
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 09 February 2023
Sec. Endocrinology of Aging
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1083722
This article is part of the Research TopicA Year in Review: Discussions in Endocrinology of AgingView all 5 articles
Purpose: To determine an alternative skeletal muscle index (a-SMI), easy diagnosis of sarcopenia in elderly patients with type 2 diabetes mellitus (T2DM).
Patients and methods: This cross-sectional study included 223 inpatients with T2DM (100 males, age range 60-89; 123 females, age range 60-87). Screening for grip strength and gait speed, measuring SMI by dual-energy X-ray absorptiometry (d-SMI) for sarcopenia diagnosis, according to the Asian Working Group for Sarcopenia (AWGS) 2019 consensus. The a-SMI was established by binary logistic regression analysis with positive screening population. To assess the conformance of the new diagnostic approach with the AWGS 2019.
Results: Sarcopenia was present in 36.3% of the study population. 59 had normal d-SMI and 81 had low d-SMI in screening patients with probable sarcopenia. In univariate analyses for all positive screening population, body mass index (BMI), 25-hydroxyvitamin D (25 - (OH) VitD), high density lipoprotein cholesterol (HDL-C), hypertension (HTN), and gender were correlates of d-SMI. Binary logistic regression analysis revealed that male (B = 2.463, 95%CI: 3.640 ~ 37.883, p = 0.000), HTN (B = 1.404, 95%CI: 1.599 ~ 10.371, p = 0.003), BMI (B = -0.344, 95%CI: 0.598 ~ 0.839, p = 0.000), 25-(OH) VitD (B = -0.058, 95%CI: 0.907 ~ 0.982, p = 0.004) were independent factors for d-SMI detection. Based on the extracted four correlates, the a-SMI was determined. The area under receiver operating characteristic (ROC) curve was 0.842, sensitivity and specificity for the new diagnostic approach were 84.0% and 84.5%. In a statistical measure of agreement between the AWGS 2019 and the new diagnostic approach, the kappa coefficient was 0.669 (p < 0.001).
Conclusion: The a-SMI - based on gender, obesity status, 25-(OH) VitD, and HTN history - can be used in the absence of the d-SMI to supplement the algorithm for sarcopenia diagnosis in elderly patients with T2DM.
Type 2 diabetes mellitus (T2DM) is an important health condition in a growing ageing population, approximately 25% of people over the age of 65 years have diabetes (1). Patients with T2DM often have accelerated muscle loss and multiple comorbidities, such as hypertension (HTN); overweight/obesity; hyperlipidemia; stroke; chronic kidney disease (CKD); and cardiovascular disease (CVD) (1, 2). T2DM is characterized by insulin resistance, inflammation, increased advanced glycation end-products (AGEs), and oxidative stress (3). These characteristics may lead to losses in skeletal muscle mass, strength and function, accelerating the development of sarcopenia (3). The previous study reported, besides microvascular and macrovascular complications, sarcopenia had been described as a new complication in the elderly population with T2DM (4). Sarcopenia was an aging and disease-related syndrome characterized by progressive and generalized loss of skeletal muscle mass, low muscle strength or low physical performance, with the risk of falls, frailty, fractures, disability, hospitalization and death (5, 6).
In detecting sarcopenia, algorithms required measuring muscle strength or physical performance and skeletal muscle mass (5, 6). To date, several imaging modalities, including dual-energy X-ray absorptiometry (DXA), computed tomography (CT), magnetic resonance imaging (MRI), bioimpedance analysis (BIA) and ultrasound (US), had been developed to measure skeletal muscle mass and achieve the diagnosis of sarcopenia (7, 8). Recently, DXA has been often used to estimate muscle mass in clinical practice, and also been the only radiological tool with accepted cutoff values to diagnose sarcopenia (9). CT and MRI were considered the gold standards, but their application was mostly limited to research (7). Radiation exposure is a major limitation. And clinical CT protocols were not standardized across hospital sites. Segmentation of continuous whole body MRI is too cumbersome and expensive for clinical practice (8). US had been always regarded as a minor tool in sarcopenia, and BIA had its weakness in low precision especially in patients who have chronic illness (9, 10). However, these imaging modalities had caveats in terms of cost, possible radiation exposure, and limited accessibility for primary care and field studies.
