mild-intensity stress can enhance cognitive functions, particularly tasks such as encoding and memory consolidation of task-relevant stimuli and implicit memory or basic declarative tasks. However, when exposed to high-intensity stress, the ability to form and retrieve explicit memories and cognitive processes involving complex reasoning becomes impaired. Exposure to stress has also been associated with tasks of attention; however, conflicting results have been reported, with some studies reporting a negative effect of mild acute psychological stress on attention (231, 232), and other findings demonstrating improved performance (including response time, vigilance and sensory intake) on attention tasks with exposure to mild acute stress (233, 234). Sandi (186) further linked chronic stress to impaired explicit memory processes which pertain to conscious retrieval of factual information in individuals. These processes are reliant on hippocampal and prefrontal functioning (235). At the same time, chronic stress appears to strengthen implicit memory, information that cannot be declared, which is more reliant on the amygdala and the striatum of the limbic system (186).
To complicate the relationship between stress and cognition further, the deleterious effects of psychosocial stress on cognition may be mitigated by certain protective factors. These include high resiliency; individuals with a strong belief in their own abilities, also known as self-efficacy; and those with a strong cognitive reserve (236, 237). The latter refers to the brain’s ability to make use of alternative networks in order to sustain baseline functioning following impairment or changes (238, 239). Cognitive reserve is closely linked to education as well as global cognitive functioning (229). Those with a weak cognitive reserve as well as those with a self-perceived lack of control appear particularly vulnerable to the effects of social stressors on cognitive performance (240, 241).
Education has also been identified as a moderator of the effects of stress on cognition. Individuals with a higher level of education and reportedly greater engagement with cognitively stimulating tasks, even in later life, have reduced risk of developing MCI and AD (229). Moreover, those with a low education level appear more at risk for developing AD in later life (227). Similarly, Tschanz et al. (242), in their population based study, found that the relationship between stressful life events and cognitive functioning was moderated by level of education as well as the amount of stressful life events experienced. Lower levels of education and greater stressful life events appeared to accelerate cognitive decline. This relationship was further moderated by age: younger persons experienced accelerated cognitive decline in relation to a greater number of stressful life events than their older counterparts. However, this study did not account for variables such as stress duration or subjective intensity which have been noted as possible mediators of stress.
Further, age and sex have been found to modulate the relationship between stress and cognitive performance (56, 113, 243). Of note, sex differences in recall performance were only pronounced in a young sample as investigated by Hidalgo et al. (113). The chosen acute stressor (TSST) in that study was significantly and negatively associated with memory performance in men while this relationship was not found amongst the women.
Within the stress literature, the majority of studies have explored relationships between psychosocial stress and cognition or between cortisol and cognition. Fewer studies have included measures of both psychosocial stress and cortisol in relation to cognitive functioning. In humans, research has demonstrated positive correlations between psychosocial stress and cortisol levels (241, 244, 245), as well as identifying negative correlations between psychosocial stress and cognitive functioning (43, 241, 244–246).
Recent findings (247) confirm results reported from previous studies (124, 241, 243, 248) demonstrating that an abundant release of glucocorticoids can modulate cognition. Sussams et al. (249) used a multi-centre longitudinal study of individuals with amnestic mild cognitive impairment (aMCI) to explore psychobiological stress and cognitive outcomes. Measures of psychological stress included the Recent Life Changes Questionnaire (RLCQ), the Perceived Stress Scale (PSS) and salivary cortisol. Scores were examined in relation to rates of cognitive decline over an 18 month follow-up period and conversion to dementia over 5.5 years. They found that, compared with controls, PSS scores were higher in the aMCI group, but there were no between-group differences in RLCQ scores or salivary cortisol measures. RLCQ scores and poorer cognitive function at baseline were associated with high salivary cortisol levels but no relationship was found between salivary cortisol and conversion rate to dementia. The authors proposed that psychological stress (as measured by the RLCQ or PSS) was not associated with adverse cognitive outcomes in an aMCI group. Instead, they postulated that this may be an indication of diminished cortisol production to psychological stress with disease progression.
Similar results were reported by another longitudinal study conducted by Ouanes et al. (250), investigating cognitively healthy older adults, which suggested that significantly high salivary cortisol levels were not produced on account of stressful life events. While stressful life events do appear to be predictive of developing dementia in later life, it is not yet clear whether stressful life events are directly correlated with poor cognitive functioning (242). Indeed, recent literature links high levels of cortisol to poor cognitive performance only (251–253). However, in the longitudinal study conducted by Sussams et al. (249), both self-report questionnaires and salivary cortisol levels were not predictive of conversion to dementia over the 5.5 year period.
Regarding acute stress, different tasks elicit different stress responses in humans (186). For example, physical stressors, such as cold water submersion and mental tasks that induce cognitive stress, trigger the sympathetic nervous system while psychosocial tasks are associated with activation of the HPA axis (71, 113).
Factors that appear to mediate the relationship between memory functioning and cortisol include the point at which memory is tested (i.e., during encoding or retrieval, etc.), level of arousal, the amount of cortisol reported, and the specific brain region upon which the memory function is dependent (43, 186, 254). Moreover, the time course of stress hormones in the human body may be responsible for memory functioning: when levels of both catecholamines and glucocorticoids are elevated, individuals are able to effectively encode and consolidate information relevant to the stressor. Information that is not relevant to the stressor is neglected and impaired. Consequently, when catecholamine levels have dropped but glucocorticoid levels remain high, consolidation processes are optimised at the expense of encoding and retrieval (186, 207, 255). It is further posited that there is a window wherein memory may be restored given normal glucocorticoid levels which restore the protective brain derived neurotrophic factor (256).
Some studies have found a dose-dependent inverted U-shaped relationship between cortisol and memory performance during retrieval (187, 247, 257). One study found this effect after administering cortisol equivalent to 0, 3, 6, 12, and 24 mg intravenously (257). Both Schilling et al. (257) and Wu and Yan (258) demonstrated how rapidly these acute doses of cortisol impair retrieval in healthy individuals (approximately 8 minutes). Other research suggests that age may be a mediating factor in which younger people are less vulnerable to the effects of cortisol on memory performance than their older counterparts (43, 259–262). These findings might elucidate the negative effect of chronically high levels of cortisol on cognitive functioning found in individuals with AD (246).
In the context of older adults, and similar to findings from animal studies, in cognitively healthy older adults, Aβ has been associated with increased cognitive decline. Further, elevated plasma cortisol levels may expedite the Aβ effect on decline in global cognition, episodic memory, and executive function (263). Further, in a study investigating 28 young adults and 32 older adults in two different conditions (a familiar learning environment for each age group and an unfamiliar learning environment for each group), older adults performed significantly worse on tasks of delayed recall and had higher cortisol levels during the unfamiliar condition (260). This relationship was inverted when older adults were tested within the familiar condition for their age group. The young adults were not significantly affected by the familiarity of the condition for both memory performance and cortisol levels. In contrast, scores on immediate recall did not appear to be affected by the condition or the cortisol levels recorded. Researchers of this same study suggested that the variation in performance between delayed and immediate recall might be due to the role of the hippocampus in delayed recall processes and the abundant supply of glucocorticoid receptors in this same brain region. This would render the hippocampus more susceptible to elevations of cortisol levels and would subsequently impair delayed recall. Overall, across conditions and despite differences in cortisol levels, the young adults performed significantly better than their older counterparts. However, the magnitude of these age-related differences might be mediated by the stress of the environment. Further, in this sample, basal salivary cortisol levels did not differ between the young and older adults.
Executive functioning performance has consistently been associated with cortisol levels, stress, and the HPA axis in humans (235, 264, 265). High cortisol awakening response (cortisol measured during the first 30 minutes following sleep) has been associated with executive dysfunction (266). It is hypothesised that because executive functioning is largely supported by the workings of the prefrontal cortex, which is additionally home to abundant glucocorticoid receptors, executive functioning is largely influenced by the experience of stress (187; 220, 222).
Particularly, the effect of cortisol on working memory performance is pronounced (266, 267). Working memory is viewed as a division of executive functioning wherein information is ‘held’ and actively worked upon to achieve a specific outcome (267). As with memory retrieval, an inverted U-shaped relationship has been observed in relation to cortisol and working memory (124, 268). Ennis et al. (269) found that higher waking cortisol levels were significantly associated with better working memory (this association was not found for episodic memory or processing speed). Additionally, sex appears to have a modulatory effect on working memory, cortisol and stress. In one study by Schoofs et al. (270), men, but not women were found to have improved working memory performance due to stress induced by the TSST. In a meta-analysis by Shields et al. (235), the opposite finding was true: men appeared more vulnerable to the effects of stress and cortisol on working memory performance than their female counterparts. This same meta-analysis found that working memory was further modulated by time between cortisol administration and assessment, with a delay impairing performance further. This contrasts earlier meta-analyses findings which found cortisol to decrease and enhance performance after delay (235).