Recently, Ken-ichiro Sasaki et al. reported that the equation based on sex, weight, and calf circumference could predict muscle mass in patients with CVD (11). Furthermore, a Japanese study developed a simple anthropometric equation, which incorporated sex, height, weight, waist circumference (WC), and calf circumference, to be potential as a reliable and an effective substitute for estimating skeletal muscle index (SMI) in the local community (12). At present, there are still many gaps in sarcopenia research, the European Working Group on Sarcopenia in Older People (EWGSOP) 2018 consensus recommended seeking accurate, affordable and simple muscle quality assessment tools, to facilitate early detection and better treatment of sarcopenia in clinical practice (5). Therefore, we aimed to determine an alternative skeletal muscle index (a-SMI) based on simple measurement indexes, and to validate the diagnostic value of new diagnostic approach for sarcopenia in elderly patients with T2DM.
We performed a cross-sectional study in Chinese hospitalized patients over the age of 60 years with T2DM. The exclusion criteria included the following: 1) acute complications of diabetes mellitus such as diabetic ketoacidosis and hyperosmolar hyperglycemia; 2) acute myocardial infarction; acute cerebrovascular disease; acute inflammation; Gastrointestinal bleeding; Malignant tumor; 3) maintenance hemodialysis; 4) hepatic dysfunction (>3-fold elevation of alanine aminotransferase, aspartate aminotransferase); 5) severe osteoarthropathy or neuromuscular disease; 6) implantation of a pacemaker; 7) inability to understand/perform the exercise tests for this study; and 8) others judged ineligible by the investigators. This study was approved by the ethics committee of the First Hospital of Qinhuangdao. All subjects provided written informed consent before study initiation.
Sarcopenia was diagnosed by measuring skeletal muscle mass, muscle strength and physical performance according to the recommended diagnostic algorithm of the Asian Working Group for Sarcopenia 2019 consensus (AWGS 2019) (6). Sarcopenia was defined as low DXA-derived SMI (d-SMI) (< 7.0 kg/m2 in males; < 5.4 kg/m2 in females) associated with either low handgrip strength (< 28 kg in males; < 18 kg in females) or low gait speed (< 1.0 m/s) (6). Subjects were divided into sarcopenic and non-sarcopenic groups on this criterion.
With the use of predesigned questionnaires, we collected the following patient data: general data such as age and gender, and comorbidities, as well as the results of DXA, biochemical and anthropometric measurements.
We diagnosed multiple diabetes comorbidities according to Standards of Medical Care in Diabetes—2014 (13). Common comorbid conditions included HTN, fractures and falls, macrovascular diabetes complications consisted of CVD, stroke, and peripheral artery disease (PAD), and microvascular complications included diabetic kidney disease, diabetic retinopathy and neuropathy (13).
Anthropometric measurements, including height, weight, and WC, were obtained while the subjects were in light clothing and not wearing shoes. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). A commonly used gait speed test is called the 6-m usual walking speed test, with speed measured manually with a stopwatch. We measured grip strength with the Jamar dynamometer (Performance health supply, inc., Cedarburg, WI, USA), and took the maximum reading of at least 2 trials using either both hands or the dominant hand with the same standard.
Peripheral venous blood samples were taken at 8:00 AM after at least 8-hours of fasting, and subjected to biochemical measurements, including fasting blood glucose (FBG), glycated hemoglobin (HbA1c), albumin (ALB), triglycerides (TG), cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), Cr-glomerular filtration rate (GFR), uric acid (UA), and 25-hydroxyvitamin D (25 - (OH) VitD).
Body composition included bone mineral density (BMD), total body fat, total body lean were measured by using DXA (MEDILINK SARL., France). The d-SMI was calculated as follows: the formula =the sum of the lean amount of the bilateral upper limbs and the bilateral lower limbs (kg)/height2 (m2).
Data were analyzed using SPSS (version 23.0 for Windows, SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean (SD) and discrete variables were expressed as counts (percentages). Mutivariate logistic regression analysis was performed to determine independent factors for d-SMI, and established an alternative formula for d-SMI. The area under receiver operating characteristic (ROC) curve analysis was calculated to predict the diagnostic value of the a-SMI, and determined the cutoff value for a-SMI diagnosis. In a statistical measure of agreement between the AWGS 2019 and the new diagnostic approach based on a-SMI. Statistical significance was established at p < 0.05.
As shown in Table 1, 81 of 223 patients (36.3%) were diagnosed as having sarcopenia in elderly patients with T2DM. Sarcopenic patients were older, and included higher numbers of males and subjects with HTN as compared with non-sarcopenic patients (p < 0.05). BMI, handgrip strength, gait speed, 25 - (OH) VitD, ALB, HDL-C, d-SMI, and BMD of right femoral neck, were significantly lower in sarcopenic patients than those in non-sarcopenic patients (p < 0.05).
All participants were screened for handgrip strength and gait speed, and 140 positive screening population were found, which was a criterion in the first step to diagnose sarcopenia. 59 had normal d-SMI and 81 had low d-SMI in screening patients with probable sarcopenia, which was further diagnosed as sarcopenia. In univariate analyses for all positive screening population, BMI, 25 - (OH) VitD, HDL-C, HTN and gender were correlates of d-SMI (Table 2).
The dependent variable was whether positive screening population had lower d-SMI (normal d-SMI = 0, lower d-SMI = 1), and the independent variables were gender (female = 0, male = 1), 25- (OH) VitD, HTN (without = 0, with =1), BMI, HDL-C. As shown in Table 3, logistic analysis showed that male (B = 2.463, 95%CI: 3.640 ~ 37.883, p = 0.000), HTN (B = 1.404, 95%CI: 1.599 ~ 10.371, p = 0.003), BMI (B = -0.344, 95%CI: 0.598 ~ 0.839, p = 0.000) and 25 - (OH) VitD (B = -0.058, 95%CI: 0.907 ~ 0.982, p = 0.004) were independent factors for d-SMI. The HDL-C (B = -1.639, 95%CI: 0.035 ~ 1.090, p > 0.05) was not introduced in this study. Based on the extracted four correlates, a calculation formula for getting an a-SMI, which was the criterion in the second step to diagnose sarcopenia, was determined as follows: a-SMI = 2.463 x gender {(female = 0) or (male = 1)}+ 1.404 x HTN {(without = 0) or (with = 1)}+ {- 0.344 x (BMI, kg/m2)}+ {-0.058 x (25 - (OH) VitD, nmol/L)}+ 11.738
The a-SMI, obtained from the diagnostic regression formula, had a high accuracy in ROC curve analysis (sensitivity 84.0%, specificity 62.7%) when the area under ROC curve was 0.837 and the cutoff value for a-SMI diagnosis was 1.77 (Figure 1).
The a-SMI was the second step in the new diagnostic criteria for sarcopenia (Figure 2). The ROC curve was adopted to analyze the predictive value of the new diagnostic approach for sarcopenia in elderly patients with T2DM. The results showed that the area under ROC curve was 0.842, sensitivity was 84.0%, and specificity was 84.5% (Figure 3).
Lastly, in a statistical measure of agreement between the AWGS 2019 and the new diagnostic approach for all participants, the kappa coefficient was 0.669 (p < 0.001), indicating the two criteria were closely agreed (Table 4).
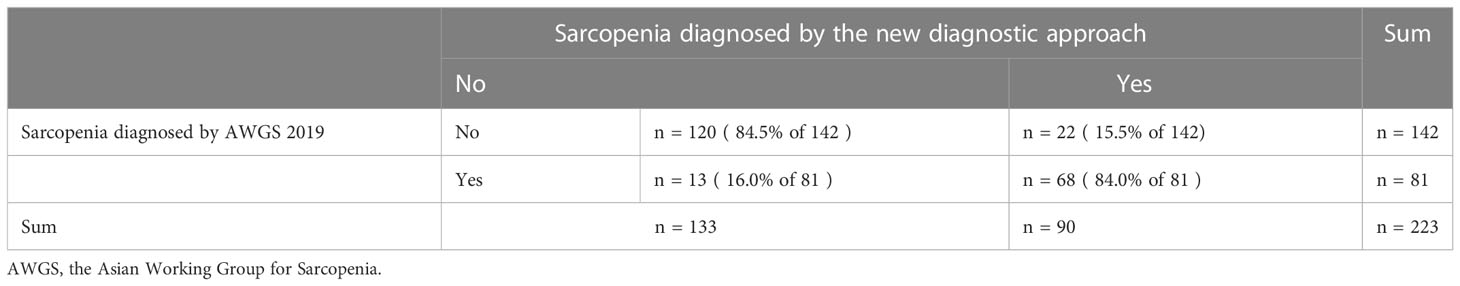
Table 4 In a statistical measure of agreement between the AWGS 2019 and the new diagnostic approach for all participants.