Finally, a broad range of cognitive functioning appears to be affected by high cortisol levels, including short term and long-term verbal memory, learning, and attention in older adults (113). Wu and Yan (258) as well as Shields et al. (267) have further explored the range of executive dysfunction associated with high chronic stress as measured by hair and salivary cortisol levels. This includes impaired spatial memory performance, poor hand-eye coordination as well as poor impulsivity control and accuracy during arithmetic tasks. Cognitive flexibility and mental rotation have additionally been identified as cognitive skills vulnerable to the effects of cortisol (247).
On reflection of the information covered in this review, there are two main points that we wish to raise in terms of clinical applicability for endocrinologists. The first of which relates to the utility of cortisol measurements and the second of which relates to awareness of the psychological, cognitive, and behavioural aspects of endocrine-related disorders.
Reviewing the existing body of cortisol-related literature has demonstrated the complexity of this biological marker of HPA axis dysregulation (different samples used to determine cortisol concentrations, the timings of when the samples are taken, varying methods of measurement, storage techniques, and a range of lifestyle and biological factors that affect cortisol concentrations, etc.). What is clear though, is that a single-morning cortisol sample provides very limited predictability of the diurnal secretion. Additionally, sex and age jointly determine the 24 hour secretory profile of cortisol. In terms of diagnostic considerations, these tenuous physiological alterations bear little relevance in diagnosing major endocrine disturbances as in the case of Cushing’s disease and conditions of adrenal failure. To aid the diagnosis of such conditions, the Endocrine Societies provide clear guidelines in this regard.
The second point we wish to raise relates to raising awareness of the psychological, cognitive, and behavioural aspects of HPA axis dysfunction and endocrine-related disorders. In addition to diagnosing and treating the causes of hypercortisolaemia and adrenal insufficiency, endocrinologists should be aware of the emotional, behavioural, and cognitive symptoms often associated with these conditions (179, 185). Furthermore, future studies in this field should aim to explore further if and how longitudinal changes associated with these endocrine disorders affect (subclinical and clinical) psychopathology.
Given the links between endocrine functioning and the associations with and effects on cognitive and emotional wellbeing, endocrinologists should enquire from their patients whether they have past or current mental health difficulties. They could then consider referrals to therapy and/or psychiatric services for assessment, psychoeducation, and management. The provision of biologically-informed psychoeducation to patients about the negative impact of stress on physiological, psychological and cognitive systems may allow for appropriate intervention and cortical inhibition of fear-based responses to non-threatening stimuli. Early intervention may help patients to manage stress levels and mitigate potential adverse effects of cortisol on the body and the brain. Treatment is not just important for short-term mental health (by preventing transition to chronic anxiety and depressive symptoms, for example), it may also be key to maintaining long-term brain health (serotonin depletion and hippocampal degeneration are likely to be affected by and also have an impact on stress levels, mood, as in the case of depression, and cognitive functioning). As we get older, the impact of stress can become more severe because the ageing brain does not recover from stress as well as when we are younger. Therefore, if an individual’s stress levels are interfering with daily functioning, helpful recommendations would include seeking therapy and professional help sooner rather than later.
Stressful events are an inevitable occurrence in daily life and our ability to overcome challenges promotes a sense of success and achievement. While it is not possible, nor a helpful or realistic aim to avoid stressors completely, humans have the capacity to control their perception of and response to stressors. In this way, maladaptive cognitive appraisals, unhelpful thoughts or unchecked beliefs about the threatening nature of potential stressors may lead to an excessive physiological stress response and cortisol dysfunction. Modern day approaches to maintaining good health include identifying and addressing modifiable risk factors. Stress is a modifiable risk factor and there is increasing evidence that effectively managing stress levels has numerous benefits for psychological and physical health.
The scientific stress literature is vast; it covers different theories of the impact of stress, how stress is responded to and coped with by animals and humans, effects of stress on the body and the brain, and the different contributors of stress in terms of acute stress and that which is prolonged and enduring. As a result of these complexities, the literature contains heterogeneous findings about the causes, nature, and subsequent effects of stress. This review has summarised and integrated literature on the relationships between physiological and psychosocial stress, the stress hormone cortisol, as controlled by the HPA axis, and the effects of stress on cognitive functioning.
The challenge of this review has been to draw concrete conclusions about the decades of research that have been conducted in this field. One of the main reasons for this difficulty is that the majority of stress-related research has been conducted at a group level. What is clear from the literature is that there is great individual variation in the exposure, reaction, experience, perception, and coping mechanisms of stress. These variations determine how stress affects the brain and what this then means for individuals’ vulnerability to disease.
Further contributors to the individual profile of stress response include genetic predisposition, sex, age at time of stressor occurrence, life history, personality traits, sociocultural environment, psychological resilience and coping mechanisms. Additionally, many studies have examined the impact of stress at particular time points, often relying on historical recollections of experiences. This method fails to take into account the significance of when adverse life events occur in relation to (brain) developmental stage. The importance of lifespan cannot be overlooked. To address the gaps in current knowledge, longitudinal studies that include a range of life stages need to be conducted. Relatedly, in older adults, elevated cortisol levels and the increased vulnerability of the brain raise further concerns about the negative impact of stress and the need for relevant interventions and stress management skills and support.
Lastly, this review has provided suggestions of clinical applicability for endocrinologists who are uniquely placed to measure outcomes related to endocrine, nervous and immune system functioning and identify areas of intervention. The key point in this regard is the importance of awareness of the psychological, cognitive, and behavioural aspects of HPA axis dysfunction and endocrine-related disorders, particularly as such symptoms may be the only manifestations of a potentially life-threatening endocrine disorder. Awareness of these aspects also lends itself to potential psychological and behavioural interventions that may provide an additional layer of support.
Review written by KJ, JS, NS and MC. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shahsavarani AM, Azad Marz Abadi E, Hakimi Kalkhoran M. 'Stress: Facts and theories through literature review'. Int J Med Rev (2015) 2(2):230–41.
2. Slavich GM. Life stress and health. Teach Psychol (2016) 43(4):346–55. doi: 10.1177/0098628316662768
3. Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. EXCLI J (2017) 16(1):1057–72. doi: 10.17179/excli2017-480
4. Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, et al. More than a feeling: a unified view of stress measurement for population science. Front Neuroendocrinol (2018) 49:146–69. doi: 10.1016/j.yfrne.2018.03.001
5. Hutmacher F. Putting stress in historical context: Why it is important that being stressed out was not a way to be a person 2,000 years ago. Front Psychol (2021) 12:539799. doi: 10.3389/fpsyg.2021.539799
7. Selye H. The significance of the adrenals for adaptation. Science (1937) 85(2201):247–8. doi: 10.1126/science.85.2201.247
9. Kemeny ME. The psychobiology of stress. Curr Dir psychol Sci (2003) 12(4):124–9. doi: 10.1111/1467-8721.01246
10. Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: Basic concepts and clinical implications. Front Behav Neurosci (2018) 12:127(127). doi: 10.3389/fnbeh.2018.00127
11. Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. psychol Bull (2004) 130(3):355–91. doi: 10.1037/0033-2909.130.3.355
12. Crielaard L, Nicolaou M, Sawyer A, Quax R, Stronks K. Understanding the impact of exposure to adverse socioeconomic conditions on chronic stress from a complexity science perspective. BMC Med (2021) 19(1). doi: 10.1186/s12916-021-02106-1
13. Baum A, Cohen L, Hall M. Control and intrusive memories as possible determinants of chronic stress. Psychosomatic Med (1993) 55(3):274–86. doi: 10.1097/00006842-199305000-00005
14. McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann New York Acad Sci (2004) 1032(1):1–7. doi: 10.1196/annals.1314.001
15. McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev (2007) 87(3):873–904. doi: 10.1152/physrev.00041.2006
16. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci (2009) 10(6):434–45. doi: 10.1038/nrn2639
17. Dhabhar FS. The short-term stress response – mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front Neuroendocrinol (2018) 49:175–92. doi: 10.1016/j.yfrne.2018.03.004
18. Lupien SJ, Juster RP, Raymond C, Marin MF. The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol (2018) 49:91–105. doi: 10.1016/j.yfrne.2018.02.001
19. Rohleder N. Stress and inflammation – the need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology (2019) 105:164–71. doi: 10.1016/j.psyneuen.2019.02.021
20. Petrie K, Milligan-Saville J, Gayed A, Deady M, Phelps A, Dell L, et al. Prevalence of PTSD and common mental disorders amongst ambulance personnel: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol (2018) 53(9):897–909. doi: 10.1007/s00127-018-1539-5
21. Dhama K, Latheef SK, Dadar M, Samad HA, Munjal A, Khandia R, et al. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci (2019) 6:91. doi: 10.3389/fmolb.2019.00091
22. Merabet N, Lucassen PJ, Crielaard L, Stronks K, Quax R, Sloot PMA, et al. How exposure to chronic stress contributes to the development of type 2 diabetes: a complexity science approach. Front Neuroendocrinol (2022) 65:100972. doi: 10.1016/j.yfrne.2021.100972
23. Almuneef M, Qayad M, Aleissa M, Albuhairan F. Adverse childhood experiences, chronic diseases, and risky health behaviors in Saudi Arabian adults: a pilot study. Child Abuse Negl (2014) 38(11):1787–93. doi: 10.1016/j.chiabu.2014.06.003
24. Harris ML, Oldmeadow C, Hure A, Luu J, Loxton D, Attia J. Stress increases the risk of type 2 diabetes onset in women: a 12-year longitudinal study using causal modelling. PloS One (2017) 12(2). doi: 10.1371/journal.pone.0172126
25. El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann Clin Biochem (2017) 54(3):308–22. doi: 10.1177/0004563216687335
26. Richter-Levin G, Xu L. How could stress lead to major depressive disorder? IBRO Rep (2018) 4:38–43. doi: 10.1016/j.ibror.2018.04.001
27. Hussenoeder FS, Conrad I, Pabst A, Luppa M, Stein J, Engel C, et al. Different areas of chronic stress and their associations with depression. Int J Environ Res Public Health (2022) 19(14):8773. doi: 10.3390/ijerph19148773
28. Planchez B, Surget A, Belzung C. Animal models of major depression: drawbacks and challenges. J Neural Transm (2019) 126(11):1383–408. doi: 10.1007/s00702-019-02084-y
29. Nandam LS, Brazel M, Zhou M, Jhaveri DJ. Cortisol and major depressive disorder–translating findings from humans to animal models and back. Front Psychiatry (2020) 10:974(10). doi: 10.3389/fpsyt.2019.00974
30. Becker M, Pinhasov A, Ornoy A. Animal models of depression: what can they teach us about the human disease? Diagnostics (2021) 11(1):123. doi: 10.3390/diagnostics11010123
31. Perić I, Costina V, Stanisavljević A, Findeisen P, Filipović D. Proteomic characterization of hippocampus of chronically socially isolated rats treated with fluoxetine: depression-like behaviour and fluoxetine mechanism of action. Neuropharmacology (2018) 135:268–83. doi: 10.1016/j.neuropharm.2018.03.034
32. Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology (2008) 33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008
33. Watt T, Kim S, Ceballos N, Norton C. People who need people: the relationship between adverse childhood experiences and mental health among college students. J Am Coll Health (2020) 70(4):1–9. doi: 10.1080/07448481.2020.1791882
34. Mall S, Mortier P, Taljaard L, Roos J, Stein DJ, Lochner C. The relationship between childhood adversity, recent stressors, and depression in college students attending a south African university. BMC Psychiatry (2018) 18:63. doi: 10.1186/s12888-017-1583-9
35. Escher CM, Sannemann L, Jessen F. Stress and alzheimer’s disease. J Neural Transm (2019) 126(9):1155–61. doi: 10.1007/s00702-019-01988-z
36. Bougea A, Anagnostouli M, Angelopoulou E, Spanou I, Chrousos G. Psychosocial and trauma-related stress and risk of dementia: a meta-analytic systematic review of longitudinal studies. J Geriatric Psychiatry Neurol (2020) 35(1):24–37. doi: 10.1177/0891988720973759
37. Stuart KE, Padgett C. A systematic review of the association between psychological stress and dementia risk in humans. J Alzheimer’s Dis (2020) 78(1):335–52. doi: 10.3233/jad-191096
38. Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet (2014) 5:88. doi: 10.3389/fgene.2014.00088
39. Hoeijmakers L, Ruigrok SR, Amelianchik A, Ivan D, van Dam AM, Lucassen PJ, et al. Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an alzheimer’s disease mouse model. Brain Behavior Immun (2017) 63:160–75. doi: 10.1016/j.bbi.2016.12.023
40. Dominguez S, Rodriguez G, Fazelinia H, Ding H, Spruce L, Seeholzer SH, et al. Sex differences in the phosphoproteomic profiles of app/ps1 mice after chronic unpredictable mild stress. J Alzheimer’s Dis (2020) 74(4):1131–42. doi: 10.3233/jad-191009
41. Han B, Wang J-H, Geng Y, Shen L, Wang H-L, Wang Y-Y, et al. Chronic stress contributes to cognitive dysfunction and hippocampal metabolic abnormalities in app/ps1 mice. Cell Physiol Biochem (2017) 41(5):1766–76. doi: 10.1159/000471869
42. Koyanagi A, Oh H, Vancampfort D, Carvalho AF, Veronese N, Stubbs B, et al. nt among 32,715 community-dwelling older adults across six low- and middle-income countries. Gerontology (2019) 65(2):155–63. doi: 10.1159/000492177
43. James KA, Grace LK, Pan CY, Combrinck MI, Thomas KGF. Psychosocial stress associated with memory performance in older south African adults. Aging Neuropsychol Cogn (2019) 27(4):553–66. doi: 10.1080/13825585.2019.1645809
44. Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, et al. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain (2010) 133(8):2217–24. doi: 10.1093/brain/awq116
45. Mehta M, Whyte E, Lenze E, Hardy S, Roumani Y, Subashan P, et al. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. Int J Geriatric Psychiatry (2008) 23(3):238–43. doi: 10.1002/gps.1868
46. Mlinac ME, Schwabenbauer A. Psychological resilience. Resilience Aging (2018), 81–104. doi: 10.1007/978-3-030-04555-5_5
47. Kalisch R, Müller MB, Tüscher O. A conceptual framework for the neurobiological study of resilience. Behav Brain Sci (2015) 38:1–79. doi: 10.1017/s0140525x1400082x
48. Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. J Pers Soc Psychol (2006) 91(4):730–49. doi: 10.1037/0022-3514.91.4.730
49. MacLeod S, Musich S, Hawkins K, Alsgaard K, Wicker ER. The impact of resilience among older adults. Geriatric Nurs (2016) 37(4):266–72. doi: 10.1016/j.gerinurse.2016.02.014
50. Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry (2019) 86(6):421–32. doi: 10.1016/j.biopsych.2019.04.028
51. Wilcoxen TE, Boughton RK, Bridge ES, Rensel MA, Schoech SJ. Age-related differences in baseline and stress-induced corticosterone in Florida scrub-jays. Gen Comp Endocrinol (2011) 173(3):461–6. doi: 10.1016/j.ygcen.2011.07.007
52. Elliott KH, O’Reilly KM, Hatch SA, Gaston AJ, Hare JF, Anderson WG. The prudent parent meets old age: A high stress response in very old seabirds supports the terminal restraint hypothesis. Hormones Behav (2014) 66(5):828–37. doi: 10.1016/j.yhbeh.2014.11.001
53. Lendvai Á.Z., Giraudeau M, Chastel O. Reproduction and modulation of the stress response: an experimental test in the house sparrow. Proc R Soc B: Biol Sci (2006) 274(1608):391–7. doi: 10.1098/rspb.2006.3735
54. Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: stress and resilience in older adults. Neurosci Biobehav Rev (2016) 68:928–45. doi: 10.1016/j.neubiorev.2016.05.036
55. Scott SB, Sliwinski MJ, Mogle JA, Almeida DM. Age, stress, and emotional complexity: Results from two studies of daily experiences. Psychol Aging (2014) 29(3):577–87. doi: 10.1037/a0037282
56. Bangasser DA, Eck SR, Telenson AM, Salvatore M. Sex differences in stress regulation of arousal and cognition. Physiol Behav (2018) 187:42–50. doi: 10.1016/j.physbeh.2017.09.025
57. Weisbrod AS, Barry ES, Graham AM, Eklund M, Grunberg NE. Decreased BDNF in female but not male rats after exposure to stress: a sex-sensitive rat model of stress? Stress (2019) 22(5):581–91. doi: 10.1080/10253890.2019.1617692
58. Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci (2007) 2(3):227–39. doi: 10.1093/scan/nsm018
59. Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcoholism: Clin Exp Res (2008) 32(7):1242–50. doi: 10.1111/j.1530-0277.2008.00679.x
60. Cohen S, Janicki-Deverts D. Who’s stressed? distributions of psychological stress in the united states in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol (2012) 42(6):1320–34. doi: 10.1111/j.1559-1816.2012.00900.x
61. Matud MP. Gender differences in stress and coping styles. Pers Individ Dif (2004) 37(7):1401–15. doi: 10.1016/j.paid.2004.01.010
62. Trzaskowski M, Mehta D, Peyrot WJ, Hawkes D, Davies D, Howard DM, et al. Quantifying between-cohort and between-sex genetic heterogeneity in major depressive disorder. Am J Med Genet Part B: Neuropsychiatr Genet (2019) 180(6):439–47. doi: 10.1002/ajmg.b.32713
63. Fonkoue IT, Michopoulos V, Park J. Sex differences in post-traumatic stress disorder risk: autonomic control and inflammation. Clin autonomic Res Off J Clin Autonomic Res Soc (2020) 30(5):409–21. doi: 10.1007/s10286-020-00729-7
64. Herbison CE, Allen K, Robinson M, Newnham J, Pennell C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev Psychopathol (2017) 29(4):1443–54. doi: 10.1017/S0954579417000372
65. Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol (2012) 233(1):102–11. doi: 10.1016/j.expneurol.2011.10.032
66. Barrero-Castillero A, Morton SU, Nelson CA, Smith VC. Psychosocial stress and adversity: Effects from the perinatal period to adulthood. Neoreviews (2019) 20(12):e686–96. doi: 10.1542/neo.20-12-e686
67. Gomes FV, Zhu X, Grace AA. Correction: the pathophysiological impact of stress on the dopamine system is dependent on the state of the critical period of vulnerability. Mol Psychiatry (2019) 25(12):3449–9. doi: 10.1038/s41380-019-0527-9
68. Nelson CA, Gabard-Durnam LJ. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci (2020) 43(3):133–43. doi: 10.1016/j.tins.2020.01.002
69. Schalinski I, Breinlinger, Hirt V, Teicher M, Odenwald M, Rockstroh B. Environmental adversities and psychotic symptoms: The impact of timing of trauma, abuse, and neglect. Schizophr Res (2019) 205:4–9. doi: 10.1016/j.schres.2017.10.034
70. Sapolsky RM. Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology, Cambridge, Massachusetts: MIT Press (2002). p. 409–50.