In the current study, we proposed a model to estimate d-SMI in positive screening population, which was calculated by a regression formula including the values of the subject’s sex, BMI, 25 - (OH) VitD, and HTN history, based on stepwise multivariate binomial logistic regression analysis. The new diagnostic approach developed in this study, was composed of handgrip strength, gait speed, and the a-SMI, could be used to supplement the algorithm for sarcopenia diagnosis in elderly patients with T2DM. For the diagnosis of sarcopenia, the prevalence of sarcopenia diagnosis by the new diagnostic method was 40.4%, and that was present in 36.3% according to the recommended diagnostic algorithm of the AWGS 2019. This indicated that the two criteria were in close agreement. And the a-SMI held potential as a reliable and an effective substitute in the absence of the d-SMI.
In our equation, a higher BMI level was associated with lower d-SMI. Previous studies reported that underweight subjects were at a higher risk of low skeletal muscle (14). A retrospective cross-sectional study included community-dwelling adults over 60 years of age suggested that obesity might have a protective effect in sarcopenic individuals (15). Several explanations had been proposed as to why obesity is not harmful to older adults (15). Overweight or obesity might be associated with a better supply of vitamins and other nutrients that ensure proper functioning (15). In addition, a higher BMI was associated with greater BMD, it might provide against osteoporotic fractures (16). Another important benefit of obesity in older adults was its association with higher levels of estrogen in this population (15). The estrogen produced by adipose tissue might exert protective effects on various organs through the effect of secretion. A Japanese study found elderly diabetes patients with low BMI and high body fat mass may be more likely to develop sarcopenia (17). Without taking body fat into account, subjects with high BMI tend to have more lean body mass (18, 19). Therefore, BMI might reflect lean mass in elderly diabetes patients (17).
Vitamin D played an important role in bone mineralization and musculoskeletal health (20). Our results had shown that 25 - (OH) VitD was significantly decreased in elderly patients with type 2 diabetic sarcopenia, and it was independent risk factor for d-SMI. It implied that 25 - (OH) VitD may play an important role in sarcopenia. The results of our study were in line with other studies where 25 - (OH) VitD was associated with muscle mass and function, increased the risk of sarcopenia in the elderly (21–23). A Korea study reported that sarcopenia was significantly associated with hypertension, particularly in the subjects with DM, and revealed that appendicular muscle mass (ASM) was independently related to SBP (24). HTN history in our study had adverse effects on d-SMI. Four possible mechanisms might explain the link between ageing muscle and HTN, including insulin resistance, inflammation, the relative paucity of myokines and alterations within the renin-angiotensin-aldosterone system (RAAS) (24–27).
Anagnostis et al. reported that the risk of sarcopenia were increased 1.72 -fold (95% CI: 1.1 ~ 2.69; p = 0.017) in male and 1.46 -fold (95% CI: 0.94 ~ 2.25; p = 0.08) in women with T2DM, and this difference between genders was not significant (28). In this study, the prevalence of sarcopenia in the T2DM patients aged 60 years was 19.7% for males and 16.6% for females. Males in our study had adverse effects on d-SMI. Testosterone levels in men decline with age, adversely affecting the distribution of muscle and adipose tissue (29). Earlier study had shown that men have more lean mass, and women have more fat mass. As people get older, men were more likely to accumulate adipose tissue around the trunk and abdomen, while women usually accumulate adipose tissue around the hips and thighs (30).