71. Allen AP, Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci Biobehav Rev (2014) 38:94–124. doi: 10.1016/j.neubiorev.2013.11.005
72. Leistner C, Menke A. Hypothalamic-pituitary-adrenal axis and stress. Handb Clin Neurol (2020) 175:55–64. doi: 10.1016/B978-0-444-64123-6.00004-7
73. Evans AN, Liu Y, Macgregor R, Huang V, Aguilera G. Regulation of hypothalamic corticotropin-releasing hormone transcription by elevated glucocorticoids. Mol Endocrinol (Baltimore Md.) (2013) 27(11):1796–807. doi: 10.1210/me.2013-1095
74. Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of ACTH and cortisol secretion and implications for disease. Endocrine Rev (2020) 41(3):470–90. doi: 10.1210/endrev/bnaa002
75. Morgan CA, Southwick S, Hazlett G, Rasmusson A, Hoyt G, Zimolo Z, et al. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry (2004) 61(8):819. doi: 10.1001/archpsyc.61.8.819
76. Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-s response to acute psychosocial stress in healthy men and women. Biol Psychol (2012) 90(2):143–9. doi: 10.1016/j.biopsycho.2012.03.003
77. Kinlein S, Wilson C, Karatsoreos I. Dysregulated hypothalamic–pituitary–adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry (2015) 6:31. doi: 10.3389/fpsyt.2015.00031
78. Kaur S, Teoh AN, Shukri NHM, Shafie SR, Bustami NA, Takahashi M, et al. Circadian rhythm and its association with birth and infant outcomes: research protocol of a prospective cohort study. BMC Pregnancy Childbirth (2020) 20(1). doi: 10.1186/s12884-020-2797-2
79. Wong SD, Wright KP, Spencer RL, Vetter C, Hicks LM, Jenni OG, et al. Development of the circadian system in early life: maternal and environmental factors. J Physiol Anthropol (2022) 41(1). doi: 10.1186/s40101-022-00294-0
80. Serón-Ferré M, Mendez N, Abarzua-Catalan L, Vilches N, Valenzuela FJ, Reynolds HE, et al. Circadian rhythms in the fetus. Mol Cell Endocrinol (2012) 349(1):68–75. doi: 10.1016/j.mce.2011.07.039
81. Ivars K, Nelson N, Theodorsson A, Theodorsson E, Ström JO, Mörelius E. Correction: development of salivary cortisol circadian rhythm and reference intervals in full-term infants. PloS One (2016) 11(3):e0151888. doi: 10.1371/journal.pone.0151888
82. Ivars K, Nelson N, Theodorsson A, Theodorsson E, Ström JO, Mörelius E. Development of salivary cortisol circadian rhythm in preterm infants. PloS One (2017) 12(8):e0182685. doi: 10.1371/journal.pone.0182685
83. Hofstra WA, de Weerd AW. How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav (2008) 13(3):438–44. doi: 10.1016/j.yebeh.2008.06.002
84. Zisapel N, Tarrasch R, Laudon M. The relationship between melatonin and cortisol rhythms: clinical implications of melatonin therapy. Drug Dev Res (2005) 65(3):119–25. doi: 10.1002/ddr.20014
85. Popp J, Schaper K, Kölsch H, Cvetanovska G, Rommel F, Klingmüller D, et al. CSF cortisol in alzheimer’s disease and mild cognitive impairment. Neurobiol Aging (2009) 30(3):498–500. doi: 10.1016/j.neurobiolaging.2007.07.007
86. Wang LY, Raskind MA, Wilkinson CW, Shofer JB, Sikkema C, Szot P, et al. Associations between CSF cortisol and CSF norepinephrine in cognitively normal controls and patients with amnestic MCI and AD dementia. Int J Geriatric Psychiatry (2018) 33(5):763–8. doi: 10.1002/gps.4856
87. Brossaud J, Ducint D, Gatta B, Molimard M, Tabarin A, Corcuff J. Urinary cortisol metabolites in corticotroph and adrenal tumours. Endocrine Abstracts (2012) 29.
88. Russell E, Koren G, Rieder M, Van Uum SHM. The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Ther Drug Monit (2014) 36(1):1. doi: 10.1097/ftd.0b013e31829daa0a
89. Torrente-Rodríguez RM, Tu J, Yang Y, Min J, Wang M, Song Y, et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mhealth system. Matter (2020) 2(4):921–37. doi: 10.1016/j.matt.2020.01.021
90. Venugopal M, Arya SK, Chornokur G, Bhansali S. A realtime and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sensors Actuators A: Phys (2011) 172(1):154–60. doi: 10.1016/j.sna.2011.04.028
91. Shukur HH, de Rijke YB, van Rossum EFC, Hussain-Alkhateeb L, Höybye C. Hair cortisol-a method to detect chronic cortisol levels in patients with prader-willi syndrome. BMC Endocrine Disord (2020) 20(1). doi: 10.1186/s12902-020-00646-w
92. Davison B, Singh GR, Oguoma VM, McFarlane J. Fingernail cortisol as a marker of chronic stress exposure in indigenous and non-indigenous young adults. Stress (2019) 23:1–10. doi: 10.1080/10253890.2019.1683159
93. Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem: Int J Biochem Lab Med (1983) 20(6):329–35. doi: 10.1177/000456328302000601
94. Schmidt NA. Salivary cortisol testing in children. Issues Compr Pediatr Nurs (1997) 20(3):183–90. doi: 10.3109/01460869709028262
95. Garde AH, Hansen Å.M. Long-term stability of salivary cortisol. Scandinavian J Clin Lab Invest (2005) 65(5):433–6. doi: 10.1080/00365510510025773
96. Kiess W, Meidert A, Dressendörfer RA, Schriever K, Kessler U, Köunig A, et al. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res (1995) 37(4):502–6. doi: 10.1203/00006450-199504000-00020
97. Pritchard BT, Stanton W, Lord R, Petocz P, Pepping GJ. Factors affecting measurement of salivary cortisol and secretory immunoglobulin a in field studies of athletes. Front Endocrinol (2017) 8:168. doi: 10.3389/fendo.2017.00168
98. al'Absi M, Lovallo WR. Caffeine's effects on the human stress axis. In: Nehlig A, editor. Coffee, tea, chocolate, and the brain. Florida, United States: CRC Press/Routledge/Taylor and Francis Group (2004). p. 113–31.
99. Lovallo WR, Whitsett TL, al’Absi M, Sung BH, Vincent AS. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosomatic Med (2005) 67(5):734–9. doi: 10.1097/01.psy.0000181270.20036.06
100. King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol (2006) 59(3):203–9. doi: 10.1016/j.ijpsycho.2005.10.008
101. Riordan FA, Thomson AP, Ratcliffe JM, Sills JA, Diver MJ, Hart CA. Admission cortisol and adrenocorticotrophic hormone levels in children with meningococcal disease: evidence of adrenal insufficiency? Crit Care Med (1999) 27(10):2257–61. doi: 10.1097/00003246-199910000-00032
102. Moffat SD, Yang An MS, Resnick SM, Diamon MP, Ferrucci L. Longitudinal change in cortisol levels across the adult life span. Journals Gerontol: Ser A (2019) 75(2):394–400. doi: 10.1093/gerona/gly279
103. Yiallouris A, Tsioutis C, Agapidaki E, Zafeiri M, Agouridis AP, Ntourakis D, et al. Adrenal aging and its implications on stress responsiveness in humans. Front Endocrinol (2019) 10:54. doi: 10.3389/fendo.2019.00054
104. Veldhuis JD, Sharma A, Roelfsema F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clinics North America (2013) 42(2):201–25. doi: 10.1016/j.ecl.2013.02.002
105. Roelfsema F, van Heemst D, Iranmanesh A, Takahashi P, Yang R, Veldhuis JD. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocrine Connections (2017) 6(7):500–9. doi: 10.1530/ec-17-0160
106. Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology (2001) 26(3):225–40. doi: 10.1016/s0306-4530(00)00043-3
107. Larsson CA, Gullberg B, Råstam L, Lindblad U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: a cross-sectional study. BMC Endocrine Disord (2009) 9(1). doi: 10.1186/1472-6823-9-16
108. de Souza-Talarico JN, de Marin MF, Sindi S, Lupien SJ. Effects of stress hormones on the brain and cognition: evidence from normal to pathological aging. Dementia Neuropsychol (2011) 5(1):8–16. doi: 10.1590/s1980-57642011dn05010003
109. Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier social stress test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology (1993) 28(1-2):76–81. doi: 10.1159/000119004
110. Patchev VK, Almeida OFX. Gender specificity in the neural regulation of the response to stress. Mol Neurobiol (1998) 16(1):63–77. doi: 10.1007/bf02740603
111. Kokras N, Hodes GE, Bangasser DA, Dalla C. Sex differences in the hypothalamic–pituitary–adrenal axis: an obstacle to antidepressant drug development? Br J Pharmacol (2019) 176(21):4090–106. doi: 10.1111/bph.14710
112. Stephens MAC, Mahon PB, McCaul ME, Wand GS. Hypothalamic–pituitary–adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology (2016) 66:47–55. doi: 10.1016/j.psyneuen.2015.12.021
113. Hidalgo V, Pulopulos MM, Puig-Perez S, Espin L, Gomez-Amor J, Salvador A. Acute stress affects free recall and recognition of pictures differently depending on age and sex. Behav Brain Res (2015) 292:393–402. doi: 10.1016/j.bbr.2015.07.011
114. Batabyal A, Bhattacharya A, Thaker M, Mukherjee S. A longitudinal study of perceived stress and cortisol responses in an undergraduate student population from India. PloS One (2021) 16(6):e0252579. doi: 10.1371/journal.pone.0252579
115. Sauro MD, Jorgensen RS, Teal Pedlow C. Stress, glucocorticoids, and memory: a meta-analytic review. Stress (2003) 6(4):235–45. doi: 10.1080/10253890310001616482
116. Hogenelst K, Soeter M, Kallen V. Ambulatory measurement of cortisol: where do we stand, and which way to follow? Sens Bio-Sensing Res (2019) 22:100249. doi: 10.1016/j.sbsr.2018.100249
117. Sánchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci (2000) 20(12):4657–68. doi: 10.1523/jneurosci.20-12-04657.2000
118. de Kloet ER, Datson NA, Revsin Y, Champagne DL, Oitzl MS. Brain Corticosteroid Receptor Function in Response to Psychosocial Stressors. In: Pfaff D., Kordon C., Chanson P., Christen Y. (eds) Hormones and Social Behaviour. Research and Perspectives in Endocrine Interactions. Berlin, Heidelberg: Springe. doi: 10.1007/978-3-540-79288-8_10
119. Shields GS, Sazma MA, McCullough AM, Yonelinas AP. The effects of acute stress on episodic memory: a meta-analysis and integrative review. psychol Bull (2017) 143(6):636–75. doi: 10.1037/bul0000100
120. Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci (2012) 15(11):1475–84. doi: 10.1038/nn.3234
121. Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J. Neurobiology of depression: an integrated view of key findings. Int J Clin Pract (2007) 61(12):2030–40. doi: 10.1111/j.1742-1241.2007.01602.x
122. Weaver ICG, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann New York Acad Sci (2004) 1024(1):182–212. doi: 10.1196/annals.1321.099
123. McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol (1995) 5(2):205–16. doi: 10.1016/0959-4388(95)80028-x
124. Wirth MM. Hormones, stress, and cognition: the effects of glucocorticoids and oxytocin on memory. Adaptive Hum Behav Physiol (2014) 1(2):177–201. doi: 10.1007/s40750-014-0010-4
125. Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NMK, Nair NPV. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology (2002) 27(3):401–16. doi: 10.1016/s0306-4530(01)00061-0
126. Joëls M, Krugers HJ. LTP after stress: up or down? Neural Plasticity (2007) 2007:1–6. doi: 10.1155/2007/93202
127. Žarković M, Stefanova E, Ćirić J, Penezić Z, Kostić V, Šumarac-Dumanović M, et al. Prolonged psychological stress suppresses cortisol secretion. Clin Endocrinol (2003) 59(6):811–6. doi: 10.1046/j.1365-2265.2003.01925.x
128. Cadegiani FA, Kater CE. Adrenal fatigue does not exist: a systematic review. BMC Endocrine Disord (2016) 16(1). doi: 10.1186/s12902-016-0128-4
129. Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology (2005) 30(10):1010–6. doi: 10.1016/j.psyneuen.2005.04.006
130. McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol (2008) 583(2-3):174–85. doi: 10.1016/j.ejphar.2007.11.071
131. McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism (2010) 59:S9–S15. doi: 10.1016/j.metabol.2010.07.012
132. Joëls M. Impact of glucocorticoids on brain function: relevance for mood disorders. Psychoneuroendocrinology (2011) 36(3):406–14. doi: 10.1016/j.psyneuen.2010.03.004
133. Gerritsen L, Comijs HC, Deeg DJH, Penninx BWJH, Geerlings MI. Salivary cortisol, APOE-ϵ4 allele and cognitive decline in a prospective study of older persons. Neurobiol Aging (2011) 32(9):1615–25. doi: 10.1016/j.neurobiolaging.2009.09.007
134. O’Donoghue MC, Murphy SE, Zamboni G, Nobre AC, Mackay CE. APOE genotype and cognition in healthy individuals at risk of alzheimer’s disease: a review. Cortex (2018) 104:103–23. doi: 10.1016/j.cortex.2018.03.025
135. Hankey G, Wardlaw J. Clinical neurology. London, United Kingdom: CRC Press (2008). doi: 10.1201/b17272
136. Podewils LJ. Physical activity, APOE genotype, and dementia risk: findings from the cardiovascular health cognition study. Am J Epidemiol (2005) 161(7):639–51. doi: 10.1093/aje/kwi092
137. Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, et al. Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. Am J Psychiatry (2008) 165(11):1456–64. doi: 10.1176/appi.ajp.2008.07091532
138. Gordon I, Ben-EliyahU S, Rosenne E, Sehayek E, Michaelson DM. Derangement in stress response of apolipoprotein e-deficient mice. Neurosci Lett (1996) 206(2):212–4. doi: 10.1016/S0304-3940(96)12470-8
139. Grootendorst J, Kempes MM, Lucassen PJ, Dalm S, de Kloet ER, Oitzl MS. Differential effect of corticosterone on spatial learning abilities in apolipoprotein e knockout and C57BL/6J mice. Brain Res (2002) 953(1-2):281–5. doi: 10.1016/s0006-8993(02)03399-1
140. Peskind ER, Wilkinson CW, Petrie EC, Schellenberg GD, Raskind MA. Increased CSF cortisol in AD is a function of APOE genotype. Neurology (2001) 56(8):1094–8. doi: 10.1212/wnl.56.8.1094
141. Raber J, Akana SF, Bhatnagar S, Dallman MF, Wong D, Mucke L. Hypothalamic–pituitary–adrenal dysfunction inapoe–/– mice: possible role in behavioral and metabolic alterations. J Neurosci (2000) 20(5):2064–71. doi: 10.1523/JNEUROSCI.20-05-02064.2000
142. Dose J, Huebbe P, Nebel A, Rimbach G. APOE genotype and stress response - a mini review. Lipids Health Dis (2016) 15(1). doi: 10.1186/s12944-016-0288-2