For the diagnosis of sarcopenia, it was necessary to measure grip strength, gait speed, and d-SMI. Grip strength and gait speed can be easily measured with small and simple instruments. We propose the a-SMI -based on gender, obesity status, 25-(OH) VitD, and HTN history - could be used in the absence of the d-SMI. Sarcopenia was diagnosed by the cutoff value of the a-SMI. Compared with MRI, CT and DXA, a-SMI can be performed in a small infirmary at a lower cost without radiation hazards. We did not define formulas by sex because we did not think it appropriate to define formulas based on data from a small number of men and women with sarcopenia. Nonetheless, the accuracy of the diagnosis of sarcopenia needs to be verified with surrogate indices and cut-off values in other populations.
There were some limitations in our study. First, due to the absence of CT and MRI (gold standards) for sarcopenia diagnosis, DXA recommended by AWGS 2019 was used in this study. The second concerns the formula, which calculated an abstract dimensionless proxy with quite different cut-off values, did not estimate muscle mass in kg or not a SMI in kg/m2. Third, it was a single-center study with a small population, limiting the ability to apply the result to the other ethnic groups, which needs to be further verified in other groups and institutions.
The present study demonstrates that the a-SMI - based on gender, obesity status, 25-(OH) VitD, and HTN history - can be used in the absence of the d-SMI to supplement the algorithm for sarcopenia diagnosis in elderly patients with T2DM. We believe that this new diagnostic approach of sarcopenia may be a useful tool for early prevention and early treatment of elderly T2DM patients with sarcopenia, thereby mitigating the adverse outcomes of T2DM patients due to sarcopenia.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the ethics committee of the First Hospital of Qinhuangdao. All subjects provided written informed consent before study initiation.
BL: Conceptualization, Funding Acquisition, Resources, Supervision, Writing - Review & Editing. LL: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft. All authors contributed to the article and approved the submitted version.
This work was supported by the People’s Livelihood Special Project of Science and Technology Department of Hebei Province (2037708D).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Diabetes Association. 12. Older adults: Standards of medical care in diabetes-2019. Diabetes Care (2019) 42(Suppl 1):S139–s147. doi: 10.2337/dc19-S012
2. Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin (2016) 32(7):1243–52. doi: 10.1185/03007995.2016.1168291
3. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab syndrome Obes Targets Ther (2019) 12:1057–72. doi: 10.2147/DMSO.S186600
4. Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc (2016) 17(9):846–51. doi: 10.1016/j.jamda.2016.07.007
5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing (2019) 48(4):16–31.
6. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc (2020) 21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012
7. Sergi G, Trevisan C, Veronese N, Lucato P, Manzato E. Imaging of sarcopenia. Eur J Radiol (2016) 85(8):1519–24. doi: 10.1016/j.ejrad.2016.04.009
8. Walowski CO, Braun W, Maisch MJ, Jensen B, Peine S, Norman K, et al. Reference values for skeletal muscle mass - current concepts and methodological considerations. Nutrients (2020) 12(3):755. doi: 10.3390/nu12030755
9. Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol (2020) 30(4):2199–208. doi: 10.1007/s00330-019-06573-2
10. Kim SW, Jung HW, Kim CH, Kim KI, Chin HJ, Lee H. A new equation to estimate muscle mass from creatinine and cystatin c. PloS One (2016) 11(2):e0148495. doi: 10.1371/journal.pone.0148495
11. Sasaki KI, Kakuma T, Sasaki M, Ishizaki Y, Fukami A, Enomoto M, et al. The prevalence of sarcopenia and subtypes in cardiovascular diseases, and a new diagnostic approach. J Cardiol (2020) 76(3):266–72. doi: 10.1016/j.jjcc.2020.03.004
12. Kawakami R, Miyachi M, Tanisawa K, Ito T, Usui C, Midorikawa T, et al. Development and validation of a simple anthropometric equation to predict appendicular skeletal muscle mass. Clin Nutr (2021) 40(11):5523–30. doi: 10.1016/j.clnu.2021.09.032
13. American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care (2014) 37 Suppl 1:S14–80. doi: 10.2337/dc14-S014
14. Lim J, Park HS. Relationship between underweight, bone mineral density and skeletal muscle index in premenopausal Korean women. Int J Clin Pract (2016) 70(6):462–8. doi: 10.1111/ijcp.12801
15. Bahat G, Kilic C, Ozkok S, Ozturk S, Karan MA. Associations of sarcopenic obesity versus sarcopenia alone with functionality. Clin Nutr (2021) 40(5):2851–9. doi: 10.1016/j.clnu.2021.04.002
16. Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: The framingham study. J Bone Miner Res (1993) 8(5):567–73. doi: 10.1002/jbmr.5650080507
17. Fukuoka Y, Narita T, Fujita H, Morii T, Sato T, Sassa MH, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig (2019) 10(2):322–30. doi: 10.1111/jdi.12908
18. Li C, Kang B, Zhang T, Gu H, Man Q, Song P, et al. High visceral fat area attenuated the negative association between high body mass index and sarcopenia in community-dwelling older Chinese people. Healthcare (Basel) (2020) 8(4):479. doi: 10.3390/healthcare8040479
19. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis (2008) 18(5):388–95. doi: 10.1016/j.numecd.2007.10.002
20. Cangussu LM, Nahas-Neto J, Orsatti CL, Bueloni-Dias FN, Nahas EA. Effect of vitamin d supplementation alone on muscle function in postmenopausal women: A randomized, double-blind, placebo-controlled clinical trial. Osteoporosis Int J established as Result cooperation between Eur Foundation Osteoporosis Natl Osteoporosis Foundation USA (2015) 26(10):2413–21. doi: 10.1007/s00198-015-3151-9
21. Visser M, Deeg DJ, Lips P. Low vitamin d and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The longitudinal aging study Amsterdam. J Clin Endocrinol Metab (2003) 88(12):5766–72. doi: 10.1210/jc.2003-030604
22. Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, van Loon LJ, De Groot LC. Low vitamin d status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr (2013) 67(10):1050–5. doi: 10.1038/ejcn.2013.144
23. Verlaan S, Maier AB, Bauer JM, Bautmans I, Brandt K, Donini LM, et al. Sufficient levels of 25-hydroxyvitamin d and protein intake required to increase muscle mass in sarcopenic older adults - the PROVIDE study. Clin Nutr (Edinburgh Scotland) (2018) 37(2):551–7. doi: 10.1016/j.clnu.2017.01.005
24. Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, et al. Sarcopenia as a determinant of blood pressure in older koreans: findings from the Korea national health and nutrition examination surveys (KNHANES) 2008-2010. PloS One (2014) 9(1):e86902. doi: 10.1371/journal.pone.0086902
25. Doğan MH, Karadag B, Ozyigit T, Kayaoglu S, Ozturk AO, Altuntas Y. Correlations between sarcopenia and hypertensive target organ damage in a Turkish cohort. Acta clinica Belgica (2012) 67(5):328–32. doi: 10.2143/ACB.67.5.2062685
26. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the national health and nutrition examination survey III. PloS One (2010) 5(5):e10805. doi: 10.1371/journal.pone.0010805
27. Burton LA, McMurdo ME, Struthers AD. Mineralocorticoid antagonism: a novel way to treat sarcopenia and physical impairment in older people? Clin Endocrinol (2011) 75(6):725–9. doi: 10.1111/j.1365-2265.2011.04148.x
28. Anagnostis P, Gkekas NK, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: A systematic review and meta-analysis. Calcif Tissue Int (2020) 107(5):453–63. doi: 10.1007/s00223-020-00742-y
29. Ji T, Li Y, Ma L. Sarcopenic obesity: An emerging public health problem. Aging Dis (2022) 13(2):379–88. doi: 10.14336/AD.2021.1006
Keywords: T2DM, sarcopenia, SMI, elderly, diagnosis
Citation: Lu L, Liu B and Yin F (2023) Alternative skeletal muscle index for sarcopenia diagnosis in elderly patients with type 2 diabetes mellitus: A pilot study. Front. Endocrinol. 14:1083722. doi: 10.3389/fendo.2023.1083722
Received: 29 October 2022; Accepted: 27 January 2023;
Published: 09 February 2023.
Edited by:
Marc R. Blackman, United States Department of Veterans Affairs, United StatesReviewed by:
Krzysztof Kusy, Poznan University of Physical Education, PolandCopyright © 2023 Lu, Liu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bowei Liu, bGl1Ym9fd2VpQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.