143. Montoliu T, Hidalgo V, Pulopulos MM, Ivorra JL, Martínez MJ. The relationship between cortisol and cognitive function in healthy older people: The moderating role of apolipoprotein e polymorphism. Neurobiol Learn Memory (2018) 155:297–305. doi: 10.1016/j.nlm.2018.08.013
144. Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, et al. Gene dose of apolipoprotein e type 4 allele and the risk of alzheimer’s disease in late onset families. Science (1993) 261(5123):921–3. doi: 10.1126/science.8346443
145. Martins CAR, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology (2005) 65(12):1888–93. doi: 10.1212/01.wnl.0000188871.74093.12
146. Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, et al. Tensor-based morphometry as a neuroimaging biomarker for alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. NeuroImage (2008) 43(3):458–69. doi: 10.1016/j.neuroimage.2008.07.013
147. Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, et al. The effects of prolonged stress and apoe genotype on memory and cortisol in older adults. Biol Psychiatry (2007) 62(5):472–8. doi: 10.1016/j.biopsych.2007.03.013
148. Shea A, Walsh C, MacMillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology (2005) 30(2):162–78. doi: 10.1016/j.psyneuen.2004.07.001
149. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci (2008) 31(9):464–8. doi: 10.1016/j.tins.2008.06.006
150. Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M. HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci (2021) 11(10):1298. doi: 10.3390/brainsci11101298
151. Bertollo A, Grolli RE, Plissari ME, Gasparin VA, Quevedo J, Réus GZ, et al. Stress and serum cortisol levels in major depressive disorder: a cross-sectional study. AIMS Neurosci (2020) 7(4):459–69. doi: 10.3934/neuroscience.2020028
152. Bijanki KR, Hodis B, Brumm MC, Harlynn EL, McCormick LM. Hippocampal and left subcallosal anterior cingulate atrophy in psychotic depression. PloS One (2014) 9(10):e110770. doi: 10.1371/journal.pone.0110770
153. Block TS, Kushner H, Kalin N, Nelson C, Belanoff J, Schatzberg A. Combined analysis of mifepristone for psychotic depression: plasma levels associated with clinical response. Biol Psychiatry (2018) 84(1):46–54. doi: 10.1016/j.biopsych.2018.01.008
154. Liu RT, Alloy LB. Stress generation in depression: a systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev (2010) 30(5):582–93. doi: 10.1016/j.cpr.2010.04.010
155. Hammen C. Generation of stress in the course of unipolar depression. J Abnormal Psychol (1991) 100(4):555–61. doi: 10.1037//0021-843x.100.4.555
156. Bahji A, Forth E, Hargreaves T, Harkness K. Genetic markers of the stress generation model: A systematic review. Psychiatry Res (2021) 304:114139. doi: 10.1016/j.psychres.2021.114139
157. Fischer JE, Calame A, Dettling AC, Zeier H, Fanconi S. Experience and endocrine stress responses in neonatal and pediatric critical care nurses and physicians. Crit Care Med (2000) 28(9):3281–8. doi: 10.1097/00003246-200009000-00027
158. Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current directions in stress and human immune function. Curr Opin Psychol (2015) 5(1):13–7. doi: 10.1016/j.copsyc.2015.03.007
159. Schultheiss OC, Wiemers US, Wolf OT. Exploring effects of hydrocortisone on implicit motivation and activity inhibition: a randomized placebo-controlled study. Adaptive Hum Behav Physiol (2016) 2(3):267–80. doi: 10.1007/s40750-016-0043-y
160. Timmers I, Kaas AL, Quaedflieg CWEM, Biggs EE, Smeets T, de Jong JR. Fear of pain and cortisol reactivity predict the strength of stress-induced hypoalgesia. Eur J Pain (2018) 22(7):1291–303. doi: 10.1002/ejp.1217
161. Het S, Schoofs D, Rohleder N, Wolf OT. Stress-induced cortisol level elevations are associated with reduced negative affect after stress. Psychosomatic Med (2012) 74(1):23–32. doi: 10.1097/psy.0b013e31823a4a25
162. Kristenson M, Garvin P, Lundberg U. The role of saliva cortisol measurement in health and disease. United Arab Emirates: Potomac MD: Bentham Books (2012). doi: 10.2174/97816080534211120101
163. Kudielka BM, von Känel R, Preckel D, Zgraggen L, Mischler K, Fischer JE. Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biol Psychol (2006) 72(2):147–53. doi: 10.1016/j.biopsycho.2005.09.001
164. Munawar M, Iftikhar PM, Hasan CA, Sohail CS, Rizvi SW. Neuropsychiatric manifestation of addison’s disease: A rare case report. Cureus (2019) 11:1–5. doi: 10.7759/cureus.4356
165. Abdel-Motleb M. The neuropsychiatric aspect of addison’s disease: a case report. Innov Clin Neurosci (2012) 9(10):34–6.
166. Crofford LJ, Pillemer SR, Kalogeras KT, Cash JM, Michelson D, Kling MA, et al. Hypothalamic–pituitary–adrenal axis perturbations in patients with fibromyalgia. Arthritis Rheumatism (1994) 37(11):1583–92. doi: 10.1002/art.1780371105
167. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology (2000) 25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9
168. Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nervous Ment Dis (1986) 174(3):145–9. doi: 10.1097/00005053-198603000-00003
169. Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nervous Ment Dis (1990) 178(6):366–9. doi: 10.1097/00005053-199006000-00004
170. Ye P, Kenyon CJ, MacKenzie SM, Nichol K, Seckl JR, Fraser R, et al. Effects of ACTH, dexamethasone, and adrenalectomy on 11β-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) gene expression in the rat central nervous system. J Endocrinol (2008) 196(2):305–11.
171. Holmes MC, Yau JLW, French KL, Seckl. JR. The effect of adrenalectomy on 5-hydroxytryptamine and corticosteroid receptor subtype messenger RNA expression in rat hippocampus. Neuroscience (1995) 64(2):327–37. doi: 10.1016/0306-4522(94)00407-V
172. de Jong IE, Oitzl MS, de Kloet ER. Adrenalectomy prevents behavioural sensitisation of mice to cocaine in a genotype-dependent manner. Behav Brain Res (2007) 177(2):329–39. doi: 10.1016/j.bbr.2006.11.015
173. Gallagher SF, Wahi M, Haines KL, Baksh K, Enriquez J, Lee TM, et al. Trends in adrenalectomy rates, indications, and physician volume: A statewide analysis of 1816 adrenalectomies. Surgery (2007) 142(6):1011–21. doi: 10.1016/j.surg.2007.09.024
174. Citton M, Viel G, Torresan F, Rossi GP, Iacobone M. Effect of unilateral adrenalectomy on the quality of life of patients with lateralized primary aldosteronism. BMC Surg (2019) 18(S1). doi: 10.1186/s12893-018-0432-1
175. Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci (1991) 11(3):585–99. doi: 10.1523/JNEUROSCI.11-03-00585.1991
176. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp (1932) 50:137–95. doi: 10.1002/j.1550-8528.1994.tb00097.x
177. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). J Neurosurg (1964) 21(4):318–47. doi: 10.3171/jns.1964.21.4.0318
178. Sonino N, Fava GA. Psychiatric disorders associated with cushing’s syndrome. CNS Drugs (2001) 15(5):361–73. doi: 10.2165/00023210-200115050-00003
179. Pivonello R, Simeoli C, De Martino MC, Cozzolino A, De Leo M, Iacuaniello D, et al.Neuropsychiatric disorders in cushing’s syndrome. Front Neurosci (2015) 9:129. doi: 10.3389/fnins.2015.00129
180. Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BMK, Colao A. Complications of cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol (2016) 4(7):611–29. doi: 10.1016/s2213-8587(16)00086-3
181. Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in cushing’s syndrome after correction of hypercortisolism. J Clin Endocrinol Metab (1997) 82(3):912–9. doi: 10.1210/jcem.82.3.3834
182. Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, et al. Mechanisms in endocrinology: Cushing’s syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur J Endocrinol (2015) 173(1):R1–R14. doi: 10.1530/eje-14-1101
183. Bourdeau I, Bard C, Noël B, Leclerc I, Cordeau M-P, Bélair M, et al. Loss of brain volume in endogenous cushing’s syndrome and its reversibility after correction of hypercortisolism. J Clin Endocrinol Metab (2002) 87(5):1949–54. doi: 10.1210/jcem.87.5.8493
184. O’Riordain DS, Farley DR, Young WF, Grant CS, van Heerden JA. Long-term outcome of bilateral adrenalectomy in patients with cushing’s syndrome. Surgery (1994) 116:1088–93.
185. Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in cushing’s syndrome. Neuroendocrinology (2010) 92(Suppl. 1):65–70. doi: 10.1159/000314317
186. Sandi C. Stress and cognition. Wiley interdisciplinary reviews. Cogn Sci (2013) 4(3):245–61. doi: 10.1002/wcs.1222
187. McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala and prefrontal cortex. Neuropsychopharmacology (2016) 41(1):3–23. doi: 10.1038/n2015.171
188. Atrooz F, Alkadhi KA, Salim S. Understanding stress: insights from rodent models. Curr Res Neurobiol (2021) 2:100013. doi: 10.1016/j.crneur.2021.100013
189. Jaggi AS, Bhatia N, Kumar N, Singh N, Anand P, Dhawan R. A review on animal models for screening potential anti-stress agents. Neurol Sci (2011) 32(6):993–1005. doi: 10.1007/s10072-011-0770-6
190. Zhu F, Nair RR, Fisher EMC, Cunningham TJ. Humanising the mouse genome piece by piece. Nat Commun (2019) 10(1). doi: 10.1038/s41467-019-09716-7
191. Hennessy MB, Willen RM, Schiml PA. Psychological stress, its reduction, and long-term consequences: what studies with laboratory animals might teach us about life in the dog shelter. Animals (2020) 10(11):2061. doi: 10.3390/ani10112061
192. Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci (2002) 3(6):453–62. doi: 10.1038/nrn849
193. Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress (2012) 15(1):11–20. doi: 10.3109/10253890.2011.572207
194. Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, et al. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry (2013) 73(7):658–66. doi: 10.1016/j.biopsych.2012.10.023
195. Mehta M, Schmauss C. Strain-specific cognitive deficits in adult mice exposed to early life stress. Behav Neurosci (2011) 125(1):29–36. doi: 10.1037/a0021952
196. Adler SM, Schmauss C. Cognitive deficits triggered by early life stress: the role of histone deacetylase 1. Neurobiol Dis (2016) 94:1–9. doi: 10.1016/j.nbd.2016.05.018
197. Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron (2012) 75(5):747–61. doi: 10.1016/j.neuron.2012.08.016
198. Lesuis SL, Hoeijmakers L, Korosi A, de Rooij SR, Swaab DF, Kessels HW, et al. Vulnerability and resilience to alzheimer’s disease: early life conditions modulate neuropathology and determine cognitive reserve. Alzheimer’s Res Ther (2018) 10(1):95. doi: 10.1186/s13195-018-0422-7
199. Tanaka T, Hirai S, Hosokawa M, Saito T, Sakuma H, Saido T, et al. Early-life stress induces the development of alzheimer’s disease pathology via angiopathy. Exp Neurol (2021) 337:113552. doi: 10.1016/j.expneurol.2020.113552
200. Liu H, Atrooz F, Salvi A, Salim S. Behavioral and cognitive impact of early life stress: Insights from an animal model. Prog Neuropsychopharmacol Biol Psychiatry (2017) 78:88–95. doi: 10.1016/j.pnpbp.2017.05.015
201. Heck AL, Handa RJ. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology (2019) 44(1):45–58. doi: 10.1038/s41386-018-0167-9
202. Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL, et al. Sex differences in the stress response in SD rats. Behav Brain Res (2015) 284:231–7. doi: 10.1016/j.bbr.2015.02.009
203. Vieira JO, Duarte JO, Costa-Ferreira W, Morais-Silva G, Marin MT, Crestani CC. Sex differences in cardiovascular, neuroendocrine and behavioral changes evoked by chronic stressors in rats. Prog Neuropsychopharmacol Biol Psychiatry (2018) 81:426–37. doi: 10.1016/j.pnpbp.2017.08.014
204. Babb JA, Masini CV, Day HEW, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic–pituitary–adrenocortical axis hormones following restraint in rats. Neuroscience (2013) 234:40–52. doi: 10.1016/j.neuroscience.2012.12.051
205. Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology (2002) 143(7):2534–40. doi: 10.1210/endo.143.7.8888
206. Babb JA, Masini CV, Day HEW, Campeau S. Habituation of hypothalamic–pituitary–adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress (2014) 17(3):224–34. doi: 10.3109/10253890.2014.905534
207. Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress (2004) 7(4):221–31. doi: 10.1080/10253890500070005
208. Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci (2015) 16(5):290–304. doi: 10.1038/nrn3918
209. Yoshii T, Oishi N, Ikoma K, Nishimura I, Sakai Y, Matsuda K, et al. Brain atrophy in the visual cortex and thalamus induced by severe stress in animal model. Sci Rep (2017) 7(1). doi: 10.1038/s41598-017-12917-z
210. Luine V, Martinez C, Villegas M, María Magariños A, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiol Behav (1996) 59(1):27–32. doi: 10.1016/0031-9384(95)02016-0
211. Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, et al. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex (2008) 19(9):1978–89. doi: 10.1093/cercor/bhn225
212. Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, et al. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci (2011) 31(40):14436–49. doi: 10.1523/jneurosci.3836-11.2011
213. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry (2012) 39(1):112–9. doi: 10.1016/j.pnpbp.2012.05.018
214. Costin BN, Wolen AR, Fitting S, Shelton KL, Miles MF. Role of adrenal glucocorticoid signaling in prefrontal cortex gene expression and acute behavioral responses to ethanol. Alcoholism: Clin Exp Res (2012) 37(1):57–66. doi: 10.1111/j.1530-0277.2012.01841.x
215. Reis FMCV, Almada RC, Fogaça MV, Brandão ML. Rapid activation of glucocorticoid receptors in the prefrontal cortex mediates the expression of contextual conditioned fear in rats. Cereb Cortex (2016) 26(6):2639–49. doi: 10.1093/cercor/bhv103
216. Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience (2009) 164(2):798–808. doi: 10.1016/j.neuroscience.2009.08.053
217. Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry (2014) 19(11):1171–8. doi: 10.1038/mp.2013.175
218. Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci (2002) 22(15):6810–8. doi: 10.1523/jneurosci.22-15-06810.2002
219. Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci (2006) 26(30):7870–4. doi: 10.1523/jneurosci.1184-06.2006
220. McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron (2013) 79(1):16–29. doi: 10.1016/j.neuron.2013.06.028
221. Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci (2012) 16(1):61–71. doi: 10.1016/j.tics.2011.12.011
222. McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry (2013) 74(9):672–9. doi: 10.1016/j.biopsych.2013.03.024
223. Pietrek C, Elbert T, Weierstall R, Müller O, Rockstroh B. Childhood adversities in relation to psychiatric disorders. Psychiatry Res (2013) 206(1):103–10. doi: 10.1016/j.psychres.2012.11.003
224. Juruena MF. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav (2014) 38:148–59. doi: 10.1016/j.yebeh.2013.10.020
225. Schiavone S, Colaianna M, Curtis L. Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress. Curr Pharm Design (2015) 21(11):1404–12. doi: 10.2174/1381612821666150105143358
226. Lu XT, Zhao YX, Zhang Y, Jiang F. Psychological stress, vascular inflammation, and atherogenesis. J Cardiovasc Pharmacol (2013) 62(1):6–12. doi: 10.1097/fjc.0b013e3182858fac
227. Seifan A, Schelke M, Obeng-Aduasare Y, Isaacson R. Early life epidemiology of alzheimer’s disease - a critical review. Neuroepidemiology (2015) 45(4):237–54. doi: 10.1159/000439568
228. Radford K, Delbaere K, Draper B, Mack HA, Daylight G, Cumming R, et al. Childhood stress and adversity is associated with late-life dementia in aboriginal australians. Am J Geriatric Psychiatry (2017) 25(10):1097–106. doi: 10.1016/j.jagp.2017.05.008
229. Sattler C, Toro P, Schönknecht P, Schröder J. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and alzheimer’s disease. Psychiatry Res (2012) 196(1):90–5. doi: 10.1016/j.psychres.2011.11.012
230. Leng Y, Wainwright NWJ, Hayat S, Stephan BCM, Matthews FE, Luben R, et al. The association between social stress and global cognitive function in a population-based study: the European prospective investigation into cancer (EPIC)-Norfolk study. psychol Med (2013) 43(3):655–66. doi: 10.1017/s0033291712001316
231. Vinski MT, Watter S. Being a grump only makes things worse: a transactional account of acute stress on mind wandering. Front Psychol (2013) 12:730(4). doi: 10.3389/fpsyg.2013.00730
232. Olver JS, Pinney M, Maruff P, Norman TR. Impairments of spatial working memory and attention following acute psychosocial stress. Stress Health (2015) 31:115–123. doi: 10.1002/smi.2533
233. Qi M, Gao H, Liu G. The effect of mild acute psychological stress on attention processing: an ERP study. Exp Brain Res (2018) 236:2061–71. doi: 10.1007/s00221-018-5283-6
234. Shields GS, Ramey MM, Slavich GM, Yonelinas AP. Determining the mechanisms through which recent life stress predicts working memory impairments: precision or capacity? Stress (2019) 22(2):280–5. doi: 10.1080/10253890.2018.1556635
235. Shields GS, Bonner JC, Moons WG. Does cortisol influence core executive functions? a meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology (2015) 58:91–103. doi: 10.1016/j.psyneuen.2015.04.017
236. He FX, Turnbull B, Kirshbaum MN, Phillips B, Klainin-Yobas P. Assessing stress, protective factors and psychological well-being among undergraduate nursing students. Nurse Educ Today (2018) 68:4–12. doi: 10.1016/j.nedt.2018.05.013
237. Panico F, Luciano SM, Sagliano L, Santangelo G, Trojano L. Cognitive reserve and coping strategies predict the level of perceived stress during COVID-19 pandemic: a cross-sectional study. Pers Individ Dif (2022) 195:111703. doi: 10.1016/j.paid.2022.111703
238. Stern Y. What is cognitive reserve? theory and research application of the reserve concept. J Int Neuropsychol Society: JINS (2002) 8(3):448–60. doi: 10.1017/S1355617702813248
239. Ihle A, Gouveia É.R., Gouveia BR, Orsholits D, Oris M, Kliegel M. Solving the puzzle of cognitive reserve effects on cognitive decline: the importance of considering functional impairment. Dementia Geriatric Cogn Disord (2020) 49(4):349–54. doi: 10.1159/000511768
240. Tun PA, Miller-Martinez D, Lachman ME, Seeman T. Social strain and executive function across the lifespan: The dark (and light) sides of social engagement. Aging Neuropsychol Cogn (2013) 20(3):320–38. doi: 10.1080/13825585.2012.707173
241. Gaffey AE, Wirth MM. Stress, rejection, and hormones: cortisol and progesterone reactivity to laboratory speech and rejection tasks in women and men. F1000Research (2014) 3:208. doi: 10.12688/f1000research.5142.2
242. Tschanz JT, Pfister R, Wanzek J, Corcoran C, Smith K, Tschanz BT, et al. Stressful life events and cognitive decline in late life: moderation by education and age. Cache County Study. Int J Geriatric Psychiatry (2013). doi: 10.1002/gps.3888
243. Herrera AY, Mather M. Actions and interactions of estradiol and glucocorticoids in cognition and the brain: Implications for aging women. Neurosci Biobehav Rev (2015) 55:36–52. doi: 10.1016/j.neubiorev.2015.04.005
244. Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatric Psychiatry (2011) 19(4):327–34. doi: 10.1097/jgp.0b013e31820119da
245. Popp J, Wolfsgruber S, Heuser I, Peters O, Hüll M, Schröder J, et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of alzheimer’s type. Neurobiol Aging (2015) 36(2):601–7. doi: 10.1016/j.neurobiolaging.2014.10.031
246. Machado A, Herrera AJ, de Pablos RM, Espinosa-Oliva AM, Sarmiento M, Ayala A, et al. Chronic stress as a risk factor for alzheimer’s disease. Rev Neurosci (2014) 25(6):785–805. doi: 10.1515/revneuro-2014-0035
247. Henry M, Thomas KGF, Ross IL. Sleep, cognition and cortisol in addison’s disease: A mechanistic relationship. Front Endocrinol (2021) 12:694046. doi: 10.3389/fendo.2021.694046
248. Calvo MG, Gutiérrez-García A. Cognition and stress. In: Stress: Concepts, cognition, emotion, and behavior (2016) (Sydney, Australia: Academic Press). p. 139–44. doi: 10.1016/b978-0-12-800951-2.00016-9
249. Sussams R, Schlotz W, Clough Z, Amin J, Simpson S, Abbott A, et al. Psychological stress, cognitive decline and the development of dementia in amnestic mild cognitive impairment. Sci Rep (2020) 10(1). doi: 10.1038/s41598-020-60607-0
250. Ouanes S, Castelao E, Gebreab S, von Gunten A, Preisig M, Popp J. Life events, salivary cortisol, and cognitive performance in nondemented subjects: a population-based study. Neurobiol Aging (2017) 51:1–8. doi: 10.1016/j.neurobiolaging.2016.11.014
251. Tatomir A, Micu C, Crivii C. The impact of stress and glucocorticoids on memory. Clujul Med (2014) 87(1):3–6. doi: 10.15386/cjm.2014.8872.871.at1cm2
252. Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Gudnason V, et al. Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology (2015) 85(11):976–83. doi: 10.1212/wnl.0000000000001931
253. Vogel S, Fernández G, Joëls M, Schwabe L. Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn Sci (2016) 20(3):192–203. doi: 10.1016/j.tics.2015.12.003
254. van Ast VA, Cornelisse S, Marin MF, Ackermann S, Garfinkel SN, Abercrombie HC. Modulatory mechanisms of cortisol effects on emotional learning and memory: Novel perspectives. Psychoneuroendocrinology (2013) 38(9):1874–82. doi: 10.1016/j.psyneuen.2013.06.012
255. Roozendaal B, McReynolds JR, van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci (2009) 29(45):14299–308. doi: 10.1523/jneurosci.3626-09.2009
256. Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience (2013) 239:196–213. doi: 10.1016/j.neuroscience.2012.08.065
257. Schilling TM, Kölsch M, Larra MF, Zech CM, Blumenthal TD, Frings C, et al. For whom the bell (curve) tolls: Cortisol rapidly affects memory retrieval by an inverted U-shaped dose–response relationship. Psychoneuroendocrinology (2013) 38(9):1565–72. doi: 10.1016/j.psyneuen.2013.01.001
258. Wu J, Yan J. Editorial: stress and cognition. Front Psychol (2017) 8:970. doi: 10.3389/fpsyg.2017.00970
259. Solas M, Aisa B, Mugueta MC, Del Río J, Tordera RM, Ramírez MJ. Interactions between age, stress and insulin on cognition: implications for alzheimer’s disease. Neuropsychopharmacology (2010) 35(8):1664–73. doi: 10.1038/n2010.13
260. Sindi S, Fiocco AJ, Juster RP, Pruessner J, Lupien SJ. When we test, do we stress? impact of the testing environment on cortisol secretion and memory performance in older adults. Psychoneuroendocrinology (2013) 38(8):1388–96. doi: 10.1016/j.psyneuen.2012.12.004
261. dos Santos AT, Leyendecker DMD, Costa ALS, de Souza-Talarico JN. Relationship between cortisol reactivity to psychosocial stress and declarative memory decline during aging: Impact of age and sex. Geriatrics Gerontol Int (2018) 18(1):169–76. doi: 10.1111/ggi.13139
262. Mikneviciute G, Ballhausen N, Rimmele U, Kliegel M. ). does older adults’ cognition particularly suffer from stress? a systematic review of acute stress effects on cognition in older age. Neurosci Biobehav Rev (2022) 132:583–602. doi: 10.1016/j.neubiorev.2021.12.009
263. Pietrzak RH, Laws SM, Lim YY, Bender SJ, Porter T, Doecke J, et al. Plasma cortisol, brain amyloid-β, and cognitive decline in preclinical alzheimer’s disease: a 6-year prospective cohort study. Biol Psychiatry: Cogn Neurosci Neuroimaging (2017) 2(1):45–52. doi: 10.1016/j.bpsc.2016.08.006
264. Diamond A. Executive functions. Annu Rev Psychol (2013) 64(1):135–68. doi: 10.1146/annurev-psych-113011-143750
265. Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci (2013) 7:123. doi: 10.3389/fnhum.2013.00123
266. Butler K, Klaus K, Edwards L, Pennington K. Elevated cortisol awakening response associated with early life stress and impaired executive function in healthy adult males. Hormones Behav (2017) 95:13–21. doi: 10.1016/j.yhbeh.2017.07.013
267. Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci Biobehav Rev (2016) 68:651–68. doi: 10.1016/j.neubiorev.2016.06.038
268. Lukasik KM, Waris O, Soveri A, Lehtonen M, Laine M. The relationship of anxiety and stress with working memory performance in a large non-depressed sample. Front Psychol (2019) 10:4(4). doi: 10.3389/fpsyg.2019.00004
269. Ennis GE, Moffat SD, Hertzog C. The cortisol awakening response and cognition across the adult lifespan. Brain Cogn (2016) 105:66–77. doi: 10.1016/j.bandc.2016.04.001
Keywords: cognition, cortisol, endocrinologist, hypothalamic - pituitary - adrenal axis, glucocorticoids, physiological stress, psychosocial stress, mental health
Citation: James KA, Stromin JI, Steenkamp N and Combrinck MI (2023) Understanding the relationships between physiological and psychosocial stress, cortisol and cognition. Front. Endocrinol. 14:1085950. doi: 10.3389/fendo.2023.1085950
Received: 31 October 2022; Accepted: 14 February 2023;
Published: 06 March 2023.
Edited by:
Hubert Vaudry, Université de Rouen, FranceReviewed by:
Damian Gabriel Zuloaga, University at Albany, United StatesCopyright © 2023 James, Stromin, Steenkamp and Combrinck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharine Ann James, a2F0aGphbWVzMUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.